Abstract
Background and aim
COVID-19 pandemic has resulted in an unprecedented increased usage of Personal protective equipment (PPE) by healthcare-workers. PPE usage causes headache in majority of users. We evaluated changes in cerebral hemodynamics among healthcare-workers using PPE.
Methods
Frontline healthcare-workers donning PPE at our tertiary center were included. Demographics, co-morbidities and blood-pressure were recorded. Transcranial Doppler (TCD) monitoring of middle cerebral artery was performed with 2-MHz probe. Mean flow velocity (MFV) and pulsatility index (PI) were recorded at baseline, after donning N95 respirator-mask, and after donning powered air-purifying respirator (PAPR), when indicated. End-tidal carbon-dioxide (ET-CO2) pressure was recorded for participants donning PAPR in addition to the N95 respirator-mask.
Results
A total of 154 healthcare-workers (mean age 29 ± 12 years, 67% women) were included. Migraine was the commonest co-morbidity in 38 (25%) individuals while 123 (80%) developed de-novo headache due to N95 mask. Donning of N95 respirator-mask resulted in significant increase in MFV (4.4 ± 10.4 cm/s, p < 0.001) and decrease in PI (0.13 ± 0.12; p < 0.001) while ET-CO2 increased by 3.1 ± 1.2 mmHg (p < 0.001). TCD monitoring in 24 (16%) participants donning PAPR and N95 respirator mask together showed normalization of PI, accompanied by normalization of ET-CO2 values within 5-min. Combined use of N95 respirator-mask and PAPR was more comfortable as compared to N95 respirator-mask alone.
Conclusion
Use of N95 respirator-mask results in significant alterations in cerebral hemodynamics. However, these effects are mitigated by the use of additional PAPR. We recommend the use of PAPR together with the N95 mask for healthcare-workers doing longer duties in the hospital wards.
Keywords: Transcranial Doppler (TCD), Personal protection equipment (PPE), Powered air-purifying respirator (PAPR), Carbon dioxide (CO2), Headache, Healthcare workers, Face-mask, N95, Respirator, Cerebral hemodynamics, Coronavirus disease, COVID-19
Highlights
-
•
Most N95 mask users develop de novo headache or worsening of pre-existing headache.
-
•
End-tidal carbon dioxide levels increase while donning N95 respirator mask.
-
•
Significant cerebral hemodynamic changes occur during donning of N95 mask.
-
•
Powered air-purifying respirator restores N95 mask induced cerebral hemodynamic changes.
1. Introduction
During the rapidly emerging coronavirus disease 2019 (COVID-19) pandemic, there has been an unprecedented rise in the usage of personal protective equipment (PPE) by healthcare workers [1]. Essentially, PPE comprises of N95 respirator, protective eyewear (goggles or face shield), gown and gloves [2]. Additionally, healthcare professionals deployed to the hospital areas where extremely high-risk aerosol-generating medical procedures are performed, it is imperative to use powered air-purifying respirators (PAPR), in addition to PPE [3]. Although, these equipment offer a high level of protection to healthcare professionals in fighting infections like COVID-19, it causes physical discomfort, resulting in poor compliance, especially when PPE is donned for prolonged duration [4,5].
Previous studies have shown that there is a significant increase in headache among frontline healthcare workers who used PPE for prolonged hours during Severe Acute Respiratory Syndrome (SARS) and COVID-19 outbreak [6,7]. One possible explanation for development of headache among PPE users was the external compression of sensitive facial skin and superficial nerves by face mask and its tight straps, especially when used for longer duration [6]. However, altered cerebral hemodynamics due to hypoxemia and carbon di-oxide (CO2) retention have also been proposed to contribute to PPE-induced headache, especially for those who develop headache within minutes of wearing N95 respirator mask [8,9]. We evaluated cerebral hemodynamic changes during donning of N95 respirator mask alone as well together with PAPR among frontline healthcare workers during COVID-19 pandemic.
2. Methods
As a part of our hospital's infectious disease policy, all frontline workers were required to be mask-fitted and wear full PPE while managing patients with suspected and confirmed COVID-19 infection. A subgroup of the medical personnel involved in performing high-risk procedures like throat swabbing, endotracheal intubation or bronchoscopy underwent training for donning PAPR due to the heightened risk of aerosolization of COVID-19 virus.
2.1. Study population
This cross-sectional study was conducted between February and April 2020 on healthcare workers who underwent evaluation for headache related to the use of PPE at our tertiary institution [7]. The study was approved by institutional review board. All participants were at least 21 years old and agreed to participate in the study by signing an informed consent form. Baseline demographics, comorbidities, medical history and duration of N95 respirator use were prospectively recorded. All participants were mask-fitted for the 3 M® N95 respirator mask, certified by the National Institute for Occupational Safety and Health to filter out 95% of particles greater than 0.3 μm in size, protecting the user from inhalation of viral particles when worn appropriately. A subgroup of the participants were trained for additional donning of the PAPR (3 M® Versaflo® TR-300 series).
Participants with history of cerebrovascular disease, atrial fibrillation, intra- or extra-cranial arterial steno-occlusive disease were excluded.
2.2. Cerebral hemodynamics evaluation
We used transcranial Doppler (TCD) to monitor non-invasively, the cerebral blood flow changes in real-time [[10], [11], [12]]. In addition, TCD portability facilitated the test performance in individual wards, while adhering to the hospital-wide infection control and segregation policy during the current COVID-19 outbreak. Serial cerebral hemodynamic changes during the donning of PPE and PAPR in selected cases were assessed using TCD monitoring.
All TCD evaluations were performed by experienced and credentialed sonographers (CB, CSH), according to American Society of Neuroimaging recommendations [13]. After confirming the presence of a sufficient temporal acoustic window with 2-MHz diagnostic TCD (Sonara Viasys Inc., US), both middle cerebral arteries (MCA) were evaluated (depth 45-55 mm) and the side with better Doppler spectra was selected for further monitoring [14]. Patients with insufficient temporal acoustic windows on both sides were excluded from the study. In order to maintain a constant insonation depth and angle, Spencer's head frame (Spencer Technologies, US) was used in all study participants for continuous monitoring of blood flow. We performed unilateral instead of bilateral MCA monitoring, since the latter was technically more challenging and time consuming especially when the participants moved or changed their head position during donning of N95 respirator and PAPR [15]. Continuous TCD spectra, for 1-min each, were recorded at baseline (breathing ambient air), 5 min after donning N95 respirator mask and 5 min after donning N95 respirator together with and PAPR. The 5-min time was chosen to avoid fluctuations in mean flow velocities (MFV) and pulsatility index (PI) due to anxiety and altered breathing pattern as well as obtain a steady state end-tidal carbon dioxide (ET-CO2) level. TCD spectra were digitally recorded for later analysis, which was performed independently by a credentialed neurosonologist (VKS). Peak systolic (PSV) and end-diastolic (EDV) flow velocities were used to calculate MFV as EDV + (PSV-EDV/3). PI was calculated as PSV - EDV/MFV [[11], [12], [13]].
2.3. Monitoring other physiological parameters
ET-CO2 was continuously monitored with a capnometer (TIDAL Wave Sp Model 715; Novametrix, Wallingford, CT). A thin plastic tube was inserted from the sides of N95 respirator mask while ensuring the air seal. ET-CO2 values at the time of TCD recordings (at baseline, 5-min after donning N95 respirator mask and 5-min after donning PAPR) were recorded.
2.4. Statistical analyses
Categorical variables are expressed by numbers (n) and percentages (%) and continuous variables as mean ± standard deviation (SD). The effects of N95 or PAPR exposure on cerebral hemodynamics were analyzed with the use of two-way repeated-measures analysis of variance (ANOVA). A p-value of less than 0.05 was considered as statistically significant. Statistical analyses were performed using SPSS statistical package program version 25.0 for Windows (SPSS Inc., 2003, Chicago, IL, USA).
3. Results
A total of 154 frontline healthcare workers agreed to participate in the study. Majority of them were female (n = 103, 66.9%) with mean age of 29.6 ± 12.1 years (Table 1 ). Migraine was the commonest co-morbidity in 38 (25%) individuals while 123 (80%) participants reported de-novo headache due to the donning of N95 mask (Table 1).
Table 1.
Baseline characteristics of the study population (n = 154).
| Variables | Values |
|---|---|
| Mean Age in years (SD) | 29.6 (12.1) |
| Female gender, n (%) | 103 (66.9) |
| Co-morbidities, n (%) | |
| Hypertension | 3 (1.9) |
| Diabetes mellitus | 0 (0.0) |
| Hyperlipidaemia | 1 (0.6) |
| Pre-existing migraine | 38 (24.7) |
| De novo headache | 123 (79.9) |
| Coronary artery disease | 0 (0.0) |
| Congestive heart failure | 0 (0.0) |
| Chronic obstructive pulmonary disease | 0 (0.0) |
Mean systolic and diastolic blood pressure at baseline were 116 ± 8 and 75 ± 6 mmHg, respectively. Mean heart rate at baseline was 68 ± 10 per minute. These values did not change significantly during the study. All participants underwent TCD monitoring at baseline and 5 min after donning N95 respirator mask. We did not encounter suboptimal acoustic window in our study participants.
3.1. Cerebral hemodynamic changes during donning N95 respirator mask
Mean MFV for MCA at baseline was 56.7 ± 18.2 cm/s, which increased to 61.1 ± 17.0 cm/s after wearing the N95 respirator mask (p < 0.001, Table 2 ). This was accompanied by corresponding reduction in PI from 0.92 ± 0.16 to 0.79 ± 0.12 (p < 0.001, Table 2). There was no statistically significant difference in MFV change between participants with history of pre-existing migraine and those without such history. Interestingly, compared to the those without de novo headaches, participants who developed de novo headaches (n = 123), MCA MFV showed a statistically significant increase after donning N95 respirator mask (62.3 ± 17.8 cm/s from 55.9 ± 18.1 cm/s at baseline; p < 0.001), accompanied by a decrease in PI (0.93 ± 0.13 at baseline to 0.79 ± 0.13 during donning of N95 respirator mask (p < 0.001),
Table 2.
Change in cerebral hemodynamic parameters after donning N95 respirator mask (n = 154).
| Parameter | Baseline | N95 | Absolute Change | Relative Change | p value |
|---|---|---|---|---|---|
| Mean MFV (SD) in cm/s | 56.7 (18.2) | 61.1 (17.0) | +4.4 (10.4) | +12.3% (29.8) | <0.001 |
| PI (SD) | 0.92 (0.16) | 0.79 (0.12) | −0.13 (0.12) | −13.3% (11.3) | <0.001 |
MFV: mean flow velocity, PI: pulsatility index, SD: standard deviation.
3.2. Cerebral hemodynamic changes during donning N95 respirator mask and PAPR together
A subgroup of 24 (15.6%) participants underwent training for PAPR, in addition to the N95 respirator mask. During initial donning of N95 respirator, MFV increased from 54.5 ± 8.7 cm/s at baseline to 61.4 ± 8.2 cm/s (p < 0.001, Table 3 ) while PI decreased from 0.92 ± 0.06 to 0.81 ± 0.06 (p < 0.001, Table 3). After donning PAPR and N95 respirator mask together, MFV increased to 59.9 ± 8.8 cm/s from baseline (p = 0.004, Table 3), while PI remained similar at 0.91 ± 0.06 compared to baseline (p = 0.694, Table 3). Serial TCD findings in a representative participant are presented in Fig. 1 .
Table 3.
Cerebral hemodynamic parameters after donning N95 respirator mask alone together with powered air purifying respirator (PAPR) (n = 24).
| Parameter | Baseline | N95 alone | Changea | P | N95 + PAPR | Changeb | p |
|---|---|---|---|---|---|---|---|
| Mean MFV (SD) in cm/s | 54.5 (8.7) | 61.4 (8.2) | +7.0 (6.9) | <0.001 | 59.9 (8.8) | +5.5 (8.4) | 0.004 |
| PI (SD) | 0.92 (0.06) | 0.81 (0.06) | −0.10 (0.04) | <0.001 | 0.91 (0.06) | 0.00 (0.04) | 0.694 |
| Mean ET-CO2 (SD) in mmHg | 37.3 (1.3) | 40.4 (1.6) | +3.1 (1.2) | <0.001 | 37.3 (1.3) | 0.0 (1.2) | 1.000 |
MFV: mean flow velocity, PI: pulsatility index, SD: standard deviation, ET-CO2: end-tidal carbon dioxide.
Absolute change between baseline and after donning N95.
Absolute change between baseline and after donning PAPR.
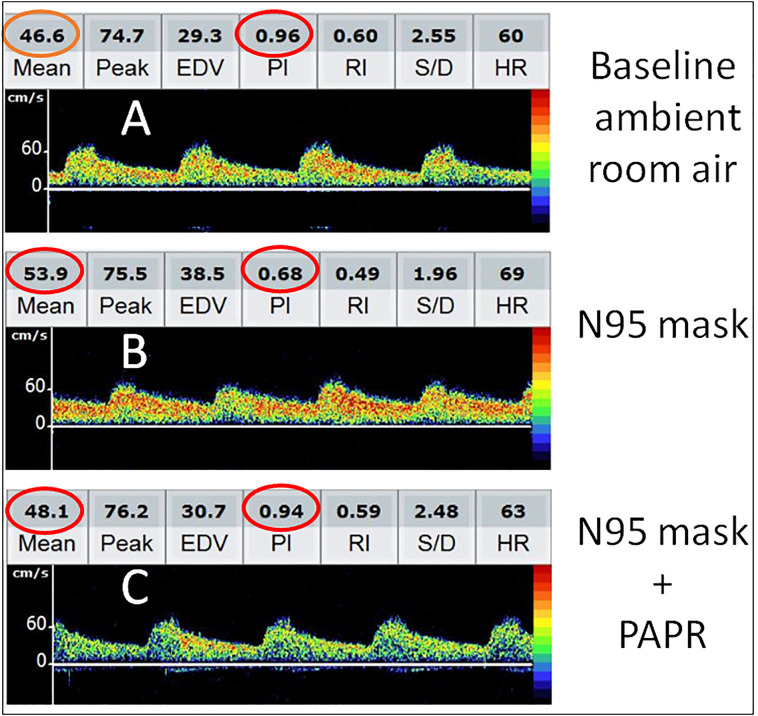
Fig. 1.
Serial transcranial Doppler (TCD) changes during donning of N95 respirator mask alone and together with powered air purifying respirator (PAPR). While breathing ambient room air, Doppler spectra from right middle cerebral artery showed mean flow velocity (MFV) 46.6 cm/s and pulsatility index (PI) 0.96 (A). After donning N95 respirator mask for 5-minutes, MFV increased to 53.9 cm/s while PI decreased to 0.68 (B). However, both MFV and PI returned to near-baseline 5-minutes after donning PAPR and N95 respirator mask together (C).
For this subgroup, serial ET-CO2 recordings showed an increase from baseline (37.3 ± 1.3 mmHg) to 40.4 ± 1.6 mmHg after wearing the N95 respirator mask (p < 0.001, Table 3). However, ET-CO2 levels returned to the baseline values after donning PAPR (Table 3, Fig. 2 ). The serial changes in TCD parameters and ET-CO2 were similar in participants who reported headache due to PPE as well those who did not.
Fig. 2.
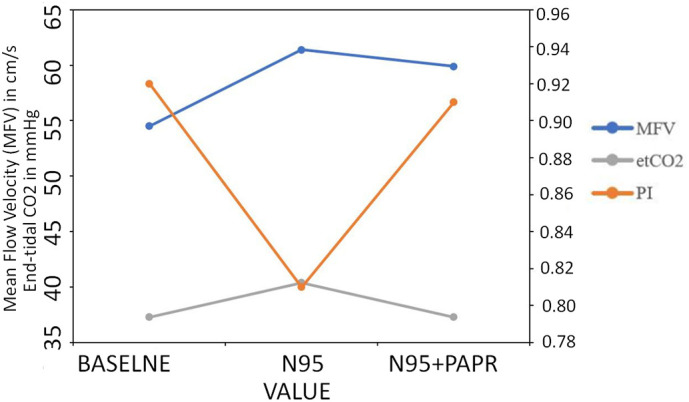
Serial changes in mean flow velocity (MFV), pulsatility index (PI) and end-tidal carbon di-oxide (ET-CO2) during baseline (ambient room air), 5-minutes after donning N95 respirator mask alone and N95 respirator mask plus powered air purifying respirator (PAPR).
4. Discussion
Our study shows that the use of N95 respirator mask results in significant alterations in cerebral hemodynamics. However, these effects are mitigated by the use of additional PAPR. Interestingly, the cerebral hemodynamic effects are dependent on the ET-CO2 level changes during the donning of N95 respirator mask alone and together with PAPR.
In this study, we used TCD measurements of MFV and PI. These parameters are reliable and validated surrogate markers of cerebral blood flow and vascular tone [12,[16], [17], [18]]. Cerebral blood flow can be altered by the change in partial pressure of CO2 (PaCO2) in arterial blood, blood pressure (BP) and the rate of cerebral metabolism. Among these factors, PaCO2 is the most important determinant of the vascular tone in intracranial circulation and a rise in PaC02 by 1 mmHg proportionately increases CBF by 3–6% [19]. We recorded TCD flow velocities, ET-CO2 values and systemic blood pressure levels at baseline, during donning of N95 respirator mask alone as well as together with PAPR in a subgroup of our study participants. Although, we did not monitor the rate of cerebral metabolism, it is extremely unlikely for this variable to change significantly during 12–15 min of participants' involvement in our study.
TCD derived PI primarily measures the downstream cerebral vascular resistance and is dependent on overall compliance of the arterial wall as well as the surrounding brain tissue, probably mediated by the release of nitric oxide [20,21]. Accordingly, CO2 induced arterial vasodilation would result in a decrease in PI [22]. Importantly, being a ratio, PI values are not altered by change in angle of insonation that may occur due to head movement during TCD monitoring. Our study documented a reduction in PI following donning of N95 mask, which was associated with a significant increase in ET-CO2. Importantly, this decrease in PI was restored within 5 min of donning PAPR, which was accompanied by a proportionate correction in ET-CO2. Thus, both N95 respirator mask and PAPR resulted in significant changes in the cerebral hemodynamic albeit in the opposite directions.
In a recent study on PPE donners, we described a high prevalence of headache [7]. While most of the participants reported de novo headaches, the headache severity and frequency worsened in those with pre-existing history of headache disorders. Although, most of the participants suffering from headache could mark the pressure points over face and scalp, suggestive of compression or irritation of skin and superficial nerves being responsible, at least in part, to their headaches. Our study demonstrates the contributory role of vasodilatation of intracranial vessels due to CO2 build-up in causing headaches related to the PPE use. Perhaps, the N95 respirator mask expands the effective dead space volume for respiration due to the tight seal around the mouth and nose, which results in hypercapnia, as observed in our subgroup with ET-C02 monitoring [23]. Interestingly, donning of PAPR led to normalization of ET-CO2 as well as cerebral hemodynamic parameters. We believe that the positive pressure generated by PAPR resulted in a relative hypocapnia by increased concentration of oxygen inside the hood and by positive pressure assisted exhalation. Although we cannot substantiate, this relative hypocapnia during PAPR could have upregulated the sympathetic activity while downregulating parasympathetic modulation. Interestingly, all participants reported the combined donning of N95 respirator mask and PAPR more comfortable as compared to the use of N95 alone.
We acknowledge certain limitations of our study. First, ET-CO2 was monitored only in a small subgroup of the study participants who donned PAPR in addition to the N95 respirator mask. However, getting a larger number of participants in this subgroup was difficult due to the strict hospital infection control guidelines about mixing of healthcare workers and safe-distancing policies. Also, PAPR training was provided only to the healthcare workers deployed in high risk hospital wards. Second, it is well known that ET-CO2 overestimates the CO2 levels as compared to arterial PaCO2 [24]. However, our aim was to evaluate the relative changes in cerebral hemodynamic parameters due to corresponding changes in ET-CO2 levels. Finally, changes in cerebral hemodynamic parameters were evaluated at 5 min after donning of N95 respirator mask and 5 min after donning additional PAPR. Whether, these changes are sustained or corrected by cerebral autoregulation remain unknown.
5. Conclusion
Our study shows that donning of N95 respirator alone or in combination with PAPR results in significant changes in cerebral hemodynamic parameters. The use of N95 respirator mask causes significant increase in MCA-MFV and ET-CO2, coupled with reduction in PI. However, these changes, which probably contribute to the new onset headache by healthcare workers due to the wearing of N95 masks, are mitigated by the use of additional PAPR. Overall, PAPR maintains a favorable intracranial physiologically as compared to N95 respirator mask alone. Further large scale studies with prolonged monitoring are recommended to confirm our findings.
Funding
None.
Financial disclosures
VKS is the current recipient of Senior Clinician Scientist Award from National Medical Research Council, Ministry of Health, Singapore.
Other authors have no financial disclosures.
References
- 1.Organization WH . World Heatlh Organization; 2020. WHO Characterizes COVID-19 as a Pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (Published 2020. Accessed 12 April 2020) [Google Scholar]
- 2.World Health Organization (WHO) Who; 2020. Rational Use of Personal Protective Equipment for Coronavirus Disease 2019 (COVID-19) (Accessed 13 March 2020) [Google Scholar]
- 3.Roberts V. To PAPR or not to PAPR? Can. J. Respir. Ther. 2014;50:87–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Or P.P., Chung J.W., Wong T.K. A study of environmental factors affecting nurses’ comfort and protection in wearing N95 respirators during bedside procedures. J. Clin. Nurs. 2018;27:e1477–e1484. doi: 10.1111/jocn.14268. [DOI] [PubMed] [Google Scholar]
- 5.Powell J.B., Kim J.H., Roberge R.J. Powered air-purifying respirator use in healthcare: effects on thermal sensations and comfort. J. Occup. Environ. Hyg. 2017;14:947–954. doi: 10.1080/15459624.2017.1358817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim E.C., Seet R.C., Lee K.H., Wilder-Smith E.P., Chuah B.Y., Ong B.K. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol. Scand. 2006;113:199–202. doi: 10.1111/j.1600-0404.2005.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong J.J.Y., Bharatendu C., Goh Y. Vol. 60. 2020. Headaches Associated with Personal Protective Equipment - A Cross-Sectional Study Among Frontline Healthcare Workers During COVID-19; pp. 864–877. [DOI] [PubMed] [Google Scholar]
- 8.Rebmann T., Carrico R., Wang J. Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses. Am. J. Infect. Control. 2013;41:1218–1223. doi: 10.1016/j.ajic.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim T., Vemuganti R. Mechanisms of Parkinson’s disease-related proteins in mediating secondary brain damage after cerebral ischemia. J. Cereb. Blood Flow Metab. 2017;37:1910–1926. doi: 10.1177/0271678X17694186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., Zhu Y.S., Hill C. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension. 2013;62:973–979. doi: 10.1161/HYPERTENSIONAHA.113.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandrov A.V., Sloan M.A., Tegeler C.H. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J. Neuroimaging. 2012;22:215–224. doi: 10.1111/j.1552-6569.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- 12.Alexandrov A.V., Sharma V.K., Lao A.Y., Tsivgoulis G., Malkoff M.D., Alexandrov A.W. Reversed Robin Hood syndrome in acute ischemic stroke patients. Stroke. 2007;38:3045–3048. doi: 10.1161/STROKEAHA.107.482810. [DOI] [PubMed] [Google Scholar]
- 13.Alexandrov A.V. Practical models of cerebral hemodynamics and waveform recognition. In: Alexandrov A.V., editor. Cerebrovascular Ultrasound in Stroke Prevention and Treatment. Wiley-Blackwell; Oxford: 2011. pp. 68–213. [Google Scholar]
- 14.Bishop C.C., Powell S., Rutt D., Browse N.L. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17:913–915. doi: 10.1161/01.str.17.5.913. [DOI] [PubMed] [Google Scholar]
- 15.Tsivgoulis G., Alexandrov A.V. Ultrasound in neurology. Continuum. (Minneap Minn). 2016;22:1655–1677. doi: 10.1212/CON.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 16.Washington C.W., Zipfel G.J. Participants in the International Multi-disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit. Care. 2011;15:312–317. doi: 10.1007/s12028-011-9594-8. [DOI] [PubMed] [Google Scholar]
- 17.Bellner J., Romner B., Reinstrup P., Kristiansson K.A., Ryding E., Brandt L. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP) Surg. Neurol. 2004;62:45–51. doi: 10.1016/j.surneu.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Cardim D., Robba C., Donnelly J. Prospective study on noninvasive assessment of intracranial pressure in traumatic brain-injured patients: comparison of four methods. J. Neurotrauma. 2016;33:792–802. doi: 10.1089/neu.2015.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brian J.E., Jr. Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88:1365–1386. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Ursino M., Giulioni M., Lodi C.A. Relationships among cerebral perfusion pressure, autoregulation, and transcranial Doppler waveform: a modeling study. J. Neurosurg. 1998;89:255–266. doi: 10.3171/jns.1998.89.2.0255. [DOI] [PubMed] [Google Scholar]
- 21.White R.P., Hindley C., Bloomfield P.M. The effect of the nitric oxide synthase inhibitor L-NMMA on basal CBF and vasoneuronal coupling in man: a PET study. J. Cereb. Blood Flow Metab. 1999;19:673–678. doi: 10.1097/00004647-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Lavi S., Gaitini D., Milloul V., Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1856–H1861. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- 23.Johnson A.T., Scott W.H., Lausted C.G., Coyne K.M., Sahota M.S., Johnson M.M. Effect of external dead volume on performance while wearing a respirator. AIHAJ. 2000;61:678–684. doi: 10.1080/15298660008984577. [DOI] [PubMed] [Google Scholar]
- 24.Tymko M.M., Hoiland R.L., Kuca T. Measuring the human ventilatory and cerebral blood flow response to CO2: a technical consideration for the end-tidal-to-arterial gas gradient. J. Appl. Physiol. 1985;2016(120):282–296. doi: 10.1152/japplphysiol.00787.2015. [DOI] [PubMed] [Google Scholar]