Abstract
Atherosclerosis, the major underlying pathology of cardiovascular disease (CVD), is characterized by accumulation and subsequent oxidative modification of lipoproteins within the artery wall, leading to inflammatory cell infiltration and lesion formation that can over time result in arterial stenosis, ischemia, and downstream adverse events. The contribution of innate and adaptive immunity to atherosclerosis development is well established, and B cells have emerged as important modulators of both pro- and anti-inflammatory effects in atherosclerosis. Murine B cells can broadly be divided into two subsets: 1) B-2 cells, which are bone marrow-derived and include conventional follicular and marginal zone B cells, and 2) B-1 cells, which are largely fetal liver-derived and persist in adults through self-renewal. B cell subsets are developmentally, functionally, and phenotypically distinct with unique subset-specific contributions to atherosclerosis development. Mechanisms whereby B cells regulate vascular inflammation and atherosclerosis will be discussed with a particular emphasis on B-1 cells. B-1 cells have a protective role in atherosclerosis that is mediated in large part by IgM antibody production. Accumulating evidence over the last several years has pointed to a previously underappreciated heterogeneity in B-1 cell populations which may have important implications for understanding atherosclerosis development and potential targeted therapeutic approaches. This heterogeneity within atheroprotective innate B cell subsets will be highlighted.
Introduction
This article is based on the 2019 Russell Ross Memorial Lecture in Vascular Biology presented at the American Heart Association Scientific Sessions Annual Conference. The paradigm-defining work of Russell Ross first established atherosclerosis as a response to injury1. This injury leads to the development of chronic vascular inflammation as elegantly put forth more than two decades later in another seminal paper by Dr. Ross2. Low-density lipoprotein (LDL) enters the subendothelial space of the artery wall and becomes modified by oxidation presenting an array of neoantigens which are central to the ensuing immunologic response. These oxidation specific epitopes (OSE) are pro-inflammatory and result in endothelial cell dysfunction, increased adhesion molecule expression and circulating leukocyte recruitment into the vascular wall3–6. Recruited monocytes and other immune cells secrete pro-inflammatory cytokines and chemokines that further contribute to a positive feedback loop of increased immune cell entry into the nascent lesion. Monocytes differentiate into macrophages upon scavenging oxidized LDL (oxLDL), eventually becoming lipid-laden foam cells. As disease progresses, the infiltration of immune cells, accumulation of dying cells, and migration of phenotypically switched smooth muscle cells collectively contribute to the formation of an atherosclerotic plaque consisting of a lipid-rich necrotic core stabilized by a collagen-rich fibrous cap. This manifests clinically as either progressive narrowing of the vessel lumen with relative tissue ischemia, or acute plaque rupture resulting in in situ thrombosis, vessel occlusion and tissue infarction. While B cells are not a prominent subtype of immune cells within this inflammatory plaque, they have a major role in regulating plaque inflammation through systemic and perivascular effects.
B cells in atherosclerosis
The pro- and anti-inflammatory roles of specific B cell subtypes in atherosclerosis has recently been elegantly reviewed by Sage et al7 and by others8,9. We focus here on antibody-producing functions of B cells in regulating inflammation in atherosclerosis as depicted in Figure 1 with a particular emphasis on IgM-producing B-1 cells. B cells are broadly divided into B-1 and B-2 subsets that are so-called due to the timing of their development; B-1 cells arise earlier in ontogeny primarily within the fetal liver and persist through self-renewal, while B-2 cells arise later and are continuously generated de novo by progenitors in adult bone marrow10. B-1 cells can be further divided into distinct B-1a and B-1b subsets based on expression of the surface marker CD5; B-1a being CD5 positive, B-1b CD5 negative. B-1 cells are present at highest frequency in serosal cavities, and at lower frequencies in the spleen, bone marrow, blood, mucosal tissues such as the gut and lung, and adipose tissues. B-1 cells have largely been found to be protective against atherosclerosis, primarily via IgM antibody production. Both B-1a and B-1b subsets secrete IgM against OSE and protect against atherosclerosis after adoptive transfer into immunodeficient hosts11,12. Additionally, Siglec-G deficient mice, in which there is specific expansion of B-1a cells and elevated OSE-IgM levels, are protected from atherosclerosis13. One unique subset of GM-CSF-producing B-1a-derived cells, termed innate response activator B cells, appears to promote atherosclerosis through the expansion of dendritic cells and the inflammatory IFN-γ producing T helper 1 (Th1) cell subset14. But in general, B-1a and B-1b cells and their secreted IgM are protective.
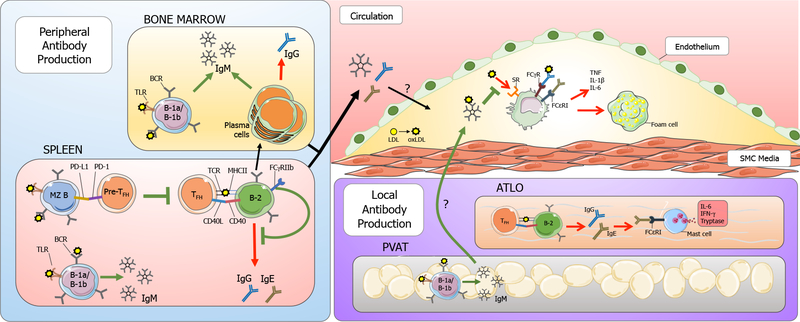
Figure 1. B cell-derived antibodies in atherosclerosis.
B cells have subset-specific roles in atherosclerosis. Peripheral antibody production occurs primarily in the spleen and bone marrow. IgM-secreting B-1 cells in the bone marrow and spleen contribute significantly to plasma IgM titers. Plasma cells in the bone marrow also produce IgM and IgG antibodies. In the spleen, follicular B-2 cells present antigens to TFH cells via MHCII, and provide co-stimulatory signaling through CD40-CD40L interaction. This can lead germinal center (GC) reactions in which B cells undergo affinity maturation and isotype switching to generate high-affinity IgG or IgE antibodies. B-2 cells can differentiate into long lived plasma cells that reside in the bone marrow. Additionally, FcγRIIb has been shown to inhibit GC-derived IgG production in B-2 cells. In response to hypercholesterolemia, marginal zone (MZ) B cells upregulate PD-L1 which interacts with PD-1 on TFH cells to suppress TFH differentiation, thus limiting pro-inflammatory TFH/B-2 cell interactions. During atherosclerosis, LDL accumulation and oxidative modification into oxLDL is pro-inflammatory and results in recruitment of monocytes and other immune cells into the subintimal space. IgM, IgG, and IgE antibodies made peripherally or locally in the perivascular adipose tissue (PVAT) and adventitial tertiary lymphoid organs (ATLO) enters the lesion with immunomodulatory effects. IgM binds oxLDL and prevents its binding through scavenger receptors (SR) on monocytes and macrophages in the lesion, thus preventing pro-inflammatory cytokine secretion and foam cell formation. IgG binding to Fcγ receptors (FcγR) and IgE binding to FcεRI on macrophages can also result in proinflammatory cytokine production. TFH and B-2 interactions in ATLO can result in IgG and IgE antibodies that are pro-inflammatory. IgE can bind FcεRI present on mast cells, resulting in release of pro-inflammatory cytokines including IL-6 and IFN-γ.
Outside of IgM production, there are several other mechanisms by which B-1 may exert their atheroprotective function. For example, B-1 cells have greater phagocytic capacity than B-2 cells15 and this may prevent atherosclerosis either directly through enhanced clearance of OSE or indirectly through modulation of adaptive immunity via antigen presentation. Additionally, B-1 cells have been shown to be a significant source of certain cytokines such as IL-1016,17, which may also exert atheroprotection through paracrine effects18–20. The remainder of this review will discuss recent studies, which have revealed a previously underappreciated rich heterogeneity in B-1 populations and their respective immunoglobulins and how these may impact protective immunity in atherosclerosis.
B-1 cells produce anti-OSE IgM antibodies
Studies using IgM allotype chimeric mice have demonstrated that ~80% of steady-state IgM is B-1 derived21. Importantly, B-1 cells are the main B cell subset contributing to the production of natural antibodies, a subset of primarily IgM isotype antibodies that form in the absence of any exogenous antigen exposure22–26, and are thus present even in germ-free mice fed an antigen-free diet27. The repertoire of natural IgM antibodies consists primarily of germline VDJ gene segments with a propensity for autoreactive specificities10,28,29. Natural IgM antibodies undergo limited somatic hypermutation and non-template-encoded N-nucleotide insertions, resulting in antibodies that are low affinity and polyreactive. In addition to natural IgM, B-1 cells can also be induced to secrete antigen-specific immune IgM in response to pathogens24,30–32.
In contrast to follicular B-2 cells of the adaptive immune response, which are strongly selected against self-reactivity and produce high affinity, class-switched antibodies in a T cell-dependent manner, B-1a and B-1b cells instead participate in type 1 and type 2 T cell-independent (TI) responses. In the TI type 1 response, signals independent of the B cell receptor (BCR), including stimulation of pattern recognition receptors (PRR) like toll-like receptors (TLR) or cytokine receptors, induces the secretion of polyclonal IgM antibodies. In the TI type 2 response, extensive BCR crosslinking to multivalent antigens, like the capsular polysaccharides of gram-negative bacteria, overrides the requirement of T cell help for production of antigen-specific immune antibodies. Studies using pneumococcal polysaccharide (PPS) immunization initially supported a simple division of labor model in which B-1a cells were responsible for natural IgM production while B-1b cells were responsible for generating PPS-specific immune IgM, memory responses, and long-lasting immunity to pathogen challenge33,34. However, alternative infection models utilizing Francisella tularensis immunization31,35 demonstrated that B-1a cells, too, can generate pathogen-specific IgM antibody and long-term immunity. Therefore, it is clear that the B-1 cell IgM response depends on a number of factors, including the antigen itself. It has been proposed that the differences between natural IgM-secreting B-1 cells versus antigen-induced IgM-secreting B-1 cells may arise from differences in B-1 ontogeny, microenvironmental niche, and responsiveness to self-antigens versus foreign antigens, as elegantly reviewed by Baumgarth and colleagues22.
Notably, a significant proportion (~30%) of B-1 derived natural IgM antibodies recognize oxidation specific epitopes6, including phosphocholine (PC) and malondialdehyde (MDA). Interestingly, these epitopes are commonly conserved on both pathogen-associated molecular patterns (PAMPs) on invading pathogens, and the damage-associated molecular patterns (DAMPs) on neo-self epitopes that arise endogenously in health and disease, particularly in atherogenesis. For example, the prototypic natural antibody, T15, recognizes the PC head group present as the immunodominant epitope on the cell wall of S. pneumoniae, the cell membrane of apoptotic cells, and on oxidized phospholipids present in atherosclerotic lesions36. Analysis of hybridomas derived from splenic B cells of hypercholesterolemic ApoE−/− mice demonstrated that there are abundant IgM antibodies specific for various epitopes of oxidized LDL (oxLDL) in vivo and that they localize to atherosclerotic lesions6,37,38. Moreover, sequencing of the antigen-binding CDR3 region of one clone, E06, demonstrated 100% similarity in the VL and VH regions to T1539. Because B-1 cells are thought to undergo positive selection for their autoreactivity40,41, it has been argued that the self-antigens that form the B-1 repertoire are “evolutionarily conserved templates” providing antibody specificities for both damaging self and pathogen-related epitopes10.
Natural anti-OSE IgM is atheroprotective
The functions of natural IgM antibodies include providing the first line of defense against exogenous pathogens21,31,34,35,42–44, providing a housekeeping role through enhancing apoptotic cell clearance45–47, and performing an immunoregulatory role by inhibiting proinflammatory pathways induced by OSE39,48,49. The immunologic responses to both apoptotic cells and OSEs are central to the pathogenesis of atherosclerosis and thereby provide several mechanisms by which IgM might impair atherogenesis.
In support of this, LDLR−/− mice deficient for secreted IgM (sIgM−/−) have increased atherosclerosis50, and delivery of polyclonal IgM during the last 4 weeks of a 29-week Western diet feed reduces lesion area51. Strategies to increase IgM production have also been shown to mitigate atherosclerosis development. Immunization with phosphatidyl serine-coated liposomes, apoptotic cells, or PPS was demonstrated to increase IgM titers to OSE, increase IgM-secreting B-1 cell number, and curb lesion development52–54. There are several possible mechanisms by which anti-OSE IgM may be atheroprotective55. First, IgM has been proposed to act as a decoy receptor for its cognate ligands as secreted IgM has been shown to inhibit BCR signaling56. In the case of anti-OSE-IgM, this feature may block the interaction of OSEs with a number of its atherogenic targets. For example, anti-OSE-IgM has been shown to block oxLDL uptake by macrophages in vitro52, suggesting that it may inhibit foam cell formation in vivo. Furthermore, transgenic mice overexpressing E06-scFv, the antigen-binding portion of the IgM antibody, have decreased oxLDL uptake by peritoneal cavity (PerC) macrophages, a reduced inflammatory phenotype in both PerC and aortic macrophages, and reduced atherosclerotic lesion and necrotic core area57. Likewise, overexpression of IK17-scFv, which recognizes MDA epitopes, is also able to reduce lipid accumulation in PerC macrophages and decrease atherosclerosis58. These studies using scFv antibodies suggest that anti-OSE antibodies mediate atheroprotection primarily through sequestering OSE and blocking its ability to induce inflammation in macrophages. However, whether endogenous anti-OSE IgM might subsequently facilitate OSE clearance through binding receptors via its Fc portion remains unclear. Studies comparing atherosclerosis in C1q-deficient and sIgM-deficient mice suggest that the protective effects of IgM in atherosclerosis are partially independent of the classical complement pathway and apoptotic cell clearance50. This also aligns with studies in which mice given sIgM−/− B-1a cells had similar lesional apoptotic cell content as mice given sIgM+/+ B-1a cells11. Whether the Fcμ or Fcα/μ receptors have a role in anti-OSE IgM-mediated atheroprotection is unexplored.
Importantly, IgM secretion is the primary mechanism of B-1-mediated atheroprotection, as adoptive transfer of sIgM-deficient B-1a cells fails to protect from atherosclerosis, unlike transfer of sIgM-sufficient B-1a cells11. As B-1-derived anti-OSE IgM has been demonstrated to be atheroprotective, it is important to understand the factors that regulate its production, as this may provide new therapeutic targets for atherosclerosis prevention.
Niche-specific differences in B-1 IgM antibody production
In mice, B-1 cells are present at highest frequency (~40% of all lymphocytes) in the serosal (peritoneal and pleural) cavities, while smaller frequencies are seen in the spleen, bone marrow, intestinal lamina propria, and lymph nodes10. In addition to lymphoid organs, IgM-secreting B-1 cells are also present in adipose tissues, including omental fat59 and the perivascular adipose tissue (PVAT), which surrounds the aorta and other major vessels60 (Figures 1 & 2). Importantly, while B-1 frequency is high in the peritoneal cavity, constitutive IgM antibody production by PerC B-1 cells occurs at a very low level during steady state61, though activation by innate stimuli can induce their rapid migration to other sites and differentiation into antibody secreting cells62–64.
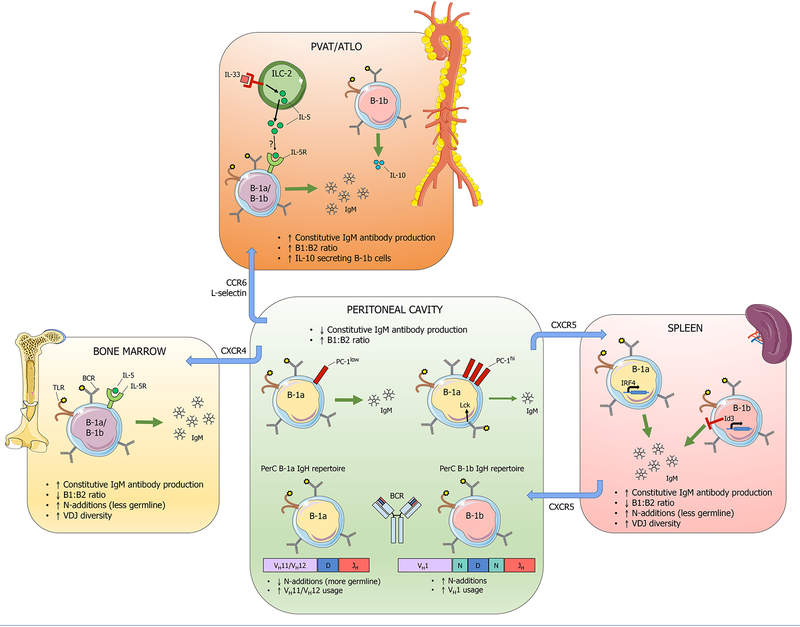
Figure 2. Niche-specific B-1 cell heterogeneity.
B-1 cells from distinct anatomical regions display functional heterogeneity in IgM antibody production, arising from both extrinsic microenvironmental factors and intrinsic differences. Peritoneal cavity (PerC) B-1 cells secrete less IgM constitutively compared to B-1 cells from spleen, bone marrow, perivascular adipose tissue (PVAT), and adventitial tertiary lymphoid organs (ATLO). In the PerC, PC-1 expression distinguishes B-1a cells with high capacity to produce IgM (PC-1lo), from those with low capacity to produce IgM (PC-1hi). The src family kinase Lck also maintains the hyporesponsiveness of PerC B-1a cells. Additional heterogeneity in the PerC B-1 population is evidenced by distinct immunoglobulin heavy chain (IgH) repertoires expressed by PerC B-1a vs B-1b cells, with PerC B-1a cells expressing more germline sequences that lack N-additions and a preference for VH11/VH12 family gene segments. In contrast, splenic B-1a cells produce more IgM constitutively in a manner dependent on the transcription factor IRF4, and the splenic B-1a IgH repertoire contains more N-additions and greater VDJ gene segment diversity. The transcription factor Id3 inhibits B-1b cell number and consequently IgM antibody production. Bone marrow (BM) B-1 cells also produce more IgM constitutively compared to PerC B-1 cells, and IL-5 has been shown to maintain the BM IgM-secreting cell population. The PVAT contains type 2 innate lymphoid cells (ILC-2) that secrete IL-5 in response to IL-33. Whether ILC-2 derived IL-5 is responsible for maintaining the high constitutive IgM production that occurs in PVAT is unknown. ATLO contain an abundance of IL-10 producing B-1b cells. B-1 cell trafficking between these compartments relies on adhesion molecules. The chemokine receptor CXCR5 mediates B-1 cell trafficking between the spleen and the PerC. CXCR4 mediates B-1 cell migration to the bone marrow. CCR6 and L-selectin mediate B-1 localization to the PVAT and aorta.
In contrast, B1 cells in lymphoid organs such as the spleen and bone marrow secrete greater amounts of IgM on a per cell basis constitutively and are the primary contributors to steady-state IgM titers64–67. Baumgarth and colleagues have further demonstrated that sorted bone marrow IgM-secreting cells produce more IgM on a per cell basis than splenic IgM-secreting cells in vitro65. Additionally, studies from our lab demonstrate that the highest B1:B2 cell ratio, after the PerC, is present in PVAT and omental fat, compared to spleen and bone marrow, raising the interesting possibility that these adipose tissues may also significantly contribute to circulating IgM levels60, and unpublished data.
IgM-secreting B-1 cells also accumulate within artery tertiary lymphoid organs (ATLOs) that form in the adventitia in aged ApoE−/− mice68,69. Studies by Habenicht and colleagues demonstrate that lymphotoxin-β receptor signaling induces smooth muscle cell expression of CXCL13 and CCL21, lymphorganogenic chemokines that result in progressive immune cell aggregation and eventual formation of ATLOs that contain high endothelial venules, B cell follicles and germinal centers, T cell zones, and other immune cells70. Interestingly, most B-1 cells within ATLOs are B-1b cells, a majority of which produce the anti-inflammatory cytokine IL-1068.
As B-1 cell IgM production appears to be heterogeneous and niche-specific, it becomes important from a therapeutic standpoint to understand the factors that contribute to this heterogeneity and what regulates their localization to sites of high antibody production. The differences in IgM antibody production by B cells from different locations appear to be driven by both intrinsic properties of those B-1 cells, as well as extrinsic factors that inhibit or activate B-1 antibody production. Current studies demonstrate that multiple factors have a role in regulating B-1 IgM production, including 1) soluble factors within a microenvironmental niche and 2) intrinsic factors such as B-1 ontogeny, the BCR repertoire, and intracellular signaling molecules.
Extrinsic mechanisms contributing to B-1 cell heterogeneity
In support of B cell-extrinsic mechanisms contributing to B-1 cell functional heterogeneity, Chace and colleagues reported that release of prostaglandin E2 by peritoneal macrophages inhibited LPS-induced IgM production by peritoneal B1 cells in vitro, suggesting that PerC macrophages may be partially responsible for the low IgM-producing capacity of PerC B-1 cells in vivo71. As certain cytokines have also been shown to be important for maintaining B-1 cell homeostasis and IgM production, differential cytokine concentration or availability within distinct niches could likely impact IgM production within a niche. For example, interleukin (IL)-5 has a critical role in maintaining peritoneal B-1a cell survival and proliferation, and consequently antibody production72,73. IgM production by bone marrow B cells was also demonstrated to be enhanced by IL-5 in vitro74. Moreover, IL-5-deficient bone marrow chimeras have decreased T15/E06 IgM titers in response to Western diet feeding and increased atherosclerosis75. IL-10 has also been demonstrated to be important for B-1 cell function, operating in concert with CXCL12 signaling to mediate B-1 cell proliferation, survival, and retention within the peritoneal cavity76. In the NZB/W mouse model of lupus, the combined actions of IL-10 and CXCL12 were demonstrated to be key for development of anti-dsDNA autoantibodies77. A fraction of serosal B-1a cells termed innate response activator B cells have additionally been demonstrated to require autocrine GM-CSF for efficient IgM production78.
Notably, the presence of fat-associated lymphoid clusters (FALCs) as sites of B-1 cell activation and antibody production has been highlighted in recent years79. FALCs form independently of canonical mechanisms leading to formation of other secondary lymphoid tissues, like the lymphotoxin-β receptor pathway and lymphoid tissue-inducer cells80. Instead, FALC formation increases with age and is inducible by inflammatory stimuli in a manner dependent on tumor-necrosis factor receptor signaling80. FALCs are found primarily in the omentum, pericardium, and mediastinum, and contain abundant numbers of type 2 innate lymphoid cells (ILC2) that produce Th2 cytokines including IL-581, and stromal cells that produce IL-3382. In a model of pleural infection, IL-5 and IL-33 were both required for the activation of FALC B1 cells and IgM production82. Therefore, it is very likely that FALC formation brings into close proximity IL-33, IL-5, and B-1 cells, and may be responsible for the greater IgM production that occurs in adipose tissues relative to that in the serosal cavities. Notably, ILC2 are also found within the PVAT and aortic adventitia, and produce IL-5 after stimulation with IL-33, suggesting that they may contribute to B-1 homeostasis and IgM production within the PVAT83.
Intrinsic mechanisms contributing to B-1 cell heterogeneity
Intrinsic differences in B-1 cell ontogeny, BCR repertoire, and intracellular signaling molecules likely also account for differences in IgM production. The source of IgM-secreting B-1 cells in various compartments remains controversial. B-1 cells in adult mice are a largely self-renewing population, derived from precursors from the fetal liver and bone marrow, in contrast to B-2 cells which are continuously generated de novo in adult bone marrow. Dorshkind and colleagues have proposed a model in which murine B-1 cell development occurs in waves, with the first wave being the embryonic yolk sac, the second wave arising from the fetal liver and bone marrow, and the third wave arising from neonatal and adult bone marrow during the first few weeks of life84. After that, de novo B-1 cell development wanes, and the adult B-1 cell pool is primarily maintained through self-renewal. However, distinct B-1a and B-1b progenitors have also been found in adult bone marrow and in adult spleen85–88. Yet, the capacity of these progenitors to regenerate the B-1 compartment has been demonstrated only after an injury to the B-1 compartment, such as lethal irradiation, making it unclear how significantly these progenitors contribute to endogenous B-1a and B-1b subsets in health. Additionally, earlier studies suggested that peritoneal B-1 cells arose from precursors within the spleen, because splenectomy reduced peritoneal B-1 number89,90, and a distinct B-1 cell progenitor was later discovered in mouse spleen91. However, in apparent contrast to this, adoptive transfer of adult peritoneal B-1 cells is able to reconstitute all B-1 compartments in adult mice, including in the spleen and bone marrow74,92,93. Though the origins and migratory capacities of B-1 cells from different sites is not completely understood, it is clear that functional heterogeneity exists within the B-1 compartments; some B-1 cells contribute to constitutive natural IgM secretion (predominantly B-1 cells within spleen and bone marrow), while serosal B-1 cells seem to be primed for rapid response to stimuli via migration and differentiation into antibody-secreting cells22.
An exciting recent realization is the heterogeneity in the B-1 population at the level of the B cell receptor (BCR) (Figure 2). Antibodies are the secreted form of the BCR, therefore the BCR determines antibody specificity. The BCR develops through combinatorial joining of VH, D, and JH gene family segments that make up the immunoglobulin heavy chain, and VL and JL segments that make up the immunoglobulin light chain. Pairing and dimerization of two heavy and two light chains forms the mature BCR. Positive and negative selection events strongly influence the development of the BCR and the overall B cell repertoire present in an animal. In addition to VDJ recombination, further heterogeneity in the BCR occurs through non-template encoded nucleotide additions (N-additions) and nucleotide excisions at the junctions between V-D and D-J segments. Antigen binding occurs at the variable regions of the heavy and light chains, and is determined by hypervariable regions called the complementarity determining regions (CDRs). The third CDR of the heavy chain (CDR-H3) extends across both V-D and D-J junctions and is therefore the most variable.
Several studies have sequenced the variable region of the heavy chain (IgH V) and shown that significant heterogeneity exists in the IgH V repertoire of B cell subsets from different locations. For example, IgH deep sequencing indicates that the B-1a repertoire (both peritoneal and splenic) is generally more restricted and favors usage of certain VH genes that encode for autoreactive specificities, compared to the IgH repertoire of splenic marginal zone, splenic follicular B-2, and peritoneal B-2 cells29. Moreover, the peritoneal B-1a repertoire is generally less diverse in comparison to the splenic B-1a repertoire regardless of age, containing more recurrent CDR-H3 nucleotide sequences, a bias towards VH11 and VH12 gene usage, and little if any non-templated nucleotide additions (N additions), indicative of a more germline antibody repertoire29. In contrast, the splenic B-1a repertoire contains more unique CDR-H3 sequences, and appears to accumulate N-additions and undergo increased somatic hypermutation with age29. Because N-additions require the enzyme Tdt, which is only expressed postnatally, it has been proposed that the peritoneal B-1a IgH repertoire is generated primarily from prenatal fetal-derived B-1a cells. However, other studies have demonstrated that N-additions increase in PerC B-1a cells with age87,94, which may arise from the contribution of postnatally developed B-1a cells, B-1a cells migrating from other compartments, or from selective antigen-induced pressures that are specific to a particular microenvironmental niche. Intriguingly, IgH sequencing analysis also indicates significant repertoire differences between peritoneal B-1a and B-1b cells, with the B-1b repertoire containing more unique CDR-H3 nucleotide sequences with more frequent N-additions, and a paucity of VH11 gene usage28. This suggests that there are underlying differences between peritoneal B-1a and B-1b cells leading to IgM repertoire differences that cannot be explained by PerC-restricted expression of antigens that drive selection of certain B cell clones in the PerC. Importantly, given the potential for postnatal diversification and selection of the IgM repertoire, defining the IgM repertoire in the context of atherosclerosis risk factors such as age and hyperlipidemia is of key importance. Our group has begun analyzing the IgM repertoire of B-1a cells from 100-week-old ApoE−/− mice that have established atherosclerosis. Our findings indicate significant differences in VDJ gene segment usage between bone marrow (BM) and PerC B-1a cells, and an increased frequency of N-additions in BM B-1a cells compared to PerC B-1a95. Moreover, analysis of CDR-H3 amino acid sequences demonstrates some sequences common to both BM and PerC B-1a cells, suggesting migration between compartments, and convergence towards certain CDR-H3 specificities in aged ApoE−/− mice.
Certain intracellular signaling pathways may also contribute to differences in IgM antibody production between B-1 cells from different compartments. For example, Holodick and colleagues reported that low-level constitutive IgM production by peritoneal B-1 cells was independent of the transcription factor IRF4, unlike splenic B-1 cells, which secrete much more IgM on a per cell basis in an IRF4-dependent manner61. Additionally, expression of the src family kinase Lck in peritoneal, but not splenic, B-1a cells was demonstrated to contribute to their hyporesponsiveness, and Lck-null mice have increased amounts of circulating natural antibodies96. Surface expression of plasma cell alloantigen 1 (PC-1) was also demonstrated to divide the peritoneal B-1a compartment based on high capacity (PC-1lo B-1a) versus low capacity (PC-1hi B-1a) to produce natural IgM97. Interestingly, PC-1lo B-1a cells were demonstrated to arise in an earlier wave of B-1a cell development from the fetal liver, while PC-1hi B-1a cells increased in number only after birth. Additionally, certain molecules including Id312, Lin28b98, PIK3CD99, IgM Fc receptor100, RasGRP1101, and CD6102 have been shown to play a role in B-1 cell development or self-renewal and serum natural IgM production. In these studies, it is important to note the difference between direct modulation of IgM production versus indirect modulation of IgM titers by changes in B-1 cell number. Finally, a recently published landmark study by Graf et al. showed using a clever transgenic system that BCR specificity itself can independently drive B-1 vs B-2 differentiation103, adding another layer of complexity to the vast array of mechanisms which regulate B cell phenotype. The factors regulating preferential expression of these molecules in a niche-, subset- or antigen-specific manner is an intriguing area for future study.
Niche-specific B-1 cell contributions to atheroprotection
Although B-1 cells in the aorta are present predominantly in the surrounding perivascular adipose tissue and adventitia and only rarely within intimal lesions themselves60,68, abundant IgM to OSE is present in human and mouse atherosclerotic lesions6,38,39. As IgM has been demonstrated to block OSE-induced pro-inflammatory signals within macrophages and this associates with decreased atherosclerosis57,58, it is thought that IgM limits plaque formation primarily through its actions within the plaque. However, how IgM gets into the lesion and whether it is supplied by systemic plasma IgM made in peripheral niches, by IgM-producing cells locally within the PVAT and adventitia, or both, remains unclear.
Of all the candidate sites for atheroprotective IgM production, the contribution of the spleen has been most widely studied. B cells in the spleen respond to atherogenesis through expansion of clones reactive to PC and oxLDL53. Splenectomy aggravates atherosclerosis in ApoE−/− mice, and adoptive transfer of splenic B cells increases circulating titers of anti-OSE IgM and IgG and reduces lesion size even below that of sham-operated controls104. Moreover, splenectomy significantly reduces the amount of IgM within aortic root lesions, suggesting that peripheral IgM production significantly contributes to lesional IgM accumulation11. These studies demonstrate that the spleen harbors a protective B cell response during atherosclerosis. However, it was not determined to which niche the adoptively transferred B cells homed nor where IgM was being generated in the absence of the spleen.
The role of local adventitial and PVAT B-1 cells in contributing to atheroprotection is not completely understood. Our lab has demonstrated that adoptively transferred splenic B cells home to the aortic adventitia in a manner dependent on Id3 and CCR6, and that this associates with decreased lesion area and reduced plaque macrophage content105. Furthermore, transfer of splenic CCR6+/+ApoE−/− B cells into sIgM−/− ApoE−/− mice attenuates atherosclerosis only if those B cells are capable of secreting IgM106. Additionally, L-selectin-deficient mice have reduced numbers of aortic B-1a and regulatory B cells, decreased T15 IgM and IL-10 in aortic supernatants, and increased atherosclerosis107. These studies suggest that B cell migration to the aorta and subsequent local IgM production is atheroprotective. The specific impact of ATLOs on atherosclerosis has not been fully elucidated.
Additionally, whether the PVAT itself, as a niche containing IgM-secreting B-1 cells, is necessary for protection from atherosclerosis remains unresolved108. Models of PVAT transplantation have yielded contrasting results, with some studies suggesting a protective role for PVAT in limiting plaque macrophage content and inflammatory cytokine expression109, and others demonstrating a pro-atherogenic role of PVAT110,111, possibly through inducing endothelial cell dysfunction112. These contrasting results may be due at least in part to differences in technique, including the source of transplanted PVAT: abdominal versus thoracic aorta. Abdominal PVAT is phenotypically more similar to white adipose tissue, while thoracic PVAT behaves more like thermogenic brown adipose tissue113. Finally, while the bone marrow and omentum are important sites of B-1 IgM production, the local B-1 response within these niches during progression of atherosclerosis and the contribution of these niches to atheroprotection is not known. B-1 circulation between all of these sites is likely dynamic and recent studies have shed light on the chemokine axis governing this traffic.
Regulation of B-1 cell trafficking
While there are clear differences in IgM production between B-1 cells from different niches as summarized in the sections above, the factors regulating B-1 cell number at sites of high antibody production, both at steady state and in the context of chronic inflammation, remain largely unexplored. Cell trafficking and localization is controlled by a number of cell surface adhesion molecules including chemokine receptors, integrins, and selectins. Parabiosis studies have demonstrated dynamic circulation of B-1 cells to the PerC between parabiotic partners62. Moreover, cell transfer studies indicate that the B-1 cell population in the spleen at steady-state is derived partially from B-1 migrants from the PerC64,114. Steady-state B-1 localization to the PerC depends on the chemokine/receptor pairs CXCR5/CXCL1362 and CXCR4/CXCL12115, as well as the integrins α4β1 and CD963. CXCR5/CXCL13 is required for both B-1 cell trafficking to the PerC via the omentum62,116, as well as B-1 cell trafficking out of the PerC into the spleen63 (Figure 2).
In addition to steady-state circulation, body cavity B-1 cells can also mobilize in response to activation by inflammatory stimuli114. Several studies have used TLR agonists or bacterial ligands to study B-1 migration. For example, intraperitoneal transfer of IgMb allotype PerC cells into congenic IgMa allotype hosts and stimulation with intraperitoneal LPS resulted in emigration of donor B-1 cells from PerC to spleen and differentiation into antibody-secreting cells64. TLR4 stimulation induces rapid down-regulation of α4, β1, and CD9 on PerC B-1 cells that coincides with B-1 egress from PerC, as treatment with CD9 or α4 blocking antibodies resulted in increased B-1 numbers in spleen and omentum concomitant with decreased PerC B1 numbers63. Absence of CXCL13 also hampers B-1 emigration from PerC in response to TLR4 agonist, further demonstrating the importance of this chemokine in B-1 trafficking63.
Importantly, a number of these studies demonstrating niche-specific deficits in B-1 cell number in the absence of chemokines or their receptors also demonstrate an associated deficit in natural IgM production, highlighting the idea that B-1 cell localization influences IgM production. For example, CXCL13−/− mice that contain diminished PerC B-1 cell numbers have reduced titers of anti-PC and T15 IgM both at steady-state and in response to intraperitoneal immunization with R36A streptococcal vaccine, though no differences in total IgM, IgG isotypes or IgA62. Additionally, our group has found that B-1a cell localization to the bone marrow depends on the chemokine receptor CXCR4, and that this influences total and anti-OSE IgM production95. Other factors have been shown to specifically influence migration toward atherosclerotic lesions and sites of local antibody production. For instance absence of L-selectin in ApoE−/− mice was associated with reduced B-1a and regulatory B cell number in the aorta and consequently reduced T15 IgM and IL-10 in aortic supernatants107. The chemokine receptor CCR6 also mediates B cell migration to the aorta in an Id3-dependent manner105, and B-1 cell localization to the PVAT (unpublished data).
Human B cell subsets and human B-1 cells
In contrast to murine B-1 cells, human B-1 cells have been harder to define. Initial studies on human B cell subsets were slower compared to their T cell counterparts, in part due to a lack of reagents that clearly distinguished B cell subsets117. CD5 was a helpful marker in a clinical setting for distinguishing lymphomas and leukemias, but it was less helpful in differentiating healthy B cells. Early studies by Liu and Banchereau in the late 1990’s used IgD and CD38 expression to classify naïve, germinal center, and memory B cells from tonsils, and named this the B mature classification system118,119. Since then, more surface markers, as well as characterization of BCR affinity, BCR mutation frequency, and functional assays including immunoglobulin secretion have added to our understanding of human B cell populations117. In light of these findings, it is clear that a much deeper heterogeneity exists in human B cell subsets that can’t be seen when using one or two surface markers. For example, CD27, which is a surface marker used to classify memory B cells, correlates with functional parameters of memory, including greater somatic hypermutation in the IgH V region, proliferation, and ability to differentiate into antibody secreting cells120–122. However, the total CD27+ B cell pool is diverse, being expressed on cells with nonmemory phenotypes123, and certain B cells with memory-like phenotype that lack CD27 expression124. It is also important to consider that surface marker expression may be dynamic, and a transitioning or polarized population may have altered expression of certain subset-specific markers, yet may be derived or related to a conventional subset.
Rothstein and colleagues identified the putative human B-1 subset using a reverse engineering approach, in which characteristics typical of murine B-1 cells, including spontaneous in vitro IgM antibody production, efficient T cell stimulation, and tonic intracellular signaling were used to identify a human B-1 equivalent125 (Figure 3). This subset was identified at low frequency in peripheral and umbilical cord blood and defined as CD20+CD3−CD27+CD43+126,127. Since its initial discovery, controversy has existed on the frequency of this population in peripheral blood, and the difference between human B-1 cells and plasmablasts or pre-plasmablasts128–130. In response, Rothstein and colleagues have extensively and carefully delineated the isolation process and gating strategy to identify human B-1 cells from peripheral blood131 and demonstrated that this subset differs from plasmablasts and pre-plasmablasts based on CD38 expression, transcriptional profiling, antibody repertoire, and other characteristics132. Yet, studies from Rothstein’s lab have also identified further heterogeneity in the CD20+CD3−CD27+CD43+ population, including differential CD11b expression, which distinguishes predominantly IgM-secreting B-1 cells (CD11b−), from predominantly IL-10-secreting B-1 cells with increased capacity to modulate T cells (CD11b+)17,133. Thus far, an equivalent to murine B-1a and B-1b subsets has not been identified within the human B-1 population. CD5, which distinguishes these subsets in mice, does not differentiate human B-1 cells; most CD5+ B cells are not CD20+CD3−CD27+CD43+ B-1 cells, and CD5 is not expressed on all B-1 cells125. Studies from our lab also provide evidence that deeper heterogeneity exists in the human B-1 subset based on chemokine receptor expression95, with expression of CXCR4 on circulating B-1 cells associating with increased amount of plasma anti-OSE IgM antibodies and negatively associating with coronary artery plaque burden and stenosis. Thus, further research characterizing human B-1 cell heterogeneity with associated B-1 cell functions may be relevant for discovery of biomarkers in human CVD.
Figure 3. Human B-1 cells.
Human B-1 cells were identified in peripheral and cord blood by Rothstein and colleagues as a CD20+CD3-CD27+CD43+ population. Functionally, this subset spontaneously secretes IgM, exhibits tonic intracellular phospholipase C gamma 2 (PLC-g2) and Syk phosphorylation, and efficiently stimulates T cell proliferation. Heterogeneity has been demonstrated within the human CD20+CD3-CD27+CD43+ B-1 population differentiated by CD11b expression. Human B-1 cells with low or no CD11b expression are better able to spontaneously secrete IgM, thus are termed “B-1 secretors.” CD11b+ human B-1 cells have been demonstrated to express higher levels of CD86 and to more efficiently stimulate allogeneic CD4+ T cell proliferation compared to B-1 secretors. CD11b+ B-1 cells also spontaneously secrete IL-10, which is able to suppress intracellular TNF-a expression by anti-CD3 stimulated T cells in vitro. CD11b+ B-1 cells are termed “B-1 orchestrators” due to their increased capacity to modulate T cell activation and proliferation. In contrast to murine B-1 cells, an equivalent B-1a/B-1b dichotomy has not been identified in the human B-1 cell subset.
Anti-OSE antibodies in human CVD
The role of B cells and their secreted antibodies in human CVD remains an evolving field. A 2013 study utilizing an integrated systems biology approach to analyze data from the Framingham Heart Study revealed that B cell activation genes were enriched in control individuals compared to those with coronary artery disease134. Moreover, natural anti-OSE antibodies and human B-1 cells decline in amount, frequency, and avidity with age135, while prevalence of atherosclerosis increases with age, leading to the interesting hypothesis that natural IgM may serve as a useful metric to predict susceptibility to age-related diseases involving OSE136. Indeed, studies in human CVD patient cohorts have demonstrated that increased amounts of circulating IgM antibodies for MDA-modified LDL are associated with less coronary artery disease and fewer cardiovascular events137–140.
Recent interest has been piqued in examining cardiovascular parameters in human patients receiving B cell-targeted immunotherapies that have been used for decades in treatment of autoimmune diseases, such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA)141. This is especially so in light of the CANTOS trial, which highlighted the important role of inflammation in CVD progression142, and in line with this, autoimmune patient populations show increased susceptibility to adverse cardiovascular events143. A recent review by Porsch and Binder examining current B cell-targeted therapies and their impact on human CVD indicates that more work in larger human cohorts is necessary to draw conclusions141. For example, the anti-CD20 B cell-depleting monoclonal antibody rituximab showed promise in a small-scale study in reducing carotid intima-media thickness (n=55 RA subjects)144. However, other studies showed no significant effect of rituximab treatment, while other B-cell immunotherapies either have no effect on CVD parameters, or have simply not yet been assessed7.
Importantly, human B-1 cells have been shown to produce IgM antibodies that are associated with CVD. Advanced glycation endproduct (AGE) modifications are glycated proteins or lipids that arise after exposure to sugar. AGE modifications have been implicated in progression of CVD associated with oxidative stress, inflammation, and endothelial cell dysfunction145. Methylglyoxal (MGO) is a reactive aldehyde that results in AGE modifications, and human B-1 cells have been shown to produce IgM antibodies against methylglyoxal (MGO)-modified apoB100 in vitro146. Moreover, low levels of MGO-specific IgM antibodies are associated with increased risk of cardiovascular events146. These data provide compelling support for the human relevance of murine findings that B-1 derived IgM against modified LDL that arise during chronic inflammatory disease are atheroprotective.
Summary and Future Directions
Ross’s fundamental work defining atherosclerosis as a response to injury and chronic inflammatory disease opened numerous avenues of study for understanding the basic mechanisms of disease pathogenesis. One of these areas with clear direct connections to therapeutic intervention is that of protective humoral immunity mediated by B-1 lymphocytes. Recent work has unveiled surprising B-1 cell heterogeneity across a wide range of cellular functions. How each of these factors differentially impact atherosclerosis will undoubtedly be key to the translation of these findings to meaningful clinical intervention. For example, there are clear important differences in B-1 cell function between different niches, but how each of these contribute to atheroprotection remains to be clarified. B-1 localization relates to another important unanswered question, which is how does immunoglobulin enter the atherosclerotic plaque? While it is possible that it crosses the luminal endothelial layer, a perhaps equally likely alternative would be via the vasa vasorum. Answering these questions may require vessel transplantation or other creative approaches. On the cell intrinsic level, differential BCR VH usage provides a wealth of opportunity for further study that could inform targeted therapeutic strategies. Modern single-cell methods and massively parallel sequencing techniques will likely continue to be of increasing value in these pursuits. Additionally, what of B-1 cell plasticity? How these cells can be induced to take on a more atheroprotective phenotype and the transcriptional machinery that governs this will be key in directing future interventions. Finally, perhaps the most important step will be translating these findings from murine studies to the functional human equivalents.
Supplementary Material
Highlights.
This article reviews and summarizes current literature that supports the following:
B-1 cells have a protective role in atherosclerosis, primarily via production of IgM antibodies against oxidation-specific epitopes.
B-1 cells are very heterogeneous, with niche-specific differences in IgM production, B cell receptor repertoire, ontogeny, and trafficking.
Heterogeneity in the B-1 subset can arise from both B-1 cell intrinsic and B-1 cell extrinsic mechanisms.
Functional heterogeneity has been discovered in the putative CD20+CD3-CD27+CD43+ human B-1 cell subset, and further research characterizing human B-1 cell heterogeneity may be relevant for biomarker discovery and the development of targeted therapies for human CVD.
Sources of Funding
The research in the author’s laboratory covered in this review was supported by National Institutes of Health grants HL107490, HL136098, and HL136275.
Nonstandard abbreviations and acronyms:
- ApoE
Apolipoprotein E
- ATLO
Artery tertiary lymphoid organ
- BCR
B cell receptor
- BM
Bone marrow
- CDR-H3
Complementarity determining region 3 of the heavy chain
- CVD
Cardiovascular disease
- FALC
Fat-associated lymphoid cluster
- IgM
Immunoglobulin M
- LDL
Low density lipoprotein
- MDA
Malondialdehyde
- N-additions
Non-template encoded nucleotide additions
- OSE
Oxidation-specific epitopes
- oxLDL
Oxidized low density lipoprotein
- PC
Phosphocholine
- PerC
Peritoneal cavity
- PPS
Pneumococcal polysaccharide
- PVAT
Perivascular adipose tissue
- sIgM
Secreted IgM
- TLR
Toll-like receptor
- VH
Heavy chain variable region
Footnotes
Disclosures
None.
References
- 1.Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976;295(7):369–377. [DOI] [PubMed] [Google Scholar]
- 2.Ross R Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. Immunity. 2013;38(6):1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108(2):235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30(12):2311–2316. [DOI] [PubMed] [Google Scholar]
- 6.Chou MY, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. The Journal of clinical investigation. 2009;119(5):1335–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sage AP, Tsiantoulas D, Binder CJ, Mallat Z. The role of B cells in atherosclerosis. Nature Reviews Cardiology. 2019;16(3):180–196. [DOI] [PubMed] [Google Scholar]
- 8.Perry HM, Bender TP, McNamara CA. B cell subsets in atherosclerosis. Frontiers in Immunology. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srikakulapu P, McNamara CA. B cells and atherosclerosis. Am J Physiol Heart Circ Physiol. 2017;312(5):H1060–H1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumgarth N The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. [DOI] [PubMed] [Google Scholar]
- 11.Kyaw T, Tay C, Krishnamurthi S, et al. B1a B Lymphocytes Are Atheroprotective by Secreting Natural IgM That Increases IgM Deposits and Reduces Necrotic Cores in Atherosclerotic Lesions. Circulation Research. 2011;109(8):830–840. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld SM, Perry HM, Gonen A, et al. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circ Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruber S, Hendrikx T, Tsiantoulas D, et al. Sialic Acid-Binding Immunoglobulin-like Lectin G Promotes Atherosclerosis and Liver Inflammation by Suppressing the Protective Functions of B-1 Cells. Cell Rep. 2016;14(10):2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilgendorf I, Theurl I, Gerhardt LM, et al. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation. 2014;129(16):1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popi AF. B-1 phagocytes: the myeloid face of B-1 cells. Ann N Y Acad Sci. 2015;1362:86–97. [DOI] [PubMed] [Google Scholar]
- 16.Alhakeem SS, Sindhava VJ, McKenna MK, et al. Role of B cell receptor signaling in IL-10 production by normal and malignant B-1 cells. Ann N Y Acad Sci. 2015;1362:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin DO, Rothstein TL. Human “orchestrator” CD11b(+) B1 cells spontaneously secrete interleukin-10 and regulate T-cell activity. Mol Med. 2012;18:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu H, Daugherty A. Regulatory B cells, interleukin-10, and atherosclerosis. Curr Opin Lipidol. 2015;26(5):470–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85(8):e17–24. [DOI] [PubMed] [Google Scholar]
- 20.Strom AC, Cross AJ, Cole JE, et al. B regulatory cells are increased in hypercholesterolaemic mice and protect from lesion development via IL-10. Thromb Haemost. 2015;114(4):835–847. [DOI] [PubMed] [Google Scholar]
- 21.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgarth N B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front Immunol. 2016;7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer seminars in immunopathology. 2005;26(4):347–362. [DOI] [PubMed] [Google Scholar]
- 24.Grönwall C, Vas J, Silverman GJ. Protective roles of natural IgM antibodies. Frontiers in Immunology. 2012;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holodick NE, Rodriguez-Zhurbenko N, Hernandez AM. Defining Natural Antibodies. Front Immunol. 2017;8:872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. Journal of immunology. 2015;194(1):13–20. [DOI] [PubMed] [Google Scholar]
- 27.Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. Eur J Immunol. 1984;14(12):1127–1130. [DOI] [PubMed] [Google Scholar]
- 28.Prohaska TA, Que X, Diehl CJ, et al. Massively Parallel Sequencing of Peritoneal and Splenic B Cell Repertoires Highlights Unique Properties of B-1 Cell Antibodies. Journal of immunology. 2018;200(5):1702–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Wang C, Yang Q, et al. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife. 2015;4:e09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21(3):379–390. [DOI] [PubMed] [Google Scholar]
- 31.Cole LE, Yang Y, Elkins KL, et al. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(11):4343–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med. 2008;205(13):3053–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alugupalli KR, Gerstein RM. Divide and conquer: division of labor by B-1 B cells. Immunity. 2005;23(1):1–2. [DOI] [PubMed] [Google Scholar]
- 34.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23(1):7–18. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Ghosn EE, Cole LE, et al. Antigen-specific antibody responses in B-1a and their relationship to natural immunity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(14):5382–5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsiantoulas D, Gruber S, Binder CJ. B-1 cell immunoglobulin directed against oxidation-specific epitopes. Front Immunol. 2012;3:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palinski W, Hörkkö S, Miller E, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. Journal of Clinical Investigation. 1996;98(3):800–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw PX, Horkko S, Tsimikas S, et al. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler Thromb Vasc Biol. 2001;21(8):1333–1339. [DOI] [PubMed] [Google Scholar]
- 39.Shaw PX xF, rkk, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. The Journal of clinical investigation. 2000;105(12):1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayakawa K, Asano M, Shinton SA, et al. Positive selection of natural autoreactive B cells. Science. 1999;285(5424):113–116. [DOI] [PubMed] [Google Scholar]
- 41.Kreslavsky T, Wong JB, Fischer M, Skok JA, Busslinger M. Control of B-1a cell development by instructive BCR signaling. Current opinion in immunology. 2018;51:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. Journal of immunology. 2003;170(7):3819–3827. [DOI] [PubMed] [Google Scholar]
- 43.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000;192(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med. 1998;188(12):2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Khanna S, Goodyear CS, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. Journal of immunology. 2009;183(2):1346–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Park Y-B, Patel E, Silverman GJ. IgM Antibodies to Apoptosis-Associated Determinants Recruit C1q and Enhance Dendritic Cell Phagocytosis of Apoptotic Cells(). Journal of immunology (Baltimore, Md : 1950). 2009;182(10):6031–6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38(4):259–264. [DOI] [PubMed] [Google Scholar]
- 48.Harmon DB, Srikakulapu P, Kaplan JL, et al. Protective Role for B-1b B Cells and IgM in Obesity-Associated Inflammation, Glucose Intolerance, and Insulin Resistance. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hörkkö S, Bird DA, Miller E, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid–protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. Journal of Clinical Investigation. 1999;103(1):117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin M Is Required for Protection Against Atherosclerosis in Low-Density Lipoprotein Receptor–Deficient Mice. Circulation. 2009;120(5):417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cesena FHY, Dimayuga PC, Yano J, et al. Immune-modulation by polyclonal IgM treatment reduces atherosclerosis in hypercholesterolemic apoE−/− mice. Atherosclerosis. 2012;220(1):59–65. [DOI] [PubMed] [Google Scholar]
- 52.Binder CJ, Horkko S, Dewan A, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9(6):736–743. [DOI] [PubMed] [Google Scholar]
- 53.Grasset EK, Duhlin A, Agardh HE, et al. Sterile inflammation in the spleen during atherosclerosis provides oxidation-specific epitopes that induce a protective B-cell response. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(16):E2030–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosseini H, Li Y, Kanellakis P, et al. Phosphatidylserine liposomes mimic apoptotic cells to attenuate atherosclerosis by expanding polyreactive IgM producing B1a lymphocytes. Cardiovasc Res. 2015;106(3):443–452. [DOI] [PubMed] [Google Scholar]
- 55.Tsiantoulas D, Diehl Cody J, Witztum Joseph L, Binder Christoph J. B Cells and Humoral Immunity in Atherosclerosis. Circulation Research. 2014;114(11):1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsiantoulas D, Kiss M, Bartolini-Gritti B, et al. Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic B cell development. Sci Rep. 2017;7(1):3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Que X, Hung MY, Yeang C, et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558(7709):301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsimikas S, Miyanohara A, Hartvigsen K, et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. Journal of the American College of Cardiology. 2011;58(16):1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones DD, Racine R, Wittmer ST, et al. The omentum is a site of protective IgM production during intracellular bacterial infection. Infect Immun. 2015;83(5):2139–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srikakulapu P, Upadhye A, Rosenfeld SM, et al. Perivascular Adipose Tissue Harbors Atheroprotective IgM-Producing B Cells. Frontiers in physiology. 2017;8:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holodick NE, Tumang JR, Rothstein TL. Immunoglobulin secretion by B1 cells: Differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. European Journal of Immunology. 2010;40(11):3007–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16(1):67–76. [DOI] [PubMed] [Google Scholar]
- 63.Ha SA, Tsuji M, Suzuki K, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203(11):2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Y, Tung JW, Ghosn EE, Herzenberg LA, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4542–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. European journal of immunology. 2012;42(1):120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holodick NE, Vizconde T, Rothstein TL. Splenic B-1a Cells Expressing CD138 Spontaneously Secrete Large Amounts of Immunoglobulin in Naive Mice. Front Immunol. 2014;5:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, Baumgarth N. Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med. 2017;214(9):2777–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srikakulapu P, Hu D, Yin C, et al. Artery Tertiary Lymphoid Organs Control Multilayered Territorialized Atherosclerosis B-Cell Responses in Aged ApoE−/− Mice. Arterioscler Thromb Vasc Biol. 2016;36(6):1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin C, Mohanta SK, Srikakulapu P, Weber C, Habenicht AJ. Artery Tertiary Lymphoid Organs: Powerhouses of Atherosclerosis Immunity. Front Immunol. 2016;7:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grabner R, Lotzer K, Dopping S, et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med. 2009;206(1):233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chace JH, Fleming AL, Gordon JA, Perandones CE, Cowdery JS. Regulation of differentiation of peritoneal B-1a (CD5+) B cells. Activated peritoneal macrophages release prostaglandin E2, which inhibits IgM secretion by peritoneal B-1a cells. Journal of immunology. 1995;154(11):5630–5636. [PubMed] [Google Scholar]
- 72.Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. Journal of immunology. 2004;172(10):6020–6029. [DOI] [PubMed] [Google Scholar]
- 73.Takatsu K, Kouro T, Nagai Y. Interleukin 5 in the link between the innate and acquired immune response. Adv Immunol. 2009;101:191–236. [DOI] [PubMed] [Google Scholar]
- 74.Reynolds AE, Kuraoka M, Kelsoe G. Natural IgM is produced by CD5- plasma cells that occupy a distinct survival niche in bone marrow. Journal of immunology. 2015;194(1):231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Binder CJ, Hartvigsen K, Chang M-K, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. Journal of Clinical Investigation. 2004;114(3):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balabanian K, Foussat A, Bouchet-Delbos L, et al. Interleukin-10 modulates the sensitivity of peritoneal B lymphocytes to chemokines with opposite effects on stromal cell-derived factor-1 and B-lymphocyte chemoattractant. Blood. 2002;99(2):427–436. [DOI] [PubMed] [Google Scholar]
- 77.Balabanian K, Couderc J, Bouchet-Delbos L, et al. Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. Journal of immunology. 2003;170(6):3392–3400. [DOI] [PubMed] [Google Scholar]
- 78.Weber GF, Chousterman BG, Hilgendorf I, et al. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J Exp Med. 2014;211(6):1243–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jackson-Jones LH, Benezech C. Control of innate-like B cell location for compartmentalised IgM production. Current opinion in immunology. 2018;50:9–13. [DOI] [PubMed] [Google Scholar]
- 80.Benezech C, Luu N-T, Walker JA, et al. Inflammation-induced formation of fat-associated lymphoid clusters. Nature immunology. 2015;16(8):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. [DOI] [PubMed] [Google Scholar]
- 82.Jackson-Jones LH, Duncan SM, Magalhaes MS, et al. Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun. 2016;7:12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perry HM, Oldham SN, Fahl SP, et al. Helix-loop-helix factor inhibitor of differentiation 3 regulates interleukin-5 expression and B-1a B cell proliferation. Arterioscler Thromb Vasc Biol. 2013;33(12):2771–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duber S, Hafner M, Krey M, et al. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114(24):4960–4967. [DOI] [PubMed] [Google Scholar]
- 86.Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009;39(9):2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Holodick NE, Vizconde T, Rothstein TL. B-1a cell diversity: nontemplated addition in B-1a cell Ig is determined by progenitor population and developmental location. Journal of immunology. 2014;192(5):2432–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nature immunology. 2006;7(3):293–301. [DOI] [PubMed] [Google Scholar]
- 89.Kretschmer K, Stopkowicz J, Scheffer S, Greten TF, Weiss S. Maintenance of peritoneal B-1a lymphocytes in the absence of the spleen. Journal of immunology. 2004;173(1):197–204. [DOI] [PubMed] [Google Scholar]
- 90.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195(6):771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2879–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forster I, Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987;17(4):521–528. [DOI] [PubMed] [Google Scholar]
- 93.Lalor PA, Herzenberg LA, Adams S, Stall AM. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol. 1989;19(3):507–513. [DOI] [PubMed] [Google Scholar]
- 94.Holodick NE, Vizconde T, Hopkins TJ, Rothstein TL. Age-Related Decline in Natural IgM Function: Diversification and Selection of the B-1a Cell Pool with Age. The Journal of Immunology. 2016:1600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Upadhye A, Srikakulapu P, Gonen A, et al. Diversification and CXCR4-Dependent Establishment of the Bone Marrow B-1a Cell Pool Governs Atheroprotective IgM Production Linked To Human Coronary Atherosclerosis. Circ Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dal Porto JM, Burke K, Cambier JC. Regulation of BCR signal transduction in B-1 cells requires the expression of the Src family kinase Lck. Immunity. 2004;21(3):443–453. [DOI] [PubMed] [Google Scholar]
- 97.Wang H, Shin DM, Abbasi S, et al. Expression of plasma cell alloantigen 1 defines layered development of B-1a B-cell subsets with distinct innate-like functions. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(49):20077–20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335(6073):1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wray-Dutra MN, Al Qureshah F, Metzler G, Oukka M, James RG, Rawlings DJ. Activated PIK3CD drives innate B cell expansion yet limits B cell-intrinsic immune responses. J Exp Med. 2018;215(10):2485–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi SC, Wang H, Tian L, et al. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. Journal of immunology. 2013;190(3):987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guo B, Rothstein TL. RasGRP1 Is an Essential Signaling Molecule for Development of B1a Cells with Autoantigen Receptors. Journal of immunology. 2016;196(6):2583–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Enyindah-Asonye G, Li Y, Xin W, et al. CD6 Receptor Regulates Intestinal Ischemia/Reperfusion-induced Injury by Modulating Natural IgM-producing B1a Cell Self-renewal. The Journal of biological chemistry. 2017;292(2):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Graf R, Seagal J, Otipoby KL, et al. BCR-dependent lineage plasticity in mature B cells. Science. 2019;363(6428):748–753. [DOI] [PubMed] [Google Scholar]
- 104.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. The Journal of clinical investigation. 2002;109(6):745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Doran AC, Lipinski MJ, Oldham SN, et al. B-Cell Aortic Homing and Atheroprotection Depend on Id3. Circulation Research. 2012;110(1):e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Srikakulapu P, McSkimming C, McNamara C. Abstract 464: Chemokine Receptor CCR6 Expression on B Cells Augments Local IgM Production and Atheroprotection. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36(suppl_1):A464–A464. [Google Scholar]
- 107.Gjurich BN, Taghavie-Moghadam PL, Ley K, Galkina EV. L-selectin deficiency decreases aortic B1a and B(reg) subsets and promotes atherosclerosis. Thrombosis and haemostasis. 2014;112(4):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qi XY, Qu SL, Xiong WH, Rom O, Chang L, Jiang ZS. Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword. Cardiovasc Diabetol. 2018;17(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ren L, Wang L, You T, et al. Perivascular adipose tissue modulates carotid plaque formation induced by disturbed flow in mice. J Vasc Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 110.McKenney-Drake ML, Rodenbeck SD, Bruning RS, et al. Epicardial Adipose Tissue Removal Potentiates Outward Remodeling and Arrests Coronary Atherogenesis. Ann Thorac Surg. 2017;103(5):1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McKenney ML, Schultz KA, Boyd JH, et al. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Horimatsu T, Patel AS, Prasad R, et al. Remote Effects of Transplanted Perivascular Adipose Tissue on Endothelial Function and Atherosclerosis. Cardiovasc Drugs Ther. 2018;32(5):503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Dam AD, Boon MR, Berbee JFP, Rensen PCN, van Harmelen V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol. 2017;816:82–92. [DOI] [PubMed] [Google Scholar]
- 114.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. Journal of immunology. 2003;171(10):5406–5414. [DOI] [PubMed] [Google Scholar]
- 115.Foussat A, Balabanian K, Amara A, et al. Production of stromal cell-derived factor 1 by mesothelial cells and effects of this chemokine on peritoneal B lymphocytes. Eur J Immunol. 2001;31(2):350–359. [DOI] [PubMed] [Google Scholar]
- 116.Berberich S, Forster R, Pabst O. The peritoneal micromilieu commits B cells to home to body cavities and the small intestine. Blood. 2007;109(11):4627–4634. [DOI] [PubMed] [Google Scholar]
- 117.Jackson SM, Wilson PC, James JA, Capra JD. Chapter 5 Human B Cell Subsets Advances in Immunology. Vol 98: Academic Press; 2008:151–224. [DOI] [PubMed] [Google Scholar]
- 118.Liu YJ, Arpin C, de Bouteiller O, et al. Sequential triggering of apoptosis, somatic mutation and isotype switch during germinal center development. Seminars in immunology. 1996;8(3):169–177. [DOI] [PubMed] [Google Scholar]
- 119.Liu YJ, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–126. [DOI] [PubMed] [Google Scholar]
- 120.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188(9):1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188(9):1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Macallan DC, Wallace DL, Zhang Y, et al. B-cell kinetics in humans: rapid turnover of peripheral blood memory cells. Blood. 2005;105(9):3633–3640. [DOI] [PubMed] [Google Scholar]
- 123.Fecteau JF, Neron S. CD40 stimulation of human peripheral B lymphocytes: distinct response from naive and memory cells. Journal of immunology. 2003;171(9):4621–4629. [DOI] [PubMed] [Google Scholar]
- 124.Fecteau JF, Cote G, Neron S. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. Journal of immunology. 2006;177(6):3728–3736. [DOI] [PubMed] [Google Scholar]
- 125.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20(+)CD27(+)CD43(+)CD70(−). The Journal of Experimental Medicine. 2011;208(1):67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Human B-1 cells take the stage. Annals of the New York Academy of Sciences. 2013;1285:97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rothstein TL, Quach TD. The human counterpart of mouse B-1 cells. Annals of the New York Academy of Sciences. 2015;1362:143–152. [DOI] [PubMed] [Google Scholar]
- 128.Covens K, Verbinnen B, Geukens N, et al. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood. 2013;121(26):5176–5183. [DOI] [PubMed] [Google Scholar]
- 129.Descatoire M, Weill JC, Reynaud CA, Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208(13):2563–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Perez-Andres M, Grosserichter-Wagener C, Teodosio C, van Dongen JJ, Orfao A, van Zelm MC. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208(13):2565–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Griffin DO, Rothstein TL. Human b1 cell frequency: isolation and analysis of human b1 cells. Front Immunol. 2012;3:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Quach TD, Rodriguez-Zhurbenko N, Hopkins TJ, et al. Distinctions among Circulating Antibody-Secreting Cell Populations, Including B-1 Cells, in Human Adult Peripheral Blood. Journal of immunology. 2016;196(3):1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Griffin DO, Rothstein TL. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208(13):2591–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huan T, Zhang B, Wang Z, et al. A Systems Biology Framework Identifies Molecular Underpinnings of Coronary Heart Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(6):1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rodriguez-Zhurbenko N, Quach TD, Hopkins TJ, Rothstein TL, Hernandez AM. Human B-1 Cells and B-1 Cell Antibodies Change With Advancing Age. Front Immunol. 2019;10:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rothstein TL. Natural Antibodies as Rheostats for Susceptibility to Chronic Diseases in the Aged. Front Immunol. 2016;7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ravandi A, Boekholdt SM, Mallat Z, et al. Relationship of IgG and IgM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: results from the EPIC-Norfolk Study. Journal of Lipid Research. 2011;52(10):1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsimikas S, Brilakis ES, Lennon RJ, et al. Relationship of IgG and IgM autoantibodies to oxidized low density lipoprotein with coronary artery disease and cardiovascular events. Journal of Lipid Research. 2007;48(2):425–433. [DOI] [PubMed] [Google Scholar]
- 139.Tsimikas S, Willeit P, Willeit J, et al. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. Journal of the American College of Cardiology. 2012;60(21):2218–2229. [DOI] [PubMed] [Google Scholar]
- 140.Iseme RA, McEvoy M, Kelly B, et al. A role for autoantibodies in atherogenesis. Cardiovasc Res. 2017;113(10):1102–1112. [DOI] [PubMed] [Google Scholar]
- 141.Porsch F, Binder CJ. Impact of B-Cell-Targeted Therapies on Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2019;39(9):1705–1714. [DOI] [PubMed] [Google Scholar]
- 142.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017. [DOI] [PubMed] [Google Scholar]
- 143.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE--mechanisms and management. Nat Rev Rheumatol. 2012;8(4):214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Novikova DS, Popkova TV, Lukina GV, et al. The Effects of Rituximab on Lipids, Arterial Stiffness and Carotid Intima-Media Thickness in Rheumatoid Arthritis. Journal of Korean medical science. 2016;31(2):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hegab Z, Gibbons S, Neyses L, Mamas MA. Role of advanced glycation end products in cardiovascular disease. World J Cardiol. 2012;4(4):90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Engelbertsen D, Vallejo J, Quach TD, et al. Low Levels of IgM Antibodies against an Advanced Glycation Endproduct-Modified Apolipoprotein B100 Peptide Predict Cardiovascular Events in Nondiabetic Subjects. Journal of immunology. 2015;195(7):3020–3025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.