Abstract
Background
Dietary protein deficiency and amino acid imbalance cause hepatic fat accumulation. We previously demonstrated that only arginine deficiency or total amino acid deficiency in a diet caused significant hepatic triglyceride (TG) accumulation in young Wistar rats. In this study, we explored the mechanisms of fatty liver formation in these models.
Methods
We fed 6-week-old male Wistar rats a control diet (containing an amino acid mixture equivalent to 15% protein), a low-total-amino acid diet (equivalent to 5% protein; 5PAA), and a low-arginine diet (only the arginine content is as low as that of the 5PAA diet) for 2 weeks.
Results
Much greater hepatic TG accumulation was observed in the low-arginine group than in the low-total-amino acid group. The lipid consumption rate and fatty acid uptake in the liver did not significantly differ between the groups. In contrast, the low-total-amino acid diet potentiated insulin sensitivity and related signaling in the liver and enhanced de novo lipogenesis. The low-arginine diet also inhibited hepatic very-low-density lipoprotein secretion without affecting hepatic insulin signaling and lipogenesis.
Conclusions
Although the arginine content of the low-arginine diet was as low as that of the low-total-amino acid diet, the two diets caused fatty liver via completely different mechanisms. Enhanced lipogenesis was the primary cause of a low-protein diet-induced fatty liver, whereas lower very-low-density lipoprotein secretion caused low-arginine diet-induced fatty liver.
Keywords: Arginine deficiency, Low-protein diet, Hepatosteatosis, Apolipoprotein A-IV, Insulin signaling
Background
Dietary protein has a strong impact on growth, body composition, and energy homeostasis. Protein overload results in a reduced body fat in humans [1, 2] and in a improve insulin sensitivity in rats [3, 4]. By contrast, dietary protein malnutrition induces hepatic fat accumulation, a symptom of kwashiorkor, in rats [5] and humans [6]. The dietary amino acid balance also affects fat deposition in the liver. For example, rats fed low-protein diets supplemented with essential amino acids, such methionine, experience excessive fat deposition in the liver, and supplementation of a low-protein diet with DL-threonine prevents hepatic fat accumulation [7].
Non-alcoholic fatty liver disease (NAFLD) represents a series of hepatic disorders triggered by excessive liver fat accumulation and is associated with central obesity and insulin resistance. While the effects of dietary fat and carbohydrates on liver fat accumulation have been extensively studied, the effects of dietary protein [8, 9] and amino acids [10] on energy homeostasis are less well understood despite their importance in the pathogenesis of NAFLD. For example, insulin is a major anabolic hormone that is intimately involved in hepatic metabolism, and its secretion is affected by dietary protein [11] and amino acid intake [12]. Protein malnutrition suppresses insulin secretion from the pancreas in response to glucose intake [9, 13]. Some amino acids, such as arginine and leucine, are also known to stimulate insulin secretion [12, 14]. Moreover, our group previously demonstrated that dietary protein malnutrition decreased plasma insulin and insulin-like growth factor 1 concentrations [9, 15], and upregulated the protein expression of insulin receptor substrate 2 (IRS2), one of the key mediators of insulin signaling, in the livers of growing rats, resulting in increased insulin sensitivity [16]. These results implied that enhanced insulin sensitivity caused by dietary protein deficiency may mediate fatty liver development.
We previously investigated the impacts of various amino acids on fat accumulation in the liver using amino acid-deficient diets, and the results showed that the liver triglyceride (TG) content could be increased only by a deficiency of arginine out of the 20 major amino acids tested [10]. Therefore, we hypothesized that lipid accumulation in the liver induced by dietary amino acid deficiency could be attributed to increased insulin sensitivity, which may be caused specifically by dietary arginine deficiency. Here, we tested this hypothesis by evaluating metabolic status and hepatic lipid flux in models of total amino acid and arginine deficiency.
Methods and materials
Materials
Anti-insulin receptor subunit β (IRβ) and anti-CD36 antibodies for immunoblotting were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The anti-IRS2 (clone 9.5.2) antibody for immunoblotting was purchased from Merck Millipore (Billerica, MA, USA). Anti-phospho-p70 S6K (Thr389), anti-p70 S6K (49D7), anti-phospho-4E-BP1 (Thr37/46), anti-4E-BP1, and anti-phospho-AMPKα (Thr172) (40H9) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). The anti-GAPDH (6C5) antibody was purchased from Abcam (Cambridge, UK). Anti-rabbit and mouse IgG horseradish peroxidase-conjugated secondary antibodies were purchased from GE Healthcare (Little Chalfont, UK).
Animals
The experiments were approved by the Animal Usage Committee of the Faculty of Agriculture of the University of Tokyo and performed in accordance with its guidelines (Permission No. P09–375). Male Wistar rats were purchased from Charles River Laboratories International (Kanagawa, Japan). The animals were housed individually in wire cages with free access to food and water. The rats were maintained at a room temperature of 23 °C ± 1 °C with 50–60% relative humidity under a 12-h light/dark cycle (light from 08:00 to 20:00). In the pre-experimental period, the rats were fed a purified diet containing 15% protein from casein.
Comparison of the effects of the low-total-amino acid and low-arginine diets
Six-week-old male Wistar rats were randomly divided into 3 groups with different diets: the amino acid mixture control diet (equivalent to 15% protein in the diet; 15PAA), low-arginine diet in which the concentration of arginine in young rodents was 33% of that in the 15PAA diet (low Arg, n = 8), and the low-total-amino acid diet (equivalent to 5% protein in the diet; 5PAA). The low-total-amino acid mixture simulated the composition of casein [17]. The diet compositions are shown in Tables 1 and 2. Rats were given ad libitum access to tap water and food. Body weight and food intake were measured daily. Fourteen days after initiation of the experimental diets, the rats were anesthetized by intraperitoneal injection of pentobarbital (30 mg/kg) 1 h after removal of the diet, and a postprandial blood sample was collected from the carotid artery. The liver, longissimus muscles, and abdominal fat were removed and weighed. The livers and muscle tissues were soaked in RNAlater (AMBION, Austin, TX, USA) or snap-frozen in liquid nitrogen and stored at − 80 °C until use. The organ samples were used to analyze tissue TG concentrations, and protein and RNA expression.
Table 1.
Composition of diet
| Component | 15P AA | 5PAA | Low Arg |
|---|---|---|---|
| g/kg diet | |||
| Amino acid mixturea | 150.0 | 50.0 | 146.5 |
| DL-methionine | 2.5 | 0.8 | 2.5 |
| Corn starch | 620.5 | 747.2 | 649.0 |
| Soy bean oil | 50.0 | 50.0 | 50.0 |
| Vitamin mixtureb | 10.0 | 10.0 | 10.0 |
| Mineral mixtureb | 40.0 | 40.0 | 40.0 |
| Choline chloride | 2.0 | 2.0 | 2.0 |
| Cellulose powder | 100.0 | 100.0 | 100.0 |
aComposition of amino acid mixture were described in Table 2
bAIN-76 prescription (Oriental Yeast Co., ltd)
Table 2.
The amino acid content of each diet
| 15PAA | 5PAA | Low Arg | |
|---|---|---|---|
| g/kg diet | |||
| Alanine | 4.08 | 1.36 | 4.08 |
| Arginine | 5.25 | 1.75 | 1.75 |
| Asparagine-H20 | 5.76 | 1.92 | 5.76 |
| Aspartate | 5.05 | 1.68 | 5.05 |
| Cystine | 0.8 | 0.27 | 0.80 |
| Glutamate | 14.65 | 4.88 | 14.65 |
| Glutamine | 14.65 | 4.88 | 14.65 |
| Glycine | 2.59 | 0.86 | 2.59 |
| Histidine | 4.06 | 1.35 | 4.06 |
| Isoleucine | 7.12 | 2.37 | 7.12 |
| Leucine | 13 | 4.33 | 13.00 |
| Lysine-HCl | 14.1 | 4.70 | 14.10 |
| Methionine | 3.89 | 1.30 | 3.89 |
| Phenylalanine | 7.2 | 2.40 | 7.20 |
| Proline | 14.98 | 4.99 | 14.98 |
| Serine | 8.09 | 2.70 | 8.09 |
| Threonine | 6.09 | 2.03 | 6.09 |
| Tryptophan | 7.76 | 0.58 | 1.73 |
| Tyrosine | 1.73 | 2.59 | 7.76 |
| Valine | 9.16 | 3.05 | 9.16 |
| Total volume | 150.00 | 50.00 | 146.50 |
We performed oral glucose tolerance tests (OGTTs) 12 d after initiation of the experimental diets. After 16 h of overnight fasting, glucose (2 g/kg) was orally administered to the rats. Blood (preprandial) was collected from the tail vein into heparinized tubes that were chilled on ice. The blood samples were subjected to centrifugation at 3000×g for 5 min at 4 °C and the supernatants were transferred to new tubes. Plasma samples were stored at − 80 °C until analysis.
Blood biochemistry
Blood biochemical parameters, including total cholesterol, TG, free fatty acids (FFA), and glucose concentrations, were determined using commercial kits (Cholesterol E-test, Triglyceride E-test, NEFA C-test, and Glucose CII test, respectively; Wako Pure Chemical Industries, Osaka, Japan). The plasma insulin concentration was measured using an insulin measurement kit (Morinaga Institute of Biological Science, Yokohama, Japan) according to the manufacturer’s instructions.
Lipid extraction and TG measurements
Lipids were extracted from frozen livers and longissimus muscles via modified Folch method [18] in a 2:1 (vol/vol) mixture of chloroform/methanol. The extracts were washed with 0.5 volumes of 0.8% KCl and centrifuged at 1500×g for 10 min, and the organic phases were recovered. The TG content in the liver and plasma was also determined using a commercial kit (Wako Pure Chemical Industries) according to the manufacturer’s instructions.
RNA extraction and reverse transcription-polymerase chain reaction
Total RNA was isolated from homogenized livers using NucleoSpin® RNA (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions. The total RNA concentration was measured with a NanoDrop® spectrophotometer (ND-1000, NanoDrop, Wilmington, DE, USA). The quality of the RNA was determined by assessing the A260/280 ratio and by agarose gel electrophoresis. The RNA was reverse-transcribed into cDNA using PrimeScript® RT Master Mix (Takara Bio, Shiga, Japan). cDNA was amplified using SYBR® Premix Ex Taq II (Takara Bio) according to the manufacturer’s protocol. We designed the primers for reverse transcription (RT) polymerase chain reaction (PCR) with the design software Primer 3. β-actin was used as an endogenous control. The following PCR primers were used: β-actin (Actb) forward, 5′-GGAGATTACTGCCCTGGCTCCTA-3′, and reverse, 5′-GACTCATCGTACTCCTGCTTGCTG-3′; MTP (Mttp) forward, 5′-AGCAACATGCCTACTTCTTACAC-3′, and reverse, 5′-TCACGGGTTCACTTTCACTG-3′; apolipoprotein A-IV (ApoA4 or Apoa4) forward, 5′-ACCCTCTTCCAGGACAAACTTG-3′, and reverse, 5′-CCTTGGTTAGATGTCCACTCAGTTG-3′; and apolipoprotein B (ApoB or Apob) forward, 5′-CCTGTCCATTCAAAACTACCACA-3′, and reverse 5′-CAATGAACGAATCAGAAGGTGA-3′.
Western blotting
Western blotting analysis was performed as previously described [9, 16]. In brief, frozen livers were homogenized in homogenizing buffer and centrifuged at 100,000×g for 1 h at 4 °C. The protein content in the supernatant was determined using a Bio-Rad Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Protein extracts were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto polyvinylidene fluoride membranes. The membranes were blocked with blocking buffer, and then incubated at 4 °C overnight with primary antibodies against IRβ, CD36, and FFA synthase (1:200 dilution), and against PI3 kinase p85, phospho-p70 S6K (Thr389), p70 S6K, phospho-4E-BP1 (Thr37/46), 4E-BP1, phospho-AMPKα (Thr172), AMPKα, phospho-acetyl-CoA carboxylase (Ser79), acetyl CoA carboxylase, and acetyl CoA carboxylase 1 (1:1000 dilution). Primary mouse anti-IRS2 and anti-GAPDH antibodies were used at dilutions of 1:1000 and 1:3000, respectively. We visualized the blots by chemiluminescence after incubating with donkey anti-rabbit IgG or sheep anti-mouse IgG conjugated to horseradish peroxidase (1:2500). The immunoreactive bands were exposed and the signals were quantified using a cooled charge-coupled device camera system (LAS-3000 Mini; Fujifilm, Kanagawa, Japan).
Very-low-density lipoprotein excretion test
Male Wistar rats were fed a casein control diet between 10:00 and 18:00 for 7 d prior to the experiment. After habituation, the rats (7.5 weeks of age, 208–229 g) were assigned to the 15PAA (n = 8), 5PAA (n = 8), and low Arg (n = 9) groups. The diet compositions are shown in Tables 1 and 2. The experimental diets were provided for 5 h from 9:00 to 14:00. One hour after removing the diets, we administered tyloxapol (200 mg/kg, dissolved in 0.9% NaCl; Triton WR-1339, Sigma-Aldrich, St. Louis, MO, USA) to all rats under isoflurane anesthesia (3–4%, 5 l/min; Dainippon Sumitomo Pharma, Osaka, Japan) via the tail vein. Blood was collected from the tail vein into chilled, heparinized tubes prior to tyloxapol injection and 30, 60, 120, and 240 min after injection to measure plasma TG concentrations. Tyloxapol inhibits endogenous lipoprotein lipase and blocks the clearance of lipid-carrying lipoproteins in the blood [19–21]. Thus, the rise in plasma TG concentration after tyloxapol injection is approximately proportional to the very-low-density lipoprotein (VLDL)-TG level. The TG concentrations in the plasma were measured with a commercial kit as described in “Lipid extraction and TG measurements.” The TG secretion rate was expressed in mg TG/dl/min.
Respiratory exchange ratio
Six-week-old male Wistar rats were assigned to the 15PAA (n = 6), 5PAA (n = 6), and low Arg (n = 6) groups. Six days after initiation of the experimental diets, animals were individually placed in a metabolic chamber for 24 h, and VO2 and VCO2 were monitored every 10 min with an OXYMAX system (Columbus Instruments, Columbus, OH, USA). Rats were allowed free access to water and food during the experiment.
Theoretically, a respiratory exchange rate (RER) of 1.0 represents the dominant consumption of carbohydrates, and a decrease in the RER to approximately 0.7 represents proportionally higher lipid consumption.
De novo lipogenesis assay
De novo lipogenesis was measured based on the method described previously with minor modifications [22]. Six-week-old male Wistar rats were assigned to the 15PAA (n = 8), 5PAA (n = 8), and low Arg (n = 8) groups (240–280 g) for 1 night (from 18:00 to 10:00). Rats were allowed free access to food. The following day, the rats of each group were further divided into D2O (Sigma Aldrich, St. Louis, MO, USA) or H2O injection groups (5 ml/kg body weight, intraperitoneally). Liver samples were collected under isoflurane anesthesia (3–4%, 5 l/min; Dainippon Sumitomo Pharma, Osaka, Japan) 24 h after injection and stored at − 80 °C until analysis. Lipids were extracted as described in “Lipid extraction and TG measurements” [18]. The extracted lipids were hydrolyzed and methylated with an FFA Methylation Kit (Nacalai Tesque, Kyoto, Japan), and the obtained products were purified with a Fatty Acid Methyl Ester Purification Kit (Nacalai Tesque). Purified FFA esters were analyzed by gas chromatography–mass spectrometry (GCMS-QP2010 Plus, SHIMADZU, Kyoto, Japan) to quantify palmitate isotopomers. The relative amounts of fatty acids were normalized by tissue weight and the difference in the total amount of methyl palmitate isotopomers in the D2O-injected group and H2O-injected group was interpreted as the de novo lipogenesis rate.
Hepatic FFA uptake assay
We performed an in vivo FFA uptake assay according to the method published in previous reports [23, 24]. Six-week-old male Wistar rats were assigned to the 15PAA (n = 8), 5PAA (n = 8), and low Arg (n = 8) groups (240–280 g) for 1 night (from 18:00 to 10:00). The following day, the rats of each group were further divided into 2 groups; one was treated with fluorescent FFA analog (0.1 mg/kg, BODIPY® FL C12, Invitrogen, Carlsbad, CA, USA) and the other was treated with only vehicle (0.1% bovine serum albumin in phosphate-buffered saline) by intravenous injection under isoflurane anesthesia (3–4%, 5 l/min; Dainippon Sumitomo Pharma, Osaka, Japan). The liver samples were collected 10 min after injection and stored at − 80 °C until analysis. Lipids were extracted [18] and reconstituted in isopropanol. Then, lipid fluorescence was measured with an ARVO X3 microplate reader (PerkinElmer, Waltham, MA, USA). All values were normalized by tissue weight, and the value of the vehicle-administered group was subtracted as background fluorescence from that of the BODIPY-administered group.
Statistical analysis
All data are presented as the mean ± standard error. The data were statistically analyzed with the Ekuseru-Toukei 2010 software package (Social Survey Research Information, Tokyo, Japan). Statistical significance was calculated using one-way analysis of variance (ANOVA) with the Bonferroni and Tukey–Kramer post-hoc tests to assess differences between groups. P < 0.05 was considered statistically significant.
Results
Diet-induced changes in the body composition of rats
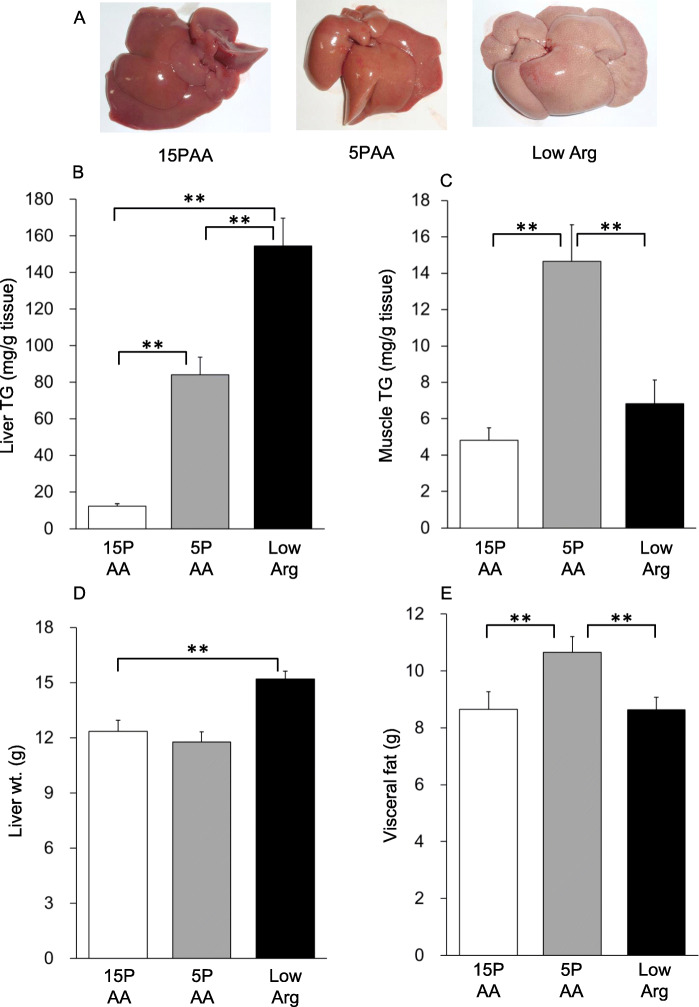
Body weight gain was slightly lower in the 5PAA group than in the 15PAA group, whereas the weight gain of the low Arg group was comparable to that of the 15PAA group (Table 3). Liver TG levels were significantly higher in the 5PAA group than in the 15PAA group (Fig. 1b, p < 0.01) as previously reported [9, 16]; however, even higher TG levels were observed in the low Arg group than in the 5PAA group (Fig. 1b, p < 0.01 vs. 15PAA and 5PAA). Macroscopic observations also supported this result; the livers of the 5PAA and low Arg groups were lighter in color than those of the 15PAA group (Fig. 1a). The TG content in the longissimus muscle and the visceral fat volume were significantly higher in the 5PAA group compared to the 15PAA group, whereas these parameters were not affected by the low Arg diet (Fig. 1c, e). Liver weight was significantly higher in the low Arg group than the 15PAA group, but this was not the case for the 5PAA group (Fig. 1d). These data suggested that, in contrast to our expectation, a low-total-amino acid diet and low-arginine diet might lead to different phenotypes, given that the low Arg diet did not completely recapitulate the effects of 5PAA administration.
Table 3.
Blood biochemical parameters in the postprandial condition after feeding the low-protein and low-arginine diets for 14 days
| 15PAA | 5PAA | Low Arg | |
|---|---|---|---|
| n | 8 | 8 | 9 |
| Body weight (g) | 286 +/− 5 a | 267 +/− 5 b | 283 +/− 5 a b |
| Total cholesterol (mg/dl) | 69.9 +/− 2.8a | 65.0 +/− 2.0a | 50.8 +/− 2.3b |
| HDL cholesterol (mg/dl) | 14.8 +/− 0.6 | 11.6 +/− 0.7 | 13.7 +/− 0.5 |
| Glucose (mg/dl) | 202 +/− 8 | 196 +/− 9 | 209 +/− 9 |
| Insulin (ng/ml) | 3.2 +/− 0.2ab | 1.9 +/− 0.4a | 4.6 +/− 0.6b |
Data are shown as the mean +/− S.E.M.
Different superscript letters denote significant differences (p < 0.05)
Fig. 1.
Effects of the 5PAA and low Arg diets on the liver. Macroscopic liver characteristics (a), hepatic (b) and longissimus muscle (c) TG contents, liver weight (d), and visceral fat weight (e) in 6-week-old male Wistar rats fed the 15PAA (n = 8), low Arg (n = 8), and 5PAA (n = 8) diets for 14 d. The values are means ± standard errors. Asterisks indicate significant differences as assessed by one-way ANOVA with Bonferroni and Tukey–Kramer post-hoc tests (*p < 0.05; **p < 0.01)
Diet-induced changes in blood biochemical parameters
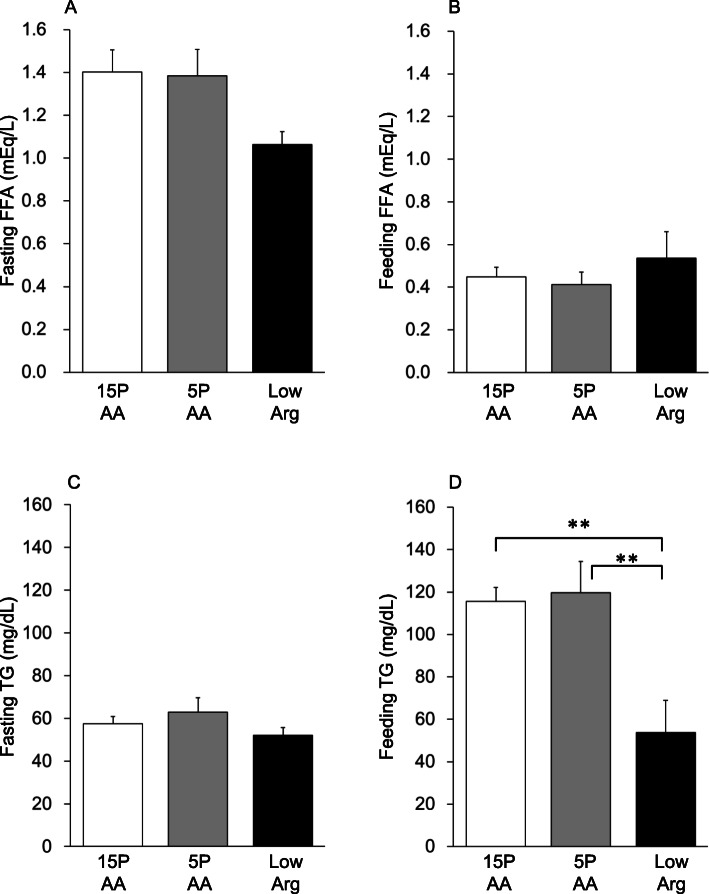
The blood glucose and insulin levels were over 190 mg/dl and 1.9 ng/ml, respectively, in the postprandial blood (Table 3, Supplementary Table 1). No differences were observed in the FFA concentrations of the fasting and postprandial blood among the 3 groups (Fig. 2a and b). By contrast, postprandial plasma TG and total cholesterol levels were significantly lower in the low Arg group than in the 15PAA group (Fig. 2d, Table 3), whereas the 5PAA diet did not affect plasma TG levels of the preprandial and postprandial blood (Fig. 2c and d). The high-density lipoprotein cholesterol concentrations were similar among the 3 groups in the preprandial and postprandial blood (Table 3).
Fig. 2.
Plasma free fatty acid and TG levels in preprandial or postprandial conditions after feeding the 5PAA and low Arg diets. Six-week-old male Wistar rats were fed the 15PAA (n = 8), low Arg (n = 8), and 5PAA (n = 8) diet for 14 d. Preprandial free fatty acid (a) and TG (c) levels and postprandial free fatty acid (b) and TG (d) levels are shown. The values are the means ± standard errors. Asterisks indicate significant differences as assessed by one-way ANOVA with Bonferroni and Tukey–Kramer post hoc tests (**p < 0.01)
Glucose tolerance
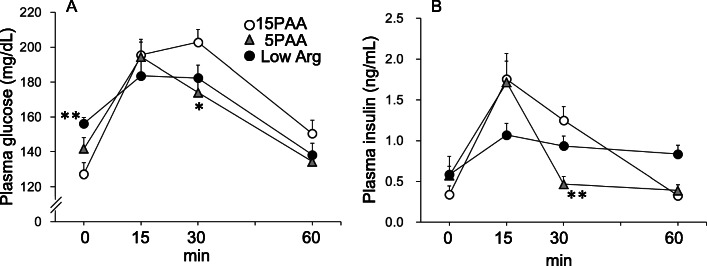
When the OGTT was performed at 12 d after initiation of the experimental diets, the plasma glucose levels of the 5PAA group were lower than those of the 15PAA group at 30 and 60 min after glucose loading (Fig. 3a). In addition, the plasma insulin levels were significantly lower in the 5PAA group than in the 15PAA group at 30 min after glucose loading (Fig. 3b). The fasting plasma glucose levels in the low Arg group were higher than in the 15PAA group (Fig. 3a), whereas the fasting plasma insulin levels were not significantly different between the low Arg and 15PAA groups (Fig. 3b). The plasma glucose level after glucose loading was not affected by the low Arg diet. The plasma insulin levels increased less in the low Arg group after glucose loading than in the 5PAA and 15PAA groups. The plasma insulin levels fell at 60 min after glucose loading in the 15PAA group but did not decrease in the low Arg group.
Fig. 3.
Effects of the 5PAA and low Arg diets on blood glucose and insulin levels after glucose loading. Six-week-old male Wistar rats were fed the 15PAA (n = 8), low Arg (n = 8), and 5PAA (n = 8) diets, and then OGTTs were performed on d 12 after initiation of the experimental diets. Plasma glucose (a) and insulin (b) concentrations are shown. The values are the means ± standard errors. Asterisks indicate significant differences as assessed by one-way ANOVA with Bonferroni and Tukey–Kramer post-hoc tests (*p < 0.05; **p < 0.01)
Hepatic insulin receptor signaling
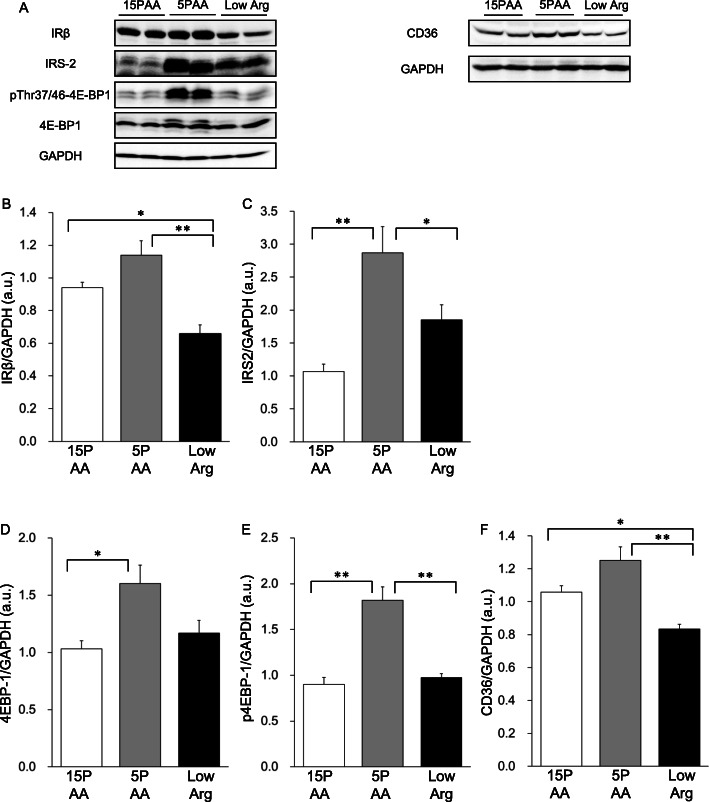
Western blotting analysis revealed that the expression of IRβ was slightly, but not significantly, higher in the livers of 5PAA-fed rats than those of the 15PAA-fed rats (Fig. 4a and b). Rats fed the 5PAA diet exhibited markedly higher expression levels of IRS2 (Fig. 4a and c) in the liver than rats fed the 15PAA or low Arg diet. Expression of the downstream effector 4E-BP1 (Fig. 4a and d) and its threonine phosphorylation were significantly higher in the 5PAA group than in the 15PAA or low Arg group (Fig. 4a and e). In contrast, low Arg-fed rats showed a lower total IRβ level than the 15PAA- and 5PAA-fed rats, and IRS2, 4E-BP1, and phosphorylated 4E-BP1 levels were comparable to those of 15PAA-fed rats. These results further indicated that the 5PAA and low Arg diets had disparate effects on liver insulin signaling.
Fig. 4.
Insulin signaling-related protein levels in rats fed diets low in protein or arginine. Six-week-old male Wistar rats were fed the 15PAA (n = 8), low Arg (n = 8), and 5PAA (n = 8) diets for 14 d. Whole-liver lysates were analyzed by immunoprecipitation and immunoblotting with antibodies against IRβ (b), IRS-2 (c), 4E-BP1 (d), phosphorylated 4E-BP1 (Thr37/46) (E), CD36 (f), and GAPDH as an internal control. Representative immunoblots (a) are shown. The values are the means ± standard errors. Asterisks indicate significant differences as assessed by one-way ANOVA with Bonferroni and Tukey–Kramer post-hoc tests (*p < 0.05; **p < 0.01)
TG secretion, de novo lipogenesis, FFA uptake, and energy consumption
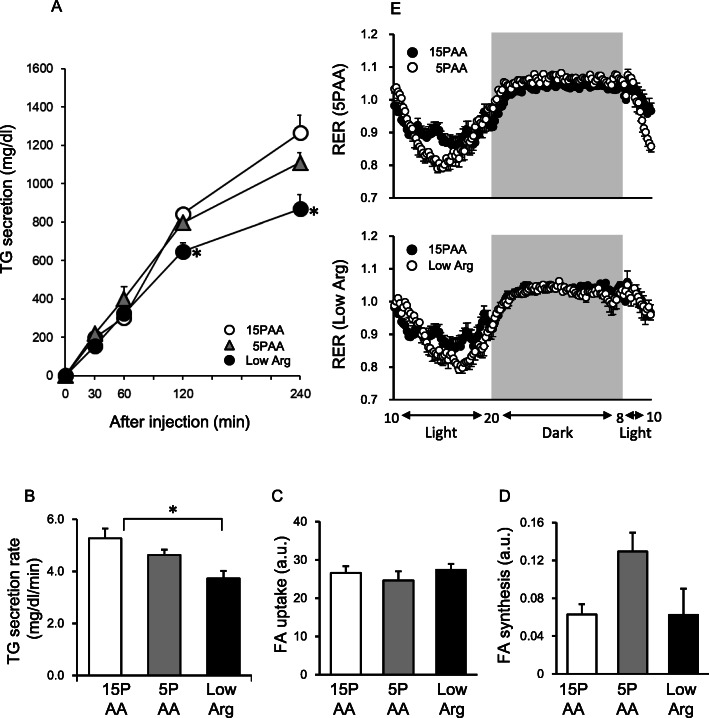
We next assessed hepatic lipid flux in rats fed the 5PAA and low Arg diets. For the hepatic TG secretion assay, the rats were fed the indicated experimental diets for 5 h after a 15-h fast, and then tyloxapol was injected into the tail vein. The rats in the 3 groups consumed approximately equal amounts of the diets during the 5 h of feeding (15PAA: 13 ± 1 g; 5PAA: 14 ± 1 g; and low Arg: 14 ± 0 g). When plasma TG concentrations were measured at 0, 30, 60, 120, and 240 min after tyloxapol injection, every group showed time-dependent increases, but the rate of increase of the low Arg group was significantly lower than that of the 15PAA group (Fig. 5a). Remarkably, the hepatic TG secretion rate of the 5PAA group did not significantly change compared to that of the 15PAA group (Fig. 5b). Analysis of hepatic FFA uptake activity using a fluorescent FFA analog showed comparable levels among the 3 groups, although the expression of CD36 in the liver, which mediates cellular FFA uptake, was significantly lower in the low Arg group than in the other groups (Fig. 4a and f, c). In addition, the de novo lipogenesis assay demonstrated that the lipid synthetic activity in the liver was enhanced only in the 5PAA group, whereas it was unchanged in the low Arg group (Fig. 5d).
Fig. 5.
VLDL secretion from the livers, lipogenesis, fatty acid uptake, and RERs of rats fed the 5PAA and low Arg diets. Rats were fed the 15PAA (n = 8), 5PAA (n = 8), and low Arg (n = 8) diets. After 5 h of feeding, rats were injected with tyloxapol (200 mg/kg), and then TG concentrations (a and c) were measured at 0, 30, 60, 120, and 240 min post-injection. The RER (b) of each rat (n = 6) was measured for 24 h. A de novo lipogenesis assay (d) was performed by measuring palmitate isotopomers after intraperitoneal injection of D2O or H2O into rats fed the 15PAA (n = 8), 5PAA (n = 8), and low Arg (n = 8) diets for 1 night (18:00–10:00). Fatty acid uptake (e) was evaluated by incorporation of a fatty acid analog, BODIPY® FL C12, into the livers of rats fed the 15PAA (n = 4), 5PAA (n = 4), and low Arg (n = 4) diets for 1 night (18:00–10:00), and fluorescence was measured in the liver lipid extractions. The values are the means ± standard errors. Asterisks indicate significant differences assessed by one-way ANOVA with Bonferroni and Tukey–Kramer post-hoc tests (*p < 0.05)
We also measured the RER of the 3 groups (Fig. 5e, Supplementary Figure 2). RER is a stoichiometric ratio of exhaled CO2 to inhaled O2, which roughly reflects the oxidized energy source. The RERs of the 3 groups measured during the night were almost comparable at around 1.0, whereas the RERs measured during the day were slightly lower in the 5PAA and low Arg groups than in the 15PAA group, indicating that the 5PAA diet and low Arg diet did not slow lipid consumption in comparison with the 15PAA diet.
Hepatic TG secretion-related gene expression in the liver
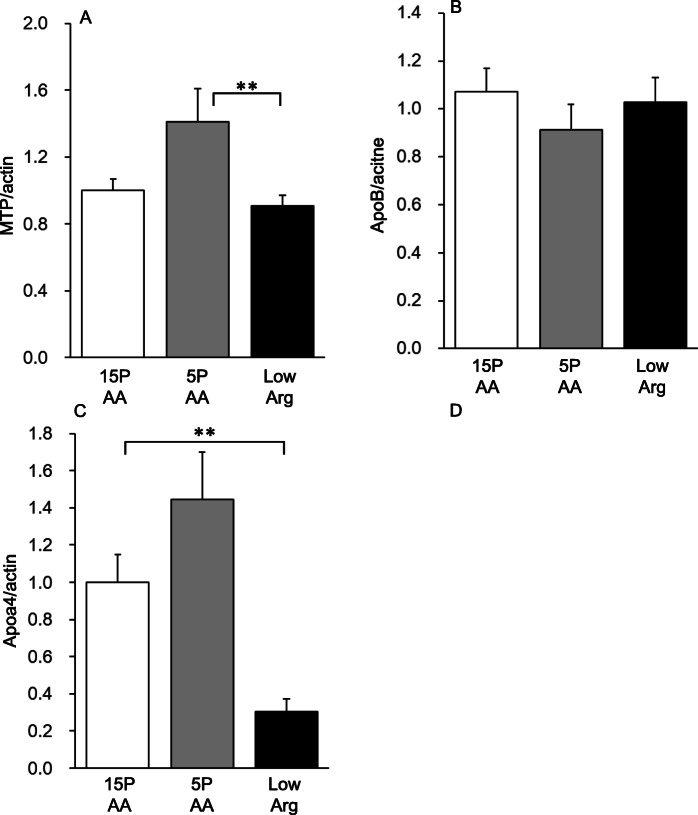
We performed microarray analysis of liver samples from the rats of the 3 groups to evaluate the effects of the diets on hepatic transcripts (Supplementary Table 2). The results showed a marked decrease (0.47-fold) in the expression of the ApoA4 gene in the livers of the low Arg group compared with that of the 15PAA livers, which was validated by quantitative PCR analysis. ApoA4, an apolipoprotein primarily expressed in the intestine in mammals, is believed to be involved in chylomicron formation [25]. However, in rodents, it is reportedly also expressed in the liver where it is associated with VLDL formation and TG secretion [20].
There were no significant differences in ApoB and Mttp mRNA levels, which are also important genes for hepatic VLDL formation, between the 15PAA and low Arg groups (Fig. 6a, b), whereas there was a tendency of Mttp mRNA levels to be slightly increased in the livers of the rats in the 5PAA group (Fig. 6A, p < 0.08 vs. 15PAA). However, the low Arg diet caused a dramatic decrease in ApoA4 mRNA levels compared with the 15PAA diet, whereas the 5PAA diet had no effect (Fig. 6c).
Fig. 6.
Effects of the 5PAA and low Arg diets on the expression of VLDL assembly-related genes. Six-week-old male Wistar rats were fed the 15PAA (n = 8), low Arg (n = 8), and 5PAA (n = 8) diets for 14 d. Total RNA was isolated from the liver and then subjected to quantitative RT-PCR. The transcript expression levels for Mttp (a), ApoB (b), and ApoA4 (c) are shown. Asterisks indicate significant differences as assessed by one-way ANOVA with Bonferroni and Tukey–Kramer post-hoc tests (**p < 0.01)
Discussion
We previously reported that both dietary total amino acid deficiency and selective arginine deficiency caused fatty liver, and activation of insulin receptor signaling is known to stimulate lipid accumulation [9, 16]. Therefore, we speculated that the phenotypes caused by a low Arg diet simply reflected those caused by the 5PAA diet, which could be mediated through enhanced insulin sensitivity. However, in the present study, we identified several distinct phenotypes in the 5PAA and low Arg groups, including the lipid accumulation level, serum lipid profile, glucose tolerance, and insulin signal activities. In addition, because liver TG content is thought to be determined by the integration of de novo lipogenesis, TG secretion, FFA uptake, and lipid oxidation [26], we evaluated these activities in rats fed the 5PAA or low Arg diet. Contrary to our expectations, these parameters also completely differed between the groups; only the 5PAA group exhibited increased lipogenesis, only the low Arg group showed attenuated TG secretion, and FFA uptake and RER scarcely differed among the groups. The RER was slightly lower, particularly during the day, in the 5PAA and low Arg groups than in the 15PAA group, which indicated that the lipid oxidation rates of the experimental groups were almost the same or slightly higher than that of the 15PAA group. However, as the RER indicates the opposite of lipid accumulation, it cannot be responsible for fatty liver development. Collectively, these results indicate that distinct mechanisms underlie the fatty liver formation induced by total amino acid deficiency and only arginine deficiency.
IRβ and IRS2 are pivotal mediators of insulin activity in the liver. We found that the 5PAA diet significantly increased the protein expression levels of total IRS2 and 4EBP1 and 4EBP1 phosphorylation (p < 0.05 in comparison with 15PAA), and tended to increase total IRβ expression in the liver. This suggests that the 5PAA diet may potentiate insulin signaling. The activation of this pathway is known to upregulate FFA synthesis [9, 27–29], and, indeed, increased de novo lipogenesis was observed in 5PAA diet-fed rats. In contrast, we recently reported that when hepatocytes were cultured in an amino acid-sufficient or -deficient medium, the intracellular TG level was increased by amino acid deficiency without the addition of any lipids or hormones, and was accompanied by enhanced lipid synthesis [10]. These results indicate that hepatocytes themselves monitor extracellular amino acid concentrations to induce lipid accumulation in a cell-autonomous manner. It is possible that both the lipogenesis enhanced by insulin signal potentiation and that stimulated directly by amino acid deficiency may lead to lipid accumulation in the liver in response to 5PAA feeding. On the other hand, the low Arg diet decreased IRβ and did not have any statistically significant effects on IRS2 and 4E-BP1 protein levels. These findings support the conclusion that the 5PAA and low Arg diets induced hepatic fat accumulation via different mechanisms. 5PAA diet-induced TG accumulation appears to be mediated through insulin signaling, whereas low Arg diet-induced accumulation was insulin-independent.
We also demonstrated that only the 5PAA diet induced visceral and muscular fat accumulation, in addition to accumulation in the liver. Some reports have shown that a low-protein diet leads to intramuscular and other fat deposition in piglets [30–32], and a high-protein diet reduces visceral fat and hepatic TG levels in humans [2, 33]. In addition, we previously found that the activation of insulin signaling in response to consumption of the 5PAA diet was specific to the liver and was not observed in the white adipose tissue or muscle [9]. Taken together, these results suggest that the dietary protein content and amino acid balance can provoke a tissue-specific reaction, and are, thus, important determinants of body fat deposition in mammals.
The assembly and secretion of VLDL are the key regulatory steps of plasma and intrahepatic TG and FFA levels owing to its glyceride-rich structure, and are closely linked to the progression of NAFLD. The 5PAA diet had no effect on postprandial plasma TG and fasting FFA levels, whereas the low Arg group had lower postprandial plasma TG and liver CD36 levels than the control group, although their fasting FFA levels were comparable. By contrast, neither the 5PAA nor low Arg diet influenced FFA uptake by the liver. The scavenger FFA receptor CD36 is known to mediate FFA uptake into the metabolic tissues, and is reportedly associated with non-alcoholic fatty liver development [23, 34]. However, in our model, changes in the hepatic CD36 level and blood TG/FFA levels did not affect FFA uptake into the liver, suggesting that the change in serum lipid concentrations was unlikely to be a direct reason for fatty liver formation.
In this study, we analyzed the VLDL secretion rate after tyloxapol injection. Tyloxapol is a non-ionic detergent that prevents the catabolism of triacylglycerol-rich lipoproteins by lipoprotein lipase [19, 35]; it is widely used for the in vivo determination of the secretion and clearance rates of VLDL [21]. We found that VLDL secretion in the 5PAA group was not significantly different to that in the 15PAA group, whereas that of the low Arg group was significantly lower. These findings suggested that the hepatic lipid accumulation caused by the low Arg diet, but not the 5PAA diet, was attributable to the downregulation of VLDL secretion. As the inhibition of VLDL secretion was observed within just 5 h of the initiation of the low Arg diet, it may be the major cause of fatty liver formation induced by the low Arg diet.
Although Mttp and ApoB are the most important elements in VLDL assembly [36], neither the 5PAA nor the low Arg diet statistically affected the expression of these genes. Instead, we discovered a decrease in the expression level of ApoA4. ApoA4, a 46-kDa protein with a lipid-binding site, is expressed in the intestine and liver of rodents [25]. It is generally believed to be expressed primarily in the small intestine in humans, although minor expression in the human liver and human hepatocyte lines has also been reported [37, 38]. Adenoviral overexpression of ApoA4 in the liver increases VLDL secretion and decreases hepatic TG levels, and ApoA4-knockout mice show lower VLDL secretion than wild-type controls [20]. Thus, ApoA4 is another important apolipoprotein for VLDL formation. Based on these findings, we believe that the downregulation of ApoA4 expression in response to the low Arg diet may attenuate VLDL secretion.
Arginine and lysine use the same membrane transporter system. Lysine is an important amino acid for growth and nutritional metabolism. It tends to be the first limiting amino acid in a variety of circumstances. Low dietary lysine in piglets drives intramuscular fat accumulation and a reduction in serum glucose levels in growing pigs [39]. In our previous study, however, feeding rats a low Arg diet did not affect serum arginine and lysine concentrations, which was presumably due to the compensatory synthesis of arginine. On the other hand, the serum levels of other amino acids, such as methionine and histidine, were significantly increased by a low Arg diet [10]. Therefore we do not consider arginine alone a signal molecule. Instead, we believe that the complex alteration of the comprehensive serum amino acid profile is important. In support of that hypothesis, in the same previous report, a nonlinear analysis based on a mathematical machine learning method revealed that the comprehensive amino acid profile correlated with TG levels in the liver although no single amino acid concentration correlated well. The molecular basis of how such information is sensed by cells remains unclear. We are currently investigating the molecular sensing mechanism and preparing a report on that topic.
We demonstrated that a mild deficiency in dietary total amino acids induces fat accumulation in visceral and ectopic, but not subcutaneous, fat. Increases in ectopic fat accumulation and intra-abdominal fat are thought to occur in parallel [40, 41]. Feeding a low-protein diet to piglets leads to the deposition of intramuscular and other fat [30, 31]. In healthy humans, a high-protein diet reduces visceral fat [2], and a high-protein/low-carbohydrate diet also lowers hepatic TG levels [33]. Therefore, dietary protein content is an important determinant of body fat deposition in mammals.
Conclusions
Our present results demonstrated that a mild deficiency in dietary total amino acids or solely in dietary arginine caused a large increase in TG accumulation in the liver via different mechanisms, although both diets lacked arginine to the same extent. The low Arg diet caused hepatic lipid accumulation by attenuating VLDL secretion, which may be caused, at least in part, by the downregulation of ApoA4 expression. Although the concrete molecular mechanisms remain elusive, understanding how these differences come about may explain the tissue-specific responses to dietary protein malnutrition with regard to insulin-like activity and lipid metabolism.
Supplementary information
Additional file 1: Figure S1 Insulin signaling-related protein levels in rats fed the 5PAA and low Arg diets. Six-week-old male Wistar rats were fed the 15PAA (n = 8), low Arg (n = 8), or 5PAA (n = 8) diet for 14 d. Whole-liver lysates were analyzed by immunoprecipitation and immunoblotting with antibodies against p85 (B), S6K (C), pS6K (D), AMPK (F), and pAMPK (G), where anti-S6K served as the internal control; or with antibodies against pS6K/S6K (E) and pAMPK (H), where anti-GAPDH served as the internal control. Figure S2 Average RER values during the light period (8:00–20:00) and dark period (20:00–8:00) were calculated individually.
Additional file 2: Table S1 Plasma glucose and insulin levels in rats fed the 5PAA and low Arg diets. Table S2 List of apolipoprotein genes.
Acknowledgements
We thank Dr. Susan Hall (University of North Carolina) for critically reading the manuscript.
Abbreviations
- 15PAA
Diet containing amino acids from casein equivalent to 15% protein
- 4E-BP1
Eukaryotic initiation factor 4E-binding protein
- 5PAA
Diet containing amino acids from casein equivalent to 5% protein
- ANOVA
Analysis of variance
- ApoA4
Apolipoprotein A-IV
- ApoB
Apolipoprotein B
- FFA
Free fatty acid
- IRβ
Insulin receptor subunit β
- IRS2
Insulin receptor substrate 2
- MTP
Microsomal triglyceride transfer protein
- NAFLD
Non-alcoholic fatty liver disease
- OGTT
Oral glucose tolerance test
- PCR
Polymerase chain reaction
- RER
Respiration exchange rate
- RT
Reverse transcription
- S6K
S6 kinase
- TG
Triglyceride
- VLDL
Very-low-density lipoprotein
Authors’ contributions
L.O., H.N., S.-I.T., and H.K. were responsible for the study concept and design. L.O., H.N., A.K., and Y.A. contributed to the acquisition of animal data. Y.Ta. and Y.To. performed western blotting and analyzed the data. L.O., H.N., S.-I.T., and H.K. assisted with data analysis and the interpretation of the findings. L.O. and H.N. drafted the manuscript with input from all authors. Y.Ta., Y.To., D.Y., F.H., and H.J. contributed to the interpretation of the results. H.K. provided critical revision of the manuscript with important intellectual input. All authors critically reviewed the content and approved the final version for publication.
Funding
This work was supported by the Program for the Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (awarded to S.-I.T. and H.K.). In addition, we are grateful for the matching of funds for research and development in the field of Knowledge Integration and Innovation from the Bio-oriented Technology Research Advancement Institution of the National Agriculture and Food Research Organization, Grants-in-Aid for Scientific Research (S) #25221204 and Scientific Research (A) #18H03972, and funding from the Core-to-Core Program (Advanced Research Networks) from the Japan Society for the Promotion of Science.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
All experiments using animals were approved by the Animal Usage Committee of the Faculty of Agriculture of the University of Tokyo, and performed in accordance with the Guidelines for Animal Experiments of The University of Tokyo (Permission No. P09–375).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lila Otani and Hiroki Nishi contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12986-020-00477-5.
References
- 1.Clifton PM, Bastiaans K, Keogh JB. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr Metab Cardiovasc Dis. 2009;19:548–554. doi: 10.1016/j.numecd.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Pesta DH, Samuel VT. A high-protein diet for reducing body fat: mechanisms and possible caveats. Nutr Metab. 2014;11:53. doi: 10.1186/1743-7075-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belobrajdic DP, McIntosh GH, Owens JA. A high-whey-protein diet reduces body weight gain and alters insulin sensitivity relative to red meat in wistar rats. J Nutr. 2004;134:1454–1458. doi: 10.1093/jn/134.6.1454. [DOI] [PubMed] [Google Scholar]
- 4.Almeida JA, Santana HAP, Motta-Santos D, Nogueira ME, Silva KKS, Miotto H, et al. High-protein diet associated with Bocaiuva supplementation decreases body fat and improves glucose tolerance in resistance-trained rats. J Med Food. 2020;23:258–265. doi: 10.1089/jmf.2019.0072. [DOI] [PubMed] [Google Scholar]
- 5.Edozien JC. Experimental kwashiorkor and marasmus. Nature. 1968;220:917–919. doi: 10.1038/220917a0. [DOI] [PubMed] [Google Scholar]
- 6.Soliman AT, Hassan AE, Aref MK, Hintz RL, Rosenfeld RG, Rogol AD. Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr Res. 1986;20:1122–1130. doi: 10.1203/00006450-198611000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Winje ME, Harper AE, Benton DA, Boldt RE, Elvehjem CA. Effect of dietary amino acid balance on fat deposition in the livers of rats fed low protein diets. J Nutr. 1954;54:155–166. doi: 10.1093/jn/54.1.155. [DOI] [PubMed] [Google Scholar]
- 8.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 9.Toyoshima Y, Tokita R, Taguchi Y, Akiyama-Akanishi N, Takenaka A, Kato H, et al. Tissue-specific effects of protein malnutrition on insulin signaling pathway and lipid accumulation in growing rats. Endocr J. 2014;61:499–512. doi: 10.1507/endocrj.ej13-0514. [DOI] [PubMed] [Google Scholar]
- 10.Nishi H, Yamanaka D, Kamei H, Goda Y, Kumano M, Toyoshima Y, et al. Importance of serum amino acid profile for induction of hepatic steatosis under protein malnutrition. Sci Rep. 2018;8:5461. doi: 10.1038/s41598-018-23640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine LS, Wright PG, Marcus F. Failure to secrete immunoreactive insulin by rats fed a low protein diet. Acta Endocrinol. 1983;102:240–245. doi: 10.1530/acta.0.1020240. [DOI] [PubMed] [Google Scholar]
- 12.Thams P, Capito K. L-arginine stimulation of glucose-induced insulin secretion through membrane depolarization and independent of nitric oxide. Eur J Endocrinol. 1999;140:87–93. doi: 10.1530/eje.0.1400087. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira F, Barbosa HCL, Stoppiglia LF, Delghingaro-Augusto V, Pereira EA, Boschero AC, et al. Decreased insulin secretion in islets from rats fed a low protein diet is associated with a reduced PKA alpha expression. J Nutr. 2004;134:63–67. doi: 10.1093/jn/134.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, et al. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem. 2003;278:2853–2858. doi: 10.1074/jbc.M210577200. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi S, Kajikawa M, Umezawa T, Kato H, Miura Y, Nam TJ, et al. Effect of dietary proteins on the plasma immunoreactive insulin-like growth factor-1/somatomedin C concentration in the rat. Br J Nutr. 1990;63:521–534. [PubMed] [Google Scholar]
- 16.Toyoshima Y, Tokita R, Ohne Y, Hakuno F, Noguchi T, Minami S, et al. Dietary protein deprivation upregulates insulin signaling and inhibits gluconeogenesis in rat liver. J Mol Endocrinol. 2010;45:329–340. doi: 10.1677/JME-10-0102. [DOI] [PubMed] [Google Scholar]
- 17.Narita K, Nagao K, Bannai M, Ichimaru T, Nakano S, Murata T, et al. Dietary deficiency of essential amino acids rapidly induces cessation of the rat estrous cycle. PLoS One. 2011;6:1. doi: 10.1371/journal.pone.0028136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of Total Lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 19.Otway S, Robinson DS. The effect of a non-ionic detergent (triton WR 1339) on the removal of triglyceride fatty acids from the blood of the rat. J Physiol. 1967;190:309–319. doi: 10.1113/jphysiol.1967.sp008210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VerHague MA, Cheng DM, Weinberg RB, Shelness GS. Apolipoprotein A-IV expression in mouse liver enhances triglyceride secretion and reduces hepatic lipid content by promoting very low density lipoprotein particle expansion. Arterioscler Thromb Vasc Biol. 2013;33:2501–2508. doi: 10.1161/ATVBAHA.113.301948. [DOI] [PubMed] [Google Scholar]
- 21.Yue JTY, Mighiu PI, Naples M, Adeli K, Lam TKT. Glycine normalizes hepatic triglyceride-rich VLDL secretion by triggering the CNS in high-fat fed rats. Circ Res. 2012;110:1345. doi: 10.1161/CIRCRESAHA.112.268276. [DOI] [PubMed] [Google Scholar]
- 22.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson CG, Tran JL, Erion DM, Vera NB, Febbraio M, Weiss EJ. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed mice. Endocrinology. 2016;157:570–585. doi: 10.1210/en.2015-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao J, Sportsman R, Harris J, Stahl A. Real-time quantification of fatty acid uptake using a novel fluorescence assay. J Lipid Res. 2005;46:597–602. doi: 10.1194/jlr.D400023-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Kohan AB, Lo CM, Liu M, Howles P, Tso P. Apolipoprotein A-IV: a protein intimately involved in metabolism. J Lipid Res. 2015;56(8):1403. doi: 10.1194/jlr.R052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koo SH. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol. 2013;19:210–215. doi: 10.3350/cmh.2013.19.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubota N, Kubota T, Itoh S, Kumagai H, Kozono H, Takamoto I, et al. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 2008;8:49–64. doi: 10.1016/j.cmet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi CM, Ueki K, Kahn R. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J Clin Invest. 2005;115:718–727. doi: 10.1172/JCI23187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest. 2006;116:101–114. doi: 10.1172/JCI25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood JD, Nute GR, Richardson RI, Whittington FM, Southwood O, Plastow G, et al. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 2004;67:651–667. doi: 10.1016/j.meatsci.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 31.da Costa N, McGillivray C, Bai Q, Wood JD, Evans G, Chang KC. Restriction of dietary energy and protein induces molecular changes in young porcine skeletal muscles. J Nutr. 2004;134:2191–2199. doi: 10.1093/jn/134.9.2191. [DOI] [PubMed] [Google Scholar]
- 32.Katsumata M. Promotion of intramuscular fat accumulation in porcine muscle by nutritional regulation. Anim Sci J. 2011;82:17–25. doi: 10.1111/j.1740-0929.2010.00844.x. [DOI] [PubMed] [Google Scholar]
- 33.Martens EA, Gatta-Cherifi B, Gonnissen HK, Westerterp-Plantenga MS. The potential of a high protein-low carbohydrate diet to preserve intrahepatic triglyceride content in healthy humans. PLoS One. 2014;9:e109617. doi: 10.1371/journal.pone.0109617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borensztajn J, Rone MS, Kotlar TJ. The inhibition in vivo of lipoprotein lipase (clearing-factor lipase) activity by triton WR-1339. Biochem J. 1976;156:539–543. doi: 10.1042/bj1560539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiwari S, Siddiqi SA. Intracellular trafficking and secretion of VLDL. Arterioscler Thromb Vasc Biol. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbosa S, Fasanella G, Carreira S, Llarena M, Fox R, Barreca C, et al. An orchestrated program regulating secretory pathway genes and cargos by the transmembrane transcription factor CREB-H. Traffic. 2013;14:382–398. doi: 10.1111/tra.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagasawa M, Hara T, Kashino A, Akasaka Y, Ide T, Murakami K. Identification of a functional peroxisome proliferator-activated receptor (PPAR) response element (PPRE) in the human apolipoprotein A-IV gene. Biochem Pharmacol. 2009;78:523–530. doi: 10.1016/j.bcp.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Katsumata M, Kobayashi H, Ashihara A, Ishida A. Effects of dietary lysine levels and lighting conditions on intramuscular fat accumulation in growing pigs. Anim Sci J. 2018;89:988–993. doi: 10.1111/asj.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab. 2004;28(Suppl 4):S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 41.Hocquette JF, Gondret F, Baeza E, Medale F, Jurie C, Pethick DW. Intramuscular fat content in meat-producing animals: development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4:303–319. doi: 10.1017/S1751731109991091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1 Insulin signaling-related protein levels in rats fed the 5PAA and low Arg diets. Six-week-old male Wistar rats were fed the 15PAA (n = 8), low Arg (n = 8), or 5PAA (n = 8) diet for 14 d. Whole-liver lysates were analyzed by immunoprecipitation and immunoblotting with antibodies against p85 (B), S6K (C), pS6K (D), AMPK (F), and pAMPK (G), where anti-S6K served as the internal control; or with antibodies against pS6K/S6K (E) and pAMPK (H), where anti-GAPDH served as the internal control. Figure S2 Average RER values during the light period (8:00–20:00) and dark period (20:00–8:00) were calculated individually.
Additional file 2: Table S1 Plasma glucose and insulin levels in rats fed the 5PAA and low Arg diets. Table S2 List of apolipoprotein genes.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.