Abstract
Pandemic coronavirus disease-2019, commonly known as COVID-19 caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a highly contagious disease with a high mortality rate. Various comorbidities and their associated symptoms accompany SARS-CoV-2 infection. Among the various comorbidities like hypertension, cardiovascular disease and chronic obstructive pulmonary disease, diabetes considered as one of the critical comorbidity, which could affect the survival of infected patients. The severity of COVID-19 disease intensifies in patients with elevated glucose level probably via amplified pro-inflammatory cytokine response, poor innate immunity and downregulated angiotensin-converting enzyme 2. Thus, the use of ACE inhibitors or angiotensin receptor blockers could worsen the glucose level in patients suffering from novel coronavirus infection. It also observed that the direct β-cell damage caused by virus, hypokalemia and cytokine and fetuin-A mediated increase in insulin resistance could also deteriorate the diabetic condition in COVID-19 patients. This review highlights the current scenario of coronavirus disease in pre-existing diabetic patients, epidemiology, molecular perception, investigations, treatment and management of COVID-19 disease in patients with pre-existing diabetes. Along with this, we have also discussed unexplored therapies and future perspectives for coronavirus infection.
Keywords: Coronavirus, COVID-19, SARS-CoV-2, Diabetes, Comorbidities, Epidemiology, Treatment, Management
Graphical abstract

1. Introduction
Coronavirus Disease-19 (COVID-19) caused by a virus of the family Coronaviridae (commonly known as Corona Virus), documented as SARS-CoV-2. According to past studies, several severe diseases like Severe Acute Respiratory Syndrome (SARS) and the Middle East respiratory syndrome (MERS) affected humans by viruses belonging to the same Coronaviridae family. Likewise, SARS-CoV-2, SARS and MERS, HKU1, NL63 and OC43 viruses also belong to the family of Coronaviridae. The first outbreak of the coronavirus reported in Wuhan city of China where bats were consumed as a food item and are assumed the foremost reason to justify the outbreak of this novel virus. There are various theories associated with the outbreak of this deadly virus. Novel coronavirus caused the disease to have an identical genome sequence to the coronavirus found in the bats which are sold in Wuhan city market [1]. The worst part about this novel virus is that it spread rapidly throughout the world, due to its high contagious nature and as per now, no effective treatment is available for which made it more detrimental. Fig. 1 demonstrate worldwide SARS-CoV-2 affected areas [2].
Fig. 1.
Worldwide severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) affected areas.
Scientists all over the globe, working hard to find suitable vaccines or treatments to treat the novel virus infection. Recently the World Health Organization (WHO) declared this novel virus infection as a pandemic. At present, the exact number of confirmed infected patients are 15,656,766 and 636,575 deaths from the COVID-19 as of July 24, 2020, 04:58 GMT (Fig. 2 ) [2,3].
Fig. 2.
Top 10 Countries affected by SARS-CoV-2 infection.
* First outbreak occurred in Wuhan City, China. (Highlighted in bold and red). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In medicinal sciences, comorbidity mainly defined as a condition present concurrently; however, individuals with an additional condition or a related medical condition. In simple term, comorbidity defines the effect of all other situations an individual patient might have other than the primary condition of interest and can be physiological or psychological. American epidemiologist A. R. Feinstein first introduced the term comorbidity in the 1970s. The combination of a prolonged disease like diabetes and a severe viral infection like COVID-19 gives a tough challenge to the medical profession to save lives. Diabetes mellitus (DM) is a chronic disease that affects the global population. Both diabetes type 1 and 2 are a family of diseases that results in elevated sugar level in the blood. When the body unable to produce a sufficient amount of the hormone insulin, which helps the body to get glucose into cells for energy, the glucose builds up in the blood. The overall diabetes occurrence in 2019 projected to be 9.3% with a probable value of 463 million people suffering from the condition [4,5]. Patients with diabetes considered as high-risk patients for acquired infections. The higher the glucose, the higher the risk of infections [6]. The vast majority of patients in the United States are not reaching glucose goals (70%) [7]. Besides, most patients with diabetes develop over time, co-morbidities that increase such risks. Hypertension, diabetes, cardiovascular disease (CVD) and obesity are few known or common conditions related to metabolic syndrome, and altogether or individually, they can be inclined towards a set of causes linked to COVID-19 pathogenesis. Coronavirus does have a potential ability to destroy islets by their angiotensin-converting enzyme 2 (ACE2) receptor present in islets and hence promote diabetes during the infection. According to studies, the virus predominantly infects human by allowing the cells to enter through the ACE2 receptor [8]. Due to insufficient genetic data, the existence of coronavirus S-protein binding resistant towards ACE2 still a mystery [9]. Dysglycemia is well known to downregulate critical mediators of the innate immune response of the host to pathogenesis. Patients associated with hyperglycaemia, diabetes and insulinopenia deliberately disabled the host innate and humoral immune system by weakening the synthesis of pro-inflammatory cytokines along with their downstream acute phase reactant.
In addition, immune responses get reduced by impairing lymphocyte and macrophage functions due to metabolic disorders which afterwards render patients more likely to be affected by the infectious disease [10]. Though limited data is there regarding COVID-19 patients with diabetes Wuhan shows 42.3% of 26 fatalities due to COVID-19, where patients have diabetes [11]. The Wuhan city of China conducted studies where it is found that diabetes was not a significant predictor of mortality in one study group of 140 patients with COVID-19 [12]. Whereas, another group of 150 patients were 68 deaths cases and 82 recovered patients were reported showed that the number of co-morbidities to be a risk factor for severe disease course [13]. Evaluation of 11 studies associating laboratory abnormalities in COVID-19 patients did not report diabetes or raised blood glucose level as a risk factor [14]. Apart from all these contradictory data and reports, Chinese Centre for Disease Control and Prevention published a report where they showed all over 72,314 cases of COVID-19 and showed increased mortality rate in people with diabetes (2.3%, overall and 7.3%, patients with diabetes) [15]. Agreeing with Current Diabetes Review, type 2 diabetes increase the incidence of infectious diseases and related comorbidities, and type 1 also do the same [16].
2. Diabetes as a threat in coronavirus infection
Diabetes is one of the standards and significant risk factor connected with mortality triggered by COVID-19. Diabetes closely characterised by impaired immunity that assumed to lead an augmented susceptibility to COVID-19 contagion, particularly in those with an elevated level of blood glucose. Cardiovascular disease, collective comorbidity towards endocrine disease comprising diabetes, is a significant donor to COVID-19 morbidity [6,17].
Prominent data of COVID-19 patients associating several other diseases such as hypertension, diabetes and CVD suggest that different studies have different prevalence rate globally, according to a report on comorbidities in COVID-19 patients by Singh et al., states that a pooled data from 10 Chinese studies (n = 2209) reported an occurrence of diabetes, hypertension and CVD in 11%, 21% followed by 7% separately [18]. Likewise, Yang et al. reports agreeing with a meta-analysis of 8 trails of 46,248 patients having diabetes, CVD and hypertension prevalence of 8%, 5% and 17% respectively [19]. A report from the Chinese Center for Disease Control and Prevention (China CDC) that investigate a study in 20,982 COVID-19 patients having pre-existing diabetes followed by CVD and hypertension in approximately 5.3%, 4.2%, and 12.8% respectively. This study was done by the Epidemiology Working Group of China CDC [20]. A report by Onder et al. from Italy state that out of the total 355 novel coronavirus infected patients 43% of the patients associated with CVD and 36% of patients having a pre-existing diabetic profile [21]. Similarity with the Italian study as reported by Bhatraju et al. claimed that out of 24 patients of COVID-19, 58.0% of patients associated with diabetes [22]. COVID-19 surveillance group of the USA CDC reports an incidence of 10.9% from the total 7162 coronavirus infected patients having diabetes, followed by 33.9% of patients in Italy (n = 481) reported by COVID-19 surveillance group, Italy [23,24]. In Table. 1 different comorbidity associated with novel coronavirus infection is shown.
Table 1.
Different comorbidities in SARS-CoV-2 infected patients.
| Sl. no | Investigator | Total number of patients | Diabetes (%) | Hypertension (%) | Cardiovascular disease (%) | Chronic obstructive pulmonary disease (%) |
|---|---|---|---|---|---|---|
| Comorbidities in coronavirus infected patients in China | ||||||
| 1. | Liu et al. [25] | 61 | 8.2 | 19.7 | 1.6 | 8.2 |
| 2. | Guan et al. [26] | 1099 | 7.4 | 15.0 | 3.8 | 1.1 |
| 3. | Huang et al. [27] | 41 | 19.5 | 14.6 | 15.0 | 2.4 |
| 4. | Chen et al. [17] | 99 | 12.1 | NR | 40.0 | 1.0 |
| 5. | Wang et al. [28] | 138 | 10.1 | 31.2 | 19.6 | 2.9 |
| 6. | Zhou et al. [29] | 191 | 19.0 | 30.0 | 8.0 | 3.0 |
| 7. | Zhang et al. [12] | 140 | 12.1 | 30.0 | 8.6 | 1.4 |
| 8. | Yang et al. [30] | 52 | 17.0 | NR | 23.0 | 8.0 |
| 9. | Wu et al. [31] | 201 | 10.9 | 19.4 | 4.0 | 2.5 |
| 10. | Guo et al. [32] | 187 | 15.0 | 32.6 | 11.2 | 2.1 |
| 11. | Liu et al. [33] | 137 | 10.2 | 9.5 | 7.3 | 1.5 |
| 12. | Chen et al. [34] | 274 | 17.0 | 34.0 | 8.0 | 7.0 |
| 13. | CDC (China) [20] | 20,982 | 5.3 | 12.8 | 4.2 | 2.4 |
| Comorbidities in coronavirus infected patients in Italy | ||||||
| 1. | Onder et al. [21] | 355 | 35.5 | NR | 42.5 | NR |
| 2. | CSG (Italy)a [23] | 481 | 33.9 | 73.8 | 30.1 | 13.7 |
| Comorbidities in coronavirus infected patients in the USA | ||||||
| 1. | Bhatraju et al. [22] | 24 | 58.0 | NR | NR | 4.0 |
| 2. | CDC (USA) [24] | 7162 | 10.9 | NR | 9.0 | 9.2 |
COVID-19 Surveillance Group of Italy.
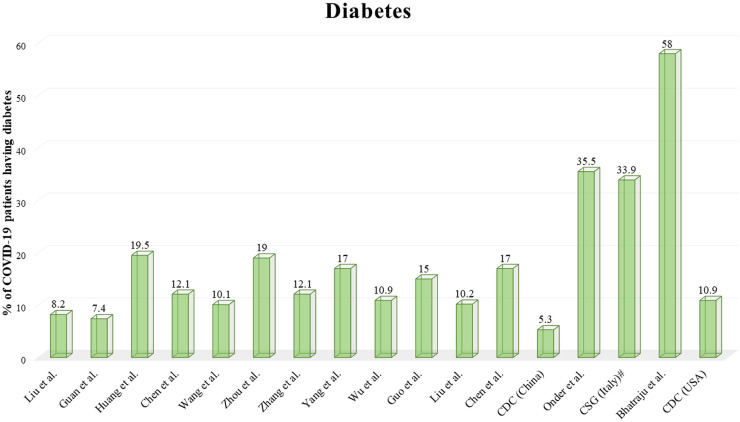
Due to less data availability, it is difficult to conclude that pre-existing diabetes in coronavirus affected patient increases the mortality rate. In Table 1, the incidence of various comorbidities in obtainable reports of patients having COVID-19 shown. In Fig. 3 a graphical representation demonstrates which elaborate the association of diabetes along with novel coronavirus infection.
Fig. 3.
Reports highlighted the percentage of COVID-19 patients having diabetes.
# COVID-19 Surveillance Group of Italy.
Only a few reports are existing that includes the severity of coronavirus infection in pre-existing diabetes patients [35]. Liu et al. report a study that includes 61 patients in which 5 patients are having a pre-existing diabetic profile (8.2%). In that report, 17.6% of cases are severe, whereas 4.5% of cases are non-severe [25]. In another study, Guan et al. reports out of 1099 COVID-19 cases 81 patients also suffering from diabetes (7.4%), in which severe and non-severe cases are 16.2% and 5.7 respectively [26]. Wu et al. state in a report that 14 patients out of 138 patients having diabetes (10.1%) as comorbidity, in which 5.9 cases are severe and 22.2% cases are non-severe [31]. In addition to these reports, Zhang et al. report a study of a total of 201 patients in which 22 are diabetic (12.1%). That further states 19.2% and 5.1% of cases are severe and non-severe, respectively [12]. Huang et al. in a study report that out of 41 total cases, 8 are having diabetes (15%) in which 25% are severe cases, and 8% are non-severe cases [27]. In a report of Centers for Disease Control and Prevention USA states that out of 7162 patients 784 having a diabetic profile (10.9%) in which 32% are severe, and 9.4% are non-severe [24]. Mention in Table. 2 highlights the incidence of diabetes in COVID-19 patients & Fig. 4 represent the severe and non-severe incidences of diabetes in COVID-19 patients.
Table 2.
Incidence of severe and non-severe cases of diabetes in SARS-CoV-2 infected patients.
| Sl. no | Investigator | Total number of patients | Patients with pre-existing diabetes | Severe cases in percentage (%) | Non-severe cases in percentage (%) |
|---|---|---|---|---|---|
| 1. | Liu et al. [25] | 61 | 5 (8.2%) | 17.6 | 4.5 |
| 2. | Guan et al. [26] | 1099 | 81 (7.4%) | 16.2 | 5.7 |
| 3. | Wang et al. [28] | 138 | 14 (10.1%) | 22.2 | 5.9 |
| 4. | Wu et al. [31] | 201 | 22 (10.9%) | 19.0 | 5.1 |
| 5. | Zhang et al. [12] | 140 | 17 (12.1%) | 13.8 | 11.0 |
| 6. | Huang et al. [27] | 41 | 8 (15%) | 25.0 | 8.0 |
| 7. | CDC (USA) [24] | 7162 | 784 (10.9%) | 32.0 | 9.4 |
Fig. 4.
Incidence of severe and non-severe cases of diabetes in SARS-CoV-2 infected patients.
The survivor, non-survivor percentage of patients having pre-existing diabetes suffering from COVID-19 is eye-catching. In a report by Yang et al. demonstrate that out of 52 patients, 9 having a diabetic profile (17%) in which 10% of cases are survivors whereas 22% of are non-survivors [30]. In another study by Zhou et al. reports that 36 cases are diabetic (19%) out of 191 cases, 14% survive, whereas 31% is non-survival cases [29]. Wu et al. in a study report that out of 88 patients, 16 patients have diabetes in that 12.5% are survivors, and 25% are non-survivors [31]. In a study of 274 patients, 47 having diabetes (17%) reported by Chen et al., in which 14% are survivor, whereas 21 are non-survivor [34]. In an extensive study by Guan et al. which reports that out of 1099 cases, 81 having a diabetic profile (7.4%) in which 6.1% are survivors and 26.9% cases are non-survivors [26]. Table 3 and Fig. 5 represent the incidence of survivor and non-survivor COVID-19 patients with diabetes.
Table 3.
Incidence of survivor and non-survivor COVID-19 patients having diabetes.
| Sl. no | Investigator | Total number of patients | Patients with pre-existing diabetes | COVID-19 survivor in percentage (%) | COVID-19 non-survivor in percentage (%) |
|---|---|---|---|---|---|
| 1. | Yang et al. [30] | 52 | 9 (17%) | 10 | 22 |
| 2. | Zhou et al. [29] | 191 | 36 (19%) | 14.0 | 31.0 |
| 3. | Wu et al. [31] | 88 | 16a (18.2%) | 12.5 | 25.0 |
| 4. | Chen et al. [34] | 274 | 47 (17%) | 14.0 | 21.0 |
| 5. | Guan et al. [26] | 1099 | 81 (7.4%) | 6.1 | 26.9 |
Patients having a profile of acute respiratory distress syndrome (ADRs).
Fig. 5.
Incidence of survivor and non-survivor COVID-19 patients with diabetes.
Death rate appears to be about threefold greater in the case of people having diabetes matched with the overall mortality of COVID-19 in China [12,13,15,19,26,[29], [30], [31],36,37]. Remarkably, diabetes used to be a more significant risk factor for severe disease and mortality in the earlier SARS, MERS coronavirus infections along with severe influenza A H1N1 pandemic in 2009 [[38], [39], [40]]. Patients with type 2 diabetes tend to be obese, and obesity is another risk factor for severe infection [41]. It was clarified from the period of influenza A H1N1 epidemic in 2009 that the disease was more severe and had a lengthier duration of approximately two-fold more patients with obesity who were then treated in intensive care units compared with a related population [[40], [41], [42]]. Specifically, a high-risk factor always associated with metabolically active abdominal obesity. A chronic low-grade abdominal obesity resulted due to abnormal secretion of adipokines and cytokines (TNF-α, interferon) may lead to induce in impaired immune response [[41], [42], [43]]. People are suffering from severe abdominal obesity as well having complications like mechanical respiratory problems, with the reduction in the ventilation of the basal lung caused increase in the risk of pneumonia in addition to resulting declined oxygen saturation of the blood. Obesity suffering people are also showing an increased incidence of asthma risk, and those patients with obesity and asthma have shown diverse symptoms, extra frequent and severe exacerbations and also affected decreased response to several asthma medications [44].
In general, patients who have diabetes, often have weakened immune response, both concerning cytokine profile as well as changes in immune-responses together with T-cell and macrophage activation [45]. Weaker glycaemic control harms several aspects of the immune response by a viral infection and in addition to the risk of potential bacterial secondary infection in the lungs [46]. According to reports, it is shown that a novel virus-infected patient having pre-existing diabetes history shows a reduction in metabolic control all over the world.
Not only diabetes, its associated diseases such as diabetes ketoacidosis, nephropathy and ischaemic heart disease can also cause the susceptibility to COVID-19 disease severity. This kind of complication results not only in an increased number of infected patients but also causes an inadequate immune response. In several cases, patients required care, such as acute dialysis. Few reports indicate that COVID-19 could be the reason behind acute cardiac injury through heart failure, leading towards deterioration in circulation [37]. Without a doubt, individuals with diabetes are always in high-risk for severe disease.
3. Epidemiology behind diabetes and COVID-19
The latest meta-analysis from China states that among 46,248 patients infected by COVID-19, most of the patients have pre-existing disease background. Hypertension remains on top (17 ± 7, 95% confidence interval (CI) 14–22% followed by diabetes (8 ± 6, 95% CI 6–11%), cardiovascular diseases (5 ± 4, 95% CI 4–7%) and respiratory diseases (2 ± 0, 95% CI 1–3%), which unexpectedly arises much later than the components of metabolic syndrome. Patients suffering from the severe disease were 2.36 times more probable to have hypertension (95% CI 1.46–3.83), 2.46 times expected to have respiratory disease (95% CI 1.76–3.44) and 3.42 times likely to have underlying cardiovascular disease (95% CI 1.88–6.22), as compared to those with the mild disease who do not require any hospitalization [19]. In a cohort of 54 severely ill patients admitted with COVID-19 pneumonia in China, 44.4%, 24.1% and 14.8% of patients had pre-existing hypertension, diabetes and coronary heart disease, respectively. In which 44.4% of patients were complicated with myocardial injury as demonstrated by elevated cardiac enzymes, and N-terminal pro-B-type natriuretic peptide. These patients are having a profile of very high mortality of 48.1% [47]. In another cohort study of 131 patients with COVID-19 infection admitted at a hospital in Wuhan, hypertension was the most common associated comorbidity (30%), followed by diabetes (19%) and coronary artery disease (8%) [48].
4. Molecular perceptions of diabetes and COVID-19
Earlier SARS virus has been reported to bind target cells through angiotensin-converting enzyme 2 (ACE2), which mainly expressed by the epithelial cells of the lung, kidneys, intestines and blood vessels. The upregulated expression in people usually treated with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type I receptor blockers (ARBs), which are also a drug of choice to treat hypertension when a patient is suffering from a metabolic disorder like diabetes. Medicines like ibuprofen and thiazolidinediones, precisely pioglitazone linked to increased ACE2 expression [49,50]. Augmented ACE2 expression, on the contrary, found to be useful and linked to reduced inflammation in the lungs, which a probable area of therapy in people living with inflammatory lung disease. In addition, individuals with different ACE2 polymorphisms would more complicate the picture. Still, this leftover an area of active research and the picture is likely to be more transparent shortly.
5. Diabetes investigation in COVID-19 patients
COVID-19 management teams around the world must be conscious about the nuances of endocrine screening and diagnosis for patients with diabetes. People infected with coronavirus must be screened for diabetes and other illnesses. At present, there is lack of proper guidelines to screen diabetes patients suffering from COVID-19, standard American Diabetes Association criteria for the diagnose of diabetes should use for the screening process [51]. Two abnormal glycaemic test reports usually required to approve a diagnosis of diabetes in infected persons. In symptomatic cases, a solo abnormal glucose value might be sufficient to diagnose diabetes. Health care personals must be aware of the possibility of stress hyperglycaemia and differentiate it from pre-existing diabetes.
In the case of type 1 diabetes, the immune cells of the body begin to destroy β-cells, which are solely liable for the production of insulin hormone in the pancreas. Data from various studies as well as coronavirus infected patients indicates that the novel virus destroys insulin-producing β-cells, which is further resulted as hyperglycemia [52]. Different viruses, including one that causes SARS, were related to autoimmune conditions, including such as type 1 diabetes [53]. There are multiple organs involved behind the regulation of blood sugar in the body and also consists of different proteins like ACE2, and coronavirus further uses it to infect the cells [54]. Blood sugar and ketones are seen in higher levels in COVID-19 infected patients. If the body is unable to produce an adequate level of insulin to breakdown the blood sugar, it utilizes ketone as another source of fuel, which further leads to diabetic ketoacidosis [55,56].
In the pancreas, β-cells produce blood sugar-lowering hormone insulin, and α-cells make the glucagon hormone that increases blood sugar. SARS-CoV-2 may infect α- and β-cells; as a result, few of them gets destroyed. The virus can also induce some protein production (chemokines and cytokines), which can trigger an immune response that can also kill the specific cells and alter insulin secretion [57]. In obese people, insulin resistance (IR) may be a crucial aspect of the incidence of COVID-19. ACE2 is the potential link between IR and COVID-19 since the virus enters the host body via ACE2. ACE2 helps in the maintenance of RAAS and abnormality of the same leads to IR and cardiovascular dysfunction. Degradation of angiotensin 2 results to the reduction of IR by decreasing oxidative stress, improving insulin signaling and enhanced insulin transport. It is important to normalize the blood glucose and insulin level, thereby reducing the expression of ACE2 and eventually COVD-19 severity [58]. It is assumed that insulin production and SARS-CoV-2 are interconnected to each other. Low levels of insulin contribute to hyperglycemia, and patients with COVID-19 exacerbates the situation. However, IR and interaction with COVID-19 are not yet fully understood. Clinical and biochemical insulin resistance markers should, therefore, be evaluated for their prognostic usefulness. In addition, where there is a correlation between insulin sensitivity and the incidence of COVID-19 is found, and attention should be given to the evaluation of medical measures to improve insulin sensitivity. Therefore, additional care and treatment are also required for patients with low insulin levels.
6. Genetic polymorphism of ACE
Spike proteins of coronavirus usually bind to the ACE receptors in the lungs. It is a peptidase enzyme and expressed in other tissues too to regulate the renin-angiotensin-aldosterone system (RAAS). This enzyme is the main component that converts Ang-I to Ang-II. Binding of the virus to the receptor helps the virus to invade to the host body as well as it causes depletion of ACE2 and degradation of lung tissues due to damaging effects of AngII [59]. Genetic polymorphism to ACE is recently observed in human populations. ACE1 enzyme shows polymorphism by insertion/deletion (I/D) in intron 16; it is associated with a change in levels of ACE in different tissues, including lungs. Even though they have genetic variation, there is no marked difference in their structural conformation. ACE polymorphism causes the blood pressure to change due to its effect on RAAS. Deletion leads to reduced levels of ACE2 in circulation. This I/D polymorphism is different in different geographical regions; e.g. in China, the northern region shows the genetic difference from the southern region [60].
Genetic mutation to this ACE2 affects its interaction with the spike proteins. Based on this polymorphism, the incidence rate in different geographic areas around the globe is different. COVID-19 vulnerability cannot be clarified exclusively by severity or even by ACE2 polymorphism. ACE2 polymorphism and Ang II lead levels were observed in severe COVID-19 patients. Multi-organ failure is another risk factor related to COVID-19. ACE2 polymorphism is associated with a change in blood pressure due to its influence on RAAS [61]. ACE2 mutation is first identified in Chinese people, with three forms of polymorphism. In Canadian teenagers, another three types of genetic polymorphism are observed. Mutations of both ACE1 and ACE2 are recorded among Brazilians. Such mutations have clinical effects, primarily cardiac complications, diabetes and complications of the cerebrovascular system. Cardiac complications among COVID-19 patients was found to be one of the comorbidities. ACE2 expression is less frequent in patients with SARS-CoV. Genetic polymorphism may explain why COVID-19 has the highest incidence rate in Europe than in Africa [[62], [63], [64], [65]].
7. Management of diabetes in SARS-CoV-2 infected patients
Endocrinopathy required long-lasting management and without proper guidance; patients should not alter the therapy or stop the medications. In the case of COVID-19 disease affected patients; the daily routine must change to decrease the chances of spreading as well as the severity of the disease [66]. Persons affected with the virus must inform the healthcare persons about their diabetic profile when they admitted to hospital for their better treatment management; especially people dealing with type 1 diabetes and those who depend on adrenal corticosteroids/mineralocorticoids for survival. If patients are not in a condition to take the oral tablets or are unable for subcutaneous insulin, they must give them intravenously. The management of diabetes itself a unique challenge, particularly for those who are on non-insulin depended diabetes mellitus (NIDDM) or type 2 diabetes. In cases with uncontrolled hyperglycaemia, it would be pragmatic to intensify insulin dosing, relatively than other oral drugs [67]. Present data give an idea that hydroxychloroquine (HCQ) is the drug of choice for the patient affected with Coronavirus due to its antiviral properties [68]. Still, there are not enough pieces of evidence present to support the uses of hydroxychloroquine as an ideal drug for the management of diabetes complicated by COVID-19 infection. Healthcare professions must alert the potential endocrine and adverse metabolic effects of the drugs, which are in use. Like corticosteroids, able to cause dysglycaemia, however long-term antiretroviral therapy may be associated with metabolic syndrome [69]. Apart from the lack of specific therapies and knowledge about potential therapeutic targets, it is challenging to treat a disease along with other comorbidities. The drug repurposing for SARS-CoV-2 also found to be an exciting option to combat with this novel virus infection for diabetic patients [70].
8. Treatment of diabetes during SARS-CoV-2 infection
Poor glycaemic control always results in a severe risk factor for different infections and adverse outcomes. Escalation of any infection (can be bacterial pneumonia) depends on the average glucose level of a human being. Treating with glucocorticoids to hyperglycaemic patients affected with COVID-19 come up with tremendous challenges to the medical professional. The problem associate with this novel virus infection results in loss of glycaemic, control due to unstable food intake and intercurrent diseases like fever and others. To keep the glucose level optimum, frequent glucose monitoring in addition to continuous change in antidiabetic medications required.
Patients having type 1 diabetes generally treated with basal-bolus or insulin pump therapy and the doses of insulin should be frequent and be monitored to avoid a situation like hypoglycaemia as well as severe hyperglycaemia and ketoacidosis mainly in patients with reduced food intake. Patients having a diabetic profile should follow some basic prevention instruction to dodge the novel coronavirus infection. This quickly spreading virus infection can stop since this is a communicable disease; several recommendations help to diminish the spread of the deadly virus ( Fig. 6 ).
Fig. 6.
General prevention advice to control coronavirus infection.
Patients are having a profile of type 2 diabetes, Sodium-glucose co-transporter-2 (SGLT2) inhibitors along with metformin, should be stopped to control moderate to severe illness. Chances of dehydration and diabetic ketoacidosis during infection is there, so additional precaution requires to be taken by the patients to avoid such incidences. Inhibitors like Dipeptidyl peptidase-4 (DPP4) linagliptin can be useful in the cases of impaired kidney function without risk of hypoglycaemia. Drugs class, like sulphonylureas, consider as one of the reasons for inducing hypoglycaemia in patients having a low-calorie intake, so before taking this class of drug, proper guidance should be followed. Uses of drugs like long-acting glucagon-like peptide-1 (GLP-1) receptor agonist should not be discontinued without the proper consultation of a physician. Patients having a profile of Type 2 Diabetes Mellitus, insulin treatment is better and vital to be started. Treatment of patients in the case of intercurrent disease a time-involved process, which is a serious issue [71]. A drug like pioglitazone a thiazolidinedione should be avoided; it may trigger the severity of the disease. In Fig. 7 illustrate the list of drug therapies recommended for COVID-19 patients who have diabetes [72].
Fig. 7.
List of drugs therapies recommended for COVID-19 patients suffering from diabetes.
*Chances of dehydration and diabetic ketoacidosis during infection is there, so additional precaution should be taken by the patients to avoid such cases.
** But the use of this class of drugs is still questionable during SARS-CoV-2 infection.
There is no current treatment available for this novel virus infection. Still, a small trial testified from France using a combination of hydroxychloroquine and azithromycin, showing decent outcomes in patients suffering from respiratory symptoms [73]. The reported study endpoint based on a negative test, which means the absence of the virus after receiving treatment, and not on the health status of the participating persons. Furthermore, a clinical trial with remdesivir versus placebo for the treatment of COVID-19 (ClinicalTrials.gov identifier NCT: NCT04280705) presently under process in the National Institute of Allergy and Infectious Diseases [74]. This trial is still in progress and needs time; due to the short duration of the intervention, findings must turn out to be accessible in a reasonable time.
Angiotensin-converting-enzyme 2 (ACE2), a membrane glycoprotein, mainly expressed in epithelial cells of the lungs, intestine, kidney and blood vessels. ACE2 plays a vital role in the breakdown of angiotensin-II (Ang-II) and to some extent, angiotensin-I (Ang-I) to peptides, angiotensin(1 – 7) and angiotensin(1 – 9), separately [75]. ACE2/Angiotensin (1–7) have significant anti-inflammatory, anti-oxidant role in the protection of lung against acute respiratory distress syndrome as well as in the case of H5N1 infection. The role of ACE2 in the relation between COVID-19 and DM is often found attractive [76]. The expression level of ACE2 found reduced in case of DM probably because of glycosylation. Due to this reason, the chances of severe lung injuries closely followed by acute respiratory distress syndrome (ARDs) with COVID-19 increases [31,75]. Some reports even also state that overexpression of ACE2 unfavourable in the case of COVID-19. SARS-CoV-2 considered ACE2 as their receptor to enter into the host pneumocytes [77]. ACE inhibitors and Angiotensin II receptor blocker (ARBs) are widely used in the treatment of DM. ACE2 expression found remarkably increased in DM suffering patients, which is an adaptive response to counter the increased levels of Ang-II and Ang-I. So the use of ACE2 stimulating drugs found detrimental in SARS-CoV-2 infected cases. At present, pioglitazone and liraglutide shown upregulation of ACE2 in animal studies [78,79]. However, none of these drugs was used in the treatment. Due to ACE2 expression, thiazolidinediones class of drugs cannot be used in patients suffering from COVID-19 along with diabetes [78].
Some reports recommend that several incidences of COVID-19 infection in patients primarily connected with the low cytosolic pH. The regulation of cell pH often a complex mechanism involving Serum lactate dehydrogenase (LDH), a cytosolic enzyme and its rising level in serum cause the cell to break down. Due to this novel virus infection, serum LDH level dramatically increased in patients. In the case of anaerobic conditions, lactate formation increases from pyruvate. Due to increased lactate level in the extracellular area, the symporter carries the lactate and H+ ion into the cell, resulting in acidic intracellular pH followed by Na+/H+ exchange activation. Even though H+ ion threw out of the cell, Na+ and Ca2+ enter into the cell. When the level of Na+ and Ca2+ increase within the cell, the cells start swelling and further leading to cell death. An SGLT2 inhibitor (Dapagliflozin) reported to have a lactate reducing profile is useful in this scenario. Reduction in lactate level is found beneficial for pH maintenance. In another mechanism, dapagliflozin also inhibits the Na+ and Ca2+ flow. It is worthy of using dapagliflozin in patients having a pre-diabetic profile to prevent the severe development of COVID-19 infection as well as a combination therapy for diabetes treatment [80].
Dipeptidyl peptidase-4 (DPP-4) was revealed as a receptor for MERS-CoV while SARS-CoV-2 appears to use preferentially angiotensin-converting enzyme 2 to enter the cell [81]. Yet, since DPP-4 inhibitors are popular glucose-lowering medications worldwide, it will be of interest to explore whether they might protect against SARS-CoV-2 infection [82,83]. And so far now, no such specified drug or vaccine has been approved for COVID-19 [84]. To get control over the pandemic, large clinical trials of drugs (like tocilizumab, chloroquine phosphate, remdesivir, ribavirin, lopinavir/ritonavir, arbidol, interferon etc.) are under progress to assess both the therapeutic activity and safety as well [85]. Despite being an immunomodulant and anti-malarial drug, chloroquine (CQ) and its hydroxy-analogue (Hydroxychloroquine) do have broad-spectrum antiviral activity. Thus, it comes under the limelight of having potential pharmacological activity for the patient of COVID-19 with diabetes though no such clinically proven reports are submitted in the support. However, there are pieces of evidence for being a highly effective drug in controlling SARS-CoV-2 in vitro. The most reliable and considerable reason for taking it under consideration is that it interrupts the glycosylation of SARS-CoV's cellular receptors by increasing endosomal pH and hence effectively blocks the viral infection [86]. As per the report of a Chinese Clinical trial of more than 100 patients depicts that CQ showed a superior effect in controlling the disease along with promoting a negative virus conversion, inhibiting pneumonia exacerbation and radiological improvement without any severe adverse effects [87].
And on the other hand, countable studies have been reported which states that HCQ improves treatment-refractory diabetic patients and glycaemic control in decompensated [88,89]. In India, CQ already being approved for the treatment of Type-2 DM along with as a therapy for those patients who cannot achieve glycaemic targets even after administrating two other glucose-lowering drugs [90]. HCQ lowers glycosylated haemoglobin (HbA1c) in diabetes patients without the rheumatic disease, the mechanism of HCQ over glycaemia remains still unknown [88]. Somewhere down the line, an improved pancreatic β-cell function has been reported by the increase in C-peptide response by the impact of CQ [89]. According to a few submitted reports of animal studies, it has been found that HCQ effectively increased insulin accumulation and reduced intracellular insulin degradation [91]. Apart from all the data and reports on glucose metabolism, considerable caution should be taken before the use of HCQ/CQ to diabetic patients and COVID-19 patients as well and if required dose adjustment can be made to prevent hypoglycaemic events. In Table. 4 summarizes the list of drugs used to treat COVID-19 and their effect on glucose along with their dose and common side effects [92,93]. More than 2200 clinical trials are currently underway seeking to find a vaccine or treatment for COVID-19 [94]. Dexamethasone, an uncommon medication, has been found potential in Covid-19 treatment [94,95]. ChAdOx1 nCoV-19 vaccine displayed an appropriate safety profile, although homologous boosting increased responses to the antibody. Such findings, along with the activation of humoral and cellular immune responses, support this candidate vaccine's wide-ranging evaluation in an ongoing phase 3 trial [96].
Table 4.
List of drugs used for COVID-19 patients and their effect on glucose.
| Sl. no. | Drug name | Drug type | Dose of drugs | Effect on glucose | Common side effects |
|---|---|---|---|---|---|
| 1. | Remdesivir | Adenosine nucleotide analogues | Loading dose of 200 mg IV infusion and maintenance dose of 100 mg IV | Effective glycaemic control | Increased liver enzymes, nausea, vomiting |
| 2. | Ribavirin | Nucleotide analogues | 400 mg every 12 h IV or oral | Effective glycaemic control | Anxiety, mood changes, blurred vision, stomach upset, loss of appetite, dry skin, dry mouth |
| 3. | Chloroquine | 4-aminoquinoline | Loading dose of 300 mg and then 100 mg daily | Effective glycaemic control | Blurred vision, abdominal cramps, hair loss, muscle weakness, headache |
| 4. | Hydroxychloroquine | 4-aminoquinoline | Loading dose of 200 mg oral dose 3 times a day and then 2 tablets per day (BID) | Effective glycaemic control | Headache, dizziness, diarrhoea, stomach cramps, vomiting |
| 5. | Camostat mesilate | Protease inhibitors | 600 mg (200 mg 3times a day) | Effective glycaemic control | Edema and urticaria, pruritus, diarrhoea |
| 6. | Darunavir/cobicistat | Protease inhibitors | 800 mg darunavir and 150 mg cobicistat daily | Effective glycaemic control | Jaundice, ocular icterus, nausea (cobicistat) darunavir data is not available |
| 7. | Lopinavir/ritonavir | Protease inhibitors | 400/100 mg BID | Effective glycaemic control | Diarrhoea, hyperlipidaemia, rash, nausea, vomiting, abdominal pain |
| 8. | Favipiravir | RNA polymerase inhibitors | Low dose: 1000 mg BID for 1 day, 400 mg BID for 4 days High dose: 1200 mg BID for 1 day, 800 mg BID for 4 days |
Effective glycaemic control | Decreased RBC cell production, increase in liver enzymes |
| 9. | Umifenovir | Fusion inhibitor | Adjuvant therapy with other antivirals. Dose regimen not available | Not known | Not known |
| 10. | Interferon-β1 | Cytokines | 250 μg subcutaneous alternate days | Worsen glucose level | Altered blood pressure, fatigue, behavioural and cognitive change, seizures |
| 11. | Aerosolized interferon α | Cytokines | 5 mU BID (nebulization) | Worsen glucose level | Hepatic enzyme abnormalities, renal failure, haemorrhage, myocardial infarction on overdose |
| 12. | Oseltamivir | Neuraminidase inhibitor | 75 mg oral BID | Effective glycaemic control | Diarrhoea, dizziness, headache, eye discomfort, nausea vomiting, nosebleed |
| 13. | Baloxivir marboxil | Viral endonuclease inhibitor | Not known | Worsen glucose level | Unavailable |
| 14. | Tocilizumab | Monoclonal antibody | 400 mg IV in 12 h | Effective glycaemic control | Sinus pain, runny nose, headache, dizziness, itching, mild muscle cramps, urinary tract infection |
| 15. | Sarilumab | Monoclonal antibody | 200 mg IV | Effective glycaemic control | Neutropenia, liver enzyme abnormalities, urinary tract infection and thrombocytopenia |
| 16. | Eculizumab | Monoclonal antibody | 900 mg IV every 7 days | Effective glycaemic control | Neutropenia, urinary tract infection, anaemia, sleep disturbances, |
| 17. | Ribavirin plus Interferon | Mixed | Not known | Not known | Not known |
| 18. | Interferon β plus Lopinavir/Ritonavir | Mixed | Not known | Not known | Not known |
In silico molecular modelling and docking studies has been widely used in identifying newer drugs or repurposing of existing drugs for the treatment of COVID-19. In several of such in silico docking studies lopinavir, remdesivir and ritonavir reported interacting with more than one protein targets of COVID-19. Notably, anti-HIV drug lopinavir, as compared with others closely followed by ritonavir, showed very interesting in silico binding with COVID-19 main protease (Mpro) but showed poor bioavailability in in vivo studies. Most of the HIV protease inhibitors are tested for their action against novel coronavirus infection solely or in a combination form along with some other drugs. Remarkably, these drugs are successful in in silico, in vitro and animal models but unfortunately failed to deliver the maximum efficacy in clinical studies [[97], [98], [99]]. Remdesivir, an analogue of adenosine, is a remdesivir-triphosphate prodrug shows its action by inhibiting RNA-dependent RNA polymerase [100]. In in silico molecular docking simulation studies, remdesivir also demonstrate promising results [101,102]. Other than this, drugs like ribavirin, chloroquine, hydroxychloroquine, camostat mesylate, darunavir, cobicistat, favipiravir, umifenovir, interferon-β1 and Oseltamivir, which are used to treat SARS-CoV-2 in patients with pre-existing diabetic profile, shows good to excellent in silico binding efficacy with the protein structure of COVID-19 Mpro [102]. In a study reported by Shah et al. [101], the protease inhibitor lopinavir interacted with the protein structure of COVID-19 Mpro (PDB: 5R81) and displays H-bond binding interactions with amino acid Glu166, His41 [101]. Nevertheless, it is failed to prove its worth in clinical trials. In the same study, remdesivir showed H-bond interaction with Glu166 and Asn142 of COVID-19 Mpro. It is assumed that the 5-cyano-3,4-dihydroxytetrahydrofuran ring scaffold present in remdesivir is responsible for decent clinical activity against the deadly SARS-CoV-2. In another in silico study, Kumar et al. [102] demonstrate a combination of lopinavir-ritonavir displays various binding interactions with different amino acids of viral protein (SARS-CoV-2 Mpro PDB ID: 6Y2F) and promising antiviral action against SARS-CoV-2. Similarly, baloxivir-marboxil also exhibited interesting in silico interactions with the same protein structure.
9. Unexplored therapies and future prospective
In the absence of any particular drug therapy, various antiviral drugs are used to treat the novel virus infection. Drugs like lopinavir, ritonavir followed by RNA polymerase inhibitors, interferon-1β and hydroxychloroquine are widely used. Some reports suggest that the binding site of the novel coronavirus receptor having a strong affinity with the ACE2. Therefore, it is assumed that the renin-angiotensin system (RAS) inhibitors could have an essential role in the treatment. In the other side, zinc nanoparticles and vitamin C like options not yet tested with currently used drugs, so it is interesting to check their effectiveness in combination with other drugs. There is still no specific vaccine available to treat this novel virus, and the process of development of a vaccine is ongoing, which is assumed to be a significant way to overcome this pandemic. Leprosy medication sepsivac showed promise in the COVID-19 trial, and its efficacy is further being evaluated. Some Rasayana botanicals defined in Ayurveda like Withania somnifera (Ashwagandha), Tinospora cordifolia (Guduchi), Asparagus racemosus (Shatavari), Phyllanthus embelica (Amalaki), and Glycyrrhiza glabra (Yashtimadhu) are potential immunomodulators, may also consider as an add-on treatment for COVID-19 patients. Repurposing of existing drugs could be considered as an excellent option in identifying novel therapies for COVID-19 treatment. The severity of this contagion also warrants the design and development of new chemical entities (NCEs).
10. Conclusion
The Coronavirus disease-19 pandemic is disastrous for world health. The combination of one medical pandemic (COVID-19) with another medical epidemic (diabetes mellitus) seems to be a severe threat to human life. Treatment of diabetes to make sure the patient stays within target ranges, keeping patients well hydrated and following the recommendations to prevent infection is crucial to stop further spread of Coronavirus disease in patients with a chronic condition like diabetes. For endocrinologists, it is a difficult challenge to deal with two or more diseases at the same time in which one is an infectious disease caused by a novel virus. Few articles have reported the relation between diabetes and novel coronavirus infection. A better understanding of the various ways in which endocrine status, and endocrine interventions, influence COVID-19, and vice versa, can help to improve therapeutic outcomes. Research in this particular area is urgently required to deliver an improved understanding concerning possible alterations in genetic predispositions across populations, fundamental pathophysiological mode of action between COVID-19 and diabetes, followed by its clinical management.
Acknowledgments
Acknowledgement
The authors are thankful to i) Manipal Academy of Higher Education (MAHE), Manipal (for Dr. T.M.A. Pai Doctoral Fellowship to Subham Das and Sumit R Birangal and Dr. T.M.A. Pai Postdoctoral Fellowship to Dr. Abhijeet Pandey), ii) All India Council for Technical Education (AICTE), Government of India, New Delhi (for National Doctoral Fellowship to Anu K R), iii) Themis Medicare Limited, Mumbai (for Junior Research Fellowship to Ajinkya N Nikam). The authors also acknowledge Manipal College of Pharmaceutical Sciences, for providing necessary facilities to complete this review. The authors also acknowledge with thanks to BioRender.
Declaration of competing interest
The authors report no conflict of interest.
References
- 1.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil. Med. Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins Coronavirus Resource Center COVID-19 map - Johns Hopkins coronavirus resource center, Johns Hopkins coronavirus Resour. Cent. 2020. https://coronavirus.jhu.edu/map.html
- 3.Worldometer . Worldometer; 2020. Coronavirus Cases; pp. 1–22. [DOI] [Google Scholar]
- 4.IDF Atlas 9th edition and other resources, (n.d.). https://diabetesatlas.org/en/resources/ (accessed April 5, 2020).
- 5.Wang W., Tang J., Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J. Med. Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves C., Casqueiro J., Casqueiro J. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J. Endocrinol. Metab. 2012;16:27. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iglay K., Hannachi H., Howie P.J., Xu J., Li X., Engel S.S., Moore L.M., Rajpathak S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 2016;32:1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 8.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:4–7. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dooley K.E., Chaisson R.E. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng S.-Q., Peng H.-J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020;9:575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.jin Zhang J., Dong X., yuan Cao Y., dong Yuan Y., bin Yang Y., qin Yan Y., Akdis C.A., dong Gao Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur. J. Allergy Clin. Immunol. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 13.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020:20–23. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 15.Z W., JM M. Characteristics of and important lessons from the coronavirus disease 2019(COVID-19) outbreak in China. Jama. 2020;2019 doi: 10.1001/jama.2020.2648. (doi:10.1001/jama.2020.2648) [DOI] [PubMed] [Google Scholar]
- 16.Diabetes and COVID-19 - how to prepare for the coronavirus with diabetes, (n.d.). https://www.endocrineweb.com/conditions/diabetes/diabetes-covid-19 (accessed April 5, 2020).
- 17.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A.K., Gupta R., Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:283–287. doi: 10.1016/j.dsx.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA - J. Am. Med. Assoc. 2019;2020:2019–2020. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 22.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., Kritek P.A., West T.E., Luks A., Gerbino A., Dale C.R., Goldman J.D., O’Mahony S., Mikacenic C. Covid-19 in critically ill patients in the Seattle region — case series. N. Engl. J. Med. 2020:1–11. doi: 10.1056/nejmoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giulia F. 2020. Characteristics of COVID-19 Patients Dying in Italy Report Based on Available Data on March 20th, 2020; pp. 4–8. [Google Scholar]
- 24.Chow N., Fleming-Dutra K., Gierke R., Hall A., Hughes M., Pilishvili T., Ritchey M., Roguski K., Skoff T., Ussery E. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. Morb. Mortal. Wkly Rep. 2020;69:382–386. doi: 10.15585/MMWR.MM6913E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C., Zhang M., Tan J., Xu Y., Song R., Song M., Wang L., Zhang W., Han B., Yang L., Wang X., Zhou G., Zhang T., Li B., Wang Y., Chen Z., Wang X. Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. MedRxiv. 2020;807 doi: 10.1101/2020.02.10.20021584. 2020.02.10.20021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. China medical treatment expert group for Covid-19, clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA - J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;6736:1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;2600:1–7. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020:1–10. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;2019 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P., Xiao W., Wang Y.N., Zhong M.H., Li C.H., Li G.C., Liu H.G. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:1–14. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung C. Clinical features of deaths in the novel coronavirus epidemic in China. Rev. Med. Virol. 2020:5–8. doi: 10.1002/rmv.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020 doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., Sun G.Z., Yang G.R., Zhang X.L., Wang L., Xu X., Xu X.P., Chan J.C.N. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006;23:623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 39.Schoen K., Horvat N., Guerreiro N.F.C., de Castro I., de Giassi K.S. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect. Dis. 2019;19:964. doi: 10.1186/s12879-019-4592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W., Chen H., Li Q., Qiu B., Wang J., Sun X., Xiang Y., Zhang J. Fasting plasma glucose is an independent predictor for severity of H1N1 pneumonia. BMC Infect. Dis. 2011;11:1–6. doi: 10.1186/1471-2334-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huttunen R., Syrjänen J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013;37:333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 42.Honce R., Schultz-Cherry S. Impact of obesity on influenza a virus pathogenesis, immune response, and evolution. Front. Immunol. 2019;10:1–15. doi: 10.3389/fimmu.2019.01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Almond M.H., Edwards M.R., Barclay W.S., Johnston S.L. Obesity and susceptibility to severe outcomes following respiratory viral infection. Thorax. 2013;68:684–686. doi: 10.1136/thoraxjnl-2012-203009. [DOI] [PubMed] [Google Scholar]
- 44.Dixon A.E., Peters U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018;12:755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferlita S., Yegiazaryan A., Noori N., Lal G., Nguyen T., To K., Venketaraman V. Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially mycobacterium tuberculosis. J. Clin. Med. 2019;8:2219. doi: 10.3390/jcm8122219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Critchley J.A., Carey I.M., Harris T., DeWilde S., Hosking F.J., Cook D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care. 2018;41:2127–2135. doi: 10.2337/dc18-0287. [DOI] [PubMed] [Google Scholar]
- 47.He X.W., Lai J.S., Cheng J., Wang M.W., Liu Y.J., Xiao Z.C., Xu C., Li S.S., Zeng H.S. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E011. doi: 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 48.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020:1–9. doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W., Xu Y., Liu B. Pioglitazone Upregulates Angiotensin Converting Enzyme 2 Expression in Insulin-Sensitive Tissues in Rats with High-Fat Diet-Induced Nonalcoholic Steatohepatitis. Sci. World J. 2014;2014 doi: 10.1155/2014/603409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Care D., Suppl S.S. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 52.Mallapaty S. Mounting clues suggest the coronavirus might trigger diabetes. Nature. 2020;583:16–17. doi: 10.1038/d41586-020-01891-8. [DOI] [PubMed] [Google Scholar]
- 53.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis, diabetes. Obes. Metab. 2020:1–7. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res. Clin. Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang L., Han Y., Nilsson-Payant B.E., Gupta V., Wang P., Duan X., Tang X., Zhu J., Zhao Z., Jaffré F., Zhang T., Kim T.W., Harschnitz O., Redmond D., Houghton S., Liu C., Naji A., Ciceri G., Guttikonda S., Bram Y., Nguyen D.H.T., Cioffi M., Chandar V., Hoagland D.A., Huang Y., Xiang J., Wang H., Lyden D., Borczuk A., Chen H.J., Studer L., Pan F.C., Ho D.D., tenOever B.R., Evans T., Schwartz R.E., Chen S. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020:125–136. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finucane F.M., Davenport C. Coronavirus and obesity: could insulin resistance mediate the severity of Covid-19 infection? Front. Public Heal. 2020;8:19–21. doi: 10.3389/fpubh.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devaux C.A., Rolain J., Raoult D. ScienceDirect ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hussain M., Jabeen N., Raza F., Shabbir S., Baig A.A., Amanullah A., Aziz B. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J. Med. Virol. 2020 doi: 10.1002/jmv.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delanghe J.R., Speeckaert M.M., De Buyzere M.L. The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin. Chim. Acta. 2020;505:192–193. doi: 10.1016/j.cca.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatami N., Ahi S., Sadeghinikoo A., Foroughian M., Javdani F., Kalani N., Fereydoni M., Keshavarz P., Hosseini A. Worldwide ACE (I/D) polymorphism may affect COVID-19 recovery rate: an ecological meta-regression. Endocrine. 2020:479–484. doi: 10.1007/s12020-020-02381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30–33. doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ciaglia E., Vecchione C., Puca A.A. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front. Pediatr. 2020;8:11–13. doi: 10.3389/fped.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laffel L. Sick-day management in type 1 diabetes. Endocrinol. Metab. Clin. N. Am. 2000;29:707–723. doi: 10.1016/S0889-8529(05)70160-2. [DOI] [PubMed] [Google Scholar]
- 67.Umpierrez G.E., Pasquel F.J. Management of inpatient hyperglycemia and diabetes in older adults. Diabetes Care. 2017;40:509–517. doi: 10.2337/dc16-0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prajapa M., Sarma P., Shekhar N., Avti P., Sinha S., Kaur H., Kumar S., Bhattacharyya H., Kumar Anusuya, Bansal S., Medhi S. Drug targets for corona virus: a systematic review. Indian J. Pharmacol. 2020;52:56–65. doi: 10.4103/ijp.IJP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yi D., Kang Y.H. Drug-induced diabetes mellitus Dongwon. J. Korean Diabetes. 2017;18:160–168. [Google Scholar]
- 70.Pandey A., Nikam A.N., Shreya A.B., Mutalik S.P., Gopalan D., Kulkarni S., Padya B.S., Fernandes G., Mutalik S., Prassl R. Potential therapeutic targets for combating SARS-CoV-2: drug repurposing, clinical trials and recent advancements. Life Sci. 2020:117883. doi: 10.1016/j.lfs.2020.117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.I., Skyler J.S., DeVries J.H., Renard E., Eckel R.H., Zimmet P., Alberti K.G., Vidal J., Geloneze B., Chan J.C., Ji L., Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.I., Skyler J.S., DeVries J.H., Renard E., Eckel R.H., Zimmet P., Alberti K.G., Vidal J., Geloneze B., Chan J.C., Ji L., Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8587:1–5. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Adaptive COVID-19 treatment trial (ACTT) - full text view - ClinicalTrials.gov, (n.d.). https://clinicaltrials.gov/ct2/show/NCT04280705 (accessed April 5, 2020).
- 75.Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012;2012 doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X., Ju X., Liang Z., Liu Q., Zhao Y., Guo F., Bai T., Han Z., Zhu J., Zhou H., Huang F., Li C., Lu H., Li N., Li D., Jin N., Penninger J.M., Jiang C. Angiotensin-converting enzyme 2 protects from lethal avian influenza a H5N1 infections. Nat. Commun. 2014;5:1–7. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (80-. ) 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Romaní-Pérez M., Outeiriño-Iglesias V., Moya C.M., Santisteban P., González-Matías L.C., Vigo E., Mallo F. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156:3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 80.Cure E., Cumhur Cure M. Can dapagliflozin have a protective effect against COVID-19 infection? A hypothesis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:405–406. doi: 10.1016/j.dsx.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Investig. 2020:2–4. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gentile S., Strollo F., Ceriello A. COVID-19 infection in Italian people with diabetes: lessons learned for our future (an experience to be used) Diabetes Res. Clin. Pract. 2020;162:108137. doi: 10.1016/j.diabres.2020.108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H., Zhou Y., Zhang M., Wang H., Zhao Q., Liu J. Updated approaches against SARS-CoV-2. Antimicrob. Agents Chemother. 2020 doi: 10.1128/AAC.00483-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.WHO Landscape analysis of COVID therapeutics as 21 march 2020. 2020. https://www.who.int/blueprint/priority-diseases/key-action/Table_of_therapeutics_Appendix_17022020.pdf?ua=1
- 86.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:1–2. doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- 88.Rekedal L.R., Massarotti E., Garg R., Bhatia R., Gleeson T., Lu B., Solomon D.H. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Care Res. 2010;62:3569–3573. doi: 10.1002/art.27703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gerstein H.C., Thorpe K.E., Taylor D.W., Haynes R.B. The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas — a randomized trial. Diabetes Res. Clin. Pract. 2002;55:209–219. doi: 10.1016/s0168-8227(01)00325-4. [DOI] [PubMed] [Google Scholar]
- 90.Kumar V., Singh M.P., Singh A.P., Pandey M.S., Kumar S., Kumar S. Efficacy and safety of hydroxychloroquine when added to stable insulin therapy in combination with metformin and glimepiride in patients with type 2 diabetes compare to sitagliptin. Int. J. Basic Clin. Pharmacol. 2018;7:1959. doi: 10.18203/2319-2003.ijbcp20183930. [DOI] [Google Scholar]
- 91.Emami J., Pasutto F.M., Mercer J.R., Jamali F. Inhibition of insulin metabolism by hydroxychloroquine and its enantiomers in cytosolic fraction of liver homogenates from healthy and diabetic rats. Life Sci. 1998;64:325–335. doi: 10.1016/S0024-3205(98)00568-2. [DOI] [PubMed] [Google Scholar]
- 92.Pal R., Bhadada S.K. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab. Syndr. 2020;14:513–517. doi: 10.1016/j.dsx.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clin. Trials Arena; 2020. Dexamethasone, an unexpected drug, shows promise in treating Covid-19. https://www.clinicaltrialsarena.com/comment/dexamethasone-covid-19/
- 95.Mahase E. Covid-19: demand for dexamethasone surges as RECOVERY trial publishes preprint. BMJ. 2020;369:m2512. doi: 10.1136/bmj.m2512. [DOI] [PubMed] [Google Scholar]
- 96.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020:1–13. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Choy K.T., Wong A.Y.L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.H., Huang X., Peiris M., Yen H.L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith T., Bushek J., Prosser T. COVID-19 drug therapy highlights: antimicrobials with potential activity against SARS-CoV-2. Clin. Drug Inf. 2020:1–21. [Google Scholar]
- 100.Elfiky A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah B., Modi P., Sagar S.R. In silico studies on therapeutic agents for COVID-19: drug repurposing approach. Life Sci. 2020;252 doi: 10.1016/j.lfs.2020.117652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumar Y., Singh H., Patel C.N. In silico prediction of potential inhibitors for the Main protease of SARS-CoV-2 using molecular docking and dynamics simulation based drug-repurposing. J. Infect. Public Health. 2020:19–21. doi: 10.1016/j.jiph.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]