Abstract
The immune system plays an important role in obesity-induced adipose tissue (AT) inflammation and the resultant metabolic dysfunction which can lead to hypertension, dyslipidemia, and insulin resistance and their downstream sequelae of type II diabetes and cardiovascular disease. While macrophages are the most abundant immune cell type in AT, other immune cells are also present, such as B cells, which play important roles in regulating AT inflammation. This brief review will overview B cell subsets, describe their localization in various adipose depots and summarize our knowledge about the function of these B cell subsets in regulating AT inflammation, obesity-induced metabolic dysfunction and atherosclerosis.
Keywords: Adipose tissue, obesity, inflammation, B cells, atherosclerosis
Graphical Abstract
Introduction
Adipose Tissue (AT) provides structural support for organs, insulation to the body and energy storage in the form of fat. Yet, AT is a complex organ, enriched not only with adipocytes, but also containing immune cells that contribute to metabolic, endocrine, and immune activities (1). AT specific immune profiles and their function are profoundly changed with obesity (2) due to activation of resident immune cells and recruitment of additional inflammatory cells. This AT inflammation, particularly when present in visceral AT (VAT) can lead to metabolic syndrome (MetS), a cluster of risk factors including insulin resistance (IR) that increase the risk of cardiovascular diseases (CVD) and type 2 diabetes (T2D), serious worldwide health threats (3,4).
Macrophages are the most abundant immune cell type in the AT of mice and humans, and particularly the M1 phenotype, are thought to be major drivers of AT inflammation (5–8). Yet, AT also contains other myeloid cells (dendritic cells and neutrophils) (9–11), and lymphoid cells including T cells (11,12), and B cells (13) which can play important roles in modulating AT inflammation. The role of macrophages and T cells in AT inflammation has been extensively reviewed (14,15). In this review, we summarize the current knowledge on AT B cells during steady-state and in cardiometabolic disorders.
Overview of B lymphocytes
B cells are one of the major lymphocyte populations and play important roles in both innate and adaptive immunity through both antibody production and cytokine secretion. B cells are primarily divided into two major classes: B-1 and B-2 cells largely based on their origin, development, anatomic niches, and requirement of T cell help for antibody production as illustrated in Figure 1. The molecular regulation of peripheral B cells is complex, beyond the scope of this review and recently extensively reviewed elsewhere (16). In brief, B-1 cells are innate-like B cells produce natural antibodies in the absence of antigen, mounting rapid T cell-independent antibody responses against multivalent microbial antigens or pathogen associate molecular patterns and neoantigens formed in the setting of oxidative stress or danger associated molecular patterns. B-1 cells can be subdivided into CD5+ B-1a and CD5− B-1b subtypes (17).
Figure 1: Schematic of murine B cell origin, development and anatomical distribution:
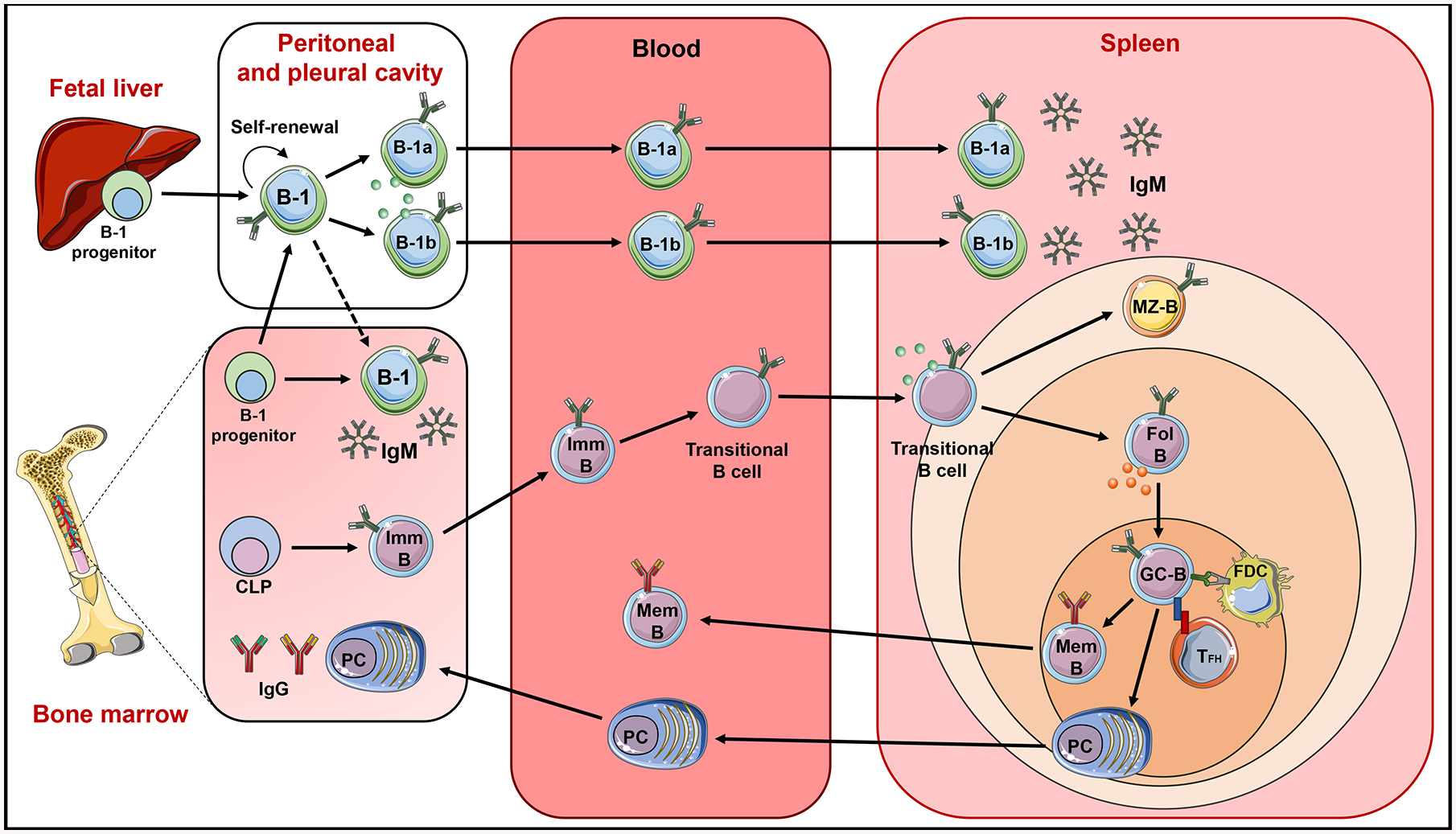
B cells are divided into two major subsets, B-1 and B-2 cells. B-2 cells arise from precursors in the bone marrow (BM). Common lymphoid progenitors (CLPs) in the BM differentiate into immature B cells (Imm-B). The immature B cells leave the BM, enter the blood stream, undergo transitional stages (T-1 and T-2), then travel to spleen to differentiate into mature marginal zone B cells (MZ-B) and follicular B cells (Fol-B). After antigen stimulation or presentation from follicular dendritic cells (FDC), and with the help of follicular helper T cell (TFh), Fol-B cells enter germinal center reactions (GC-B) followed by differentiation into memory B cells (Mem-B) and antigen specific antibody secreting long lived plasma cells (PC). These memory B cells enter the circulation and PCs migrate to BM and stay for longer periods. B-1 B cells develop from B-1 precursors in the fetal liver and adult BM, migrate to and reside in the peritoneal and pleural cavity. B-1 cells are divided into B-1a and B-1b cells. After activation, peritoneal B-1 cells migrate to the spleen and perhaps BM to secrete IgM. B cells can produce pro-inflammatory cytokines (green circles) and anti-inflammatory cytokines (red circles).
B-2 cells are conventional B cells derived from progenitors in the bone marrow, ultimately traveling to secondary lymphoid organs such as spleen and lymph nodes (LN) where they mature, differentiating into marginal zone B cells and follicular B cells. Marginal zone B cells reside within the splenic marginal sinus and participate in the first line defense against blood-borne pathogens (18,19). Follicular B cells become activated by antigen stimulation and follicular helper T cells (TFH), undergo germinal center (GC) reactions and differentiate into memory B cells or plasma cells. These plasma cells and memory B cells participate in long-lived protective humoral immunity (20).
In addition to antibody mediated responses, some B cells can regulate immune responses by secreting cytokines. When B cells secrete anti-inflammatory cytokines such as interleukin (IL)-10, IL-35 and transforming growth factor-beta, they are termed regulatory B cells (Bregs) (21–27). Bregs are not necessarily a different subset but they are a functionally different phenotype derived from either B-1 or B-2 cells. Bregs can suppress both Th1 and Th2 polarized immune cells, inhibit macrophage/dendritic cell antigen presentation, and proinflammatory cytokine production (28). This could explain antibody independent immunoregulatory functions of B cells in several experimental models of infection and autoimmunity (29–32).
Localization of B cell subsets in different AT types at homeostasis
In addition to lymphoid tissues, B cells reside in non-lymphoid organs including AT and function to maintain homeostasis at steady state. In mice, the numbers of B-1 and B-2 cells vary between different compartments. B-2 cells vastly outnumber B-1 cells in the spleen and BM with the ratio of B-1 to B-2 cells in these lymphoid tissues of ~0.01:1. In contrast, the B-1 to B-2 cell ratio is much higher in AT, with omental VAT (~0.8:1) being the highest and nearly equivalent to that in the peritoneal cavity (~1:1). Notably perivascular AT (PVAT) (~0.3:1), epidydimal VAT (~0.3:1) and brown AT (BAT) (~0.2:1) also have much higher B-1 to B-2 cell ratio than subcutaneous AT (SAT), spleen and bone marrow (33) (Srikakulapu P, unpublished data), suggesting the B cell-mediated immune responses in AT may be unique from those in spleen and bone marrow. Data on human B-1 to B-2 cell ratios in AT is much more limited due to a less defined definition of human B-1 cells and the challenges inherent in obtaining AT samples from visceral and perivascular depots in humans (Figure 2).
Figure 2: Relative abundance of adipocyte and B cell subtypes in AT depots in mice and humans:
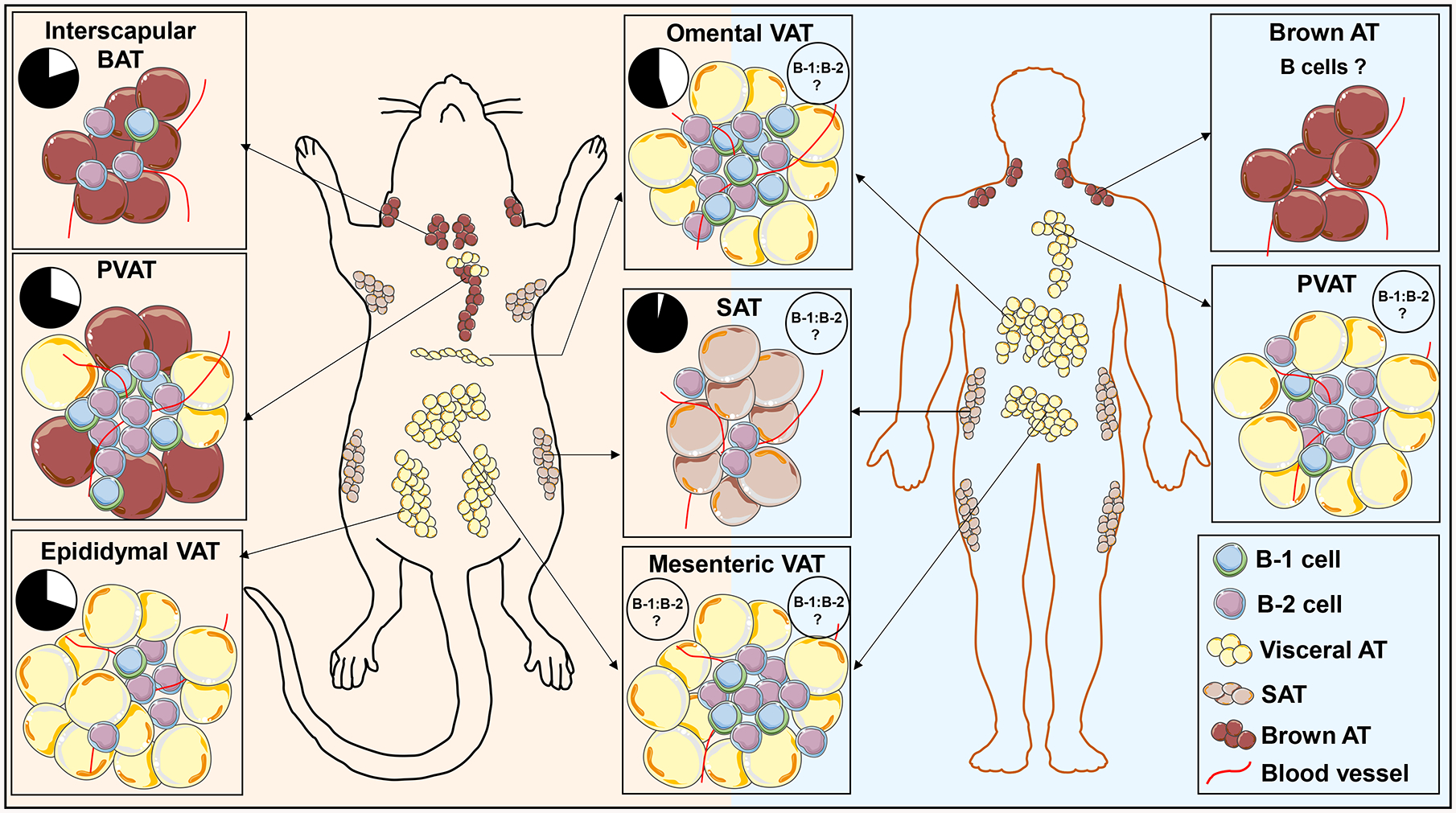
Both mice and humans store fat in distinct white fat depots within the abdomen/thoracic region (called VAT), under the skin (SAT), and in brown AT (BAT). Notable differences between mice and humans include prominent epididymal VAT in mice, absent in humans, more brown adipocytes in murine PVAT and potentially fewer B-1 cells in omental and PVAT in humans, although data on these depots in humans is limited. Pie charts represents relative abundance of B-1 (white) and B-2 cells (black) as percentages of total B cells at homeostatic condition in mice (left) and human (right).
Even within the same type of AT (VAT vs SAT), immune cell numbers may vary by anatomic location. For example, while VAT is found in various locations throughout the body (omental, epididymal/gonadal, mesenteric, epicardial, pericardial and perivascular (34), the abundance of B cells and the B-1 to B-2 ratio is significantly greater in the omentum relative to other visceral depots in mice (35). Omental VAT has unique immune functions. In humans, omental VAT is connected to the spleen, stomach, pancreas and colon, and hangs over other abdominal organs and provides protection (36,37). However, in mice, omental VAT is a small strip of fat anterior and slightly ventral to the stomach (38). Omental VAT is involved in the attenuation of peritonitis, promotes surgical wound healing, promotes vascularization, limits infection and inflammation (39). Due to these features, omental VAT is also often called “the policeman of the abdomen” (40). Omental VAT harbors high numbers of immune cells in the form of cell clusters called milky spots (MS) (Figure 3). MS contains high numbers of B cells particularly innate B-1 cells and macrophages in both humans and mice (35). In addition to residing there in high numbers, B-1 cells continuously traffic to and from the pleural and peritoneal cavities via the omental VAT. This B-1 cell trafficking is largely controlled by the chemokine C-X-C ligand-13 (CXCL13) produced by resident macrophages (41). Another type of lymphoid aggregate, termed fat associated lymphoid cluster (FALC), was originally identified in mesenteric AT in both mice and humans (42). These FALCs are non-classical lymphoid tissues associated with adipocytes and have since been found in several AT depots including mesenteric, mediastinal, gonadal, pericardial VAT and PVAT (33,42–45). The frequency and size of FALCs varies between different AT in mice (42,44). Unlike LNs, FALCs are not encapsulated and are in direct contact with surrounding adipocytes (42). The organization of immune cells in FALCs differ from the secondary lymphoid organs. There is no clear B and T cell compartmentalization areas in FALCs. FALCs from mesenteric AT are composed of a tight cluster of B220+ B cells, with variable numbers of CD4+ T cells and CD11b+ myeloid cells (42,44). We and others have identified the presence of FALCs in PVAT at steady state and in atherosclerotic conditions (33,45). In contrast, SAT harbors fewer numbers of immune cells compared to other ATs such as omental VAT (35) and FALCs are not detectable in SAT (44). In addition to white AT, B cells are also present in BAT in mice and increase in percentage in response to high fat diet (HFD), although their role in BAT is poorly understood (46). It would stand to reason that these unique adipose depot-specific B cell features (such as B cell number, B-1 to B-2 ratio, architecture) may impact on B cell functions in specific ATs. A better understanding of the role of B cells in AT inflammation and disease may provide important clues.
Figure 3: Mouse omental VAT with milky spots:
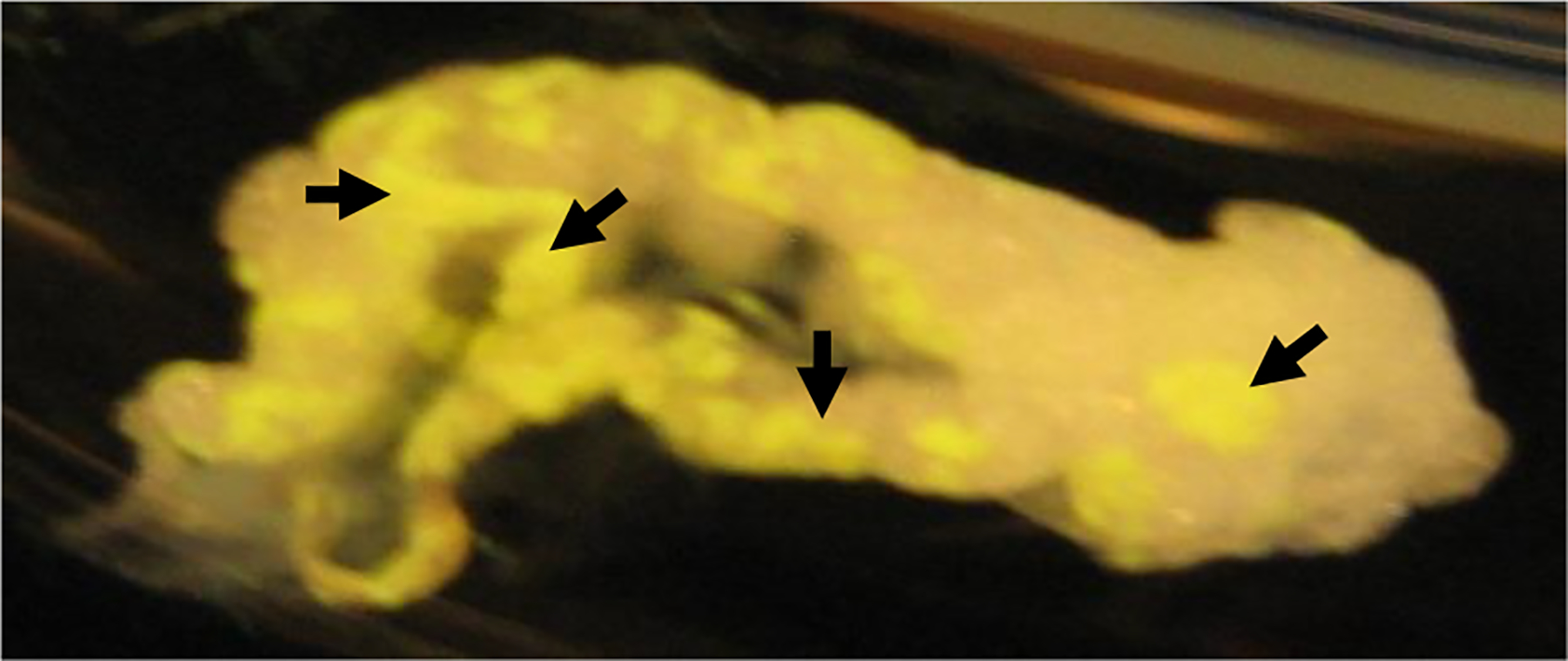
Fluorescent labelled latex beads were injected into mouse peritoneal cavity. Phagocytic cells such as macrophages and B-1 cells in peritoneal cavity engulf these fluorescent labelled latex beads and migrate to milky spots (fluorescent yellow regions) in the omental VAT. After 2 hrs of injection, omental VAT was collected and photographed. Black head arrows show milky spot areas in the omental VAT.
B-2 cells promote AT inflammation in HFD-fed and aged mice
It is well established that chronic AT inflammation is a characteristic feature of obesity in both rodents and humans, and many studies have demonstrated that this chronic inflammatory state is a key contributor to decreased insulin sensitivity and the MetS (47–51). Macrophages and different T cell subtypes have been particularly well studied, and several secretory factors that can cause decreased insulin sensitivity are known (52–58). However, much less is known about the role of B cells in this process despite the fact that B cells are one of the first immune cells to accumulate in AT in response to HFD (59). Studies demonstrated that three weeks after HFD, C57BL6/J mice have a 3-fold increase in B cells in their epididymal VAT depot, and no change in T cell or macrophage numbers. Elevated B cell numbers largely persisted over the next 9 weeks while T cell numbers increased after 6 weeks of diet and macrophages did not increase in number until 12 weeks after diet, coincident with an increase in IR (59). The function of these B cells early after HFD was initially speculated to be protective against inflammation, although subsequent studies suggested an inflammatory role for B-2 cells in HFD-induced AT expansion (13,60,61).
In addition to HFD, aging has been linked to AT B cell-mediated inflammation through VAT accumulation of follicular B-2 cells (62) and age-associated B cells that can produce pro-inflammatory IgG and cytokines have been identified (63–65). Specific depletion of VAT resident B cells in aged mice using anti-CD20 monoclonal antibody directly injected into the visceral depot reduced FALC associated B cells in VAT but not in other tissues like spleen and restored insulin sensitivity in mice, providing evidence that local VAT B cells impair AT metabolic homeostasis (63). Moreover, expression of Oct coactivator B (OcaB), a B cell specific transcriptional coactivator important for transcription of the variable part of kappa light chain (66), is increased in VAT with age in both humans and mice. Global deletion of OcaB reduced B-2 cell numbers in both epididymal VAT and SAT, reduced proinflammatory IgG2c antibody levels, and improved insulin sensitivity in mice (62) providing further evidence of age-related B-2 cell-mediated effects on VAT inflammation and dysmetabolism.
Pro-inflammatory cytokine mediated AT inflammation
Elegant studies by Winer et.al. confirms the early accumulation of B cells in epididymal VAT in response to HFD, yet contrary to earlier speculation that these B cells protect against diet-induced inflammation, they and others have reported that B cells promote inflammation. Global B cell deficiency attenuated HFD-induced AT inflammation and IR (13,60,61,67). Epididymal VAT specific B cells from obese mice secreted pro-inflammatory cytokines such as IL-6 and interferon-gamma (INF-γ) and regulated activation of T cells and macrophages in VAT. Consistent with these findings, B cell-deficiency in HFD-fed mice result in lower concentrations of epididymal VAT-derived inflammatory cytokines such as IFN-γ (13,60) and reduction in HFD-induced IR (61). Introduction of splenic B cells from obese mice into B cell deficient mice worsened glucose tolerance (13,60,61). Given that more than 90% of the CD19+ B cell population in the spleen are B-2 cells, the worsening of the glucose tolerance is likely due to B-2 cells which activate local T cells and macrophages (61).
Not only are B-2 effects in promoting inflammation seen in epididymal VAT, but B cell infiltration into mesenteric VAT tissue and the liver in response to HFD also occurs early, predating T cell and macrophage infiltration. Expression levels of pro-inflammatory cytokines (IL-6, tumor necrosis factor-alpha (TNF-α) and macrophage chemoattractant protein-1 (MCP-1)) are reduced in mesenteric VAT of HFD-fed B cell deficient mice compared to HFD-fed wild-type mice. In addition, mesenteric VAT B cells from HFD-fed mice induce an inflammatory macrophage phenotype and expression of pro-inflammatory cytokines in vitro (68), implying that B cells are involved in macrophage activation followed by pro-inflammatory cytokine production. These data suggest that B cells may also play a role in obesity-linked non-alcoholic fatty liver disease (68).
Consistent with murine data, circulating B cells from obese and obese diabetic individuals secrete higher amounts of pro-inflammatory cytokines IL-6 and TNF-α in comparison to healthy individuals (69). Suggesting that obesity factor alone is enough and there is no additional effect of diabetes is required to produce pro-inflammatory cytokines from circulatory B cells. Also, B cells from diabetic individuals secrete high levels of pro-inflammatory IL-8 as compared to B cells from non-diabetic individuals (70) and serum IL-8 levels correlate with body mass index in obese patients (71). Given that IL-8 promotes neutrophil recruitment, B cells possibly promote neutrophil recruitment into VAT via local IL-8 secretion and result in disease progression. Understanding these pathways may be beneficial to develop therapeutics.
IgG mediated AT inflammation
In addition to promoting inflammation through cytokine production, B cell mediated-inflammation has also been linked to production of pro-inflammatory immunoglobulins. Pro-inflammatory IgG2c concentrations in serum and epididymal VAT are elevated in obese mice and B cell infiltration in AT is associated with increased IgG production. Moreover, adoptive transfer of IgG from HFD-fed mice into B cell-deficient mice induces IR (13). At the molecular level, the Fc portion of IgG binds to Fc-gamma receptors (FcγRs) expressed on macrophages to induce the secretion of pro-inflammatory TNF-α (13). IgG and FcγR also regulates antigen presentation by dendritic cells (72). Thus, a logical mechanism mediating the pro-inflammatory effects of IgG may be through binding to FcγRs expressed on macrophages and inducing inflammatory cytokine secretion. Initial studies suggested this may be the case as mice with FcγR-chain deficiency had reduced HFD-induced obesity, AT inflammation and IR (73). However, follow-on studies demonstrated that though deficiency in FcγRs reduced the activity of macrophages upon IgG stimulation, FcγR and complement protein C3 deficiency did not provide protection against HFD-induced glucose intolerance or AT inflammation (74) leaving the specific mechanisms whereby IgG may promote AT inflammation incompletely resolved.
Similar to mice, human omental VAT contains significantly more B-2 cells than SAT (35), although much less is known about immune mechanisms in human VAT as it is more difficult to study humans VAT due to the need for invasive techniques to acquire the tissue. There is evidence that B cells produce IgG and cytokines in human SAT. Crown-like structures consisting of macrophages and T cells have also been identified in human SAT (75) and B cells have been shown to be present in these crown-like structures suggesting that these B cells may be involved in regulating inflammation in SAT of humans (76). In support of this concept, Frasca et.al. demonstrated that B cell IgG antibodies secretion occurs in SAT and that B cells in SAT also express genes linked to the production of autoimmune IgG antibodies such as the transcription factor T-bet, the membrane marker CD11c and cytokines known to promote GC formation, isotype class switch, and plasma cell differentiation. Moreover, they identified high numbers of GC B cells and TFH cells in SAT compared to peripheral blood mononuclear cells in obese subjects suggesting that antibody production may occur locally in SAT (77).
Taken together, there is clear evidence for HFD induced early accumulation of B cells and age-related B cell effects leading to VAT inflammatory IgG and cytokine accumulation and IR. However, identification of the specific AT-derived self-antigens or local B cell activators in obesity and aging is needed.
Potential (self-) antigens in obesity, AT inflammation and IR
Evidence for specific antigens involved in obesity-related AT inflammation and IR is limited. However, there is a speculation about possible potential antigens coming from dietary components in the context of obesity related AT inflammation and IR (78). HFD induces stress to intestinal epithelium reducing barrier function (79). It has been demonstrated by adding ovalbumin to the diet of HFD-fed mice that HFD promotes intestinal absorption which can include gut bacterial antigenic components such as lipopolysaccharide (80,81), inducing inflammatory immune responses in nearby lymphoid tissues. Mice previously sensitized with ovalbumin antigen show significantly increased inflammatory responses with high numbers of T cell (in particular CD4+ T cells) accumulated, increased inflammatory cytokine gene expression in mesenteric VAT than SAT, and impaired glucose tolerance compared to the unsensitized control group after HFD feeding with 1% dietary ovalbumin (82). T cells in mice are primed/activated in mesenteric LNs or mesenteric VAT during sensitization to activate adaptive B cell responses (83,84). In addition, HFD induces changes in immunoglobulin heavy chain repertoire in AT B cells via increased somatic hyper mutations in variable region of the heavy chain, suggesting that AT B cells may undergone affinity maturation in response to dietary antigens with the help of local T cells (85). However, how these AT B cells respond to dietary and bacterial surface antigens through toll like receptors or B cell receptors in the context of AT inflammation during obesity and aging is unknown. Increased levels of circulatory IgG to gut bacterial antigens was seen in HFD-fed mice and similar findings were noted in obese diabetic individuals in comparison to low-fat diet fed mice and lean healthy individuals respectively (86). Studies from our lab showed evidence of increased B-1b cell proliferation in omental VAT after lipopolysaccharide injection compared to saline injected control mice (35), suggesting that the dietary or gut microbial antigens may regulate B cell biology in AT. Recent studies demonstrate that B-1 cells respond and proliferate more efficiently to TLR signals, while, B-2 cells proliferate after BCR signals and this proliferation is significantly enhanced after TLR signals (87). This suggests that B-1 and B-2 cells respond to antigens by different mechanisms. However, how AT B cell subsets respond to bacterial or HFD-induced antigens via TLR or BCR in AT inflammation needs to be explored. Further studies are needed to understand whether microbial-specific B cell responses occur in AT and whether gut microbial antigens and self-antigens have common antigenic features in obesity.
Human studies identified IgG autoantibodies in the serum of insulin resistant obese males. The top three antigen targets identified were Golgi SNAP receptor complex member 1, Bruton agammaglobulinemia tyrosine kinase and glial fibrillary acidic protein (13). Though the function of these antigen targets is unknown, presence of significantly high titers of glial fibrillary acidic protein autoantibodies were previously identified in both type 1 diabetes and T2D patient’s plasma samples compared to healthy donors (88). Increased numbers of B cells within islets (89) and islet-associated antigen specific autoantibodies against glial fibrillary acidic protein, glutamic acid decarboxylase-65 and IA-2 are identified in T2D individuals compared to healthy control individuals (88,90,91). This IgG autoantibody data suggests the possibility of having some common pathogenesis between obesity induced T2D and classical autoimmune disease type 1 diabetes in the context of self-antigens and autoantibodies. Murine mechanistic studies will be helpful to determine whether these self-antigens observed in T2D individuals have the capacity to induce disease in healthy controls. In addition, future studies to identify potential dominant antigens in the context of obesity related AT inflammation, diabetes and IR could lead to antigen specific targeted therapies. Population-based correlation studies showed that obesity is an important provocation factor to aggravate other classical autoimmune disease conditions (92), suggesting that obesity may be involved in progression of autoimmune disease by inducing/increasing auto-antibody production.
Role of co-stimulatory signals in obesity and AT inflammation
In adaptive immune responses, antigen presenting cells such as macrophages, dendritic cells and B cells present antigenic peptides via surface major histocompatibility complex class-II molecule to T cell receptor on T helper cells to initiate antigen specific immune response. In addition to this primary signal, there are other co-stimulatory signals that are required to complete this process. For instance, in B-T cell interaction, the engagement of the B cell receptor by the antigen in combination with co-stimulatory signals from T helper cells are required to deliver survival signals that rescue B cells which display high-affinity immunoglobulins on their surface from apoptosis. Here we discuss co-stimulatory signals linked to AT inflammation.
CD40 – CD40L:
A well-characterized interaction occurs between the CD40 receptor and its ligand (CD40L) and this co-stimulatory signal is important in HFD induced obesity, AT inflammation and IR in mice and humans (93–95). Global knock out or antibody mediated blockade of CD40L ameliorates HFD induced obesity, AT inflammation and IR in mice (94). In addition, upon targeting CD40L-CD40 downstream adaptor proteins such as TNF receptor associated factors (TRAFs) by genetic deletion, blocking or inhibition of CD40-TRAF6 interaction, there is reduction in diet-induced obesity, AT inflammation, and glucose tolerance. Also, there was prevalence of anti-inflammatory environment and reduction in T cell and macrophage accumulation in epididymal VAT (96,97), suggesting that blocking CD40-TRAF6 interaction may be an important therapeutic target to reduce obesity induced AT inflammation and IR. Whether these effects are B cell-mediated is unknown.
Surprisingly, mice with global deletion of CD40, had worsened AT inflammation and IR; an effect thought to be mediated by increased CD8+ T cell and F4/80+ macrophage numbers in epididymal VAT compared to control (98,99). This effect was not due to loss of CD40 on macrophages, although the cell type involved in this worsening of AT inflammation following CD40 global knockout is unknown (100). Effects could be due to existence of alternate CD40L receptors such as αIIbβ3 on platelets (101), Mac-1 on macrophages (102) and α5β1 on monocytes (103) activation of alternative pathways that could lead to AT inflammation in CD40−/− mice. As CD40-CD40L co-stimulatory signaling between T and B cells is important for memory B cell and plasma cell differentiation, future studies are required to understand the role of B cell specific CD40 in AT inflammation.
CD28 – CD80/CD86 and CTLA-4 – CD80/CD86:
Another important co-stimulatory signal for antigen presenting cell – T cell interaction is CD28 (expressed on T helper cell) or cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) (expressed on activated T cells and regulatory T cells) and their ligands CD80/CD86 (expressed on antigen presenting cells including B cells). CTLA-4Ig treatment reduced epididymal VAT and SAT weight, adipocyte size, promoted M2 polarization and reduced HFD-induced IR (104). Yet, CD80/CD86 double knockout reduced regulatory T cell development and proliferation in epididymal VAT, spleen, LN and blood and worsened obesity-induced VAT inflammation and IR in both HFD and normal chow diet conditions. Although, it is unclear if B cells are involved in this effect (105).
B-1 cells limit HFD-induced AT inflammation
In addition to B-2 cells, B-1 cells are also present in AT (35,106). However, in contrast to B-2 cell-derived IgG which aggravates inflammation, B-1 cell-derived natural IgM blocks inflammation (33,107–109). We and others have demonstrated that B-1 cells attenuate VAT inflammation, glucose intolerance, and IR in diet-induced obese mice (35,106). Shen L, et al., found that the percentage of B-1a cells in the peritoneal cavity and the VAT of mice were reduced after HFD feeding. Adoptive transfer of B-1a cells into B cell-deficient mice improved glucose tolerance. The protective effect of the B-1a cells was mediated via IL-10 production and polyclonal IgM (106). In vitro studies demonstrated that B-1a-derived IL-10 not only promoted macrophage IL-10 production but reduced IL-6 and TNF-α production in macrophages (106,110), consistent with a previous report that B-1a cells induce M2 polarization in macrophages (111).
While most studies have focused on CD5+ B-1a cells, recent evidence suggests an equally important role for the CD5− B-1 cell, the B-1b cell in IgM production and attenuation of inflammation in AT. B-1a cells produced more IgM than B-1b cells in response to lipopolysaccharide stimulation in vitro. However, adoptive transfer studies demonstrated that B-1b cells produce more IgM than B-1a cells in vivo (112). We identified the helix-loop-helix transcription factor Id3 as an important regulator of plasma and AT IgM levels and B-1b cell numbers. Mice that lack Id3 specifically in B cells (ID3BKO) have increased B-1b cell numbers and IgM in several compartments including AT (35,112). Diet-induced obese ID3BKO mice had reduced levels of VAT inflammatory cytokines and a significant reduction in HFD-induced M1 macrophage production of TNF-α and MCP-1. Moreover, the production of these cytokines by M1 macrophage was significantly inhibited by B-1b cell conditioned media, providing evidence that B-1b cells in AT can serve to limit diet-induced AT inflammation. Adoptive transfer of Id3 deficient B-1b cells to B and T cell deficient Rag1−/− mice resulted in recruitment to VAT, local IgM secretion and attenuated diet-induced glucose intolerance and VAT IR. In contrast, adoptive transfer of B-1b B cells unable to secrete IgM, had no effect on glucose tolerance, providing evidence that B-1b cell effects on metabolism are due, at least in part, to the secretion of IgM. B-1 cell-derived IgM is considered a pattern recognition receptor that can bind to danger associated molecular patterns and inhibit inflammatory signaling (109,113). Notably, B-1 cells are also enriched in human omental VAT and IgM antibodies to oxidation-specific epitopes produced in the omental VAT inversely correlated with plasma MCP-1 levels in humans (35).
Breg cells limit AT inflammation and IR
In addition to the anti-inflammatory effects of B-1 cell-derived IgM, B-1 cells as well as other B-1 and B-2 derived subtypes can provide suppression of immune responses in AT via IL-10 production. B cell types that produce IL-10, termed Breg cells, are present in AT (114,115). Studies from diabetic patients showed that the % of IL-10+ B cells were significantly reduced in circulation of diabetic patients compared to non-diabetic control donors. In addition, circulating B cells from diabetic patients secrete low levels of anti-inflammatory IL-10 cytokine compared to B cells from non-diabetic donors (70). Similarly, B cell derived IL-10 levels were significantly reduced in obese diabetic patients after stimulation with anti-CD40+B cell receptor compared to healthy controls. Suggesting that B cells in obese diabetic patients have reduced capacity to produce anti-inflammatory IL-10 cytokine (69), secretion of which by Breg cells actually confers protection to diabetes in humans. Supporting to this concept, Nishimura S, et al., identified CD19+ CD45R+ CD22+ CD5− IgM+ IgD+ B cell as IL-10 producers (Breg cells) and demonstrated that B cell-specific IL-10 deletion enhanced, whereas adoptive transfer of these AT Breg cells ameliorated adipose inflammation and IR in diet-induced obese mice (114). VAT in lean mice harbors another type of IL-10 producing cell which are primarily CD5+ B-1a cells. These Breg numbers reduce in VAT after splenectomy, suggesting that the spleen supports maintenance of VAT resident Bregs. Moreover, adoptive transfer of CD5+ B-1a cells attenuate HFD induced IR in mice by recruiting more B-1a cells into VAT and secreting IL-10 locally (116). The expression levels of B cell markers (CD19) and IL10 in human SAT inversely correlated with body mass index, suggesting that B cell IL-10 may impact on obesity in humans (114). Consistent with these findings, Garcia-Hernandez, et al., reported a reduced percentage of CD19+CD27+CD38High and CD19+CD24HighCD38High IL-10+ Breg cells in AT and circulation of overweight and obesity individuals compared to individuals with normal weight. These overweight individuals had elevated production of IL-17 and IFN-γ in their cultured CD4+ T cells compared to individuals with normal-weight (115).
Taken together, studies provide clear evidence that the role of B cells in AT inflammation, T2D and IR is subset dependent; with B-2 cells aggravating and B-1 and Bregs attenuating AT inflammation and metabolic dysfunction. Yet, the regulation of the accumulation of pathogenic and protective B cells in AT is incompletely understood.
Regulation of B cell accumulation in AT
B cell accumulation in AT could be enhanced through the enhancement of B cell survival and proliferation or through chemokine-mediated recruitment. Camell et.al. demonstrated that nucleotide-binding domain and leucine-rich repeat containing protein (Nlrp) 3 inflammasome, important for inflammatory IL-1β and IL-18 production from innate immune cells such as macrophages, induced VAT resident B cell accumulation and expansion in FALCs. Age associated B cells in FALCs express IL1-R and blocking of IL-1β reduces this pro-inflammatory AT B cell proliferation in aged mice (63). Several studies provide evidence that adipocyte and stromal vascular cell-derived chemokines, and the respective chemokine receptors expressed on B cells are involved in B cell recruitment into AT to increase inflammation. Leukotriene B4 receptor (LTB4R1) expression on B-2 cells, for instance, is important for B-2 cell chemotaxis, localization in VAT, and B-2 cell-induced IR via T cell activation in VAT (61). Total B cell recruitment into FALCs in mesenteric VAT (44) and B-1 cell recruitment into omental VAT (41) is mediated by the CXCL13 - C-X-C chemokine receptor-5 (CXCR5) axis. Consistent with this data, global CXCR5 deficiency specifically reduced IgM secreting cells in omental and epididymal VAT but not in PVAT and SAT compared to CXCR5 wild type mice (Srikakulapu P, unpublished data). We have previously shown that another C-C chemokine receptor-6 (CCR6), expression on B cells, is important for their recruitment to the aorta including PVAT (117) and CCR6 deficient mice show reduced B cell numbers particularly in PVAT but not in other ATs (118) (Srikakulapu P, unpublished data), suggesting that specific chemokine/chemokine receptor axes regulate B cell recruitment into different VAT anatomic locations. In addition, splenic B cells when co-cultured with adipocytes differentiate into pro-inflammatory B cell type, suggesting that adipocyte derived mediators induce a pro-inflammatory B cell phenotype and these mediators such as chemokines may be involved in B cell recruitment into VAT (119). Though the studies done so far show total B cell recruitment to AT, more such studies to delineate specific chemokine-receptor axes which regulate B cell subset specific recruitment into AT during inflammation are needed.
AT B cells in atherosclerosis
Studies have clearly demonstrated that B-2 cells aggravate while B-1 cells attenuate atherosclerosis (33,112,120–122). Obesity, is associated with CVD (123,124) through both direct and indirect mechanisms (Figure 4). Obese AT is a source of inflammatory cytokines that can promote MetS, diabetes and the associated dyslipidemia, all of which increase CVD risk (34). We have outlined mechanisms whereby AT B cells regulate local and systemic inflammation in the sections above. However, another important way in which B cells in AT can impact on CVD is through local mechanisms. In humans, large B cell clusters develop in epicardial AT in subjects with coronary artery disease (CAD) compared to those without CAD. Similarly, in mice, B cell numbers significantly increase in pericardial AT, but not in heart tissue, after 3 days of myocardial infarction (MI). This B cell increase is controlled by local B cell proliferation in pericardial AT in response to MI. In addition, these B cells produce granulocyte-macrophage colony-stimulating factors to recruit and maintain dendritic cells in pericardial AT and increase fibrosis post-MI. This post-MI fibrosis is reversed upon B cell depletion or surgical removal of pericardial AT (125), indicating the role of AT B cells in pathogenesis. In contrast, presence of IL-10 producing B cells in pericardial AT provide protection from acute MI in mice and most of these IL-10 producing B cells are of the CD5+ B-1a subtype. Interestingly, this IL-10 producing B-1a cell enrichment in pericardial AT is mediated by the B cell specific chemokine CXCL13 and by the cytokine IL-33 (126), which is important for B-1 cell expansion and IgM production (127,128). These results suggest that MI can be regulated/balanced by both pro- and anti-inflammatory B cells in pericardial AT FALCs.
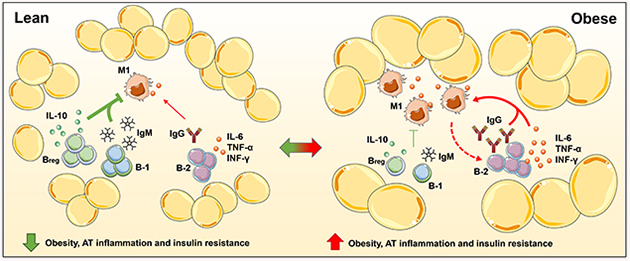
Figure 4: B cell subtype regulation of inflammatory mediators in adipose tissue and potential impact on atherosclerosis:
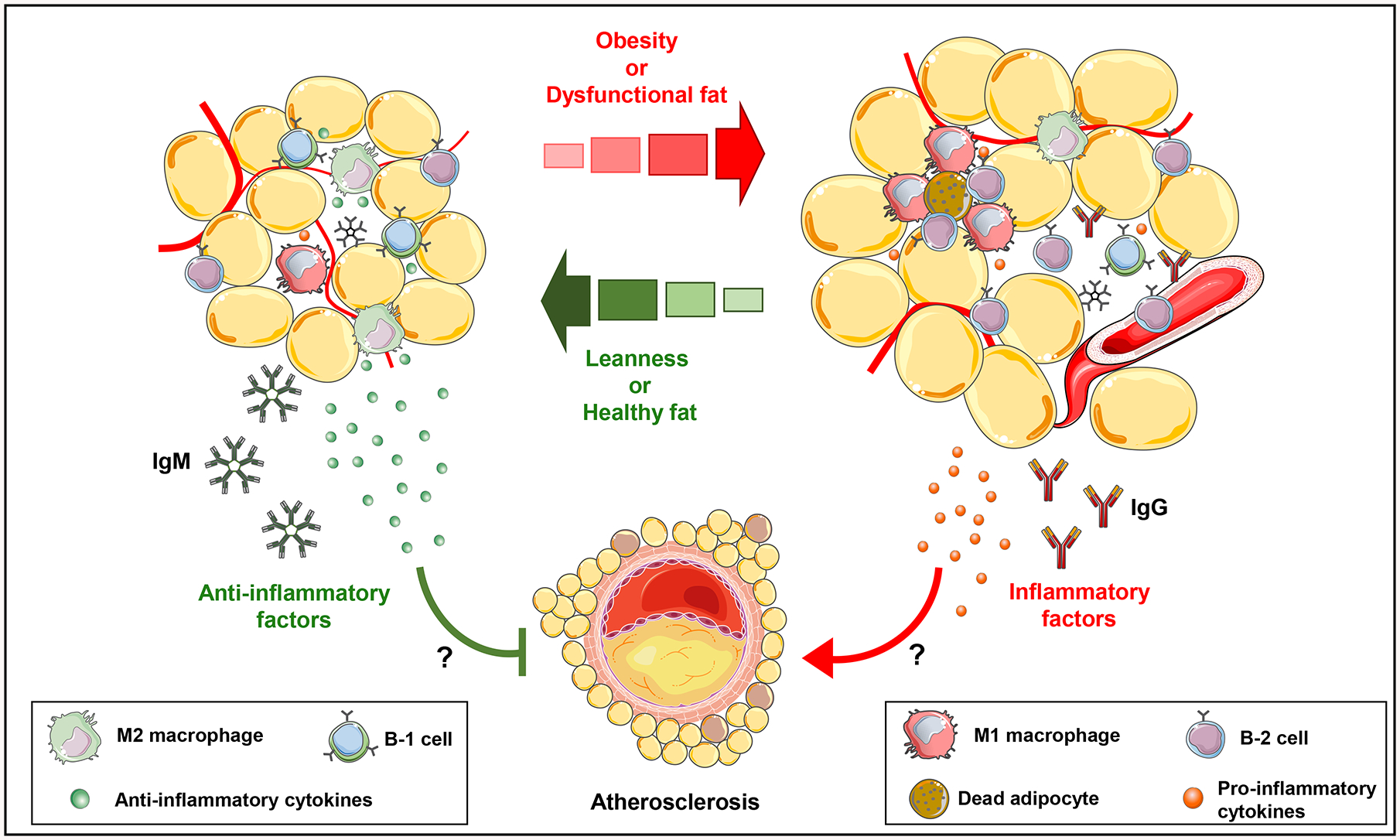
In lean mice, AT resident B-1 cells and M-2 macrophages secrete anti-inflammatory cytokines and natural IgM that attenuate AT inflammation and potentially atherosclerosis. In contrast, in obese mice, AT becomes dysfunctional followed by recruitment of inflammatory immune cells. Recruited B-2 cells and M-1 macrophages secrete pro-inflammatory cytokines and IgG that promote AT inflammation and potentially atherosclerosis. The impact of AT B cells may be systemic or local via effects in the PVAT.
Another important AT is PVAT, which is in direct contact with the aortic adventitia and adipocytes in PVAT secrete both pro inflammatory and anti-inflammatory cytokines can regulate atherogenesis (129). PVAT transplantation experiments in mice revealed that PVAT-derived MCP-1 increases (130) while PVAT derived low-density lipoprotein receptor-related protein-1 attenuates lesion development in wire injury model (131). Evidence suggests both pro- and anti-inflammatory processes in PVAT that can impact on underlying vascular lesions. Interestingly, in mice the outer layer of the vessel wall including the PVAT harbors both atherogenic B-2 cells and atheroprotective B-1 cells (128). These local B-1 cells can be induced to proliferate by IL-5 and they secrete atheroprotective IgM locally (33,128). Whether local B-1 cells in PVAT can reduce inflammation remains an unanswered and important question particularly given the role of B-1b-derived IgM in inhibiting M1 macrophage production of MCP-1, the pro-inflammatory cytokine implicated in PVAT-induced vascular pathology. Equally important is whether PVAT adjacent to human blood vessels might contributed to atherosclerosis. Indeed, PVAT adjacent to human blood vessels is more inflamed than PVAT adjacent to non-diseased vessel segments (132). Immunofluorescent staining of human coronary artery including PVAT revealed B cell-rich FALC in PVAT adjacent to an advanced plaque (33) and B cell density in PVAT near plaque, positively correlates with arterial dysfunction (133). Flow cytometry of coronary artery PVAT from hearts explanted at the time of transplantation revealed the presence of both B-1 and B-2 cells (33), raising the intriguing possibility that the relative abundance and activity of B-1 and B-2 cells in PVAT may regulate inflammation and atherosclerosis in human coronary arteries
Translational B cell targeted therapies
B cell depleting antibodies such as Rituximab (anti-CD20 antibody) and Belimumab (blocks the B cell activating factor - B cell activating factor receptor interaction) are well known monoclonal antibodies that impair mature B cell development, antibody production and reduce autoimmune diseases (134). However, with the exception of a few murine studies (13,63) to date there have been no published clinical trials showing the possible protective effect of B cell depletion therapies or therapies targeting co-stimulatory molecules on B cells in obesity induced T2D and IR. Also, it is important to remember that though B cell depletion therapies have a beneficial effect in autoimmune diseases, in the context of obesity, T2D and IR, reducing B cell derived protective IgM and IL-10 levels would increase AT inflammation. As such, monitoring the effect of B cell depletion or co-stimulatory checkpoint inhibitor therapies on IR, T2D and CVD in subjects receiving these therapies for autoimmune disease or cancer could provide evidence to support prospective trials of these therapies for cardiometabolic disease.
Conclusions
In summary, in both mice and humans, of all types of AT harbor B cells, although the number and frequency of specific subsets of B cells varies by AT type and location. Importantly, B cell effects in AT are subset-dependent: with B-2 cells promoting inflammation in HFD-fed and aged mice through both inflammatory cytokine production and IgG production; and B-1 cells and Bregs inhibiting inflammation through production of natural IgM and IL-10 respectively. The mechanisms that regulate subset-specific B cell accumulation in AT, specific antigens targeted by B cell-derived immunoglobulins, the role of co-stimulatory molecules or B cell targeted therapies are incompletely understood. As such, many important unanswered questions remain. What additional factors regulate the number of B-1 cells in AT and are they depot-specific? Could AT B-1 cells be induced to produce more protective IgM and could this limit diet-induced inflammation and dysmetabolism in humans? What are the relevant antigens or danger associated molecular patterns in AT? Are there sex-specific effects on AT specific B cell responses during steady state and obesity-induced inflammation? Are local LNs involved in B cell adaptive immunity in response to AT inflammation? Could bolstering PVAT B-1 cell number impact on CVD? Answers to these and the many other remaining questions about B cells in AT may inform novel strategies to limit AT inflammation and its damaging effects on health.
Highlights.
B cells reside in all types and locations of ATs in both mice and humans and have a markedly higher ratio of B-1:B-2 cells than spleen and bone marrow.
Mechanisms regulating B cell accumulation and effects in AT are subset-dependent.
B-2 cells promoting inflammation in HFD-fed and aged mice through both inflammatory cytokine production and IgG production.
B-1 cells and Bregs inhibit inflammation through production of natural IgM and IL-10 respectively.
Specific antigens targeted by B cell-derived immunoglobulins, and the role of co-stimulatory molecules or B cell targeted therapies are incompletely understood. However, future studies to expand knowledge in these areas may hold promise for novel immunotherapy for adipose inflammation and its attendance cardiometabolic diseases.
Sources of Funding
The research in the author’s laboratory covered in this review was supported by National Institutes of Health grants HL107490, HL136098, and HL136275 (CAM) and an AHA Career Development Award (18CDA34110392) to PS.
Non-standard abbreviations and acronyms
- AT
adipose tissue
- VAT
visceral adipose tissue
- MetS
metabolic syndrome
- IR
insulin resistance
- CVD
cardiovascular diseases
- T2D
type 2 diabetes
- LN
lymph nodes
- TFH
follicular helper T cells
- GC
germinal center
- IL
interleukin
- TGF-β
transforming growth factor-beta
- Bregs
regulatory B cells
- PVAT
perivascular adipose tissue
- BAT
brown adipose tissue
- SAT
subcutaneous adipose tissue
- MS
milky spots
- CXCL13
chemokine C-X-C ligand-13
- FALC
fat associated lymphoid cluster
- HFD
high fat diet
- INF-γ
interferon gamma
- TNF-α
tumor necrosis factor-alpha
- FcγR
Fc-gamma receptor
Footnotes
Disclosure
None
References
- 1.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Molecules and cells 2014;37:365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochimica et biophysica acta 2014;1842:446–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy P, Lent-Schochet D, Ramakrishnan N, McLaughlin M, Jialal I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clinica chimica acta; international journal of clinical chemistry 2019;496:35–44. [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet (London, England) 2005;365:1415–28. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Zhao J, Meng H, Zhang X. Adipose Tissue-Resident Immune Cells in Obesity and Type 2 Diabetes. Frontiers in immunology 2019;10:1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends in endocrinology and metabolism: TEM 2012;23:407–15. [DOI] [PubMed] [Google Scholar]
- 7.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. The Journal of endocrinology 2014;222:R113–27. [DOI] [PubMed] [Google Scholar]
- 8.Boutens L, Hooiveld GJ, Dhingra S, Cramer RA, Netea MG, Stienstra R. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia 2018;61:942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe Y, Nagai Y, Honda H et al. Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2019;33:11821–11835. [DOI] [PubMed] [Google Scholar]
- 10.Stefanovic-Racic M, Yang X, Turner MS et al. Dendritic cells promote macrophage infiltration and comprise a substantial proportion of obesity-associated increases in CD11c+ cells in adipose tissue and liver. Diabetes 2012;61:2330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertola A, Ciucci T, Rousseau D et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 2012;61:2238–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priceman SJ, Kujawski M, Shen S et al. Regulation of adipose tissue T cell subsets by Stat3 is crucial for diet-induced obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America 2013;110:13079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winer DA, Winer S, Shen L et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature medicine 2011;17:610–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russo L, Lumeng CN. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018;155:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Wu H. T Cells in Adipose Tissue: Critical Players in Immunometabolism. Frontiers in immunology 2018;9:2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boothby MR, Hodges E, Thomas JW. Molecular regulation of peripheral B cells and their progeny in immunity. Genes & development 2019;33:26–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srikakulapu P, McNamara CA. B cells and atherosclerosis. American journal of physiology Heart and circulatory physiology 2017;312:H1060–h1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan AT, Roghanian A, Cragg MS. B cells--masters of the immunoverse. The international journal of biochemistry & cell biology 2011;43:280–5. [DOI] [PubMed] [Google Scholar]
- 19.Baumgarth N B-cell immunophenotyping. Methods in cell biology 2004;75:643–62. [DOI] [PubMed] [Google Scholar]
- 20.Hamel KM, Liarski VM, Clark MR. Germinal center B-cells. Autoimmunity 2012;45:333–47. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002;16:219–30. [DOI] [PubMed] [Google Scholar]
- 22.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nature immunology 2002;3:944–50. [DOI] [PubMed] [Google Scholar]
- 23.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. The Journal of experimental medicine 2003;197:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. Journal of immunology (Baltimore, Md : 1950) 2001;167:1081–9. [DOI] [PubMed] [Google Scholar]
- 25.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. Journal of immunology (Baltimore, Md : 1950) 2003;170:5897–911. [DOI] [PubMed] [Google Scholar]
- 26.Shen P, Roch T, Lampropoulou V et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014;507:366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang RX, Yu CR, Dambuza IM et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nature medicine 2014;20:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacological reviews 2003;55:241–69. [DOI] [PubMed] [Google Scholar]
- 29.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015;42:607–12. [DOI] [PubMed] [Google Scholar]
- 30.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Current opinion in immunology 2008;20:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunological reviews 2008;224:201–14. [DOI] [PubMed] [Google Scholar]
- 32.Mizoguchi A, Bhan AK. A case for regulatory B cells. Journal of immunology (Baltimore, Md : 1950) 2006;176:705–10. [DOI] [PubMed] [Google Scholar]
- 33.Srikakulapu P, Upadhye A, Rosenfeld SM et al. Perivascular Adipose Tissue Harbors Atheroprotective IgM-Producing B Cells. Front Physiol 2017;8:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oikonomou EK, Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nature reviews Cardiology 2019;16:83–99. [DOI] [PubMed] [Google Scholar]
- 35.Harmon DB, Srikakulapu P, Kaplan JL et al. Protective Role for B-1b B Cells and IgM in Obesity-Associated Inflammation, Glucose Intolerance, and Insulin Resistance. Arteriosclerosis, thrombosis, and vascular biology 2016;36:682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams R, White H. The greater omentum: its applicability to cancer surgery and cancer therapy. Current problems in surgery 1986;23:789–865. [DOI] [PubMed] [Google Scholar]
- 37.Wilkosz S, Ireland G, Khwaja N et al. A comparative study of the structure of human and murine greater omentum. Anatomy and embryology 2005;209:251–61. [DOI] [PubMed] [Google Scholar]
- 38.Cohen CA, Shea AA, Heffron CL, Schmelz EM, Roberts PC. Intra-abdominal fat depots represent distinct immunomodulatory microenvironments: a murine model. PloS one 2013;8:e66477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meza-Perez S, Randall TD. Immunological Functions of the Omentum. Trends in immunology 2017;38:526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morison R Remarks ON SOME FUNCTIONS OF THE OMENTUM. British medical journal 1906;1:76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 2002;16:67–76. [DOI] [PubMed] [Google Scholar]
- 42.Moro K, Yamada T, Tanabe M et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 2010;463:540–4. [DOI] [PubMed] [Google Scholar]
- 43.Elewa YH, Ichii O, Otsuka S, Hashimoto Y, Kon Y. Characterization of mouse mediastinal fat-associated lymphoid clusters. Cell and tissue research 2014;357:731–41. [DOI] [PubMed] [Google Scholar]
- 44.Benezech C, Luu NT, Walker JA et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nature immunology 2015;16:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newland SA, Mohanta S, Clement M et al. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nature communications 2017;8:15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson KR, Flaherty DK, Hasty AH. Obesity Alters B Cell and Macrophage Populations in Brown Adipose Tissue. Obesity (Silver Spring, Md) 2017;25:1881–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation 1995;95:2409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science (New York, NY) 1996;271:665–8. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Barnes GT, Yang Q et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation 2003;112:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2003;27 Suppl 3:S49–52. [DOI] [PubMed] [Google Scholar]
- 51.Holland WL, Bikman BT, Wang LP et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. The Journal of clinical investigation 2011;121:1858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007;56:16–23. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura S, Manabe I, Nagasaki M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature medicine 2009;15:914–20. [DOI] [PubMed] [Google Scholar]
- 54.Winer S, Chan Y, Paltser G et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nature medicine 2009;15:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morris DL, Cho KW, Delproposto JL et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 2013;62:2762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cipolletta D, Feuerer M, Li A et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012;486:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh DY, Morinaga H, Talukdar S, Bae EJ, Olefsky JM. Increased macrophage migration into adipose tissue in obese mice. Diabetes 2012;61:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feuerer M, Herrero L, Cipolletta D et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature medicine 2009;15:930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochemical and biophysical research communications 2009;384:482–5. [DOI] [PubMed] [Google Scholar]
- 60.DeFuria J, Belkina AC, Jagannathan-Bogdan M et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences of the United States of America 2013;110:5133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ying W, Wollam J, Ofrecio JM et al. Adipose tissue B2 cells promote insulin resistance through leukotriene LTB4/LTB4R1 signaling. The Journal of clinical investigation 2017;127:1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carter S, Miard S, Caron A et al. Loss of OcaB Prevents Age-Induced Fat Accretion and Insulin Resistance by Altering B-Lymphocyte Transition and Promoting Energy Expenditure. Diabetes 2018;67:1285–1296. [DOI] [PubMed] [Google Scholar]
- 63.Camell CD, Gunther P, Lee A et al. Aging Induces an Nlrp3 Inflammasome-Dependent Expansion of Adipose B Cells That Impairs Metabolic Homeostasis. Cell metabolism 2019;30:1024–1039.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rubtsov AV, Rubtsova K, Fischer A et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood 2011;118:1305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 2011;118:1294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casellas R, Jankovic M, Meyer G et al. OcaB is required for normal transcription and V(D)J recombination of a subset of immunoglobulin kappa genes. Cell 2002;110:575–85. [DOI] [PubMed] [Google Scholar]
- 67.Ying W, Tseng A, Chang RC et al. miR-150 regulates obesity-associated insulin resistance by controlling B cell functions. Scientific reports 2016;6:20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Z, Xu J, Tan J et al. Mesenteric adipose tissue B lymphocytes promote local and hepatic inflammation in non-alcoholic fatty liver disease mice. Journal of cellular and molecular medicine 2019;23:3375–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhai X, Qian G, Wang Y et al. Elevated B Cell Activation is Associated with Type 2 Diabetes Development in Obese Subjects. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2016;38:1257–66. [DOI] [PubMed] [Google Scholar]
- 70.Jagannathan M, McDonnell M, Liang Y et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia 2010;53:1461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharabiani MT, Vermeulen R, Scoccianti C et al. Immunologic profile of excessive body weight. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals 2011;16:243–51. [DOI] [PubMed] [Google Scholar]
- 72.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity 2005;23:503–14. [DOI] [PubMed] [Google Scholar]
- 73.van Beek L, Vroegrijk IO, Katiraei S et al. FcRgamma-chain deficiency reduces the development of diet-induced obesity. Obesity (Silver Spring, Md) 2015;23:2435–44. [DOI] [PubMed] [Google Scholar]
- 74.van Dam AD, van Beek L, Pronk ACM et al. IgG is elevated in obese white adipose tissue but does not induce glucose intolerance via Fcgamma-receptor or complement. International journal of obesity (2005) 2018;42:260–269. [DOI] [PubMed] [Google Scholar]
- 75.Apovian CM, Bigornia S, Mott M et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arteriosclerosis, thrombosis, and vascular biology 2008;28:1654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonnell ME, Ganley-Leal LM, Mehta A et al. B lymphocytes in human subcutaneous adipose crown-like structures. Obesity (Silver Spring, Md) 2012;20:1372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frasca D, Diaz A, Romero M, Thaller S, Blomberg BB. Secretion of autoimmune antibodies in the human subcutaneous adipose tissue. PloS one 2018;13:e0197472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsai S, Clemente-Casares X, Revelo XS, Winer S, Winer DA. Are obesity-related insulin resistance and type 2 diabetes autoimmune diseases? Diabetes 2015;64:1886–97. [DOI] [PubMed] [Google Scholar]
- 79.Kvietys PR, Specian RD, Grisham MB, Tso P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. The American journal of physiology 1991;261:G384–91. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Ghoshal S, Ward M, de Villiers W, Woodward J, Eckhardt E. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PloS one 2009;4:e8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. Journal of lipid research 2009;50:90–7. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y, Li J, Tang L et al. T-lymphocyte responses to intestinally absorbed antigens can contribute to adipose tissue inflammation and glucose intolerance during high fat feeding. PloS one 2010;5:e13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim CS, Lee SC, Kim YM et al. Visceral fat accumulation induced by a high-fat diet causes the atrophy of mesenteric lymph nodes in obese mice. Obesity (Silver Spring, Md) 2008;16:1261–9. [DOI] [PubMed] [Google Scholar]
- 84.Magnuson AM, Regan DP, Fouts JK, Booth AD, Dow SW, Foster MT. Diet-induced obesity causes visceral, but not subcutaneous, lymph node hyperplasia via increases in specific immune cell populations. Cell proliferation 2017;50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pham TD, Chng MHY, Roskin KM et al. High-fat diet induces systemic B-cell repertoire changes associated with insulin resistance. Mucosal immunology 2017;10:1468–1479. [DOI] [PubMed] [Google Scholar]
- 86.Mohammed N, Tang L, Jahangiri A, de Villiers W, Eckhardt E. Elevated IgG levels against specific bacterial antigens in obese patients with diabetes and in mice with diet-induced obesity and glucose intolerance. Metabolism: clinical and experimental 2012;61:1211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Savage HP, Klasener K, Smith FL, Luo Z, Reth M, Baumgarth N. TLR induces reorganization of the IgM-BCR complex regulating murine B-1 cell responses to infections. eLife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gomez-Tourino I, Camina-Darriba F, Otero-Romero I et al. Autoantibodies to glial fibrillary acid protein and S100beta in diabetic patients. Diabetic medicine : a journal of the British Diabetic Association 2010;27:246–8. [DOI] [PubMed] [Google Scholar]
- 89.Butcher MJ, Hallinger D, Garcia E et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia 2014;57:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pietropaolo M, Barinas-Mitchell E, Pietropaolo SL, Kuller LH, Trucco M. Evidence of islet cell autoimmunity in elderly patients with type 2 diabetes. Diabetes 2000;49:32–8. [DOI] [PubMed] [Google Scholar]
- 91.Turner R, Stratton I, Horton V et al. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet (London, England) 1997;350:1288–93. [DOI] [PubMed] [Google Scholar]
- 92.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmunity reviews 2014;13:981–1000. [DOI] [PubMed] [Google Scholar]
- 93.Poggi M, Jager J, Paulmyer-Lacroix O et al. The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes. Diabetologia 2009;52:1152–63. [DOI] [PubMed] [Google Scholar]
- 94.Poggi M, Engel D, Christ A et al. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arteriosclerosis, thrombosis, and vascular biology 2011;31:2251–60. [DOI] [PubMed] [Google Scholar]
- 95.Chatzigeorgiou A, Phieler J, Gebler J, Bornstein SR, Chavakis T. CD40L stimulates the crosstalk between adipocytes and inflammatory cells. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 2013;45:741–7. [DOI] [PubMed] [Google Scholar]
- 96.Chatzigeorgiou A, Seijkens T, Zarzycka B et al. Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance. Proceedings of the National Academy of Sciences of the United States of America 2014;111:2686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van den Berg SM, Seijkens TT, Kusters PJ et al. Blocking CD40-TRAF6 interactions by small-molecule inhibitor 6860766 ameliorates the complications of diet-induced obesity in mice. International journal of obesity (2005) 2015;39:782–90. [DOI] [PubMed] [Google Scholar]
- 98.Yi Z, Stunz LL, Bishop GA. CD40-mediated maintenance of immune homeostasis in the adipose tissue microenvironment. Diabetes 2014;63:2751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolf D, Jehle F, Michel NA et al. Coinhibitory suppression of T cell activation by CD40 protects against obesity and adipose tissue inflammation in mice. Circulation 2014;129:2414–25. [DOI] [PubMed] [Google Scholar]
- 100.Aarts S, Reiche ME, den Toom M et al. Macrophage CD40 plays a minor role in obesity-induced metabolic dysfunction. PloS one 2018;13:e0202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andre P, Prasad KS, Denis CV et al. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nature medicine 2002;8:247–52. [DOI] [PubMed] [Google Scholar]
- 102.Zirlik A, Maier C, Gerdes N et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation 2007;115:1571–80. [DOI] [PubMed] [Google Scholar]
- 103.Leveille C, Bouillon M, Guo W et al. CD40 ligand binds to alpha5beta1 integrin and triggers cell signaling. The Journal of biological chemistry 2007;282:5143–51. [DOI] [PubMed] [Google Scholar]
- 104.Fujii M, Inoguchi T, Batchuluun B et al. CTLA-4Ig immunotherapy of obesity-induced insulin resistance by manipulation of macrophage polarization in adipose tissues. Biochemical and biophysical research communications 2013;438:103–9. [DOI] [PubMed] [Google Scholar]
- 105.Zhong J, Rao X, Braunstein Z et al. T-cell costimulation protects obesity-induced adipose inflammation and insulin resistance. Diabetes 2014;63:1289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shen L, Chng MH, Alonso MN, Yuan R, Winer DA, Engleman EG. B-1a lymphocytes attenuate insulin resistance. Diabetes 2015;64:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chou MY, Fogelstrand L, Hartvigsen K et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. The Journal of clinical investigation 2009;119:1335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chou MY, Hartvigsen K, Hansen LF et al. Oxidation-specific epitopes are important targets of innate immunity. Journal of internal medicine 2008;263:479–88. [DOI] [PubMed] [Google Scholar]
- 109.Miller YI, Choi SH, Wiesner P et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circulation research 2011;108:235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barbeiro DF, Barbeiro HV, Faintuch J et al. B-1 cells temper endotoxemic inflammatory responses. Immunobiology 2011;216:302–8. [DOI] [PubMed] [Google Scholar]
- 111.Wong SC, Puaux AL, Chittezhath M et al. Macrophage polarization to a unique phenotype driven by B cells. European journal of immunology 2010;40:2296–307. [DOI] [PubMed] [Google Scholar]
- 112.Rosenfeld SM, Perry HM, Gonen A et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circulation research 2015;117:e28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Witztum JL, Lichtman AH. The influence of innate and adaptive immune responses on atherosclerosis. Annual review of pathology 2014;9:73–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishimura S, Manabe I, Takaki S et al. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell metabolism 2013;18:759–766. [DOI] [PubMed] [Google Scholar]
- 115.Garcia-Hernandez MH, Rodriguez-Varela E, Garcia-Jacobo RE et al. Frequency of regulatory B cells in adipose tissue and peripheral blood from individuals with overweight, obesity and normal-weight. Obesity research & clinical practice 2018;12:513–519. [DOI] [PubMed] [Google Scholar]
- 116.Wu L, Parekh VV, Hsiao J, Kitamura D, Van Kaer L. Spleen supports a pool of innate-like B cells in white adipose tissue that protects against obesity-associated insulin resistance. Proceedings of the National Academy of Sciences of the United States of America 2014;111:E4638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Doran AC, Lipinski MJ, Oldham SN et al. B-cell aortic homing and atheroprotection depend on Id3. Circulation research 2012;110:e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Srikakulapu PM C; McNamara C Chemokine Receptor CCR6 Expression on B Cells Augments Local IgM Production and Atheroprotection. Arteriosclerosis, thrombosis, and vascular biology 2016:A464. [Google Scholar]
- 119.Frasca D, Diaz A, Romero M, Vazquez T, Blomberg BB. Obesity induces pro-inflammatory B cells and impairs B cell function in old mice. Mechanisms of ageing and development 2017;162:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ait-Oufella H, Herbin O, Bouaziz JD et al. B cell depletion reduces the development of atherosclerosis in mice. The Journal of experimental medicine 2010;207:1579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kyaw T, Tay C, Khan A et al. Conventional B2 B cell depletion ameliorates whereas its adoptive transfer aggravates atherosclerosis. Journal of immunology (Baltimore, Md : 1950) 2010;185:4410–9. [DOI] [PubMed] [Google Scholar]
- 122.Kyaw T, Tay C, Hosseini H et al. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PloS one 2012;7:e29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Neeland IJ, Turer AT, Ayers CR et al. Body fat distribution and incident cardiovascular disease in obese adults. Journal of the American College of Cardiology 2015;65:2150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dale CE, Fatemifar G, Palmer TM et al. Causal Associations of Adiposity and Body Fat Distribution With Coronary Heart Disease, Stroke Subtypes, and Type 2 Diabetes Mellitus: A Mendelian Randomization Analysis. Circulation 2017;135:2373–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Horckmans M, Bianchini M, Santovito D et al. Pericardial Adipose Tissue Regulates Granulopoiesis, Fibrosis, and Cardiac Function After Myocardial Infarction. Circulation 2018;137:948–960. [DOI] [PubMed] [Google Scholar]
- 126.Wu L, Dalal R, Cao CD et al. IL-10-producing B cells are enriched in murine pericardial adipose tissues and ameliorate the outcome of acute myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America 2019;116:21673–21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, Liew FY. IL-33 activates B1 cells and exacerbates contact sensitivity. Journal of immunology (Baltimore, Md : 1950) 2011;186:2584–91. [DOI] [PubMed] [Google Scholar]
- 128.Perry HM, Oldham SN, Fahl SP et al. Helix-loop-helix factor inhibitor of differentiation 3 regulates interleukin-5 expression and B-1a B cell proliferation. Arteriosclerosis, thrombosis, and vascular biology 2013;33:2771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Current opinion in pharmacology 2010;10:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Manka D, Chatterjee TK, Stoll LL et al. Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1. Arteriosclerosis, thrombosis, and vascular biology 2014;34:1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Konaniah ES, Kuhel DG, Basford JE, Weintraub NL, Hui DY. Deficiency of LRP1 in Mature Adipocytes Promotes Diet-Induced Inflammation and Atherosclerosis-Brief Report. Arteriosclerosis, thrombosis, and vascular biology 2017;37:1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henrichot E, Juge-Aubry CE, Pernin A et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arteriosclerosis, thrombosis, and vascular biology 2005;25:2594–9. [DOI] [PubMed] [Google Scholar]
- 133.Farias-Itao DS, Pasqualucci CA, Nishizawa A et al. B Lymphocytes and Macrophages in the Perivascular Adipose Tissue Are Associated With Coronary Atherosclerosis: An Autopsy Study. Journal of the American Heart Association 2019;8:e013793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Porsch F, Binder CJ. Impact of B-Cell-Targeted Therapies on Cardiovascular Disease. Arteriosclerosis, thrombosis, and vascular biology 2019;39:1705–1714. [DOI] [PubMed] [Google Scholar]


