Abstract
Background
In chronic thromboembolic pulmonary hypertension (CTEPH) impaired pulmonary hemodynamics lead to right heart failure. Natriuretic peptides reflect hemodynamic disease severity. Pregnancy-associated plasma protein-A (PAPP-A) might address another aspect of CTEPH - chronic tissue injury and inflammation. This study assessed dynamics of PAPP-A in CTEPH patients who undergo therapy with pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty (BPA).
Methods
The study included a total of 125 CTEPH patients scheduled for treatment (55 PEA/ 70 BPA) and a control group of 58 patients with pulmonary hypertension other than CTEPH. Biomarker measurement was performed at baseline and follow-up in the CTEPH cohort, prior to each BPA in the BPA cohort and once in the control group.
Results
The median PAPP-A level was slightly higher (p = 0.05) in CTEPH patients [13.8 (11.0–18.6) mU/L], than in the control group [12.6 (8.6–16.5) mU/L], without a difference between the BPA and PEA group (p = 0.437) and without a correlation to mean pulmonary artery pressure (p = 0.188), pulmonary vascular resistance (p = 0.893), cardiac index (p = 0.821) and right atrial pressure (p = 0.596). PEA and BPA therapy decreased the mean pulmonary artery pressure (p < 0.001) and pulmonary vascular resistance (p < 0.001) and improved the WHO-functional-class (baseline: I:0/II:25/III:80/IV:20 vs. follow-up: I:55/II:58/III:10/IV:2). PAPP-A levels decreased after PEA [13.5 (9.5–17.5) vs. 11.3 (9.8–13.6) mU/L; p = 0.003) and BPA treatment [14.3 (11.2–18.9) vs. 11.1 (9.7–13.3) mU/L; p < 0.001). The decrease of PAPP-A levels is delayed in comparison to N-terminal pro-B-type natriuretic peptide.
Conclusion
PAPP-A is overexpressed in CTEPH and decrease significantly after surgical or interventional therapy, however without association to hemodynamics. Further investigation is needed to define the underlying mechanism of PAPP-A expression and changes after therapy in CTEPH.
Keywords: PAPP-A, Pregnancy-associated plasma protein A, Pappalysin-1, BPA, PEA, CTEPH, Vascular remodeling
Introduction
In chronic thromboembolic pulmonary hypertension (CTEPH) insufficient thrombus resolution and vascular remodeling lead to chronic obstructions of the pulmonary arteries [1]. The corresponding impaired pulmonary hemodynamics burden the right heart, cause right heart remodeling and ultimately failure [1]. Pulmonary endarterectomy (PEA), medical treatment targeting pulmonary hypertension (PH) and balloon pulmonary angioplasty (BPA) are specific treatment modalities [1]. Impaired pulmonary hemodynamics in CTEPH correlate with non-invasively measured blood biomarkers such as natriuretic peptides and render such markers as indicators for disease severity and therapy response [2]. Considering the multifaceted pathophysiology of CTEPH, biomarkers not primarily reflecting hemodynamics might further provide information about individual disease mechanisms facilitating treatment decisions. Pregnancy-associated plasma protein-A (PAPP-A), clinically established in the pregnancy first-trimester-screening, was identified as key regulator of insulin-like growth factor (IGF)/IGF-binding-protein pathways via cleaving of IGF-binding-protein [3]. This pathway has been reported in the context of atherosclerosis, coronary artery disease, heart failure and non-cardiac conditions [4]. Its role in PH, especially CTEPH, has not been investigated so far.
The current study aimed to evaluate PAPP-A levels in CTEPH and to explore the potential modification of PAPP-A levels by PEA or BPA treatment.
Methods
We analyzed 125 consecutive patients with CTEPH and 58 controls (Patients with PH and suspected CTEPH that was excluded after diagnostic workup). Standardized diagnostic and therapeutic work-up of CTEPH patients has been published earlier [2]. The CTEPH group of the study included 55 patients that underwent PEA and 70 in whom BPA was performed. In PEA patients, biospecimen were obtained at baseline and 12 months after surgery (12-MFU), in BPA at baseline, before each staged procedure and 6 months after the final procedure (6-MFU). In control patients, biomaterial was obtained at enrollment. The biomaterial included venous blood samples that were aliquoted and frozen at − 80 °C. The study was approved by the respective local ethics committee and each patient gave written informed consent. PAPP-A was measured in frozen serum samples using an automated immunofluorescence assay on the Kryptor compact plus instrument (PAPP-A Thermo Scientific, BRAHMS GmbH, Henningsdorf, Germany).
Variables are expressed as median (IQR), mean ± SD or number (%) as appropriate. Comparative analyses used the Student t-test, Mann-Whitney-U-test, Wilcoxon signed-rank test, X2-test and Fisher-Yates test. Bivariate Pearson correlation assessed associations of PAPP-A with pulmonary hemodynamics and other biomarkers. All p-values are seen as descriptive. Statistical analyses were performed with R3.5.1 software package (R Foundation for Statistical Computing, Vienna, Austria).
Results
The comparative analysis of hemodynamic findings revealed a higher pulmonary artery pressure (meanPAP; 43.1 ± 9.7 vs. 39.9 ± 10.9 mmHg; p = 0.041) and pulmonary vascular resistance (PVR; 6.76(5.27–9.61) vs. 4.63(3.15–10.3) WU; p = 0.006) in CTEPH patients compared to the PH control group at baseline. A comprehensive illustration of baseline characteristics of the CTEPH cohort and the control group is provided in Table 1.
Table 1.
Baseline characteristics of the CTEPH cohort and the control group
| CTEPH (total) | CTEPH (BPA) | CTEPH (PEA) | Pulmonary Hypertension other than CTEPH (Controls) | PEA vs. BPA | CTEPH vs. Controls | ||
|---|---|---|---|---|---|---|---|
| n = 125 | n = 70 | n = 55 | n = 58 | (p-value) | (p-value) | ||
| Data availability | |||||||
| Demographics | |||||||
| Age, y; mean ± SD | 183/183 | 59.3 ± 14.3 | 60.83 ± 13.5 | 57.35 ± 15.2 | 62.28 ± 14.6 | 0.277 | 0.14 |
| Female sex; n (%) | 183/183 | 52 (41.6%) | 30 (42.86%) | 22 (40%) | 35 (60.34%) | 0.89 | 0.028 |
| Body mass index, kg/m2; mean ± SD | 183/183 | 25.94 ± 4.5 | 25.09 ± 3.7 | 27.02 ± 5.1 | 30.89 ± 7.9 | 0.046 | < 0.001 |
| History and risk factors | |||||||
| Smoker; n (%) | 181/183 | 55 (44.72%) | 31 (45.59%) | 24 (43.64%) | 31 (53.45%) | 0.973 | 0.348 |
| Diabetes mellitus; n (%) | 183/183 | 6 (4.8%) | 4 (5.71%) | 2 (3.64%) | 16 (27.59%) | 0.694 | < 0.001 |
| Dyslipidemia; n (%) | 182/183 | 23 (18.55%) | 17 (24.29%) | 6 (11.11%) | 14 (24.14%) | 0.101 | 0.499 |
| Arterial hypertension; n (%) | 182/183 | 59 (47.58%) | 36 (51.43%) | 23 (42.59%) | 36 (62.07%) | 0.426 | 0.096 |
| Chronic renal failure; n (%) | 183/183 | 26 (20.8%) | 13 (18.57%) | 13 (23.64%) | 15 (25.86%) | 0.638 | 0.566 |
| Coronary artery disease; n (%) | 182/183 | 20 (16.13%) | 14 (20.29%) | 6 (10.91%) | 12 (20.69%) | 0.244 | 0.586 |
| History of cancer; n (%) | 183/183 | 18 (14.4%) | 13 (18.57%) | 5 (9.09%) | 16 (27.59%) | 0.214 | 0.054 |
| History of acute pulmonary embolism; n (%) | 182/183 | 110 (88.71%) | 56 (81.16%) | 54 (98.18%) | 45 (77.59%) | 0.003 | 0.081 |
| Chronic obstructive pulmonary disease; n (%) | 175/183 | 8 (6.84%) | 4 (6.35%) | 4 (7.41%) | 12 (20.69%) | 1 | 0.014 |
| History of splenectomy; n (%) | 183/183 | 9 (7.2%) | 6 (8.57%) | 3 (5.45%) | 3 (5.17%) | 0.73 | 0.755 |
| Chronic inflammatory disease; n (%) | 183/183 | 3 (2.4%) | 1 (1.43%) | 2 (3.64%) | 6 (10.34%) | 0.582 | 0.030 |
| Laboratory parameters | |||||||
| Ceatinine, μmol/l; mean ± SD | 183/183 | 0.97 ± 0.3 | 0.95 ± 0.3 | 1 ± 0.3 | 0.93 ± 0.4 | 0.298 | 0.058 |
| eGFR, ml/min; mean ± SD | 183/183 | 82.5 ± 25.7 | 83.62 ± 26.6 | 81.08 ± 24.7 | 86.63 ± 34.5 | 0.603 | 0.558 |
| NT-proBNP, ng/l; median (IQR) | 176/183 | 845 (184.2–1860) | 743.7 (197.2–1470) | 1094 (149.775–2078.25) | 412 (181.8–1454.5) | 0.296 | 0.282 |
| PAPP-A, mU/L | 183/183 | 13.8 (11.0–18.6) | 14.5 (11.2–18.9) | 13.7 (10.4–17.6) | 12.6 (8.6–16.5) | 0.437 | 0.051 |
| Symptoms and medication | |||||||
| Guanylate cyclase stimulator; n (%) | 183/183 | 65 (52%) | 49 (70%) | 16 (29.09%) | 8 (13.79%) | < 0.001 | < 0.001 |
| WHO-functional class (I-IV) | 183/183 | I:0;II:25;III:80;IV:20 | I:0;II:11;III:49;IV:10 | I:0;II:14;III:31;IV:10 | I:0;II:7;III:40;IV:11 | ||
| Examination results | |||||||
| LVEF, %; median (IQR) | 158/183 | 55 (55–60) | 55 (55–59.25) | 55 (55–60) | 55 (55–55) | 0.416 | < 0.001 |
| TAPSE, mm; mean ± SD | 153/183 | 19.08 ± 5.3 | 18.68 ± 4.8 | 19.54 ± 5.8 | 19.5 ± 5.3 | 0.449 | 0.788 |
| 6-min-walk distance, m; mean ± SD | 85/183 | 405.18 ± 99.1 | 404.52 ± 91.8 | 409.44 ± 144.7 | 329.56 ± 122.3 | 0.312 | 0.01 |
| Hemodynamics | |||||||
| RAP, mmHg; median (IQR) | 108/183 | 7 (5–9) | 7 (5–9) | 7 (5–8) | 7.5 (4.5–11.75) | 0.977 | 0.764 |
| MeanPAP, mmHg; mean ± SD | 181/183 | 43.09 ± 9.7 | 42.44 ± 9.1 | 43.93 ± 10.6 | 39.86 ± 10.9 | 0.384 | 0.041 |
| PVR, WU (IQR) | 172/183 | 6.76 (5.27–9.61) | 6.76 (5.27–8.56) | 7.065 (5.3075–11.8075) | 4.63 (3.15–10.265) | 0.184 | 0.006 |
| CI, L/min/m2; mean ± SD | 169/183 | 2.5 ± 0.6 | 2.61 ± 0.7 | 2.33 ± 0.6 | 2.58 ± 0.8 | 0.015 | 0.705 |
| PCWP, mmHg; median (IQR) | 179/183 | 9 (8–12) | 9 (8–11) | 9 (8–13) | 11 (9–13) | 0.332 | 0.004 |
Values represent N (%) or mean ± SD or median (IQR)
Abbreviations: BPA Balloon pulmonary angioplasty, CI cardiac index, GFR glomerular filtration rate, hs-cTnT high-sensitivity cardiac troponin T, LVEF left ventricular ejection fraction, NT-proBNP N-terminal pro-B-type natriuretic peptide, PAP pulmonary artery pressure, PCWP Pulmonary capillary wedge pressure, PVR pulmonary vascular resistance, RAP right atrial pressure, TAPSE Tricuspid Annular Plane Systolic Excursion
PAPP-A levels at baseline were comparable in CTEPH patients [13.8(IQR 11.0–18.6) mU/L] and PH controls [12.6(IQR 8.6–16.5) mU/L] (p = 0.051). No relevant correlation between PAPP-A and hemodynamic parameters such as meanPAP (r = 0.120; p = 0.188), pulmonary vascular resistance (PVR) (r = 0.013; p = 0.893) or NT-proBNP (r = 0.128; p = 0.169) was observed in CTEPH patients. Nevertheless, PAPP-A correlated with C-reactive protein (r = 0.259; p = 0.004).
Surgical and interventional treatment led to an improvement of pulmonary hemodynamics and a decrease of natriuretic peptides, which is illustrated in Table 2.
Table 2.
Comparison of hemodynamic findings and NT-proBNP levels between baseline and follow-up in CTEPH patients
| Baseline mean ± SD or median (IQR) |
Follow-up mean ± SD or median (IQR) |
p-value | |
|---|---|---|---|
| PEA | |||
| MeanPAP; mmHg | 43.9 ± 10.6 | 22.3 ± 7.5 | p < 0.001 |
| PVR; WU | 7.1 (5.3–11.8) | 2.5 (1.8–3.5) | p < 0.001 |
| NT-proBNP; ng/L | 1094 (150–2078) | 192 (102–382) | p < 0.001 |
| BPA | |||
| MeanPAP; mmHg | 42.4 ± 9.1 | 31.7 ± 9.6 | p < 0.001 |
| PVR; WU | 7.1 (5.3–11.8) | 3.9 (3.1–5.3) | p < 0.001 |
| NT-proBNP; ng/L | 744 (197–1470) | 121 (70–238) | p < 0.001 |
Values represent as mean ± SD or median (IQR)
Abbreviations: BPA Balloon pulmonary angioplasty, NT-proBNP N-terminal pro-B-type natriuretic peptide, meanPAP mean pulmonary artery pressure, PEA pulmonary endarterectomy, PVR pulmonary vascular resistance
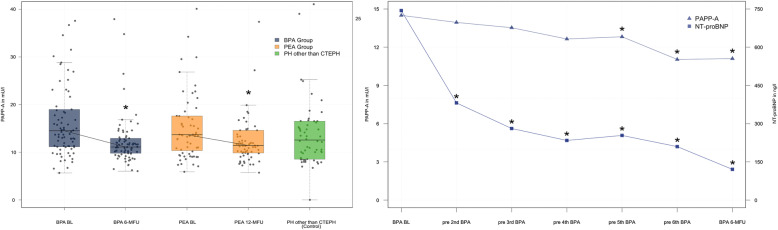
The PAPP-A levels did not differ between the PEA and BPA treatment group (13.7 (10.4–17.6) vs. 14.5 (11.2–18.9) mU/L; p = 0.437) at baseline (Fig. 1, left panel). PAPP-A levels decreased significantly after treatment from 13.7 (10.4–17.6) to 11.4 (9.9–14.6) mU/L (p = 0.003) after PEA and 14.5 (11.2–18.9) to 11.1 (9.8–12.9) mU/L (p < 0.001) after BPA therapy (Fig. 1, left panel).
Fig. 1.
Impact of treatment on pregnancy-associated plasma protein A (PAPP-A) levels in patients with chronic thromboembolic pulmonary hypertension (CTEPH). The left panel shows PAPPA-A levels in CTEPH patients before undergoing treatment with pulmonary endarterectomy (PEA BL) or balloon pulmonary angioplasty (BPA BL) and 12 months after PEA (PEA 12-MFU) respectively 6 months after the final BPA (BPA 6-MFU) procedure compared to controls of patients with pulmonary hypertension in whom CTEPH was excluded (PH Controls). The right panel shows the time dependent effect of the staged BPA procedure on PAPP-A levels. For comparison, data on NT-proBNP as a biomarker reflecting hemodynamics is provided. * Indicates p-value < 0.05 comparing difference in PAPP-A level at procedure compared to the baseline level
BPA is a staged procedure (median 6 procedures/patient) with only a limited number of pulmonary segments treated per session. PAPP-A levels decreased continuously reaching a significant change after 4 procedures in contrast to the hemodynamic marker NT-proBNP as a comparator that was significantly lowered already after the first procedure (Fig. 1, right panel).
Discussion
Key findings of this study are: (1) PAPP-A levels might be associated with CTEPH and decrease after interventional or surgical treatment. (2) The PAPP-A treatment response shows a slow and continuous lowering in marker levels in contrast to the rapid improvement in hemodynamics reflected by biomarkers such as NT-proBNP.
The PAPP-A levels in CTEPH patients, which are modifiable by treatment. Seem not to be mediated by hemodynamics and their improvement after treatment raising the question about the origin and role of PAPP-A in CTEPH.
Acute pulmonary embolism impairs pulmonary vascular homeostasis. The mechanisms leading to development of CTEPH in a subset of PE patients are not fully understood. Endothelial damage, dysfunction and inflammation are known to be involved in vascular remodeling. The IGF-I/IGF-receptor signaling promotes inflammation, anti-apoptosis and proliferation in various cell types such as endothelial and smooth muscle cells [5, 6]. Yang et al. reported a key role of the IGF-I/IGF-receptor signaling in neonatal PH, revealing an upregulation of IGF-I expression in pulmonary endothelial and smooth muscle cells under experimental hypoxia [7, 8]. Further, Harrington et al. identified PAPP-A as a promotor of atherosclerotic plaque progression and plaque vulnerability. This processes seemed to be driven by PAPP-A-mediated proinflammatory effects of macrophage cytokines and a consecutive upregulation of the IGF-I/IGF-receptor axis [9].
One might hypothesize, that an overexpression of PAPP-A might thus reflect chronic vascular remodeling in CTEPH. The potential use as a biomarker indicating disease mechanisms other than hemodynamics is further supported by the availability of robust automated measurement technology due to the routine use in the context of pregnancy. Further, as the IGF pathway plays a relevant role in certain cancer entities, PAPP-A has already been discussed as treatment target that led to e.g. development of monoclonal PAPP-A antibodies [10].
The present clinical study is based on relatively small cohort and therefore the results only allow to hypothesize about the potential role of PAPP-A. However, it is the first analysis showing an association of PAPP-A with CTEPH. Especially the modification of PAPP-A levels by treatment not primary mediated via hemodynamic improvement should stimulate further investigations to confirm the present results from a small cohort and further analyze the specific role of PAPP-A in the pathophysiology of CTEPH and a potential clinical use as biomarker or even treatment target.
Acknowledgements
We thank Dimitri Grün for statistical support and Claudia Brüderle for help with data management of the study cohort. The present analyses include data from the doctoral thesis of FR.
Abbreviations
- 12-MFU
Follow-Up twelve month after PEA
- 6-MFU
Follow-Up six month after last BPA
- BPA
Balloon pulmonary angioplasty
- CTEPH
Chronic thromboembolic pulmonary hypertension
- eGFR
estimated glomerular filtration rate
- IGF(−I)
Insulin-like growth factor (− 1)
- IQR
Interquartile range
- meanPAP
Mean pulmonary artery pressure
- NT-proBNP
N-terminal pro brain natriuretic peptide
- PAPP-A
Pregnancy-associated plasma protein A or Pappalysin-1
- PCWP
Pulmonary capillary wedge pressure
- PEA
Pulmonary endarterectomy
- PH
Pulmonary hypertension
- PVR
Pulmonary vascular resistance
- SD
Standard deviation
- WU
Wood units
Authors’ contributions
SDK: data management, statistical analysis and interpretation, first draft of the manuscript. FR: data management, statistical analysis and interpretation, first draft of the manuscript. CBW treatment and follow-up of patients, data interpretation, proofreading of the manuscript. SG treatment and follow-up of patients, data interpretation, proofreading of the manuscript. MH treatment and follow-up of patients, data interpretation, proofreading of the manuscript. EM treatment and follow-up of patients, data interpretation, proofreading of the manuscript. LM data management, proofreading of the manuscript. CWH conceptualization of the study, acquisition of funding, proofreading of the manuscript. CL conceptualization of the study data interpretation, proofreading of the manuscript. TK conceptualization of the study data interpretation, proofreading of the manuscript. The author(s) read and approved the final manuscript.
Funding
This research project is based on a cohort that is part of the Kerckhoff Biomarker Registry (BioReg) that is financially supported by the Kerckhoff Heart Research Institute (KHFI) and the German Center for Cardiovascular Research e.V. (DZHK). The research project is associated to the Collaborative Research Center (SFB) 1213 funded by the German Research Foundation (DFG). The sponsors had no influence on the study design, statistical analyses or draft of the paper.
Availability of data and materials
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. Sharing the underlying data is not in line with the written informed consent of the patients in this study. The data will be shared on reasonable request to the corresponding author.
Ethics approval and consent to participate
The study was approved by the local ethics committee of the Facility of Medicine of the Justus-Liebig-University in Giessen, Germany in July of 2014 (referral numbers 43/14 and 44/14). All participating patients were comprehensively informed by a physician and gave written informed consent.
Consent for publication
All participants gave written informed consent to be included in the study. This manuscript does not include any individual person’s data in any form, including individual details, images or videos. Therefore, no dedicated consent for publication is needed.
Competing interests
CBW received consultant honoraria and/or speaker fees from Actelion, Bayer AG, MSD, Pfizer and BTG; CWH received lecture or consulting honoraria from BRAHMS/ Thermo Fisher; EM received lecture or consulting honoraria from Actelion, Bayer, MSD, GSK, Pfizer and MSD; CL received lecture or consulting honoraria from Abbott, Astra Zeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, Daiichi-Sankyo and Pfizer-Bristol-Myers Squibb. TK received speaker fees from Abbott and Brahms; SDK, FR and LM have nothing to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Christoph Liebetrau and Till Keller contributed equally as last authors.
References
- 1.Wilkens H, Konstantinides S, Lang IM, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): updated recommendations from the Cologne Consensus Conference 2018. Int J Cardiol. 2018;272(xxxx):69–78. doi: 10.1016/j.ijcard.2018.08.079. [DOI] [PubMed] [Google Scholar]
- 2.Kriechbaum SD, Wiedenroth CB, Wolter J-SS, et al. N-terminal pro-B-type natriuretic peptide for monitoring after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2018;37(5):639–646. doi: 10.1016/j.healun.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence JB, Oxvig C, Overgaard MT, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A. 1999;96(6):3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funayama A, Shishido T, Netsu S, et al. Serum pregnancy-associated plasma protein A in patients with heart failure. J Card Fail. 2011;17(10):819–826. doi: 10.1016/j.cardfail.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Troncoso R, Ibarra C, Vicencio JM, Jaimovich E, Lavandero S. New insights into IGF-1 signaling in the heart. Trends Endocrinol Metab. 2014;25(3):128–137. doi: 10.1016/j.tem.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Pfäffle R, Kiess W, Klammt J. Downstream insulin-like growth factor. Endocr Dev. 2012;23:42–51. doi: 10.1159/000341745. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Sun M, Ramchandran R, Raj JU. IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: role of epigenetic regulation. Vasc Pharmacol. 2015;73:20–31. doi: 10.1016/j.vph.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun M, Ramchandran R, Chen J, Yang Q, Raj JU. Smooth muscle insulin-like growth factor-1 mediates hypoxia-induced pulmonary hypertension in neonatal mice. Am J Respir Cell Mol Biol. 2016;55(6):779–791. doi: 10.1165/rcmb.2015-0388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100(12):1696–1702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- 10.Becker MA, Haluska P, Bale LK, Oxvig C, Conover CA. A novel neutralizing antibody targeting pregnancy-associated plasma protein-a inhibits ovarian cancer growth and ascites accumulation in patient mouse tumorgrafts. Mol Cancer Ther. 2015;14(4):973–981. doi: 10.1158/1535-7163.MCT-14-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study. Sharing the underlying data is not in line with the written informed consent of the patients in this study. The data will be shared on reasonable request to the corresponding author.