Summary
Community transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or COVID-19) in the United States on February 26, 2020, and the rapid spread that followed forced patients, providers, payors, and policy makers to adapt to an unprecedented, nearly instant, and enormous demand for virtual care. Although few US ophthalmology practices incorporated telemedicine prior to COVID-19, its use has now become the norm. Regarding the use of synchronous patient-to-provider virtual visits (SPPVV) in pediatric ophthalmology, we have pooled our collective experience at three academic practices across the country to describe initial workflows, technology solutions, use cases, and barriers to care.
The Center for Medicare and Medicaid Services defines telemedecine as an “exchange of medical information from one site to another through electronic communication to improve a patient's health.” Although telemedicine has existed in various forms since the 1950s, the usability and ubiquity of modern technology catalyzed its growth over the last decade.1 , 2 However, lack of parity in coverage, parity in reimbursement, privacy concerns, state licensing regulations, and inertia in the adoption of new healthcare delivery methodology prevented its being widely adopted in the United States. The arrival of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, or COVID-19) obliterated these barriers as executive orders forced patients, providers, and payors to adapt to an unprecedented, nearly instant, and enormous demand for virtual care. Few US ophthalmology practices incorporated telemedicine prior to COVID-19, but its use has now become the norm. Within pediatric ophthalmology and strabismus, rapid tele-ophthalmology adoption has been fueled by both patient demand and the amenability of obtaining subspecialty-specific critical examination elements virtually. Telemedicine is broadly divided into two types, according to the technology used: synchronous (real-time data exchange) and asynchronous (store-and-forward). Each of these types is further subdivided into patient-to-provider and provider-to-provider interactions. Regarding the use of synchronous patient-to-provider virtual visits (SPPVV) in pediatric ophthalmology, we have pooled our collective experience at three academic practices across the country to describe initial workflows, technology solutions, use cases, and barriers to care. SPPVV are often referred to as “vitual visits,” “synchronous telemedicine,” or, more broadly, as “telemedicine,” both colloquially and in the literature, though, these terms lack specificity.
Workflows
Identifying Appropriate Patients for SPPVV
Success with virtual visits starts with selecting appropriate patients. Prior to COVID-19, providers would identify patients during an office visit who might benefit from follow-up via SPPVV. To provide quality care in the era of COVID-19, each practice needs an algorithmic selection process. We recommend a synthesis of objective criteria (eg, new vs established patients, age, and diagnosis) and subjective assessment of a subset of patients by the provider teams. The following workflow suggestions are based on our collective experience (with approximately 3,700 SPPVVs) and may be modified to align with an individual practice.
Scheduling the SPPVV
Based on the provider's recommendation, practice representatives call the patient to offer a SPPVV. These visits are each scheduled for 30 minutes on dedicated half-days free from clinic appointments. Interpreters are scheduled as needed if not available on-demand in the SPPVV software. Staff members send the patient instructions on how to prepare for the SPPVV. In order of preference, these are sent via electronic-health-record (EHR) secure messaging, electronic mail (email), or the postal service.
Prior to the SPPVV
A technician or orthoptist calls the patient to review instructions and to ensure that the patient is prepared to check visual acuity prior to the SPPVV. Consent for the SPPVV is obtained verbally and documented in the chart unless the SPPVV software incorporates consent. The patient is instructed on how to sign in at the appointment time or the patient receives a link to join the SPPVV through email or short message service (SMS). One day prior to the appointment, the patient is called to confirm the appointment time and to remind the family that the visual acuity should be checked in advance. The patient is encouraged to check their internet connection and device compatibility with the SPPVV software. Some practices elect to collect examination data (eg, visual acuity, ocular motility photographs, external photographs) and upload these to the chart at this time.
Day of the SPPVV
SPPVV appointments are “arrived” in the EHR. At some institutions, all subsequent steps are performed by the provider, but a delegated team workflow will be described here. Either automated SPPVV software or the provider's assistant (technician, orthoptist, resident, or fellow) initiates the SPPVV, either by inviting the patient into the meeting via email or SMS texting or by admitting the patient from the virtual waiting room, depending on the software platform. Backup workflows are essential. If unable to connect, the assistant calls the patient and troubleshoots the preferred platform. If still unsuccessful, connection via a backup video platform is attempted. If no video connection is possible, the encounter is conducted via telephone. The assistant completes the virtual rooming process and initial patient workup. If not documented previously, the assistant notes the visual acuity obtained at home, including method and reliability, as judged by the caregiver. If necessary, visual acuity can be rechecked with coaching by the assistant. The assistant then notifies the provider that the patient is ready via designation in the EHR and, as needed for convenience, via SMS if the assistant and provider are working in separate locations. The provider enters the SPPVV and completes the history, examination, and management discussion with the patient. Medications are reconciled and e-prescribed. Treatment instructions are entered and distributed via secure EHR messaging immediately or via email or postal mail after the visit. After completing the SPPVV and disconnecting with the patient, the provider finishes documentation in the EHR including follow-up instructions and coding to current telemedicine guidelines.
Day after the SPPVV
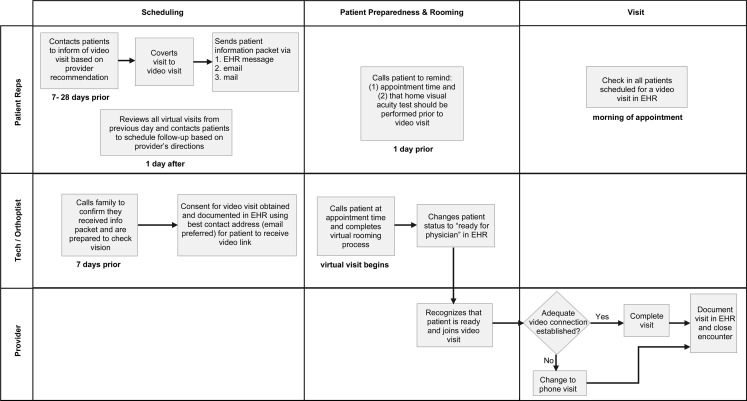
Patient representatives contact all SPPVV patients and no-shows from the previous day to schedule follow-up and send any instructions that were unable to be sent via EHR secure messaging. Figure 1 demonstrates this workflow.
Fig 1.
Synchronous provider-to-patient workflow diagram. Rep, clinic representative; Tech, ophthalmic technician.
Technology Solutions
Telemedicine workflows require specific technology solutions. There are three main types of telemedicine visits: combined audio and video, telephone (ie, audio only), and electronic communication. During the COVID-19 public health crisis, the Health and Human Services Office for Civil Rights decided to waive penalties against healthcare providers for the use of common communication technologies, regardless of compliance with the Health Insurance Portability and Accountability Act of 1996, in order to afford patients rapid access to their healthcare providers.2
For SPPVV specifically, numerous platforms exist, including FaceTime (Apple Inc), WhatsApp (Facebook Inc.), Skype (Skype Communications SARL), Google Hangouts (Google), Doxy.me (Doxy.me LLC), Doximity Dialer (Doximity Inc), and Zoom (Zoom Video Communications Inc). Each solution has unique advantages and disadvantages regarding familiarity, functionality, and privacy, but all allow for simultaneous interactive audio and video between the provider and the patient. Programs such as FaceTime, WhatsApp, Skype, and Google Hangouts offer the advantage of familiarity to patients and providers as well as the ability to add multiple people in a group call, but they cannot be used unless all parties have the same program or account. Furthermore, all reveal the personal phone number or username of both parties, significantly jeopardizing the privacy of the provider. Platforms such as Doxyme and Zoom, on the other hand, are less familiar to patients, but their functionalities are conducive to telemedicine. For instance, patients receive a private link to enter into their appointment and can do so from any device with internet access, allowing both parties to conceal contact information. These platforms have a “virtual waiting room” feature that allows multiple patients to be scheduled throughout the day, allowing the provider to move between virtual encounters as she would in a clinic experience. Additionally, the free version of Zoom offers two particularly helpful features. A third party may be added to the call, such as a family member, trainee, scribe, or interpreter. All parties may also “share” their screens. Clinical photographs, laboratory studies, imaging, or an entertaining video to grasp the attention of a young child may be shared. These two features are also available on the “professional” (subscription-based) version of Doxyme. See Table 1 for more examples of the many free and subscription-based programs available.
Table 1.
Comparison of freely available audio-video platforms that may be leveraged for synchronous patient-to-provider virtual visits during the COVID-19 perioda
| Facetime | Skype | Google Hangouts |
Doximity Dialer |
Doxy.me | Zoom | ||
|---|---|---|---|---|---|---|---|
| Concealed phone number/user name | x | x | x | ||||
| Can use between different devices | x | x | x | x | x | x | |
| Patient does not need an account | x | x | x | ||||
| Can add multiple parties to call | x | x | x | x | x | xb | |
| Share screen available | x | x | x | ||||
| Can record/ save video or photos | x | x | |||||
| Automatic closed caption available | x | ||||||
| Virtual waiting room | x | ||||||
| HIPAA compliant |
Regulations surrounding the US Health Insurance Portability and Accountability Act of 1996 (HIPAA) may prevent use of some of these platforms as the perceived need wanes.
Limited to 40 minutes per meeting.
If video is not available to the patient or provider, telephone visits may be performed in a variety of ways. The caller's number may be concealed by entering ∗67 prior to the phone number on the phone's dial pad or by using apps such as Google Voice or Doximity Dialer. Through Google Voice, a provider may obtain a new phone number that can be used for calling, text messages, and voicemail. Using Doximity Dialer, patients see only the provider's office number when the provider calls their personal phone. Electronic communications between the patient and provider are most successfully carried out through secure patient messaging functionality available in many electronic medical records or through secure email messaging. These are usually, but not always, asynchronous.
Optimizing the SPPVV Experience
A successful SPPVV begins with both the patient and the provider viewing the visit as a professional encounter rather than a video chat with friends and family.3 This professional approach requires the care team to be on time for the SPPVV, dress as they would for an office encounter, provide a backdrop that is free of clutter, and ensure privacy. Similarly, patients must approach the SPPVV with the same rigor to optimize quality and experience. Good lighting, illuminating the face of both the patient and the provider, is essential. Provider lighting enhances nonverbal communication to approximate an in-person conversation. Patient lighting promotes an accurate and complete virtual physical examination. For both parties, facing a window allows for natural illumination and avoids backlighting. Camera positioning and stability is also critical. The camera should be placed at eye level to allow the patient to see the provider's face and to allow the provider to see the patient's eyes and adnexa. Camera stability facilitates video fidelity by eliminating distracting movement and optimizing resolution. Finally, eye contact between physician and patient during SPPVV is critical to ensuring a human “connection.” When obtaining history or counseling, providers should “speak into the camera” to simulate a natural face-to-face conversation.
Use Cases
Prior to the COVID-19 period, we curated our SPPVV for specific cases and specific patients (Shah AS, et al. J AAPOS 2018;22:e14-e15 [Abstract 044]). We began with postoperative strabismus patients. With experience, we expanded to other extraocular postoperative visits for chalazia, dermoids, and eyelid lesion excisions. We then incorporated follow-up monitoring for conditions such as viral and allergic conjunctivitis. The COVID-19 pandemic quickly forced us to pivot toward broader use as a tool for healthcare delivery. Thus, new, established, and postoperative patients have been assessed via SPPVV, while allowing for “conversion” to an in-clinic visit within an appropriate time frame if concerns cannot be addressed virtually.
As with any in-person visit, each SPPVV necessitates a patient-centered approach that varies by diagnosis. Strabismus and oculoplastic visits are naturally amenable to obtaining significant examination elements. Anterior segment diagnoses are more difficult to evaluate, but virtual observations of eyelid positioning, blepharospasm, conjunctival injection, irregular corneal light reflections, and opacities obscuring the iris and pupil may guide the clinician. Posterior segment pathology is challenging or impossible to evaluate via SPPVV without augmented technology solutions. Surrogates for pathology such as remote visual acuity testing, Amsler grid testing, and photographs highlighting red reflexes may help triage the condition. For all cases, SPPVV permits integrating a thorough history with clinical experience to ensure that patients receive targeted recommendations and follow-up such as sensorimotor evaluation, fundus imaging, neuroimaging, care instructions, medication prescriptions, and surgical counseling.
Virtual Examination Techniques
With the virtual eye examination, there is a learning curve for both the provider and the patient. A successful examination requires both careful observation by the provider and the engagement of patient and caregiver. As providers, we begin the in-person evaluation of any patient from the first impression in the waiting room. We glean meaningful information from how the patient appears externally, navigates the environment, and makes eye contact. This opportunity may be leveraged during the SPPVV and can be enhanced by showing children videos or pictures via screen-share during SPPVV. It can also be enhanced by asking caregivers to hand the child a toy or to engage siblings in play. For older children and adults, observation during reading or another visual task may also help the provider obtain valuable information, such as the distance where the problem is occurring or the position of gaze that is troublesome. Caregivers and patients themselves become the provider's virtual hands to administer visual acuity testing, provide illumination, perform cover testing, move targets or initiate doll's head maneuvers for ocular motility evaluations, and assist with gross visual field examination. Our collective experience with the virtual ophthalmological examination has suggested several tips for collecting meaningful physical examination data effectively (Figure 2 ).
Fig 2.
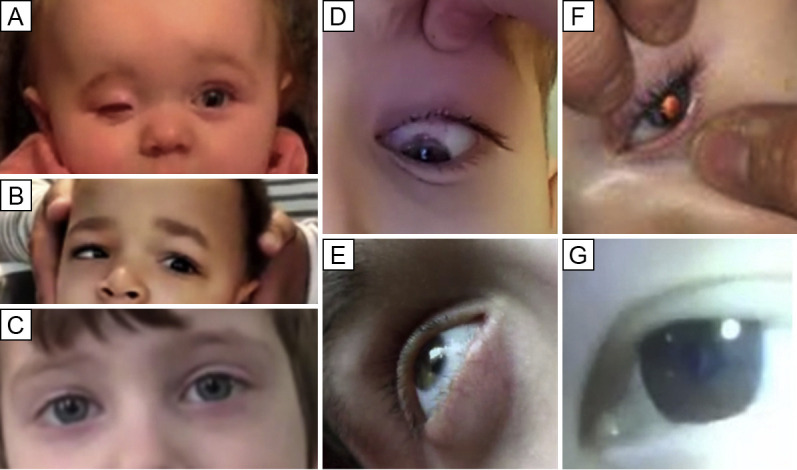
Screen-capture images from synchronous patient-to-provider virtual visits illustrating the findings associated with various virtual examination techniques. A, A 10-month-old child with right anophthalmia being treated with a hydrogel expander. Eyelid positioning and orbital expansion can be estimated through observation. B, A 23-month-old child with a left abducens nerve palsy demonstrated by a gentle doll's head maneuver performed by his mother. C, A 5-year-old child 3 months after botulinum toxin injection for an acute abducens nerve palsy caused by an ependymoma. Light reflex evaluation and parental cross cover testing showed no misalignment in primary gaze. D, An 11-year-old boy elevates his eyelid during evaluation of the superior limbus for a flare of vernal keratoconjunctivitis. E, A 16-year-old, developmentally delayed young man with tearing 3 weeks after cataract surgery. Oblique viewing and illumination highlight the anterior chamber, which appears free of hypopyon or significant fibrinous debris. There is also a lack of perilimbal injection. F, A 9-month-old girl with a congenital cataract showing maintained central red reflex with flash photography, indicating no significant progression of the lens opacity located inferior temporally (arrow) in comparison to the last office visit evaluation. G, A 16-year-old young woman with eczema diagnosed with a central cataract in the setting of subacutely diminished vision.
First, visual acuity may be measured in young children by observing fixation-and-follow behaviors and objection to occlusion. For older children and adults, age-appropriate visual acuity applications are recommended by the American Academy of Ophthalmology.4
Second, the external examination is ascertained by virtual observation. Ptosis and lagophthalmos measurements and orbicularis strength may be estimated by observing the density of the skin creases when the patient squeezes the eyes closed. Levator function may be assessed with the help of a caregiver moving a target up while holding the patient's head and fixing the brow. Torticollis can be estimated by observing the patient fixate on a target.
Third, ocular motility evaluations in young children are best conducted with toys moved by caregivers or siblings while gently stabilizing the head in primary gaze or by doll's head maneuvers implemented by the caregiver while the child watches the examiner or a video via screen-share. Instructing older children and adults to move the eyes in the different directions of gaze without moving the head typically suffices.
Fourth, ocular alignment may be estimated by light reflex testing from a caregiver's flashlight or room lighting. With brief coaching, patients or caregivers are usually able to perform cover-uncover and cross-cover testing to allow estimations of tropias and phorias.
Fifth, pupils may be observed for symmetry, size, and reaction to accommodation. Patients or caregivers may be able to illuminate the pupils for better observation. A swinging flashlight test for an afferent pupillary defect is possible with an adept caregiver's assistance, though we have found this to be a difficult task to teach.
Sixth, visual fields may be estimated by asking the patient to fixate on a single target and describe the peripheral scene. Alternatively, a caregiver might be instructed in performing confrontation visual fields, though this is often difficult to accomplish.
Seventh, the anterior segment may be visualized. Careful observation of the eyelids for discharge, scruff, erythema, or blepharospasm may inform the provider about light sensitivity. Evaluation of the conjunctiva for injection and the corneal light reflex clarity may inform the provider about ocular surface abnormalities, such as abrasions or dry eye, and the lids may be everted in cooperative patients. Having the patient look to the side of the camera, with good illumination of the face, can give insight into the depth of the anterior chamber and even the clarity of the chamber akin to the penlight illumination one may use in the office to visualize a conical reflection on the nasal cornea from temporal illumination.
Finally, as more providers perform SPPVV, innovations in gathering virtual examination data will accelerate, and technologies will enable more detailed information to be collected remotely, such as intraocular pressure and fundus imaging.
Barriers to Providing SPPVVs
Although telemedicine is a major innovation in healthcare delivery, there are significant barriers to optimizing telemedicine practices in pediatric ophthalmology to meet the National Academy of Medicine's standard of quality (Table 2 ).7
Table 2.
| Barriers | Details |
|---|---|
| Implementation costs | Lack of explicit economic framework, billing and reimbursement clarity; costs of home testing devices |
| Project reliability, sustainability, and applicability in all settings | Workload required to implement programs in our current state without guarantee of long-term sustainability, effectiveness, and acceptance |
| Clarity surrounding legal, ethical, privacy, and security issues | Concern about the medicolegal ramifications of diagnostic error Data security and confidentiality |
| Lack of evidence regarding clinical and economic benefit | Research is needed to evaluate patient-centered outcomes of newly implemented telehealth programs |
| Lack of strategic alignment between stakeholders | Different interests, concerns, and priorities of professionals implementing programs, administrators promoting implementation, patients in need of care, etc Strategic planning should incorporate a patient-centered approach (how patients view telemedicine and what they hope to gain) |
| Resistance to changing comfortable practices and familiar workflows to new uncertain and unstandardized models | Programs must quickly adapt in response to technological changes; information systems and platforms evolving rapidly, requiring rapid training and adoption by clinicians |
| Unintended consequences, such as inequitable access and exacerbation of disparities and barriers to care | Need for specific implementation strategies that consider language barriers, digital literacy, patient and family-centered approach:
|
Cost and Infrastructure
Compared to other medical specialties, the telemedical infrastructure required for ophthalmology is complex, as it may require remote testing equipment such as tonometers, binocular function and motility testing software, and ancillary imaging modalities in order to provide safe, effective, and efficient care. The Icare HOME tonometer (Icare USA, Raleigh, NC) is FDA approved, and remote intraocular pressure monitoring qualifies for reimbursement under CMS codes for remote physiologic monitoring. Beyond tonometry, there is no clarity from payors as to whether they will reimburse additional testing or devices. Alternatively, some have proposed a hybrid approach where focused testing is performed in-person while the remainder of the visit is conducted via telemedicine. Costs to establish telehealth programs may be significant but should be considered in the context of potential societal and patient savings from reduced transportation, employment leave, childcare, and time spent in healthcare environments that may reduce stress and exposure to communicable diseases. Whereas most tele-ophthalmology prior to COVID-19 consisted of asynchronous remote image interpretation for consultations between providers for programs such as ROP and diabetic retinopathy screening, now synchronous triage, diagnosis, treatment, and follow-up models are rapidly evolving directly between patients and providers.
Consensus, Validation, and Standardization
There is no clinically or financially validated dominant pathway for integrating synchronous telemedicine into pediatric ophthalmic care, and it is unlikely that there will be a “one-size-fits-all” approach. Home monitoring of parameters like intraocular pressure or visual acuity may become a powerful way to empower patients and caregivers to take control of their chronic conditions. Flexible clinicians, agile technologies, and rigorous research will be needed to validate and to standardize these new models for care. To permanently shoehorn the quality and reimbursement metrics that currently exist for office visits into policy for virtual encounters would be tremendously shortsighted.
Access
Telemedicine has been identified as an important strategy to improve access to subspecialty care.8 The hope is to facilitate more equitable access to medical services by eliminating barriers to care. However, technical progress alone will not eliminate disparities; it may exacerbate them. Unchecked, telemedicine may exacerbate existing disparities in delivery and outcomes. Deliberate implementation strategies must be designed for equity. One must ask, for example, To whom are we offering this service? Are we able to provide care in multiple languages and at all levels of socioeconomic and educational diversity? Can we pivot to back-up technologies, such as audio only, if smart devices are not available in the home or if the internet connection does not have enough bandwidth to support synchronous audio-video interactions? In our experience, the same patients that require additional resources and support for traditional clinic appointments are most likely to be left behind in a sophisticated technological care model. Accounting for the full spectrum of patient and caregiver needs will be crucial to ensure equitable, patient-centered virtual care.9
Conclusions
Our patients and our practices have adapted quickly to the COVID-19 pandemic. We predict that the achievability of quality care for many visits within our specialty combined with positive patient experiences and expectations for ongoing agile care delivery will fuel the virtual visit revolution well beyond the end of the current pandemic. Telemedicine is here to stay.10 , 11 As pediatric ophthalmologists continue to adapt to the current crisis, we hope that this overview serves as a reference for practitioners to implement and improve telemedical workflows to ensure that vulnerable children are not left behind and to reduce the consequences of deferred care during this pandemic. To meet the challenges and opportunities for our patients and practices, AAPOS has formed a Telemedicine Task Force (see eSupplement 1 at jaapos.org for a complete list of members). Likewise, we have formed the Pediatric Tele-Ophthalmology Consortium (PTOC) to research best practices and drive innovation for the next generation of healthcare delivery. We invite interested collaborators to contact us.
Supplementary Data
References
- 1.Board on Health Care Services; Institute of Medicine . National Academies Press (US); Washington (DC): 2012 Nov 20. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary.https://www.ncbi.nlm.nih.gov/books/NBK207141/ 3, The evolution of telehealth: where have we been and where are we going? Available at: [PubMed] [Google Scholar]
- 2.United States Department of Health and Human Services FAQs on Telehealth and HIPAA during the COVID-19 nationwide public health emergency. https://www.hhs.gov/sites/default/files/telehealth-faqs-508.pdf Available at:
- 3.Bowe T., Hunter D.G., Mantagos I.S., et al. Virtual visits in ophthalmology: timely advice for implementation during the COVID-19 public health crisis. Telemed J E Health. 2020 doi: 10.1089/tmj.2020.0121. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Home Eye Test for Children and Adults. American Academy of Ophthalmology. San Francisco, CA. https://www.aao.org/eye-health/tips-prevention/home-eye-test-children-adults Available at:
- 5.Saigí-Rubió F., Torrent-Sellens J., Ramos I., Sáez C., Kotzeva A., Villalobos J. PAHO; Washington, DC: 2016. Framework for the implementation of a telemedicine service. [Google Scholar]
- 6.Roig F., Saigí F. Barreras para la normalización de la telemedicina en un sistema de salud basado en la concertación de servicios. Gac Sanit. 2011;25:397–402. doi: 10.1016/j.gaceta.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine . National Academy Press; Washington, DC: 2001. Crossing the quality chasm: a new health system for the 21st century. [PubMed] [Google Scholar]
- 8.Marcin J.P., Shaikh U., Steinhorn R.H. Addressing health disparities in rural communities using telehealth. Pediatr Res. 2016;79:169–176. doi: 10.1038/pr.2015.192. [DOI] [PubMed] [Google Scholar]
- 9.Ray K.N., Ashcraft L.E., Mehrotra A., Miller E., Kahn J.M. Family perspectives on telemedicine for pediatric subspecialty care. Telemed J E Health. 2017;23:852-62. doi: 10.1089/tmj.2016.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harpaz J. 5 reasons why telehealth is here to stay (COVID-19 and beyond). Forbes Magazine. May 4, 2020. https://www.forbes.com/sites/joeharpaz/2020/05/04/5-reasons-why-telehealth-here-to-stay-covid19/#6fc78ab153fb Available at:
- 11.Brody J.E. A pandemic benefit: the expansion of telemedicine. The New York Times. May 11, 2020. https://www.nytimes.com/2020/05/11/well/live/coronavirus-telemedicine-telehealth.html Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.