Abstract
Pancreatic ductal adenocarcinoma (PDAC) has an extremely poor response to chemo- and (modest-dose conventionally fractionated) radio-therapy. Emerging evidence suggests that pancreatic stellate cells (PSCs) secrete deoxycytidine, which confers resistance to gemcitabine. In particular, deoxycytidine was detected by analysis of metabolites in fractionated media from different mouse PSCs, showing that it caused PDAC cells chemoresistance by reducing the capacity of deoxycytidine kinase (dCK) for gemcitabine phosphorylation. However, data on human models are missing and dCK expression was not associated with clinical efficacy of gemcitabine. We recently established co-culture models of hetero-spheroids including primary human PSCs and PDAC cells showing their importance as a platform to test the effects of cancer- and stroma-targeted drugs. Here, we discuss the limitations of previous studies and the potential use of above-mentioned models to study molecular mechanisms underlying chemo- and radio-resistance.
Keywords: Pancreatic cancer, Therapy resistance, Stellate cells, Deoxycytidine
To the Editor
We read with great interest the recent article by Dalin and collaborators [1] on liquid chromatography-mass spectrometry analysis and cell viability assays in different models of PDAC cells and PSCs, showing that secretion of deoxycytidine by PSCs conferred resistance to gemcitabine.
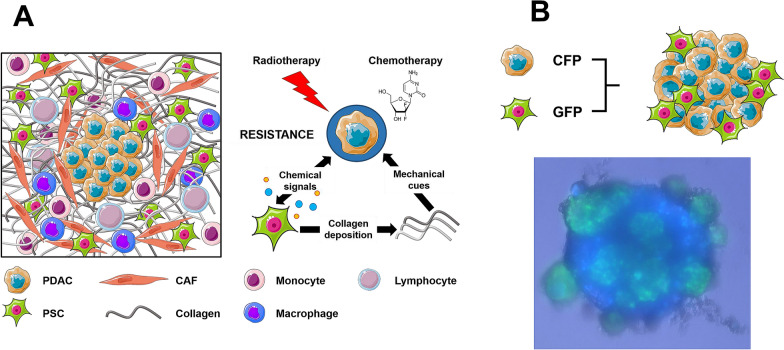
PDAC is typically characterized by features associated with poor prognosis: therapy resistance, early metastasis and recurrence [2]. Approximately 80% of PDAC volume is stroma comprising a liquid milieu of cytokines/growth factors and extracellular vesicles, a cellular component (PSCs, fibroblasts, endothelial and immune cells), and an extracellular matrix. These components are interconnected and their communication with cancer cells might affect aggressive behavior and therapy response (Fig. 1a).
Fig. 1.
Schematic overview of the surrounding TME in pancreatic cancer. a The TME of PDAC is comprised of several components, including CAFs, immune cells and extracellular matrix proteins, like collagen. Activated PSCs and collagen are able to promote resistance against chemo- and radiation treatment via paracrine signaling and mechanical cues, respectively. b Representative fluorescent image of co-cultured PSC/PDAC (1:2) spheroid. Green fluorescent protein-expressing PSCs were co-cultured with cyan fluorescent protein-expressing primary PDAC cells. Image was taken 48 h post-seeding. CAF Cancer-associated Fibroblasts, PDAC Pancreatic Ductal Adenocarcinoma, PSC Pancreatic Stellate Cells, CFP Cyan Fluorescent Protein, GFP Green Fluorescent protein. Some material of this figure was adapted from images made by Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License, at https://smart.servier.com
Despite inherent and acquired resistance against most chemotherapeutic drugs, cytotoxic chemotherapy remains the mainstay of the treatment for PDAC patients, the majority of whom present with advanced-stage disease. Gemcitabine resistance is multifactorial and PSCs have been implicated in several of the underlying processes [3]. Dalin and collaborators [1] performed an elegant analysis of metabolites in fractionated media from different mouse PSCs, showing that deoxycytidine is present and protects PDAC cells from gemcitabine cytotoxicity, raising the question of whether deoxycytidine affects key oncogenic pathways as well. Notably, mouse macrophages are also able to secrete deoxycytidine [4], but hepatic stellate cells do not. No data are available from primary human PSCs from cancer patients. Primary human PSCs and commonly used PSC-cultures differ both phenotypically and in their interactions with PDAC cells [5], emphasizing the importance of appropriate PSC-PDAC co-culture models, with paired primary cancer cells and PSCs. Moreover, when a PDAC organoid was assessed for the influence of PSC conditioned media, no significant protection against gemcitabine cytotoxicity was observed [1]. This interesting observation suggests that under particular physiological conditions and in 3D models, the microenvironment could behave differently. This motivates efforts to investigate these findings in even more appropriate tumor-TME preclinical models.
We recently established PSC/PDAC spheroids to be used as an important tool for screening of cancer- and stroma-targeted drugs (Fig. 1b). These co-culture spheroids exhibited higher resistance to gemcitabine compared to PDAC-only spheroids, whereas c-MET inhibitors tivantinib, PHA-665752 and crizotinib were equally effective in both spheroid models [6]. The potential of targeting c-MET receptor as a valuable therapeutic strategy in selected cases of PDAC was also shown in a recent in vivo study which demonstrated that a triple combination of gemcitabine with HGF and c-MET inhibitors was the most effective strategy to both reduce the size of primary tumours as well as to completely eliminate metastases [7].
In order to combat the drug- and radioresistance mediated by PSCs in PDAC [8], these novel 3D preclinical model studies are highly preferred to investigate the interaction between pharmacological and radiotherapeutic strategies, for example through testing of combinations of radiosensitizing and cytotoxic agents with radiation. Similar studies have been performed using 3D preclinical models in glioblastoma [9].
Another important question arises concerning the hypothetical role of dCK in gemcitabine resistance. Treatment with PSC media did not reduce intracellular levels of gemcitabine, suggesting no uptake competition, but rescued dCK-catalyzed formation of intracellular deoxycytidine triphosphate (dCTP) levels [1]. It would be of interest to determine the formation of gemcitabine-diphosphate, which inhibits ribonucleotide-reductase, responsible for synthesizing deoxynucleotides required for DNA synthesis/repair. A decreased dCTP would indeed result in reduced feedback inhibition of dCK, and potentiate gemcitabine activation, favoring gemcitabine-triphosphate in its competition with dCTP for incorporation into DNA [10]. Data on the synthesis of gemcitabine-triphosphate and its incorporation into DNA are therefore warranted to further elucidate the mechanism of resistance.
Finally, dCK expression is not clearly associated with clinical efficacy of gemcitabine in PDAC [5]. Further studies on the complex TME comprising distinct cell types, but also hypoxic and stromal dense areas [2], might explain differential effects on pyrimidine metabolism and chemo- as well as radio-resistance.
In conclusion, we look forward to additional studies on optimized preclinical models evaluating the effects of chemotherapy and radiotherapy in PDAC and unravelling the mechanisms behind treatment failure.
Abbreviations
- dCK
Deoxycytidine kinase
- dCTP
Deoxcytidine triphosphate
- PDAC
Pancreatic ductal adenocarcinoma
- PSCs
Pancreatic stellate cells
- TME
Tumor microenvironment
Authors’ contribution
PPC and AG performed the experiments and wrote the initial draft; concept and supervision (EG, GJP), contribution to the draft (OF, MD, PS), final editing (EG, GJP). All authors read and approved the final manuscript.
Funding
This work was partially supported by the following grants: Zabawas Foundation—Cancer Center Amsterdam Foundation CCA2019-5-55 (P.S.), CCA2015-1-19 (E.G., G.J.P), CCA2018-5-48 (E.G.), KWF Dutch Cancer Society—KWF project#11957 (E.G.), and Associazione Italiana per la Ricerca sul Cancro—AIRC/ Start-Up grant (E.G.), National Institute for Medical Research Development (NIMAD, Grant number: 957652) and the Polish National Science Center project 2018/31/B/NZ7/02909 (E.G., G.J.P.).
Competing interests
MD reports research grants from Varian Medical Systems outside the scope of this work. No writing assistance was utilized during production of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dalin S, Sullivan MR, Lau AN, Grauman-Boss B, Mueller HS, Kreidl E, et al. Deoxycytidine release from pancreatic stellate cells promotes gemcitabine resistance. Cancer Res. 2019;79(22):5723–5733. doi: 10.1158/0008-5472.CAN-19-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovannetti E, van der Borden CL, Frampton AE, Ali A, Firuzi O, Peters GJ. Never let it go: stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol. 2017;44:43–59. doi: 10.1016/j.semcancer.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Amrutkar M, Aasrum M, Verbeke CS, Gladhaug IP. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer. 2019;19(1):596. doi: 10.1186/s12885-019-5803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halbrook CJ, Pontious C, Kovalenko I, Lapienyte L, Dreyer S, Lee HJ, et al. Macrophage-Released Pyrimidines Inhibit Gemcitabine Therapy in Pancreatic Cancer. Cell Metab. 2019;29(6):1390-9 e6. doi: 10.1016/j.cmet.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenggenhager D, Amrutkar M, Santha P, Aasrum M, Lohr JM, Gladhaug IP, et al. Commonly used pancreatic stellate cell cultures differ phenotypically and in their interactions with pancreatic cancer cells. Cells. 2019;8(1):23. doi: 10.3390/cells8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firuzi O, Che PP, El Hassouni B, Buijs M, Coppola S, Lohr M, et al. Role of c-MET inhibitors in overcoming drug resistance in spheroid models of primary human pancreatic cancer and stellate cells. Cancers. 2019;11(5):638. doi: 10.3390/cancers11050638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, Pang TCY, Liu AC, Pothula SP, Mekapogu AR, Perera CJ, et al. Targeting the HGF/c-MET pathway in advanced pancreatic cancer: a key element of treatment that limits primary tumour growth and eliminates metastasis. Br J Cancer. 2020;122(10):1486–1495. doi: 10.1038/s41416-020-0782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res. 2011;71(10):3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan RS, Fedrigo CA, Brands E, Dik R, Stalpers LJ, Baumert BG, et al. The allosteric AKT inhibitor MK2206 shows a synergistic interaction with chemotherapy and radiotherapy in glioblastoma spheroid cultures. BMC Cancer. 2017;17(1):204. doi: 10.1186/s12885-017-3193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Hassouni B, Li Petri G, Liu DSK, Cascioferro S, Parrino B, Hassan W, et al. Pharmacogenetics of treatments for pancreatic cancer. Expert Opin Drug Metab Toxicol. 2019;15(6):437–447. doi: 10.1080/17425255.2019.1620731. [DOI] [PubMed] [Google Scholar]