In murine cortical collecting duct cells, prostaglandin E2 (PGE2) stimulates transepithelial sodium transport mediated by the epithelial sodium channel (ENaC). PGE2 is synthesized and secreted by the cells and acts on basolateral prostaglandin E receptor type 4 (EP4).
Abstract
Prostaglandin E2 (PGE2) is the most abundant prostanoid in the kidney, affecting a wide range of renal functions. Conflicting data have been reported regarding the effects of PGE2 on tubular water and ion transport. The amiloride-sensitive epithelial sodium channel (ENaC) is rate limiting for transepithelial sodium transport in the aldosterone-sensitive distal nephron. The aim of the present study was to explore a potential role of PGE2 in regulating ENaC in cortical collecting duct (CCD) cells. Short-circuit current (ISC) measurements were performed using the murine mCCDcl1 cell line known to express characteristic properties of CCD principal cells and to be responsive to physiological concentrations of aldosterone and vasopressin. PGE2 stimulated amiloride-sensitive ISC via basolateral prostaglandin E receptors type 4 (EP4) with an EC50 of ∼7.1 nM. The rapid stimulatory effect of PGE2 on ISC resembled that of vasopressin. A maximum response was reached within minutes, coinciding with an increased abundance of β-ENaC at the apical plasma membrane and elevated cytosolic cAMP levels. The effects of PGE2 and vasopressin were nonadditive, indicating similar signaling cascades. Exposing mCCDcl1 cells to aldosterone caused a much slower (∼2 h) increase of the amiloride-sensitive ISC. Interestingly, the rapid effect of PGE2 was preserved even after aldosterone stimulation. Furthermore, application of arachidonic acid also increased the amiloride-sensitive ISC involving basolateral EP4 receptors. Exposure to arachidonic acid resulted in elevated PGE2 in the basolateral medium in a cyclooxygenase 1 (COX-1)–dependent manner. These data suggest that in the cortical collecting duct, locally produced and secreted PGE2 can stimulate ENaC-mediated transepithelial sodium transport.
Introduction
In the kidney the amiloride-sensitive epithelial sodium channel (ENaC) is localized to the apical membrane of tubular epithelial cells lining the so-called aldosterone-sensitive distal nephron (ASDN). This comprises the late distal convoluted tubule, the connecting tubule, and the collecting duct (CD). In the ASDN, transepithelial sodium transport critically depends on the activity and abundance of ENaC. The precise regulation of ENaC is essential for the fine-tuning of urinary sodium excretion to match dietary sodium intake. Thus, ENaC regulation plays a key role for total body sodium balance and is critical for the long-term control of extracellular fluid volume and arterial blood pressure. ENaC is regulated by several hormones, including aldosterone and vasopressin, as well as by several local factors (Garty and Palmer, 1997; Loffing and Korbmacher, 2009; Bankir et al., 2010; Rossier, 2014; Kleyman et al., 2018).
Prostanoids are derivatives of arachidonic acid and are produced and secreted by many different cells. Prostanoids have complex effects on renal function (Grantham and Orloff, 1968; Breyer and Breyer, 2000b) and contribute to the regulation of sodium and water excretion, renin secretion, renal blood flow, and glomerular filtration (Hao and Breyer, 2007). Because they are rapidly metabolized, these derivatives are thought to act within close proximity to the site of their synthesis in either an autocrine or paracrine manner (Fenton and Knepper, 2007). Prostaglandin E2 (PGE2), derived via cyclooxygenase (COX), is the most abundant prostanoid in the kidney. There are conflicting reports regarding the effects of PGE2 on sodium and water transport within the collecting duct (Breyer and Breyer 2000a). Several studies suggest that in the renal medulla, PGE2 reduces sodium absorption (Stokes and Kokko, 1977; Iino and Imai, 1978). In contrast, inhibition of prostaglandin synthesis has been reported to be associated with increased urinary sodium excretion in conscious dogs, probably due to diminished sodium reabsorption in the collecting duct (Kirschenbaum and Stein, 1976). Moreover, PGE2 may be able to stimulate water and sodium absorption within the cortical CD (CCD) to maintain blood pressure in volume-contracted states (Hao and Breyer, 2008).
PGE2 exerts its diverse effects by binding to four distinct G protein–coupled receptors: EP1–EP4 (Hao and Breyer, 2007). The expression pattern of these receptors determines local effects of PGE2 (Breyer and Breyer, 2000b). EP2 and EP4 receptors are Gαs-coupled receptors, and ligand binding to these receptors stimulates adenylyl cyclase (AC), raising cytosolic cAMP concentration. In addition, alternative EP4 receptor pathways may play a role in mediating downstream effects (Fujino and Regan, 2006; Li et al., 2017). EP3 receptor is coupled to Gαi/o and has an inhibitory effect on AC, thereby lowering cytosolic cAMP concentration. The EP1 receptor is Gαq/11 coupled and promotes signaling via inositol 1,4,5-trisphosphate (IP3) and diacylglycerol resulting in elevated intracellular Ca2+ concentration and activation of protein kinase C (PKC; Narumiya et al., 1999; Breyer and Breyer, 2001).
Findings in animal models of nephrogenic diabetes insipidus indicate a likely role of prostaglandins in regulating renal water transport. In these animals EP2 and EP4 receptor activation alleviated urine-concentrating defects (Li et al., 2009; Olesen et al., 2011). Conversely, nephron-specific or collecting duct-specific knockout of EP4 receptor in mice promoted urine-concentrating defects (Gao et al., 2015). Interestingly, in in vitro studies of the collecting duct activation of EP2 and EP4 receptors promoted apical targeting and phosphorylation of aquaporin-2 water channels, reminiscent of the effect of vasopressin (Olesen et al., 2011, 2016; Gao et al., 2015). Vasopressin-dependent coupling between amiloride-sensitive sodium transport and water flow has been demonstrated in a mouse CCD cell line (mCCDcl1; Gaeggeler et al., 2011) as well as in isolated perfused rat CCDs (Reif et al., 1986). Presently, it is not known whether a potential modulatory effect of PGE2 on tubular water transport is associated with an effect of PGE2 on ENaC-mediated sodium absorption in the CCD.
The aim of the present study was to investigate whether PGE2 modifies ENaC-mediated transepithelial sodium transport in mCCDcl1 cells. This cell line provides a highly differentiated and hormone-responsive model of CCD principal cells (Gaeggeler et al., 2005, 2011). It is well suited to study the regulation of electrogenic transepithelial ion transport by hormonal and local mediators (Edinger et al., 2014; Mansley et al., 2015, 2018, 2019). We sought to identify the prostaglandin receptors present in this model and explored the interplay between PGE2 and two key hormones that promote salt and water reabsorption in the CCD, namely aldosterone and vasopressin. Finally, we investigated whether PGE2 is synthesized and secreted by mCCDcl1 cells.
Materials and methods
Cell culture
Mouse CCD cells (mCCDcl1) were kindly provided by Bernard Rossier (University of Lausanne, Lausanne, Switzerland) and cultured as described previously (Gaeggeler et al., 2005; Mansley et al., 2015, 2018). Cells were routinely passaged every 7 d (passage 25–34) and maintained in cell culture dishes at 37°C in a 5% CO2 atmosphere in Dulbecco’s modified Eagle’s Medium (DMEM)/Ham's F12 (1:1 vol/vol) medium supplemented with 2% FBS, 1 nM triiodothyronine, 60 nM sodium selenite, 10 ng·ml−1 epidermal growth factor, 5 µg·ml−1 human apotransferrin, 50 nM dexamethasone, 5 µg·ml−1 insulin, 100 U·ml−1 penicillin, and 100 µg·ml−1 streptomycin. For experimental procedures, cells were seeded onto Millicell-PCF culture plate inserts (EMD Millipore) with a membrane pore size of 0.4 µm and an effective surface area of either 0.6 cm2 or 4.2 cm2 and grown to form a polarized epithelial monolayer. At day 5 after seeding, the cell culture medium was replaced by a medium devoid of FBS, apotransferrin, and epidermal growth factor. Finally, 24 h before experiments, dexamethasone was removed from the medium.
Chemicals and solutions
DMEM/Ham's F12 (1:1 vol/vol) without phenol red was from Life Technologies and FBS from PAA and Bio&Sell. SC-560, AH23848, AH6809, GW627368X, and Sulprostone were from Cayman Chemical (Biozol); PGE2 and indomethacin were from Enzo Life Sciences. TCS2510 was from Tocris. Lumiracoxib was kindly provided by Prof. Dr. K. Brune (Institute of Experimental and Clinical Pharmacology and Toxicology, Friedrich-Alexander University, Erlangen, Germany). All other drugs were ordered from Sigma-Aldrich.
Transepithelial measurements
Experimental procedures were essentially the same as described previously (Bertog et al., 2008; Mansley et al., 2015, 2018). Briefly, transepithelial voltage (Vte) and resistance (Rte) were routinely checked using a commercially available epithelial volt-ohm meter and a set of two sticks “STX” electrodes (World Precision Instruments). On days 9–11, inserts with confluent mCCDcl1 cell monolayers were transferred to Ussing chambers for continuous equivalent short-circuit current (ISC) measurements using a CVC6 clamp device (Fiebig) as described previously (Bertog et al., 1999). Alternatively, modified Ussing chambers were used and kept in an incubator gassed with 5% CO2, and the temperature was maintained at 37°C (Mansley et al., 2015, 2018). These miniaturized chambers were designed to minimize mechanical perturbations and to reduce the bath volumes in the apical and basolateral compartment to 0.35–0.6 ml and 0.55–1.0 ml, respectively. Both experimental approaches showed similar results in the transepithelial parameters investigated. Rte was evaluated every 2–30 s by measuring voltage deflections induced by 400-ms symmetrical square current pulses of ± 3–5 µA. Using Rte and open-circuit Vte, the equivalent ISC was calculated according to Ohm’s law. Conventionally, a lumen-negative Vte corresponds to a positive ISC which may be due to electrogenic cation absorption, electrogenic anion secretion or a combination of both. After transfer into Ussing chambers, cells were allowed to equilibrate for 30 to 60 min before manipulations took place. At the end of each experiment, amiloride (10 µM) was applied to the apical compartment to determine the ENaC-mediated ISC component.
Reverse transcription (RT) PCR
Total RNA was extracted from mCCDcl1 cells following transepithelial measurements using NucleoSpin RNA Kit-XS (Macherey-Nagel) according to the manufacturer’s instructions. Lysates from two 0.6-cm2 cell culture plate inserts were pooled to enhance the RNA concentration.
For prostanoid receptors (EP1–EP4), RT and PCR amplification were performed using an RNA Reverse Transcription System Kit (Promega). Specific primers (Table 1) for murine EP1–EP4 receptors and β-actin were designed as described previously (Arakawa et al., 1996) and synthesized by Invitrogen. PCR cycling conditions were 95°C for 2 min, followed by 32 repeats of 95°C for 30 s, 60°C for 45 s, and 72°C for 1 min. Final extension time was 4 min at 72°C.
Table 1. Primer pairs of target genes used for RT-PCR in this study.
| cDNA | Sense primer (5′→3′) | Antisense primer (5′→3′) |
|---|---|---|
| EP1 | CGCAGGGTTCACGCACACGA | CACTGTGCCGGGAACTACGC |
| EP2 | AGGACTTCGATGGCAGAGGAGAC | CAGCCCCTTACACTTCTCCAATG |
| EP3 | CCGGGCACGTGGTGCTTCAT | TAGCAGCAGATAAACCCAGG |
| EP4 | TTCCGCTCGTGGTGCGAGTGTTC | GAGGTGGTGTCTGCTTGGGTCAG |
| COX-1 | CCTCTTTCCAGGAGCTCACA | CGGGTAGAACTCTAAAGCATCG |
| COX-2 | GGGAGTCTGGAACATTGTGAA | GCACATTGTAAGTAGGTGGACTGT |
| mPGES1 | AGCACACTGCTGGTCATCAA | CTCCACATCTGGGTCACTCC |
| mPGES2 | GACCCTGTACCAGTACAAGAC | GAGGAGTCATTGAGCTGTTGC |
| cPGES | CGAATTTTGACCGTTTCTCTG | TGAATCATCATCTGCTCCATCT |
| β-Actin | TCACCCACACTGTGCCCATCTAC | GAGTACTTGCGCTCAGGAGGAGC |
For all other primers, RT was performed with 0.5 µg RNA using QuantiTect Reverse Transcription Kit (Qiagen) as per the manufacturer’s protocol. Specific primers for the COX isoenzymes 1 and 2 (COX-1 and COX-2) and for the cytosolic PGE2 synthase (cPGES) were designed using universal probe library system (Roche), whereas specific primers for the microsomal PGE synthases-1 and -2 (mPGES1 and, mPGES2) were designed as described previously (mPGES1: Soodvilai et al., 2009; mPGES2: Yang et al., 2006a). Primers were obtained from biomers.net. PCR was performed using 10 pM specific primers in PCR buffer (buffer Y; Peqlab). Samples were denatured for 5 min at 95°C, followed by 35 repeats of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min. Final extension time was 10 min at 72°C. All reactions were performed in a MJ-Research PTC-200 Peltier Thermo Cycler (Biozym). Amplified PCR products were separated by agarose gel electrophoresis (1.5% universal agarose; Bio&Sell) and stained by ethidium bromide. Bands of PCR products were extracted and sequenced (LGC Genomics). For sequence comparison we used the standard nucleotide BLAST software (National Center for Biotechnology Information, National Library of Medicine).
Biotinylation assay and Western blotting
To detect β-ENaC in Western blot experiments, a previously described custom-made antibody was used in a 1:2,000 dilution (Krueger et al., 2009; Nesterov et al., 2016; Mansley et al., 2018). Horseradish peroxidase–coupled goat anti-rabbit antibodies were obtained from Santa Cruz Biotech and used in a dilution of 1:50,000.
Cell surface proteins were labeled using a biotinylation protocol similar to that previously described for surface biotinylation of lung epithelial cell monolayers (Woollhead and Baines, 2006). For biotinylation experiments, mCCDcl1 cells were grown on permeable supports (Millicell-PCF inserts, membrane size 4.2 cm2; EMD Millipore) for 10 d. All biotinylation steps were performed at 4°C. Cells were chilled to 4°C by washing three times with ice-cold PBS containing 0.7 mM MgCl2 and 0.5 mM CaCl2 (PBS-CM). Cells were kept on ice, and biotinylation of the apical cell surface was achieved by adding borate buffer containing 85 mM NaCl, 5 mM KCl, and 15 mM Na2B4O7, pH 9.0, containing 0.5 mg·ml−1 EZ-link sulfo-NHS-SS-Biotin (Pierce). The basolateral compartment was exposed to PBS-CM + 10% FBS. Cells were kept on ice and rocked for 30 min. Cells were washed once with PBS-CM and then the reaction quenched by exposing both the apical and basolateral surface of cells to PBS-CM + 10% FBS for a further 30 min. Cells were washed twice with PBS-CM and then scraped into 200 µl lysis buffer (0.4% deoxycholic acid, 1% Triton X-100, 50 mM EGTA, and 10 mM Tris, pH 7.4) including a protease inhibitor cocktail (Roche). A small sample was taken to determine protein content by BCA assay. Biotinylated proteins were captured by exposing the sample to 50 µl ImmunoPure immobilized Neutravidin agarose beads (Pierce), which had been washed twice with PBS-CM and subsequently with lysis buffer. 250 µg sample was added to the washed beads, and tubes were incubated on a rotor overnight at 4°C. Samples were centrifuged for 2 min at 8,000 rpm (6,200 ×g) at 4°C, and the supernatant was collected separately for the detection of intracellular proteins. Neutravidin beads were washed and centrifuged four times and the biotinylated fraction was finally resuspended in 45 µl of 2× reducing SDS-PAGE sample buffer (Rotiload 1; Roth).
All protein samples were heated for 5 min at 95°C before loading on SDS gels. Proteins were separated by 10% SDS-PAGE, transferred to polyvinylidene diflouride membranes by semidry electroblotting, and probed with the indicated antibodies. Chemiluminescent signals were detected using Super Signal West Femto Chemiluminescent Substrate (Pierce).
Measurement of PGE2 and cAMP concentrations
For PGE2 measurements, an enzyme immunoassay was used according to the manufacturer’s protocol (Prostaglandin E2 Express EIA Kit—Monoclonal; Cayman Chemical Company). PGE2 concentrations were determined in diluted basolateral cell culture medium after incubating mCCDcl1 cells with arachidonic acid, COX inhibitors, or vehicle at 37°C for 10 min.
For cAMP measurements, mCCDcl1 cells were lysed to release intracellular cAMP, which was quantified using an enzyme immunoassay according to the manufacturer’s instructions (cAMP Biotrak competitive enzyme immunoassay system; GE Healthcare). Cytosolic cAMP concentrations were determined in cells exposed to PGE2 or vehicle at 37°C for 10 min. Protein concentrations were determined with a bicinchoninic acid assay (BCA Protein Assay Kit; Thermo Scientific).
All samples were measured as duplicates.
Data analysis
Data were analyzed using PRISM 5.04 for Windows (GraphPad Software). Summarized data are presented as mean values ± SE (SEM). Multiple comparisons were subjected to one-way ANOVA followed by ad hoc post-tests as specified in the figure legends; otherwise, Student's t tests were used. P values < 0.05 were required to reject the null hypothesis; *, **, and *** represent P values < 0.05, 0.01, and 0.001, respectively, and “ns” represents P values ≥ 0.05. Numbers in parentheses in the figures signify the number of samples studied.
Results
Basolateral application of PGE2 stimulates ENaC-mediated transepithelial sodium transport in mCCDcl1 cells
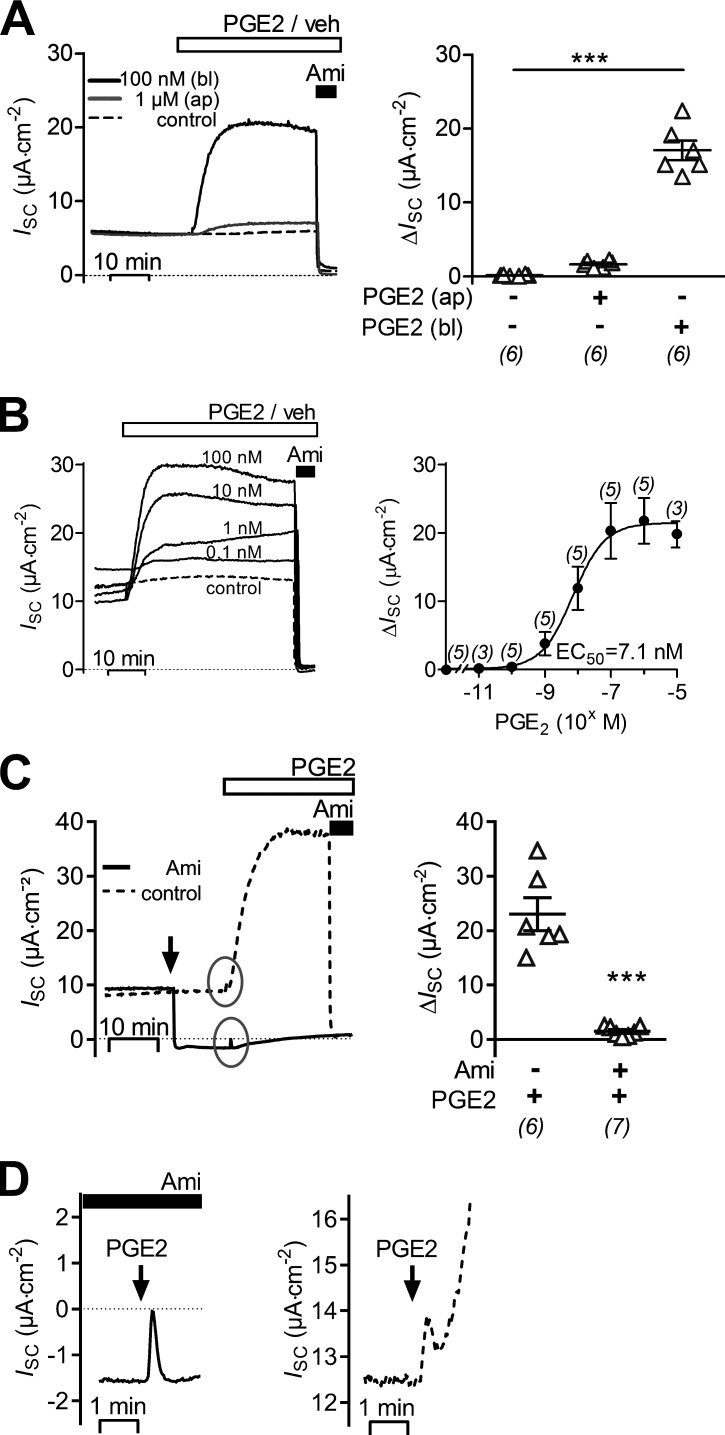
Basolateral application of 100 nM PGE2 caused a sustained increase of ISC (Fig. 1 A), which rose from a baseline value of 6.7 ± 0.7 μA·cm−2 to a maximal value of 23.7 ± 2.1 μA·cm−2 (n = 6, P < 0.001) within 5–10 min. This ISC increase of 17.0 ± 1.3 μA·cm−2 was associated with a decrease in Rte from 6.49 ± 0.41 to 2.61 ± 0.18 kΩ·cm2 (P < 0.001). In contrast, in matched vehicle-treated control cells ISC and Rte remained stable (6.3 ± 0.9 μA·cm−2 and 6.40 ± 0.54 kΩ·cm2 versus 6.4 ± 0.9 μA·cm−2 and 6.34 ± 0.54 kΩ·cm2; n = 6). Importantly, apical application of PGE2 in a concentration of up to 1 µM had negligible effects on ISC (Fig. 1 A). This indicates that the stimulatory effect of PGE2 is mediated by a basolateral receptor. Apical application of amiloride (10 µM) at the end of the experiments almost completely inhibited baseline ISC in vehicle-treated cells and the stimulated ISC in PGE2-treated cells. The inhibitory effect of amiloride on ISC was accompanied by a 1.7-fold Rte increase in vehicle-treated cells (from 6.34 ± 0.54 kΩ·cm2 to 10.56 ± 0.45 kΩ·cm2, n = 6; P < 0.001) and a 2.6-fold Rte increase in PGE2-treated cells (from 2.61 ± 0.18 kΩ·cm2 to 6.69 ± 0.18 kΩ·cm2, n = 6; P < 0.001). Thus, the stimulatory effect of basolateral PGE2 on ISC can be attributed to an increase in ENaC-mediated transepithelial sodium transport most likely due to an activation of ENaC activity. The latter conclusion is supported by the associated decrease in Rte most likely reflecting an increased sodium conductance of the apical cell membrane. As illustrated in Fig. 1 B, the stimulatory effect of basolateral PGE2 was concentration dependent, with a half-maximal effective concentration (EC50) of ∼7.1 nM. Furthermore, the PGE2-induced increase in ISC was prevented when apical application of amiloride preceded addition of PGE2 (Fig. 1 C), which confirms that the stimulatory effect of PGE2 is dependent on ENaC function. Interestingly, a small and transient ISC peak response to PGE2 was consistently observed in the presence of amiloride (Fig. 1 C, left panel; and Fig. 1 D, left panel). It was not an experimental artifact, because it was not observed in vehicle-treated controls. This amiloride-insensitive ISC component is most likely due to a transient chloride secretory response mediated by Ca2+-activated chloride channels (Sandrasagra et al., 2004; Mansley et al., 2015). A close inspection of the initial phase of the ISC response to PGE2 revealed that a similar initial ISC peak was also detectable in the absence of amiloride (Fig. 1 D, right panel). However, this ISC peak response was highly variable and in most recordings was at least partially concealed by the overlapping rapid onset of the much larger stimulatory effect of PGE2 on the amiloride-sensitive ENaC-mediated ISC. Therefore, it was not feasible to study this peak response systematically. Taken together, these data indicate that in mCCDcl1 cells basolateral PGE2 predominantly stimulates ENaC-mediated transepithelial sodium transport most likely by increasing apical ENaC activity.
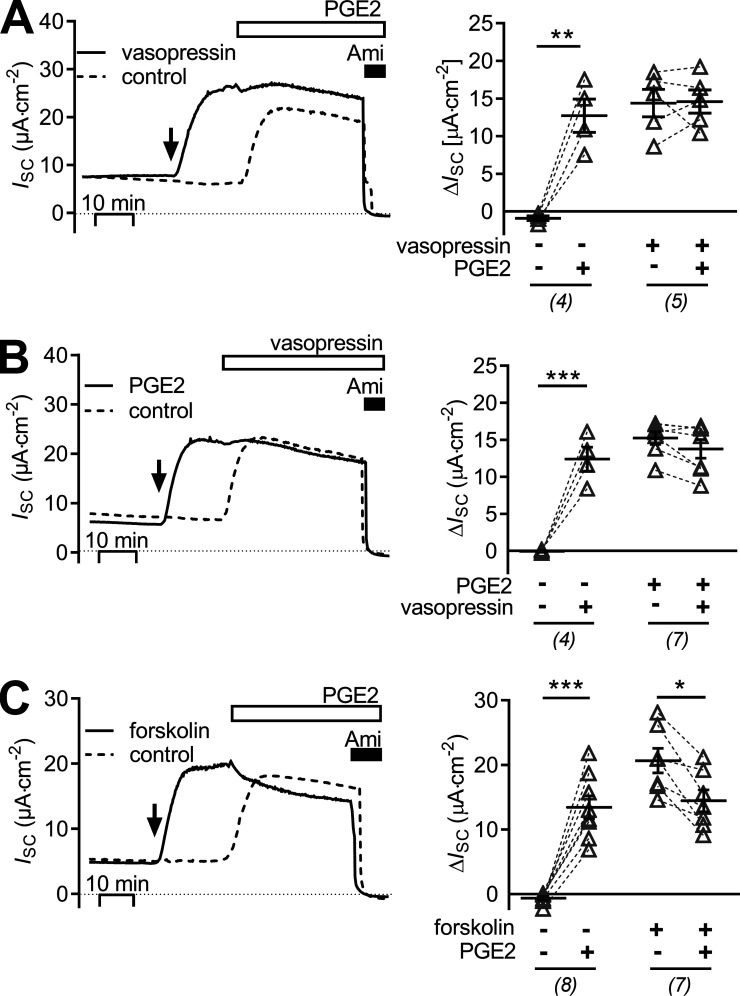
Figure 1.
Basolateral PGE2 stimulates amiloride-sensitive ISC in mCCDcl1 cells. (A–C) Representative traces of continuous ISC recordings from mCCDcl1 cells are shown in the left panels and data from similar experiments are summarized in the corresponding right panels. During the time periods indicated by the open horizontal bar, PGE2 or vehicle (veh) was present. Apical application of 10 µM amiloride (Ami) is indicated by a black horizontal bar. (A) ISC recordings with basolateral PGE2 (bl; 100 nM), apical PGE2 (ap; 1 µM) or vehicle (control) are represented by a black, gray, or dashed line, respectively. (B) ISC recordings are shown with basolateral application of PGE2 (black lines) in concentrations from 0.1–100 nM or with vehicle (dashed line). A nonlinear fit for the ISC response upon PGE2 application in concentrations of 10 pM to 10 µM was used to estimate EC50. (C) Basolateral PGE2 (100 nM) was applied in the absence (control; dashed line) or presence of 10 µM amiloride (black line). Arrow indicates time point of apical addition of amiloride or vehicle (control). In the control PGE2 recording, apical amiloride (10 µM) was added at the end of the experiment. To summarize data from different experiments as ΔISC values, the individual ISC values were corrected by subtracting the corresponding baseline ISC value. Gray circles highlight the initial phases of the ISC responses to PGE2. Summary data (right panels) are presented as individual data points and/or mean values ± SEM. Numbers of experiments are given in parenthesis. Statistical analysis was determined using one-way ANOVA with Tukey's multiple comparison test or Student’s t test where appropriate; ***, P < 0.001. (D) The initial phases of the responses to basolateral PGE2 encircled in C are shown enlarged using expanded scales. Arrows indicate the time point of PGE2 application in the continuous presence (black bar, left panel) or absence (right panel) of amiloride.
PGE2 increases the abundance of β-ENaC at the plasma membrane
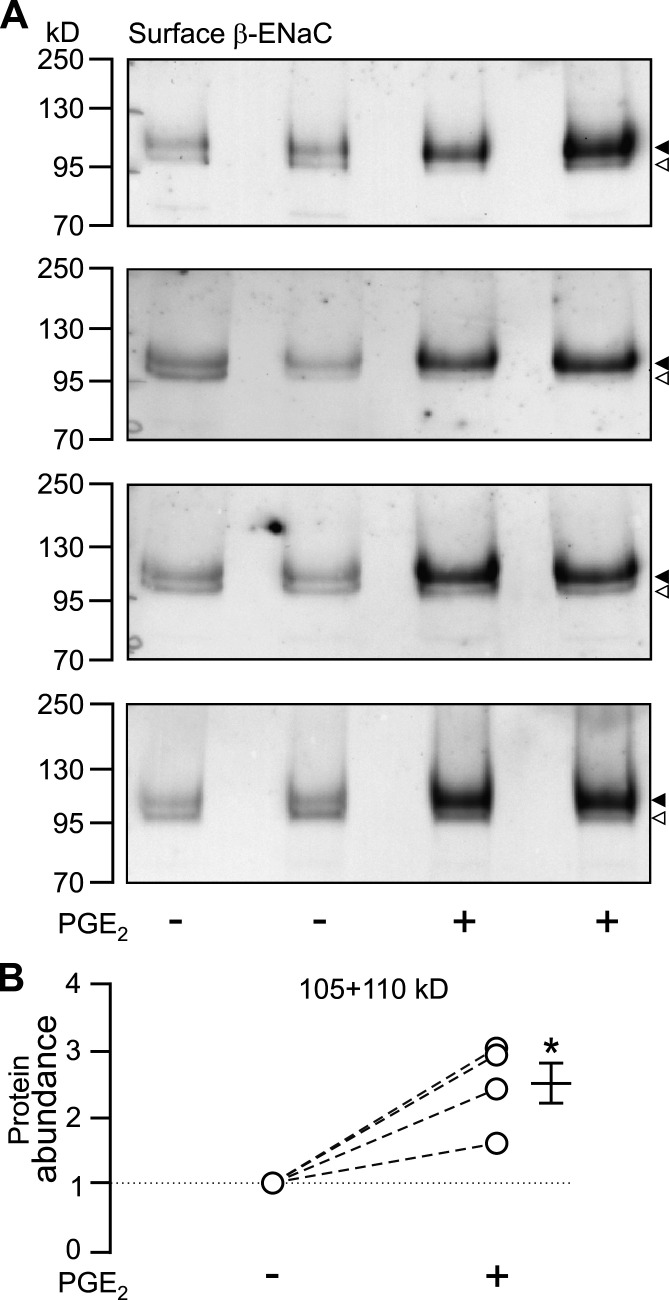
An increase in the activity of ENaC can be caused by increasing the open probability of the channel (PO), by increasing the number of channels at the cell surface (N), or by a combination of both factors. Using a biotinylation assay and Western blot analysis with antibodies directed against mouse β-ENaC, an increase (∼2.5 times) of β-ENaC protein was detected at the apical surface of cells treated with 100 nM PGE2 on the basolateral side compared with vehicle-treated cells (Fig. 2, A and B). In the corresponding cytosolic fractions, a difference in the abundance of β-ENaC was not detected (data not shown). These data indicate that the stimulatory effect of PGE2 on ENaC can be attributed at least in part to an increased channel expression at the apical plasma membrane of mCCDcl1 cells.
Figure 2.
PGE2 elevates the abundance of β-ENaC at the cell surface. (A) β-ENaC was detected by Western blot analysis using biotinylated cell surface proteins isolated from mCCDcl1 cells exposed on the basolateral side for 30 min to 100 nM PGE2 (+) or vehicle (−). The four blots represent data from four individual experiments using two filters of mCCDcl1 cells per experimental group. Arrowheads to the right of the blots indicate band sizes of ∼110 kD (filled) and ∼105 kD (open) as expected for glycosylated and nongylcosylated β-ENaC. (B) The blots shown in A were analyzed by densitometry, and the signals of the lower and upper bands of each lane were combined (105 + 110 kD). In each experiment, two matched mCCDcl1 samples were used per group (± PGE2) and an average value was determined for each group. For each experiment, the average value from the PGE2-treated cells was normalized to the average value of the nontreated cells (corresponding values are connected by a dashed line). The mean ± SEM value represents the average change in protein abundance compared with vehicle treatment. Statistical analysis was performed using Student's t test; *, P < 0.05 versus solvent vehicle controls.
PGE2 mediates its effects via basolateral EP4 receptors
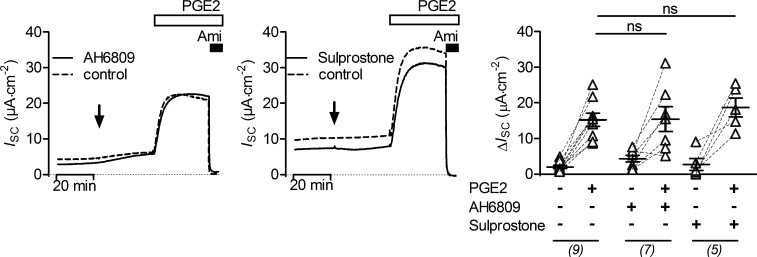
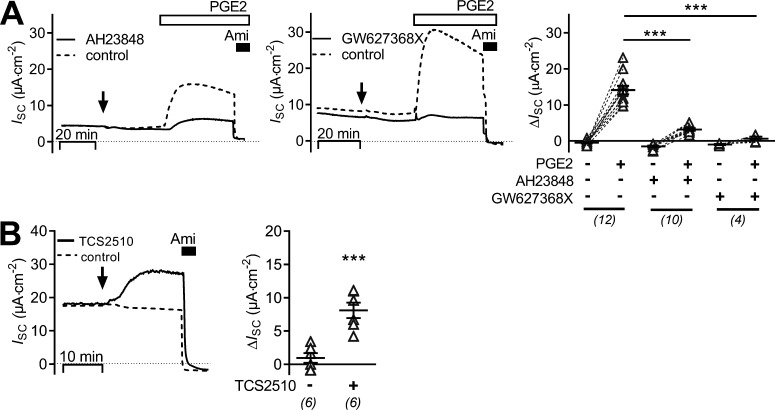
To identify the prostanoid receptor responsible for the stimulatory effect of PGE2 on ENaC activity in mCCDcl1 cells, a pharmacological approach with known receptor agonists and antagonists was used. Basolateral addition of an EP2 receptor antagonist (AH6809; 10 µM), which also shows some affinity to murine EP1 receptor (Kiriyama et al., 1997), or of sulprostone (100 nM), an agonist of EP1 and EP3 receptors, had no effect on baseline ISC and did not alter the stimulatory response to PGE2 (Fig. 3). In contrast, the EP4 receptor antagonists AH23848 (100 µM) or GW627368X (2 µM) largely diminished or nearly abolished the PGE2-mediated stimulatory response, respectively (Fig. 4 A). The small transient peak response to PGE2 appeared to be preserved in the presence of the EP4 receptor antagonist GW627368X (Fig. 4 A, middle panel). Basolateral application of TCS2510 (100 nM), an EP4 receptor agonist, stimulated the amiloride-sensitive current in a similar manner as PGE2 (Fig. 4 B). These data indicate that PGE2 stimulates ENaC activity via a basolateral Gαs-coupled EP4 receptor in mCCDcl1 cells.
Figure 3.
EP1, EP2, and EP3 receptors are not involved in mediating the stimulatory effect of PGE2 on ENaC. In the left and middle panels, representative ISC recordings are shown. At the time point indicated by an arrow, cells were exposed to 10 µM AH6809 (EP2 receptor antagonist; left panel, solid line), 100 nM sulprostone (EP1/EP3 receptor agonist; middle panel, solid line), or vehicle in matched control recordings (control, dashed line). Approximately 30 min later, all cells were exposed to 100 nM basolateral PGE2 and subsequently to apical amiloride (Ami; 10 µM) as indicated. Summary data (right panel) are presented as ΔISC values, which were determined by subtracting the corresponding baseline ISC from the ISC reached in the presence (+) or absence (−) of PGE2, AH6809, and sulprostone as indicated. Data are presented as individual values and their mean ± SEM; paired data are connected by dashed lines. Numbers of experiments are given in parenthesis. ns, P > 0.05, one-way ANOVA with Tukey's multiple comparison test.
Figure 4.
PGE2 stimulates ENaC activity via basolateral EP4 receptors. (A) The left and middle panel show representative ISC recordings in which vehicle (control; dashed lines) or EP4 receptor antagonists (100 µM AH23848 or 2 µM GW627368X; solid lines) were added basolaterally at the time point indicated by an arrow. Approximately 30 min later, all cells were exposed to 100 nM basolateral PGE2 and subsequently to apical amiloride (Ami; 10 µM) as indicated. Summary data (right panel) are presented as ΔISC values, which were determined by subtracting the corresponding baseline ISC from the ISC reached in the presence (+) or absence (−) of PGE2, AH23848, and GW627368X as indicated. (B) Representative ISC recordings (left panel) from cells exposed basolaterally to the EP4 receptor agonist TCS2510 (solid line; 100 nM) or vehicle (control, dashed line) at the time point indicated by the arrow. Apical amiloride (10 µM) was applied as indicated. Summary data (right panel) are presented as ΔISC values determined by subtracting the corresponding baseline ISC from the ISC reached after treating cells with TCS2510 (+) or vehicle (−). Data are presented as individual values and their mean ± SEM; paired data are connected by dashed lines. Numbers of experiments are given in parentheses. ***, P < 0.001, one-way ANOVA with Tukey's multiple comparison test.
The stimulatory effect of PGE2 on ENaC is similar to that of vasopressin and forskolin and is associated with an increase in intracellular cAMP
It has previously been reported that in mCCDcl1 cells, ENaC-mediated transepithelial sodium transport can be stimulated by vasopressin via a basolateral V2 receptor (Gaeggeler et al., 2011). Therefore, experiments were performed to compare the effect of vasopressin with that of PGE2 and to investigate a possible interdependence of these effects. First, the stimulatory effect of vasopressin was confirmed (Fig. 5, A and B). Basolateral application of 25 pM vasopressin increased ISC to a similar extent as 100 nM basolateral PGE2 in matched experiments. Interestingly, exposure to PGE2 after prestimulation with vasopressin had no additional stimulatory effect on ISC (Fig. 5 A). Similarly, when cells were initially exposed to PGE2, subsequent application of vasopressin failed to stimulate ISC further (Fig. 5 B). These findings suggest that the effects of PGE2 and vasopressin involve the same signaling pathway, i.e., cAMP/PKA. To confirm this, further experiments were performed with forskolin, a known activator of AC. Forskolin (10 µM) increased ISC in a similar manner as vasopressin and PGE2 (Fig. 5 C). After exposure to forskolin, application of PGE2 (100 nM) had no additional stimulatory effect. To provide evidence that PGE2 causes an increase in intracellular cAMP, an enzyme immunoassay was used. Indeed, in PGE2-treated mCCDcl1 cells cytosolic cAMP concentration was elevated to 137.6 ± 47.2 fmol/µg whole-cell protein compared with 9.5 ± 3.8 fmol/µg whole-cell protein in matched vehicle-treated control cells (n = 3).
Figure 5.
The stimulatory effect of PGE2 is similar and not additive to those of vasopressin and forskolin. (A–C) Representative traces of continuous ISC recordings from mCCDcl1 cells are shown in the left panels, and data from similar experiments are summarized in the corresponding right panels. (A) At the time point indicated by an arrow, cells were exposed to 25 pM basolateral vasopressin (solid line) or vehicle in matched control recordings (control, dashed line). About 20 min later all cells were exposed to 100 nM basolateral PGE2 and subsequently to apical amiloride (Ami; 10 µM) as indicated. (B) At the time point indicated by an arrow, cells were exposed to 100 nM basolateral PGE2 (solid line) or vehicle in matched control recordings (control, dashed line). Approximately 20 min later, all cells were exposed to 25 pM basolateral vasopressin and subsequently to apical amiloride (10 µM) as indicated. (C) At the time point indicated by an arrow, cells were exposed to 10 µM forskolin (solid line) to stimulate adenylyl cyclase or to vehicle in matched control recordings (control, dashed line). Approximately 20 min later, all cells were exposed to 100 nM basolateral PGE2 and subsequently to apical amiloride (10 µM) as indicated. Summary data (right panel) are presented as ΔISC values, which were determined by subtracting the corresponding baseline ISC from the ISC reached in the presence (+) or absence (−) of PGE2, vasopressin, and forskolin as indicated. Data are presented as individual values and their mean ± SEM. Paired data are connected by dashed lines. Numbers of experiments are given in parentheses. *, P < 0.05; **, P < 0.01; ***, P < 0.001, paired Student’s t test.
The stimulatory effect of PGE2 on ENaC activity is preserved in cells pretreated with aldosterone
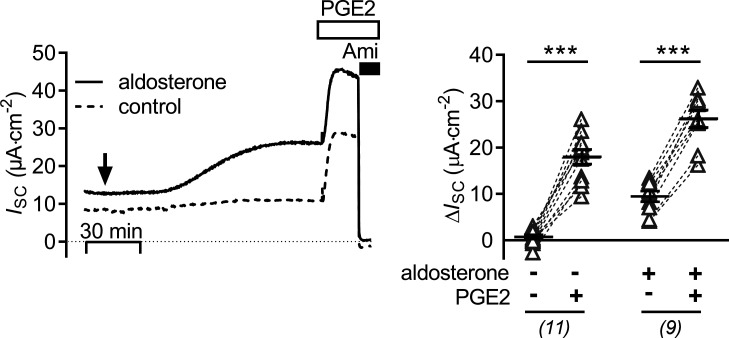
As shown previously (Gaeggeler et al., 2005; Mansley et al., 2018), mCCDcl1 cells treated with a physiological concentration of aldosterone (3 nM) responded with a sustained increase of ISC from 8.7 ± 0.9 μA·cm−2 to 17.9 ± 1.6 μA·cm−2 (n = 9, P < 0.001; Fig. 6) reaching a new plateau within ∼2 h. Basolateral application of PGE2 (100 nM) at the plateau of the aldosterone response caused a further rapid (within 5–10 min) increase in ISC by 16.8 ± 1.1 μA·cm−2. This stimulatory effect of PGE2 in aldosterone-treated cells was similar to that in matched vehicle-treated control cells, averaging 17.3 ± 1.5 μA·cm−2 (n = 11; Fig. 6). These results indicate that the stimulatory effect of PGE2 on ENaC is independent of the signaling pathways mediating the stimulatory effect of aldosterone.
Figure 6.
The stimulatory effect of PGE2 upon ENaC activity is preserved in cells pretreated with aldosterone. The left panel shows representative ISC recordings in which vehicle (control; dashed line) or aldosterone (3 nM; solid line) were added bilaterally at the time point indicated by an arrow. Approximately 2 h later, cells were exposed to 100 nM basolateral PGE2 and subsequently to apical amiloride (Ami; 10 µM) as indicated. Summary data (right panel) are presented as ΔISC values, which were determined by subtracting the corresponding baseline ISC from the ISC reached in the presence (+) or absence (−) of aldosterone and PGE2 as indicated. Data are presented as individual values and their mean ± SEM. Paired data are connected by dashed lines. Numbers of experiments are given in parentheses. ***, P < 0.001, paired Student’s t test.
PGE2 is generated and secreted by mCCDcl1 cells exposed to the precursor arachidonic acid and stimulates ENaC activity in an autocrine manner
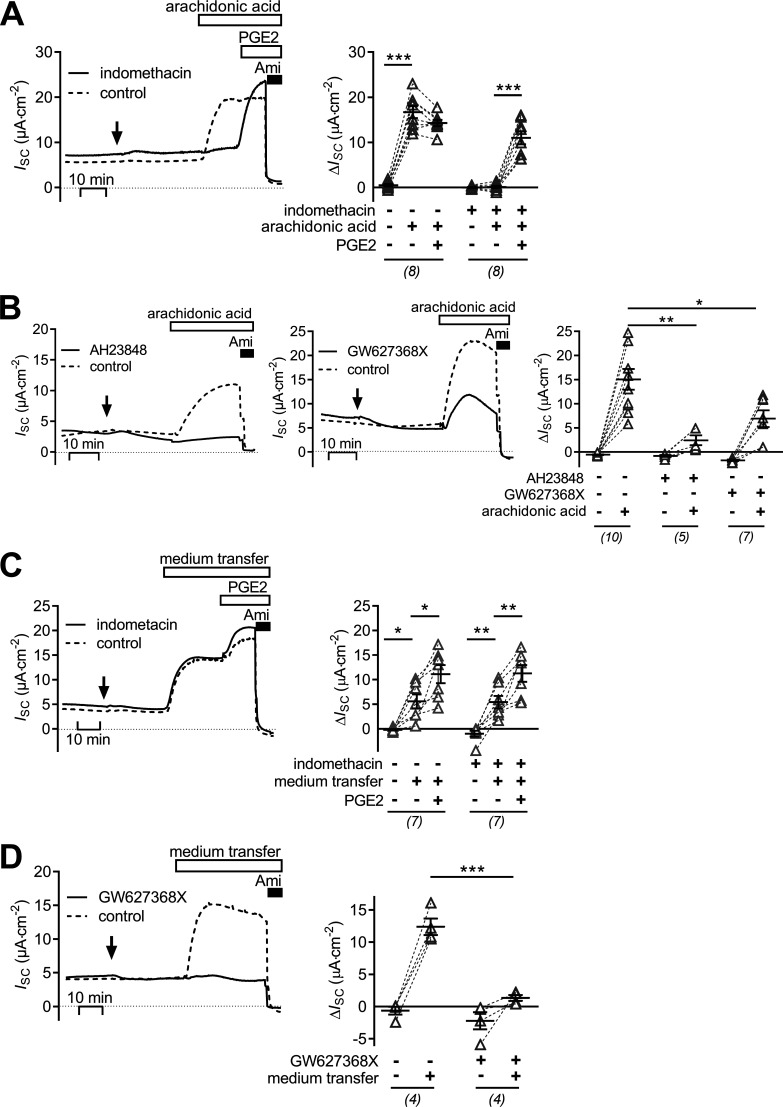
The finding that exogenously applied PGE2 stimulates ENaC activity in mCCDcl1 cells raised the question whether endogenously generated prostanoids may elicit a similar response. To promote the synthesis of endogenous PGE2, its precursor arachidonic acid was applied at a concentration of 50 µM. At this concentration, arachidonic acid is not rate limiting for the biosynthesis of PGE2 (Bonvalet et al., 1987). Apical application of arachidonic acid increased ISC from 9.8 ± 1.5 μA·cm−2 to 25.1 ± 2.4 μA·cm−2 (n = 8, P > 0.001; Fig. 7 A, dashed line). The stimulated ISC was inhibited by amiloride, which indicates that the stimulatory effect of arachidonic acid is due to increased ENaC-mediated transepithelial sodium transport. The stimulatory effect of arachidonic acid was similar to that of PGE2, and subsequent application of PGE2 (100 nM) to the basolateral bath did not increase ISC further (ΔISC = −1.5 ± 0.6 μA·cm−2). Importantly, treating mCCDcl1 cells with the COX-1/2 inhibitor indomethacin (50 µM) completely prevented the stimulatory response to arachidonic acid (ΔISC = 0.3 ± 0.2 μA·cm−2, n = 8; Fig. 7 A, solid line). In contrast, the stimulatory effect of subsequently applied PGE2 was fully preserved (ΔISC =10.7 ± 1.3 μA·cm−2, n = 8). Thus, mCCDcl1 cells are capable of synthesizing and releasing a COX-derived prostanoid, most likely PGE2, which can stimulate ENaC activity in an autocrine manner, preventing a further stimulation by exogenous PGE2. This hypothesis is further supported by the finding that the stimulatory effect of arachidonic acid was largely reduced in the presence of the EP4 receptor antagonists AH23848 (100 µM) and GW627368X (2 µM) in the basolateral solution (Fig. 7 B). In mCCDcl1 cells, we did not observe an inhibitory effect of arachidonic acid on ENaC-mediated ISC. In contrast, ENaC inhibition via cytochrome P450 epoxygenase-dependent pathways has been reported in microdissected rat CCD (Wei et al., 2004) and mpkCCD cells (Pavlov et al., 2011). The lack of an inhibitory effect in mCCDcl1 cells cannot be attributed to an insufficient concentration of arachidonic acid used in our experiments because in rat CCD, the concentration required to inhibit ENaC activity by 50% was ∼2 µM.
Figure 7.
Stimulation of ENaC activity in mCCDcl1 cells by arachidonic acid is mediated via basolateral EP4 receptors and constitutively active COX. The left panel in A and the left and middle panels in B show representative traces of continuous ISC recordings. 50 µM indomethacin (A) or 100 µM AH23848 or 2 µM GW627368X (B; solid lines) was added basolaterally at the time point indicated by an arrow. Dashed lines indicate matched control recordings treated with the respective vehicle (control). About 30 min later cells were exposed to 50 µM apical arachidonic acid and in A followed by basolateral 100 nM PGE2. Finally, apical 10 µM amiloride was added as indicated. Summary data (right panel) are presented as ΔISC values, which were determined by subtracting the corresponding baseline ISC from the ISC reached in the presence (+) or absence (−) of indomethacin, arachidonic acid, PGE2, AH23848, and GW627368X as indicated. The left panels in C and D show representative traces of continuous ISC recordings in which vehicle (control; dashed lines), 50 µM indomethacin, or 2 µM GW627368X (solid lines) was added basolaterally at the time point indicated by an arrow. Approximately 30 min later, medium from the basolateral compartment of a second set of cells (donor cells; not depicted) which were treated with apical 50 µM arachidonic acid was transferred to the basolateral compartment (indicated as medium transfer) in a 1:1 (vol/vol) ratio. This was followed in C by basolateral 100 nM PGE2. Finally, as indicated, 10 µM amiloride was applied to all cells. Summary data (right panel) are presented as ΔISC values, which were determined by subtracting the corresponding baseline ISC from the ISC reached in the presence (+) or absence (−) of indomethacin, GW627368X, PGE2, and medium transfer as indicated. Summary data are presented as individual values and their mean ± SEM. Paired data are connected by dashed lines. Numbers of experiments are given in parentheses. ***, P < 0.001; **, P < 0.01; *, P < 0.05, one-way ANOVA with Tukey's multiple comparison test or unpaired Student’s t test, where appropriate.
In additional experiments cell culture medium was collected from the basolateral bath of cells treated with apical arachidonic acid (donor cells). The collected medium was transferred to the basolateral compartment of nontreated mCCDcl1 cells (receiver cells) in a 1:1 (vol/vol) ratio. This resulted in a significant stimulation of ISC in the receiver cells (ΔISC = 5.6 ± 1.4 μA·cm−2, n = 7). Subsequent application of PGE2 had an additional but less pronounced stimulatory effect on ISC. The stimulatory effect of medium from donor cells was preserved when COX-1/2 activity in the receiver cells was inhibited by indomethacin (ΔISC 5.4 ± 1.3 μA·cm−2, n = 7; Fig. 7 C). This rules out the possibility that the stimulatory effect of the transferred medium was mediated by a contamination with arachidonic acid. Finally, the stimulatory effect of the transferred medium on ENaC activity was largely abolished when the receiver cells were pretreated with the EP4 receptor antagonist GW627368X in the basolateral bath (ΔISC = of 1.4 ± 0.5 μA·cm−2). In contrast, medium transfer increased ISC by 12.4 ± 1.3 μA·cm−2 in nontreated matched control cells (n = 4, P < 0.001; Fig. 7 D).
Stimulation of ENaC in mCCDcl1 cells by arachidonic acid requires COX-1 activity
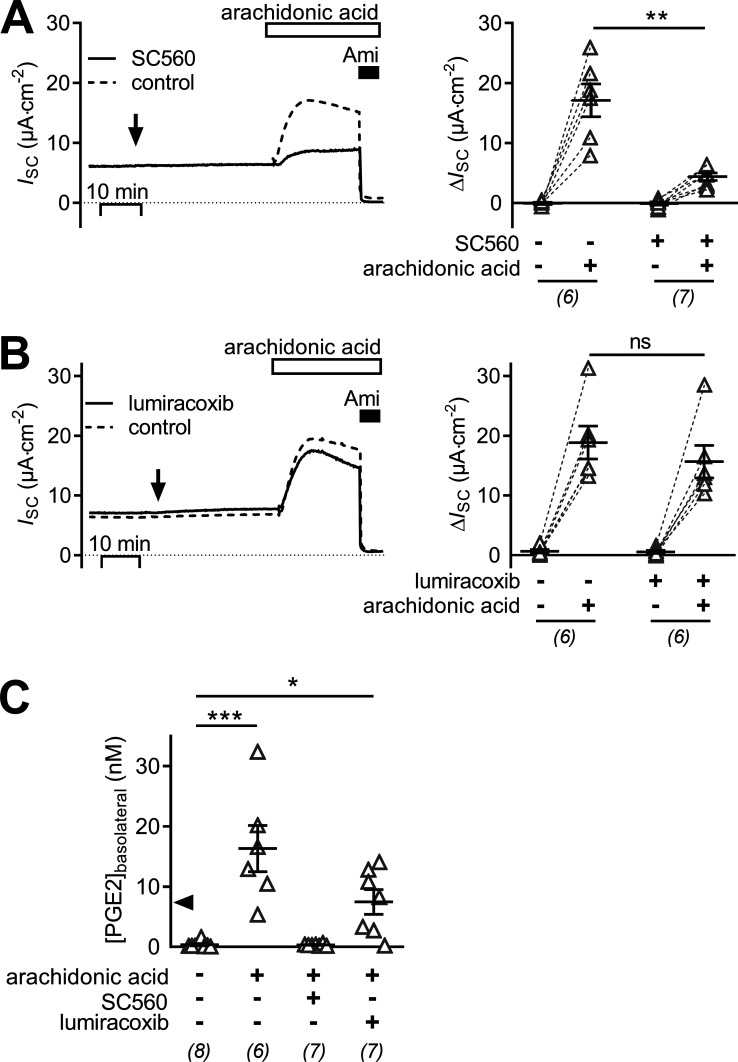
Similar to treating mCCDcl1 cells with the nonselective COX-1/2 inhibitor indomethacin (Fig. 7 A), treating cells with the selective COX-1 inhibitor SC-560 (0.14 µM; Fig. 8 A) largely prevented the stimulatory effect of arachidonic acid on ENaC activity. The arachidonic acid–induced ΔISC was reduced to 4.4 ± 0.6 μA·cm2 (n = 6) compared with the effect of arachidonic acid in matched controls with a ΔISC of 17.1 ± 2.7 μA·cm2 (n = 7, P < 0.05). In contrast, lumiracoxib (2 µM; Fig. 8 B), a highly selective COX-2 inhibitor, did not prevent the stimulatory effect of arachidonic acid. Indeed, in cells treated with lumiracoxib, the effect of arachidonic acid was preserved with a ΔISC of 15.7 ± 2.7 μA·cm−2 (n = 6), which was not different from the ΔISC of 18.9 ± 2.7 μA·cm−2 (n = 6) elicited by arachidonic acid in vehicle-treated control cells. These data indicate that COX-1 is the dominant isoenzyme involved in PGE2 generation in mCCDcl1 cells. To confirm synthesis and basolateral secretion of PGE2, its concentration was measured in the basolateral culture medium of mCCDcl1 cells. The concentration of PGE2 in the basolateral medium of mCCDcl1 cells exposed to apical arachidonic acid was significantly higher (16.3 ± 3.8 nmol·l−1, n = 6) than that of vehicle-treated control cells (0.3 ± 0.2 nmol·l−1, n = 8, P < 0.01; Fig. 8 C). The arachidonic acid–dependent increase in basolateral PGE2 concentration was abolished by the COX-1 inhibitor SC-560 (0.3 ± 0.1 nmol·l−1, n = 7, P < 0.001), but not by the COX-2 inhibitor lumiracoxib (7.4 ± 5.4 nmol·l−1, n = 7, P > 0.05), which is consistent with the ISC data reported above.
Figure 8.
Stimulation of ENaC in mCCDcl1 cells by arachidonic acid requires COX-1 activity. (A and B) Representative traces of continuous ISC recordings from mCCDcl1 cells are shown in the left panels, and data from similar experiments are summarized in the corresponding right panels. At the time point indicated by an arrow, cells were exposed to 0.14 µM SC560 (COX-1 selective inhibitor, solid line) or vehicle in matched control recordings (A; control, dashed line) or 2 µM lumiracoxib (COX-2 selective inhibitor, solid line) or vehicle in matched control recordings (B; control, dashed line). Approximately 30 min later, all cells were exposed to 50 µM apical arachidonic acid and subsequently to apical amiloride (Ami; 10 µM) as indicated. Summary data (right panel) are presented as ΔISC values, which were determined by subtracting the corresponding baseline ISC from the ISC reached in the presence (+) or absence (−) of SC560, lumiracoxib, and arachidonic acid as indicated. (C) Concentration of PGE2 measured in basolateral cell culture medium collected from cells in the presence (+) or absence (−) of 50 µM arachidonic acid, 0.14 µM SC560 and 2 µM lumiracoxib as indicated. The solid arrowhead indicates the EC50 value for the ISC response upon PGE2 as determined in Fig. 1. Data are presented as individual values and their mean ± SEM. Numbers of experiments are given in parentheses. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, P ≥ 0.05, one-way ANOVA with Dunn's multiple comparison test.
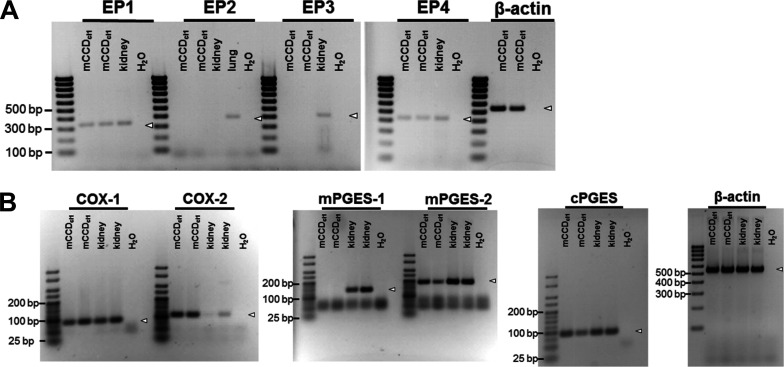
Transcripts for EP receptors and for enzymes of the PGE2 biosynthetic pathway were detected in mCCDcl1 cells
RT-PCR experiments revealed the presence of transcripts for EP4 and EP1 receptors in mCCDcl1 cells. In contrast, transcripts for EP2 or EP3 receptors were not detected (Fig. 9 A). Moreover, transcripts for several enzymes (COX-1, COX-2, mPGES2, and cPGES) involved in the biochemical pathway of PGE2 synthesis were detected not including mPGES1 (Fig. 9 B). Targets were confirmed by extracting and sequencing the PCR bands of interest.
Figure 9.
Transcripts for EP receptors and for enzymes of the PGE2 biosynthetic pathway were detected in mCCDcl1 cells. Specific primers were used to detect the transcript expression of the prostanoid receptors EP1–EP4 (A), as well as key enzymes involved in PGE2 synthesis, namely COX-1, COX-2, mPGES1, mPGES2, and cPGES (B). Transcript expression was determined in RNA extracted from mCCDcl1 cells, as well as from mouse cortical kidney and lung homogenates. H2O and primers recognizing β-actin served as no-template and loading control, respectively. Expected individual transcript sizes are indicated by open arrowheads.
Discussion
The present study provides evidence that basolateral, but not apical application of PGE2 stimulates ENaC-dependent transepithelial sodium transport in mCCDcl1 cells. Moreover, it demonstrates that this stimulatory effect is mediated by EP4 receptor signaling and is due to increased ENaC activity resulting, at least in part, from enhanced channel expression at the apical cell surface. Finally, experiments with the PGE2 precursor arachidonic acid indicate that mCCDcl1 cells can synthesize and release PGE2 to stimulate transepithelial sodium transport in an autocrine manner.
The sustained stimulatory effect of PGE2 on ENaC-mediated ISC in mCCDcl1 cells is reminiscent of similar effects previously observed in Xenopus laevis A6 (Kokko et al., 1997; Matsumoto et al., 1997) and canine MDCK renal epithelial cells (Wegmann and Nüsing, 2003). This challenges the view that PGE2 mainly inhibits sodium absorption in the collecting duct. Interestingly, an inhibitory effect of PGE2 on sodium absorption was not observed in isolated perfused rat CCD (Chen et al., 1991). Moreover, the inhibitory effects of PGE2 on Na+ transport in microdissected rabbit tubules (Stokes and Kokko, 1977; Iino and Imai, 1978) and primary cultures of rabbit principal CCD cells (Ling et al., 1992) were short-term, occurring within a few minutes of basolateral PGE2 application. In contrast, the stimulatory effect observed in mCCDcl1 cells in the present study reached a maximum within ∼10 min and was sustained. In this context, it is of interest that a biphasic action of PGE2 has been reported in Xenopus A6 renal epithelial cells with an acute inhibition (3–6 min) and a delayed stimulation (10–50 min) of ENaC by basolateral PGE2 (Kokko et al., 1994, 1997; Worrell et al., 2001). The acute inhibition is probably caused by an increase in intracellular Ca2+ signaling. In contrast, the delayed stimulatory effect is due to an increase in intracellular cAMP leading to an increase in the number of channels expressed in the apical membrane (Kokko et al., 1994). This latter interpretation is in good agreement with our observation in mCCDcl1 cells.
In a previous study, our group reported that PGE2 stimulates Cl− secretion in murine M-1 CCD cells (Sandrasagra et al., 2004). In this latter study, no stimulatory effect of PGE2 on the amiloride-sensitive ISC component was observed. A possible explanation for this discrepancy is that in M-1 cells, ENaC-dependent ISC is usually maximally stimulated under baseline conditions, which makes it difficult to detect any additional stimulatory effects on ENaC. A prestimulation of ENaC by deoxycorticosterone acetate in the majority of experiments may be a reason why no stimulatory effect of 10–100 nM PGE2 was detected in isolated rabbit collecting tubules (Stokes and Kokko, 1977; Iino and Imai, 1978). Moreover, in our previous study (Sandrasagra et al., 2004), we focused on the short-term effects of PGE2 and may have overlooked a delayed stimulatory response. In mCCDcl1 cells the sustained ISC increase in response to PGE2 is clearly due to a stimulatory effect on ENaC. This is evidenced by the finding that the response could not be elicited in the presence of amiloride and that the PGE2-stimulated ISC was completely inhibited by amiloride. In contrast, a minor rapid and transient ISC peak preceding the sustained stimulatory response to PGE2 was preserved in the presence of amiloride or in the presence of the EP4 receptor antagonist GW627368X. This suggests that the transient peak response is not mediated by EP4 and reflects electrogenic Cl− secretion reminiscent of the Cl− secretory response observed in M-1 cells (Sandrasagra et al., 2004). Thus, under certain conditions, mCCDcl1 cells, like M-1 cells and mouse inner medullary collecting duct cells (Rajagopal et al., 2014), may have the ability to secrete Cl− in response to PGE2. However, in mCCDcl1 cells, the predominant effect of PGE2 is a substantial and sustained stimulation of ENaC-dependent Na+ absorption. Importantly, the estimated EC50 of ∼7.1 nM for this stimulatory PGE2 effect is within a range that may be relevant in vivo (Bonvalet et al., 1987).
The present study provides pharmacological evidence that the stimulatory effect of PGE2 on ENaC is mediated by EP4 receptors in the basolateral membrane of mCCDcl1 cells. This conclusion is further supported by the finding that PGE2 caused an increase in intracellular cAMP as expected for a Gαs-coupled receptor. In agreement with the functional data, transcripts for EP4 receptor were detected in mCCDcl1 cells consistent with previous reports of EP4 receptor expression in the collecting duct (Jensen et al., 2001; Hao and Breyer, 2008). In contrast, transcripts for EP2 or EP3 receptors were not detected in mCCDcl1 cells. The absence of EP2 receptor transcripts is plausible, because renal EP2 receptor expression is thought to be limited to interstitial cells and the vasculature (Li et al., 2017). The lack of EP3 receptor expression in mCCDcl1 cells was unexpected and may not reflect the situation in the native collecting duct, where EP3 receptor is expressed, but its cellular localization and precise physiological role is less clear (Hao and Breyer, 2008). Of interest is the detection of EP1 receptor transcripts in mCCDcl1 cells consistent with the reported expression of EP1 receptor in the collecting duct (Guan et al., 1998) and M-1 cells (Sandrasagra et al., 2004). EP1 receptor is a Gαq/11-coupled receptor, and its activation leads to a rise in cytosolic Ca2+ via the IP3/diacylglycerol pathway (Narumiya et al., 1999; Breyer and Breyer, 2000b). An EP1-mediated increase in intracellular calcium is thought to be the mechanism by which PGE2 inhibits sodium absorption in rabbit and mouse CCD (Hébert et al., 1991; Guan et al., 1998; Nasrallah et al., 2018). Thus, whether PGE2 inhibits or stimulates sodium absorption in the collecting duct may depend on the relative expression of EP1 versus EP4 receptors which may vary in different parts of the collecting duct according to physiological needs. It has been speculated that in M-1 cells the initial Cl− secretory response to PGE2 is mediated through EP1 receptor activation and subsequent stimulation of Ca2+-activated chloride channels (Sandrasagra et al., 2004). This may also be the mechanism underlying the small ENaC-independent transient ISC response to PGE2 occasionally observed in mCCDcl1 cells. Presently, it is unclear whether Cl− secretion is physiologically relevant in CCD cells or becomes relevant under pathophysiological conditions like in polycystic kidney disease, where it is thought to contribute to cyst enlargement (Liu et al., 2012; Blanco and Wallace, 2013). In contrast, it is well established that ENaC function in the CCD and its appropriate regulation play a key role in maintaining sodium homeostasis. Thus, the reported stimulatory effect of PGE2 on ENaC-mediated transepithelial sodium transport is potentially important under certain physiological and pathophysiological conditions.
PGE2 is the most abundant prostanoid detected in the kidney, but the physiologically relevant sites for the synthesis of PGE2 remain to be defined (Hao and Breyer, 2008). The present study provides evidence that mCCDcl1 cells can synthesize and secrete PGE2. Indeed, apical exposure to the PGE2 precursor arachidonic acid caused substantial secretion of PGE2, which reached a concentration of ∼16 nM in the basolateral culture medium of treated cells compared with ∼0.3 nM in the basolateral medium of control cells. From the PGE2 concentration reached in the basolateral medium of treated cells it can be estimated that confluent mCCDcl1 cells, covering one tissue culture insert, secrete ∼4 µg PGE2 over a 30-min period. This corresponds to a secretion rate of ∼79 fg PGE2/ng cellular protein per 30 min. It is conceivable that this secretion rate may be achieved in vivo, because synthesis rates ranging from ∼800 to >8,000 fg·ng protein−1·30 min−1 have been reported for PGE2 synthesis in the collecting duct (Bonvalet et al., 1987; Liu et al., 2014). In light of the EC50 value of ∼7.1 nM, the concentration of PGE2 of ∼16 nM explains the robust stimulatory effect observed in medium transfer experiments when receiver cells were exposed to a 1:1 dilution of the basolateral medium from donor cells. It also explains why the stimulatory effect was submaximal and why subsequent exposure to 100 nM PGE2 further stimulated ISC in these experiments (Fig. 7 C). In contrast, in mCCDcl1 cells exposed to arachidonic acid on the apical side subsequent exposure to 100 nM PGE2 on the basolateral side had no additional stimulatory effect (Fig. 7 A). This is plausible, because the local PGE2 concentration reached within the vicinity of the basolateral membrane of mCCDcl1 cells exposed to apical arachidonic acid is probably much higher than the ∼16 nM measured in the bulk medium. Thus, autocrine secretion of PGE2 may well be sufficient to achieve a maximal EP4 receptor-mediated stimulatory effect on the amiloride-sensitive ISC (Fig. 7).
In the renal cortex, COX-1 and downstream prostaglandin synthases are constitutively expressed, while basal expression of COX-2 is low (Yang et al., 1998; Murakami et al., 2002; Tanikawa et al., 2002; Hao and Breyer, 2008). This study provides evidence that transcripts for COX-1, mPGES2, and cPGES, but not mPGES1, are expressed in mCCDcl1 cells. Once arachidonic acid is processed by COX-1, the biosynthesis of PGE2 can be achieved by mPGES2 or cPGES. The stimulatory effect of arachidonic acid on ENaC-dependent ISC was abolished in mCCDcl1 cells treated with the nonselective COX-1/2 inhibitor indomethacin or the selective COX-1 inhibitor SC560. In contrast, the selective COX-2 inhibitor lumiracoxib did not prevent the stimulatory effect of arachidonic acid. These findings indicate that COX-1 plays a major role in PGE2 synthesis in mCCDcl1 cells, which is consistent with the finding that COX-1 is the primary isoform in native CCD (Vitzthum et al., 2002) with a preferential localization in CCD principal cells (Câmpean et al., 2003).
cPGES transcripts are ubiquitously expressed in mouse epithelial cells of the connecting tubule and collecting duct (Chen et al., 2017; Ransick et al., 2019), and immunoreactive cPGES has been detected in cultured mouse inner medullary collecting duct cells (Zhang et al., 2003). Therefore, its detection in mCCDcl1 cells is not surprising. Interestingly, mPGES2 is thought to be primarily expressed in intercalated cells (Yang et al., 2006a), whereas mPGES1 has been detected in principal cells (Chen et al., 2017; Wang et al., 2018; Ransick et al., 2019). The mCCDcl1 cell line used in this study is a clonal cell line derived from microdissected mouse CCD and retains features typical for CCD principal cells (Gaeggeler et al., 2005, 2011; Gonzalez-Rodriguez et al., 2007; Mansley et al., 2015, 2018, 2019). Thus, at first sight it may appear surprising that transcripts for mPGES2 but not for mPGES1 were detected in mCCDcl1 cells. However, mPGES1 expression is induced by cytokines and inflammatory stimuli (Murakami et al., 2002; Hao and Breyer, 2008). Therefore, basal expression of mPGES1 in CCD principal cells is probably low and may have been below the detection limit in mCCDcl1 cells. Low basal expression of mPGES1 is also consistent with the finding that single-cell transcriptome analysis of major renal collecting duct cells types in mouse revealed a weaker expression of mPGES1 in CCD principal cells compared with cPGES (Chen et al., 2017). Interestingly, it has been reported that mCCDcl1 cells show some plasticity consistent with the ability to transition between principal and intercalated cells (Assmus et al., 2018). There is little doubt that the ENaC-dependent ISC observed in the present study is generated by mCCDcl1 cells that predominantly behave like principal cells. However, at present it is unclear whether PGE2 synthesis and secretion occurs in the same cells responsible for the amiloride-sensitive ISC or whether this occurs in distinct interspersed cells with an intercalated phenotype that may be present in the mCCDcl1 monolayer. It is tempting to speculate that the latter scenario may provide a mechanism by which intercalated cells can regulate principal cell ion transport function in a paracrine manner. Whether this occurs in native tissue and is physiologically relevant remains to be determined.
The biotinylation experiments performed in this study indicate that PGE2 stimulates ENaC activity at least in part by increasing the abundance of ENaC at the apical plasma membrane. This is consistent with the finding that PGE2 via EP4 receptor increases cytosolic cAMP in mCCDcl1 cells, as increased surface abundance of ENaC has previously been observed in response to an elevated cytosolic cAMP concentration (Snyder, 2000; Butterworth et al., 2005). Indeed, it is well known that vasopressin can stimulate ENaC-mediated sodium transport (Schafer and Troutman, 1990; Ecelbarger et al., 2000; Ecelbarger et al., 2001; Nicco et al., 2001) via the Gαs-coupled V2-receptor and subsequent activation of the AC/cAMP/PKA pathway (Schafer and Hawk, 1992; Loffing and Korbmacher, 2009; Roos et al., 2013). This involves an increased abundance of the β- and γ-ENaC subunits at the apical plasma membrane, possibly due to the insertion of ENaC-containing vesicles into the plasma membrane from a subapical pool (Snyder, 2000; Butterworth et al., 2005, 2012). Additional mechanisms probably contribute to stimulate ENaC activity following the activation of the AC/cAMP/PKA pathway (e.g., phosphorylation events; Yang et al., 2006b) and an increase in channel open probability (Bugaj et al., 2009). Thus, the stimulatory effect of PGE2 on ENaC, like that of vasopressin, is probably more complex than simply increasing channel surface expression (Kortenoeven et al., 2015).
The conclusion that the PGE2 effect on ENaC is mediated by an activation of the AC/cAMP/PKA pathway is further supported by the observation that the effects of PGE2 and vasopressin were nonadditive in mCCDcl1 cells. This indicates that the signaling pathways of PGE2 and vasopressin converge. Importantly, the stimulatory effect of PGE2 on ENaC activity was preserved when mCCDcl1 cells were prestimulated with aldosterone. In contrast to the rapid effects of PGE2 and vasopressin, the stimulatory effect of aldosterone was much slower. This slower time course of the aldosterone response is consistent with observations in isolated perfused rat CCD (Reif et al., 1986). It is not surprising, because the response to aldosterone is mediated by the mineralocorticoid receptor and involves highly complex regulatory mechanisms that are not yet fully understood but include transcriptional regulation of the channel and of regulatory proteins (Loffing and Korbmacher, 2009; Rossier, 2014).
It has been postulated that the well-documented synergistic stimulation of sodium transport by vasopressin and aldosterone (Tomita et al., 1985; Reif et al., 1986; Chen et al., 1990, 1991; Schafer and Hawk, 1992; Snyder et al., 2004; Bugaj et al., 2009) is important to achieve maximal sodium reabsorption and urine concentration due to the simultaneous stimulation of water permeability by vasopressin (Reif et al., 1986). At present, the role of PGE2 in regulating sodium and water transport in the distal nephron and collecting duct is less clear. It is commonly thought that prostanoids synthesized along the renal tubular system cause natriuresis by reducing medullary sodium and water reabsorption. This may be particularly relevant under conditions of elevated salt intake (Hao and Breyer, 2007) or when tubular fluid flow is high (Flores et al., 2012). Inhibition of sodium and water reabsorption by prostanoids may be due to various mechanisms such as their ability to blunt the action of vasopressin, promote medullary blood flow, and directly inhibit sodium transport in the distal nephron by reducing the activity of the Na+/K+-ATPase or ENaC (Stokes and Kokko, 1977; Hébert et al., 1990; Zeidel, 1993; Guan et al., 1998; Hao and Breyer, 2007; Flores et al., 2012). On the other hand, PGE2 increased water flux and elevated sodium absorption in primary cultures of freshly isolated tubules (Canessa and Schafer, 1992; Wang et al., 2016). Interestingly, the expression pattern of enzymes and receptors associated with prostanoid signaling may be altered under conditions of low salt intake or volume contraction (Hao and Breyer, 2008; Li et al., 2017; Wang et al., 2018). For example, in rabbits, salt restriction markedly stimulated PGE2 biosynthesis in the outer medulla and cortex (Davila et al., 1978; Stahl et al., 1979). This may indicate that PGE2 is needed to minimize renal sodium excretion. Thus, there is evidence that the effect of PGE2 on transepithelial sodium and water flux depends on the physiological or pathophysiologic setting. A different responsiveness to prostanoids may also explain why indomethacin decreased blood pressure in animals on a low-sodium diet but increased blood pressure in sodium-repleted rats (Stahl et al., 1981).
In conclusion, our results demonstrate that PGE2 can stimulate ENaC in mCCDcl1 cells, which depends on EP4 receptor activation and a rise in cytosolic cAMP. Furthermore, these cells can synthesize and secrete PGE2, which acts in an autocrine/paracrine manner to stimulate ENaC-mediated sodium transport. The (patho-)physiological implications of these findings remain to be elucidated. However, the findings suggest that under certain conditions, locally generated PGE2 may stimulate sodium absorption in the ASDN. Conversely, it is conceivable that pharmacological inhibition of PGE2 synthesis may attenuate sodium reabsorption in the ASDN in states with increased local production of PGE2. Additional studies are needed in native tissue and genetically modified animal models to explore a possible dual regulatory role of PGE2 associated with its ability to inhibit or stimulate sodium absorption in collecting duct cells.
Acknowledgments
David A. Eisner served as editor.
Part of the present work was performed in fulfilment of the requirements for obtaining the degree “Dr. med.” (C. Niklas) and “Dr. rer. nat.” (R. Nacken), and was published in abstract form (Niklas et al. 2011 Acta Physiol 201 [Suppl 682]: P101; Niklas et al. 2010, Acta Physiol 201 [Suppl 677]: O-TUE-3-6).
The technical assistance of Lorenz Reeh and Jessica Rinke is gratefully acknowledged.
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), project number 387509280, SFB 1350), the Alexander von Humboldt Foundation (3.3-GRO/1143730 STP), the Interdisziplinäres Zentrum für Klinische Forschung of Friedrich-Alexander University (IZKF, TP-A33), and the Bayerische Forschungsstiftung (PDOK-74-10).
The authors declare no competing financial interests.
Author contributions: C. Korbmacher and M. Bertog contributed to conception of research; M.K. Mansley, C. Niklas, K. Mandery, R. Nacken, H. Glaeser, and M. Bertog performed experiments and analyzed data; M.K. Mansley, C. Niklas, M. Fromm, C. Korbmacher, and M. Bertog interpreted results of experiments; M.K. Mansley and M. Bertog prepared figures; M.K. Mansley, C. Korbmacher and M. Bertog drafted and edited manuscript; and all authors approved final version of the manuscript.
References
- Arakawa T., Laneuville O., Miller C.A., Lakkides K.M., Wingerd B.A., DeWitt D.L., and Smith W.L.. 1996. Prostanoid receptors of murine NIH 3T3 and RAW 264.7 cells. Structure and expression of the murine prostaglandin EP4 receptor gene. J. Biol. Chem. 271:29569–29575. 10.1074/jbc.271.47.29569 [DOI] [PubMed] [Google Scholar]
- Assmus A.M., Mansley M.K., Mullins L.J., Peter A., and Mullins J.J.. 2018. mCCDcl1 cells show plasticity consistent with the ability to transition between principal and intercalated cells. Am. J. Physiol. Renal Physiol. 314:F820–F831. 10.1152/ajprenal.00354.2017 [DOI] [PubMed] [Google Scholar]
- Bankir L., Bichet D.G., and Bouby N.. 2010. Vasopressin V2 receptors, ENaC, and sodium reabsorption: a risk factor for hypertension? Am. J. Physiol. Renal Physiol. 299:F917–F928. 10.1152/ajprenal.00413.2010 [DOI] [PubMed] [Google Scholar]
- Bertog M., Letz B., Kong W., Steinhoff M., Higgins M.A., Bielfeld-Ackermann A., Frömter E., Bunnett N.W., and Korbmacher C.. 1999. Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells. J. Physiol. 521:3–17. 10.1111/j.1469-7793.1999.00003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertog M., Cuffe J.E., Pradervand S., Hummler E., Hartner A., Porst M., Hilgers K.F., Rossier B.C., and Korbmacher C.. 2008. Aldosterone responsiveness of the epithelial sodium channel (ENaC) in colon is increased in a mouse model for Liddle’s syndrome. J. Physiol. 586:459–475. 10.1113/jphysiol.2007.140459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G., and Wallace D.P.. 2013. Novel role of ouabain as a cystogenic factor in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 305:F797–F812. 10.1152/ajprenal.00248.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvalet J.P., Pradelles P., and Farman N.. 1987. Segmental synthesis and actions of prostaglandins along the nephron. Am. J. Physiol. 253:F377–F387. [DOI] [PubMed] [Google Scholar]
- Breyer M.D., and Breyer R.M.. 2000a Prostaglandin E receptors and the kidney. Am. J. Physiol. Renal Physiol. 279:F12–F23. 10.1152/ajprenal.2000.279.1.F12 [DOI] [PubMed] [Google Scholar]
- Breyer M.D., and Breyer R.M.. 2000b Prostaglandin receptors: their role in regulating renal function. Curr. Opin. Nephrol. Hypertens. 9:23–29. 10.1097/00041552-200001000-00005 [DOI] [PubMed] [Google Scholar]
- Breyer M.D., and Breyer R.M.. 2001. G protein-coupled prostanoid receptors and the kidney. Annu. Rev. Physiol. 63:579–605. 10.1146/annurev.physiol.63.1.579 [DOI] [PubMed] [Google Scholar]
- Bugaj V., Pochynyuk O., and Stockand J.D.. 2009. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am. J. Physiol. Renal Physiol. 297:F1411–F1418. 10.1152/ajprenal.00371.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth M.B., Edinger R.S., Johnson J.P., and Frizzell R.A.. 2005. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J. Gen. Physiol. 125:81–101. 10.1085/jgp.200409124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth M.B., Edinger R.S., Silvis M.R., Gallo L.I., Liang X., Apodaca G., Frizzell R.A., and Johnson J.P.. 2012. Rab11b regulates the trafficking and recycling of the epithelial sodium channel (ENaC). Am. J. Physiol. Renal Physiol. 302:F581–F590. 10.1152/ajprenal.00304.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Câmpean V., Theilig F., Paliege A., Breyer M., and Bachmann S.. 2003. Key enzymes for renal prostaglandin synthesis: site-specific expression in rodent kidney (rat, mouse). Am. J. Physiol. Renal Physiol. 285:F19–F32. 10.1152/ajprenal.00443.2002 [DOI] [PubMed] [Google Scholar]
- Canessa C.M., and Schafer J.A.. 1992. AVP stimulates Na+ transport in primary cultures of rabbit cortical collecting duct cells. Am. J. Physiol. 262:F454–F461. [DOI] [PubMed] [Google Scholar]
- Chen L., Williams S.K., and Schafer J.A.. 1990. Differences in synergistic actions of vasopressin and deoxycorticosterone in rat and rabbit CCD. Am. J. Physiol. 259:F147–F156. [DOI] [PubMed] [Google Scholar]
- Chen L., Reif M.C., and Schafer J.A.. 1991. Clonidine and PGE2 have different effects on Na+ and water transport in rat and rabbit CCD. Am. J. Physiol. 261:F126–F136. [DOI] [PubMed] [Google Scholar]
- Chen L., Lee J.W., Chou C.L., Nair A.V., Battistone M.A., Păunescu T.G., Merkulova M., Breton S., Verlander J.W., Wall S.M., et al. . 2017. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc. Natl. Acad. Sci. USA. 114:E9989–E9998. 10.1073/pnas.1710964114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila D., Davila T., Oliw E., and Anggård E.. 1978. The influence of dietary sodium on urinary prostaglandin excretion. Acta Physiol. Scand. 103:100–106. 10.1111/j.1748-1716.1978.tb06195.x [DOI] [PubMed] [Google Scholar]
- Ecelbarger C.A., Kim G.H., Terris J., Masilamani S., Mitchell C., Reyes I., Verbalis J.G., and Knepper M.A.. 2000. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am. J. Physiol. Renal Physiol. 279:F46–F53. 10.1152/ajprenal.2000.279.1.F46 [DOI] [PubMed] [Google Scholar]
- Ecelbarger C.A., Kim G.H., Wade J.B., and Knepper M.A.. 2001. Regulation of the abundance of renal sodium transporters and channels by vasopressin. Exp. Neurol. 171:227–234. 10.1006/exnr.2001.7775 [DOI] [PubMed] [Google Scholar]
- Edinger R.S., Coronnello C., Bodnar A.J., Labarca M., Bhalla V., LaFramboise W.A., Benos P.V., Ho J., Johnson J.P., and Butterworth M.B.. 2014. Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport. J. Am. Soc. Nephrol. 25:2445–2457. 10.1681/ASN.2013090931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton R.A., and Knepper M.A.. 2007. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol. Rev. 87:1083–1112. 10.1152/physrev.00053.2006 [DOI] [PubMed] [Google Scholar]
- Flores D., Liu Y., Liu W., Satlin L.M., and Rohatgi R.. 2012. Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct. Am. J. Physiol. Renal Physiol. 303:F632–F638. 10.1152/ajprenal.00169.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino H., and Regan J.W.. 2006. EP(4) prostanoid receptor coupling to a pertussis toxin-sensitive inhibitory G protein. Mol. Pharmacol. 69:5–10. 10.1124/mol.105.017749 [DOI] [PubMed] [Google Scholar]
- Gaeggeler H.P., Gonzalez-Rodriguez E., Jaeger N.F., Loffing-Cueni D., Norregaard R., Loffing J., Horisberger J.D., and Rossier B.C.. 2005. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J. Am. Soc. Nephrol. 16:878–891. 10.1681/ASN.2004121110 [DOI] [PubMed] [Google Scholar]
- Gaeggeler H.P., Guillod Y., Loffing-Cueni D., Loffing J., and Rossier B.C.. 2011. Vasopressin-dependent coupling between sodium transport and water flow in a mouse cortical collecting duct cell line. Kidney Int. 79:843–852. 10.1038/ki.2010.486 [DOI] [PubMed] [Google Scholar]
- Gao M., Cao R., Du S., Jia X., Zheng S., Huang S., Han Q., Liu J., Zhang X., Miao Y., et al. . 2015. Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc. Natl. Acad. Sci. USA. 112:8397–8402. 10.1073/pnas.1509565112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H., and Palmer L.G.. 1997. Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77:359–396. 10.1152/physrev.1997.77.2.359 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rodriguez E., Gaeggeler H.P., and Rossier B.C.. 2007. IGF-1 vs insulin: respective roles in modulating sodium transport via the PI-3 kinase/Sgk1 pathway in a cortical collecting duct cell line. Kidney Int. 71:116–125. 10.1038/sj.ki.5002018 [DOI] [PubMed] [Google Scholar]
- Grantham J.J., and Orloff J.. 1968. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3′,5′-monophosphate, and theophylline. J. Clin. Invest. 47:1154–1161. 10.1172/JCI105804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y., Zhang Y., Breyer R.M., Fowler B., Davis L., Hébert R.L., and Breyer M.D.. 1998. Prostaglandin E2 inhibits renal collecting duct Na+ absorption by activating the EP1 receptor. J. Clin. Invest. 102:194–201. 10.1172/JCI2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C.M., and Breyer M.D.. 2007. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 71:1105–1115. 10.1038/sj.ki.5002192 [DOI] [PubMed] [Google Scholar]
- Hao C.M., and Breyer M.D.. 2008. Physiological regulation of prostaglandins in the kidney. Annu. Rev. Physiol. 70:357–377. 10.1146/annurev.physiol.70.113006.100614 [DOI] [PubMed] [Google Scholar]
- Hébert R.L., Jacobson H.R., and Breyer M.D.. 1990. PGE2 inhibits AVP-induced water flow in cortical collecting ducts by protein kinase C activation. Am. J. Physiol. 259:F318–F325. [DOI] [PubMed] [Google Scholar]
- Hébert R.L., Jacobson H.R., and Breyer M.D.. 1991. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J. Clin. Invest. 87:1992–1998. 10.1172/JCI115227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y., and Imai M.. 1978. Effects of prostaglandins on Na transport in isolated collecting tubules. Pflugers Arch. 373:125–132. 10.1007/BF00584850 [DOI] [PubMed] [Google Scholar]
- Jensen B.L., Stubbe J., Hansen P.B., Andreasen D., and Skøtt O.. 2001. Localization of prostaglandin E(2) EP2 and EP4 receptors in the rat kidney. Am. J. Physiol. Renal Physiol. 280:F1001–F1009. 10.1152/ajprenal.2001.280.6.F1001 [DOI] [PubMed] [Google Scholar]
- Kiriyama M., Ushikubi F., Kobayashi T., Hirata M., Sugimoto Y., and Narumiya S.. 1997. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 122:217–224. 10.1038/sj.bjp.0701367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum M.A., and Stein J.H.. 1976. The effect of inhibition of prostaglandin synthesis on urinary sodium excretion in the conscious dog. J. Clin. Invest. 57:517–521. 10.1172/JCI108304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman T.R., Kashlan O.B., and Hughey R.P.. 2018. Epithelial Na+ Channel Regulation by Extracellular and Intracellular Factors. Annu. Rev. Physiol. 80:263–281. 10.1146/annurev-physiol-021317-121143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko K.E., Matsumoto P.S., Ling B.N., and Eaton D.C.. 1994. Effects of prostaglandin E2 on amiloride-blockable Na+ channels in a distal nephron cell line (A6). Am. J. Physiol. 267:C1414–C1425. 10.1152/ajpcell.1994.267.5.C1414 [DOI] [PubMed] [Google Scholar]
- Kokko K.E., Matsumoto P.S., Zhang Z.R., Ling B.N., and Eaton D.C.. 1997. Prostaglandin E2 increases 7-pS Cl- channel density in the apical membrane of A6 distal nephron cells. Am. J. Physiol. 273:C548–C557. 10.1152/ajpcell.1997.273.2.C548 [DOI] [PubMed] [Google Scholar]
- Kortenoeven M.L., Pedersen N.B., Rosenbaek L.L., and Fenton R.A.. 2015. Vasopressin regulation of sodium transport in the distal nephron and collecting duct. Am. J. Physiol. Renal Physiol. 309:F280–F299. 10.1152/ajprenal.00093.2015 [DOI] [PubMed] [Google Scholar]
- Krueger B., Haerteis S., Yang L., Hartner A., Rauh R., Korbmacher C., and Diakov A.. 2009. Cholesterol depletion of the plasma membrane prevents activation of the epithelial sodium channel (ENaC) by SGK1. Cell. Physiol. Biochem. 24:605–618. 10.1159/000257516 [DOI] [PubMed] [Google Scholar]
- Li J.H., Chou C.L., Li B., Gavrilova O., Eisner C., Schnermann J., Anderson S.A., Deng C.X., Knepper M.A., and Wess J.. 2009. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J. Clin. Invest. 119:3115–3126. 10.1172/JCI39680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wei Y., Zheng F., Guan Y., and Zhang X.. 2017. Prostaglandin E2 in the Regulation of Water Transport in Renal Collecting Ducts. Int. J. Mol. Sci. 18:2539 10.3390/ijms18122539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B.N., Kokko K.E., and Eaton D.C.. 1992. Inhibition of apical Na+ channels in rabbit cortical collecting tubules by basolateral prostaglandin E2 is modulated by protein kinase C. J. Clin. Invest. 90:1328–1334. 10.1172/JCI115998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Rajagopal M., Lee K., Battini L., Flores D., Gusella G.L., Pao A.C., and Rohatgi R.. 2012. Prostaglandin E(2) mediates proliferation and chloride secretion in ADPKD cystic renal epithelia. Am. J. Physiol. Renal Physiol. 303:F1425–F1434. 10.1152/ajprenal.00010.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Flores D., Carrisoza-Gaytán R., and Rohatgi R.. 2014. Biomechanical regulation of cyclooxygenase-2 in the renal collecting duct. Am. J. Physiol. Renal Physiol. 306:F214–F223. 10.1152/ajprenal.00327.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffing J., and Korbmacher C.. 2009. Regulated sodium transport in the renal connecting tubule (CNT) via the epithelial sodium channel (ENaC). Pflugers Arch. 458:111–135. 10.1007/s00424-009-0656-0 [DOI] [PubMed] [Google Scholar]
- Mansley M.K., Neuhuber W., Korbmacher C., and Bertog M.. 2015. Norepinephrine stimulates the epithelial Na+ channel in cortical collecting duct cells via α2-adrenoceptors. Am. J. Physiol. Renal Physiol. 308:F450–F458. 10.1152/ajprenal.00548.2014 [DOI] [PubMed] [Google Scholar]
- Mansley M.K., Korbmacher C., and Bertog M.. 2018. Inhibitors of the proteasome stimulate the epithelial sodium channel (ENaC) through SGK1 and mimic the effect of aldosterone. Pflugers Arch. 470:295–304. 10.1007/s00424-017-2060-5 [DOI] [PubMed] [Google Scholar]
- Mansley M.K., Roe A.J., Francis S.L., Gill J.H., Bailey M.A., and Wilson S.M.. 2019. Trichostatin A blocks aldosterone-induced Na+ transport and control of serum- and glucocorticoid-inducible kinase 1 in cortical collecting duct cells. Br. J. Pharmacol. 176:4708–4719. 10.1111/bph.14837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto P.S., Mo L., and Wills N.K.. 1997. Osmotic regulation of Na+ transport across A6 epithelium: interactions with prostaglandin E2 and cyclic AMP. J. Membr. Biol. 160:27–38. 10.1007/s002329900292 [DOI] [PubMed] [Google Scholar]
- Murakami M., Nakatani Y., Tanioka T., and Kudo I.. 2002. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat. 68–69:383–399. 10.1016/S0090-6980(02)00043-6 [DOI] [PubMed] [Google Scholar]
- Narumiya S., Sugimoto Y., and Ushikubi F.. 1999. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79:1193–1226. 10.1152/physrev.1999.79.4.1193 [DOI] [PubMed] [Google Scholar]
- Nasrallah R., Zimpelmann J., Eckert D., Ghossein J., Geddes S., Beique J.C., Thibodeau J.F., Kennedy C.R.J., Burns K.D., and Hébert R.L.. 2018. PGE2 EP1 receptor inhibits vasopressin-dependent water reabsorption and sodium transport in mouse collecting duct. Lab. Invest. 98:360–370. 10.1038/labinvest.2017.133 [DOI] [PubMed] [Google Scholar]
- Nesterov V., Krueger B., Bertog M., Dahlmann A., Palmisano R., and Korbmacher C.. 2016. In Liddle Syndrome, Epithelial Sodium Channel Is Hyperactive Mainly in the Early Part of the Aldosterone-Sensitive Distal Nephron. Hypertension. 67:1256–1262. 10.1161/HYPERTENSIONAHA.115.07061 [DOI] [PubMed] [Google Scholar]
- Nicco C., Wittner M., DiStefano A., Jounier S., Bankir L., and Bouby N.. 2001. Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension. 38:1143–1149. 10.1161/hy1001.092641 [DOI] [PubMed] [Google Scholar]
- Olesen E.T., Rützler M.R., Moeller H.B., Praetorius H.A., and Fenton R.A.. 2011. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc. Natl. Acad. Sci. USA. 108:12949–12954. 10.1073/pnas.1104691108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen E.T., Moeller H.B., Assentoft M., MacAulay N., and Fenton R.A.. 2016. The vasopressin type 2 receptor and prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma membrane targeting through a cAMP-independent pathway. Am. J. Physiol. Renal Physiol. 311:F935–F944. 10.1152/ajprenal.00559.2015 [DOI] [PubMed] [Google Scholar]
- Pavlov T.S., Ilatovskaya D.V., Levchenko V., Mattson D.L., Roman R.J., and Staruschenko A.. 2011. Effects of cytochrome P-450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC). Am. J. Physiol. Renal Physiol. 301:F672–F681. 10.1152/ajprenal.00597.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal M., Thomas S.V., Kathpalia P.P., Chen Y., and Pao A.C.. 2014. Prostaglandin E2 induces chloride secretion through crosstalk between cAMP and calcium signaling in mouse inner medullary collecting duct cells. Am. J. Physiol. Cell Physiol. 306:C263–C278. 10.1152/ajpcell.00381.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransick A., Lindström N.O., Liu J., Zhu Q., Guo J.-J., Alvarado G.F., Kim A.D., Black H.G., Kim J., and McMahon A.P.. 2019. Single-Cell Profiling Reveals Sex, Lineage, and Regional Diversity in the Mouse Kidney. Dev. Cell. 51:399–413.e7. 10.1016/j.devcel.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif M.C., Troutman S.L., and Schafer J.A.. 1986. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J. Clin. Invest. 77:1291–1298. 10.1172/JCI112433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos K.P., Bugaj V., Mironova E., Stockand J.D., Ramkumar N., Rees S., and Kohan D.E.. 2013. Adenylyl cyclase VI mediates vasopressin-stimulated ENaC activity. J. Am. Soc. Nephrol. 24:218–227. 10.1681/ASN.2012050449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier B.C. 2014. Epithelial sodium channel (ENaC) and the control of blood pressure. Curr. Opin. Pharmacol. 15:33–46. 10.1016/j.coph.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Sandrasagra S., Cuffe J.E., Regardsoe E.L., and Korbmacher C.. 2004. PGE2 stimulates Cl- secretion in murine M-1 cortical collecting duct cells in an autocrine manner. Pflugers Arch. 448:411–421. 10.1007/s00424-004-1260-y [DOI] [PubMed] [Google Scholar]
- Schafer J.A., and Hawk C.T.. 1992. Regulation of Na+ channels in the cortical collecting duct by AVP and mineralocorticoids. Kidney Int. 41:255–268. 10.1038/ki.1992.37 [DOI] [PubMed] [Google Scholar]
- Schafer J.A., and Troutman S.L.. 1990. cAMP mediates the increase in apical membrane Na+ conductance produced in rat CCD by vasopressin. Am. J. Physiol. 259:F823–F831. [DOI] [PubMed] [Google Scholar]
- Snyder P.M. 2000. Liddle’s syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na(+) channel to the cell surface. J. Clin. Invest. 105:45–53. 10.1172/JCI7869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder P.M., Olson D.R., Kabra R., Zhou R., and Steines J.C.. 2004. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J. Biol. Chem. 279:45753–45758. 10.1074/jbc.M407858200 [DOI] [PubMed] [Google Scholar]
- Soodvilai S., Jia Z., Wang M.H., Dong Z., and Yang T.. 2009. mPGES-1 deletion impairs diuretic response to acute water loading. Am. J. Physiol. Renal Physiol. 296:F1129–F1135. 10.1152/ajprenal.90478.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl R.A., Attallah A.A., Bloch D.L., and Lee J.B.. 1979. Stimulation of rabbit renal PGE2 biosynthesis by dietary sodium restriction. Am. J. Physiol. 237:F344–F349. [DOI] [PubMed] [Google Scholar]
- Stahl R., Dienemann H., Besserer K., Kneissler U., and Helmchen U.. 1981. Effect of indomethacin on blood pressure in rats with renovascular hypertension: dependence on plasma renin activity. Klin. Wochenschr. 59:245–246. 10.1007/BF01476582 [DOI] [PubMed] [Google Scholar]
- Stokes J.B., and Kokko J.P.. 1977. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J. Clin. Invest. 59:1099–1104. 10.1172/JCI108733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa N., Ohmiya Y., Ohkubo H., Hashimoto K., Kangawa K., Kojima M., Ito S., and Watanabe K.. 2002. Identification and characterization of a novel type of membrane-associated prostaglandin E synthase. Biochem. Biophys. Res. Commun. 291:884–889. 10.1006/bbrc.2002.6531 [DOI] [PubMed] [Google Scholar]
- Tomita K., Pisano J.J., and Knepper M.A.. 1985. Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J. Clin. Invest. 76:132–136. 10.1172/JCI111935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitzthum H., Abt I., Einhellig S., and Kurtz A.. 2002. Gene expression of prostanoid forming enzymes along the rat nephron. Kidney Int. 62:1570–1581. 10.1046/j.1523-1755.2002.00615.x [DOI] [PubMed] [Google Scholar]
- Wang F., Lu X., Peng K., Fang H., Zhou L., Su J., Nau A., Yang K.T., Ichihara A., Lu A., et al. . 2016. Antidiuretic Action of Collecting Duct (Pro)Renin Receptor Downstream of Vasopressin and PGE2 Receptor EP4. J. Am. Soc. Nephrol. 27:3022–3034. 10.1681/ASN.2015050592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Liu M., Zhang X., Yang G., and Chen L.. 2018. Physiological and pathophysiological implications of PGE2 and the PGE2 synthases in the kidney. Prostaglandins Other Lipid Mediat. 134:1–6. 10.1016/j.prostaglandins.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Wegmann M., and Nüsing R.M.. 2003. Prostaglandin E2 stimulates sodium reabsorption in MDCK C7 cells, a renal collecting duct principal cell model. Prostaglandins Leukot. Essent. Fatty Acids. 69:315–322. 10.1016/j.plefa.2003.06.002 [DOI] [PubMed] [Google Scholar]
- Wei Y., Lin D.H., Kemp R., Yaddanapudi G.S., Nasjletti A., Falck J.R., and Wang W.H.. 2004. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J. Gen. Physiol. 124:719–727. 10.1085/jgp.200409140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollhead A.M., and Baines D.L.. 2006. Forskolin-induced cell shrinkage and apical translocation of functional enhanced green fluorescent protein-human alphaENaC in H441 lung epithelial cell monolayers. J. Biol. Chem. 281:5158–5168. 10.1074/jbc.M509947200 [DOI] [PubMed] [Google Scholar]
- Worrell R.T., Bao H.F., Denson D.D., and Eaton D.C.. 2001. Contrasting effects of cPLA2 on epithelial Na+ transport. Am. J. Physiol. Cell Physiol. 281:C147–C156. 10.1152/ajpcell.2001.281.1.C147 [DOI] [PubMed] [Google Scholar]
- Yang T., Singh I., Pham H., Sun D., Smart A., Schnermann J.B., and Briggs J.P.. 1998. Regulation of cyclooxygenase expression in the kidney by dietary salt intake. Am. J. Physiol. 274:F481–F489. [DOI] [PubMed] [Google Scholar]
- Yang G., Chen L., Zhang Y., Zhang X., Wu J., Li S., Wei M., Zhang Z., Breyer M.D., and Guan Y.. 2006a Expression of mouse membrane-associated prostaglandin E2 synthase-2 (mPGES-2) along the urogenital tract. Biochim. Biophys. Acta. 1761:1459–1468. 10.1016/j.bbalip.2006.06.018 [DOI] [PubMed] [Google Scholar]
- Yang L.M., Rinke R., and Korbmacher C.. 2006b Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel’s beta- and gamma-subunit. J. Biol. Chem. 281:9859–9868. 10.1074/jbc.M512046200 [DOI] [PubMed] [Google Scholar]
- Zeidel M.L. 1993. Hormonal regulation of inner medullary collecting duct sodium transport. Am. J. Physiol. 265:F159–F173. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Schneider A., Rao R., Lu W.J., Fan X., Davis L., Breyer R.M., Breyer M.D., and Guan Y.. 2003. Genomic structure and genitourinary expression of mouse cytosolic prostaglandin E(2) synthase gene. Biochim. Biophys. Acta. 1634:15–23. 10.1016/j.bbalip.2003.08.003 [DOI] [PubMed] [Google Scholar]