Highlights
-
•
Lymphocyte subsets are severe indicators in COVID-19 patients.
-
•
Total T cell counts were positively correlated with the time of symptom onset.
-
•
T lymphocyte subsets were associated with the occurrence of composite endpoint events.
Keywords: Coronavirus disease 2019, Pneumonia, Lymphocyte subsets, Immune parameters
Abstract
Objective
To investigate the characteristics and predictive roles of lymphocyte subsets in COVID-19 patients.
Method
We evaluated lymphocyte subsets and other clinical features of COVID-19 patients, and analyzed their potential impacts on COVID-19 outcomes.
Results
1. Lymphocyte subset counts in the peripheral blood of patients with COVID-19 were significantly reduced, especially in patients with severe disease. 2. In patients with non-severe disease, the time from symptom onset to hospital admission was positively correlated with total T cell counts. 3. Among COVID-19 patients who did not reach the composite endpoint, lymphocyte subset counts were higher than in patients who had reached the composite endpoint. 4. The Kaplan-Meier survival curves showed significant differences in COVID-19 patients, classified by the levels of total, CD8+, and CD4+ T cells at admission.
Conclusion
Our study showed that total, CD8+, and CD4+ T cell counts in patients with COVID-19 were significantly reduced, especially in patients with severe disease. Lower T lymphocyte subsets were significantly associated with a higher occurrence of composite endpoint events. These subsets may help identify patients with a high risk of composite endpoint events.
The outbreak of coronavirus disease 2019 (COVID-19) in early 2020 has now become an unprecedented global health crisis (Zhu et al., 2020, Li et al., 2020). On March 11, 2020, the World Health Organization announced that COVID-19 had the characteristics of a global pandemic (World Health Organization, 2019a). The pandemic has since accelerated at an exponential rate. Currently, COVID-19 has spread to more than 200 countries and regions around the world. As of 6 June 2020, there were 6 772 520 confirmed cases and 393 843 deaths from COVID-19 (World Health Organization, 2019b; Onder et al., 2020).
Previous studies on the clinical characteristics of COVID-19 have shown that patients with COVID-19 may have mild or severe symptoms of acute respiratory infection. Patients with mild disease have symptoms such as fever, dry cough, fatigue and abnormal chest CT, but their prognosis is good. In contrast, some patients’ conditions progress rapidly and develop into acute respiratory distress syndrome (ARDS) or even multiple organ failure (MOF) in a very short time (Wu and McGoogan, 2020). According to different research reports, the mortality rate is between 2.3% and 15% (Wu and McGoogan, 2020; Huang et al., 2020). Immune factors may play an important role in the rapid progression of this disease. Therefore, studying the role of immune factors is important for the early identification of patients with severe COVID-19 and timely intervention is very important for control of the disease.
In this study, we retrospectively reviewed the clinical and laboratory data of 90 patients with COVID-19 diagnosed in the Central Hospital of Wuhan from January to March 2020. We examined the association of lymphocyte subsets with the progression and prognosis of COVID-19 patients.
Methods
Study design and participants
This was a retrospective single-center study, recruiting patients admitted to the Central Hospital of Wuhan, which is close to the Huanan seafood market and was one of the first designated hospitals to receive COVID-19 patients.
The retrospective analysis involved patients diagnosed with COVID-19 from January to March, 2020. All patients were positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) according to quantitative RT-PCR (qRT-PCR). Patients who were still hospitalized after March 16, 2020 were excluded. Ultimately, 90 confirmed COVID-19 patients were included in the analyses.
Patients were divided into a non-severe cohort and a severe cohort according to their condition. All COVID-19 patients were classified as having non-severe to severe disease at admission according to the COVID-19 Guidelines (seventh edition) produced by the National Health Commission of the People’s Republic of China. With regard to the classification criteria, patients with mild disease presented with mild symptoms and exhibited normal radiology findings in both lungs. Patients with moderate (typical) disease presented typical symptoms (fever, cough, and other respiratory symptoms) and radiological manifestations suggesting pneumonia. Patients with severe disease presented with any one of the following: 1) respiratory distress, defined as a respiratory rate ≥ 30 breaths per minute; 2) a pulse oxygen saturation ≤ 93% on room air; or 3) an oxygenation index (PaO2/FiO2) ≤ 300 mmHg. Critically ill patients presented with any one of the following: 1) respiratory failure for which invasive ventilation was necessary; 2) signs of shock (circulatory failure); or 3) failure of any other organ such that ICU care was necessary. The non-severe cohort included patients with mild or moderate disease, and the severe cohort included patients with severe disease or who were critically ill. This study was approved by the ethics committees of the Central Hospital of Wuhan. As this study was a retrospective analysis of electronic data, no informed consent was obtained.
The composite endpoint included admission to the intensive care unit (ICU), mechanical ventilation, or death. Based on this, patients were divided into a composite endpoint group and a non-composite endpoint group.
Data collection
Data were collected on age, sex, smoking history, the time from symptom onset to hospital admission, symptoms from onset to hospital admission (fever, mild shortness of breath, cough, expectoration, fatigue, diarrhea, myalgia), complications during hospitalization (ARDS, bacterial infection, septic shock, liver damage), laboratory findings at admission (lymphocyte subset counts, leukocyte counts, neutrophil counts, lymphocyte counts, monocyte counts, hemoglobin concentrations, platelet counts, hypersensitive C-reactive protein levels, procalcitonin levels, lactate dehydrogenase levels, partially activated prothrombin time, prothrombin time, fibrinogen levels, D-dimer levels and PaO2/FiO2 levels), and computed tomography (CT) scans at admission.
Statistical analysis
SPSS 22.0 statistical analysis software was used for data analysis. The Shapiro-Wilk normality test and the variance homogeneity test were used for measurement data. The measurement data with normal distribution were described as means ± standard deviations, and the skewed data with non-normal distribution were described as medians (P25–P75). The differences between the two endpoint groups were compared.
The measurement data with normal distribution and homogeneity of variance were compared between groups using a two-independent-samples t-test. Measurement data satisfying the normal distribution but not the homogeneity of variance criteria were compared between groups using the Satterthwaite t-test. According to the applicable conditions, the chi-square test, corrected chi-square test, and Fisher’s exact probability method were used. The Spearman rank test was used to analyse the correlations between measurement data. The influence of lymphocyte subsets on composite endpoint events was analysed by stepwise forward logistic regression. ROC curves were used to calculate the cut-off points for classifying the T lymphocyte subsets. Finally, Kaplan-Meier survival curves were used to analyse the influence of immune parameters on the occurrence of composite endpoint events; α = 0.05 was considered significant.
Results
Comparison of clinical features between severe and non-severe patients with COVID-19
A total of 61 patients (67.78%) had fever before admission. Most of the patients had pneumonia symptoms, including fatigue (33.33%), expectoration (31.1%), cough (30%), and mild shortness of breath (23.33%). Only 11.11% of the patients had diarrhea. Compared with patients with non-severe disease, those with severe disease were more likely to have fever, fatigue, expectoration, and myalgia (Table 1 ).
Table 1.
Baseline clinical features of 90 patients with COVID-19.
| Variables | No. (%) | ||||
|---|---|---|---|---|---|
| Total | Non-severe | Severe | t/χ2/Fisher | p-value | |
| (n = 90) | (n = 70) | (n = 20) | |||
| Age, mean ± SD | 51.82 ± 17.56 | 50.33 ± 17.65 | 57.1 ± 16.69 | 1.521 | 0.132 |
| Male | 49 (54.44) | 36 (51.43) | 13 (65.00) | 1.155a | 0.282 |
| Smoking history | |||||
| Never a smoker | 81 (90.00) | 64 (91.43) | 17 (85.00) | 1.404b | 0.526 |
| Ex-smoker | 6 (6.67) | 4 (5.71) | 2 (10.00) | – | |
| Current smoker | 3 (3.33) | 2 (2.86) | 1 (5.00) | – | |
| Signs and symptoms at admission | |||||
| Fever | 61 (67.78) | 43 (61.43) | 18 (90.00) | 5.814a | 0.016 |
| Mild shortness of breath | 21 (23.33) | 16 (22.86) | 5 (25.00) | 0.000c | 1.000 |
| Cough | 27 (30.00) | 24 (34.29) | 3 (15.00) | 0.755a | 0.097 |
| Expectoration | 28 (31.11) | 18 (25.71) | 10 (50.00) | 4.281a | 0.039 |
| Fatigue | 30 (33.33) | 18 (25.71) | 12 (60.00) | 8.229a | 0.004 |
| Diarrhea | 10 (11.11) | 9 (12.86) | 1 (5.00) | 0.340c | 0.560 |
| Myalgia | 11 (12.22) | 5 (7.14) | 6 (30.00) | 5.594c | 0.018 |
| Complications during hospitalization | |||||
| Acute respiratory distress syndrome | 11 (12.22) | 0 (0.00) | 11 (55.00) | 38.884c | <0.001 |
| Bacterial infection | 5 (5.56) | 0 (0.00) | 5 (25.00) | 14.071c | <0.001 |
| Septic shock | 2 (2.22) | 0 (0.00) | 2 (10.00) | b | 0.047 |
| Liver damage | 14 (15.56) | 7 (10.00) | 7 (35.00) | 5.620c | 0.018 |
| CT findings | |||||
| Unilateral pneumonia | 14 (15.56) | 14 (20.00) | 0 (0.00) | 3.337c | 0.068 |
| Bilateral pneumonia | 66 (73.33) | 46 (65.71) | 20 (100.00) | 9.351a | 0.002 |
a: chi-square test, b: Fisher’s exact probability method, c: corrected chi-square test.
There were no significant differences in age, sex, or smoking history between severe and non-severe groups. All patients underwent chest CT examination on admission. The most common abnormality was bilateral pneumonia (66; 73.3%) (Table 1). During hospitalization, the incidence of complications (acute respiratory distress syndrome, bacterial infection, septic shock, liver damage) in the severe group was higher than that in the non-severe group.
Compared with those of non-severe COVID-19 patients, the laboratory parameters for severe COVID-19 patients on admission, including hematological indicators (WBC, lymphocyte, and platelet counts), coagulation function parameters (fibrinogen and D-dimer levels), infection-related biomarkers (CRP, procalcitonin, and LDH levels) and PaO2/FiO2 level showed extensive and significant differences (Table 2 ).
Table 2.
Laboratory findings in 90 patients with COVID-19 at admission.
| Total | Non-severe | Severe | ||
|---|---|---|---|---|
| Laboratory finding | (n = 90) | (n = 70) | (n = 20) | p-value |
| WBCs, × 109/L | 5.95 ± 2.58 | 5.57 ± 1.56 | 7.27 ± 4.46 | 0.008 |
| Neutrophil count, × 109/L | 3.32 (2.48–4.31) | 3.24(2.47–4.18) | 3.88(2.76–9.97) | 0.093 |
| Lymphocyte count, × 109/L | 1.30 (0.89–1.86) | 1.50(1.08–2.05) | 0.63(0.48–1.12) | <0.001 |
| Monocyte count, × 109/L | 0.34 (0.27–0.48) | 0.34(0.28–0.48) | 0.35(0.24–0.46) | 0.927 |
| Hemoglobin, g/L | 134.50 (123.00–144.00) | 135.00 (123.00–144.00) | 133.00 (126.00–144.50) | 0.900 |
| Platelet count, × 109/L | 189.50 (143.00–248.00) | 209.00 (159.00–259.00) | 165.00 (125.00–178.00) | 0.003 |
| APTT, s | 26.80 (24.80–30.30) | 26.70 (24.90–30.30) | 28.00 (23.55–32.60) | 0.917 |
| PT, s | 11.50 (10.80–12.00) | 11.40 (10.70–11.90) | 11.85 (11.30–12.55) | 0.051 |
| D-dimer, ug/mL.FEU | 0.46 (0.19–1.00) | 0.38 (0.16–0.73) | 1.60 (0.51–3.12) | <0.001 |
| Fibrinogen, g/L | 2.44 (2.12–3.11) | 2.30 (2.10–2.68) | 3.15 (2.64–3.89) | <0.001 |
| CRP, mg/dL | 0.36 (0.08–3.68) | 0.17 (0.06–0.95) | 6.28 (2.49–10.49) | <0.001 |
| Procalcitonin, ng/mL | 0.05 (0.04–0.10) | 0.04 (0.04–0.06) | 0.17 (0.08–0.45) | <0.001 |
| LDH, U/L | 186.00 (138.00–223.00) | 160.00 (134.00–200.00) | 220.50 (188.00–364.50) | <0.001 |
| PaO2/FiO2, mmHg | 345.50 (301.00–381.00) | 355.00 (336.00–394.00) | 216.00 (197.50–260.00) | <0.001 |
The data are shown as median value (interquartile range).
Abbreviations: WBC white blood cell; APTT activated partial thromboplastin time; PT prothrombin time; CRP C-reactive protein; LDH lactate dehydrogenase.
Comparison of lymphocyte subsets between severe and non-severe groups
Among the non-severe COVID-19 patients, the median total T cell, CD8+ T cell, CD4+ T cell, NK cell, and B cell counts were 1170, 430, 670, 210, and 150, respectively, while the median values decreased to 380, 180, 180, 80, and 80, respectively, in severe COVID-19 patients. The counts of total T cells, CD8+ T cells, CD4+ T cells, NK cells, and B cells were significantly lower in patients with severe disease than in patients with non-severe disease (Figure 1 ).
Figure 1.
Comparison of lymphocyte subsets between severe and non-severe groups: severe group (n = 70); non-severe group (n = 20). A: total T cells; B: CD8+ T cells; C: CD4+ T cells; D: B cells; E: NK cells. ***p < 0.001.
Correlations between lymphocyte subsets and the time from symptom onset to hospital admission
In patients with non-severe disease, the time from symptom onset to hospital admission was positively correlated with total T cell counts (r = 0.251; p < 0.05), while other lymphocyte subsets showed no significant correlation with the time from symptom onset to hospital admission (Table 3 , Figure 2 )
Table 3.
Correlations between lymphocyte subsets and time from symptom onset to hospital admission.
| Time from symptom onset to hospital admission |
||||
|---|---|---|---|---|
| Non-severe (n = 70) |
Severe (n = 20) |
|||
| rs | p | rs | p | |
| Total T cells | 0.251 | 0.036 | ―0.166 | 0.485 |
| CD8+ T cells | 0.240 | 0.058 | ―0.329 | 0.157 |
| CD4+ T cells | 0.266 | 0.061 | ―0.106 | 0.656 |
| NK cells | ―0.032 | 0.795 | ―0.118 | 0.629 |
| B cells | 0.179 | 0.139 | ―0.464 | 0.059 |
| CD4+/CD8+ | ―0.018 | 0.885 | 0.053 | 0.825 |
Figure 2.
Correlations between total T cells and the time from symptom onset to hospital admission in non-severe patients (n = 70).
Treatment and prognosis
During hospitalization, patient treatments mainly included antiviral therapy (81.1%), antibiotic therapy (82.2%), glucocorticoids (35.6%), and immunoglobulin (35.6%). The common antiviral treatments included arbidol (67.8%), oseltamivir (24.4%), lopinavir and ritonavir (5.6%), and interferon (16.7%), with more than one-third of patients taking more than one antiviral drug. High-flow oxygen therapy was required in 13 patients (14.4%). Invasive mechanical ventilation was required in five patients (5.6%), while 10 patients (11.1%) were admitted to the ICU. As of March 16, 87 (96.7%) patients were discharged, and three (3.3%) died.
Comparison of lymphocyte subsets between composite endpoint and non-composite endpoint groups
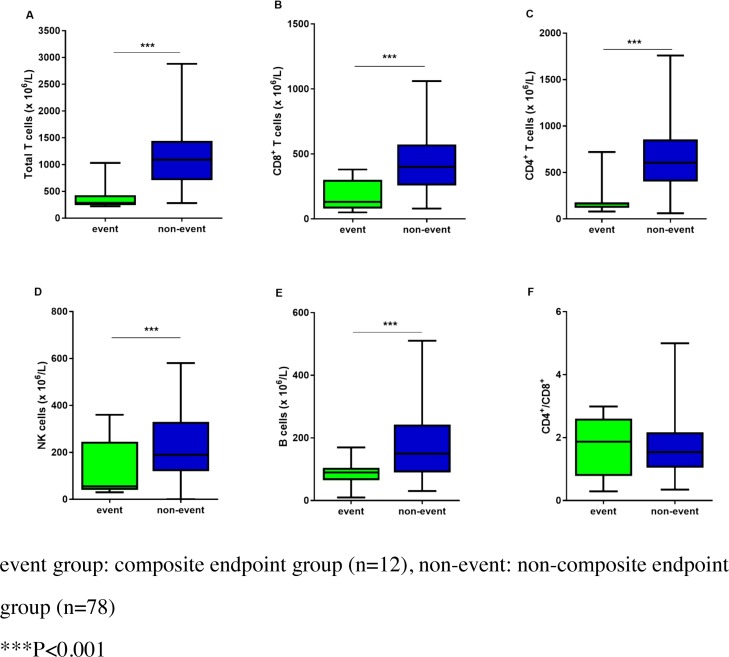
Among the COVID-19 patients who did not reach the composite endpoint, the median total T cell, CD8+ T cell, CD4+ T cell, NK cell, and B cell counts were 1090, 400, 610, 190, and 150, respectively, while the median values decreased to 290, 130, 170, 60, and 90, respectively, in patients who reached the composite endpoint. The counts of total T cells, CD8+ T cells, CD4+ T cells, NK cells, and B cells were significantly lower in patients who reached the composite endpoint than in patients who did not reach it (Figure 3 ).
Figure 3.
Comparison of lymphocyte subsets between composite endpoint and non-composite endpoint groups: composite endpoint group (n = 12); non-composite endpoint group (n = 78). A: total T cells; B: CD8+ T cells; C: CD4+ T cells; D: B cells; E: NK cells. ***p < 0.001.
Total T cell counts can be used as a predictive factor for the composite endpoint in COVID-19
Stepwise forward logistic regression was used to measure the potential association between lymphocyte subsets and composite endpoints. We found that lower total T cell counts were associated with a higher risk of composite endpoint events (OR = 0.001, 95% CI 0.000–0.038, p = 0.001) (Table 4 ). In addition, total, CD8+, and CD4+ T cells were selected as candidate markers for predicting composite endpoints. Our results showed that T lymphocyte subsets were highly correlated with the composite endpoint in COVID-19 patients. The area under the ROC curve (AUC) and the ROC curve were used to evaluate the prediction of composite endpoints in COVID-19 patients.
Table 4.
Association between lymphocyte subsets and composite endpoint events by stepwise forward logistic regression.
| B | S.E. | Wald | Exp(B) | p | 95% CI | |
|---|---|---|---|---|---|---|
| Total T cells | ―7.542 | 2.185 | 11.911 | 0.001 | 0.001 | 0.000–0.038 |
| Intercept | 2.861 | 1.087 | 6.932 | 17.474 | 0.008 |
The area under the ROC curve was 0.935 (95% CI 0.857–1.000) for total T cell counts, 0.864 (95% CI 0.761–0.967) for CD8+ T cell counts, and 0.926 (95% CI 0.825–1.000) for CD4+ T cell counts. Cut-off values, including those for total T cells (585), CD8+ T cells (325), and CD4+ T cells (202), were calculated according to the ROC curve (Figure 4). The Kaplan-Meier survival curves showed that lower levels of total, CD8+, and CD4+ T cells at admission were associated with higher occurrences of the composite endpoint events in COVID-19 patients (Figure 5 ).
Figure 4.
ROC analysis of parameters predicting the composite endpoint in COVID-19 patients.
Figure 5.
Kaplan-Meier analysis of composite endpoint events in COVID-19 patients according to total, CD8+, and CD4+ T cell counts.
Discussion
This study assessed the severity and progression of COVID-19, alongside data for T lymphocyte subsets, resulting in some novel findings. First, lymphocyte subsets were associated with COVID-19 severity and progression. Second, in patients with non-severe disease, total T cell counts recovered as the number of days past disease onset increased. Finally, total, CD8+, and CD4+ T cell counts could serve as risk predictors for fatal outcomes of COVID-19.
Our study described a cohort of 90 COVID-19 patients who were hospitalized in the Central Hospital of Wuhan. In line with the COVID-19 Guidelines (seventh version), 20 out of 90 patients were included in the severe group, accounting for approximately 22.2% of the total cases — similar to the situations reported in previous studies (Wu and McGoogan, 2020). The clinical features of patients with COVID-19 have recently been reported (Wu and McGoogan, 2020; Huang et al., 2020, Chen et al., 2020a). The most common clinical manifestations at the onset of illness in our study included fever, fatigue, expectoration, cough, and mild shortness of breath. Severe cases were more likely than non-severe cases to have fever, expectoration, and fatigue, and to develop ARDS.
Our study indicated that the total, CD8+, and CD4+ T cell counts in the peripheral blood of patients with COVID-19 were significantly reduced, especially in patients with severe disease. Postmortem findings have shown that lymphocyte counts — including those for CD8+ and CD4+ T cells — in the spleen and lymph nodes are significantly reduced. Increases in levels of the cytokine IL-6 were more significant in patients with severe disease than in those with non-severe disease, and were negatively correlated with the number of T subsets (data not shown), which is consistent with previous results (Bo et al., 2020). This may be related to a systemic inflammatory response caused by a cytokine storm, similar to that observed in SARS patients.
In COVID-19 patients, T cell overactivation and T cell exhaustion may exist simultaneously. CD4+ T cells are highly active, the absolute numbers of CD4+ T cells are decreased, and secretion of IFN-r is weakened; in addition, T cell exhaustion is more obvious in patients with severe disease than in those with non-severe disease (Chen et al., 2020b). In viral infections, co-expression of Tim-3 and PD-1 may indicate severe exhaustion of T cells. The levels of PD-1 and Tim-3 in T cells of patients with COVID-19 are significantly higher than those in T cells of healthy controls. Increased expression of PD-1 and Tim-3 in T cells can be used as a marker for progression from the prodromal to the obviously symptomatic stage, further indicating T cell exhaustion (Bo et al., 2020). Excessive activation of T cells, characterized by increases in Th17 populations and high cytotoxicity of CD8+ T cells, may be part of the cause of immune injury in patients (Xu et al., 2020). It has been reported that after SARS-CoV-2 infection, CD4+ T lymphocytes are rapidly activated into pathogenic T helper (Th) 1 cells. Next, the pathogenic Th1 cells become activated and secrete proinflammatory cytokines, such as IL-6. Inflammatory monocytes are activated and proliferate, and the activated immune cells enter the pulmonary circulation, leading to serious lung injury. Blocking GM-CSF or IL-6 may inhibit immunopathological injury (Zhou et al., 2020).
Therefore, monitoring of lymphocyte subsets and cytokines can provide early warnings of severe disease in patients. According to an expert consensus statement from Shanghai, the early warning indicators of severe disease in patients include CD4+ T lymphocyte numbers < 250/μL and significantly increased levels of IL-6 in blood. In addition, it is recommended to detect lymphocyte subsets and IL-6 at least once every 3 days. During clinical treatment, if IL-6 levels are increased, patients can be given the IL-6 receptor monoclonal antibody tocilizumab. If lymphocyte or CD4+ T cell counts are reduced, patients can be treated with thymosin.
In this study, we observed that in patients with non-severe disease, the number of days past the time from symptom onset to hospital admission was correlated with higher total T cell counts. In severe patients, it was observed that the more days past onset at admission, the lower the lymphocyte subset counts, but the differences were not statistically significant. The possible reasons for these non-significant results were: 1) the number of selected cases in this study was relatively small; 2) the total number of lymphocytes was low in patients with severe disease. Some studies on immune indicators have observed that changes in T lymphocytes in the course of disease are closely related to the severity of COVID-19 (Wang et al., 2020, He et al., 2020). Studies have also shown that immune factors after coronavirus infection play an important role in the progression of the disease. The acquired immunity to the coronavirus produced by the body is necessary for effective viral clearance. It has been reported that the number of CD4+ T cells may help predict the clearance of viral RNA, with fewer CD4+ T cells before treatment leading to slower clearance of the virus (Yun et al., 2020).
We found that the lymphocyte subset counts in patients who reached the composite endpoint were significantly lower than in patients who did not reach the composite endpoint. Lower total T cell counts were associated with a higher risk of composite endpoint events. Our data also showed that the numbers of total, CD8+, and CD4+ T cells were closely related to the occurrence of composite endpoint events in these patients, suggesting that T cell counts could act as independent risk factors for the clinical prognosis of these patients.
Our study had some limitations. First, it was a retrospective study, so there could have been systematic selection bias. Second, due to the shortage of medical resources and the urgency of time during the outbreak of the epidemic in Wuhan, there was no opportunity for detection of viral load in patients. We suggest that a multicenter follow-up study with a larger cohort is urgently needed.
Conclusions
In conclusion, our study showed that the total, CD8+, and CD4+ T cell counts in the peripheral blood of patients with COVID-19 were significantly reduced, especially in patients with severe disease. The total, CD8+, and CD4+ T cell counts were significantly associated with a higher occurrence of composite endpoint events. Our findings showed significant differences in T cell counts between the non-severe and severe groups, which may help to identify patients with a high risk of composite endpoint events.
Ethical approval
This study was approved by the ethics committees of the Central Hospital of Wuhan. As this study was a retrospective analysis of electronic data, no informed consent was obtained.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by the Wuhan Municipal Health Commission (grant number WX17Q06).
References
- Bo D., Chenhui W., Yingjun T. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R., Lu Z., Zhang L. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2019. Coronavirus Diseases 2019 (COVID-19) Situation Report-51.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar]
- World Health Organization . 2019. Coronavirus Diseases 2019 (COVID-19) Situation Report-140.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200608-covid-19-sitrep-140.pdf?sfvrsn=2f310900_2 [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Ling, Shui-Bao Xu, Yi-Xiao Lin. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J. 2020;133(2020-02–25) doi: 10.1097/CM90000000000000774. http://rs.yiigle.com/yufabiao/1182667.htm (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Yonggang, Fu Binqing, Zheng Xiaohu. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. medRxiv. 2020 doi: 10.1101/2020.02.12.945576. [DOI] [Google Scholar]
- Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]