Summary
Impaired function of pancreatic islet cells is a major cause of metabolic dysregulation and disease in humans. Despite this, it remains challenging to directly link physiological dysfunction in islet cells to precise changes in gene expression. Here we show that single-cell RNA sequencing combined with electrophysiological measurements of exocytosis and channel activity (patch-seq), can be used to link endocrine physiology and transcriptomes at the single-cell level. We collected 1,369 patch-seq cells from the pancreata of 34 human donors with and without diabetes. An analysis of function and gene expression networks identified a gene set associated with functional heterogeneity in β–cells which can be used to predict electrophysiology. We also report transcriptional programs underlying dysfunction in type 2 diabetes and extend this approach to cryopreserved cells from donors with type 1 diabetes, generating a valuable resource for understanding islet cell heterogeneity in health and disease.
eTOC:
Camunas-Soler, Dai et al. identify genes associated with islet-cell function and dysfunction in diabetes by combining single-cell RNA sequencing and patch-clamp electrophysiology in human islet cells.
Introduction
Cells use exocytosis to secrete a wide variety of molecules, including proteins, hormones and neurotransmitters. From islet cells in the pancreas to follicle cells in the reproductive system, endocrine cells control systemic metabolic homeostasis by secreting hormones that regulate metabolism, growth and development (Burgoyne and Morgan, 2003; Williams, 2016). Exocytosis can be monitored at the single-cell level by using patch-clamp electrophysiology to measure changes in membrane capacitance as vesicles fuse with the cell membrane and release their content. It is of great interest to understand the connection between dynamic physiological processes such as exocytosis with the generally slower changes in the transcriptomes of cells, which reflect the cell’s gene expression and genome regulatory processes. Single-cell RNA sequencing (scRNA-seq) allows us to profile the molecular identity of individual cells and detect changes in gene expression that arise in pathological settings. This technique has provided increasingly accurate cell type classifications in diverse organs based on their transcriptional fingerprint (La Manno et al., 2016; The Tabula Muris Consortium et al., 2018; Villani et al., 2017). However, it remains challenging to directly attribute physiological properties in a cell to its measured transcriptomic phenotype, and to establish unambiguous links with cellular dysfunction in disease (Stuart and Satija, 2019; Wang and Kaestner, 2018).
The islets of Langerhans control circulating glucose levels in the body through regulated exocytosis of the two principal glucoregulatory hormones, insulin and glucagon. Several single-cell genomics studies revealed significant variability across islet cell-types, as well as the existence of transcriptionally distinct cellular subpopulations (Baron et al., 2016; Enge et al., 2017; Muraro et al., 2016; Segerstolpe et al., 2016). This heterogeneity has been confirmed through identification of surface markers and mass-spectrometry signatures (Bader et al., 2016; van der Meulen et al., 2017; Wang et al., 2016a). Prior seminal physiologic studies established that islet cells, and especially insulin-producing β-cells, have heterogeneous function (Pipeleers, 1992; Salomon and Meda, 1986; Stefan et al., 1987), while impaired β-cell function and reduced exocytosis are hallmark features of early-stage type 2 diabetes (T2D) (Alejandro et al., 2015; Ashcroft and Rorsman, 2012; Kahn et al., 2006). However, the ability to link islet-cell molecular heterogeneity directly to such physiologic properties remains limited (Bakhti et al., 2019; Wang and Kaestner, 2018). Moreover, the connection between genes up- or down-regulated in T2D (Segerstolpe et al., 2016) to functional consequences in islets remains unclear, and major gaps persist in our mechanistic understanding of T2D ‘risk’ candidate genes identified by genone-wide association studies (Mahajan et al., 2018; Prasad and Groop, 2016; Tritschler et al., 2017).
To address such limitations, we combine here the use of whole-cell patch-clamp measurements and scRNA-seq (together referred to as patch-seq, Cadwell et al., 2016; Földy et al., 2016; Fuzik et al., 2016) in dispersed islet cells. This approach enabled us to simultaneously measure the cellular function and transcriptomes of human endocrine cells, allowing us to link gene expression and quantitative physiologic measurements of vesicle exocytosis and ion-channel activity. We collected 1,021 patch-seq cells from 28 donors with no diabetes (ND) or with T2D, most with short disease duration, and dissected function-to-gene expression relationships across islet cell-types. We discovered a subset of 484 genes that predicts multiple β-cell electrophysiological phenotypes, and we validated putative novel regulators of β-cell physiology through genetic loss-of-function experiments. We also identified pathways associated with reduced exocytosis in β-cells from donors with T2D, and found that these are distinct from those observed in nondiabetic donors. In β-cells from individuals with T2D we also identified an upregulation of genes linked to a high exocytosis phenotype compared to β-cells in nondiabetic, and propose a genetic mechanism underlying physiological dysfunction of β-cells during T2D. For example, we show that the transcription factor ETV1 likely plays a major role in β-cell dysfunction in humans with T2D. Finally, we demonstrate that our approach can also be applied to cryopreserved human islet samples. Using 348 cells from rare cryopreserved islets of donors with T1D, we obtained profiles from α-, γ-, δ-, duct, and surviving β–cells. Our work maps islet cell function and its transcriptome at the single-cell level, providing a valuable resource for exploring islet physiological and genetic dysfunction in health and disease.
Results
Patch-seq in endocrine cells to simultaneously assess physiology and the transcriptome
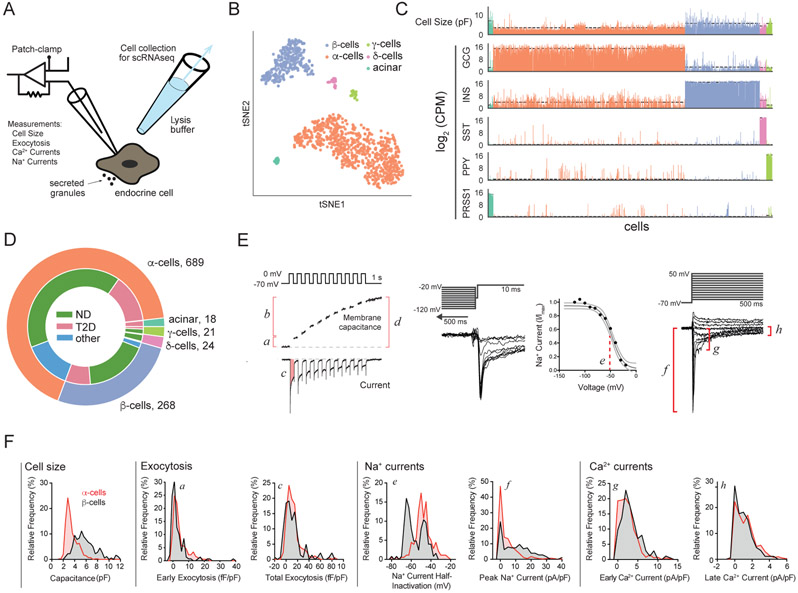
To achieve patch-clamp followed by scRNAseq (patch-seq) in individual human islet cells, we initially isolated cells from 28 donors, including 18 with no diabetes (ND), 7 with T2D, and 3 additional donors that were excluded from further analysis due to the reasons specified in Supp Table S1. We established patch-seq as a two-step process: (i) we performed electrophysiological measurements using whole-cell patch-clamp, and (ii) within 5 min from “break-in” we collected cellular content using a larger secondary pipette filled with lysis buffer (Fig 1A, Methods). This allowed intracellular access for whole-cell recording and exocytosis measurements and was followed by recovery of full-length transcriptomes using Smartseq2 that were sequenced to an average depth of 1-2 million reads (Supp Fig 1A-C, Methods). A total of 1,021 cells (80%) passed quality control for both electrophysiology and sequencing and were classified into major cell types based on the expression of key marker genes in a tSNE projection (Fig 1B, Methods). We obtained representatives of all major islet cell types (α-, β-, δ-, and γ-cells), and non-islet types such as acinar cells (Fig 1C,D). For each cell we measured parameters representing cell size, exocytosis, Na+ channel currents, and Ca2+ channel currents (Fig 1E,F). Thus, we obtained a broad survey of electrophysiological activity of all major islet cell-types in both ND and T2D settings (Supp Fig 1D-K). In addition to expected cell-type differences in size measured by whole-cell capacitance (Fig 1C,F), these data demonstrate substantial variation in exocytosis and channel activity in different pancreatic cell types and disease states, including altered exocytotic function in both α- and β-cells in T2D (Supp Fig 1D-K).
Figure 1. Patch-seq measurements in endocrine cells.
(A) Schematic of patch-seq. (B) tSNE projection of patch-seq cells clustered by gene expression of over-dispersed genes. (C) Cell size (measured as membrane capacitance) for each cell and expression of key marker genes. Color code as in panel B. Dashed line indicates average value per cell type. (D) Patch-seq cells collected in this study. (E) In each cell we measured: a- early exocytosis; b- late exocytosis; c- Ca2+ integral, d- total exocytosis, e- Na+ current half-inactivation; f peak Na+ current; g- early Ca2+ current; h- late Ca2+ current (not shown: cell size, reversal potential, and Na+ and Ca2+ conductance). (F) Distribution of selected parameters demonstrating functional heterogeneity of α– (red) and β– (black) cells. Inset letters (a-h) correspond to parameters in panel E. Distribution of exocytotic responses in a and c at 1 mM glucose (α–cells) or 5-10 mM glucose (β–cells). See also Supp. Fig. S1, S2 and Supp. Table S1.
To rigorously assess the robustness of patch-seq, we also collected an additional 3,518 cells by FACS for scRNAseq without patch clamping from 14 of these donors (8 ND, 6 T2D; Supp Fig 1A and Methods). The transcriptomes of cells after patch-seq or FACS-purification from the same donors led to comparable quality metrics (Supp Fig 2A). While we observed a difference in the number of genes with detectable transcripts, the values from cells after patch-seq or FACS were within the range of previously published datasets (Supp Fig 2B; Segerstolpe et al., 2016). Analysis of genes required to maintain fundamental cellular functions (hereafter, ‘housekeeping’), islet identity, and immediate early genes (IEG) showed that most differences are driven by (i) varying sensitivity to genes expressed at low levels (drop-out of genes with low expression), and (ii) varying expression of stress-response genes likely reflecting steps like islet shipping and dispersion (Supp Fig 2C). Gene expression in patch-clamped cells showed a high number of islet-specific transcripts and low IEG activation (Supp Fig 2C) like in prior scRNA-seq studies of human islets (Segerstolpe et al., 2016). A pseudo-bulk average analysis of our patch-seq and FACS dataset confirmed these results (Supp Fig 2D,E). We further investigated the effects of cell culture by collecting a subset of patch-seq cells on the same day of dispersion. We found an initial increase in IEG transcripts that decreased after 1 day in culture, while transcripts encoding islet-specific genes recovered (Supp Fig 2F,G). This recovery was also observed for cells obtained from donors with T2D (Supp Fig 2H). Finally, patch-seq itself did not appear to impact gene expression since cells collected with or without patch-clamp overlapped in a tSNE representation (Supp Fig 2I).
Patch-seq identifies novel genetic regulators of human β-cell physiology
We applied the patch-seq approach to find genes associated with electrical and exocytotic function in β-cells. In these cells, we found that electrophysiological properties cluster by ‘functional group’ (exocytosis, Ca2+ and Na+ currents) and are uncorrelated with other parameters such as donor age, sex or BMI (Fig 2A; Supp Fig 3A). We first correlated the transcriptome of β-cells to total exocytosis at stimulatory glucose levels, which is representative of the total secretory capacity of a β-cell and a key indicator of β-cell function and dysfunction in T2D (Ferdaoussi et al., 2015; Gembal et al., 1992). In this way, we found genes positively- or negatively-associated with β-cell exocytotic capacity (Fig 2B). Among top correlates, we found several genes linked to pathways thought to regulate insulin secretion, including β-cell transcription factors (MAFA, ETV1), molecules associated with insulin granules (SLC30A8, VAMP2, SCG2, INS), metabolic enzymes (PDK4, PDHA1, GYG1), and ion channels (ABCC9, KCNH2, KCNMB2, NALCN) including the L-type Ca2+ channel encoded by CACNA1C (Rorsman and Braun, 2013; Lu et al., 2002; Ait-Lounis et al., 2010; Zhang et al., 2005; Chimienti et al., 2004). Gene set enrichment analysis (GSEA) using correlation scores confirmed many of these pathways and revealed additional enriched categories, such as neuronal regulators, transcription factors, and regulators of cell-polarity or stress (Fig 2C; Supp Fig 3B). Islet transcription factors with weaker but significant association to exocytosis included PAX6, FOXO1 and NKX6-1 (Supp Fig 3B).
Figure 2. Correlation of β–cell exocytosis to single-cell gene expression and pathway analysis.
(A) Spearman correlation of electrophysiological parameters shows clustering of each functional group and low cross-correlation across clusters. All parameters are normalized to cell size. (B) Heatmap of top genes correlated and anticorrelated to total exocytosis in β–cells from ND at 5-10 mM glucose. Cells are sorted by exocytotic response from highest (left, dark green) to lowest (right, yellow). Gene expression is shown as a z-score after smoothing (n = 20 cells). Metadata for each cell is shown at bottom (BMI, Donor, Sex). (C) Top enriched pathways in genes correlated (red) and anticorrelated (blue) to total exocytosis (KEGG and Reactome databases, False Discovery Rate (FDR) < 0.1). (D) Summarized map of cellular location and pathways with genes correlated (green) and anticorrelated (red) to exocytosis. (E) Exocytosis in β–cells measured at 10 mM glucose following target gene knockdown compared to control cells from same donors (348 cells, n ≧3 donors per knockdown experiment). ** P < 0.01, **** P < 0.0001 (Mann-Whitney-U test and BH correction). See also Supp. Fig. S3 and Supp. Tables S2, S3, S4.
The majority of genes whose expression correlated significantly with β-cell exocytosis are islet-specific and included candidate T2D risk genes like YWHAG (Fernandez-Tajes et al., 2019). Top gene correlates to exocytosis also included regulators of oxidation and detoxification that could mitigate T2D-associated stress (Otter and Lammert, 2016), such as glutathione peroxidase and transferases (GPX3, GSTK1 and GSTA4: Supp. Table S2). We integrated these results into a map that combined correlations of transcript levels with four exocytosis measures (early, total, late, and normalized to Ca2+; Fig 2D), permitting visualization and analysis of gene expression significantly correlated with exocytotic function in β-cells.
This analysis nominated multiple genes - without previous known roles in β-cells - as candidate regulators of physiological function, including OGDHL, FAM159B, TSPAN1, RGS9 and GYG1. Prior studies have linked OGDHL to metabolism, while FAM159B and TSPAN1 encode membrane proteins and RGS9 specifies a regulator of G-protein signalling (Bunik et al., 2008; Danielsson et al., 2014; Uhlen et al., 2015). To test our predictions, we performed siRNA knock-down followed by patch-clamp for this set of genes in islets from an additional 8 ND donors (Supp Table S3; Fig 2E). Knockdown of each gene, confirmed by qPCR (Supp Table S4), was followed by electrophysiological characterization at 10 mM glucose and insulin immunostaining to confirm cell-type. In 4/4 cases of genes positively correlated to exocytosis (OGDHL, FAM159B, TSPAN1 and RGS9), we observed significant reduction of the exocytotic responses after knock-down (Fig 2E). By contrast, knock-down of GYG1, whose transcript levels are anticorrelated with exocytosis, did not lead to significant changes of exocytosis, possibly reflecting that β-cell activity is already maximally-stimulated at these high-glucose conditions. Together, these data show that patch-seq analysis robustly identified previously unknown regulators of human β-cell physiology.
Prediction of β-cell electrophysiology from gene expression
To identify and investigate additional genes linked by patch-seq to β-cell excitability, we broadened our analysis to include additional electrophysiological parameters (Ca2+ currents, Na+ currents), and selected transcripts showing consistent positive or negative correlations to multiple parameters as likely regulators of β-cell physiology in nondiabetic states (Fig 3A). This analysis identified several key genes, including genes previously associated with β–cell transcriptional heterogeneity (Baron et al., 2016; Dorrell et al., 2016; Rui et al., 2017; Segerstolpe et al., 2016). For instance, transcripts encoding retinol binding protein 4 (RBP4) correlated negatively with cell size, Na+ currents and total exocytosis, while transcripts encoding the β-cell surface protein FAM159B correlated positively to exocytosis, Ca2+ entry and Na+ currents. A tSNE projection using these highly correlated genes shows a gradient of functional measures across β-cells that overlaps with patterns of gene expression (Fig 3B). To understand further how genetic pathways are connected to each major group of physiological function (exocytosis, Ca2+ currents, and Na+ currents), we repeated GSEA on their averaged correlation scores (Supp. Fig 3C). Results for overall exocytosis are consistent with those reported above (Fig. 2C); by contrast, GSEA further identified that Na+ and Ca2+ currents are linked to pathways related to increased excitability and circadian rhythms respectively (Perelis et al., 2015). Several of the observed pathways identified by GSEA on averaged correlation scores also overlap with those recently implicated in islet dysfunction in T2D using genomic data (Fernandez-Tajes et al., 2019).
Figure 3. Gene networks associated with β–cell functional heterogeneity.
(A) Genes with significant correlation to electrophysiology (z-score > 2 for n > 5 parameters, see Methods). Significant positive/negative correlations are indicated in red/blue. (B) tSNE projection of β–cells from ND using genes in panel A. Spearman rank correlation between each parameter and gene is shown as mean and error in last digit (see Methods). (C) Network of genes connecting different functional groups (‘PS genes’). Genes (small dots) connecting different functional groups (large dots) are selected if they show significant correlations (z-score > 2) to at least one functional parameter in each group. Edge color indicates positive/negative correlations (red/blue), and gene color identifies clusters connected to the same functional groups. (E) Predictions of the k-NN model using ‘PS genes’ for training (black) and validation (red) sets. Spearman correlation and P for each parameter are indicated as inset. (F) Performance of the model using the ‘PS genes’ (dark gray), measured as Spearman correlation between experimental and predicted values. Comparison to model using 484 random genes of equivalent expression in β–cells (10,000 permutations, 95% CI interval in light gray). Asterisk indicates parameters with P < 0.05 in validation set. Smaller spider plots show performance of the ‘PS gene set’ (gray) versus top correlated genes to exocytosis (pink), Ca2+ (green), Na+ (orange), cell size (blue). See also Supp. Fig. S4 and Supp. Table S5.
We then asked whether genes that correlate to multiple groups of physiological function (“functional groups”) could be used to develop predictive algorithms that link transcriptional signatures to β-cell function. To do so, we generated a network of genes with significant correlation to more than one functional group (e.g. Ca2+ and exocytosis) and selected those with highest median expression (Fig 3C, Supp Table S5). This list of highly-connected genes, that we termed our Predictive Set (PS), contains genes: (i) previously linked to β–cell excitability, (ii) with heterogenous expression in β–cell subpopulations and in T2D (FFAR4, FXYD2, ID4), and (iii) with unknown function in β–cells (Supp Table S5). We then attempted to predict the measured electrophysiology of each cell from the average values of its nearest neighbors (NN) in PS gene expression (n = 484 genes, k-NN with 5 neighbors) (see Methods). We obtained significant correlations between the experimentally measured patch-clamp parameters and the predictions from the k-NN model (Fig 3D; Supp Fig 4A, black). We compared the performance of PS genes to randomly selected genes of equivalent expression (n = 10,000 permutations), and found that the PS genes performed significantly better for all parameters (Fig 3E). We also tested our model by comparing its predictive power to that obtained using the genes most correlated to each functional group. We found that PS genes showed consistent performance across all measured parameters, whereas group-specific gene sets only performed well for their subset of parameters (Fig 3E, Supp Fig 4B). Finally, we validated the predictive power of PS genes with a ‘test’ set of data including gene expression and functional parameters of cells withheld from the analysis that generated the PS gene set. This analysis demonstrated appropriate recovery of significant correlations for 5 of the measured functional parameters (Fig 3D; Supp Fig 4A, red, and Fig 3E). Thus, patch-seq and network analysis combined with machine learning generated unprecedented algorithms that reliably link gene expression to β-cell function.
Markers of β–cell heterogeneity correlate to β–cell excitability
Within the identified ‘PS gene set’ we found that transcripts encoding RBP4 are significantly correlated with β-cell functional heterogeneity. Prior studies have noted heterogeneous expression of RBP4 in β-cell subsets, but did not establish direct links to functional heterogeneity (Baron et al., 2016; Dorrell et al., 2016; Rui et al., 2017; Segerstolpe et al., 2016). Other studies show that RBP4 is an adipokine with roles in homeostatic regulation of metabolism (Broch et al., 2007; Tritschler et al., 2017). We observe that RBP4 transcript levels have significant correlation with multiple β-cell functional phenotypes; for example, it is the ‘PS gene’ with strongest anti-correlation to β-cell Na+ channel activity. RBP4+ β-cells have decreased exocytosis and Na+ currents despite having normal Ca2+ current activity (Fig 4A). Consistent with this, we observed that RBP4+ β-cells also have significantly reduced expression of key regulators of stimulus-secretion coupling, like KCNJ8, ABCC9 and SCN3A (Fig 4B). In rodent β-cells, Scn3a encodes the principal physiologically-relevant Na+ channel (Zhang et al., 2003). Together, these results provide evidence that RBP4 expression is a marker of β-cells with reduced function, and show that previously-identified markers of β-cell transcriptomic heterogeneity identify subpopulations with heterogeneous function (Dorrell et al., 2016; Johnston et al., 2016).
Figure 4. Functional and transcriptomic differences in β–cell subpopulations.
(A) Electrophysiological function in RBP4 subpopulations of β–cells. A significant decrease of Na+ currents and exocytosis is observed for RBP4+ cells. (B) Gene expression values (top) and % β–cells with detectable expression (bottom) of Na+, ATP-sensitive K+ channels and Ca2+ channels. *** P < 0.001, ** P < 0.01 * P < 0.05 (Mann-Whitney-U test with BH correction).
In T2D β-cells induce genes linked to increased and decreased exocytosis
Islets from the 7 donors with T2D (Supp Table 1) showed reduced insulin content and secretion (Fig 5A), and impaired β-cell exocytosis (Fig 5B, Supp Fig 5A). All β-cells were obtained from donors with recent T2D onset (<9 years), and who were not receiving insulin treatment (as we did not record any β-cells for R241, the only donor on insulin treatment: see Methods). To identify genetic drivers of β-cell dysfunction in T2D, we first assessed transcripts that we previously identified to be significantly correlated to exocytosis in β-cells from ND (Fig 2B, Supp Table 2). Unexpectedly, we found that genes that positively correlate with exocytosis in β-cells from ND are up-regulated in T2D, while genes that negatively correlate with exocytosis in β-cells from ND had reduced expression in T2D (Fig 5C). We found that this transcriptomic shift is also observed in β-cells from obese ND individuals (Supp Fig 5B). These data suggest that β-cells attempt to alter transcript levels to increase functionality, perhaps in response to increased insulin demand associated with T2D and obesity. But in T2D this ultimately fails to maintain an increase of β-cell function, since their exocytotic response is significantly impaired compared to ND controls (Fig 5B).
Figure 5. Functional and transcriptomic changes in β–cells early in T2D.
(A) Insulin content and secretion (as % content) for donors included in this study. * P < 0.05, *** P < 0.001 (Student’s t-test; Two-way ANOVA and Tukey post-test) (B) Measured exocytosis for β–cells in ND and T2D. ** P < 0.01 (Mann-Whitney-U test and BH correction). (C) Enrichment analyis in T2D of genes found to be correlated (red) or anticorrelated (blue) to exocytosis in cells from ND. Data shows median log fold-change in gene expression between T2D and ND for each subset of genes. Error is SEM. Central bar shows range of log2 fold change obtained by sampling random genes of equivalent expression (10,000 iterations, 400 genes, 5-95% range). (D) Gene correlation map of exocytosis. Scatter plot shows correlation to exocytosis in ND (x-axis) and T2D (y-axis) for each gene. Genes with significant correlations (z-score>2) are colored according to their fold-enrichment in T2D cells (red upregulated in T2D, blue downregulated in T2D), and size is proportional to relative change in % expression. Regions of interest are highlighted with dotted boxes. Genes with non-significant correlations shown in gray. (E) Enriched pathways for genes correlated to exocytosis in ND (i), T2D (ii) and genes anticorrelated to exocytosis in ND (iii), T2D (iv). Enrichment is shown as log10(FDR). Left bar indicates top category of each pathway. (F) Distribution of ETV1 expression (left) and enrichment of a subset of genes that change between ND and T2D. (G) Model showing the hypothesized role of ETV1, STAT3 and immune pathways in β–cell dysfunction in early T2D. Based on (Suriben et al., 2015). (H) Exocytosis in β–cells measured at 5 mM glucose following ETV-1 knockdown compared to control cells from same donors (n = 3 donors both for ND and T2D, *** P <0.001 Kruskal-Wallis test and Dunn’s post-test). See also Supp. Fig. S5.
To find genes that could underlie this effect, we performed correlation analysis in cells from donors with T2D, and integrated the ND and T2D results in a correlation map of exocytosis with the overall changes in gene expression (Fig 5D, Supp Table 2). Next, we performed a pathway analysis for each of 4 gene subsets (ND/T2D and correlated/anticorrelated to exocytosis) (Fig 5E, Supp Fig 5C). Our data shows that the pathways associated with reduced exocytosis in T2D are distinct from those associated with low exocytosis in ND. While we found that low exocytosis in β-cells from ND is linked to metabolic pathways and glucose metabolism, the dysfunction in T2D is related to immune response, cell cycle pathways and altered transcription factor expression. For instance, we observe induction of pathways like NFκB signalling, cell cycle checkpoints, and auto-degradation of the ubiquitin ligase COP1. Genes in T2D that show increased expression associated with reduced β-cell exocytosis included known regulators of immune and inflammatory pathways like NFKBIA, IL6R and IRAK1, and the transcription factors ETV1 and STAT3 (Fig 5D-F, Supp Fig 5D). In particular, we found ETV1 to be significantly enriched in β-cells from donors with T2D, and to show ‘reversed’ correlations to exocytosis in T2D and ND. Overall we found that in T2D, increased ETV1 mRNA levels associated with reduced β-cell exocytosis (Fig 5D, yellow box). These results were consistent across several measured exocytosis parameters (Supp Fig 5E,F). Hence, our results suggest that dysfunctional cells in T2D express higher levels of ETV1 than functional ones, whereas the opposite happens in ND.
The ETV transcription factors impair insulin secretion in hyperglycaemic mouse models, and are negatively regulated through COP1–dependent targeted degradation (Fig. 5G) (Suriben et al., 2015). We tested genes previously reported to show ETV-dependent expression in mouse islets (Suriben et al., 2015), and found several human orthologs with correlating expression in single β-cells, including MAFA, SLC30A8 and STAT3. These results indicate that optimal insulin secretion may require balanced expression of ETV1 and its downstream targets, and that an imbalance may lead to dysregulated secretion during early T2D (Fig 5G). To test the differential role of ETV1 in β-cell physiology, we performed siRNA knock-down of ETV1 followed by patch-clamp in an additional set of human islets. We found that knock-down of ETV1 rescued exocytosis in β-cells from donors with T2D, whereas it did not alter exocytosis in β-cells from ND (Fig 5H and Supp. Fig. 5G), further supporting a significant role for ETV1 in β-cell dysfunction in T2D.
Transcriptional and physiological heterogeneity observed in α cells
Cell size and Na+ channel properties are often used to identify α and β cells in rodents (Briant et al., 2017; Zhang et al., 2014); however this is not generally applicable to humans, making it difficult to distinguish islet cell types at the time of patch-clamping. For example, α-cells (definitively identified post hoc from scRNA-seq) were frequently mis-classified as possible β-cells during our initial patch-clamp measures of capacitance that indicated cell size (>4 pF; Fig 1F). To improve cell-type identification prior to scRNAseq, we implemented a machine learning algorithm based in ‘random forests’ (Breiman, 2001) to classify cell types from their electrophysiological profile. We trained and validated the model in cells from ND donors using 10-fold cross-validation, obtaining an AUC of 0.95 in the validation dataset (Fig 6A). This model correctly identified 85% (92%) of α-cells (β-cells), misidentifying 15% of α-cells and 8% of β-cells (Fig 6B). Features from every functional group contributed to the classifier (Fig 6C), and the model also performed robustly in cells from T2D donors (AUC = 0.93) (Fig 6A). Compared with a capacitance-based cell size cut-off at the time of patch-clamping, our model significantly improved the a priori identification of α-cells, and reduced their mis-classification as β-cells (Fig 6D). Analysis of α-cell transcriptomes revealed significant transcriptional heterogeneity in islets from multiple human donors, including those with T2D. In particular, a subset of cells showed enrichment of regulators of α-cell maturation (LOXL4, MAFB, ARX), regulators of reduced ER stress (DDIT3, XBP1, PPP1R15A), transcription factors governing endocrine fate (FEV, ISL1), receptors involved in glucose homeostasis (FFAR1, GPAR119), and secretory pathways (SCG2 (Fig 6E; Supp Fig 6A,B). This transcriptional heterogeneity correlated to electrophysiological features including Na+ currents and cell size (Fig 6E); further work will be required to understand the relationship of this heterogeneity to α-cell dysfunction in T2D. Thus, in addition to improving prospective identification of patch-clamped human α-cells, our studies provide evidence for molecular heterogeneity associated with functional heterogeneity in α-cells.
Figure 6. Transcriptomic and electrophysiological heterogeneity in α–cells.
(A) ROC curve of cell type prediction using random forests for the validation dataset in ND (red, blue) and in T2D (green, purple). (B) Confusion matrix showing accuracy of predictions in ND validation dataset. (C) Contribution of each feature to the random forest model. (D) Comparison of α–cell identification from predictions at the time of patch-clamping (simple cell size cut-off) versus the model. (E) t-SNE plots showing heterogeneity in gene expression of α–cells using over-dispersed genes and normalized electrophysiological measurements. See also Supp. Fig. S6.
Application of patch-seq to rare cryo-preserved samples: Islet cells in T1D
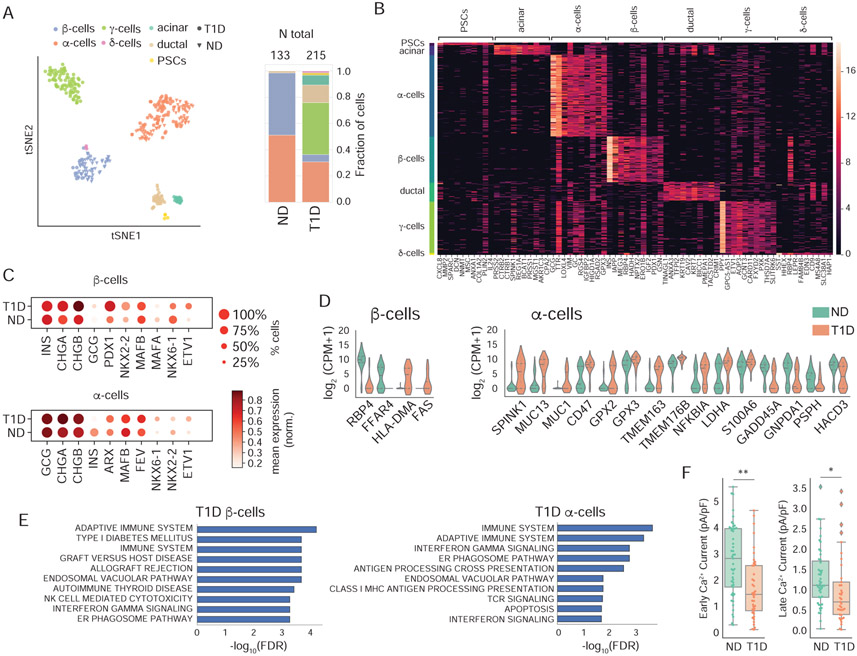
Tissue banking programs can provide unique access to cells from subjects with specific disorders. Hence we investigated whether the patch-seq approach could be extended to rare cryopreserved islet samples (Manning Fox et al., 2015). We found that single-cell transcriptomes from cryo-banked islets had comparable quality metrics to those of fresh tissue (Supp Fig 7A), allowing us to investigate cells from three donors with T1D and three controls matched for BMI, age, sex and storage-time (Supp Table S6). We performed patch-seq in 348 cells from these samples (Fig 7A; Supp Table S6) and used a logistical regression model to identify marker genes for each population (Fig 7B, Supp Fig 7B). Samples from donors with T1D contained an unanticipated variety of cell types (Fig 7B), including an enrichment for pancreatic polypeptide-secreting γ-cells and ductal cells (Supp Fig 7C).
Figure 7. Patch-seq in cells from cryopreserved T1D islets.
(A) Left: tSNE projection of patch-seq cells clustered by gene expression of over-dispersed genes. Right: Cell types and total number of cells obtained for ND and T1D. (B) Marker genes for each cell type. (C) Expression of key identity genes on α– and β–cells from donors with T1D and ND matched controls. (D) Representative genes obtained in a differential expression analysis between T1D and ND for β– and α–cells. (E) Pathways enriched in upregulated genes in T1D α– and β–cells. (F) Distribution of calcium parameters showing statistically significant differences between α–cells of donors with T1D and ND. ** P < 0.01, * P < 0.05 (Mann-Whitney-U test with BH correction). See also Supp. Fig. S7 and Supp. Tables S6, S7.
We obtained 11 β-cells from two donors with T1D, consistent with prior observations of β-cell survival years after T1D onset (Keenan et al., 2010; Morgan and Richardson, 2018) (Supp Fig 7C). These β-cells had similar electrophysiological profiles as cryopreserved β-cells from ND controls (Supp Fig 7D), and appeared to maintain equivalent expression of hallmark β-cell genes (INS, PDX1, MAFB) (Fig 7C). Differential expression analysis showed decreased expression of RBP4 and FFAR4 and transcript enrichment of genes related to immune activation and allograft rejection (HLA-DMA, FAS) (Fig 7D, 7E). In α-cells from donors with T1D we found increased NKX6.1 and decreased NKX2.2 mRNA (Fig 7C; Supp Fig 7E) along with decreased Ca2+ channel activity (Fig 7F; Supp Fig 7D), in general agreement with recent reports using bulk RNA-seq and immunohistochemistry (Brissova et al., 2018; Chakravarthy et al., 2017), although we did not detect a consistent decrease in Ca2+ channel gene expression as previously reported (Supp. Fig. 7E). Our analysis of α-cells in T1D also showed transcript enrichment of mucin (MUC) and other genes typically associated with ductal cells, and FEV1, a reported endocrine progenitor cell marker in mice (Fig. 7C,D; Supp Table S7) (Byrnes et al., 2018; Liu et al., 2018). Together, our data demonstrates that patch-seq can be used in rare cryo-stored tissue types, and supports the view that the transcriptomic and functional signatures of surviving β-cells may be preserved in T1D, while α-cells lose characteristic functional and transcriptomic phenotypes, consistent with the observation of impaired glucagon regulation in T1D.
Discussion
Success in identifying the genetic basis of islet function and dysfunction in diseases like diabetes has been limited by an inability to connect islet cell physiologic function with transcriptome regulation. Elucidating mechanisms underlying diabetes also suffers from limited human cell and tissue availability that hinders single-cell-based investigations, particularly when considering T1D. Multi-modal single-cell technologies will help address these issues by increasing the depth of available data and by making it possible to directly link transcriptomes to additional read-outs in individual cells (Macaulay et al., 2017; Stuart and Satija, 2019). One integrated approach previously used in studies of rodent brain slices is dual patch-clamp electrophysiology and single-cell sequencing (patch-seq) (Cadwell et al., 2016; Földy et al., 2016; Fuzik et al., 2016). Like neurons, endocrine-cell secretion is coupled to dynamic electrophysiological mechanisms that culminate in regulated exocytosis of secretory vesicles. Methods that allow simultaneous measurements of transcriptomes and secretory capacity hold promise for understanding the mechanisms by which gene expression enables physiological function in these cells.
Here we describe development of patch-seq for endocrine cell-types, and report paired physiologic-transcriptomic data for more than 1,300 human islet cells. This depth of data allowed us to link transcriptional and physiological heterogeneity at single-cell resolution, and to identify genes and pathways regulating exocytosis of human islet β- and α-cells. With a patch seq-derived minimal Predictive Set (PS) of genes and machine-based learning tools, we developed algorithms that correctly predicted hallmark physiological functions like exocytosis from gene expression, and accurately classified live isolated islet cell types. For studies of islets from T1D and T2D donors, we used patch-seq to identify molecular mechanisms underlying β- and α-cell dysfunction in these diseases. Thus, our study provides a powerful resource and toolset for simultaneous multiplex phenotyping of human islet cells, in health or disease.
Patch-seq analysis identified key genes and pathways regulating β–cell exocytosis, including both known and previously-uncharacterized regulators and mechanisms controlling insulin secretion such as cell adhesion molecules and neuroactive ligand receptors. Adhesion molecules have previously-demonstrated roles in β–cell attachment and motility (Dahl et al., 1996; Kaido et al., 2004), and are thought to govern β-cell polarity and function through control of directed insulin exocytosis into the vascular axis (Geron et al., 2015; Parnaud et al., 2015; Gan et al., 2018). Among neuronal regulators, we identified pairs of factors such as neurexin 1 (NRXN1) and neuroligin 1 (NLGN1), which are known to bind in a heteromeric complex (Suckow et al., 2008). The identification of binding partners in our gene-function correlation analysis confirms the robustness of this approach to identify unanticipated candidate regulators of islet-cell function. Similarly, examination of tissue-specificity reveals that several of the encoded proteins correlating to β-cell exocytosis are also enriched in other excitable cell-types (GPRASP1, RGS9 and MRAP2 in retinal tissue, GPRIN1 in brain, KCNH2 in cardiomyocytes) (Uhlen et al., 2015), indicating shared mechanisms of signal transduction, and showing that the analytical tools developed here could be applicable to patch-seq studies in other excitable cells and endocrine tissues.
Regulation of islet exocytosis by metabolism is complex (Newgard, 2017). We observed that multiple expected metabolic pathways positively correlated with β-cell functional measurements (e.g., glucose flux and metabolism, circadian rhythm), but also observed unexpected negative correlations of β-cell exocytosis to pathways like glycolysis and pyruvate metabolism. We integrated several of the observed metabolic correlations to β-cell exocytosis in a functional map (Fig 2D), providing support for the hypothesis that glucose is preferentially used for TCA anaplerosis through pyruvate carboxylase (PC) to facilitate insulin secretion (Alves et al., 2015). These findings are also sustained by measures of CPT1A and SLC16A9 expression, which mediate the rate-limiting step of fatty-acid oxidation and carnitine efflux, respectively (Aichler et al., 2017; Newgard, 2017). Overall, the observation of glucose homeostasis pathways across our analysis also resonates with the importance of metabolites in shifting transcriptional states of β-cells, recently dubbed “metabolic memory” (Rosen et al., 2018; Sharma and Rando, 2017).
Another useful outcome here is our development of a network analysis tool that links a predictive set (PS) of 484 genes with applied machine-learning techniques. We used this to predict β-cell function solely from RNA transcript abundance. Genetic regulators of β-cell activity, including many uncharacterized in islet biology, were identified in the predictive set, and included antioxidant molecules (GPX3, GSTK1), surface proteins (TSPAN1, TSPAN2), and β-cell enriched molecules (NPTX2, FAM159B). To validate predictions from patch-seq correlations and this network analysis, we used genetic loss-of-function approaches, which provided evidence that previously uncharacterized genes like OGDHL, RGS9, TSPAN1 and FAM159B are positive regulators of β-cell exocytosis. Prior studies have shown that FAM159B may be regulated by the β-cell transcription factor SIX3 (Arda et al., 2016), and is a marker of β-cell subsets (Dorrell et al., 2016). Like FAM159B, other genes linked by our analysis to differential β-cell function were also found to be expressed in β-cell sub-populations (ID2, ID4, RBP4) (Baron et al., 2016; Dorrell et al., 2016; Rui et al., 2017; Segerstolpe et al., 2016). For RBP4, we provide evidence that ND RBP4neg β-cells, compared to ND RBP4+ β-cells, have relatively superior electrophysiological function (also see discussion of T1D below). Overall, our data shows that β-cells exhibit significant transcriptomic and electrophysiological heterogeneity, supporting the idea that β-cells comprise a spectrum of cellular states. Further studies are needed to address whether this heterogeneity corresponds to dynamic or static states and the existence of mechanisms allowing cells to switch between them, or possibly to a hierarchical physiological organization of β-cells (Johnston et al., 2016). Similarly further application of the PS gene signature to FACS datasets in disease settings (T2D) would enable high-throughput functional phenotyping of β-cells.
Understanding the basis of islet adaptation or dysfunction in diabetes is another important result described here. Our work shows that the pathways associated to reduced β-cell exocytosis in T2D significantly differ from those correlated to low exocytosis in cells from ND. Furthermore, the observation that β-cells from donors with T2D or obesity show increased expression of genes that are correlated to a high exocytosis phenotype in ND, suggests molecular mechanisms for functional compensation by β-cells in early-stage T2D. However, our analysis also reveals as-yet unexplained complexity in β–cell responses; for example, ETV1 and STAT3 mRNA are positively-correlated with ND β–cell functional phenotypes, and also with β-cell dysfunction in T2D. To address this, we performed genetic loss-of-function experiments that showed recovery of exocytotic capacity after knock-down of ETV1 in β-cells from donors with T2D. Recent studies suggest that β-cell ETV1 and STAT3 protein degradation are regulated by the ubiquitin ligase complex (Dallavalle et al., 2016; Suriben et al., 2015). Our work suggests that ubiquitination and proteosomal degradation pathways in β-cells, including a pathway stimulating COP1 auto-degradation, are associated with dysfunction in T2D. Thus, in addition to transcript-based mechanisms, our results suggest that post-translational mechanisms involving factors like COP1, ETV1 and STAT3 may govern β-cell dysfunction or responses in T2D (O’Shea and Plenge, 2012; Saarimäki-Vire et al., 2017; Suriben et al., 2015).
Patch-seq also advances our understanding and ability to study human α-cells. Cell size and Na+ channel properties are key identifiers used to distinguish rodent α- and β-cells (Briant et al., 2017; Zhang et al., 2014), but human α-cell heterogeneity results in overlap of these electrophysiological properties. Using patch-seq data, we developed additional models for improved cell-type identification of live human islet α-cells and β-cells. We also show that specific phenotypes like Na+ current activities and cell size can vary significantly in α-cells, and that this functional heterogeneity corresponds well with expression of transcripts specifying islet cell lineage (ARX, MAFB, FEV) or governing cellular stress (DDIT3, PPP1R15A). Prior studies have reported the impact of the ER-stress response in β-cells subpopulations (Baron et al., 2016; Muraro et al., 2016), and in α-cells (Akiyama et al., 2013; Burcelin et al., 2008; Korsunsky et al., 2019), suggesting that varying levels of ER-stress could drive altered or dysfunctional states in both islet cells types. We noted α-cell subpopulations expressing markers indicating either low- or high ER-stress, both in ND and in T2D settings. Thus, patch-seq merges electrophysiological and transcriptomic data to document, and suggest a basis for, heterogeneous function and transcriptome regulation in human α-cells.
Phenotyping live islet cells from donors with T1D and other, rarer, forms of diabetes is a significant challenge that stems from the paucity of available tissue and low islet cell recovery; this has retarded understanding of islet and diabetes biology (Brissova et al., 2018; Wang et al., 2016b). Likewise, procuring and studying appropriate control tissue for studies of T1D islets, matched in key properties like donor age, is an enduring challenge. Electrophysiological studies of islets from only one donor with T1D have been reported, suggesting that surviving β-cells have normal function and α-cells are dysfunctional (Walker et al., 2011). Similarly, to date only islets from one donor with T1D have been studied using scRNA-seq (Wang et al., 2016b). Thus, our extension of patch-seq to studies of cryo-stored islets from ND and T1D donors represents a significant innovation. Here we show that library quality from cryopreserved islets is comparable to that of control fresh islet cells. Moreover, our detection of α-cell dysfunction and normal function of surviving β-cells using patch-seq with T1D islets correlates well with findings from a recent study using different methods (Brissova et al., 2018). It is important to note that β-cells from donors with T1D studied here were from islets obtained >11 years (and up to 31 years) following diagnosis. Thus, further studies are needed to investigate potential β-cell dysfunction that may occur at earlier stages of disease progression. Patch-seq revealed greater α-cell heterogeneity in T1D compared to ND islets, including evidence of impaired maintenance of α-cell fate, and we speculate that this might contribute to the well-recognized impairment of glucagon secretion observed in T1D (Unger and Cherrington, 2012). Moreover, transcriptomic profiling here reveals that functional preservation in β-cells from donors with T1D corresponds to undetectable expression of RBP4, consistent with work by others (Brissova et al., 2018; Rui et al., 2017). By contrast, we show that ductal cells in T1D, which some suggest represent a potential source of new β-cells (Corritore et al., 2016), lack detectable endocrine physiological phenotypes. Whether from pancreatic cell reprogramming or other sources (Chakravarthy et al., 2017; Thorel et al., 2010), rigorous evaluation of “replacement” β-cells with patch-seq should emerge as a new benchmark to assess functional and transcriptional resemblance to bona fide β-cells.
In conclusion, the approaches presented here enable a precise molecular characterization of endocrine physiology and represent significant advances in the development of multimodal scRNAseq technologies by increasing cell throughput and extending analyses of simultaneously-measured transcriptomic data and physiological features. Our results help clarify observed inherent islet cellular heterogeneity, and provide valuable tools for understanding the molecular mechanisms underlying normal islet function and dysfunction in diabetes. This work also sets a framework that could be applied to other cell types where regulated exocytosis and secretion are hallmark features of cellular identity.
Limitations of the study
Our patch-seq characterization is performed after islet isolation and dispersion into single cells. Although we performed controls to assess the effect of single-cell dispersion and patch-seq, translation of our findings to in vivo conditions will require further studies. Similarly, by single-cell dissociation, contextual information from neighboring cells and tissue structure is lost. Patch-seq directly in live pancreas slices could bridge our results to islet structure, although this still poses significant technical challenges in a ribonuclease rich tissue such as the pancreas. Throughout this work, we used voltage-clamp recordings of ion channels and measurements of exocytosis as a proxy for islet-cell function. Although these are established markers of islet-cell excitability and function, further experiments recording action potential firing, Ca2+ signalling, and hormone secretion assays would strengthen gene candidates identified here and their mechanistic role in β-cell physiology. The PS gene model is derived using patch-seq cells from nondiabetic donors. Its refinement through larger datasets that include more diabetic donors should improve the generality of the model and applicability for β-cell benchmarking in biomedical settings. For T1D studies we used cryopreserved islets. An advantage to this is that it allowed us to performe all experiments using matched controls to minimize confounding effects, however it would be of interest to confirm these results in independent studies using fresh tissue.
STAR Methods
Resource availability
Lead Contact and materials availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Stephen R. Quake (steve@quake-lab.org).
Data and Code Availability
Raw sequencing reads are available in the NCBI Gene Expression Omnibus (GEO) and Sequence Read Archive (SRA) under accession number GSE124742. The code and scripts generated during this study as well as preprocessed datasets are available at https://github.com/jcamunas/patchseq.
Experimental Model and Subject Details
Islet isolation, cryopreservation, and insulin secretion
Donor organs were obtained with written consent and research ethics approval at the University of Alberta (Pro00013094, Pro00001754), perfused via the pancreatic ductal system with buffer containing Collagenase Gold 800 (VitaCyte, Indianapolis, IN) and Thermolysin (Roche Diagnostics, Mannheim, Germany), then digested with a Ricordi Islet Isolator (Biorep Diabetes, Miami, FL) and purified by density centrifugation. Donor characteristics are described in Supp Tables S1,S3,S6. Full details of our human islet isolation protocol, equipment setup, quality control, cryopreservation, and static glucose-stimulated insulin secretion assays have been deposited in the protocols.io repository (Lyon et al. 2019).
Method Details
Electrophysiological phenotyping
Two-hundred hand-picked islets per donor were dissociated to single cells using enzyme-free Hanks’-based Cell Dissociation Buffer (Thermo Fisher Scientific, Cat#13150-016, for donors R229~R242) or Hanks’ Balanced Salt Solution and StemPro accutase (Thermo Fisher Scientific, Cat#A11105-01, for donors R243~R269, cryo-T1D donors and matched controls) and cultured in low glucose (5.5 mmol/L) DMEM with L-glutamine, 110 mg/L sodium pyruvate, 10% FBS, and 100 U/mL penicillin/ streptomycin for 1-3 days. Media were then changed to bath solution containing (in mM): 118 NaCl, 20 TEA, 5.6 KCl, 1.2 MgCl2·6H2O, 2.6 CaCl2, 5 HEPES, and either 1, 5 or 10 glucose (pH 7.4 with NaOH) in a heated chamber (32–35°C). For whole-cell patch-clamping, fire polished thin wall borosilicate pipettes coated with Sylgard (3-5 MOhm) contained intracellular solution with (in mM): 125 Cs-glutamate, 10 CsCl, 10 NaCl, 1 MgCl2·6H2O, 0.05 EGTA, 5 HEPES, 0.1 cAMP, and 3 MgATP (pH 7.15 with CsOH). Electrophysiological measures were collected using a HEKA EPC10 amplifier and PatchMaster Software (HEKA Instruments Inc, Lambrecht/Pfalz, Germany) and protocols shown in Fig 1E within 5 minutes of break-in. We measured cell size as total membrane capacitance. Membrane fusion was stimulated with a series of ten 500 ms depolarizations from −70 to 0 mV and normalized to initial cell size. Early and late exocytotic responses reflect plasma membrane fusion of the readily releasable and reserve granules, respectively, while total exocytosis was taken as a measure of exocytotic capacity. In β-cells early Ca2+ currents reflect mixed L- and P/Q-type currents, while late Ca2+ currents reflect primarily P/Q-type currents. The integrated current over a 500 ms depolarization is a measure of Ca2+ charge entry, and used for normalization of exocytosis to Ca2+ influx. Finally, we measured the peak and voltage-dependence of half-inactivation of Na+ currents since these make important contributions to cellular excitability. Quality control was assessed by the stability of seal (>10 GOhm) and access resistance (<15 MOhm) over the course of the experiment and was similar between the freshly isolated and cryopreserved cells. Data were analysed using FitMaster (HEKA Instruments Inc) and Prism 6.0h (GraphPad Software Inc., San Diego, CA). Immediately following recordings, the patch pipette was withdrawn and replaced with a wide-bore (0.2-0.5 MOhm) collecting pipette containing lysis buffer without ERCC mix. Cells were then collected by gentle suction and visual confirmation, and then transferred into 8-strip PCR tubes containing 4 μl lysis buffer (with ERRC spike-in) on ice and stored at −80°C until cDNA and library preparation (see below).
siRNA transfection.
Dissociated human islets cells were transfected with scramble siRNA (Cat# 1027284, Qiagen, Toronto, Canada) or siRNA from ThermoFisher Scientific against OGDHL (ID# s31422), TSPAN1 (ID# s19659), FAM159B (ID# 264018), RGS9 (ID# s16736), and GYG1 (ID# s6360) together with a fluorescent marker (Allstars Neg.siRNA AF488, Qiagen, Cat# 1027292) using DharmaFECT 1 (GE Healthcare) according to manufacturer’s protocol. In patch-clamp studies, the visible fluorescence was checked to identify positively transfected cells. Total RNA was prepared by TRIzol (Invitrogen) according to manufacturer’s protocol. The cDNA was prepared from 100-200 ng of total RNA using 5xAll-In-One RT Master mix (Applied Biological Materials Inc). Real-time PCR was performed using Fast SYBR Green Master Mix, 7900HT Fast Real-Time PCR systems (Applied Biosystems) and primers designed to flank an intron of each gene to confirm knockdown (Supp Table S4).
Islet dispersion and FACS
Human islets were washed once in cold PBS and dissociated into single cells by enzymatic digestion with Accumax (Invitrogen), followed by digestion using freshly prepared Dispase (Fisher Scientific). Cells were filtered using a 70 μm cell strainer, quantified, and stained with LIVE/DEAD Fixable near-IR dead cell dye (Life Technologies, L10119) as a viability marker. Cells were then blocked with mouse IgG in FACS buffer (2% FBS, 10mM EGTA, in PBS), followed by staining with appropriate antibodies at 1:100 (v/v) final concentration. The following combination of antibodies was used to select endocrine cells: HPi2-Alexa-405 (Novus, NBP1-18946AF405), HPx1-Alexa-647 (Novus, NBP1-18951AF647), CD133/1-APC (Miltenyl Biotec, 130-113-668), CD133/2-APC (Miltenyl Biotec, 130-098-129), CD31-APC-Cy7 (BioLegend, 303119). Cells were then sorted using a Sony SH800 cell-sorter and a 100 μm nozzle following doublet removal. Single cells were sorted directly into 384-well plates (Bio-Rad HSP3841) containing 0.4 μL of lysis buffer with dNTPs (Invitrogen) and ERCC spike-in control (ThermoFisher). Plates were centrifuged and placed on dry ice immediately before storage at −80°C.
Single-cell RNA-Seq
For both patch-seq cells and FACS-collected cells, we generated cDNA and sequencing libraries using the SmartSeq-2 protocol as previously described (Picelli et al., 2014). For patch-seq cells, we first assembled the 8-strip tubes into 96-well plates for increased throughput (Bio-Rad, RC9601 and MSA5001). For FACS-collected cells we proceeded directly with the obtained 96-well or 384-well plates. Briefly, mRNAs were primed with an anchored oligo-dT and reverse transcribed using an LNA-containing template switching oligo, followed by PCR amplification (21 cycles). Libraries were then generated from the amplified cDNA by tagmentation with Tn5. Libraries were sequenced either in a NextSeq 500 or NovaSeq platform (Illumina) using paired-end reads (75 bp) to an average depth of 1 million reads per cell.
Quantification and Statistical Analysis
Processing and quality control of single-cell RNA-seq data
Sequencing reads were aligned to the human genome (GRCh38 genome with supplementary ERCC sequences) using STAR (Dobin et al., 2013), and gene counts determined using htseq-count (intersection-nonempty) using a GTF annotation with Ensembl 89 release genes (Anders et al., 2015). Analysis of splicing was performed based on the splice junctions called by STAR. Gene expression was normalized to counts per million (cpm) after removal of counts corresponding to ERCC spike-ins, and transformed to log2 values after addition of a pseudocount.
We filtered patch-clamped cells based on stringent QC criteria: >1,500 genes, >100,000 human mapped reads, >40% uniquely mapped reads (STAR), <40% unmapped reads (STAR), and more than 25% of total reads mapped to exons (ht-seq). This filtering retained 89% of all patch-clamped cells (1126/1275). We also filtered cells with low expression of ACTB and GAPDH (<3SD below mean) (29 cells), and potential doublets determined from high levels of hormone coexpression (log2CPM>13 for more than one marker gene: INS, GCG, PPY, SST, PRSS1) (76 cells). In this way, we obtained 1,021 high-quality patch-seq transcriptomes for further analysis. For cryopreserved cells, we applied the same QC filtering and obtained equivalent percentages in each step. This provided an additional set of 348 cryopreserved patch-seq cells (411 initial cells).
Clustering and cell type determination
Initial clustering of cell types was performed by selection of over-dispersed genes (top 2000 genes based on coefficient of variation), followed by dimensionality reduction by PCA (10 PCs), and tSNE projection (perplexity=30, learning rate=200) using the python package scanpy (Wolf et al., 2018). Clusters were selected based on the Louvain algorithm for community detection or hdbscan, and cell types assigned based on the expression of key marker genes. Selection of marker genes for the T1D clusters was performed using a logistic regression model (Wolf et al., 2018). Downstream analysis was performed using custom python and R scripts.
Correlation between electrophysiology and gene expression
The relationship between electrophysiological parameters and gene expression was measured using rank correlation statistics. Spearman’s correlations were computed in different groups of cells according to cell type, disease condition and experimental protocol (e.g. β–cell, ND, high-glucose condition). We first removed low expressed genes by selecting genes with mean expression log2(CPM)>1, corresponding to a total of 3000-8000 genes for dataset. Outliers in electrophysiology were removed by quantile filtering of the highest and lowest 3% of cells for each parameter. We observed outlier cells to be consistent across sets of co-measured parameters (exocytosis, Ca2+, Na+ currents) suggesting that they might reflect technical noise. Measurements of exocytosis and ionic currents were normalized to initial cell capacitance to account for effects of cell size. For exocytosis measurements, we also collapsed negative values to zero to reduce their effect on the measured correlations, as fluctuations around zero are unlikely to be relevant to functional responses (i.e. exocytosis). Finally, all current measurements (Ca2+, Na+) were transformed to positive defined values regardless of their flow direction across the cell membrane, so positive correlations are representative of genes associated with larger responses in electrophysiology.
Spearman tie-corrected correlations were computed for each gene and significance was tested by bootstrapping (1,000 iterations). Reported values are mean, standard deviation, and equivalent z-score from the bootstrapped values. To verify that correlations determined on this way report genes that show significant variation across each electrophysiological parameter, we recomputed correlations after performing a median smoothing of the data. In this case, we sorted all cells according to each electrophysiological parameter and performed a median average with a rolling-window corresponding to 10% of the cells. We then computed correlations and confidence intervals using the same bootstrap approach, finding overlapping gene lists and significance values. We also verified that a bootstrap of donors (instead of cells), provided an overlapping list of genes for total exocytosis in β–cells from ND. To further refine the final list of genes for knock-down validation we focused on genes observed in >30% of cells. Analysis was implemented in python scripts.
Electrophysiological predictions using PS gene set
For patch-seq electrophysiological prediction, we split β-cells from ND into a training set (80%) and test set (20%). We used the training set to perform the bootstrapped correlation analysis between electrophysiological parameters and gene expression as detailed above. We selected genes showing significant correlations (∣z-score∣>2) with at least two electrophysiological parameters belonging to different functional groups, and retained genes with highest median expression (observed in >50% of cells). In this way, we obtained our final PS gene set (484 genes, Supp Table S5). We then built a k-nearest neighbors (k-NN) model (Pedregosa et al., 2011), to determine the k closest cells in PS gene expression to each cell (k=5, metric spearman correlation) using the training set. We used the k-NN model to infer the electrophysiological parameters of each cell from the averaged values of the identified neighbors (after masking cells for which an electrophysiological parameter could not be measured). The test set was used for final validation by predicting their electrophysiological parameters using the k-NN model build with the training dataset.
Cell type identification from electrophysiological data
We selected all α- and β-cells from ND and T2D donors as determined from the single-cell RNA sequencing analysis. Cells from ND donors were split in a training (80%) and test set (20%). We performed random undersampling to balance both types of cells in the train dataset. We also excluded electrophysiological parameters that are not measured for a significant number of cells (>8%), keeping the following 9 parameters (Cell size, Total Exocytosis, Early exocytosis, Late exocytosis, Ca2+ entry, Early Ca2+ current, Late Ca2+ current, Peak Na+ current , Na+ conductance). We then trained a random forest classifier and determined the best set of parameters using 10-fold cross-validation and recursive feature elimination. The performance of the finalized model was evaluated in the test set (ND donors), and in all cells from donors with T2D.
Statistical analysis
No statistical analysis was used to predetermine sample size and samples were not blinded for analysis. Samples obtained from three donors were excluded from all analysis due to the reasons specified in Supp. Table S1. Differential expression was performed using a non-parametric Mann-Whitney U test. For comparisons involving few donors or showing bias in sex representation (e.g. α-cells from donors with T1D), we repeated the analysis with VOOM-LIMMA using sex as a covariate. All P-values were corrected for multiple hypothesis testing using Benjami-Hochberg (BH) and significance was determined from a permutation test by shuffling the labels and using False Discovery Rate (FDR) < 0.05. Statistical differences between electrophysiological parameters was determined using a non-parametric Mann-Whitney U and corrected for multiple hypothesis testing using BH across all measured parameters unless otherwise stated.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD133/2-APC | Miltenyl Biotec | 130-098-129 |
| CD31-APC-Cy7 | BioLegend | 303119 |
| HPi2-Alexa-405 | Novus Biologicals | NBP1-18946AF405 |
| HPx1-Alexa-647 | Novus Biologicals | NBP1-18951AF647 |
| ChromPure Mouse IgG | Jackson ImmunoResearch | 015-000-003 |
| CD133/1-APC | Miltenyl Biotec | 130-113-668 |
| Biological Samples | ||
| Human pancreatic samples | Albeta Diabetes Institute IsletCore | |
| Critical Commercial Assays | ||
| KAPA HiFi HotStart ReadyMix | KAPA Biosystems | KK2601 |
| Nextera XT | Illumina | FC-131-1096 |
| LIVE/DEAD Fixable near-IR dead cell dye | Life Technologies | L10119 |
| ERCC spike-in control | ThermoFisher | 4456740 |
| TRIzol Reagent | ThermoFisher | 15596018 |
| 5X All-In-One RT Master Mix | Applied Biological Materials (abm) Inc. | G486 |
| Invitrogen ultrapure distilled water | ThermoFisher | 10977-015 |
| 10% Triton | Sigma-Aldrich | 93443 |
| Recombination RNase inhibitor | Clontech | 2313A |
| SUPERase-In RNase Inhibitor | ThermoFisher | AM2696 |
| 10 mM dNTP | ThermoFisher | 18427088 |
| DharmaFECT 1 | Dharmacon | T-2001-03 |
| Fast SYBR Green Master Mix | ThermoFisher | 4385612 |
| DMEM medium | ThermoFisher | 11885092 |
| All chemicals | Sigma-Aldrich | |
| Deposited Data | ||
| Single cell mRNA-seq data | Enge et al., 2017 | GEO:GSE81547 |
| Single cell mRNA-seq data | Segerstolpe et al., 2016 | ENA:ERP017126, ArrayExpress: MTAB-5060 |
| Single cell mRNA-seq data | This paper | GEO:GSE124742 |
| Processed patch-seq datasets | This paper | https://github.com/jcamunas/patchseq |
| Oligonucleotides | ||
| SmartSeq2 OligodT: 5′–AAGCAGTGGTATCAACGCAGAGTACT30VN-3′ | Picelli et al., 2014 | |
| SmartSeq2 TSO: 5′-AAGCAGTGGTATCAACGCAGAGTACATrGrG3′ | Picelli et al., 2014 | |
| SmartSeq2 ISPCR: 5′-AAGCAGTGGTATCAACGCAGAGT-3′ | Picelli et al., 2014 | |
| siRNA (OGDHL, TSPAN1, FAM159B, RGS9, GYG1, ETV1) - see Method Details | ThermoFisher Scientific | IDs # s31422, s19659, s264018, s16736, s6360, s4855 |
| scramble siRNA | Qiagen | 1027284 |
| Primers for qPCR (OGDHL, TSPAN1, FAM159B, RGS9, GYG1, ETV1) - see Supp. Table S4 | Integrated DNA Technologies (IDT) | |
| Software and Algorithms | ||
| STAR | Dobin et al., 2013 | https://github.com/alexdobin/STAR |
| HTSeq | Anders et al., 2015 | https://github.com/simon-anders/htseq |
| Scanpy | Wolf et al, 2019 | https://github.com/theislab/scanpy |
| Custom analysis software | This paper | https://github.com/jcamunas/patchseq |
| PatchMaster software (recording) | HEKA Elektronik | |
| Fitmaster software (analysis) | HEKA Elektronik | |
| Sigmaplot software (analysis) | SYSTAT | |
Context & Significance:
Understanding the molecular mechanisms behind the dysfunction of pancreatic islet cells during diabetes could lead to better therapies. Recently key cell types in the islets have been found to exist as different subpopulations; that is, for example, not all beta cells (the source of insulin) are the same in a given pancreas. But how these different subpopulations may be functionally different has been unclear. Here Camunas-Soler, Dai et al. address this issue by combining single-cell RNA sequencing (as a measure of phenotype) with single-cell electrophysiological measurements (as a measure of function) in islet cells from individuals with or without diabetes. The authors report induction of genes suggestive of compensation mechanisms in β-cells, and identify transcriptomic patterns associated with dysfunction in T1D and T2D.
Bullet points:
Patch-seq links single cell transcriptomes with physiology in 1,369 human islet cells.
A set of 484 genes can predict β-cell electrophysiology.
Identified transcriptomic programs for early β-cell compensation, and impairment in T2D.
Applying this approach to cryopreserved islets reveals a-cell dysfunctional states in T1D.
Acknowledgments
This work was supported by funding from the Chan Zuckerberg Biohub and the California Institute for Regenerative Medicine to SRQ; by a Foundation Grant from the Canadian Institutes of Health Research (CIHR: 148451) to PEM; and by grants from the U.S. National Institutes of Health (1U01DK10830001 1R01DK107507, 1R01DK108817 and P30 DK116074) to SKK, (1U01DK120447-01) to SKK, SRQ, and PEM, and from JDRF (2-SRA-2019-698-S-B) to SKK and PEM. Support from the Human Islet Research Core in the Stanford Diabetes Research Center is gratefully acknowledged. Human islet isolation at the Alberta Diabetes Institute IsletCore was initially subsidized by the Alberta Diabetes Foundation.
We thank Dr. S.H. Kim (Kim lab, Stanford) and Dylan Hendersson (Chan Zuckerberg Biohub) for help in sample processing, and Dr. Norma Neff (Chan Zuckerberg Biohub) and Jennifer Okamoto (Chan Zuckerberg Biohub) for sequencing expertise. We thank Dr. Linford Briant (University of Oxford) for helpful discussions on islet cell type modelling; and Dr. Jocelyn Manning Fox (University of Alberta) for critical reading of the manuscript. We thank the organ procurement organizations across Canada, particularly the Human Organ Procurement and Exchange (HOPE) program in Edmonton and the Trillium Gift of Life Network (TGLN) in Ontario, for their work in obtaining human pancreas for research. We thank Drs. Rita Bottino (Allegheny Health Network) and Alvin C. Powers (Vanderbilt University) for kindly providing islets used in validation studies. We also thank Dr. Manning Fox and Mrs. Nancy Smith (University of Alberta) for their contributions to human islet isolations. Finally, we are indebted to organ donors and their families for their generous support of scientific research.
Footnotes
Declaration of Interests
The authors disclose no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aichler M, Borgmann D, Krumsiek J, Buck A, MacDonald PE, Fox JEM, Lyon J, Light PE, Keipert S, Jastroch M, et al. (2017). N-acyl Taurines and Acylcarnitines Cause an Imbalance in Insulin Synthesis and Secretion Provoking β Cell Dysfunction in Type 2 Diabetes. Cell Metabolism 25, 1334–1347.e4. [DOI] [PubMed] [Google Scholar]
- Ait-Lounis A, Bonal C, Seguin-Estevez Q, Schmid CD, Bucher P, Herrera PL, Durand B, Meda P, and Reith W (2010). The Transcription Factor Rfx3 Regulates -Cell Differentiation, Function, and Glucokinase Expression. Diabetes 59, 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama M, Liew CW, Lu S, Hu J, Martinez R, Hambro B, Kennedy RT, and Kulkarni RN (2013). X-Box Binding Protein 1 Is Essential for Insulin Regulation of Pancreatic -Cell Function. Diabetes 62,2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Meéneur C, and Bernal-Mizrachi E (2015). Natural history of β-cell adaptation and failure in type 2 diabetes. Molecular Aspects of Medicine 42, 19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves TC, Pongratz RL, Zhao X, Yarborough O, Sereda S, Shirihai O, Cline GW, Mason G, and Kibbey RG (2015). Integrated, Step-Wise, Mass-Isotopomeric Flux Analysis of the TCA Cycle. Cell Metabolism 22, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Pyl PT, and Huber W (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arda HE, Li L, Tsai J, Torre EA, Rosli Y, Peiris H, Spitale RC, Dai C, Gu X, Qu K, et al. (2016). Age-Dependent Pancreatic Gene Regulation Reveals Mechanisms Governing Human β Cell Function. Cell Metabolism 23, 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, and Rorsman P (2012). Diabetes Mellitus and the β Cell: The Last Ten Years. Cell 148, 1160–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader E, Migliorini A, Gegg M, Moruzzi N, Gerdes J, Roscioni SS, Bakhti M, Brandl E, Irmler M, Beckers J, et al. (2016). Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 535, 430–434. [DOI] [PubMed] [Google Scholar]
- Bakhti M, Böttcher A, and Lickert H (2019). Modelling the endocrine pancreas in health and disease. Nature Reviews Endocrinology 15, 155–171. [DOI] [PubMed] [Google Scholar]
- Baron M, Veres A, Wolock SL, Faust AL, Gaujoux R, Vetere A, Ryu JH, Wagner BK, Shen-Orr SS, Klein AM, et al. (2016). A Single-Cell Transcriptomic Map of the Human and Mouse Pancreas Reveals Inter- and Intra-cell Population Structure. Cell Systems 3, 346–360.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S (1988). Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes 37, 616–621. [DOI] [PubMed] [Google Scholar]
- Breiman L (2001). Random Forests. Machine Learning 45, 5–32. [Google Scholar]
- Briant LJB, Zhang Q, Vergari E, Kellard JA, Rodriguez B, Ashcroft FM, and Rorsman P (2017). Functional identification of islet cell types by electrophysiological fingerprinting. Journal of The Royal Society Interface 14, 20160999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Haliyur R, Saunders D, Shrestha S, Dai C, Blodgett DM, Bottino R, Campbell-Thompson M, Aramandla R, Poffenberger G, et al. (2018). α Cell Function and Gene Expression Are Compromised in Type 1 Diabetes. Cell Reports 22, 2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broch M, Vendrell J, Ricart W, Richart C, and Fernandez-Real J-M (2007). Circulating Retinol-Binding Protein-4, Insulin Sensitivity, Insulin Secretion, and Insulin Disposition Index in Obese and Nonobese Subjects. Diabetes Care 30, 1802–1806. [DOI] [PubMed] [Google Scholar]
- Bunik V, Kaehne T, Degtyarev D, Shcherbakova T, and Reiser G (2008). Novel isoenzyme of 2-oxoglutarate dehydrogenase is identified in brain, but not in heart: Novel 2-oxoglutarate dehydrogenase. FEBS Journal 275, 4990–5006. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Knauf C, and Cani PD (2008). Pancreatic α-cell dysfunction in diabetes. Diabetes & Metabolism 34, S49–S55. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, and Morgan A (2003). Secretory Granule Exocytosis. Physiological Reviews 83, 581–632. [DOI] [PubMed] [Google Scholar]
- Byrnes LE, Wong DM, Subramaniam M, Meyer NP, Gilchrist CL, Knox SM, Tward AD, Ye CJ, and Sneddon JB (2018). Lineage dynamics of murine pancreatic development at single-cell resolution. Nature Communications 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, Reimer J, Shen S, Bethge M, Tolias KF, et al. (2016). Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nature Biotechnology 34, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy H, Gu X, Enge M, Dai X, Wang Y, Damond N, Downie C, Liu K, Wang J, Xing Y, et al. (2017). Converting Adult Pancreatic Islet α Cells into β Cells by Targeting Both Dnmt1 and Arx. Cell Metabolism 25, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chmelova H, Cohrs CM, Chouinard JA, Jahn SR, Stertmann J, Uphues I, and Speier S (2016). Alterations in β-Cell Calcium Dynamics and Efficacy Outweigh Islet Mass Adaptation in Compensation of Insulin Resistance and Prediabetes Onset. Diabetes 65, 2676–2685. [DOI] [PubMed] [Google Scholar]
- Chimienti F, Devergnas S, Favier A, and Seve M (2004). Identification and Cloning of a -Cell-Specific Zinc Transporter, ZnT-8, Localized Into Insulin Secretory Granules. Diabetes 53, 2330–2337. [DOI] [PubMed] [Google Scholar]
- Corritore E, Lee Y-S, Sokal EM, and Lysy PA (2016). β-cell replacement sources for type 1 diabetes: a focus on pancreatic ductal cells. Therapeutic Advances in Endocrinology and Metabolism 7, 182–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl U, Sjødin A, and Semb H (1996). Cadherins regulate aggregation of pancreatic beta-cells in vivo. Development 122, 2895–2902. [DOI] [PubMed] [Google Scholar]
- Dallavalle C, Albino D, Civenni G, Merulla J, Ostano P, Mello-Grand M, Rossi S, Losa M, D’Ambrosio G, Sessa F, et al. (2016). MicroRNA-424 impairs ubiquitination to activate STAT3 and promote prostate tumor progression. Journal of Clinical Investigation 126, 4585–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson A, Pontén F, Fagerberg L, Hallström BM, Schwenk JM, Uhlén M, Korsgren O, and Lindskog C (2014). The Human Pancreas Proteome Defined by Transcriptomics and Antibody-Based Profiling. PLoS ONE 9, e115421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Schug J, Canaday PS, Russ HA, Tarlow BD, Grompe MT, Horton T, Hebrok M, Streeter PR, Kaestner KH, et al. (2016). Human islets contain four distinct subtypes of β cells. Nature Communications 7, 11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge M, Arda HE, Mignardi M, Beausang J, Bottino R, Kim SK, and Quake SR (2017). Single-Cell Analysis of Human Pancreas Reveals Transcriptional Signatures of Aging and Somatic Mutation Patterns. Cell 171, 321–330.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdaoussi M, Dai X, Jensen MV, Wang R, Peterson BS, Huang C, Ilkayeva O, Smith N, Miller N, Hajmrle C, et al. (2015). Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. Journal of Clinical Investigation 125, 3847–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Tajes J, Gaulton KJ, van de Bunt M, Torres J, Turner M, Mahajan A, Gloyn AL, Lage K, and McCarthy MI (2019). Developing a network view of type 2 diabetes risk pathways through integration of genetic, genomic and functional data. Genome Medicine 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Földy C, Darmanis S, Aoto J, Malenka RC, Quake SR, and Südhof TC (2016). Single-cell RNAseq reveals cell adhesion molecule profiles in electrophysiologically defined neurons. Proceedings of the National Academy of Sciences 113, E5222–E5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzik J, Zeisel A, Máté Z, Calvigioni D, Yanagawa Y, Szabá G, Linnarsson S, and Harkany T (2016). Integration of electrophysiological recordings with single-cell RNA-seq data identifies neuronal subtypes. Nature Biotechnology 34, 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WJ, Do OH, Cottle L, Ma W, Kosobrodova E, Cooper-White J, Bilek M, and Thorn P (2018). Local Integrin Activation in Pancreatic β Cells Targets Insulin Secretion to the Vasculature. Cell Reports 24, 2819–2826.e3. [DOI] [PubMed] [Google Scholar]
- Gembal M, Gilon P, and Henquin JC (1992). Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. Journal of Clinical Investigation 89, 1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geron E, Boura-Halfon S, Schejter ED, and Shilo B-Z (2015). The Edges of Pancreatic Islet β Cells Constitute Adhesive and Signaling Microdomains. Cell Reports 10, 317–325. [DOI] [PubMed] [Google Scholar]
- Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, Piemonti L, Marchetti P, Bugliani M, Bosco D, et al. (2016). Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metabolism 24, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, and Utzschneider KM (2006). Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846. [DOI] [PubMed] [Google Scholar]
- Kaido T, Perez B, Yebra M, Hill J, Cirulli V, Hayek A, and Montgomery AM (2004). Alphav-integrin utilization in human beta-cell adhesion, spreading, and motility. J. Biol. Chem 279, 17731–17737. [DOI] [PubMed] [Google Scholar]
- Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, and King GL (2010). Residual Insulin Production and Pancreatic -Cell Turnover After 50 Years of Diabetes: Joslin Medalist Study. Diabetes 59, 2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsunsky I, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh P-R, and Raychaudhuri S (2019). Fast, sensitive, and accurate integration of single cell data with Harmony. Nature Methods 16, 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Manno G, Gyllborg D, Codeluppi S, Nishimura K, Salto C, Zeisel A, Borm LE, Stott SRW, Toledo EM, Villaescusa JC, et al. (2016). Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 167, 566–580.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Mignardi M, Jones R, Enge M, Kim SK, Quake SR, and Zou J (2018). Modeling Spatial Correlation of Transcripts With Application to Developing Pancreas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, and Sherry AD (2002). 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proceedings of the National Academy of Sciences 99, 2708–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon J, Spigelman A, MacDonald P, and Manning J ADI IsletCore Protocols for the Isolation, Assessment and Cryopreservation of Human Pancreatic Islets of Langerhans for Research Purposes v1 (protocols.io.x3mfqk6). [Google Scholar]
- Macaulay IC, Ponting CP, and Voet T (2017). Single-Cell Multiomics: Multiple Measurements from Single Cells. Trends in Genetics 33, 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, Payne AJ, Steinthorsdottir V, Scott RA, Grarup N, et al. (2018). Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nature Genetics 50, 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning Fox JE, Lyon J, Dai XQ, Wright RC, Hayward J, van de Bunt M, Kin T, Shapiro AMJ, McCarthy MI, Gloyn AL, et al. (2015). Human islet function following 20 years of cryogenic biobanking. Diabetologia 58, 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen T, Mawla AM, DiGruccio MR, Adams MW, Nies V, Dólleman S, Liu S, Ackermann AM, Cáceres E, Hunter AE, et al. (2017). Virgin Beta Cells Persist throughout Life at a Neogenic Niche within Pancreatic Islets. Cell Metabolism 25, 911–926.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NG, and Richardson SJ (2018). Fifty years of pancreatic islet pathology in human type 1 diabetes: insights gained and progress made. Diabetologia 61, 2499–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro MJ, Dharmadhikari G, Grün D, Groen N, Dielen T, Jansen E, van Gurp L, Engelse MA, Carlotti F, de Koning EJP, et al. (2016). A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Systems 3, 385–394.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB (2017). Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metabolism 25, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, and Plenge R (2012). JAK and STAT Signaling Molecules in Immunoregulation and Immune-Mediated Disease. Immunity 36, 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter S, and Lammert E (2016). Exciting Times for Pancreatic Islets: Glutamate Signaling in Endocrine Cells. Trends in Endocrinology & Metabolism 27, 177–188. [DOI] [PubMed] [Google Scholar]
- Parnaud G, Lavallard V, Bedat B, Matthey-Doret D, Morel P, Berney T, and Bosco D (2015). Cadherin Engagement Improves Insulin Secretion of Single Human β-Cells. Diabetes 64, 887–896. [DOI] [PubMed] [Google Scholar]
- Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, et al. (2011). Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research 12, 2825–2830. [Google Scholar]
- Perelis M, Marcheva B, Moynihan Ramsey K, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C, et al. (2015). Pancreatic cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science 350, aac4250–aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picelli S, Faridani OR, Björklund ÅK, Winberg G, Sagasser S, and Sandberg R (2014). Full-length RNA-seq from single cells using Smart-seq2. Nature Protocols 9, 171–181. [DOI] [PubMed] [Google Scholar]
- Pipeleers DG (1992). Heterogeneity in pancreatic beta-cell population. Diabetes 41, 777–781. [DOI] [PubMed] [Google Scholar]
- Prasad RB, and Groop L (2016). Single-Cell Sequencing of Human Pancreatic Islets—New Kids on the Block. Cell Metabolism 24, 523–524. [DOI] [PubMed] [Google Scholar]
- Rorsman P, and Braun M (2013). Regulation of Insulin Secretion in Human Pancreatic Islets. Annual Review of Physiology 75, 155–179. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Kaestner KH, Natarajan R, Patti M-E, Sallari R, Sander M, and Susztak K (2018). Epigenetics and Epigenomics: Implications for Diabetes and Obesity. Diabetes 67, 1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui J, Deng S, Arazi A, Perdigoto AL, Liu Z, and Herold KC (2017). β Cells that Resist Immunological Attack Develop during Progression of Autoimmune Diabetes in NOD Mice. Cell Metabolism 25, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarimäki-Vire J, Balboa D, Russell MA, Saarikettu J, Kinnunen M, Keskitalo S, Malhi A, Valensisi C, Andrus C, Eurola S, et al. (2017). An Activating STAT3 Mutation Causes Neonatal Diabetes through Premature Induction of Pancreatic Differentiation. Cell Reports 19, 281–294. [DOI] [PubMed] [Google Scholar]
- Segerstolpe Å, Palasantza A, Eliasson P, Andersson E-M, Andréasson A-C, Sun X, Picelli S, Sabirsh A, Clausen M, Bjursell MK, et al. (2016). Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metabolism 24, 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, and Rando OJ (2017). Metabolic Inputs into the Epigenome. Cell Metabolism 25, 544–558. [DOI] [PubMed] [Google Scholar]
- Stuart T, and Satija R (2019). Integrative single-cell analysis. Nature Reviews Genetics 20, 257–272. [DOI] [PubMed] [Google Scholar]
- Suckow AT, Comoletti D, Waldrop MA, Mosedale M, Egodage S, Taylor P, and Chessler SD (2008). Expression of Neurexin, Neuroligin, and Their Cytoplasmic Binding Partners in the Pancreatic β-Cells and the Involvement of Neuroligin in Insulin Secretion. Endocrinology 149, 6006–6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suriben R, Kaihara KA, Paolino M, Reichelt M, Kummerfeld SK, Modrusan Z, Dugger DL, Newton K, Sagolla M, Webster JD, et al. (2015). β-Cell Insulin Secretion Requires the Ubiquitin Ligase COP1. Cell 163, 1457–1467. [DOI] [PubMed] [Google Scholar]
- The Tabula Muris Consortium, Overall coordination, Logistical coordination, Organ collection and processing, Library preparation and sequencing, Computational data analysis, Cell type annotation, Writing group, Supplemental text writing group, and Principal investigators (2018). Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, and Herrera PL (2010). Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritschler S, Theis FJ, Lickert H, and Böttcher A (2017). Systematic single-cell analysis provides new insights into heterogeneity and plasticity of the pancreas. Molecular Metabolism 6, 974–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, et al. (2015). Tissue-based map of the human proteome. Science 347, 1260419–1260419. [DOI] [PubMed] [Google Scholar]
- Unger RH, and Cherrington AD (2012). Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. Journal of Clinical Investigation 122, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A-C, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, et al. (2017). Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 356, eaah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JN, Johnson PRV, Shigeto M, Hughes SJ, Clark A, and Rorsman P (2011). Glucose-responsive beta cells in islets isolated from a patient with long-standing type 1 diabetes mellitus. Diabetologia 54, 200–202. [DOI] [PubMed] [Google Scholar]
- Wang YJ, and Kaestner KH (2018). Single-Cell RNA-Seq of the Pancreatic Islets--a Promise Not yet Fulfilled? Cell Metab 29, 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Golson ML, Schug J, Traum D, Liu C, Vivek K, Dorrell C, Naji A, Powers AC, Chang K-M, et al. (2016a). Single-Cell Mass Cytometry Analysis of the Human Endocrine Pancreas. Cell Metabolism 24, 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Schug J, Won K-J, Liu C, Naji A, Avrahami D, Golson ML, and Kaestner KH (2016b). Single-Cell Transcriptomics of the Human Endocrine Pancreas. Diabetes 65, 3028–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R (2016). Williams textbook of endocrinology (Philadelphia, PA: Elsevier; ). [Google Scholar]
- Wolf FA, Angerer P, and Theis FJ (2018). SCANPY: large-scale single-cell gene expression data analysis. Genome Biology 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, et al. (2005). MafA Is a Key Regulator of Glucose-Stimulated Insulin Secretion. Molecular and Cellular Biology 25, 4969–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Goforth P, Bertram R, Sherman A, and Satin L (2003). The Ca2+ Dynamics of Isolated Mouse β-Cells and Islets: Implications for Mathematical Models. Biophysical Journal 84, 2852–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Chibalina MV, Bengtsson M, Groschner LN, Ramracheya R, Rorsman NJG, Leiss V, Nassar MA, Welling A, Gribble FM, et al. (2014). Na + current properties in islet α- and β-cells reflect cell-specific Scn3a and Scn9a expression: Na + channels in pancreatic islets. The Journal of Physiology 592, 4677–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing reads are available in the NCBI Gene Expression Omnibus (GEO) and Sequence Read Archive (SRA) under accession number GSE124742. The code and scripts generated during this study as well as preprocessed datasets are available at https://github.com/jcamunas/patchseq.