The anticonvulsant retigabine targets the neuronal M-channel but has adverse clinical effects due to its poor KV7 subtype specificity. Larsson et al. reveal that the selectivity of retigabine is improved by coapplication with the endocannabinoid arachidonoyl-L-serine.
Abstract
Retigabine is unique among anticonvulsant drugs by targeting the neuronal M-channel, which is composed of KV7.2/KV7.3 and contributes to the negative neuronal resting membrane potential. Unfortunately, retigabine causes adverse effects, which limits its clinical use. Adverse effects may be reduced by developing M-channel activators with improved KV7 subtype selectivity. The aim of this study was to evaluate the prospect of endocannabinoids as M-channel activators, either in isolation or combined with retigabine. Human KV7 channels were expressed in Xenopus laevis oocytes. The effect of extracellular application of compounds with different properties was studied using two-electrode voltage clamp electrophysiology. Site-directed mutagenesis was used to construct channels with mutated residues to aid in the mechanistic understanding of these effects. We find that arachidonoyl-L-serine (ARA-S), a weak endocannabinoid, potently activates the human M-channel expressed in Xenopus oocytes. Importantly, we show that ARA-S activates the M-channel via a different mechanism and displays a different KV7 subtype selectivity compared with retigabine. We demonstrate that coapplication of ARA-S and retigabine at low concentrations retains the effect on the M-channel while limiting effects on other KV7 subtypes. Our findings suggest that improved KV7 subtype selectivity of M-channel activators can be achieved through strategically combining compounds with different subtype selectivity.
Introduction
Epilepsy is a disorder of the nervous system that affects an estimated 50 million people worldwide. Although the majority of patients with epilepsy are adequately helped by available treatment (Brodie et al., 2012), over 30% continue to have seizures (Brodie et al., 2012), which motivates major efforts to develop new anti-epileptic treatment strategies. Retigabine was approved by the US Food and Drug Administration in 2011 as the first anti-epileptic drug targeting the neuronal M-channel (Main et al., 2000; Gunthorpe et al., 2012), a potassium channel important for dampening neuronal excitability by contributing to the negative resting membrane potential. Retigabine showed anti-excitable and anti-epileptic effects in cellular and animal models as well as in clinical trials (Rostock et al., 1996; Rundfeldt, 1997; Brodie et al., 2010; French et al., 2011). Retigabine was used as an adjunctive treatment in patients with partial-onset seizures and had advantages, such as less drug interaction with other anticonvulsants, because it is not metabolized through the P-450 system (Hempel et al., 1999; Łuszczki, 2009). Unfortunately, retigabine was associated with adverse effects including bladder dysfunction and changes in retinal pigmentation (Splinter, 2012; Garin Shkolnik et al., 2014), which limited its clinical use. Retigabine was withdrawn from the market in 2017. Extensive studies using retigabine have established the M-channel as a target for new anti-epileptic drugs but have also highlighted the need to develop strategies to reduce adverse effects of anticonvulsants.
Metabolic instability of retigabine (Groseclose and Castellino, 2019), retigabine effects on M-channels in peripheral nervous tissue (Tykocki et al., 2019), and retigabine off-target effects on other KV7 channel subtypes (Rode et al., 2010) all likely contribute to adverse effects produced by the drug. The human KV7 (hKV7; also called KCNQ) channel family contains a total of five voltage-sensitive potassium channels, hKV7.1 to hKV7.5, which are expressed in various tissues in the body (Robbins, 2001). The M-channel is primarily composed of hKV7.2 and hKV7.3 subunits (Wang et al., 1998), which coassemble as heterotetramers. In some neuronal compartments, hKV7.5 contributes to the M-current (Shah et al., 2002). Retigabine’s anti-excitable effect is caused by retigabine binding to a tryptophan in the ion-conducting pore domain of the M-channel (W236 in hKV7.2 and W265 in hKV7.3; Schenzer et al., 2005; Wuttke et al., 2005; Kim et al., 2015), stabilizing the open conformation of the channel. Although hKV7.1 lacks this tryptophan (rendering it insensitive to retigabine), the tryptophan is conserved in hKV7.2–5, which are all activated by retigabine (Schenzer et al., 2005). This poses a potential risk of off-target effects on smooth muscle cells, outer hair cells, and retinal pigment epithelial cells, which have been shown to express hKV7.4 and/or hKV7.5 channels (Holt et al., 2007; Xu et al., 2007; Zhang et al., 2011; Brueggemann et al., 2014; Chadha et al., 2014; Provence et al., 2018).
A means to reduce the adverse effects of retigabine is to improve the metabolic stability and subtype selectivity of retigabine. However, developing retigabine analogues that better differentiate between hKV7 subtypes is challenging because of conserved residues in the retigabine binding site. Moreover, promising retigabine analogues with improved hKV7 subtype selectivity characterized in heterologous cell systems (Kumar et al., 2016) require extensive additional studies before their possible clinical potential is shown. Because retigabine and other M-channel activators could potentially be tolerated in low doses as anti-epileptic drugs, a parallel strategy would be to develop combined treatments to preserve the anticonvulsive M-channel effect while limiting adverse effects. In support of this, Manville and Abbott (2018) elegantly demonstrated that significant activating effects on the M-channel are achieved by a low concentration of retigabine if coapplied with components of herbal extracts binding to a site overlapping with the retigabine site. We hypothesize that the concept of combined targeting of the M-channel with dual drugs could be further developed if retigabine is strategically combined with M-channel activators with KV7 subtype selectivity different from that of retigabine. Specifically, we propose that the subtype selectivity of such a combination treatment could be improved if retigabine is used in conjunction with M-channel activators that act on a site distinct from the retigabine site. In line with this notion, improved drug selectivity with potentially fewer adverse effects is one of the highlighted advantages of positive allosteric modulators targeting ligand-gated ion channels and G protein–coupled receptors (Abdel-Magid, 2015; Changeux and Christopoulos, 2016; Foster and Conn, 2017). These positive allosteric modulators are developed to bind to a site that is distinct from and less conserved than that of the natural ligand, with the aim of enhancing the response of a specific receptor to its ligand.
In this work, we test the potential of endocannabinoids as a novel class of M-channel activators that could be used in conjunction with retigabine. Common endocannabinoids, such as 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide; AEA), are composed of an arachidonic acid tail connected to a head group and signal via cannabinoid receptors (Devane et al., 1992; Mechoulam et al., 1995). In addition, several endogenous arachidonic acid–based compounds related to 2-AG and AEA have been identified (Bisogno et al., 2000; Huang et al., 2001; Milman et al., 2006), with weak to strong potential to activate cannabinoid receptors. In this study, we refer to all these endogenous ligands as “endocannabinoids.” Besides the neurobehavioral effects of endocannabinoids mediated through the cannabinoid receptors, there is increasing evidence that endocannabinoids have a broader regulating function in the nervous system through interaction with so-called noncanonical targets. For instance, 2-AG has been shown to increase the firing of dopaminergic neurons by inhibiting A-type potassium channels (Gantz and Bean, 2017). Also, endothelium-dependent relaxation by AEA has been suggested to be caused by direct activation of BK channels (Bondarenko et al., 2017). If also the M-channel is a noncanonical target of endocannabinoids, the different chemical properties of retigabine and endocannabinoids make it likely that endocannabinoids act on a site different from that of retigabine.
We find that specific members of the endocannabinoid family activate the human M-channel (formed by coexpressed hKV7.2 and hKV7.3) expressed in Xenopus laevis oocytes. Especially N-arachidonoyl-L-serine (ARA-S), a weak endogenous activator of cannabinoid receptor type 1 (Milman et al., 2006), induced large activating effects at low concentrations. We uncovered that ARA-S acts through a different mechanism and displays a different pattern of hKV7 subtype selectivity compared with that of retigabine. Finally, we demonstrate how to use this by combining low concentrations of ARA-S and retigabine for pronounced activation of the M-channel while limiting activation of the other hKV7 subtypes. Altogether, our study suggests that effective activation of the M-channel with improved hKV7 subtype selectivity can be achieved by strategically combining M-channel activators with additive effects on the M-channel and nonadditive effects on the other hKV7 subtypes.
Materials and methods
All animal experiments were approved by the Linköping Animal Care and Use Committee (Permit #1941) and conform to national and international guidelines.
Test compounds
All chemicals were purchased from Sigma-Aldrich if not stated otherwise. 2-AG, AEA, ARA-S, N-arachidonoyl dopamine (NADA), N-arachidonoyl-γ-aminobutyric acid (NAGABA), arachidonoyl serinol (ARA-Serinol), and N-oleoyl-L-serine (OLE-S) were bought from Cayman Chemicals. Retigabine dihydrocloride was bought from Alomone Labs. N-docosahexaenoyl-L-serine (DOC-S), N-linoleoyl-L-serine (LIN-S), N-arachidonoyl-D-serine, and N-arachidoyl-L-serine (arachidoyl-S) were synthesized in house. The synthesis methods have been partly described previously (Silverå Ejneby et al., 2018). Most of the reagents for synthesis of new compounds were from Sigma-Aldrich except O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate, which was from Fluka (Fisher Scientific GTH AB). Preparative liquid chromatography (LC) was run on a Waters system with an XSELECT Phenyl-Hexyl column (250 × 19 mm, 5 μm), under neutral condition using gradient CH3CN/water as eluent (A, water phase: 95: 5 water/CH3CN, 10 mM NH4OAc; B, organic phase: 90: 10 CH3CN/water, 10 mM NH4OAc). NMR spectra were recorded on a Varian Avance 300 MHz with solvent indicated. Chemical shift was reported in ppm on the δ scale and referenced to solvents peak (CDCl3: δH = 7.26 ppm, δC = 77.16 ppm; methanol-d4: δH = 3.30 ppm, δC = 49.50 ppm).
To the saturated or unsaturated aliphatic acid and 2.20 equiv triethylamine in acetonitrile (20 ml) and/or dimethylformamide (DMF; 2–4 ml) was added 1.05 equiv O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate, and the reaction mixture was stirred at room temperature (rt) for ∼1 h. D-serine or L-serine (1.20 equiv) was added at rt and stirred over 1–7 nights. The solution was concentrated, and 5 ml water was added. The mixture was extracted with ethyl acetate (EA; 5 ml × 3). The organic layers were combined, concentrated, and purified using preparative LC (40–100%). The desired fractions were combined and concentrated to remove most of the acetonitrile. Another 5 ml water was added to the residue. The resulting solution was adjusted to pH at ∼5 using 1 N HCl aqueous solution and extracted with EA (15 ml × 2). The organic layers were concentrated to give the desired product.
DOC-S
Following the general procedure with docosahexaenoic acid (100 mg, 0.304 mmol), L-serine as starting materials, and DMF (2 ml) as solvent, the reaction mixture was stirred overnight at rt, filtered, and purified using preparative LC without extraction to give the product as a syrup (16.8 mg, 13% yield). 1HNMR (CDCl3/CD3OD 3:2, 300 MHz): δ 5.43–5.14 (m, 12H), 4.48 (t, J = 4.2 Hz, 1H), 3.89 (dd, J = 11.4, 4.2 Hz, 1H), 3.76 (dd, J = 11.4, 4.2 Hz, 1H), 2.85–2.57 (m, 12H), 2.39–2.30 (m, 2H), 2.28–2.20 (m, 2H), 2.06–1.92 (m, 2H), 0.90 (t, J = 7.5 Hz, 3H). 13CNMR (CDCl3/CD3OD 3:2, 75 MHz): δ 173.4, 172.2, 131.9, 129.2, 128.4, 128.2, 128.15, 128.1, 128.0, 127.96, 127.9, 127.8, 126.9, 62.4, 54.4, 35.9, 25.5, 25.43, 25.41, 23.1, 20.4, 14.1. MS (ESI−): m/z calcd for C25H36NO4 (M-H−) 414.26, found 414.51.
LIN-S
Following the general procedure with linoleic acid (280 mg, 0.998 mmol), L-serine (126.1 mg, 1.200 mmol) as starting materials, and acetonitrile (20 ml) as solvent, the reaction mixture was stirred over seven nights at rt, purified using preparative LC to give the product as a syrup (11.1 mg, 3%). 1HNMR (CDCl3, 300 MHz): δ 6.65 (br d, J = 6.6 Hz, 1H, NH), 5.45–5.25 (m, 4H), 4.50 (m, 1H), 4.14 (dd, J = 11.4, 3.6 Hz, 1H), 3.85 (dd, J = 11.4, 3.6 Hz, 1H), 2.82–2.70 (m, 2H), 2.30 (d, J = 7.5 Hz, 2H), 2.15–2.00 (m, 4H), 1.70–1.60 (m, 2H), 1.41–1.20 (m, 14H), 0.87 (t, J = 6.9 Hz, 3H). 13CNMR (CD3OD/CDCl3 2:1, 75 MHz): δ 175.8, 173.1, 130.7, 130.6, 128.75, 128.69, 62.8, 55.5, 36.8, 32.3, 30.4, 30.11, 30.05, 30.0, 29.9, 27.9, 26.5, 26.3, 23.3, 14.3. MS (ESI−): m/z calcd for C21H36NO4 (M-H−) 366.26, found 366.41.
Arachidoyl-S
Following the general procedure with arachidic acid (312.5 mg, 1.000 mmol), L-serine (126.1 mg, 1.200 mmol) as starting materials, acetonitrile (20 ml) and DMF (4 ml) as solvents, the reaction mixture was stirred over seven nights at rt. The solubility of this compound is poor. Part of the reaction mixture was purified using preparative LC to give the product as a white solid (5.0 mg). The yield was not calculated. 1HNMR (CDCl3, 300 MHz): δ 4.41 (dd, J = 4.8, 4.2 Hz, 1H), 3.89–3.75 (m, 2H), 2.29–2.22 (m, 2H), 1.35–1.25 (m, 34H), 0.89 (t, J = 7.2 Hz, 3H). MS (ESI−): m/z calcd for C23H44NO4 (M-H−) 398.33, found 398.67.
N-arachidonoyl-D-serine
Following the general procedure with docosahexaenoic acid (50 mg, 0.164 mmol), D-serine (20.7 mg, 0.197 mmol) as starting materials, and DMF (4 ml) as solvent, the reaction mixture was stirred overnight at rt, filtered, and purified using preparative LC without extraction to give the product as a syrup (21.5 mg, 33% yield). 1HNMR (CDCl3:CD3OD 85:15, 300 MHz): δ 5.40–5.20 (m, 8H), 4.49 (t, J = 3.6 Hz, 1H), 3.89 (dd, J = 11.1, 3.6 Hz, 1H), 3.77 (dd, J = 11.1, 3.6 Hz, 1H), 2.82–2.68 (m, 6H), 2.21 (t, J = 7.8 Hz, 2H), 2.10–1.90 (m, 4H), 1.72–1.58 (m, 2H), 1.36–1.16 (m, 6H), 0.82 (t, J = 6.6 Hz, 3H). 13CNMR (CDCl3:CD3OD 85:15, 75 MHz): δ 174.1, 172.4, 130.5, 129.0, 128.8, 128.6, 128.3, 128.2, 127.9, 127.5, 62.6, 54.5, 35.7, 31.5, 29.3, 27.2, 26.7, 25.6, 25.0, 22.5, 14.0. MS (ESI−): m/z calcd for C23H36NO4 (M-H−) 390.26, found 390.60.
All compounds were either delivered as or diluted as ethanol stock solutions except for 2-AG, which was delivered in acetonitrile. Stock solutions were stored at −20°C except for 2-AG, which was stored at −80°C.
Two-electrode voltage clamp electrophysiology on Xenopus oocytes
Xenopus oocytes were surgically isolated and prepared at Linköping University alternatively purchased from Ecocyte Bioscience. Isolated Xenopus oocytes were injected with 50 nl RNA. Each oocyte was injected with 2.5 ng of hKV7.2 (GenBank accession no. NM_004518) and 2.5 ng of hKV7.3 (GenBank accession no. NM_004519) RNA for hKV7.2/3. For other channels, 2.5–50 ng of RNA was injected per oocyte (GenBank accession nos. NM_000218 for hKV7.1; NM_004700 for hKV7.4; and NM_001160133 for hKV7.5). Sequence alterations were introduced using site-directed mutagenesis (QuikChange II XL with 10 XL Gold cells; Agilent), and constructs were sequenced at the Core Facility at Linköping University to ensure correct sequence. cRNA was prepared using a T7 mMessage mMachine transcription kit (Invitrogen). RNA concentration was quantified using spectrophotometry (NanoDrop 2000c, Thermo Scientific). Injected oocytes were incubated at 8°C or 16°C for 2–4 d before being used for experiments. The two-electrode voltage clamp recordings were performed using a Dagan CA-1B Amplifier (Dagan). Currents were filtered at 500 Hz and sampled at 5 kHz. The holding voltage was generally set to −80 mV. Activation curves were generally generated in steps between −100 and +50 mV in increments of 10 mV (2-s duration). The tail voltage was generally set to −30 mV. In experiments where a large shift in half-maximal activation (V50) occurred, a prepulse to −140 mV, −120 mV, or −100 mV (2-s duration) was included before the test voltage steps to close the channels. The control solution contained 88 mM NaCl, 1 mM KCl, 15 mM HEPES, 0.4 mM CaCl2, and 0.8 mM MgCl2. pH was set to 7.4 using NaOH. When experiments were performed at lower or higher pH, pH was set the same day as the experiment using HCl or NaOH, respectively. The two-electrode voltage clamp recordings were performed at rt. Control and test solutions (with endocannabinoids or/and retigabine or ICA73) were applied continuously using a pump (model ISM597D; Labinett Lab AB) with a perfusion rate of 0.5 ml/min during all recordings. Test solution was applied during an application protocol (depolarizing steps to −40 mV every 10 s) to ensure steady-state effects. If no effect was observed during application, the compound was applied for a minimum of 5 min.
Electrophysiological analysis
GraphPad Prism 6 and 7 were used for the electrophysiological analysis. Tail currents were measured shortly after stepping to the tail voltage and plotted against the preceding test voltage to approximate voltage dependence of the channels. A Boltzmann function was fitted to the data to generate the conductance versus voltage (G(V)) curve:
| (1) |
where Bottom is the minimal conductance, Top is the maximal conductance, V50 is the midpoint (i.e., the voltage needed to reach half the maximal conductance determined from the fit), and s is the slope of the curve. s was constrained to be equal for control and test curves in each oocyte. The difference in V50 induced by the test compound in each oocyte (i.e., ΔV50) was used to quantify the shift in the voltage dependence for channel opening. In some figures, G(V) curves have been normalized to the GMAX of control for clarity of the shift of voltage dependence. Basic biophysical properties of studied channels are provided in Table S1.
To plot the concentration dependence or pH dependence of the effect (ΔE) on V50 or GMAX as a function of the compound or H+ concentration (c), the following concentration-response curve was fitted to the data:
| (2) |
where EMAX is the maximal effect on V50 or GMAX, EC50 the concentration needed to cause 50% of the maximal effect, and N the Hill coefficient. ARA-S effects on opening and closing kinetics were determined by fitting a single exponential function to the first 500 ms of the current triggered by a depolarizing or hyperpolarizing pulse (as specified in the text) and determining the ratio of the time constant, τ, in the presence and absence of ARA-S. These experiments were performed in high K+ solution (100 mM K+).
Statistical analysis
Average values are expressed as mean ± SEM. Statistical analyses were done using one-way ANOVA followed by a multiple comparison test when comparing multiple data points. Dunnett’s multiple comparisons test was used when compared to defined reference data. Statistical analyses were done using Student’s t test when comparing two data points against each other or against a hypothetical value of zero. P < 0.05 was considered statistically significant. In case of a large number of Student’s t tests, the significance level was adjusted with a Bonferroni correction to reduce the risk of a type 1 error.
Online supplementary material
Fig. S1 shows that specific endocannabinoids do not activate the hKV7.2/3 channel. Fig. S2 shows the effect of ARA-S and NAGABA on hKV7.2/3. Fig. S3 shows the effect of N-arachidonoyl-D-serine on hKV7.2/3. Fig. S4 shows that ARA-S activates homomeric hKV7.2 and hKV7.3 tryptophan mutants. Fig. S5 shows that the specific charge-neutralizing mutation in hKV7.2 reduces ARA-S effect. Fig. S6 shows that the specific charge-neutralizing mutation in hKV7.3 reduces ARA-S effect. Fig. S7 shows that coapplication of low concentrations of ARA-S and retigabine limits the off-target effect on other hKV7 subtypes. Fig. S8 shows that coapplication of low concentrations of ARA-S and ICA73 limits the off-target effect on other hKV7 subtypes. Table S1 summarizes the biophysical properties of used constructs.
Figure S1.
Specific endocannabinoids do not activate the hKV7.2/3 channel. (a–c) Representative current traces and corresponding G(V) curves for hKV7.2/3 before and after application of 100 µM indicated endocannabinoids. Arrows indicate an activating voltage step to −40, −60, and −50 mV, respectively. Insert of used voltage clamp protocol.
Figure S2.
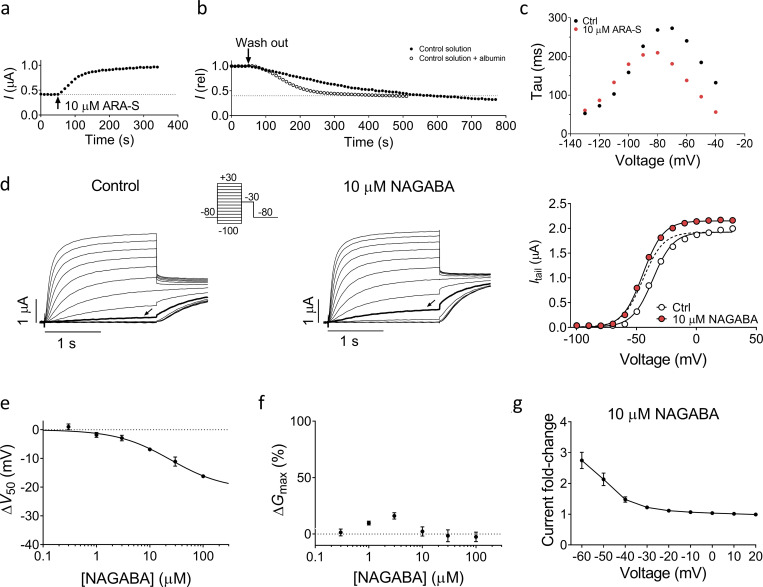
Effect of ARA-S and NAGABA on hKV7.2/3. (a and b) Representative example of the wash-in (a) and washout (b) of 10 µM ARA-S with and without supplement of 100 mg/l BSA on hKV7.2/3. The peak current after a 2-s depolarizing step to −40 mV is plotted. Dashed lines indicate basal current level before application of ARA-S. (c) Representative effect of 10 µM ARA-S on the kinetics of hKV7.2/3. Data acquired in high K+ solution (100 mM K+) by activating the channel to +20 mV followed by deactivating pulses from −40 to −130 mV in 10-mV steps. τ, determined by fitting a single exponential to the current generated by the deactivation pulse, was plotted toward the voltage of the deactivation pulse. (d) Representative current traces and corresponding G(V) curves for hKV7.2/3 before and after application of 10 µM NAGABA. Arrows indicate an activating voltage step to −50 mV. Dashed line shows the curve for 10 µM NAGABA normalized to GMAX of control. Insert of used voltage clamp protocol. (e) Concentration-response relation for NAGABA effect on V50. Data shown as mean ± SEM; n = 4–7. ΔV50:MAX (maximal shift in V50) = −21 mV; EC50 = 24 µM. (f) Concentration-response relation for NAGABA effect on GMAX. Data shown as mean ± SEM; n = 3–7. Concentration-response curve could not be converged. (g) Mean hKV7.2/3 current fold increase at different voltages induced by 10 µM NAGABA. Data shown as mean ± SEM; n = 7.
Figure S3.
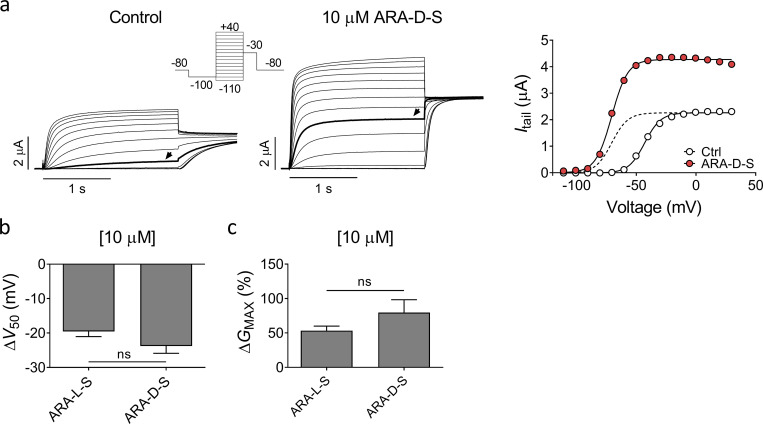
Effect of N-arachidonoyl-D-serine on hKV7.2/3. (a) Representative current traces and corresponding G(V) curve of hKV7.2/3 before and after application of 10 µM N-arachidonoyl-D-serine (ARA-D-S). Arrows in the current families indicate an activating voltage step to −50 mV. Insert of used voltage clamp protocol. Dashed line in the G(V) curve shows the curve for 10 µM ARA-D-S normalized to GMAX of control. (b and c) Mean shift in V50 (b) and increase of GMAX (c) induced by 10 µM ARA-S or 10 µM ARA-D-S on hKV7.2/3. Data shown as mean ± SEM; n = 5–8. Statistics denote Student’s t test.
Figure S4.
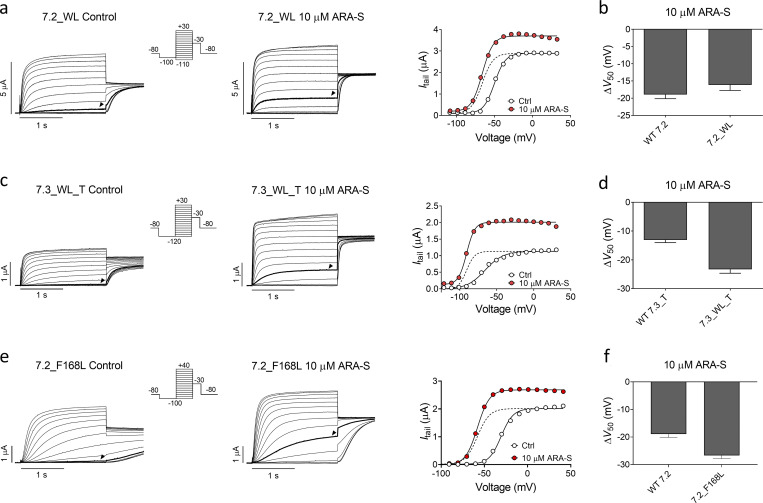
ARA-S activates homomeric hKV7.2 and hKV7.3 tryptophan mutants. (a) Representative current traces and corresponding G(V) curve of hKV7.2_W236L before and after application of 10 µM ARA-S. Arrowheads in the current families indicate an activating voltage step to −60 mV. Insert of used voltage clamp protocol. Dashed line in the G(V) curve shows the curve for 10 µM ARA-S normalized to GMAX of control. (b) Mean shift in V50 induced by 10 µM ARA-S on WT hKV7.2 and hKV7.2_W236L. Data shown as mean ± SEM; n = 6–10. (c and d) Same as in a and b, but for WT hKV7.3_A315T and hKV7.3_W265L_A315T. n = 4–9. Arrowheads in the current families indicate an activating voltage step to −80 mV. (e and f) Same as in a and b, but for WT hKV7.2 and hKV7.2_F168L. n = 6–10. Arrowheads in the current families indicate an activating voltage step to −50 mV. WL, tryptophan to leucine mutation.
Figure S5.
Specific charge-neutralizing mutation in hKV7.2 reduces ARA-S effect. Representative current traces and corresponding G(V) curve for indicated mutants before and after application of 10 µM ARA-S. Insert of used voltage clamp protocol. Note the unstable current amplitude of hKV7.2_R198Q, which prevented GMAX determination.
Figure S6.
Specific charge-neutralizing mutation in hKV7.3 reduces ARA-S effect. Representative current traces and corresponding G(V) curve for indicated mutants before and after application of 10 µM ARA-S. Insert of used voltage clamp protocol. Note the unstable current amplitude of hKV7.3_R227Q_A315T, which prevented GMAX determination.
Figure S7.
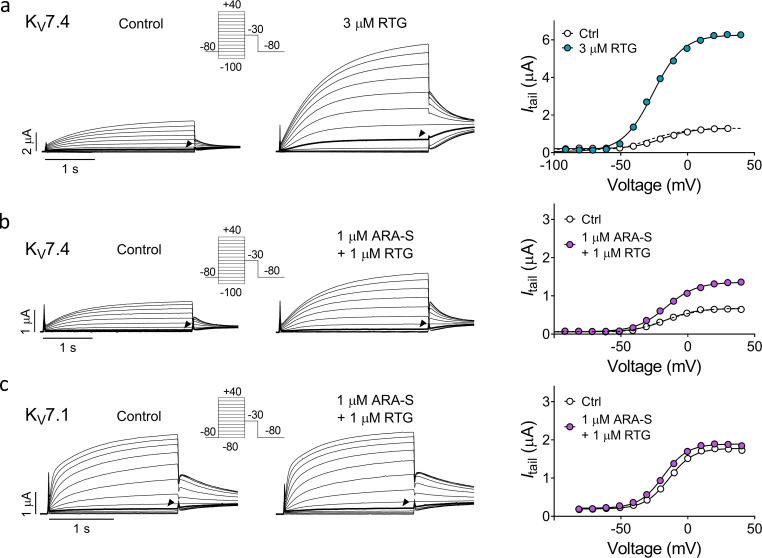
Coapplication of low concentrations of ARA-S and retigabine limits the off-target effect on other hKV7 subtypes. (a and b) Representative current traces and corresponding G(V) curves for hKV7.4 before and after application of either 3 µM retigabine (RTG; a) or coapplication of 1 µM ARA-S and 1 µM retigabine (b). Arrowheads indicate an activating voltage step to −40 mV. Insert of used voltage clamp protocols. (c) Representative current traces and corresponding G(V) curves for hKV7.1 before and after coapplication of 1 µM ARA-S and 1 µM retigabine. Arrowheads indicate an activating voltage step to −40 mV. Insert of used voltage clamp protocols. Dashed lines in the G(V) curves show the curve for each test compound normalized to GMAX of control.
Figure S8.
Coapplication of low concentrations of ARA-S and ICA73 limits the off-target effect on other hKV7 subtypes. (a) Mean shift in V50 induced by 10 µM ARA-S, 20 µM ICA73 (structure shown), or 2 µM ARA-S + 6 µM ICA73 coapplied on indicated channels. Data shown as mean ± SEM; n = 4 or 5. Dashed line denotes a shift in V50 of −20 mV. (b and c) Mean increase in GMAX of hKV7.1 or hKV7.4 induced by indicated treatment. Data shown as mean ± SEM; n = 4 or 5. Statistics denote Student’s t test.
Results
ARA-S and NAGABA activate hKV7.2/3
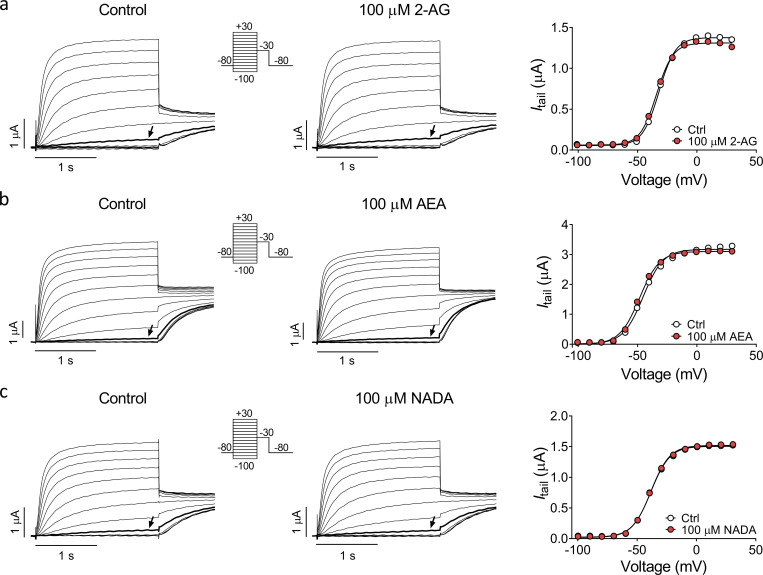
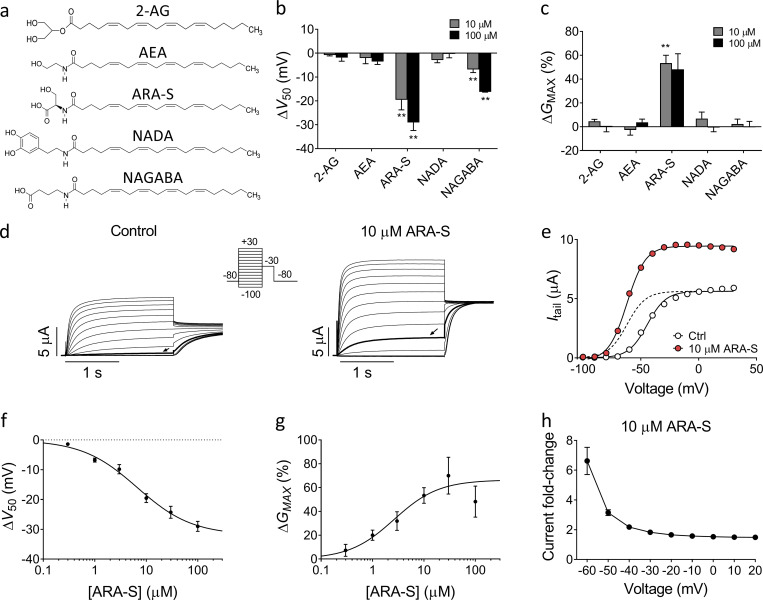
To study whether endocannabinoids target the M-channel, we initially tested the effect of five arachidonic acid–based endocannabinoids on hKV7.2 and hKV7.3 coexpressed in Xenopus oocytes (hereafter referred to as hKV7.2/3): 2-AG, AEA, ARA-S, NADA, and NAGABA (Fig. 1 a). All five compounds share an arachidonic acid tail (which makes them likely to be among the more prevalent endocannabinoids because of the high abundance of arachidonic acid in membranes; Di Marzo et al., 2004) yet contain diverse head groups (which may indicate different functional effects; Bohannon et al., 2020). Each compound was applied at two different concentrations, 10 µM and 100 µM. From this initial screen, we identified two compounds that activated the hKV7.2/3 channel. 10 µM and 100 µM ARA-S and NAGABA significantly shifted the voltage for V50 toward negative voltages (Fig. 1 b), thus allowing the channel to open at more negative voltages. ARA-S also significantly increased the maximal conductance (GMAX; Fig. 1 c). In contrast, 2-AG, AEA, and NADA had no effect on V50 or GMAX of the hKV7.2/3 channel (Fig. 1, b and c; and Fig. S1, a–c).
Figure 1.
Specific endocannabinoids activate the hKV7.2/3 channel expressed in Xenopus oocytes. (a) Molecular structure of 2-AG, AEA, ARA-S, NADA, and NAGABA. (b and c) Mean shift in V50 (ΔV50; b) and mean increase in GMAX (ΔGMAX; c) induced by 10 or 100 µM of indicated endocannabinoid on hKV7.2/3. Data shown as mean ± SEM; n = 4–8. Statistics denote Student’s t test compared with a hypothetical value of 0 with a Bonferroni corrected significance value of P < 0.005. ** indicates P < 0.001. (d) Representative current traces of hKV7.2/3 before and after application of 10 µM ARA-S. Arrow indicates an activating voltage step to −60 mV. Insert of used voltage clamp protocol. (e) Representative G(V) curve for the effect of 10 µM ARA-S for the cell shown in d. Dashed line shows the curve for 10 µM ARA-S normalized to GMAX of control. (f) Concentration-response relation for ARA-S effect on V50. Data shown as mean ± SEM; n = 4–11. ΔV50:MAX (maximal shift in V50) = −32 mV; EC50 = 7 µM. (g) Concentration-response relation for ARA-S effect on GMAX. Data shown as mean ± SEM; n = 4–10. ΔGMAX:MAX (maximal increase of GMAX) = 66%; EC50 = 3 µM. (h) Mean hKV7.2/3 current fold increase at different voltages induced by 10 µM ARA-S. Data shown as mean ± SEM; n = 8.
ARA-S potently modulates the hKV7.2/3 channel
We investigated the activating effects of ARA-S and NAGABA further. 10 µM ARA-S induced a clear increase in the overall current amplitude of hKV7.2/3 (Fig. 1 d). The onset of the ARA-S effect was relatively fast, with stable effects achieved within 2 min (Fig. S2 a). The washout of the ARA-S effect using control solution was relatively slow, with roughly 10 min of washout required for full reversibility (Fig. S2 b). The washout was faster when albumin, which binds endocannabinoids (Bojesen and Hansen, 2003), was added to the control solution (Fig. S2 b). ARA-S sped up channel-opening kinetics and slowed down the closing kinetics (Fig. S2 c; for opening at +20 mV, τARA-S/τctrl = 0.76 ± 0.02; for closing at −100 mV, τARA-S/τctrl = 1.45 ± 0.11; n = 3). The conductance versus voltage (G(V)) curve clearly demonstrates the ability of ARA-S to shift V50 and increase GMAX (Fig. 1 e). On average, 10 µM ARA-S shifted V50 by −19.5 ± 1.5 mV and increased GMAX by 53% ± 7%. The shift in V50 induced by ARA-S was significant at concentrations as low as 300 nM (−1.4 ± 0.4 mV, P = 0.03 using Student’s t test; Fig. 1 f). The concentration-response curve for the V50 effect of ARA-S suggests an estimated maximal shift of V50 by −31.9 ± 4.3 mV with 50% of the maximal shift (EC50) caused by 7 µM (Fig. 1 f). The concentration-response curve for the GMAX effect of ARA-S suggests an estimated maximal increase of GMAX by 66% ± 8%, with an EC50 of 3 µM (Fig. 1 g). Both the shift in V50 and the increase in GMAX occur at similar concentrations and together contribute to the overall increase of the current. On average, 10 µM ARA-S induced a sevenfold increase in the current at −60 mV (Fig. 1 h), suggesting that ARA-S effectively activates hKV7.2/3 at voltages around the resting membrane potential of neurons.
NAGABA produced overall similar but smaller effects compared with ARA-S (Fig. S2, d–g). On average, 10 µM NAGABA shifted V50 by −6.9 ± 0.5 mV but did not significantly increase GMAX. The concentration-response curve for the V50 effect of NAGABA suggests an estimated maximal shift of V50 by −20.8 ± 5.2 mV with an EC50 of 24 µM (Fig. S2 e). A concentration-response curve for the GMAX effect of NAGABA could not be obtained as there was no consistent effect of NAGABA on GMAX (Fig. S2 f). On average, 10 µM NAGABA induced a threefold increase in the current at −60 mV (Fig. S2 g).
The charge of the endocannabinoid head group is essential for the activating effect
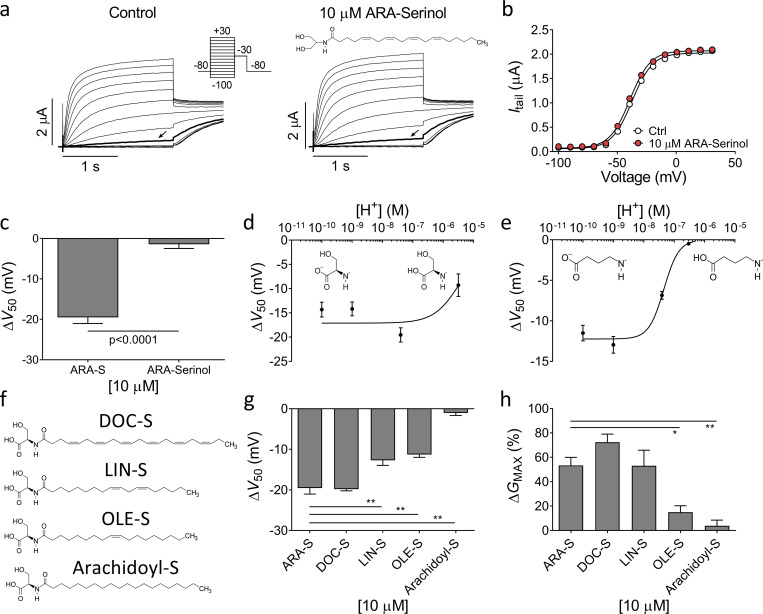
We tested which molecular determinants of the studied endocannabinoids are necessary to induce activating effects on the hKV7.2/3 channel. Modifying either the head or the lipid tail of the endocannabinoid could potentially change the effect of the compound. For instance, the head group of both ARA-S and NAGABA contains a carboxyl group, which can lose a proton to become negatively charged depending on the acid dissociation constant (pKa) of the head group and the pH of the surrounding solution. To test whether the negative charge of the head group is important for the ARA-S effect, we tested ARA-Serinol, which has a head group that lacks the carboxyl group of ARA-S and therefore is uncharged (Fig. 2 a). 10 µM ARA-Serinol had no significant effect on V50 or GMAX of hKV7.2/3 (Fig. 2, a–c; P > 0.05 using Student’s t test), suggesting that the carboxyl group is required for the activating effect.
Figure 2.
Importance of the negative charge of the head group and composition of lipid tail for endocannabinoid effect on hKV7.2/3. (a) Representative current traces of hKV7.2/3 before and after application of 10 µM ARA-Serinol. Arrow indicates an activating voltage step to −50 mV. Insert of used voltage clamp protocol and molecular structure of ARA-Serinol. (b) Representative G(V) curve for the effect of 10 µM ARA-Serinol for the cell shown in a. (c) Mean ΔV50 induced by 10 µM ARA-S or 10 µM ARA-Serinol. Data shown as mean ± SEM; n = 4–8. (d) pH response relation for 10 µM ARA-S effect on V50. Data shown as mean ± SEM; n = 3–8. Apparent pKa = 5.4. Inserts indicate the structure of deprotonated and protonated ARA-S head. (e) pH response relation for 10 µM NAGABA effect on V50. Data shown as mean ± SEM; n = 4–7. Apparent pKa = 7.3. Inserts indicate the structure of deprotonated and protonated NAGABA head. (f) Molecular structure of DOC-S, LIN-S, OLE-S, and arachidoyl-S. (g and h) Mean ΔV50 (g) and ΔGMAX (h) induced by 10 µM indicated serine endocannabinoid analogues. Data shown as mean ± SEM; n = 5–9. Statistics denote one-way ANOVA with Dunnett’s multiple comparison test with ARA-S set as control. * indicates P < 0.01; ** indicates P < 0.001.
We tested the effect of ARA-S and NAGABA on V50 at different extracellular pH to alter the degree of carboxyl group protonation. For ARA-S, the effect on V50 was not increased when pH levels were increased from pH 7.4 to pH 9.0 or 10 (Fig. 2 d). However, the effect was reduced when pH was decreased from pH 7.4 to pH 5.5 (Fig. 2 d). The estimated apparent pKa for ARA-S at the channel is 5.4 (Fig. 2 d), which suggests that ARA-S is fully deprotonated at physiological pH. In contrast, the effect of NAGABA was increased when pH was increased from pH 7.4 to pH 9.0 or 10, and the effect of NAGABA was eliminated when pH was decreased from pH 7.4 to pH 6.5 (Fig. 2 e). The estimated apparent pKa for NAGABA at the channel is 7.3 (Fig. 2 e), suggesting that only around 50% of the NAGABA molecules are deprotonated at physiological pH. The difference in estimated pKa could partly explain the difference in effect of ARA-S and NAGABA on V50 at pH 7.4.
Since the head group of ARA-S contains a serine moiety, ARA-S could be either L or D enantiomer. The naturally occurring enantiomer (Milman et al., 2006) tested in the initial experiments was the L enantiomer of ARA-S. To test if the chirality affects the effect of ARA-S on hKV7.2/3, we tested the D enantiomer of ARA-S. The D enantiomer of ARA-S induced effects comparable to those of the L enantiomer on V50 and GMAX of hKV7.2/3 (Fig. S3, a–c). Altogether, these findings suggest that the negative charge of the endocannabinoid head group is important for the activating effect on the hKV7.2/3 channel, but not the chirality of the head. The importance of the negative charge could explain why 2-AG, AEA, and NADA do not activate the hKV7.2/3 channel, as none of these compounds can be negatively charged.
The composition of the fatty acid tail alters the effect
We next tested the impact of the fatty acid tail on the effect of ARA-S on hKV7.2/3. We kept the serine head but exchanged the arachidonic acid tail of ARA-S to different lipid tails. We tested the polyunsaturated docosahexaenoic and linoleic tails (DOC-S and LIN-S), the monounsaturated oleic tail (OLE-S), and the saturated arachidic tail (arachidoyl-S; Fig. 2 f). At a concentration of 10 µM, exchanging the arachidonic tail for the docosahexaenoic tail preserved the ability to shift V50 and increase GMAX (Fig. 2, g and h). The linoleic tail reduced the ability to shift V50 but preserved the ability to increase GMAX (Fig. 2, g and h). The oleic tail reduced the ability to shift both V50 and increase GMAX (Fig. 2, g and h). The arachidic tail eliminated the activating effect on hKV7.2/3 (Fig. 2, g and h). Altogether, these data suggest that the composition of the lipid tail alters the effect on hKV7.2/3. Decreasing the number of double bonds in the fatty acid tail tended to decrease the effect of the serine compounds on hKV7.2/3. It should be noted that arachidoyl-S was difficult to dissolve, suggesting that the absence of effect of arachidoyl-S could be caused in part by poor solubility.
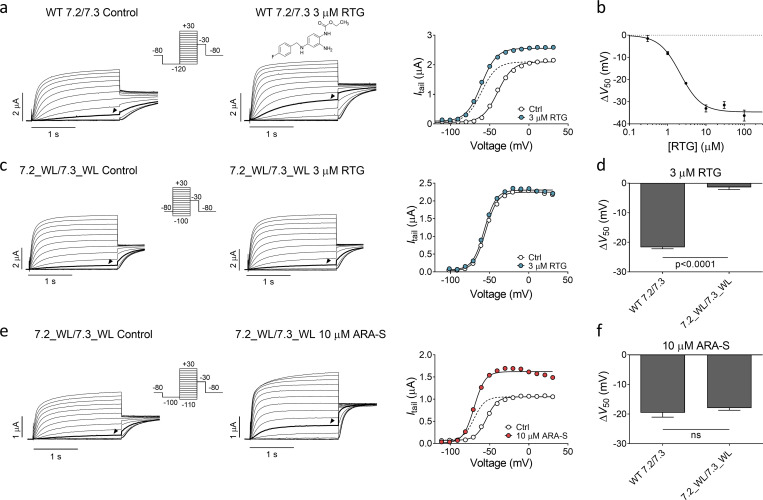
ARA-S and retigabine activates hKV7.2/3 through different mechanisms
As previously described, retigabine (structure in Fig. 3 a) shifts V50 of hKV7.2/3 to negative voltages and increases the overall current (Main et al., 2000). In our experiments, retigabine shifted V50 of hKV7.2/3 with an estimated EC50 of 2 µM and a ΔV50 of −21.7 ± 0.5 mV induced by 3 µM retigabine (Fig. 3, a and b). As has been previously demonstrated (Schenzer et al., 2005), removing the tryptophan in S5 that is important for retigabine effects (by constructing the hKV7.2_W236L and hKV7.3_W265L mutants and coexpressing them as hKV7.2_W236L/hKV7.3_W265L) rendered the channel insensitive to 3 µM retigabine (Fig. 3, c and d). ARA-S, on the other hand, retained its effect on the hKV7.2_W236L/hKV7.3_W265L channel (Fig. 3, e and f) as well as on homomeric hKV7.2_W236L and hKV7.3_W265L channels (Fig. S4, a–d). These experiments demonstrate that the tryptophan in S5 is not important for the activating effect of ARA-S and suggest that ARA-S and retigabine activate the channel through distinct mechanisms. Moreover, ARA-S retained its effect on the hKV7.2_F168L channel (Fig. S4, e and f), which was previously shown to render the channel insensitive to the M-channel activator ICA73 (ICA-069673) by removing the phenylalanine in S3 (Wang et al., 2017; Wang et al., 2018). This suggests that ARA-S activates the channel through a mechanism different from that of ICA73.
Figure 3.
ARA-S and retigabine activate hKV7.2/3 through different mechanisms. (a) Representative current traces and corresponding G(V) curve of hKV7.2/3 before and after application of 3 µM retigabine (RTG). Arrowheads in the current families indicate an activating voltage step to −50 mV. Insert of used voltage clamp protocol. Dashed line shows the curve for 3 µM retigabine normalized to GMAX of control. (b) Concentration-response relation for retigabine effect on V50. Data shown as mean ± SEM; n = 4–7. ΔV50:MAX (maximal shift in V50) = −35 mV; EC50 = 2 µM. (c) Same as in a, but for hKV7.2_W236L/hKV7.3_W265L. Arrowheads indicate an activating voltage step to −60 mV. (d) Mean shift in V50 induced by 3 µM retigabine on WT hKV7.2/3 or hKV7.2_W236L/hKV7.3_W265L. Data shown as mean ± SEM; n = 4 or 5. Statistics denote Student’s t test. (e) Same as in c, but for the effect of 10 µM ARA-S on hKV7.2_W236L/hKV7.3_W265L. Arrowheads indicate an activating voltage step to −60 mV. (f) Mean shift in V50 induced by 10 µM ARA-S on WT hKV7.2/3 or hKV7.2_W236L/hKV7.3_W265L. Data shown as mean ± SEM; n = 5–8. Statistics denote Student’s t test. ns, nonsignificant; WL, tryptophan to leucine mutation.
Positively charged residues in S4 and S6 are important for ARA-S–induced activation
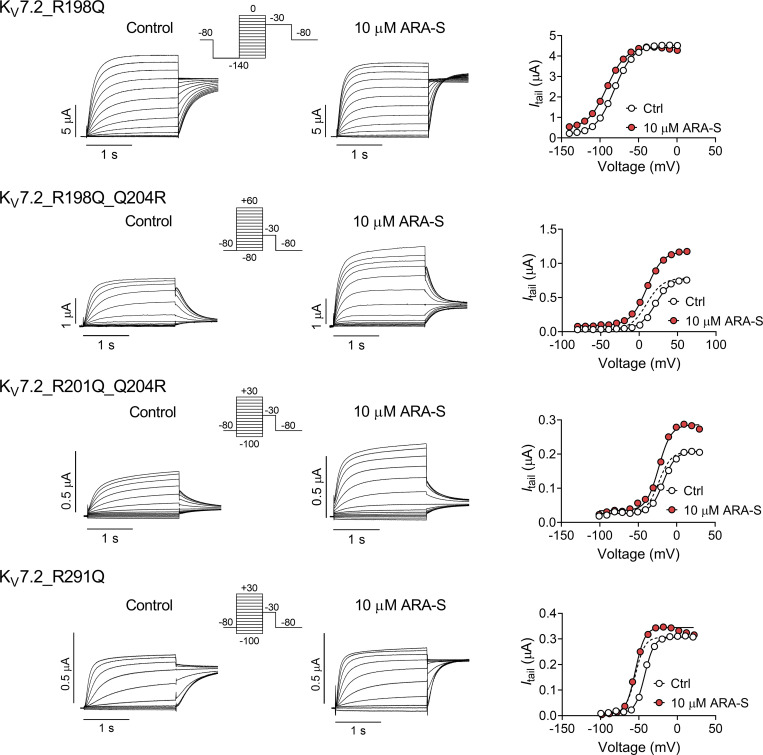
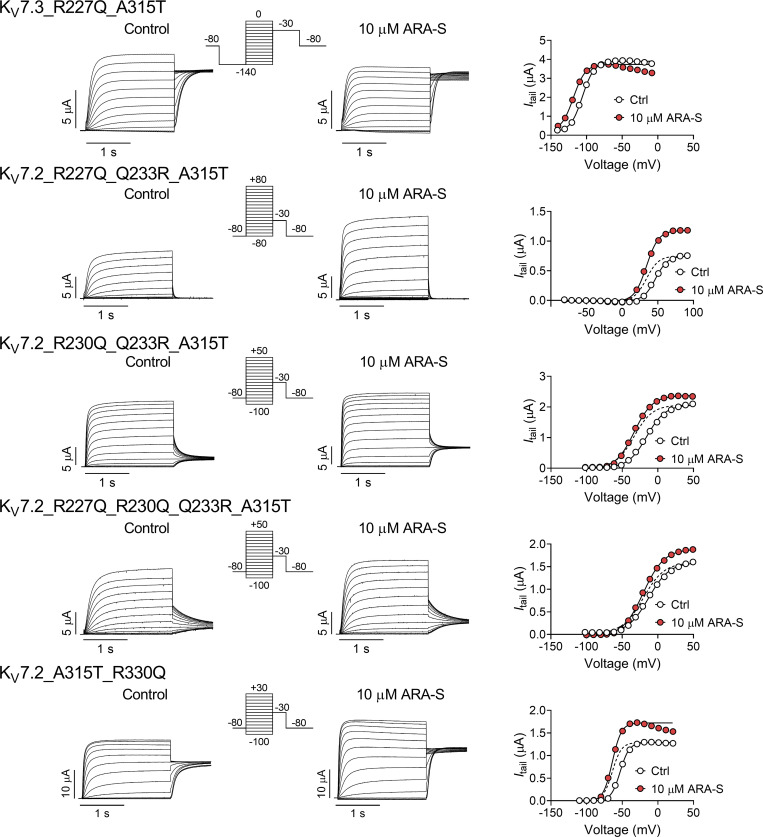
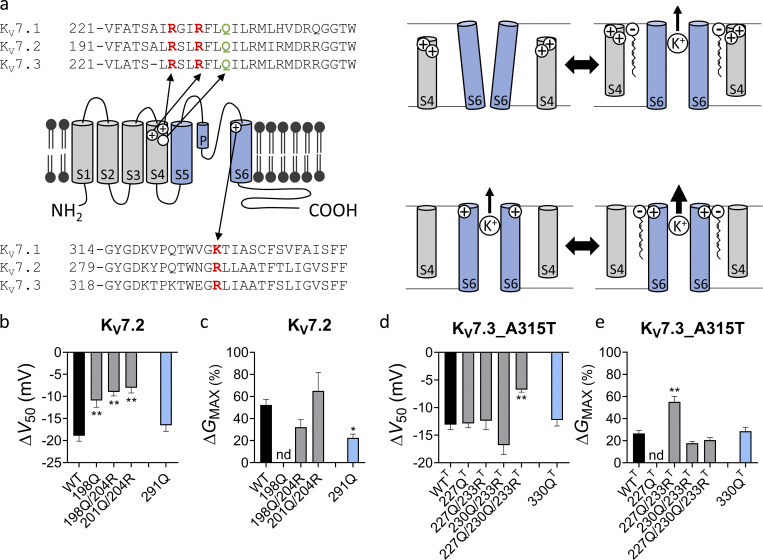
In previous work, we showed that polyunsaturated fatty acids and their analogues activate KV channels (Liin et al., 2015; Liin et al., 2016; Elinder and Liin, 2017). In hKV7.1, we have suggested that the activation is caused by two distinct interactions between polyunsaturated fatty acids and the channel (Liin et al., 2018): (1) interaction with the outermost arginines in S4 of the voltage-sensing domain facilitates outward S4 movement to shift V50 toward more negative voltages (illustrated in Fig. 4 a, top row), and (2) interaction with a lysine in S6 of the pore domain increases GMAX, possibly by stabilizing the conducting selectivity filter (illustrated in Fig. 4 a, bottom row). Both the negative charge of the fatty acid and the polyunsaturation of the lipid tail are important for these effects (Börjesson et al., 2008; Börjesson and Elinder, 2011; Liin et al., 2015; Bohannon et al., 2019). Because of the similarity in chemical properties of polyunsaturated fatty acids and endocannabinoids and their overall similar pattern of chemical properties required to activate KV7 channels, we tested whether the corresponding residues in hKV7.2 and hKV7.3 are important for the ARA-S effect. In hKV7.2, neutralization of either the first or second S4 arginine (R198 and R201, respectively) significantly reduced the ability of 10 µM ARA-S to shift V50 (Fig. 4 b and Fig. S5). Note that the Q204R mutation was introduced together with the R201Q mutation, as the R201Q single mutant is voltage insensitive (Miceli et al., 2008). Guided by studies in hKV7.1 (Panaghie and Abbott, 2007; Wu et al., 2010), we found that voltage sensitivity of the R201Q mutant was retained by introducing a compensatory positive charge at the Q204 position, which allowed probing of ARA-S effects on V50 of this mutant. The Q204R mutation per se did not appear to alter the ARA-S effect on V50 (compare effects on R198Q and R198Q/Q204R in Fig. 4 b). In contrast, neutralization of R291 in S6 (corresponding to the lysine in S6 of hKV7.1) significantly reduced the ability of 10 µM ARA-S to increase GMAX (Fig. 4 c and Fig. S5). In hKV7.3, neutralization of either the first or second S4 arginine (R227 and R230, respectively) alone did not alter the ARA-S effect on V50 (Fig. 4 d and Fig. S6). However, simultaneous neutralization of R227 and R230 significantly reduced the ability of 10 µM ARA-S to shift V50 (Fig. 4 d and Fig. S6). As with hKV7.2, a compensatory positive charge at the Q233 position was introduced to retain voltage sensitivity.
Figure 4.
Positively charged residues in S4 and S6 are important for ARA-S activation of hKV7.2 and hKV7.3. (a) Schematic side view of one hKV7 subunit. Transmembrane segments S1–S4, forming the voltage sensor domain, are in gray, and transmembrane segments S5 and S6, forming the pore domain, are in blue. P denotes the pore helix. Amino acid sequences for hKV7.1, hKV7.2, and hKV7.3 are shown above (S4) and below (S6) the channel. Residues mutated to study the ARA-S mechanism of action are indicated in the schematic channel model and sequences. These residues were selected based on previous work identifying residues important for polyunsaturated fatty acid effects on hKV7.1 (Liin et al., 2018). A similar mechanism of action was hypothesized for ARA-S. Top right: the negative charge of the ARA-S head interacts with the first and/or second top arginines of S4 (often called “R1” and “R2,” indicated in red in the S4 sequence) to facilitate the outward movement of S4. S4 movement triggers channel opening. Lower right: In addition, the negative charge of the ARA-S head interacts with the lysine/arginine in the top of S6 (indicated in red in the S6 sequence) to stabilize the selectivity filter in the pore, which increases the overall K+ conductance. Note that hKV7 channels have a glutamine in the natural spot for the third S4 arginine (“Q3,” indicated in green in the S4 sequence). For certain constructs, an arginine was introduced in the Q3 position to restore voltage sensitivity or shift the voltage sensitivity to more WT-like voltages. (b and c) Mean ΔV50 (b) and ΔGMAX (c) induced by 10 µM ARA-S on hKV7.2 with indicated amino acid substitutions. Data shown as mean ± SEM; n = 6–10. Statistics denote one-way ANOVA with Dunnett’s multiple comparison test with WT set as control. The Q204R mutation was introduced to retain voltage sensitivity (see main text for details). nd denotes not determined, as GMAX could not be reliably determined for the R198Q mutant. (d and e) Same as in b and c, but for hKV7.3_A315T with indicated amino acid substitutions (T denotes the substitution of A315T in the constructs). n = 4–9. Statistics denote one-way ANOVA with Dunnett’s multiple comparison test with WTT set as control. The Q233R mutation was introduced to retain voltage sensitivity (see main text for details). nd denotes not determined, as GMAX could not be reliably determined for the R227Q mutant. * indicates P < 0.05; ** indicates P < 0.001.
For hKV7.3, the effect of mutation on GMAX was less clear, with no significant reduction in the ability of 10 µM ARA-S to increase GMAX by the R330Q mutation (Fig. 4 e and Fig. S6). However, interpretation of alteration in the GMAX effect in hKV7.3 is possibly complicated by the A315T mutation introduced to enable studies of homotetrameric hKV7.3 channels. The A315T mutations have been proposed to increase the K+ current generated by hKV7.3 by stabilizing the conducting pore (Choveau et al., 2012a, 2012b), possibly together with increasing membrane expression (Gómez-Posada et al., 2010). Because the ARA-S effect on GMAX is expected to be dependent on the intrinsic open probability of the channel (Liin et al., 2018), possible varying intrinsic open probability of the hKV7.3 mutants will impact the ability of ARA-S to increase GMAX. Altogether, experiments on the set of mutants with neutralized positive residues at specific positions suggest that positively charged amino acids in S4 and S6 are important for the ability of ARA-S to shift V50 (in both hKV7.2 and hKV7.3) and increase GMAX (in hKV7.2), respectively.
ARA-S displays a different KV7 subtype selectivity than retigabine
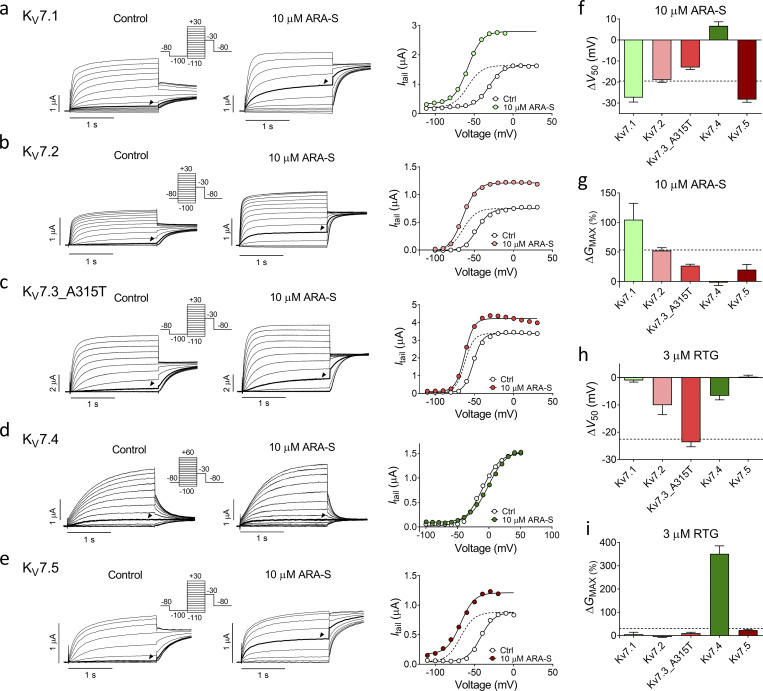
Retigabine was previously shown to activate all members within the hKV7 family except hKV7.1 (Tatulian et al., 2001; Schenzer et al., 2005). Because our results indicate that the activation of hKV7.2/3 induced by ARA-S and retigabine occurs via different mechanisms, ARA-S might show a different hKV7 subtype selectivity than retigabine. We tested the effect of ARA-S on each member of the hKV7 channel family expressed as homomeric channels in Xenopus oocytes. 10 µM ARA-S significantly activated hKV7.1, hKV7.2, hKV7.3_A315T, and hKV7.5 by shifting V50 toward more negative voltages and increasing GMAX (Fig. 5, a–c and e–g). In contrast, ARA-S had a slight inhibiting effect on hKV7.4. 10 µM ARA-S shifted V50 of hKV7.4 by +6.7 ± 2.0 mV (Fig. 5 dFig. 5, d and f; P < 0.009 with Student’s t test) but had no effect on GMAX (Fig. 5 g). Fig. 5, h and i shows the corresponding effects of 3 µM retigabine on V50 and GMAX, which are in overall concordance with previously reported effects for this retigabine concentration (Schenzer et al., 2005). Altogether, these experiments reveal that ARA-S has a different subtype selectivity in its effect on the hKV7.1 and hKV7.4 channels compared with retigabine.
Figure 5.
ARA-S activates all KV7 family members but KV7.4. (a–e) Representative current traces and corresponding G(V) curves for hKV7.1, hKV7.2, hKV7.3_A315T, hKV7.4, and hKV7.5 before and after application of 10 µM ARA-S. Arrowheads indicate an activating voltage step to −60 mV except for KV7.1 and KV7.4, for which the arrowheads indicate an activating voltage step to −50 mV and −30 mV, respectively. Dashed line in the G(V) curve shows the curve for 10 µM ARA-S normalized to GMAX of control. (f and g) Mean shift in V50 (f) and increase in GMAX (g) induced by 10 µM ARA-S. Data shown as mean ± SEM; n = 9–13. The effect of ARA-S on GMAX of hKV7.1 and hKV7.5 might be underestimated because of a tendency of decreasing tail currents in the presence of ARA-S at the most positive voltages (e.g., Fig. 5e), the reason for which remains unknown. (h and i) Same as in f and g, but for 3 µM retigabine (RTG). n = 4–5. Dashed lines in f–i denote effect of indicated treatment on hKV7.2/3.
Significant activating effects on hKV7.2/3 when coapplying low concentrations of ARA-S and retigabine
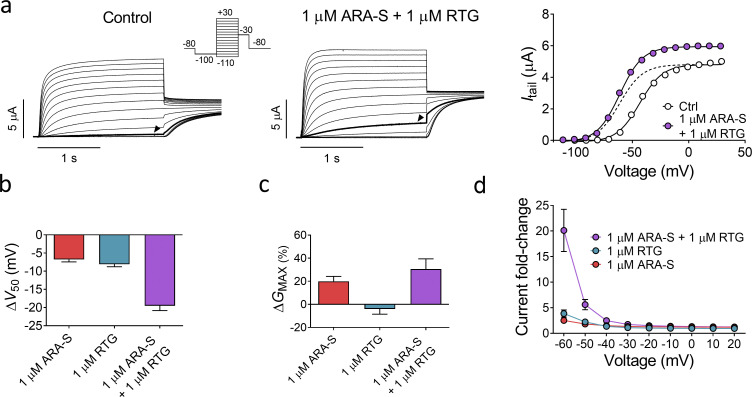
We hypothesized that one could leverage upon the similar effect of ARA-S and retigabine on the M-channel and the different effect on the other hKV7 subtypes by coapplying low concentrations of ARA-S and retigabine for retained M-channel effect with limited off-target effects. As a first step, we compared the effect of low concentrations of ARA-S or retigabine alone with both compounds in combination on hKV7.2/3. 1 µM ARA-S alone shifted V50 of hKV7.2/3 by −6.7 ± 0.7 mV and increased GMAX by 20% ± 4% (Fig. 6). 1 µM retigabine alone shifted V50 of hKV7.2/3 by −8.1 ± 0.7 mV and did not affect GMAX (Fig. 6). In contrast, coapplication of 1 µM ARA-S and 1 µM retigabine shifted V50 by −19.5 ± 1.3 mV and increased GMAX by 31% ± 9% (Fig. 6). The improved effect of coapplication of low concentrations of ARA-S and retigabine was clear when comparing the magnitude of hKV7.2/3 current fold increase at negative voltages. On average, coapplication of 1 µM ARA-S and 1 µM retigabine induced a 20-fold increase of the current at −60 mV, compared with a three- to fourfold increase for 1 µM of either of the compounds individually (Fig. 6 d). Notably, the 20-fold increase of the current at −60 mV induced by coapplication is almost three times the increase of the current induced by 10 µM ARA-S alone (compare with Fig. 1 h). Thus, the combined activating effect of low concentrations of ARA-S and retigabine substantially increased the current generated by hKV7.2/3 at voltages corresponding to the resting membrane potential of neurons.
Figure 6.
Coapplication of low concentrations of ARA-S and retigabine improves the activating effect on hKV7.2/3. (a) Representative current traces and corresponding G(V) curves for hKV7.2/3 before and after coapplication of 1 µM ARA-S and 1 µM retigabine (RTG). Arrowheads indicate an activating voltage step to −60 mV. Insert of used voltage clamp protocols. Dashed line in the G(V) curve shows the curve for coapplication of 1 µM ARA-S and 1 µM retigabine normalized to GMAX of control. (b and c) Mean shift in V50 (b) and increase in GMAX (c) of hKV7.2/3 induced by indicated treatment. Data shown as mean ± SEM; n = 7–10. (d) Mean hKV7.2/3 current fold increase at different voltages induced by indicated treatment. Data shown as mean ± SEM; n = 7–10.
Improved subtype selectivity when combining low concentrations of ARA-S and retigabine
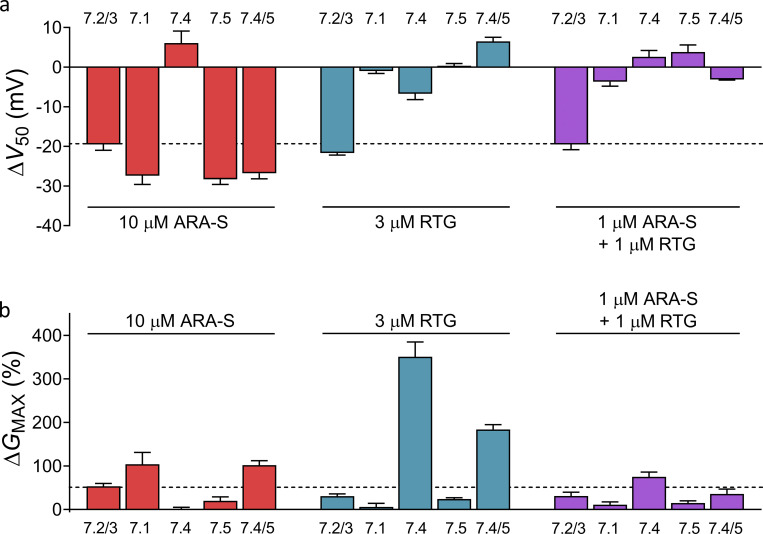
As a second step, we compared the off-target effect of coapplying ARA-S and retigabine on hKV7.1, hKV7.4, hKV7.5, and hKV7.4/5 channels with the effects of the compounds individually. hKV7.4/5 was included in this analysis because heterotetramers of hKV7.4 and hKV7.5 have been implicated in regulation of smooth muscle contraction (Brueggemann et al., 2014; Chadha et al., 2014; Provence et al., 2018). We compared the effect of either 10 µM ARA-S or 3 µM retigabine individually with that of coapplication of 1 µM ARA-S and 1 µM retigabine, as these three treatments all shifted V50 of the hKV7.2/3 channel by about −20 mV (Fig. 7 a). We will refer to these three treatments as ARA-Shigh, retigabinehigh, and coapplication. As was shown in Fig. 5, ARA-Shigh primarily induced off-target effects on hKV7.1 and hKV7.5 by shifting V50 by about −30 mV (Fig. 7 a). ARA-Shigh induced similar off-target effects on hKV7.4/5 by shifting V50 by about −25 mV (Fig. 7 a). Retigabinehigh, on the other hand, primarily induced off-target effects on hKV7.4 by increasing GMAX by 350% (Fig. 7 b and Fig. S7 a). Retigabinehigh also induced off-target effects on hKV7.4/5 by increasing GMAX by 180% (Fig. 7 b). In contrast, all off-target effects were reduced during coapplication: coapplication shifted V50 of hKV7.1, hKV7.5, and hKV7.4/5 by less than 4 mV (Fig. 7 a and Fig. S7 c) and reduced the increase in GMAX of hKV7.4 and hKV7.4/5 to 75% and 35%, respectively (Fig. 7 b and Fig. S7 b).
Figure 7.
Coapplication of low concentrations of ARA-S and retigabine limits the off-target effect on other hKV7 subtypes. (a) Mean shift in V50 induced by 10 µM ARA-S, 3 µM retigabine (RTG), or 1 µM ARA-S + 1 µM retigabine coapplied on indicated channels. Data shown as mean ± SEM; n = 4–13. Dashed line denotes a shift in V50 of −20 mV, which was the approximate effect of each treatment on hKV7.2/3. (b) Mean increase in GMAX induced by 10 µM ARA-S, 3 µM retigabine, or 1 µM ARA-S + 1 µM retigabine coapplied on indicated channels. Data shown as mean ± SEM; n = 4–13. Dashed line denotes an increase in GMAX of 50%, which was the approximate effect of 10 µM ARA-S on hKV7.2/3.
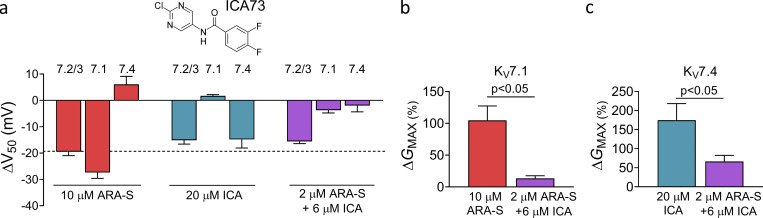
We also tested the concept of coapplication using ICA73 (structure in Fig. S8 a). Although 20 µM of ICA73 alone (referred to as ICA73high) activated hKV7.2/3 by shifting V50 by −15 mV (Fig. S8 a), ICA73high also induced off-target effects on hKV7.4 by shifting V50 by −15 mV and increasing GMAX by 175% (Fig. S8, a and c). Like ICA73high, coapplication of 2 µM ARA-S and 6 µM ICA73 shifted V50 of hKV7.2/3 by about −15 mV (Fig. S8 a). However, coapplication did not shift V50 of hKV7.4 (P > 0.05 with Student’s t test) and reduced the increase in GMAX of hKV7.4 to 66% (Fig. S8, a and c). Altogether, these experiments demonstrate that ARA-S can be combined with either retigabine or ICA73 to preserve the activating effect on the hKV7.2/3 channel and limit off-target effects on other hKV7 subtypes.
Discussion
In this study, we make three important observations. First, we uncover that specific endogenous compounds belonging to the endocannabinoid family activate the hKV7.2/3 channel expressed in Xenopus oocytes. ARA-S in particular was identified as a potent hKV7.2/3 activator, which activated the channel by both shifting the voltage dependence of channel opening toward negative voltages and increasing the maximal conductance. Second, we found that ARA-S activates hKV7.2/3 through a different mechanism than retigabine and that these two compounds differ in their hKV7 subtype selectivity. The activating effect of retigabine on hKV7.2/3 has been extensively studied. Retigabine activates hKV7.2/3 by binding to an internal site in the pore domain, in which a tryptophan is critical for binding, to stabilize the open channel (Schenzer et al., 2005; Wuttke et al., 2005; Kim et al., 2015). For endocannabinoids, we here describe that conserved positively charged residues in S4 and S6, distinct from the retigabine site but corresponding to those previously shown to be important for polyunsaturated fatty acid effects in hKV7.1 (Liin et al., 2018), are important for ARA-S–induced activation. We also describe that the negative charge of the endocannabinoid head is critical for the effect on hKV7.2/3, which could explain why 2-AG, AEA, and NADA are ineffective, and that a saturated tail appears to eliminate the effect. Given the corresponding important channel residues identified and the shared chemical properties of polyunsaturated fatty acids and endocannabinoids, we find it likely that endocannabinoids act through a similar mechanism to that previously described for polyunsaturated fatty acids (Liin et al., 2018). Thus, we hypothesize that the negative charge of the endocannabinoid head group is required to enable electrostatic interaction with S4 arginines (to facilitate outward S4 movement) and with the S6 arginine (to induce structural rearrangements in the pore that enhance potassium conductance; Liin et al., 2018). The flexibility of a polyunsaturated tail to adopt bent conformations has been reported to allow intimate and high-affinity interaction of polyunsaturated fatty acids with other voltage-gated ion channels (Tian et al., 2016; Yazdi et al., 2016). Possibly, the more rigid nature of monounsaturated and saturated endocannabinoid tails limits bent conformations needed for interaction with hKV7.2/3. Future studies are needed to further resolve molecular details for endocannabinoid interactions with hKV7.2/3. We note that the electrostatic nature of the proposed interaction between the negative endocannabinoid head group and positive residues in the channel is reminiscent of the interaction between PIP2 (phosphatidylinositol 4,5-bisphosphate) and KV7 channels (Zaydman and Cui, 2014; Taylor and Sanders, 2017). However, whereas PIP2 interacts with positively charged residues in the inner half of the channel (because PIP2 is localized to the inner leaflet of the plasma membrane), we propose that endocannabinoids primarily act on residues in the outer half of the channel. Third, we demonstrate how to leverage upon the different hKV7 subtype selectivities through coapplication of ARA-S and retigabine for efficient activation of hKV7.2/3 while limiting the off-target effect on hKV7.1, hKV7.4, hKV7.5, and hKV7.4/5. Beneficial effects of coapplication were also achieved by combining ARA-S and ICA73.
Can coapplication of low doses of two compounds targeting the M-channel be a viable approach to reach adequate anti-convulsant effect while reducing adverse effects usually caused by higher doses of these compounds? Manville and Abbott (2018) lend support to efficient anti-convulsant effect in an animal epilepsy model of coadministration of multiple compounds targeting the M-channel. Our experiments on hKV7 channels expressed in Xenopus oocytes show that combined application of low concentrations of ARA-S and retigabine effectively activates the M-channel and that strategic combination of M-channel activators with different subtype selectivity limits off-target effects on other hKV7 subtypes. Developing new anticonvulsants is a costly and exhaustive process. Withdrawal of a drug that has passed critical safety testing is a major setback. Our strategy of targeting the M-channel with dual compounds acting through a distinct mechanism, here tested in Xenopus oocytes, might offer ways for promising drugs with some degree of off-target effect to be tolerated clinically. Therefore, we encourage further exploration of this concept in more complex experimental systems. Such studies need to address important questions, such as which M-channel activators should ideally be combined (considering critical aspects such as ion channel selectivity, apparent affinity, and efficacy) and the balance between anti-excitable effects in neurons and possible off-target effects in other cell types. Clinical challenges with combined treatment using two or more drugs include possible varying pharmacodynamic profiles of the drugs used and possible drug interaction. However, as ∼20% of patients suffering from epilepsy are treated using two or more drugs (Brodie et al., 2012), these are challenges already faced by current treatment strategies.
We note that coapplication of 1 µM ARA-S and 1 µM retigabine induced hKV7.2/3 effects that were slightly larger than expected from additive effects (Fig. 6, b–d). In particular, the increase of current amplitude at −60 mV was unexpectedly large upon coapplication (Fig. 6 d). Manville and Abbott (2018) described synergistic effects on hKV7.2/3 upon coapplication of retigabine and herbal extracts. They proposed that the ability of such compounds to simultaneously occupy a shared binding pocket in combination with prominent effects on different subunits in the hKV7.2/3 channel (hKV7.3 for retigabine and hKV7.2 for herbal components) underlies synergistic effects (Manville and Abbott, 2018). We observed larger ARA-S effects on hKV7.2 than on hKV7.3. However, because ARA-S and retigabine use different binding sites, it is not clear if different preference for different hKV7.2/3 subunits contributed to the apparent synergy in ARA-S and retigabine effects. Alternatively, presence of one of the compounds may facilitate action of the other compound (e.g., by improving affinity or efficacy). However, at this point, we are careful with interpretation of additive versus synergistic effects in our data, as the most prominent indication of synergy was observed for current amplitudes at −60 mV, which is at the foot of the G(V) curve, meaning that small variations in V50 between cells may impact the magnitude of current increase. Further characterization of additive/synergistic effects and possible underlying mechanisms is worth exploring in future studies.
Because ARA-S is an endogenous compound, this raises several questions. For example, does ARA-S have physiological or pathophysiological effects in the body at the concentrations used in this study, and could these effects be tuned by regulating the concentration of ARA-S? Although all five arachidonic acid–based endocannabinoids studied in this work have been detected in neuronal tissue from mammals (Devane et al., 1992; Kondo et al., 1998; Bisogno et al., 2000; Huang et al., 2001; Milman et al., 2006), the understanding of the endogenous functions of some of them is less clear. The endocannabinoids 2-AG and AEA have been shown to signal noncanonically through other pathways than cannabinoid receptors (Bondarenko et al., 2017; Gantz and Bean, 2017). ARA-S has been isolated from bovine brain (Milman et al., 2006), but it is not known if ARA-S is present in human tissue and, if so, at what concentrations. This makes it difficult to evaluate whether ARA-S might have physiological or pathophysiological functions in the human body. Future studies will hopefully shed light on whether ARA-S endogenously reaches concentrations triggering noncanonical signaling through interaction with ion channels such as hKV7.2/3, the BK channel (Godlewski et al., 2009), and the N-type CaV channel (Guo et al., 2008).
To conclude, this study shows that specific members within the endocannabinoid family, most notably ARA-S, target and activate the neuronal M-channel. The pronounced activation of the M-channel by low concentrations of ARA-S highlights ARA-S as an interesting model compound for future development of new treatment strategies for epilepsy targeting the M-channel. The successful combination of ARA-S and retigabine (as well as ARA-S and ICA73) to achieve M-channel activation with improved hKV7 subtype selectivity allows for possibilities to use such ARA-S mimetic compounds either alone or in conjunction with other M-channel activators. Future studies are required to study the concept of combined treatment in more complex systems.
Supplementary Material
summarizes the biophysical properties of used constructs.
Acknowledgments
Joseph A. Mindell served as editor.
We thank Dr. H. Peter Larsson at the University of Miami and Dr. Fredrik Elinder at Linköping University for valuable comments on the manuscript. We thank Dr. Peter Konradsson at Linköping University for valuable discussion regarding synthesis strategies. The clones for human KV7.1–7.5 were kind gifts from Dr. Nicole Schmitt at the University of Copenhagen, Copenhagen, Denmark.
This work was supported by the Swedish Society for Medical Research and the Swedish Research Council (2017-02040).
A patent application (#62/032,739) including a description of the interaction of charged lipophilic compounds with the KV7.1 channel has been submitted by the University of Miami, with S.I. Liin identified as one of the inventors.
Author contributions: J.E. Larsson: concept and design, acquisition of data, analysis and interpretation of data, and drafting and revising of the article. U. Karlsson: concept and design, interpretation of data, and drafting and revising the article. X. Wu: contributing new compounds and drafting and revising the article. S.I. Liin: concept and design, acquisition of data, analysis and interpretation of data, and drafting and revising the article.
References
- Abdel-Magid A.F. 2015. Allosteric modulators: an emerging concept in drug discovery. ACS Med. Chem. Lett. 6:104–107. 10.1021/ml5005365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T., Melck D., Bobrov MYu N.M., Gretskaya N.M., Bezuglov V.V., De Petrocellis L., and Di Marzo V.. 2000. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 351:817–824. 10.1042/bj3510817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon B.M., Perez M.E., Liin S.I., and Larsson H.P.. 2019. ω-6 and ω-9 polyunsaturated fatty acids with double bonds near the carboxyl head have the highest affinity and largest effects on the cardiac IKs potassium channel. Acta Physiol. (Oxf.). 225 e13186 10.1111/apha.13186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon B.M., Wu X., Wu X., Perez M.E., Liin S.I., and Larsson H.P.. 2020. Polyunsaturated fatty acids produce a range of activators for heterogeneous IKs channel dysfunction. J. Gen. Physiol. 152 e201912396 10.1085/jgp.201912396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen I.N., and Hansen H.S.. 2003. Binding of anandamide to bovine serum albumin. J. Lipid Res. 44:1790–1794. 10.1194/jlr.M300170-JLR200 [DOI] [PubMed] [Google Scholar]
- Bondarenko A.I., Panasiuk O., Okhai I., Montecucco F., Brandt K.J., and Mach F.. 2017. Direct activation of Ca2+ and voltage-gated potassium channels of large conductance by anandamide in endothelial cells does not support the presence of endothelial atypical cannabinoid receptor. Eur. J. Pharmacol. 805:14–24. 10.1016/j.ejphar.2017.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börjesson S.I., and Elinder F.. 2011. An electrostatic potassium channel opener targeting the final voltage sensor transition. J. Gen. Physiol. 137:563–577. 10.1085/jgp.201110599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börjesson S.I., Hammarström S., and Elinder F.. 2008. Lipoelectric modification of ion channel voltage gating by polyunsaturated fatty acids. Biophys. J. 95:2242–2253. 10.1529/biophysj.108.130757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie M.J., Lerche H., Gil-Nagel A., Elger C., Hall S., Shin P., Nohria V., and Mansbach H.; RESTORE 2 Study Group . 2010. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 75:1817–1824. 10.1212/WNL.0b013e3181fd6170 [DOI] [PubMed] [Google Scholar]
- Brodie M.J., Barry S.J., Bamagous G.A., Norrie J.D., and Kwan P.. 2012. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 78:1548–1554. 10.1212/WNL.0b013e3182563b19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann L.I., Mackie A.R., Cribbs L.L., Freda J., Tripathi A., Majetschak M., and Byron K.L.. 2014. Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J. Biol. Chem. 289:2099–2111. 10.1074/jbc.M113.527820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha P.S., Jepps T.A., Carr G., Stott J.B., Zhu H.L., Cole W.C., and Greenwood I.A.. 2014. Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler. Thromb. Vasc. Biol. 34:887–893. 10.1161/ATVBAHA.114.303405 [DOI] [PubMed] [Google Scholar]
- Changeux J.P., and Christopoulos A.. 2016. Allosteric Modulation as a Unifying Mechanism for Receptor Function and Regulation. Cell. 166:1084–1102. 10.1016/j.cell.2016.08.015 [DOI] [PubMed] [Google Scholar]
- Choveau F.S., Bierbower S.M., and Shapiro M.S.. 2012a Pore helix-S6 interactions are critical in governing current amplitudes of KCNQ3 K+ channels. Biophys. J. 102:2499–2509. 10.1016/j.bpj.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choveau F.S., Hernandez C.C., Bierbower S.M., and Shapiro M.S.. 2012b Pore determinants of KCNQ3 K+ current expression. Biophys. J. 102:2489–2498. 10.1016/j.bpj.2012.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., and Mechoulam R.. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 258:1946–1949. 10.1126/science.1470919 [DOI] [PubMed] [Google Scholar]
- Di Marzo V., Bifulco M., and De Petrocellis L.. 2004. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 3:771–784. 10.1038/nrd1495 [DOI] [PubMed] [Google Scholar]
- Elinder F., and Liin S.I.. 2017. Actions and Mechanisms of Polyunsaturated Fatty Acids on Voltage-Gated Ion Channels. Front. Physiol. 8:43 10.3389/fphys.2017.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D.J., and Conn P.J.. 2017. Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron. 94:431–446. 10.1016/j.neuron.2017.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J.A., Abou-Khalil B.W., Leroy R.F., Yacubian E.M., Shin P., Hall S., Mansbach H., and Nohria V.; RESTORE 1/Study 301 Investigators . 2011. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 76:1555–1563. 10.1212/WNL.0b013e3182194bd3 [DOI] [PubMed] [Google Scholar]
- Gantz S.C., and Bean B.P.. 2017. Cell-Autonomous Excitation of Midbrain Dopamine Neurons by Endocannabinoid-Dependent Lipid Signaling. Neuron. 93:1375–1387.e2. 10.1016/j.neuron.2017.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin Shkolnik T., Feuerman H., Didkovsky E., Kaplan I., Bergman R., Pavlovsky L., and Hodak E.. 2014. Blue-gray mucocutaneous discoloration: a new adverse effect of ezogabine. JAMA Dermatol. 150:984–989. 10.1001/jamadermatol.2013.8895 [DOI] [PubMed] [Google Scholar]
- Godlewski G., Offertáler L., Osei-Hyiaman D., Mo F.M., Harvey-White J., Liu J., Davis M.I., Zhang L., Razdan R.K., Milman G., et al. 2009. The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J. Pharmacol. Exp. Ther. 328:351–361. 10.1124/jpet.108.144717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Posada J.C., Etxeberría A., Roura-Ferrer M., Areso P., Masin M., Murrell-Lagnado R.D., and Villarroel A.. 2010. A pore residue of the KCNQ3 potassium M-channel subunit controls surface expression. J. Neurosci. 30:9316–9323. 10.1523/JNEUROSCI.0851-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseclose M.R., and Castellino S.. 2019. An Investigation into Retigabine (Ezogabine) Associated Dyspigmentation in Rat Eyes by MALDI Imaging Mass Spectrometry. Chem. Res. Toxicol. 32:294–303. 10.1021/acs.chemrestox.8b00313 [DOI] [PubMed] [Google Scholar]
- Gunthorpe M.J., Large C.H., and Sankar R.. 2012. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 53:412–424. 10.1111/j.1528-1167.2011.03365.x [DOI] [PubMed] [Google Scholar]
- Guo J., Williams D.J., and Ikeda S.R.. 2008. N-arachidonoyl L-serine, a putative endocannabinoid, alters the activation of N-type Ca2+ channels in sympathetic neurons. J. Neurophysiol. 100:1147–1151. 10.1152/jn.01204.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel R., Schupke H., McNeilly P.J., Heinecke K., Kronbach C., Grunwald C., Zimmermann G., Griesinger C., Engel J., and Kronbach T.. 1999. Metabolism of retigabine (D-23129), a novel anticonvulsant. Drug Metab. Dispos. 27:613–622. [PubMed] [Google Scholar]
- Holt J.R., Stauffer E.A., Abraham D., and Géléoc G.S.. 2007. Dominant-negative inhibition of M-like potassium conductances in hair cells of the mouse inner ear. J. Neurosci. 27:8940–8951. 10.1523/JNEUROSCI.2085-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.M., Bisogno T., Petros T.J., Chang S.Y., Zavitsanos P.A., Zipkin R.E., Sivakumar R., Coop A., Maeda D.Y., De Petrocellis L., et al. 2001. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J. Biol. Chem. 276:42639–42644. 10.1074/jbc.M107351200 [DOI] [PubMed] [Google Scholar]
- Kim R.Y., Yau M.C., Galpin J.D., Seebohm G., Ahern C.A., Pless S.A., and Kurata H.T.. 2015. Atomic basis for therapeutic activation of neuronal potassium channels. Nat. Commun. 6:8116 10.1038/ncomms9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Kondo H., Nakane S., Kodaka T., Tokumura A., Waku K., and Sugiura T.. 1998. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor agonist: identification as one of the major species of monoacylglycerols in various rat tissues, and evidence for its generation through CA2+-dependent and -independent mechanisms. FEBS Lett. 429:152–156. 10.1016/S0014-5793(98)00581-X [DOI] [PubMed] [Google Scholar]
- Kumar M., Reed N., Liu R., Aizenman E., Wipf P., and Tzounopoulos T.. 2016. Synthesis and Evaluation of Potent KCNQ2/3-Specific Channel Activators. Mol. Pharmacol. 89:667–677. 10.1124/mol.115.103200 [DOI] [PubMed] [Google Scholar]
- Liin S.I., Silverå Ejneby M., Barro-Soria R., Skarsfeldt M.A., Larsson J.E., Starck Härlin F., Parkkari T., Bentzen B.H., Schmitt N., Larsson H.P., et al. 2015. Polyunsaturated fatty acid analogs act antiarrhythmically on the cardiac IKs channel. Proc. Natl. Acad. Sci. USA. 112:5714–5719. 10.1073/pnas.1503488112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liin S.I., Karlsson U., Bentzen B.H., Schmitt N., and Elinder F.. 2016. Polyunsaturated fatty acids are potent openers of human M-channels expressed in Xenopus laevis oocytes. Acta Physiol. (Oxf.). 218:28–37. [DOI] [PubMed] [Google Scholar]
- Liin S.I., Yazdi S., Ramentol R., Barro-Soria R., and Larsson H.P.. 2018. Mechanisms Underlying the Dual Effect of Polyunsaturated Fatty Acid Analogs on Kv7.1. Cell Rep. 24:2908–2918. 10.1016/j.celrep.2018.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łuszczki J.J. 2009. Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions. Pharmacol. Rep. 61:197–216. 10.1016/S1734-1140(09)70024-6 [DOI] [PubMed] [Google Scholar]
- Main M.J., Cryan J.E., Dupere J.R., Cox B., Clare J.J., and Burbidge S.A.. 2000. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol. Pharmacol. 58:253–262. 10.1124/mol.58.2.253 [DOI] [PubMed] [Google Scholar]
- Manville R.W., and Abbott G.W.. 2018. Ancient and modern anticonvulsants act synergistically in a KCNQ potassium channel binding pocket. Nat. Commun. 9:3845 10.1038/s41467-018-06339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R., et al. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50:83–90. 10.1016/0006-2952(95)00109-D [DOI] [PubMed] [Google Scholar]
- Miceli F., Soldovieri M.V., Hernandez C.C., Shapiro M.S., Annunziato L., and Taglialatela M.. 2008. Gating consequences of charge neutralization of arginine residues in the S4 segment of K(v)7.2, an epilepsy-linked K+ channel subunit. Biophys. J. 95:2254–2264. 10.1529/biophysj.107.128371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G., Maor Y., Abu-Lafi S., Horowitz M., Gallily R., Batkai S., Mo F.M., Offertaler L., Pacher P., Kunos G., et al. 2006. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc. Natl. Acad. Sci. USA. 103:2428–2433. 10.1073/pnas.0510676103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaghie G., and Abbott G.W.. 2007. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J. Gen. Physiol. 129:121–133. 10.1085/jgp.200609612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provence A., Angoli D., and Petkov G.V.. 2018. KV7 Channel Pharmacological Activation by the Novel Activator ML213: Role for Heteromeric KV7.4/KV7.5 Channels in Guinea Pig Detrusor Smooth Muscle Function. J. Pharmacol. Exp. Ther. 364:131–144. 10.1124/jpet.117.243162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. 2001. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol. Ther. 90:1–19. 10.1016/S0163-7258(01)00116-4 [DOI] [PubMed] [Google Scholar]
- Rode F., Svalø J., Sheykhzade M., and Rønn L.C.. 2010. Functional effects of the KCNQ modulators retigabine and XE991 in the rat urinary bladder. Eur. J. Pharmacol. 638:121–127. 10.1016/j.ejphar.2010.03.050 [DOI] [PubMed] [Google Scholar]
- Rostock A., Tober C., Rundfeldt C., Bartsch R., Engel J., Polymeropoulos E.E., Kutscher B., Löscher W., Hönack D., White H.S., et al. 1996. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 23:211–223. 10.1016/0920-1211(95)00101-8 [DOI] [PubMed] [Google Scholar]
- Rundfeldt C. 1997. The new anticonvulsant retigabine (D-23129) acts as an opener of K+ channels in neuronal cells. Eur. J. Pharmacol. 336:243–249. 10.1016/S0014-2999(97)01249-1 [DOI] [PubMed] [Google Scholar]
- Schenzer A., Friedrich T., Pusch M., Saftig P., Jentsch T.J., Grötzinger J., and Schwake M.. 2005. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J. Neurosci. 25:5051–5060. 10.1523/JNEUROSCI.0128-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M., Mistry M., Marsh S.J., Brown D.A., and Delmas P.. 2002. Molecular correlates of the M-current in cultured rat hippocampal neurons. J. Physiol. 544:29–37. 10.1113/jphysiol.2002.028571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverå Ejneby M., Wu X., Ottosson N.E., Münger E.P., Lundström I., Konradsson P., and Elinder F.. 2018. Atom-by-atom tuning of the electrostatic potassium-channel modulator dehydroabietic acid. J. Gen. Physiol. 150:731–750. 10.1085/jgp.201711965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter M.Y. 2012. Ezogabine (retigabine) and its role in the treatment of partial-onset seizures: a review. Clin. Ther. 34:1845–56.e1. 10.1016/j.clinthera.2012.07.009 [DOI] [PubMed] [Google Scholar]
- Tatulian L., Delmas P., Abogadie F.C., and Brown D.A.. 2001. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J. Neurosci. 21:5535–5545. 10.1523/JNEUROSCI.21-15-05535.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K.C., and Sanders C.R.. 2017. Regulation of KCNQ/Kv7 family voltage-gated K+ channels by lipids. Biochim. Biophys. Acta Biomembr. 1859:586–597. 10.1016/j.bbamem.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Aursnes M., Hansen T.V., Tungen J.E., Galpin J.D., Leisle L., Ahern C.A., Xu R., Heinemann S.H., and Hoshi T.. 2016. Atomic determinants of BK channel activation by polyunsaturated fatty acids. Proc. Natl. Acad. Sci. USA. 113:13905–13910. 10.1073/pnas.1615562113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykocki N.R., Heppner T.J., Dalsgaard T., Bonev A.D., and Nelson M.T.. 2019. The KV 7 channel activator retigabine suppresses mouse urinary bladder afferent nerve activity without affecting detrusor smooth muscle K+ channel currents. J. Physiol. 597:935–950. 10.1113/JP277021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.S., Pan Z., Shi W., Brown B.S., Wymore R.S., Cohen I.S., Dixon J.E., and McKinnon D.. 1998. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 282:1890–1893. 10.1126/science.282.5395.1890 [DOI] [PubMed] [Google Scholar]
- Wang A.W., Yang R., and Kurata H.T.. 2017. Sequence determinants of subtype-specific actions of KCNQ channel openers. J. Physiol. 595:663–676. 10.1113/JP272762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.K., Lamothe S.M., Wang A.W., Yang R.Y., and Kurata H.T.. 2018. Pore- and voltage sensor-targeted KCNQ openers have distinct state-dependent actions. J. Gen. Physiol. 150:1722–1734. 10.1085/jgp.201812070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Pan H., Delaloye K., and Cui J.. 2010. KCNE1 remodels the voltage sensor of Kv7.1 to modulate channel function. Biophys. J. 99:3599–3608. 10.1016/j.bpj.2010.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuttke T.V., Seebohm G., Bail S., Maljevic S., and Lerche H.. 2005. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to its activation gate. Mol. Pharmacol. 67:1009–1017. 10.1124/mol.104.010793 [DOI] [PubMed] [Google Scholar]
- Xu T., Nie L., Zhang Y., Mo J., Feng W., Wei D., Petrov E., Calisto L.E., Kachar B., Beisel K.W., et al. 2007. Roles of alternative splicing in the functional properties of inner ear-specific KCNQ4 channels. J. Biol. Chem. 282:23899–23909. 10.1074/jbc.M702108200 [DOI] [PubMed] [Google Scholar]
- Yazdi S., Stein M., Elinder F., Andersson M., and Lindahl E.. 2016. The Molecular Basis of Polyunsaturated Fatty Acid Interactions with the Shaker Voltage-Gated Potassium Channel. PLOS Comput. Biol. 12 e1004704 10.1371/journal.pcbi.1004704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaydman M.A., and Cui J.. 2014. PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front. Physiol. 5:195 10.3389/fphys.2014.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yang D., and Hughes B.A.. 2011. KCNQ5/K(v)7.5 potassium channel expression and subcellular localization in primate retinal pigment epithelium and neural retina. Am. J. Physiol. Cell Physiol. 301:C1017–C1026. 10.1152/ajpcell.00185.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
summarizes the biophysical properties of used constructs.