Abstract
Background
Dopamine replacement medication has positive effects on existing motor skills for people with Parkinson disease (PD), but may have detrimental effects on the learning of motor skills necessary for effective rehabilitation according to the dopamine overdose hypothesis.
Objectives
This study aimed to determine whether dopamine replacement medication (i.e. levodopa) affects: learning of a novel upper extremity task, decrements in skill following withdrawal of practice, the rate of learning, and the transfer of movement skill to untrained upper extremity tasks compared to training “off” medication, in people with PD.
Methods
Participants with mild-moderate PD (Hoehn and Yahr stage 2) were randomized to train “on” (n=12) or “off” (n=11) levodopa medication. Participants practiced 10 blocks of five trials of a functional motor task with their non-dominant upper extremity over three consecutive days (acquisition period), followed by a single block of five trials two and nine days later. Participants were also assessed “on” levodopa with two transfer tasks (the nine-hole peg test and a functional dexterity task) prior to any practice and nine days after the end of the acquisition period.
Results
Participants who practiced “on” levodopa medication learned the upper extremity task to a greater extent that those who practiced “off” medication, as determined by retained performance two days after practice. Skill decrement and skill transfer were not significantly different between groups. Rate of learning was unable to be modelled in this sample.
Conclusions
Levodopa medication improved the learning of an upper extremity task in people with mild-moderate PD.
Keywords: Parkinson disease, motor learning, upper extremity, levodopa medication, transfer, rehabilitation
1. Introduction
Parkinson disease (PD)1 is associated with symptomatic declines in motor function due to loss of dopaminergic neurons within the basal ganglia [1]. Most people with PD report difficulties performing upper extremity activities that interfere with activities of daily living (e.g., writing and dressing) [2]. There is, however, little research evaluating the effect of upper extremity rehabilitation for people with PD. Two recent large randomized controlled trials (RCTs) have investigated the effect of functional upper limb training involving handwriting [3] and hand dexterity [4] training for 4–6 weeks in people with PD while “on” medication. While both studies showed improvements in upper extremity task performance following intervention compared to a control group, performance following a period of no training (i.e. retention) were mixed [3, 4], which may be due to difficulties with motor learning in this population [5, 6].
Motor learning is most commonly defined as a relatively permanent change in motor performance due to experience/practice [7]. Key features of the acquisition phase include how quickly and how much motor performance changes, as well as how much performance at retention is different from the start or end of practice. Studies of motor learning in neurologically-intact healthy participants and individuals with PD generally show that people with PD learn new motor tasks more slowly and to a lesser extent than healthy adults [5, 8]. A recent meta-analysis demonstrated that movement time improved in both people with and without PD following practice of reaching tasks (one to 10 acquisition sessions, depending on the study), though learning effects were smaller in people with PD compared to people without PD, particularly at retention [8]. One possible mechanism relates to the neural circuitry involved in the early stages of motor learning, namely cortico-striatal and cortico-cerebellar pathways [9, 10]. It is speculated that people with PD partially compensate for lack of cortico-striatal activation with excessive cortico-cerebellar activation during this stage of learning [11]. Thus, the notable deficits in retaining newly learned motor skills in people with PD following a period of no practice arises from the cortico-striatal deficits in people with PD, as the striatum is responsible for the consolidation of learned tasks during late stages of motor learning [6, 10, 12]. Further, reductions in long term potentiation of the primary motor cortex may also limit the ability of people with PD to form new motor memories and fully automate newly learned motor tasks [6, 13].
In addition to variations in learning based on the task type and practice features, medications used to treat PD may also influence motor learning. Ironically, exogenous dopamine replacement medication (i.e., levodopa) may have positive effects on existing motor skills but has been theorized to have potentially detrimental effects on (re)learning motor skills in the context of rehabilitation. Studies that have suggested that learning deficits are larger in persons with PD when they learned tasks “on” levodopa [14, 15] compared to “off” form the basis for what has been termed “the dopamine overdose hypothesis”.
Examination of the pattern of striatal degradation may provide insight into the physiologic and anatomic basis for the dopamine overdose hypothesis. PD-associated degradation of the striatum appears to follow a sequential pattern with the dorsal and lateral (sensorimotor) portions of the striatum degenerating early in the disease, presumably when patients are yet undiagnosed or at Hoehn and Yahr stages 1–2 [16], while the ventral (associative) region is relatively spared. Paradoxically, the pattern of recruitment of striatal structures for motor sequence learning proceeds in a ventral to dorsal direction [12, 17], suggesting that any dopamine overdose will most likely affect the early stages of acquisition. Although dopamine replacement medications are prescribed to replace lost dopamine in the earlier degenerating sensorimotor areas of the striatum, these may actually be “overdosing” the relatively spared associative striatum, a neuroanatomical correlate for motor learning [18, 19], particularly during early disease stages [14]. While this overdose hypothesis has been tested in specific motor learning paradigms such as reversal learning and motor sequence learning, its relevance to functional tasks performed in rehabilitation is not clear [8]. This is due in part due to the fact that few motor learning studies have utilized functional tasks, or evaluated motor learning with clinically relevant delays until retention testing following practice (i.e. 1 week).
The primary aim of this pilot randomized controlled trial (RCT) was to determine whether dopamine replacement medication affected learning of a functional upper extremity task. Given that motor learning is typically defined as a relatively permanent change in performance due to experience [7], task performance was re-evaluated following a two-day retention period after acquisition, compared to baseline task performance. Secondary aims were to determine whether dopamine replacement medication affected i) decrements in acquired skill two and nine days after acquisition, relative to task performance at the end of practice; ii) rate of skill acquisition during practice; and iii) transfer of acquired skill to untrained upper extremity tasks. Based on the dopamine overdose hypothesis [18], we hypothesized that skill learning, skill decrement, rate of skill acquisition, and skill transfer would be worse in individuals with PD who practiced the upper extremity task “on” levodopa compared to those who practiced “off”.
2. Material and methods
2.1. Design
A pilot RCT involving people with PD practicing an upper extremity functional motor task [20] was undertaken during January to December 2016. The study was approved by the University of Utah Institutional Review Board and participants gave written informed consent prior to study enrollment. This trial was prospectively registered on ClinicalTrials.gov (NCT02600858) and adhered to CONSORT guidelines.
A computer-generated randomization schedule stratified according to each participant’s Hoehn and Yahr disease stage (threshold of 2) was generated prior to recruitment. Immediately following pretest assessment), the project manager randomly allocated participants to practice the functional motor task “on” or “off” levodopa according to the randomization schedule. Outcomes were assessed and intervention delivered at the University of Utah or in participants’ homes.
2.2. Participants
Participants were recruited via advertisements posted at a Movement Disorders Center, local PD support groups and through a PD wellness exercise group in Salt Lake City, USA. Individuals were included if they had idiopathic PD diagnosed by a neurologist, were aged 50–80 years, in Hoehn and Yahr stages 1–3, and had been on a stable antiparkinsonian medication regime for one month prior to pretest assessment as well as throughout the study. Individuals were excluded if they were not taking levodopa medication, or had prior deep brain stimulation, a Montreal Cognitive Assessment score <18 [21], or any health conditions that would interfere with safe participation.
To determine participants’ responsiveness to levodopa, PD severity as determined by the motor section of the Movement Disorders Society revised version of the Unified Parkinson Disease Rating Scale (MDS-UPDRS) [22] was scored at pretest “off” medication following overnight withdrawal of levodopa, then subsequently “on” medication 30–60 minutes after participants had taken their usual morning dose of levodopa. Participants continued to take other antiparkinsonian medications as usual, e.g. dopamine agonists. This was by design, given the multiple days of practice and the longer half-life of dopamine agonist medications, to minimize participant burden. As participants were not withdrawn from all antiparkinsonian medication, a difference that exceeded the standard error of measurement of the MDS-UPDRS motor section was taken as being responsive to levodopa for the purposes of study inclusion [23].
2.3. Intervention
The “off” medication group practiced the functional motor task in the morning following overnight withdrawal of levodopa. The “on” medication group practiced the task while their antiparkinsonian medications were working optimally, usually 60 minutes following ingestion of their morning dose. Both groups practiced the motor task over three consecutive days, (i.e., acquisition) with care being taken to practice the task at the same time each day to control for the effects of fatigue.
The upper extremity task used in this study is well-documented among clinical and nonclinical samples as a motor skill learning paradigm, such that repeated exposure to the task results in improved performance, even after long retention periods [20, 24, 25]. This task involves learning a basic motor sequence and mimics functional movements involved in feeding oneself, a critical activity of daily living [26]. Participants sat behind a desk with the training board placed 15 cm from their torso. Four cups were secured to this board with the proximal “home” cup aligned with the participant’s non-dominant shoulder (see Figure 1). Using their non-dominant hand, participants transported two raw kidney beans within each spoonful from the “home” cup half filled with beans to a target cup distal to their body as quickly as possible in a unidirectional arc starting with the cup ipsilateral to their non-dominant arm and working to the contralateral cup. Each trial consisted of 15 repetitions (i.e., five arcs to each of the three target cups). Trials started when participants picked up the tablespoon and stopped when they touched the spoon back to the wooden board upon completion of a trial. The time taken to complete each trial was timed in seconds with a handheld stopwatch. If participants made an error (e.g., placing the wrong number of beans into a target cup), they had to correct the error before continuing with the remainder of the trial; thus, any errors resulted in increased trial time. Because the dominant hand is typically used in daily life to handle a spoon [27], participants in this study were instructed to use their non-dominant hand. We have already demonstrated in older adults that the non-dominant hand is initially much slower on this task than the dominant hand [28], and that with practice, performance with the non-dominant hand can improve significantly [25] and these improvements are retained for at least one month [29]. Thus, using the non-dominant hand minimized any ceiling effects on the learning task. That being said, this task mimics an activity of daily living and is therefore not entirely novel to the participant, yet is likely novel to the non-dominant hand based on our previous data [28]. This further points to our hypothesis that the “on” levodopa group would have worse motor learning outcomes than the “off” group, since the learning of novel tasks may be selectively impaired by levodopa [18]. Participants were given no information about their performance strategy during practice. Practice consisted of ten blocks of five trials and lasted approximately 60–90 minutes per acquisition day. Participants were re-tested on a single block (five trials) of the task two (early retention) and nine (late retention) days following the end of acquisition. These retention blocks were used to determine motor skill learning and skill decrement [25, 28]. Participants completed these retention blocks according to their group allocation, i.e. “on” or “off” levodopa.
Figure 1.
Functional motor task performed with the nondominant upper extremity.
2.4. Outcome measures
The primary outcome was motor skill learning, which compared the average trial times of the first block of acquisition on Day 1 (referred to as ‘baseline’) and the early retention block. Secondary outcomes were skill decrement, rate of skill acquisition, and skill transfer. Skill decrement compared average trial time of the last block of acquisition and both retention blocks (to measure early and late decrements). The exponential decay function, y = a + be-x/c, was used to determine rate of skill acquisition [25], where a is the final trial time value that the exponential decay function approaches (i.e. asymptote), b is the scale of learning from the first trial time to the value a, x is the trial number, and 1/c is the number of trials needed to obtain asymptote (i.e. 1 – e−1); thus, c indicates rate of learning. Transfer, i.e. the extent to which practicing the motor task would enhance performance on other untrained upper extremity tasks, was determined with the nine-hole peg test (9HPT) [30] and a functional dexterity task [20], the latter with precedence for transfer effects following this motor learning paradigm [20, 31]. The 9HPT required participants to pick up a peg from a bowl, transfer it to one of the nine holes and repeat until all nine pegs were in a hole, then return each peg to the bowl. Timing started when participants touched the first peg and stopped when participants released the ninth peg into the bowl. The functional dexterity task required participants to fasten then unfasten 10 buttons (2.5 cm diameter, center spaced 5 cm apart) sewn on plain weave cotton fabric secured to a wooden board. The row of buttons was aligned with the participant’s non-dominant shoulder and the button-holed side of the fabric was unfolded on the table lateral to that arm. Participants had to completely fasten each button consecutively, starting with the button distal to their body and working their way along the row to the most proximal button, and once the tenth button was fastened, completely unfasten each button in the reverse order until they could reopen the fabric. Timing started when participants touched the fabric to start fastening the buttons and stopped when they had completely unfastened the last button. Participants performed two trials of each task as quickly as possible, with the average time across both trials used for analysis. All participants were assessed on the transfer tasks by a trained physical therapist blinded to participants’ group allocation during pretest (i.e. 3–4 days prior to the start of acquisition) and again at posttest nine days after the end of acquisition (i.e. the same day as late retention) while “on” their prescribed dopamine replacement medication, regardless of group allocation.
2.5. Data analysis
Power calculations showed that a sample size of 15 participants per group was required to detect a 22% improvement in task performance at early retention (baseline mean 75.3 s, SD 14.4 s, power 0.8, alpha 0.05), allowing for 10% drop-out [25].
Visual inspection of the data revealed missing data due to two participants who were unable to complete all 10 blocks of training on each day of acquisition, along with some outliers in trial time due to participant inattention. The mean trial time from the first block of each day of acquisition was calculated for each individual, and extreme outliers exceeding three times the mean from this first block on each day of acquisition were excluded. Total data loss was 2.2%. An intention-to-treat analysis was used with the last observation carried forward for missing data.
Separate 2 × 2 repeated measures analyses of variance (RM-ANOVA) were used to test the primary and secondary aims. Group (“on” versus “off” levodopa) was modelled as the between-subject factor and time was modelled as the within-subject factor. Assumptions of homoscedasticity were confirmed using Levene’s test (p=.40). For the primary aim, the timepoints of interest were baseline and early retention. Depending on the secondary aim, the timepoints of interest were end of acquisition and early or late retention (for skill decrement), and pretest and posttest (for the transfer tasks). Tukey HSD tests were used to determine whether significant interactions were due to differences in group and/or time. Data were analyzed by an investigator blinded to group allocation (SYS) using JMP Pro v13 (SAS Institute, Cary NC).
3. Results
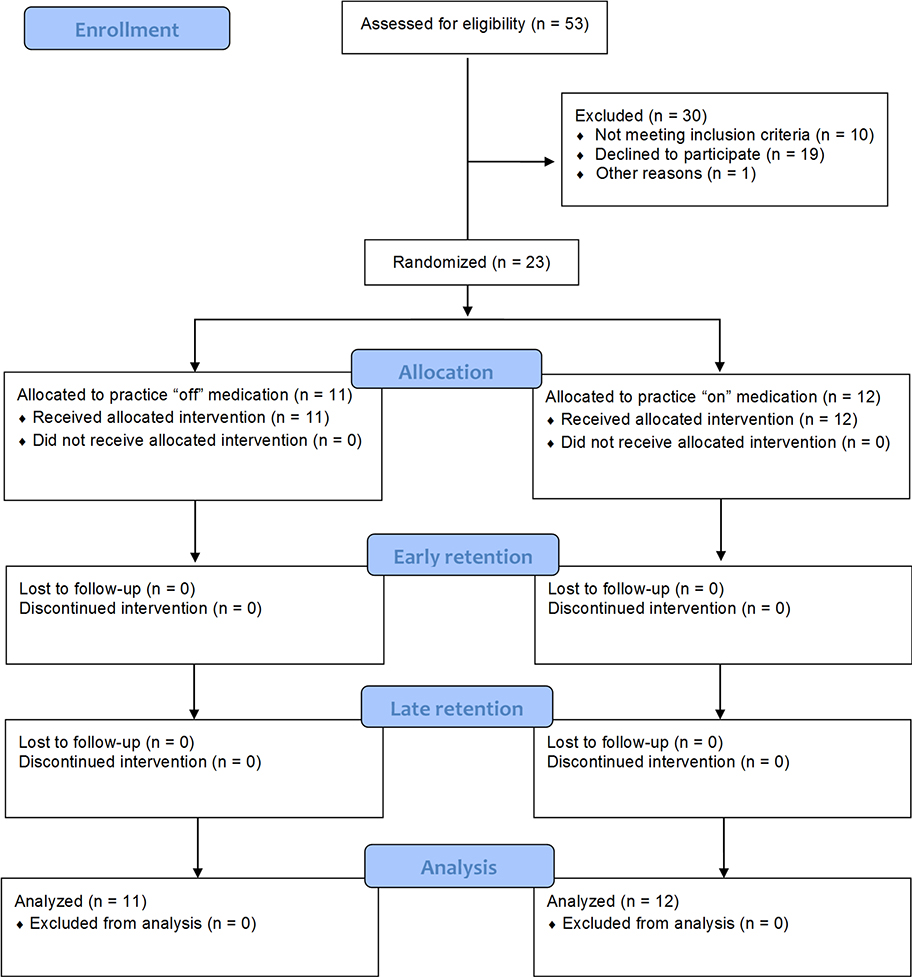
3.1. Participant flow
A total of 23 participants were randomized to practice the functional motor task “on” (n=12) or “off” (n=11) levodopa (Table 1), with participants completing all allocated acquisition and retention sessions (Figure 2). All participants were right-hand dominant and thus practiced the functional motor task with their left hand. Thirty percent (n=7) of all participants had a more symptomatic right hand, 39% (n=9) had a more symptomatic left hand, and the remainder did not have a significant difference in symptom presentation between hands (Table 1). Two participants (one in each group) were unable to complete all 150 acquisition trials due to fatigue. One participant in the “off” levodopa group completed 76% of acquisition, and one participant in the “on” levodopa group completed 82% of acquisition. These participants were included in all analyses using an intention-to-treat approach.
Table 1.
Participant characteristics by group allocation and for the entire sample at pretest.
| Outcome | “On” levodopa practice (n = 12) | “Off” levodopa practice (n = 11) | All (n = 23) | Between-group difference (p) |
|---|---|---|---|---|
| Age (years) | 71.7 (5.1) | 70.3 (8.6) | 71.0 (6.9) | .63 |
| Sex (M) | 5 (42%) | 6 (55%) | 11 (48%) | .54 |
| MoCA (0–30)† | 26.8 (2.1) | 26.5 (2.1) | 26.6 (2.0) | .74 |
| Disease duration (years) | 5.3 (4.6) | 3.4 (3.4) | 4.4 (4.1) | .26 |
| LED (mg) | 599.9 (421.5) | 575.0 (276.4) | 588.0 (351.7) | .75 |
| MDS-UPDRS (0–132): “off” | 47.7 (10.2) | 43.5 (6.6) | 45.7 (8.7) | .27 |
| “on” | 32.9 (10.9) | 27.9 (5.1) | 30.5 (8.8) | .17 |
| HY stage (2)‡ | 12 (100%) | 11 (100%) | 23 (100%) | 1.0 |
| Self-reported hand dominance, right | 12 (100%) | 11 (100%) | 23 (100%) | 1.0 |
| More symptomatic UE, n/group size (%)§ | .31 | |||
| Right | 5/12 (42%) | 2/11 (18%) | 7/23 (30%) | |
| Left | 4/12 (33%) | 5/11 (45%) | 9/23 (39%) | |
Data presented as mean (SD) or n (%).
HY: Hoehn and Yahr; LED: levodopa equivalent dose; MDS-UPDRS: Movement Disorder Society sponsored version of the Unified Parkinson’s Disease Rating Scale; MoCA: Montreal Cognitive Assessment; UE: upper extremity.
Higher score indicates better performance
Hoehn and Yahr (HY) stage was the same regardless of whether the participant was tested “off” or “on” levodopa.
Upper extremity (UE) motor symptoms calculated as the sum of the following items for either the right or left sides from the MDS-UPDRS when tested OFF medication: 3.3 rigidity, 3.4 finger tapping, 3.5 hand movements, 3.6 hand pronation-supination, 3.15 postural tremor, 3.16 kinetic tremor, 3.17 rest tremor. Using these items, one side was determined to be more symptomatic if it was scored ≥2 points higher than the other side.
Figure 2.
Participant flow through the pilot randomized controlled trial.
3.2. Impact of levodopa on skill learning (primary outcome)
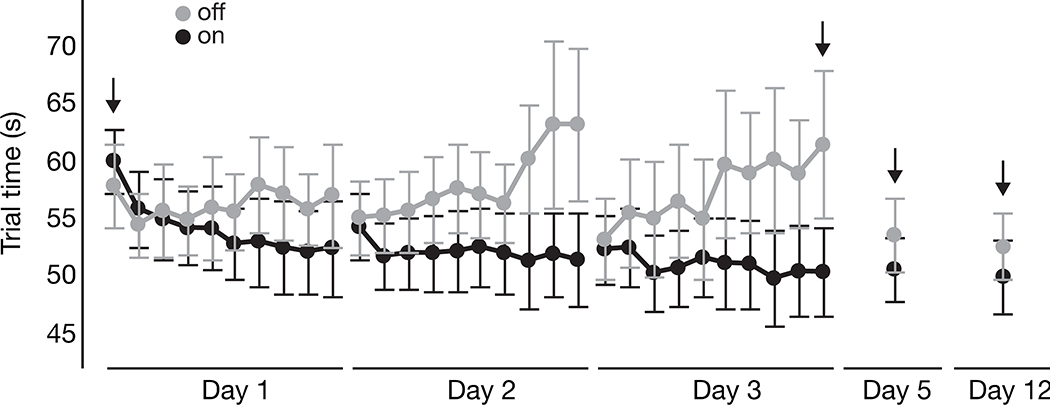
As shown in Figure 3, the “on” levodopa group tended to improve performance on the motor task over the acquisition period, while the “off” levodopa group’s performance got worse (i.e. longer trial times) during each practice session, despite starting at the same level of performance each day. The two participants with incomplete acquisition are not included in Figure 3, with their data are reported separately in Figure 4. Moreover, those who practiced “on” levodopa had improved performance at early retention compared to those who practiced “off”. There was a significant group (“on” versus “off”) x time (baseline versus early retention) interaction for skill learning (F1,21=5.15; p=.03). Tukey HSD tests indicated that only the “on” levodopa group improved from baseline to early retention (p<.0001), whereas the “off” levodopa group did not (p=.14). Descriptive statistics for all blocks of interest are provided in Table 2.
Figure 3.
Average trial time for each block of the acquisition period (Days 1–3) and the two retention sessions (Days 5 and 12). Arrows indicate blocks of interest for the primary and secondary outcomes. Gray = “off” levodopa group; Black = “on” levodopa” group. Error bars indicate standard error. Lower trial times indicate better performance.
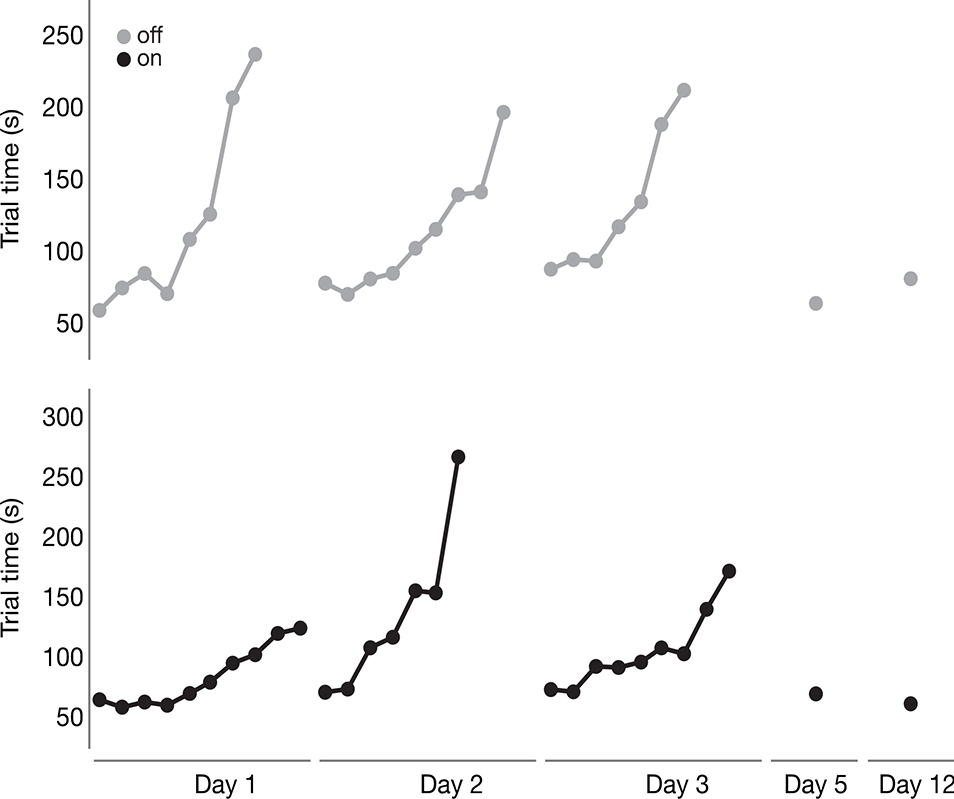
Figure 4.
Average trial time data are shown for the two participants who were unable to complete all 10 blocks of training on each day of acquisition (one from the “off” levodopa group, shown on top, and one from the “on” levodopa group, shown on the bottom). Similar to Figure 3, average trial time for each block of the acquisition period (Days 1–3) and the two retention sessions (Day 5 and Day 12) are shown. Note that most acquisition days have less than 10 blocks, indicating incomplete acquisition.
Table 2.
Mean (SD) of raw scores, reported in seconds, for the trained motor task for each group at baseline (first block of the acquisition period), end of acquisition (last block of the acquisition period), early retention and late retention.
| Group | Time Period | |||
|---|---|---|---|---|
| Baseline | End of acquisition | Early retention | Late retention | |
| (Day 1) | (Day 3) | (Day 5) | (Day 12) | |
| “Off” levodopa practice | 57.86 (10.83) | 74.98 (49.15) | 54.41 (10.25) | 55.03 (12.11) |
| “On” levodopa practice | 60.36 (8.93) | 58.38 (30.54) | 52.14 (10.30) | 50.87 (10.40) |
3.3. Impact of levodopa on skill decrement, rate of skill acquisition and skill transfer (secondary outcomes)
As the “off” levodopa group showed substantial declines in performance within each day of the acquisition period, there was little skill ‘decrement’ from the end of acquisition to early and late retention periods given the poor performance at the end of acquisition on Day 3 (Figure 3). Nevertheless, there were no significant group x time (end of acquisition versus retention) interactions for early (F1,21=0.99; p=.33) or late (F1,21=0.86; p=.36) skill decrement.
We were unable to statistically compare rates of skill acquisition between groups as originally proposed in our clinical trial registration, given that the three-parameter exponential decay model to measure rate of skill acquisition could not be applied to our data. Very low R2 values indicated poor goodness-of-fit to the typical ‘acquisition curve’ [32] (e.g. R2=0.003 for the “off” group on Day 1), due largely to the marked increases in trial time (i.e. decrements in performance) within each day of acquisition for the “off” levodopa group (refer to Figure 3). However, a more exploratory analysis using RM-ANOVA revealed a group x block interaction for Day 1 (F1,9=2.16; p=.03), Day 2 (F1,9=2.81; p=.004), and Day 3 (F1,9=4.04; p=.0001), providing statistical evidence of group differences in the acquisition phase of this study. Some key post hoc findings from this exploratory analysis were the similarities between the groups in Block 1 for all three days of acquisition (all p≥.99) and clear differences between the groups in Block 10 for Days 2 and 3 (all p<.0001), indicating significant decrements in performance while practicing “off” levodopa.
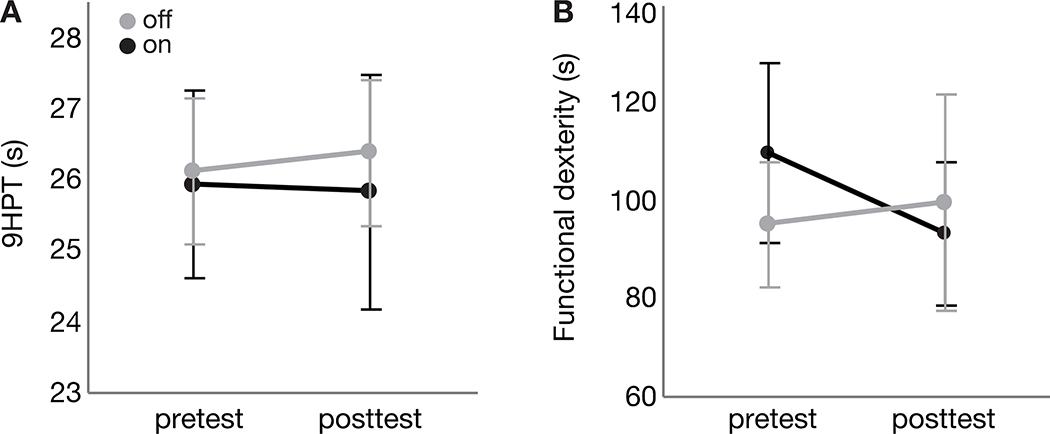
Average performance for each group at pretest and posttest for the 9HPT (Figure 5A) and the functional dexterity task (Figure 5B) are reported in Figure 5 and Table 3. Contrary to our hypothesis, there were no significant group x time interactions for either transfer task, nor any main effects of group or time (all p>.13).
Figure 5.
Average trial time for pretest (3–4 days prior to start of acquisition) and posttest (9 days following end of acquisition) are shown for the (A) nine hole peg test (9HPT) and (B) functional dexterity task. Gray = “off” levodopa group; Black = “on” levodopa” group. Note that both groups performed the 9HPT and functional dexterity task “on” levodopa, regardless of group allocation. Error bars indicate standard error. Lower times indicate better performance.
Table 3.
Mean (SD) of raw scores, reported in seconds, for the transfer tasks for each group prior to and following practice of the motor task.
| Task | 9HPT | Functional dexterity task | |||
|---|---|---|---|---|---|
| Time period | Pre practice | Post practice | Pre practice | Post practice | |
| Group | “Off” levodopa practice | 26.1 (3.4) | 26.4 (3.4) | 95.2 (43.3) | 99.6 (73.3) |
| “On” levodopa practice | 25.9 (4.6) | 25.8 (5.8) | 109.8 (64.0) | 93.3 (50.9) | |
9HPT: Nine hole peg test.
4. Discussion
This pilot RCT of dopamine replacement medication on upper extremity motor learning showed that levodopa positively impacted learning in people with mild to moderate PD, with the “on” training group showing improved performance at retention compared to those who trained “off” levodopa, thereby contradicting our hypothesis.
People with PD successfully acquired and retained performance of a novel upper extremity task following practice “on” levodopa, but less so “off” levodopa. Although a dopamine overdose hypothesis has been suggested based on the results of prior upper extremity motor learning studies [14, 15], our results add to growing evidence suggesting that the influence of dopamine replacement medication on the learning of motor tasks is more complicated [33, 34]. Our results demonstrating learning at early retention concur with previous studies showing that people with PD are capable of acquiring new skills with practice [5], underscoring the value of rehabilitation for these individuals to improve and/or maintain performance. Similar to a longer duration upper extremity motor learning study [3], our PD participants retained newly acquired skills following a short period of no practice, with greater skill retention demonstrated in those who trained “on” levodopa. This suggests that the learning task (functional reaching) may not be novel enough to demonstrate a dopamine overdose effect [18].
This training did not, however, appear to generalize to the 9HPT or the functional dexterity task. It is noted, though, that neither the “on” nor “off” levodopa group showed significant differences in these transfer tasks from pretest to posttest on average. This is similar to data from a larger cohort of older adults without PD (i.e. ‘healthy controls’) who showed no transfer overall to functional dexterity task following the same dose of training on functional reaching [31]. In that study, age was a signficant predictor of transfer, with ‘old old’ adults showing little to no transfer while ‘young old’ adults did, such that any transfer effect was washed out when grouped together [31]. The RCT nature and small sample sizes of the current study precluded us from analyzing individual differences in transfer, but we mention it here in light of earlier findings that suggest that age-related factors, rather than PD- or levodopa-specific factors, may explain the lack of transfer at the group level. It is also noted that this study tested for transfer between functionally different motor tasks (reaching vs. dexterity), suggesting an expectation of ‘far’ transfer [35] between tasks that are not very similar [20, 36, 37]. Transfer between different conditions of the same task (i.e., ‘near’ transfer) has been reported in PD, with more transfer “on” levodopa compared to “off” in lower-extremity training [34], thereby warranting future studies to determine the extent to which transfer in PD is dependent on effector (lower- vs. upper-extremity), task similarity (near vs. far transfer), age, and/or levodopa.
Unlike recent lower extremity motor learning paradigms in PD that demonstrated no significant difference between practicing “on” or “off” levodopa [38, 39], the results of the current study concur with other studies examining both upper [33] and lower [34] extremity motor learning paradigms demonstrating better learning when a motor task is practiced “on” levodopa. These differences may be due to the known benefits of levodopa on acquired motor skills [18], the effector used in this study, and severity of disease in this sample. Firstly, cardinal PD impairments such as bradykinesia and tremor have greater impact on acquired upper extremity motor skills than lower extremity tasks such as balance-demanding activities. These cardinal impairments are responsive to dopamine replacement medication [40] whereas impaired balance and gait that affect lower extremity task performance are predominantly influenced by non-dopaminergic pathways [41]. It is also plausible that both groups had comparable acquisition early on Day 1 (see Fig. 3) and the marked differences in performance between the groups during the later portions of each practice session reflect the deleterious effects of levodopa withdrawal on an already-acquired skill rather than any effect on learning. Similarly, if the “off” levodopa group had been tested on their typical medication at retention, the group differences at Days 5 and 12 may have been attenuated, given the benefits of levodopa on acquired motor skills [18, 42]. However, the presence of group differences at retention but not at the start of any other day (see Figure 3 and exploratory analysis during acquisition phase) suggest that, at least to some extent, practicing “on” levodopa positively impacted long-term learning. These improvements in learning when practicing “on” levodopa may also be partially attributable to levodopa’s effect on increasing motivation and effort [43], unlike the “off” levodopa group who struggled with the motoric components of the task and became frustrated with the difficulty of the task and lack of improvement during the acquisition phase. Lastly, in this and other studies [33, 34], participants most likely had mild-moderate PD, whereas the dopamine overdose hypothesis appears to be most pronounced in very early disease [14] before levodopa is commonly prescribed. Taken together, these results suggest that the motoric benefits of levodopa [42] on acquired skills [18] outweigh any decrements in learning new skills while “off” levodopa in people with mild-moderate PD who are already taking medication.
One unexpected outcome was the substantial decline in task performance over the course of each day of acquisition. The increases in trial time limited our ability to use standard approaches to fitting acquisition curves [32], given the lack of a nonlinear decrease in trial time, particularly for the “off” levodopa group. Recent work has developed new methods for dissociating such decrements in performance (that may arise from fatigue or other factors) from any underlying learning [44]. Interestingly, worse motor performance over time during skill acquisition does not appear to impair longer-term learning in chronic stroke survivors [32], suggesting that learning processes are still underway regardless of decrements in performance. Given the tendency for the “off” levodopa group to worsen performance within-session yet show improved performance the following day of acquisition (see Figure 3), future studies can apply this novel approach to dissociate performance changes due to learning from those associated with fatigue or attentional factors.
4.1. Limitations and Future Directions
We utilized an RCT design to limit threats to internal validity. However, an RCT does not remove the influence of factors such as sample size, group imbalance despite randomization, or issues inherent to motor learning studies such as the interaction of participant with task difficulty. Our small sample of mild-moderate participants may have contributed to variability that may have concealed between-group differences on the secondary outcomes, as we were unable to recruit the intended number of participants to fully power the study. Additionally, while all participants trained using their non-dominant hand, an uneven number of participants in each group trained using their more symptomatic upper extremity, despite random assignment.
The influence of cognitive function on motor learning is unclear [24, 45] and not well examined within this study as our participants did not have significant cognitive impairment, based on their performance on the Montreal Cognitive Assessment. Future motor learning research should utilize large samples of people with PD with varying degrees of cognitive impairment in an effort to uncover specific cognitive domains that may be predictive of motor learning capability [24].
Although upper extremity tasks are impaired in people with PD compared to healthy individuals [46], practicing upper limb tasks may still be beneficial [3]. Our experimental task has previously been used in neurologically-impaired and neurotypical samples [20, 25] and now, for the first time, in PD. Participants in the “off” levodopa group were not withdrawn from all antiparkinsonian medications to minimize participant burden, as the long half-life of dopamine agonists would require these participants to take no antiparkinsonian medication for multiple consecutive days. Yet the motoric difficulty seen in individuals randomly assigned to train “off” levodopa medication over each day of acquisition may have confounded some of the skill acquisition that may have been occurring. This observation, along with these participants’ change in “on” versus “off” levodopa scores on the MDS-UPDRS [23], suggest that these participants were functionally “off” medication. Clinically this provides an additional rationale to consider task practice while “on” medication.
4.2. Conclusion
People with PD demonstrated significantly better acquisition and retention of a functional upper extremity motor task following practice “on”, but were less successful at learning this task when they practiced “off”, levodopa. The improved retention in the “on” levodopa group argues against the adverse effects of dopamine on motor learning in people with mild-moderate PD.
Acknowledgements
The authors wish to thank Amy Ballard, Shelby Dibble, Kirsten Gorski, Jaclyn Hill, Orin Ryan and Jane Saviers-Steiger for their assistance with data collection.
Funding sources: This work was supported by the University of Utah Office of Research (2015–16 grant), the American Parkinson Disease Association (2015–16 postdoctoral fellowship grant), and the National Institutes of Health (K01AG047926).
Role of the funding source
The funders had no role in the conduct of the research or in the decision to publish the findings.
Footnotes
Abbreviations: MDS-UPDRS: Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale; PD: Parkinson disease; RCT: randomized controlled trial; RM-ANOVA: repeated measures analysis of variance; 9HPT: nine-hole peg test.
REFERENCES
- 1.Jankovic J Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. [DOI] [PubMed] [Google Scholar]
- 2.Dural A, Atay MB, Akbostanci C, Kucukdeveci A. Impairment, disability, and life satisfaction in Parkinson’s disease. Disability and rehabilitation. 2003;25(7):318–23. [DOI] [PubMed] [Google Scholar]
- 3.Nackaerts E, Heremans E, Vervoort G, Smits-Engelsman BC, Swinnen SP, Vandenberghe W, et al. Relearning of writing skills in Parkinson’s disease after intensive amplitude training. Movement disorders : official journal of the Movement Disorder Society. 2016;31(8):1209–16. [DOI] [PubMed] [Google Scholar]
- 4.Vanbellingen T, Nyffeler T, Nigg J, Janssens J, Hoppe J, Nef T, et al. Home based training for dexterity in Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord. 2017;41:92–8. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwboer A, Rochester L, Müncks L, Swinnen SP. Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord. 2009;15(Supplement 3):S53–8. [DOI] [PubMed] [Google Scholar]
- 6.Marinelli L, Quartarone A, Hallett M, Frazzitta G, Ghilardi MF. The many facets of motor learning and their relevance for Parkinson’s disease. Clin Neurophysiol. 2017;128(7):1127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. 5th ed. Champaign, IL: Human Kinetics; 2011. [Google Scholar]
- 8.Felix K, Gain K, Paiva E, Whitney K, Jenkins ME, Spaulding SJ. Upper extremity motor learning among individuals with Parkinson’s disease: a meta-analysis evaluating movement time in simple tasks. Parkinson’s disease. 2012;2012:589152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyon J Motor sequence learning and movement disorders. Current Opinion in Neurology. 2008;21(4):478–83. [DOI] [PubMed] [Google Scholar]
- 10.Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199(1):61–75. [DOI] [PubMed] [Google Scholar]
- 11.Mentis MJ, Dhawan V, Nakamura T, Ghilardi MF, Feigin A, Edwards C, et al. Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology. 2003;60(4):612–9. [DOI] [PubMed] [Google Scholar]
- 12.Lehericy S, Benali H, Van de Moortele PF, Pelegrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA. 2005;102(35):12566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, et al. Learning and consolidation of visuo-motor adaptation in Parkinson’s disease. Parkinsonism & Related Disorders. 2009;15(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dan X, King BR, Doyon J, Chan P. Motor Sequence Learning and Consolidation in Unilateral De Novo Patients with Parkinson’s Disease. PloS one. 2015;10(7):e0134291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwak Y, Müller MLTM, Bohnen NI, Dayalu P, Seidler RD. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson’s disease. J Neurophysiol. 2010;103(2):942–9. [DOI] [PubMed] [Google Scholar]
- 16.Keus SH, Bloem BR, Hendriks EJ, Bredero-Cohen AB, Munneke M. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Movement Disorders. 2007;22(4):451–60. [DOI] [PubMed] [Google Scholar]
- 17.Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Current Opinion in Neurobiology. 2005;15(2):161–7. [DOI] [PubMed] [Google Scholar]
- 18.Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R. Dopamine overdose hypothesis: evidence and clinical implications. Movement disorders : official journal of the Movement Disorder Society. 2013;28(14):1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cools R, Ivry RB, D’Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cogn Neurosci. 2006;18(12):1973–83. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer SY, Patterson CB, Lang CE. Transfer of training between distinct motor tasks after stroke: Implications for task-specific approaches to upper-extremity neurorehabilitation. Neurorehabil Neural Repair. 2013;27(7):602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society. 2008;23(15):2129–70. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Martin P, Rodriguez-Blazquez C, Alvarez-Sanchez M, Arakaki T, Bergareche-Yarza A, Chade A, et al. Expanded and independent validation of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). Journal of neurology. 2013;260(1):228–36. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer SY, Duff K. Within-session and one-week practice effects on a motor task in amnestic mild cognitive impairment. Journal of clinical and experimental neuropsychology. 2017;39(5):473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer SY, Dibble LE, Duff K. Efficacy and feasibility of functional upper extremity task-specific training for older adults with and without cognitive impairment. Neurorehabil Neural Repair. 2015;29(7):636–44. [DOI] [PubMed] [Google Scholar]
- 26.Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. The Gerontologist. 1970;10(1):20–30. [DOI] [PubMed] [Google Scholar]
- 27.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer SY. Preserved motor asymmetry in late adulthood: is measuring chronological age enough? Neuroscience. 2015;294:51–9. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer SY, Duff K. Rapid Responsiveness to Practice Predicts Longer-Term Retention of Upper Extremity Motor Skill in Non-Demented Older Adults. Frontiers in aging neuroscience. 2015;7(214). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathiowetz V, Weber K, Kashman N, Volland G. Adult Norms for the Nine Hole Peg Test of Finger Dexterity. Occup Ther J Res. 1985;5:24–33. [DOI] [PubMed] [Google Scholar]
- 31.Walter CS, Hengge CR, Lindauer BE, Schaefer SY. Declines in motor transfer following upper extremity task-specific training in older adults. Experimental gerontology. 2019;116:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadden KP, Asis K, Mang CS, Neva JL, Peters S, Lakhani B, et al. Predicting motor sequence learning in individuals with chronic stroke. Neurorehabil Neural Repair. 2017;31(1):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beigi M, Wilkinson L, Gobet F, Parton A, Jahanshahi M. Levodopa medication improves incidental sequence learning in Parkinson’s disease. Neuropsychologia. 2016;93(Pt A):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson DS, Horak FB. The effect of levodopa on improvements in protective stepping in people with Parkinson’s disease. Neurorehabil Neural Repair. 2016;30(10):931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnett SM, Ceci SJ. When and where do we apply what we learn? A taxonomy for far transfer. Psychological bulletin. 2002;128(4):612–37. [DOI] [PubMed] [Google Scholar]
- 36.Gagné RM, Baker KE, Foster H. On the relation between similarity and transfer of training in the learning of discriminative motor tasks. Psychological Review. 1950;57(2):67–79. [Google Scholar]
- 37.Osgood CE. The similarity paradox in human learning: a resolution. Psychological Review. 1949;56(3):132–43. [DOI] [PubMed] [Google Scholar]
- 38.Hayes H, Hunsaker N, Boyd L, Shultz B, Schenkenberg T, White A, et al. Does dopamine replacement medication affect postural sequence learning in Parkinson disease? Motor control. 2015;19(4):325–40. [DOI] [PubMed] [Google Scholar]
- 39.Paul SS, Schaefer SY, Olivier GN, Walter CS, Lohse KR, Dibble LE. Dopamine replacement medication does not influence implicit learning of a stepping task in people with Parkinson’s disease. Neurorehabil Neural Repair. 2018;32(12):1031–42. [DOI] [PubMed] [Google Scholar]
- 40.Zach H, Dirkx M, Pasman JW, Bloem BR, Helmich RC. The patient’s perspective: The effect of levodopa on Parkinson symptoms. Parkinsonism Relat Disord. 2017;35:48–54. [DOI] [PubMed] [Google Scholar]
- 41.Bohnen NI, Cham R. Postural control, gait, and dopamine functions in parkinsonian movement disorders. Clin Geriatr Med. 2006;22(4):797–812. [DOI] [PubMed] [Google Scholar]
- 42.Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F. Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clinical interventions in aging. 2010;5:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chong TT, Bonnelle V, Manohar S, Veromann KR, Muhammed K, Tofaris GK, et al. Dopamine enhances willingness to exert effort for reward in Parkinson’s disease. Cortex. 2015;69:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park H, Schweighofer N. Nonlinear mixed-effects model reveals a distinction between learning and performance in intensive reach training post-stroke. J Neuroeng Rehabil. 2017;14(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muslimović D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson’s disease. Brain. 2007;130(11):2887–97. [DOI] [PubMed] [Google Scholar]
- 46.Heremans E, Nackaerts E, Broeder S, Vervoort G, Swinnen SP, Nieuwboer A. Handwriting impairments in people with Parkinson’s disease and freezing of gait. Neurorehabil Neural Repair. 2016. [DOI] [PubMed] [Google Scholar]