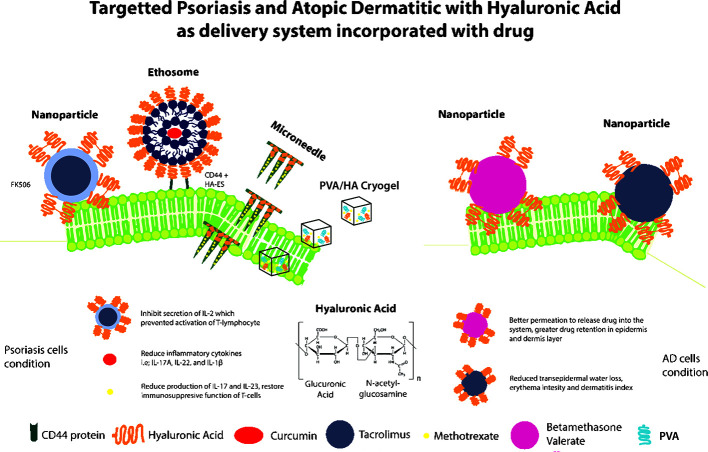
Abstract
Hyaluronic acid (HA), a major component of extracellular matrix has been widely applied in pharmaceutical and cosmetic industries due to its reported pharmacological properties. Various types of HA drug delivery system including nanoparticles, cryogel-based formulations, microneedle patches, and nano-emulsions were developed. There are studies reporting that several HA-based transdermal delivery systems exhibit excellent biocompatibility, enhanced permeability and efficient localized release of anti-psoriasis drugs and have shown to inhibit psoriasis-associated skin inflammation. Similarly HA is found in abundant at epidermis of atopic dermatitis (AD) suggesting its role in atopic AD pathology. Anti-allergenic effect of atopic eczema can be achieved through the inhibition of CD44 and protein kinase C alpha (PKCα) interaction by HA. Herein, we aim to evaluate the current innovation on HA drug delivery system and the other potential applications of HA in inflammatory skin diseases, focusing on atopic dermatitis and psoriasis. HA is typically integrated into different delivery systems including nanoparticles, liposomes, ethosomes and microneedle patches in supporting drug penetration through the stratum corneum layer of the skin. For instance, ethosomes and microneedle delivery system such as curcumin-loaded HA-modified ethosomes were developed to enhance skin retention and delivery of curcumin to CD44-expressing psoriatic cells whereas methotrexate-loaded HA-based microneedle was shown to enhance skin penetration of methotrexate to alleviate psoriasis-like skin inflammation. HA-based nanoparticles and pluronic F-127 based dual responsive (pH/temperature) hydrogels had been described to enhance drug permeation through and into the intact skin for AD treatment.
Keywords: hyaluronic acid, inflammatory skin diseases, psoriasis, atopic dermatitis, drug delivery, CD44 receptor
Graphical Abstract.
HA-mediated Drug Delivery System Targeting Inflammatory Skin Diseases (Psoriasis and Atopic Dermatitis).
Introduction
Skin as the first line of defense against infection is made up of three main layers which vary in functions and anatomy: epidermis (outer layer), dermis (middle layer) and hypodermis (bottom layer) (Prost-Squarcioni, 2006). As it interacts with environment, skin acts as a protector against diseases, excessive water loss, UV damage, mechanical injury, and also helps in regulate body temperature (Logger et al., 2019). Skin and subcutaneous diseases are the 4th leading cause of disability globally in 2013 excluding mortality, with dermatitis being the greatest burden among all costing approximately 9.3 million disability-adjusted life years (Karimkhani et al., 2017). Psoriasis is reported to affect 2% of worldwide population due to genetic and environmental factors (Mohd Affandi et al., 2018). Psoriasis is an inflammatory skin diseases which is chronic with strong genetic predisposition and autoimmune pathogenic traits (Du et al., 2019) that is causing burden to patient either physically or physiologically (Boehncke and Schön, 2015). For mild to moderate psoriasis, topical corticosteroids are the first-line treatment. However, long term application of topical corticosteroids can produce adverse effects such as epidermal thinning, atrophy, ulceration and facial erythema (Coondoo et al., 2014).
Atopic dermatitis (AD), a common chronic inflammatory skin disease poses a significant threat to both patient and the economy. It also affects the quality of individual life as well as their families (Kapur et al., 2018). The disease is believed to arise due to the complex interplay between skin barrier malfunction, immune dysregulation and infectious agents from environment factor (Kapur et al., 2018). In Canada, it was reported that an estimated cost of $1.4 billion annually is incurred to assist AD patient in getting treatment, like emollients application, as well as frequent physician visits which impacted the economy (Barbeau and Lalonde, 2006). AD has significant impact on the patients quality of life as there will be constant itchiness which can lead to skin trauma and sleep deprivation (Kapur et al., 2018).
CD44, a receptor for HA is found to be highly expressed in psoriatic skin. The concentration is negatively correlated with HA distribution (Zhang et al., 2019). It was found that anti-allergenic effect can be achieved through the inhibition of CD44 and PKCα interaction by HA (Kim et al., 2008). Based on the understanding of the characteristic of HA and HA-CD44 pathway, HA is widely used as a nano-drug delivery system to enhance the therapeutic effect of drug at the lesion site (Taetz et al., 2009). HA assisted drug delivery systems that has been described include nanoparticle, ethosomes and microneedle. It is an important vehicle to enhance drug-skin penetration and an important vehicle to retain moisture in AD in view of barrier dysfunction. The objective of this review is to review the role of HA as a drug delivery system in inflammatory skin diseases namely psoriasis and atopic dermatitis.
Structural and Functional Characteristics of Hyaluronic Acid
Hyaluronic acid (HA), a major component of extracellular matrix has been widely applied in pharmaceutical and cosmetic industries due to its reported pharmacological properties which includes anti-aging (Bukhari et al., 2018), anti-inflammatory (Chen, 2018), skin repairing (Narurkar et al., 2016), tissue regeneration (Bukhari et al., 2018) and wound recovery properties (Chen, 2018). HA possess good biocompatibility, high moisture retention, and tuneable viscoelastic properties (Sze et al., 2016). It is a natural unbranched polymer composed of repeating disaccharide units of D-glucuronic acid and N-acetylglucosamine linked by a glucuronidic β (1–3) bond. HA with different molecular weights exhibit distinct properties. For instance, low molecular weight HA (LMW-HA) is reported to be associated with promoting angiogenesis whereas high molecular weight HA (HMW-HA) inhibits angiogenesis. The appropriate balance between synthesis and degradation of HA is vital in the regulation of various biological functions including cell proliferation, migration, differentiation (Bychkov and Kuzmina, 2015), vasculogenesis and angiogenesis (Pardue et al., 2008), as well as regulating cell adhesion and motility (Kouvidi et al., 2011), as determined by their molecular weight. LMW-HA on the other hand exerts opposite effect as compared to HMW-HA where it demonstrates biological activities such as antioxidant properties and pronounced free radical scavenging has been developed in recent years (Ke et al., 2011). In addition, LMW-HA has also shown to induce inflammatory genes in T-24 carcinoma cells, in eosinophils (Ohkawara et al., 2000) as well as dendritic cells (Termeer et al., 2000). Both sizes of HA are safe and efficacious, and the market is expecting a continuing emergence of HA-based products in the coming years (Tabassum and Hamdani, 2014).
Psoriasis
Psoriasis is a chronic inflammatory autoimmune skin disease characterized by marked epidermal proliferation and abnormal differentiation with activation of both innate and acquired immunity (Rendon and Schäkel, 2019). This autoimmune disorder is multifactorial, and inflammation is known to play a major role in its development. Research have showed that activated Th1 and Th17 T cells (CD4+ T cells), CD8+ T cells, as well as increased levels of cytokines such as IL-17, IL-23, TNF-α and IL-27, have been directly implicated in psoriasis immunopathogenesis (Luger and Loser, 2018). The immunology and genetics studies of psoriasis have provided insights into the heterogeneity and regulatory pathways that govern psoriasis, and assist in the development of therapy based on patients biogenetic markers and identify new avenues for treatment based on a more complete understanding of the immunological mechanisms. Topical administration is one of the important approaches to treat psoriasis. Drugs are applied directly to the affected skin lesions to inhibit inflammatory symptoms of psoriasis. Topical administration of drugs with narrow therapeutic window can reduce systemic absorption compared to drugs delivered through oral and intravenous routes which help to reduce adverse systemic effects. Though topically applied drugs provide prolonged duration of action, the stratum corneum limits the amount of drugs being percutaneously absorbed, resulting in poor clinical efficacy. Hence, novel and improved percutaneous delivery system to overcome the stratum corneum barrier of psoriatic skin is vital to antipsoriatic drug for topical therapy.
HA as a Drug Delivery System in Psoriasis
Currently, HA is widely incorporated as part of the transdermal delivery system to enhance drug penetration in psoriasis treatment. Various forms of drug delivery systems including HA-based nanoparticles, ethosomes, cryogels, and microneedle patches have been developed to enhance drug permeation through and into the intact skin for psoriasis treatment. The excellent solubility properties of HA resulted in its development as one of the most important topical carriers for the localized delivery of drugs to the skin and also as a drug delivery agent for ophthalmic, nasal, pulmonary, parenteral and topical routes of administration (Brown and Jones, 2005). HA acts as mucoadhesive, retaining the drug at specific site of action/absorption. It also can modify the in vivo release and absorption rate of the therapeutic agent and it is able to localize delivery of drug to the epidermis. The types of psoriasis drugs that have been incorporated into these HA-based delivery systems include methotrexate, tacrolimus, and corticosteroids, all which are first-line treatments for moderate to severe psoriasis.
One of the novel nano-topical drug delivery system developed using HA-modified ethosomes target CD44 in the inflamed epidermis (Zhang et al., 2019). Ethosomes are novel deformable liposomes derived from dispersing liposomes in small-chain biocompatible alcohols and they are demonstrated to be more superior than classic liposomes which significantly increase skin retention of drugs (Touitou et al., 2000). The incorporation of curcumin in HA-modified ethosomes target CD44 in the inflamed epidermis (Zhang et al., 2019). Recent studies have found that CD44 protein is highly expressed in the epidermis of psoriatic inflamed skin, suggesting that CD44 can serve as a potential target of novel active-targeting nanocarriers for topical administration to increase skin drug retention and enhance drug efficacy (Lindqvist et al., 2012). The HA-modified ethosomes showed specific adhesion CD44 in imiquimod-induced psoriasis-like inflamed skin, and that the increased topical drug delivery of curcumin reduced TNF-α, IL-17A, IL-17F, IL-22, and IL-1β mRNA levels; and lower CCR6 protein expression.
Another formulated hybrid nanoparticle system based on the combination of amphiphilic conjugations of HA–Cholesterol-self-assembled nanoparticles and hydrotropic nicotinamide was developed to enhance the permeation of tacrolimus (FK506) in the treatment of psoriasis (Wan et al., 2017). Commercial FK506 is an ointment formulation use to treat atopic dermatitis, but it is applied to other skin diseases like psoriasis (Malecic and Young, 2016). FK506 is a type of macrolide immunosuppressive drug which prevents activation of T-lymphocyte through inhibition of IL-2, a cytokine that is responsible for the development and persistence of psoriasis lesions (Goebel et al., 2011). However, topical treatment of tacrolimus ointment on hyperkeratotic psoriatic plaques did not promote FK506 deposition due to its high hydrophobicity and high molecular weight (Zonnevdd et al., 1998). The combination of HA–Cholesterol nanoparticles exhibited a significant synergistic effect on the permeation of FK506 ointment and that it presented a synergistic effect on antipsoriasis which might increase the therapeutic effect and minimize systemic side effects (Wan et al., 2017).
Synthetic biodegradable polymers have significant versatility and diverse biomedical applications owing to their tailorable designs or modifications. Poly(vinyl alcohol) (PVA) is a water soluble biodegradable synthetic polymer with good biocompatibility, and it can be physically cross-linked by the freeze-thaw method (Stauffer and Peppast, 1992) to form hydrogels useful in pharmaceutical formulations. The development of PVA/HA cryogels loaded with methotrexate showed good in vivo biocompatibility after systemic and topical administration in laboratory animals. The pH-responsive swelling and releasing abilities of these PVA/HA cryogels allow these systems to be developed as topical formulations for psoriasis therapy.
Meanwhile, development of pH-responsive biodegradable poly-L-glutamic acid (PGA)-fluocinolone acetonide (FLUO) conjugate allows the controlled release of the FLUO to reduce skin inflammation (Dolz-Pérez et al., 2020). However, PGA-FLUO showed limitations in the release of drug in the epidermis which bring about the application of PGA-FLUO within hyaluronic acid (HA)-poly-L-glutamate cross polymer (HA-CP) resulted in slower and sustained drug transfer in the epidermis allowing sufficient residence time for drug activity (Dolz-Pérez et al., 2020).
Microneedle patch is a highly efficient and versatile device which attracted extensive scientific and industrial interests in the past decades due to prominent properties including painless penetration, low cost, excellent therapeutic efficacy, and relative safety. The robust microneedle enabling transdermal delivery has a paramount potential to create advanced functional devices with superior nature for biomedical applications. Methotrexate, an analogue of folic acid has been used as a drug to treat psoriasis skin condition (Czarnecka-Operacz and Sadowska-Przytocka, 2014). Methotrexate has been shown to down-regulate interleukin-17 and interferon-c, inhibit proliferation of effector T cells and restore the immunosuppressive function of regulatory T cells (Chen, 2018). Nevertheless, its high molecular weight, hydrophilicity and inability to maintain a stable form at physiological pH restricted the permeability of methotrexate by passive diffusion through the stratum corneum (Alvarez-Figueroa et al., 2001). The development of methotrexate-loaded HA-based microneedle patch successfully penetrated imiquimod (IMQ)-induced thickened epidermis in mice and alleviated the psoriasis-like skin inflammation (Du et al., 2019). In addition, the methotrexate-loaded HA-based microneedle patches were significantly more efficacious than taking the same dose of drug orally. This significantly reduced the amount of methotrexate required for the microneedle patch delivery system required for a comparable amelioration, which in turn lowered its systemic toxicity (Du et al., 2019).
HA as a Biomarker in Psoriasis
Serum HA had been utilized as a biomarker for psoriasis as studies reported that concentration of serum HA increases significantly in patients with serious cutaneous psoriasis (Yamamoto et al., 1997). This is due to the ability of low molecular weight HA (LMWHA) in inducing inflammatory cytokine gene expression and serving as a ligand for toll-like receptors (TLRs). In addition, presence of IL-1β, tumor necrosis factor-α (TNF-α) and degradative enzymes such as metalloproteinases will lead to an increase in hyaluronan synthetases (HAS) 1–3 and hyaluronidases that synthesize LMWHA by degrading HMWHA (Mehta et al., 2011; Hellman et al., 2019). In psoriasis, TLRs play a vital role as innate immune responses through activation of TLR7 and TLR9 and also the secretion of interleukin-23 (IL-23) via autoimmune plasmacytoid dendritic cell activation which releases interferon-a, that further signals Tip-DC (tumor necrosis factor-a and inducible nitric oxide synthase-producing dendritic cell) activation (Yamamoto, 2015). Other TLRs including TLR2 and TLR4 contribute to the pathogenesis of psoriasis plaque, guttate (Garcia-Rodriguez et al., 2013) and also psoriatic arthritis (Carrasco et al., 2011). HA is highly abundant in psoriatic skin, and it signals through TLR4 and TLR2. Meanwhile the binding of HA on CD44 also induces secretion of IL-6 and inflammation in T cells and neutrophils (Jiang et al., 2011).
Atopic Dermatitis
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease affecting infants and children (Siegfried and Hebert, 2015). Factors causing AD includes defect in terminal epithelial differentiation, immune dysregulation, altered skin microbiome and altered composition of stratum corneum (SC) intracellular lipids leading to barrier dysfunction (Bieber et al., 2017; Nakatsuji et al., 2017). Impairment of the SC permeability barrier functions cause dehydration due to an increase of transepidermal water loss, followed by inflammation due to the increasing release of cytokines, chemokines and interleukines (Damiani et al., 2019). Skin hydration, anti-bacterial measures and topical immunosuppressant therapy using topical corticosteroids calcineurin inhibitors are the common approaches in the management of AD (Sathishkumar and Moss, 2016).
HA’s Role in Atopic Dermatitis
HA is found to be related to inflammatory skin dermatoses (Ohtani et al., 2009; Barnes et al., 2012) The synthesis of HA is regulated by hyaluronan synthases (HAS). In normal circumstances, HAS1 is found to be regulating epidermal differentiation. However, role of HAS3 becomes apparent during pathological condition (Malaisse et al., 2014). The earlier studies provide conflicting evidences on HA role in epidermal differentiation (Maytin et al., 2004; Passi et al., 2004; Bourguignon et al., 2006; Farwick et al., 2011). Latest evidence by Malaisse et al. (2014) acknowledge the fact that though HA present at high level, it is not necessary for homeostatic epidermal differentiation (Malaisse et al., 2014). However, HA become important in pathological condition. During inflammation, HAS3 is upregulated while HAS1 is downregulated. Heparin binding- epidermal growth factor. (HB-EGF) is found to be possibly linked to HAS expression in AD lesions. However, how HAS involved in the pathomechanism of keratinocyte inflammation is still unclear and thus requiring further studies (Malaisse et al., 2014).
HA in Medical Device Moisturizers
The basic principle AD treatment is optimal skin care that address the skin barrier defect. Moisturizers restore the ability of lipid bilayer to restore, retain and redistribute water. They penetrate and contribute to the reorganization of the structure of the skin layers. Thus, moisturizers play an important role in AD management (Giam et al., 2016). Moisturizers can be categorized into three main groups, namely humectant, occlusive and emollients. These products are formulated in various delivery system including gel, foam, cream, ointment and lotion. They also contain various composition to enhance its efficacy. Humectants work by attracting fluids from the deeper epidermis to SC and has a biological similarities to the natural moisturizing factors of the corneocytes (Giam et al., 2016). HA being a natural component of the skin, has been one of the most favorite humectant used in almost all nutricosmetic products with moisturizing properties (Bukhari et al., 2018). It helps in attracting and retaining fluid, and skin barrier repair (Maytin et al., 2004; Goh et al., 2019). However it is rarely used primarily as a sole ingredient, but rather working synergistically with other category of moisturizer to achieve its role in skin repair (Draelos, 2011; Frankel et al., 2011; Pacha and Hebert, 2012; Micali et al., 2018). The molecular weight of HA shall be considered, as HA with a molecular weight higher than 50 kDa may reduce skin penetration. Thus, HMWHA is used to form a film to impede epidermal water loss, while LMWHA is utilized to improve skin penetration to restore a sustained physiological and hydrated microenvironment for optimize skin rejuvenation and tissue repair (Symonette et al., 2014).
HA as a Drug Delivery System in Atopic Dermatitis
Similar to psoriasis, various forms of drug delivery systems including HA-based nanoparticles and pluronic F-127 based dual responsive (pH/temperature) hydrogels have been described to enhance drug permeation through and into the intact skin for AD treatment. The types of drugs that have been incorporated into these HA-based delivery systems include tacrolimus, bethamethasone valerate and gallic acid, a principal component of traditional Chinese medicine Cortex Moutan.
Topical calcineurin inhibitor, namely tacrolimus ointment (Protropic®) and pimecrolimus cream (Elidel®) are steroid sparing agents for the treatment of atopic dermatitis. However the potency of tacrolimus 0.1% was only found to be equivalent to medium potency topical steroid(Eichenfield et al., 2014), possibly due to the limited skin penetration due to its large molecular size. A novel HA-coated tacrolimus-loaded nanoparticles (HA-TCS-CS-NPs) was described (Zhuo et al., 2018). It was noted that the physiochemical properties had been optimized, in vivo drug release was more sustained, drug permeation and retention had been improvised, and anti-AD efficacy in AD mice model was found to be superior in comparison with tacrolimus solution (Zhuo et al., 2018). However, this study did not compare their nanoparticles with the marketed Protropic®. Similar technology had been used to encapsulate betamethasone valerate (BMV), a medium potency topical corticosteroid. It was found that (HA-BMV-CS-NP) demonstrated optimum physicochemical characteristics, including fine particle size, high zeta potential, high entrapment efficacy and loading capacity. It also demonstrated higher drug permeation and drug retention(Pandey et al., 2019).
Pluronic F-127 (PF127) is a non-ionic triblock copolymer of poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide), thermo-responsive polymer capable to form gel from its aqueous solution. Chitosan is a cationic pH-responsive biopolymer that swells in acidic pH and shrinks in basic pH, while HA is an anionic pH-responsive biopolymer that can swell in basic pH and shrink in acidic pH (Chatterjee et al., 2019). The conjugation of these polymers formed a dual-responsive hydrogels [PF127/HA-Ala-Chito(oligo)]. It had been used to incorporate gallic acid, the principal component of traditional Chinese medicine Cortex Moutan, recommended in the treatment of atopic dermatitis. This anti-inflammatory and anti-allergic herbal medicine is claimed to have similar effect like corticosteroids without their side effects. PF127/HA-Ala-chito(oligo) drug delivery system was found to have improve rheological properties, mechanical stability and pH responsiveness. This drug delivery system was found to exhibit better gallic acid release under neutral pH condition. The mechanical stability and sustain drug release property despite increasing acidity in the external environment. However, further modification of this drug delivery system is required to involve non-toxic biomaterials (Chatterjee et al., 2019).
Conclusion
HA is widely utilized in dermatology as it is a natural, non-immunogenic polysaccharide with good biocompatibility and degradability (Huang and Huang, 2018). The moisturizing properties of HA made it exist in almost all moisturizing and antiaging cosmetics. The identification of CD44 receptors and HA on both psoriasis and AD epidermis had led to many researchers exploring this component as a possible new way for drug delivery Research on this field flourishes further even more with the improve understanding of nanomedicine. However, the current research are mainly in the pre-clinical phase. There are still large gaps in terms of best material to be utilized to improve the safety and efficacy of drug delivery. The mechanism of HA based drug delivery system is still unclear. The role of HA and its receptor on both AD and psoriasis is also yet to be well defined. Thus, more work needs to be done on all aspect of HA and its receptors on patho-mechanism of inflammatory skin diseases and its potential role in improvising future drug delivery.
Author Contributions
KNH, ZWL, and WHY conceived the idea and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ministry of Education (MOE) Fundamental Research Grant Scheme (FRGS/1/2019/STG05/TAYLOR/03/3) awarded to ZWL and (FRGS/1/2019/SKK08/TAYLOR/02/2) awarded to WHY.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
HA, hyaluronic acid; AD, atopic dermatitis; LMWHA, low molecular weight hyaluronic acid; HMWHA, high molecular weight hyaluronic acid; IL, interleukin; SC, stratum corneum; PKCα; protein kinase C alpha; HaCat, human keratinocyte cell line.
References
- Alvarez-Figueroa M. J., Delgado-Charro M. B., Blanco-Méndez J. (2001). Passive and iontophoretic transdermal penetration of methotrexate. Int. J. Pharm. 212, 101–107. 10.1016/s0378-5173(00)00599-8 [DOI] [PubMed] [Google Scholar]
- Barbeau M., Lalonde H. (2006). Burden of atopic dermatitis in Canada. Int. J. Dermatol. 45, 31–36. 10.1111/j.1365-4632.2004.02345.x [DOI] [PubMed] [Google Scholar]
- Barnes L., Carraux P., Saurat J. H., Kaya G. (2012). Increased expression of CD44 and hyaluronate synthase 3 is associated with accumulation of hyaluronate in spongiotic epidermis. J. Invest. Dermatol. 132 (3), 736. 10.1038/jid.2011.384 [DOI] [PubMed] [Google Scholar]
- Bieber T., D’Erme A. M., Akdis C. A., Traidl-Hoffmann C., Lauener R., Schäppi G., et al. (2017). Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J. Allergy Clin. Immunol. 139, S58–S64. 10.1016/j.jaci.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Boehncke W. H., Schön M. P. (2015). Psoriasis. Lancet 386, 983–994. 10.1016/S0140-6736(14)61909-7 [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Ramez M., Gilad E., Singleton P. A., Man M. Q., Crumrine D. A., et al. (2006). Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J. Invest. Dermatol. 126 (6), 1356–1365. 10.1038/sj.jid.5700260 [DOI] [PubMed] [Google Scholar]
- Brown M. B., Jones S. A. (2005). Hyaluronic acid: a unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereology JEADV 19 (3), 308–318. 10.1111/j.1468-3083.2004.01180.x [DOI] [PubMed] [Google Scholar]
- Bukhari S. N. A., Roswandi N. L., Waqas M., Habib H., Hussain F., Khan S., et al. (2018). Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 120, 1682–1695. 10.1016/j.ijbiomac.2018.09.188 [DOI] [PubMed] [Google Scholar]
- Bychkov S. M., Kuzmina S. A. (2015). “The Biological Role of Hyaluronic Acid,” in Hyaluronic Acid (Chichester, UK: John Wiley & Sons, Ltd; ), 9–75. 10.1002/9781118695920.ch2. [Google Scholar]
- Carrasco S., Neves F., Fonseca M., Gonçalves C., Saad C., Sampaio-Barros P., et al. (2011). Toll-like receptor (TLR) 2 is upregulated on peripheral blood monocytes of patients with psoriatic arthritis: a role for a gram-positive inflammatory Trigger. Clin. Exp. Rheumatol. 29, 958–962. [PubMed] [Google Scholar]
- Chatterjee S., Hui P. C., Kan C., Wang W. (2019). Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 9 (1), 1–13. 10.1038/s41598-019-48254-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z. (2018). What’s new about the mechanism of methotrexate action in psoriasis? Br. J. Dermatol. 179, 818–819. 10.1111/bjd.16908 [DOI] [PubMed] [Google Scholar]
- Coondoo A., Phiske M., Verma S., Lahiri K. (2014). Side-effects of topical steroids: A long overdue revisit. Indian Dermatol. Online J. 5, 425. 10.4103/2229-5178.142483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecka-Operacz M., Sadowska-Przytocka A. (2014). The possibilities and principles of methotrexate treatment of psoriasis - The updated knowledge. Postep. Dermatologii i Alergol. 31, 392–400. 10.5114/pdia.2014.47121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani G., Eggenhöffner R., Pigatto P. D. M., Bragazzi N. L. (2019). Nanotechnology meets atopic dermatitis: Current solutions, challenges and future prospects. Insights and implications from a systematic review of the literature. Bioact. Mater. 4, 380–386. 10.1016/j.bioactmat.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolz-Pérez I., Sallam M. A., Masiá E., Morelló-Bolumar D., Pérez del Caz M. D., Graff P., et al. (2020). Polypeptide-corticosteroid conjugates as a topical treatment approach to psoriasis. J. Control. Release. 318, 210–222. 10.1016/j.jconrel.2019.12.016 [DOI] [PubMed] [Google Scholar]
- Draelos Z. D. (2011). A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis. J. Cosmet. Dermatol. 10 (3), 185–188. 10.1111/j.1473-2165.2011.00568.x [DOI] [PubMed] [Google Scholar]
- Du H., Liu P., Zhu J., Lan J., Li Y., Zhang L., et al. (2019). Hyaluronic Acid-Based Dissolving Microneedle Patch Loaded with Methotrexate for Improved Treatment of Psoriasis. ACS Appl. Mater. Interfaces. 11 (46), 43588–43598. 10.1021/acsami.9b15668 [DOI] [PubMed] [Google Scholar]
- Eichenfield L. F., Tom W. L., Berger T. G., Krol A., Paller A. S., Schwarzenberger K., et al. (2014). Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 71 (1), 116–132. 10.1016/j.jaad.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwick M., Gauglitz G., Pavicic T., Köhler T., Wegmann M., Schwach-Abdellaoui K., et al. (2011). Fifty-kDa hyaluronic acid upregulates some epidermal genes without changing TNF-α expression in reconstituted epidermis. Skin Pharmacol. Physiol. 24 (4), 210–217. 10.1159/000324296 [DOI] [PubMed] [Google Scholar]
- Frankel A., Sohn A., Patel R. V., Lebwohl M. (2011). Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis. J. Drugs Dermatol. 10 (6), 666. 10.1016/j.jaad.2010.09.266 [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez S., Arias-Santiago S., Perandrés-López R., Castellote L., Zumaquero E., Navarro P., et al. (2013). Increased gene expression of Toll-like receptor 4 on peripheral blood mononuclear cells in patients with psoriasis. J. Eur. Acad. Dermatol. Venereol. 27, 242–250. 10.1111/j.1468-3083.2011.04372.x [DOI] [PubMed] [Google Scholar]
- Giam Y. C., Hebert A. A., Dizon M. V., Van Bever H., Tiongco-Recto M., Kim K.-H., et al. (2016). A review on the role of moisturizers for atopic dermatitis. Asia Pac. Allergy 6 (2), 120–128. 10.5415/apallergy.2016.6.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel A. S. B., Neubert R. H. H., Wohlrab J. (2011). Dermal targeting of tacrolimus using colloidal carrier systems. Int. J. Pharm. 404, 159–168. 10.1016/j.ijpharm.2010.11.029 [DOI] [PubMed] [Google Scholar]
- Goh J. X. H., Tan L. T.-H., Yew H. C., Pusparajah P., Lingham P., Long C. M., et al. (2019). Hydration effects of moisturizing gel on normal skin: A pilot study. Prog. Drug Discovery Biomed. Sci. 2 (1), a0000023. 10.36877/pddbs.a0000023 [DOI] [Google Scholar]
- Hellman U., Engström-Laurent A., Larsson A., Lindqvist U. (2019). Hyaluronan concentration and molecular mass in psoriatic arthritis: biomarkers of disease severity, resistance to treatment, and outcome. Scand. J. Rheumatol. 48, 284–293. 10.1080/03009742.2019.1577490 [DOI] [PubMed] [Google Scholar]
- Huang G., Huang H. (2018). Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release. 278, 122–126. 10.1016/j.jconrel.2018.04.015 [DOI] [PubMed] [Google Scholar]
- Jiang D., Liang J., Noble P. W. (2011). Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 91, 221–264. 10.1152/physrev.00052.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S., Watson W., Carr S. (2018). Atopic dermatitis. Allergy Asthma Clin. Immunol. 14 (suppl 2), 44–52. 10.1186/s13223-018-0281-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimkhani C., Dellavalle R. P., Coffeng L. E., Flohr C., Hay R. J., Langan S. M., et al. (2017). Global skin disease morbidity and mortality an update from the global burden of disease study 2013. JAMA Dermatol. 153, 406–412. 10.1001/jamadermatol.2016.5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke C., Sun L., Qiao D., Wang D., Zeng X. (2011). Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 49 (10), 2670–2675. 10.1016/j.fct.2011.07.020 [DOI] [PubMed] [Google Scholar]
- Kim Y., Lee Y. S., Hahn J. H., Choe J., Kwon H. J., Ro J. Y., et al. (2008). Hyaluronic acid targets CD44 and inhibits FcϵRI signaling involving PKCδ, Rac1, ROS, and MAPK to exert anti-allergic effect. Mol. Immunol. 45, 2537–2547. 10.1016/j.molimm.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Kouvidi K., Berdiaki A., Nikitovic D., Katonis P., Afratis N., Hascall V. C., et al. (2011). Role of receptor for hyaluronic acid-mediated motility (RHAMM) in low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell adhesion. J. Biol. Chem. 286 (44), 38509–38520. 10.1074/jbc.M111.275875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist U., Pihl-Lundin I., Engström-Laurent A. (2012). Dermal distribution of hyaluronan in psoriatic arthritis: Coexistence of CD44, MMP3 and MMP9. Acta Derm. Venereol. 92 (4), 372–377. 10.2340/00015555-1286 [DOI] [PubMed] [Google Scholar]
- Logger J., Olydam J., Woliner-van der Weg W., van Erp P. (2019). Noninvasive Skin Barrier Assessment: Multiparametric Approach and Pilot Study. Cosmetics 6:20. 10.3390/cosmetics6010020 [DOI] [Google Scholar]
- Luger T. A., Loser K. (2018). Novel insights into the pathogenesis of psoriasis. Clin. Immunol. (Orlando Fla.) 186, 43–45. 10.1016/j.clim.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Malaisse J., Bourguignon V., De Vuyst E., Lambert De Rouvroit C., Nikkels A. F., Flamion B., et al. (2014). Hyaluronan metabolism in human keratinocytes and atopic dermatitis skin is driven by a balance of hyaluronan synthases 1 and 3. J. Invest. Dermatol. 134, 2174–2182. 10.1038/jid.2014.147 [DOI] [PubMed] [Google Scholar]
- Malecic N., Young H. (2016). Tacrolimus for the management of psoriasis: Clinical utility and place in therapy. Psoriasis Targets Ther. 6, 153–163. 10.2147/PTT.S101233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maytin E. V., Chung H. H., Seetharaman V. M. (2004). Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. Am. J. Pathol. 165, 1331–1341. 10.1016/S0002-9440(10)63391-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N. N., Yu Y. D., Saboury B., Foroughi N., Krishnamoorthy P., Raper A., et al. (2011). Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): A pilot study. Arch. Dermatol. 147, 1031–1039. 10.1001/archdermatol.2011.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micali G., Paternò V., Cannarella R., Dinotta F., Lacarrubba F. (2018). Evidence-based treatment of atopic dermatitis with topical moisturizers. G. Ital. di Dermatologia e Venereol. 153, 396–402. 10.23736/S0392-0488.18.05898-4 [DOI] [PubMed] [Google Scholar]
- Mohd Affandi A., Khan I., Ngah Saaya N. (2018). Epidemiology and Clinical Features of Adult Patients with Psoriasis in Malaysia: 10-Year Review from the Malaysian Psoriasis Registry, (2007-2016). Dermatol. Res. Pract. 2018, 1–8. 10.1155/2018/4371471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T., Chen T. H., Narala S., Chun K. A., Two A. M., Yun T., et al. (2017). Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 9 eaah4680. 10.1126/scitranslmed.aah4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narurkar V. A., Cohen J. L., Dayan S., Kaminer M. S., Rivkin A., Shamban A., et al. (2016). A Comprehensive Approach to Multimodal Facial Aesthetic Treatment: Injection Techniques and Treatment Characteristics From the HARMONY Study. Dermatologic Surg. 42 (Suppl 2), S177–S191. 10.1097/DSS.0000000000000743 [DOI] [PubMed] [Google Scholar]
- Ohkawara Y., Tamura G., Iwasaki T., Tanaka A., Kikuchi T., Shirato K. (2000). Activation and transforming growth factor-β production in eosinophils by hyaluronan. Am. J. Respir. Cell Mol. Biol. 23, 444–451. 10.1165/ajrcmb.23.4.3875 [DOI] [PubMed] [Google Scholar]
- Ohtani T., Memezawa A., Okuyama R., Sayo T., Sugiyama Y., Inoue S., et al. (2009). Increased hyaluronan production and decreased E-cadherin expression by cytokine-stimulated keratinocytes lead to spongiosis formation. J. Invest. Dermatol. 129 (6), 1412–1420. 10.1038/jid.2008.394 [DOI] [PubMed] [Google Scholar]
- Pacha O., Hebert A. A. (2012). Treating atopic dermatitis: Safety, efficacy, and patient acceptability of a ceramide hyaluronic acid emollient foam. Clin. Cosmet. Investig. Dermatol. 5, 39. 10.2147/CCID.S23269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M., Choudhury H., Gunasegaran T. A. P., Nathan S. S., Md S., Gorain B., et al. (2019). Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: fabrication, characterisation, in vitro release kinetics, and dermal targeting. Drug Deliv. Transl. Res. 9, 520–533. 10.1007/s13346-018-0480-1 [DOI] [PubMed] [Google Scholar]
- Pardue E. L., Ibrahim S., Ramamurthi A. (2008). Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis 4 (4), 203–214. 10.4161/org.4.4.6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passi A., Sadeghi P., Kawamura H., Anand S., Sato N., White L. E., et al. (2004). Hyaluronan suppresses epidermal differentiation in organotypic cultures of rat keratinocytes. Exp. Cell Res. 296 (2), 123–134. 10.1016/j.yexcr.2004.01.031 [DOI] [PubMed] [Google Scholar]
- Prost-Squarcioni C. (2006). Histologie de la peau et des follicules pileux. Medecine/Sciences 22, 131–137. 10.1051/medsci/2006222131 [DOI] [PubMed] [Google Scholar]
- Rendon A., Schäkel K. (2019). Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 20 (6), 1475. 10.3390/ijms20061475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar D., Moss C. (2016). Topical therapy in atopic dermatitis in children. Indian J. Dermatol. 61, 656–661. 10.4103/0019-5154.193677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried E., Hebert A. (2015). Diagnosis of Atopic Dermatitis: Mimics, Overlaps, and Complications. J. Clin. Med. 4, 884–917. 10.3390/jcm4050884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer S. R., Peppast N. A. (1992). Poly(vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer 33 (18), 3932–3936. 10.1016/0032-3861(92)90385-A [DOI] [Google Scholar]
- Symonette C. J., Kaur Mann A., Tan X. C., Tolg C., Ma J., Perera F., et al. (2014). Hyaluronan-Phosphatidylethanolamine Polymers Form Pericellular Coats on Keratinocytes and Promote Basal Keratinocyte Proliferation. BioMed. Res. Int. 2014. 10.1155/2014/727459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze J. H., Brownlie J. C., Love C. A. (2016). Biotechnological production of hyaluronic acid: a mini review. 3 Biotech. 6, 1–9. 10.1007/s13205-016-0379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabassum N., Hamdani M. (2014). Plants used to treat skin diseases. Pharmacogn. Rev. 8, 52–60. 10.4103/0973-7847.125531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetz S., Bochot A., Surace C., Arpicco S., Renoir J. M., Schaefer U. F., et al. (2009). Hyaluronic acid-modified DOTAP/DOPE liposomes for the targeted delivery of anti-telomerase siRNA to CD44-expressing lung cancer cells. Oligonucleotides 19, 103–115. 10.1089/oli.2008.0168 [DOI] [PubMed] [Google Scholar]
- Termeer C. C., Hennies J., Voith U., Ahrens T., M. Weiss J., Prehm P., et al. (2000). Oligosaccharides of Hyaluronan Are Potent Activators of Dendritic Cells. J. Immunol. 165, 1863–1870. 10.4049/jimmunol.165.4.1863 [DOI] [PubMed] [Google Scholar]
- Touitou E., Dayan N., Bergelson L., Godin B., Eliaz M. (2000). Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J. Controlled release 65 (3), 403–418. 10.1016/s0168-3659(99)00222-9 [DOI] [PubMed] [Google Scholar]
- Wan T., Pan W., Long Y., Yu K., Liu S., Ruan W., et al. (2017). Effects of nanoparticles with hydrotropic nicotinamide on tacrolimus: Permeability through psoriatic skin and antipsoriatic and antiproliferative activities. Int. J. Nanomedicine. 12, 1485. 10.2147/IJN.S126210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Matsuuchi M., Irimajiri J., Katayama I., Nishioka K. (1997). John Libbey Eurotext - European Journal of Dermatology - Elevated circulating hyaluronan in patients with generalized pustular psoriasis. Eur. J. Dermatol. 7 (6), 409–411. [Google Scholar]
- Yamamoto T. (2015). Hyaluronic acid in psoriasis. J. Eur. Acad. Dermatol. Venereol. 29, 2487–2487. 10.1111/jdv.12581 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xia Q., Li Y., He Z., Li Z., Guo T., et al. (2019). CD44 assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: A new strategy for clustering drug in inflammatory skin. Theranostics 9 (1), 48. 10.7150/thno.29715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo F., Abourehab M. A. S., Hussain Z. (2018). Hyaluronic acid decorated tacrolimus-loaded nanoparticles: Efficient approach to maximize dermal targeting and anti-dermatitis efficacy. Carbohydr. Polym. 197, 478–489. 10.1016/j.carbpol.2018.06.023 [DOI] [PubMed] [Google Scholar]
- Zonnevdd I. M., Rubins A., Jablonska S., Dobozy A., Ruzicka T., Kind P., et al. (1998). Topical tacrolimus is not effective in chronic plaque psoriasis: A pilot study. Arch. Dermatol. 134, 1101–1102. 10.1001/archderm.134.9.1101 [DOI] [PubMed] [Google Scholar]