Abstract
Background
Concurrent chemoradiotherapy is the standard of care for locally advanced cervical cancer. Concurrent chemoradiotherapy with programmed blockade of the cell death-1/programmed cell death-ligand 1 pathway may promote a more immunogenic environment through increased phagocytosis, cell death, and antigen presentation, leading to enhanced immune-mediated tumor surveillance.
Primary Objective
The CALLA trial is designed to determine the efficacy and safety of the programmed cell death-ligand 1 blocking antibody, durvalumab, with and following concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in women with locally advanced cervical cancer.
Study Hypothesis
Durvalumab concurrent with and following concurrent chemoradiotherapy will improve progression-free survival in patients with International Federation of Gynecology and Obstetrics (FIGO) 2009 stage IB2 to IVA cervical cancer compared with concurrent chemoradiotherapy alone.
Trial Design
CALLA is a phase III, randomized, multicenter, international, double-blind, placebo-controlled study. Patients will be randomized 1:1 to receive either durvalumab (1500 mg intravenously (IV)) or placebo every 4 weeks for 24 cycles. All patients will receive external beam radiotherapy with cisplatin (40 mg/m2) IV or carboplatin (area under the curve 2) IV once a week for 5 weeks, followed by image-guided brachytherapy.
Major Inclusion/Exclusion Criteria
The study will enroll immunotherapy-naïve adult patients with histologically confirmed cervical adenocarcinoma, cervical squamous, or adenosquamous carcinoma FIGO 2009 stages IB2–IIB node positive and stage IIIA–IVA with any node stage. Patients will have had no prior definitive surgical, radiation, or systemic therapy for cervical cancer.
Primary Endpoint
The primary endpoint is progression-free survival (assessed by the investigator according to Response Evaluation Criteria in Solid Tumors v1.1, histopathological confirmation of local tumor progression or death).
Sample Size
Approximately 714 patients will be randomized 1:1 to receive either durvalumab + concurrent chemoradiotherapy or placebo + concurrent chemoradiotherapy.
Estimated Dates for Completing Accrual and Presenting Results
Patient enrollment is continuing globally with an estimated completion date of April 2024.
Trial Registration
Keywords: ureter; intestine, large; peritoneal neoplasms; pain; uterus
Highlights.
CALLA will explore the potential of durvalumab, with and following concurrent radiotherapy, to improve outcomes.
As this is one of the largest trials in this patient population, CALLA has the potential to change clinical practice.
CALLA is a robust, prospective, placebo-controlled randomized trial with a strong commitment to global enrollment.
INTRODUCTION
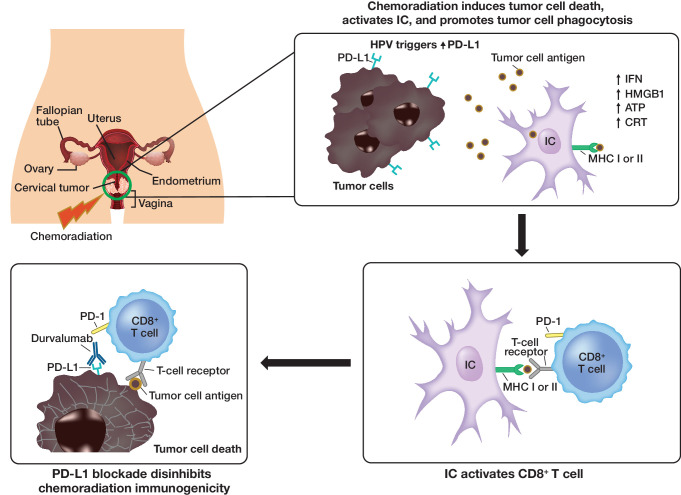
Concurrent chemoradiotherapy (comprising external beam radiotherapy and brachytherapy, with concurrent cisplatin or carboplatin) is the standard of care for the treatment of locally advanced cervical cancer. Chemotherapy added to radiation improves overall survival (hazard ratio (HR)=0.71; p<0.0001) and progression-free survival (HR=0.61; p<0.0001) compared with radiotherapy alone.1 Concurrent chemoradiotherapy in combination with blockade of the programmed cell death-1/programmed cell death-ligand 1 pathway may induce an increased immunogenic environment by initiating DNA breaks, cell death, phagocytosis, and antigen presentation, thereby leading to reactivation of immune-mediated tumor surveillance and enhanced anti-tumor activity (Figure 1).2
Figure 1.
Concurrent chemoradiotherapy + anti-programmed cell death-1/PD-L1 therapies: proposed mechanism of action. Data from pre-clinical and early clinical studies provide the rationale for adding anti-PD-1/PD-L1 therapies to CCRT to improve anti-tumor responses by recruiting the immune system. ATP, adenosine triphosphate; CD8, cluster of differentiation 8; CCRT, concurrent chemotherapy and radiation therapy; CRT,chemotherapy and radiation therapy; HMGB1, high-mobility group box 1; HPV, human papilloma virus; IC, immune cell; IFN, interferon; MHC, major histocompatibility complex; PD-1, programmed cell death-1; PD-L1, programmed cell death-ligand 1.
Cervical cancer itself is a highly immunogenic disease due to chronic human papillomavirus (HPV) infection. HPV is the primary causative agent for the majority of cervical cancer cases, with more than 90% of squamous cervical cancers containing HPV DNA. In the presence of a chronic HPV infection, a natural immune response develops with activation of the adaptive immune system. HPV proteins E6 and E7 also promote neoantigen generation, further stimulating the innate immune response.3 This is supported by programmed cell death-1 expression, which increases with higher grades of cervical intra-epithelial neoplasia,4 with 88% of cases of locally advanced cervical cancer ≥1% positive for programmed cell death-ligand 1 (PD-L1).5
In cervical cancer, immunotherapy has proven activity in second-line, metastatic disease and pembrolizumab has been granted accelerated approval for the treatment of patients with advanced, PD-L1-positive cervical cancer with disease progression while receiving, or after, chemotherapy. The KEYNOTE-158 trial treated 98 women with cervical squamous cell cancer with pembrolizumab. That study had an objective response rate of 12% (12/98), with three patients having a complete response. This checkpoint inhibitor was well tolerated and had a reasonable toxicity profile.6
Durvalumab is a selective, high-affinity, human immunoglobulin G1 monoclonal antibody that blocks PD-L1 binding to PD-L1 and CD80 (B7.1), allowing T cells to recognize and kill tumor cells.7 In patients with non-small cell lung cancer, the PACIFIC trial (NCT02125461) showed that durvalumab administered within 42 days of concurrent chemoradiotherapy significantly improved median progression-free survival compared with placebo (16.8 months (95% CI 13.0 to 18.1) vs 5.6 months (95% CI 4.6 to 7.8); stratified HR for disease progression or death, 0.52; 95% CI 0.42 to 0.65; p<0.001).7 Similar findings in favor of durvalumab compared with placebo were found for duration of response (72.8% vs 46.8% of patients had ongoing response at 18 months, respectively) and median time to death or distant metastasis (23.2 vs 14.6 months, respectively; p<0.001).7 Durvalumab with concurrent chemoradiotherapy was also shown to be well tolerated with a manageable safety profile.7
Although women with early-stage, node-negative cervical cancer have favorable outcomes with available therapy, an important unmet medical need remains in high-risk groups. The prognosis of patients with stage II and IVA node-positive cervical carcinoma is poor and remains a therapeutic dilemma and constitutes an unmet need. Patients with para-aortic lymph node metastases at diagnosis are a group with particularly poor prognosis.8 Similarly, patients with more advanced local disease such as stage III and stage IVA disease, while potentially curative, have a high rate of relapse and poor overall survival, with a 3-year overall survival ranging between 29% and 52%.9 The CALLA study will explore the potential of durvalumab, when administered with and following concurrent chemoradiotherapy, to improve outcomes in patients with International Federation of Gynecology and Obstetrics (FIGO) 2009 stage IB2 to IVA cervical cancer compared with concurrent chemoradiotherapy alone.
METHODS
Trial Design
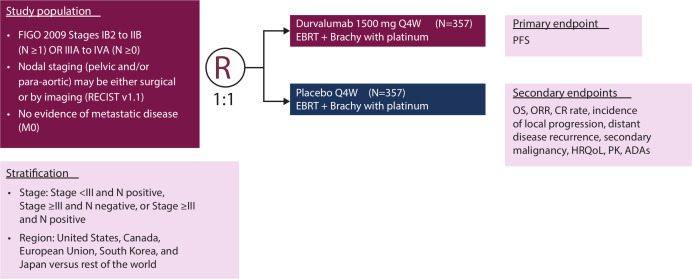
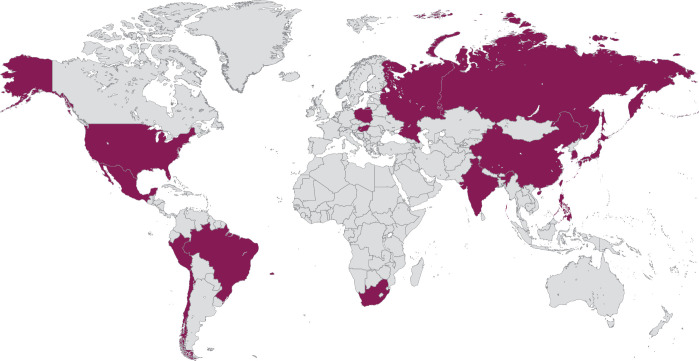
CALLA (NCT03830866) is a phase III, randomized, multicenter, international, double-blind, placebo-controlled study in which patients will be randomized 1:1 to receive either durvalumab (1500 mg intravenously (IV)) or infusion of matching placebo every 4 weeks for 24 cycles (Figure 2). All patients will receive external beam radiotherapy followed by image-guided brachytherapy with cisplatin (40 mg/m2) IV or carboplatin (area under the curve 2) IV once weekly for 5 weeks (sixth dose optional). Study enrollment is planned at about 140 sites globally in 15 countries (Figure 3). As the participating institutions are global, the radiation in the trial has a specific quality assurance workflow to ensure standardized radiotherapy for all treated patients. The radiation process consists of modern techniques, including the use of intensity-modulated radiation therapy, and image-guided brachytherapy. Funding for this study is provided by AstraZeneca.
Figure 2.
CALLA study design. CALLA is a phase III, randomized, double-blind, placebo-controlled, multicenter study. ADA, anti-drug antibody; Brachy, brachytherapy; CR, complete response; EBRT, external beam radiotherapy; FIGO, International Federation of Gynecology and Obstetrics; HRQoL, health-related quality of life; M, metastasis; N, node; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PK, pharmacokinetics; Q4W, once every 4 weeks; R, randomization; RECIST, Response Evaluation Criteria in Solid Tumors.
Figure 3.
Planned study sites. Approximately 131 sites including 114 sites outside the United States are planned.
Participants
The study will enroll immunotherapy-naïve adult patients aged ≥18 years with histologically confirmed cervical adenocarcinoma, cervical squamous, or adenosquamous carcinoma (FIGO) 2009 stages IB2–IIB node-positive and stages IIIA–IVA with any node stage and no prior definitive surgical, radiation, or systemic therapy for cervical cancer. Key patient inclusion and exclusion criteria are shown in Table 1.
Table 1.
Key inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| Women aged ≥18 years*, body weight >30 kg | Diagnosis of small cell (neuroendocrine) histology or mucinous adenocarcinoma cervical cancer |
Histologically confirmed cervical adenocarcinoma, cervical squamous carcinoma, or cervical adenosquamous carcinoma with:
|
Intent to administer a fertility-sparing treatment regimen |
| WHO/ECOG PS of 0 or one at enrollment and randomization | Prior hysterectomy (including supracervical hysterectomy) and patients who intend to have a hysterectomy as part of their initial cervical cancer therapy |
| ≥1 lesion that qualifies as a RECIST v1.1 tumor lesions, not previously irradiated, at baseline assessed (by CT scan or MRI) within 28 days before randomization | Evidence of metastatic disease per RECIST v1.1, including lymph nodes ≥15 mm (short axis) above the L1 cephalad body or outside the planned radiation field |
| No prior chemotherapy or radiotherapy for cervical cancer and immunotherapy naïve | History of another primary malignancy, active primary immunodeficiency, or allogeneic organ transplantation |
| Sustainability and fitness for concurrent chemoradiotherapy as determined by the investigator | Active or prior documented autoimmune or inflammatory disorders |
| Uncontrolled inter-current illness | |
| Prior chemotherapy or radiation therapy for the management of cervical cancer | |
| Any concurrent chemotherapy, IP, biologic, or hormonal therapy for cancer treatment |
*For female patients aged <20 years and enrolled in Japan, written informed consent should be obtained from the patient and her legally acceptable representative.
CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; IP, intra-peritoneal; M, metastasis; MRI, magnetic resonance imaging; N, node; PS, performance status; RECIST, Response Evaluation Criteria in Solid Tumors; WHO, World Health Organization.
Primary Endpoints
The CALLA study will evaluate the primary endpoint of progression-free survival based on a composite of investigator assessment of disease progression according to the Response Evaluation Criteria in Solid Tumors v1.1, histopathological confirmation of local tumor progression, or death. Secondary endpoints include progression-free survival (3 year); progression-free survival in PD-L1-positive patients; objective response rate; complete response rate, and duration of response in patients with a complete response rate; incidence of local progression, distant disease progression, and secondary malignancy (Table 2). Health-related quality of life and safety will also be evaluated.
Table 2.
Study endpoints and outcome measures
| Endpoints | Outcome measures |
| Primary endpoint | |
| Progression-free survival | Time from date of randomization until tumor progression or death due to any cause, as confirmed by investigator assessment per RECIST v1.1 or per histopathological confirmation of local tumor progression |
| Secondary endpoints | |
| Overall survival | Time from date of randomization until date of death due to any cause |
| Progression-free survival (3 year) | Proportion of patients alive and progression free at 3 years |
| Progression-free survival in programmed death ligand-1 positive patients | Time from date of randomization until tumor progression or death due to any cause, as confirmed by investigator assessment per RECIST v1.1 or per histopathological confirmation of local tumor progression in patients who are programmed death ligand-1 positive |
| Objective response rate | Percentage of evaluable patients with an investigator-assessed visit response of complete response rate or partial response |
| Complete response rate | Disappearance of all target and non-target lesions as determined at the 20-week assessment |
| Duration of response in patients with complete response rate | Time from date of first detection of complete response rate as determined at the 20-week assessment until the date of objective disease progression per RECIST v1.1 or per histopathologic confirmation of local tumor progression |
| Incidence of local progression, distant disease progression, and secondary malignancy as the first documented progression event | Number and percentage of patients who develop local progression, distant disease recurrence, or secondary malignancy |
| Health-related quality of life | Change from baseline in EORTC 30-item Core Quality of Life Questionnaire and EORTC cervical cancer module of the Core Quality of Life Questionnaire |
| Pharmacokinetics | Blood concentration of durvalumab when used in combination with concurrent chemoradiotherapy |
| Immunogenicity | Presence of anti-drug antibodies |
| Safety endpoint | |
| Safety and tolerability | AEs, laboratory findings, vital signs, physical examinations |
| Exploratory endpoints | |
| Candidate markers likely to correlate with clinical benefit | Analysis of blood/tissue samples to assess exploratory biomarkers, which may include, but is not limited to ctDNA, mRNA signatures, CD8 by IHC, and tumor mutational burden |
| Patient-reported outcomes | Specific treatment-related Patient-reported Outcomes Version of the Common Terminology Criteria for Adverse Events symptoms Patients Global Impression of Change, Patients Global Impression of Severity, and European Organisation for Research and Treatment of Cancer; EuroQoL 5-Dimensional 5-Level Questionnaire |
AE, adverse event; CD8, cluster of differentiation 8; ctDNA, circulating tumor DNA; DNA, deoxyribonucleic acid; EORTC, European Organisation for Research and Treatment of Cancer; IHC, immunochemistry; mRNA, messenger ribonucleic acid; RECIST, Response Evaluation Criteria in Solid Tumors.
Sample Size
Approximately 714 patients will be randomized 1:1 to durvalumab + concurrent chemoradiotherapy or placebo + concurrent chemoradiotherapy.
Randomization and Blinding
For randomization, patients will be stratified by disease stage status (FIGO 2009 stage <III and node positive, FIGO 2009 stage ≥III and node negative, or FIGO 2009 stage ≥III and node positive) and region (Canada, European Union, Japan, South Korea, United States vs rest of the world). Participants and investigators will remain blinded throughout the course of the study.
Statistical Methods
The progression-free survival, overall survival, progression-free survival in PD-L1-positive patients (and duration of response in patients with a complete response rate) will be summarized using Kaplan-Meier curves plotted by treatment arm. Progression-free survival and overall survival will be analyzed using a stratified log-rank test, stratified by disease stage and region. Progression-free survival (3 year) will be summarized using the Kaplan-Meier estimate of progression-free survival at 3 years. The objective response rate and complete response rate will be presented as an OR with an associated CI and p value. The summaries of objective response rate and complete response rate will be presented as number and percentage of patients. Time to first local progression, time to first distant disease progression or first secondary malignancy, and time to death will be analyzed separately using competing risk methodology, including cumulative incidence plots by treatment arm for the cause of interest and the competing risk.
DISCUSSION
CALLA is a phase III, randomized, multicenter, international, double-blind, placebo-controlled study, which will randomize approximately 714 immunotherapy-naïve adult patients with histologically confirmed cervical adenocarcinoma, cervical squamous, or adenosquamous carcinoma FIGO 2009 stages IB2–IIB node-positive and stages IIIA–IVA with any node stage and no prior definitive surgical, radiation, or systemic therapy for cervical cancer, making it one of the largest trials in patients with cervical cancer.10
It is expected that durvalumab, when administered in combination with concurrent chemoradiotherapy and as maintenance therapy following concurrent chemoradiotherapy, will significantly prolong progression-free survival, in comparison with concurrent chemoradiotherapy and placebo, thus improving prognosis in patients with FIGO stage IB2 and IVA cervical cancer. The clinical activity associated with potentiating the pro-inflammatory effects of concurrent chemoradiotherapy suggests that administering durvalumab in combination with concurrent chemoradiotherapy may improve clinical outcomes, including increasing the response rate to concurrent chemoradiotherapy, improving the complete response rate, and decreasing the number of patients who progress when receiving concurrent chemoradiotherapy.2 Safety observations in other tumor types have shown that concurrent administration of concurrent chemoradiotherapy and immunotherapy is generally well tolerated.11–13 Therefore, the CALLA trial was started to evaluate the efficacy and safety of concurrent administration of durvalumab and concurrent chemoradiotherapy in patients with cervical cancer.
In addition, the quality assurance process of radiotherapy on the CALLA study is noteworthy. A radiation therapy committee serves to ensure that the radiotherapy specified in the protocol meets the standard of care, answers any questions sites might have, and reviews plans during the trial. As the participating institutions are global, the radiation in the trial has a specific quality assurance workflow to ensure standardized radiotherapy for all patients treated. All institutions will undergo a radiation-credentialing process with the radiotherapy plan reviewed for each patient in the trial. The radiation process in CALLA consists of modern techniques, including the use of intensity-modulated radiation therapy, and image-guided brachytherapy.
Since the National Cancer Institute issued a consensus statement changing the standard of care for locally advanced cervical cancer to concurrent chemotherapy with radiation more than 20 years ago, treatment remains largely unchanged.1 CALLA, as a robust, prospective, placebo-controlled, randomized trial, has the potential to have an immense effect. Additionally, since the burden of cervical cancer is global, the CALLA trial is unique with its strong commitment to global enrollment with more than 130 sites outside the United States. The CALLA trial has the potential to change clinical practice in cervical cancer with its ability to reach patients throughout the world.
Acknowledgments
Medical writing support, which as in accordance with Good Publication Practice (GPP3) guidelines, was provided by Sherri Baber, MBA, MSIR of Parexel (Hackensack, New Jersey, USA) and was funded by AstraZeneca.
Footnotes
Contributors: All authors made substantial contributions to the conception, analysis, interpretation of data, and the drafting and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding: This study was funded by AstraZeneca.
Competing interests: Outside the submitted work, JM, reports personal fees from Varian Medical Systems, personal fees from GOG Foundation, personal fees from NRG Oncology, personal fees from AstraZeneca, grants from NRG Oncology, other from GOG Foundation. ATN, ML report personal fees from AstraZeneca during the conduct of the study and personal fees from AstraZeneca outside the submitted work. MM reports personal fees from AstraZeneca and personal fees from Incyte Corporation during the conduct of the study and personal fees from AstraZeneca and Incyte Corporation outside the submitted work. MCL reports personal fees from AZ/MedImmune during the conduct of the study and personal fees from AZ/MedImmune outside the submitted work. BJM reports personal fees from Abbvie, Advaxis, Agenus, Amgen, AstraZeneca, Biodesix, Clovis, Conjupro, Genmab, Gradalis, ImmunoGen, Immunomedics, Incyte, Janssen/Johnson&Johnson, Mateon (formally Oxigene), Merck, Myriad, Perthera, Pfizer, Precison Oncology, Puma, Roche/Genentech, Samumed, Takeda, TESARO, Inc. and VBL outside the submitted work. ATN, ML, MM and MCL are all employees of AstraZeneca.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: There are no data in this work
References
- 1. Green JA, Kirwan JM, Tierney JF, et al. . Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 2001;358:781–6. 10.1016/S0140-6736(01)05965-7 [DOI] [PubMed] [Google Scholar]
- 2. Menderes G, Black J, Schwab CL, et al. . Immunotherapy and targeted therapy for cervical cancer: an update. Expert Rev Anticancer Ther 2016;16:83–98. 10.1586/14737140.2016.1121108 [DOI] [PubMed] [Google Scholar]
- 3. Qin Y, Ekmekcioglu S, Forget M-A, et al. . Cervical cancer neoantigen landscape and immune activity is associated with human papillomavirus master regulators. Front Immunol 2017;8:689. 10.3389/fimmu.2017.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang W, Lu Y-P, Yang Y-Z, et al. . Expressions of programmed death (PD)-1 and PD-1 ligand (PD-L1) in cervical intraepithelial neoplasia and cervical squamous cell carcinomas are of prognostic value and associated with human papillomavirus status. J Obstet Gynaecol Res 2017;43:1602–12. 10.1111/jog.13411 [DOI] [PubMed] [Google Scholar]
- 5. Enwere EK, Kornaga EN, Dean M, et al. . Expression of PD-L1 and presence of CD8-positive T cells in pre-treatment specimens of locally advanced cervical cancer. Mod Pathol 2017;30:577–86. 10.1038/modpathol.2016.221 [DOI] [PubMed] [Google Scholar]
- 6. Schellens JHM, Marabelle A, Zeigenfuss S, et al. . Pembrolizumab for previously treated advanced cervical squamous cell cancer: preliminary results from the phase 2 KEYNOTE-158 study. JCO 2017;35:5514 10.1200/JCO.2017.35.15_suppl.5514 [DOI] [Google Scholar]
- 7. Antonia SJ, Villegas A, Daniel D, et al. . Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 8. Macdonald OK, Chen J, Dodson M, et al. . Prognostic significance of histology and positive lymph node involvement following radical hysterectomy in carcinoma of the cervix. Am J Clin Oncol 2009;32:411–6. 10.1097/COC.0b013e31819142dc [DOI] [PubMed] [Google Scholar]
- 9. Surveillance, Epidemiology, and End Results (SEER) Seer is an authoritative source for cancer statistics in the United States. Available: www.seer.cancer.gov [Accessed 11 Nov 2019].
- 10. Monk BJ, Mayadev J, Nunes AT, et al. . CALLA: efficacy and safety of durvalumab with and following concurrent chemoradiotherapy (CCRT) versus CCRT alone in women with locally advanced cervical cancer: a phase III, randomized, double-blind, multicenter study. J Clin Oncol 2019;37 10.1200/JCO.2019.37.15_suppl.TPS5597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jabbour SK, Berman AT, Decker RH, et al. . Prospective phase I multi-institutional trial of PD-1 blockade with pembrolizumab during concurrent chemoradiation for locally advanced, unresectable non-small cell lung cancer. J Clin Oncol 2019;37 10.1200/JCO.2019.37.15_suppl.8511 [DOI] [Google Scholar]
- 12. Langer CJ, Gadgeel SM, Borghaei H, et al. . Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497–508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rizvi NA, Hellmann MD, Brahmer JR, et al. . Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016;34:2969–79. 10.1200/JCO.2016.66.9861 [DOI] [PMC free article] [PubMed] [Google Scholar]