Abstract
The world is facing lockdown for the first time in decades due to the novel coronavirus COVID-19 (SARS-CoV-2) pandemic. This has led to massive global economic disruption, placed additional strain on local and global public health resources and, above all, threatened human health. We conducted a review of peer-reviewed and unpublished data, written in English, reporting on the current COVID-19 pandemic. This data includes previously used strategies against infectious disease, recent clinical trials and FDA-approved diagnostic and treatment strategies. The literature was obtained through a systematic search using PubMed, Web of Sciences, and FDA, NIH and WHO websites. Of the 98 references included in the review, the majority focused on pathogen and host targeting, symptomatic treatment and convalescent plasma utilization. Other sources investigated vaccinations in the pipeline for the possible prevention of COVID-19 infection. The results demonstrate various conventional as well as potentially advanced in vitro diagnostic approaches (IVD) for the diagnosis of COVID-19. Mixed results have been observed so far when utilising these approaches for the treatment of COVID-19 infection. Some treatments have been found highly effective in specific regions of the world while others have not altered the disease process. The responsiveness of currently available options is not conclusive. The novelty of this disease, the rapidity of its global outbreak and the unavailability of vaccines have contributed to the global public’s fear. It is concluded that the exploration of a range of diagnostic and treatment strategies for the management of COVID-19 is the need of the hour.
Key Words: COVID-19, SARS-CoV-2, diagnosis, treatment strategies, pandemic, public health
INTRODUCTION
SARS-CoV2, a deadly virus belonging to the family Coronaviridae, primarily targets the pulmonary system and manifests with symptoms of moderate to high fever, dry cough and difficulty breathing, pneumonia, and respiratory distress. The emergence and outbreak of microbial infections depends upon several factors including the mutability of microbes, the distraction of human and microbial environments, and human experiments on new microbes. The epicentre of COVID-19 infection was in Wuhan, in the city of Hubei, China, from where the disease surged on December 29, 2019, and spread quickly to the rest of the China (1,2). The World Health Organisation reported COVID-19 as pandemic on March 11, 2020, and declared a Public Health Emergency of International Concern against COVID-19 (3).
Up to today, 8 July 2020, the COVID-19 outbreak has spread to 215 countries as reported by the World Health Organisation. Almost 539,026 deaths have been reported around the globe, with nearly 11,635,939 cases are reported as confirmed cases of COVID-19 infection. The highest number of deaths has been reported in the USA (129,963), followed by Brazil (65,487), Italy (34,869), Mexico (31,119), Spain (28,388), India (20,642), Iran (11,931) and China (4648). In USA, a total of 2,923,432 people have been infected, which makes it the most affected area in this pandemic (4). These numbers are growing on a daily basis. Unsurprisingly, this sharp upsurge in the number of infected patients has instigated widespread panic among the population. Scientists around the globe have been looking for possible detection and treatment strategies since the beginning of the outbreak. In this regard, the first genome of COVID-19 was published by Prof. Yong-Zhen Zhang and colleagues on 10 January 2020 (2). However, there is only limited data available on the clinical diagnosis, treatment and control of this deadly virus.
Owing to the devastating conditions of the COVID-19 outbreak, the unavailability of a vaccine and its deadly influence on human health, it is important to understand the available detection tools recently approved by the FDA or health authorities of other advanced countries and possible treatment strategies to fight this lethal virus. We therefore conducted a comprehensive review on potential detection and treatment strategies related to COVID-19. This review will provide a brief description of the clinical diagnosis and therapeutic drug delivery options. Furthermore, this review will provide information on COVID-19 as a basis for further, extensive research. It may also provide a good background for various government and private organisations to develop diagnostic, treatment and preventive strategies against COVID-19 and other such viruses to support public health. Moreover, we aimed to increase knowledge of the clinical expression of COVID-19 infection to further establish and reinforce a timely diagnosis and treatment of the infection in order to reduce mortality rate.
DIAGNOSTICS
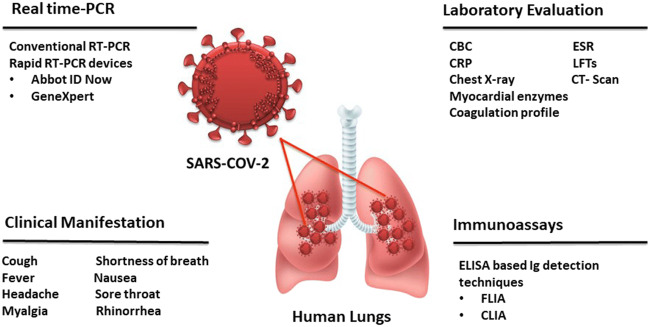
Due to the unavailability of satisfactory treatment and vaccination, the management of COVID-19 depends upon the timely diagnosis and standard (symptomatic) treatment yet. Thus, for timely detection of virus, efficient diagnostic strategies (enlisted in Fig. 1) should be required to opt. The detail of these diagnostics along with clinical manifestation is also discussed below.
Fig. 1.
Diagnostic approaches for COVID-19
Advance In Vitro Diagnostic Approaches
In vitro diagnostic (IVD) approach relies on either the principle of viral genetic material detection or the host antibody, against COVID-19, detection, in the host specimen. The detailed comparison between these two COVID-19 diagnostic approaches is stated in Table I.
Table I.
Comparison Between IVD-RT-PCR-Based Technique and Immunoassay-Based Techniques
| Parameter | Polymerase chain reaction (PCR)–based techniques | Immunoassay-based kits |
|---|---|---|
| Sampling | Nasopharyngeal swab, saliva | Blood (10–20 μL) |
| Component to be detected |
Envelop (E) gene |
Antibodies (IgM-IgG) |
| Stage at which infection detected | Asymptomatic or subclinical infection can be detected | Detection is usually when the immune system starts responding to infection |
| Average result time |
Within few hours Rapid device (Xpert®)—app. 40–45 min |
10–20 min CLIA-based kit (designed by DIAZYME)—50 tests/h |
| Specificity | High (5) | High (5) |
| Cost | High | Low |
| Results validity | High sensitivity to SARS-COV-2 viral genes (5) | Possibility of false negative results as IgG level appears in blood approximately 20 days post-infection* (7) |
PCR- Polymerase chain reaction; CLIA- Chemiluminescence immunoassay; IgM- Immunoglobulin M; IgG- Immunoglobulin G; RT-PCR- Reverse Transcriptase- Polymerase chain reaction; SARS-COV-2- Severe Acute Respiratory Syndrome Corona Virus-2
*But recommended to be used when there is fall short in RT-PCR facility
Viral Nucleic Acid Detection
Real-Time Polymerase Chain Reaction
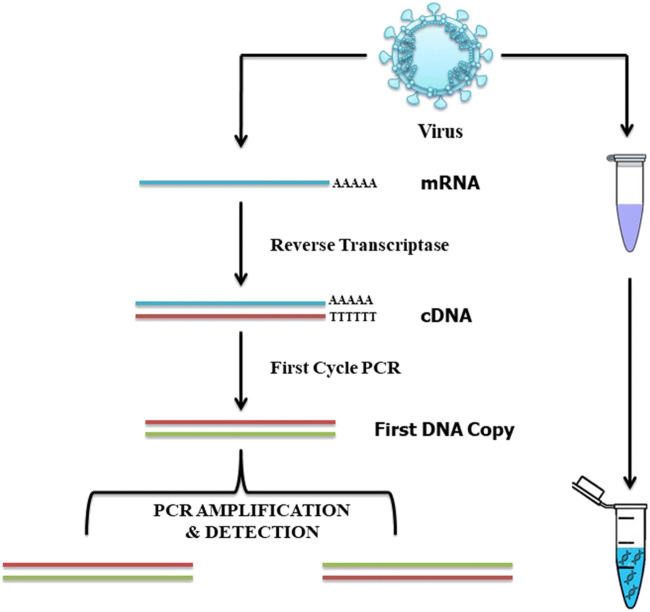
For the confirmed diagnosis of COVID-19, viral genetic material identification in the patient’s body is strongly recommended. Real-time reverse transcription polymerase chain reaction (RT-PCR) can be used for the detection of COVID-19 nucleic acids in nasopharyngeal swabs, lower respiratory tract secretions, sputum, blood, faeces and other specimens. A graphical illustration of real-time RT-PCR is shown in Fig. 2. At the start of 2020, a German scientist suggested using the RT-PCR test for the detection of SARS-CoV-2 as it has high accuracy and precision (8). Conventional RT-PCR for virus detection can sometimes be associated with false negative results, particularly in the early stages of infection. To bypass this issue, more advanced RT-PCR diagnostics are needed (9).
Fig. 2.
Graphical illustration of RT-PCR
NGS (next generation sequencing) is an alternative approach that can be used for this purpose; fortunately, it is more advanced and rapid compared with PCR (10). The mutation of the pathogen can also be checked by NGS. In order to obtain accurate results, the sample should be taken from the lower respiratory tract (2).
RT-PCR-Based Rapid Test Devices
Recently, the FDA has given approval for the first rapid detection test kit for COVID-19 detection for use under an Emergency Use Authorisation (EUA) developed by Cepheid. It can detect COVID-19 within approximately 45 min. Samples are collected via nasopharyngeal swab or nasal wash and are prepared in less than 1 min. The technique used in this is RT-PCR and the kit has the required probes, primers and internal controls with it. The GeneXpert® instrument system (mentioned in Table II), present in qualified laboratories in the USA, is currently used to perform this test (12).
Table II.
Recommended COVID-19 Nucleic Acid Detection Techniques
| Organization conducted the study | Test principle | Sensitivity of test | Manufacturer/commercially available devices | Reference |
|---|---|---|---|---|
| Huazhong University of Science and Technology, Wuhan, China |
Chest CT RT-PCR |
Chest CT 97% RT-PCR 59% |
- | (11) |
| Fred Hutchinson Cancer Research Center, WA, USA | Real-time RT-PCR | 84.6% |
Xpert® Xpress by Cepheid Roche Molecular System |
(12) |
| University of California, San Francisco, USA | CRISPR | Not reported |
Cepheid Sherlock Biosciences Mammoth Biosciences |
(13) |
| Wuhan Institute of Virology, China | Isothermal nucleic acid amplification technology | 95% | Abbott® ID Now | (7,14,15) |
CT- Computed Tomography; RT-PCR- Reverse Transcriptase- Polymerase chain reaction; CRISPR- Clustered Regularly Interspaced Short Palindromic Release
Isothermal Nucleic Acid Amplification Assay
Due to confrontation of inadequate RT-PCR sensitivity for COVID-19 diagnosis, more advanced nucleic acid detection techniques were recently investigated including isothermal technique. It was found suitable for the detection of very low quantity of viral RNA (genetic material) and it can affectively be used as an alternative technique to RT-PCR (16). Two companies have developed the rapid detection devices on the basis of these techniques, stated in Table II. An isothermal nucleic acid amplification–based device developed by Abbott under the trade name of ID Now® has obtained FDA EUA (7).
CRISPR Nucleic Acid Assay
Another advanced technology for nucleic acid detection is the use of Clustered Regularly Interspaced Short Palindromic Release (CRISPR) using myriad bacterial enzymes such as Cas-12, to detect viral genome. In comparison with real-time RT-PCR, this technique ensures rapidity and better sensitivity for COVID-19 nucleic acid detection (13).
Antibody Detection Immunoassays
Lateral Flow Immunoassay–Based Kits
Just like the rapid pregnancy strip test, lateral flow immunoassay–based techniques for COVID-19 have been developed for rapid diagnosis (17). This mode of diagnosis requires a significantly low quantity of sample (10 μL of blood) along with a detection time of less than 15 min. A US-based company, BioMedomics, has developed an IgM-IgG-based rapid test kit and obtained approval from the FDA for distribution in the USA. In addition, several other companies have also developed serological testing strips (18). Though the standard COVID-19 test is nucleic acid detection by PCR, these antibody detection-based techniques have comparable sensitivity and selectivity to RT-PCR and are recommended to be used when RT-PCR facilities are not available (19).
Chemiluminescence Immunoassay–Based Kits
Recently, Diazyme in the USA developed a chemiluminescence immunoassay (CLIA)–based IgM and IgG rapid detection test and also gained FDA EUA. It runs on an automated analyser, with a throughput of 50 tests/h (20). On a similar detection principle, the Chinese company Snibe has developed the MAGLUMI CLIA analyser for the detection of IgM and IgG in patient samples (21).
Conventional Diagnostic Approaches
Physical Examination
This strategy involves the diagnosis of the disease based upon physical signs and symptoms. Some patients have only mild symptoms with fatigue, and low fever in the absence of pneumonia, whereas patients with severe cases demonstrate dyspnoea, lung crackles and dullness to percussion, as reported by Jin et al. in 2020 (2). Patients with severe disease may progress to acute respiratory distress syndrome, dyspnoea (after 1 week), septic shock, bleeding disorders, difficult to correct metabolic acidosis and even multiple organ failure. Those in severe or critical condition could show a low to moderate fever (22).
Imaging Examination
CT imaging of the patient is strongly recommended for the diagnosis of patients, as reported by the researchers at Zhongnan Hospital of Wuhan University. The sensitivity of CT scan, however, for COVID-19 diagnosis is appeared to be 97.2% (23). It has been reported that CT imaging of lesions may exhibit major distributions in the subpleural region or may barely exist along bronchial vascular bundles. A quantitative analysis demonstrated the existence of randomly one to two and often more than three lesions. Their morphology was observed to be nodular, lumpy or patchy. Moreover, the density was found to be mostly uneven with a condensed bronchial wall. Other associated indications vary from air-filled bronchi, enlarged lymph nodes (mediastinal) and rarely pleural effusion (2,24).
Laboratory Examination
Haematological Examination
By assessing haematological variations, COVID-19 can be provisionally diagnosed. As reported by Jin and colleagues, initially the number of lymphocytes decreases, and the number of monocytes remains normal or increases, whereas the total leukocyte count remains normal or decreases with advancement of the disease. In cases where a significant decline is observed in CD4+ and CD8+ T cells or when the lymphocyte absolute value falls below 0.8 × 109/L, attention must be given with routine rechecking of blood counts every 3 days (2). Similarly, elevation in muscle and liver enzymes, i.e. myoglobin and lactate dehydrogenase, is observed in some patients (22). Elevation in C-reactive protein and a higher erythrocyte sedimentation rate with normal levels of pro-calcitonin are also seen in many cases. Elevated D-dimer levels with decreased peripheral blood lymphocytes are observed in severely ill patients (25). Moreover, among critical patients, troponin levels are also elevated. Inflammatory factor levels are also observed to be increased in both severe and critical patients (2).
Pathological Changes Observed from Biopsies and Autopsies
During the recent COVID-19 pandemic, autopsy and biopsy studies confirmed that the pathological basis of this disease is alveolar damage, with the organisation of exudates in alveoli and fibrosis in the lungs. Lymphocytopenia and necrosis can be observed in the spleen and lymph nodes. Necrosis can also be observed in myocardial tissues, but the observed heart and liver changes can be attributed or limited to underlying diseases (22,26). However, these invasive techniques are not recommended for routine diagnosis.
Additional Laboratory Testing
For monitoring the improvement of a patient’s condition, other laboratory tests such as blood gas analysis (oxygenation), liver and kidney function tests, myocardial enzymes, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), percutaneous nephrostomy (PCN), lactate dehydrogenase (LDH), D-dimer, urinalysis, coagulation image, 11 items of the tuberculosis (TB) subgroup, inflammatory factors (IL-6, IL-10, TNF-α), complement and anti-acid staining can commonly be used. Moreover, CRP and PCN can be assessed. The existence of a bacterial infection in the lungs should be determined as these inflammatory factors are commonly elevated in such sort of infection (2,22,25).
TREATMENT STRATEGIES
Pathogen-Targeting Strategies
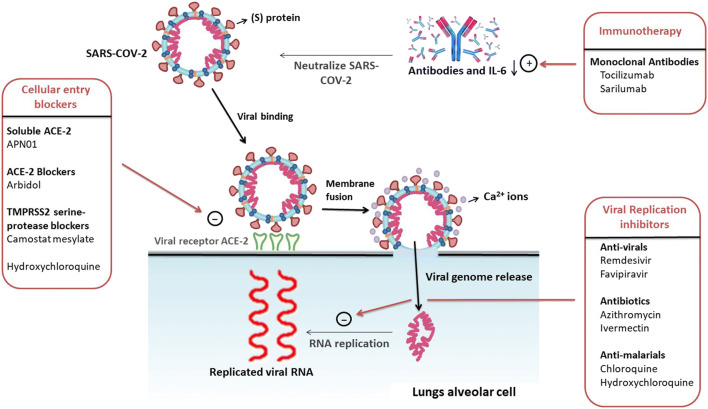
Despite the rapid progression of the COVID-19 pandemic, unfortunately, anti-virals or other drugs still are yet to be approved for the treatment of COVID-19. However, the sudden outbreak has compelled the repurposing of already available drugs (described in Fig. 3) that have been documented to possess activity against HIV, MERS and SARS-COV-1.
Fig. 3.
Mechanism of action of major repurposing agents against COVID-19
Anti-Virals
COVID-19 is, in fact, an RNA betacoronavirus, and its genomic sequence and various enzymes are similar (80–90%) to those of the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronaviruses. The use of existing anti-viral may become the mainstay to reduce viral load and treat the infection. Multiple pre-existing anti-viral agents have been tried to assess their in vitro anti-COVID-19 properties (27). Protease inhibitors possess considerable inhibitory activity against RNA viruses. Thus, on the basis of lopinavir-ritonavir in vitro inhibitory activity reported against acute respiratory distress syndrome caused by SARS and MERS, an open-label, randomised control trial (ChiCTR2000029539) was conducted in China (28). Unfortunately, the results were not significantly different between the two randomised groups (one group treated with lopinavir-ritonavir and other with standard care protocols) (29). Favipiravir, remdesivir and ribavirin, i.e. nucleoside structural analogues, have also been reported to possess in vitro inhibitory activity against COVID-19. Recently, a study was conducted on five anti-viral agents to determine their activity regarding COVID-19 inhibition, i.e. preventing viral incorporation and multiplication inside Vero E6 cells. Among them, remdesivir was found to possess significant anti-viral activity at lower values of the half-maximal effective concentration (EC50), i.e. 0.77 μM, along with the anti-malarial agent chloroquine, with an EC50 value of 1.13 μM (30).
Remdesivir, developed by Gilead Sciences, a US-based enterprise, has historically been used for eliminating Ebola virus (31). It is a broad-spectrum investigational anti-viral agent, currently progressing through a double-blinded placebo control trial as well as a phase III open-label clinical trial, due to the abovementioned success in vitro Vero E6 cell experiments. In addition, it is currently being used on COVID-19 patients in non-trial areas of the USA and other countries (NCT04292899) (32). Due to its promising effects against COVID-19, Gilead Sciences have agreed to a pact to allow Pakistan’s national pharmaceutical company, Ferozsons Laboratories Ltd., to carry out production for the country’s utilisation and export (33). It is, fortunately, the only recommended anti-viral drug which can be used in patients with low oxygen saturation (below 94%) and critical patients on artificial ventilation. But its use in mild symptomatic patients is not recommended. The recently conducted trial in hospitalised patients has clearly demonstrated a significantly reduced mortality rate (from 11 to 7.1%) with remdesivir use (34). Due to adequate evidence from the literature, a small monocular agent having anti-viral activity against influenza virus strains, adenovirus, rhinovirus and several other RNA and DNA viruses, Arbidol, also appears to possess significant anti-COVID-19 activity and is going through a phase IV clinical trial at present (NCT04260594) (35). In addition to that, another randomised control trial has been conducted in China comparing the cure rate and 7-day recovery rates in patients randomly allocated to two groups, one receiving favipiravir and the other receiving Arbidol treatment. The clinical recovery rate of favipiravir was found to be superior, i.e. 61.21%, and was better than that of Arbidol (51.67%) (36). Most recently, another broad spectral anti-viral drug, N-hydroxycytidine and its prodrug, has been found to have promising anti-viral ability against various strains of the Coronaviridae family, i.e. SARS-CoV-2, MERS-COV and SARS-COV, and has qualified as candidates for human clinical trials. Furthermore, these studies utilised human airway epithelial cells (HAE) and Calu-3 cells, in addition to Vero E6 cells (37).
Anti-Malarials
Chloroquine, an old drug renowned for its anti-malarial and anti-rheumatic uses, has been found to exhibit substantial anti-COVID-19 activity, at a low EC50, i.e. 1.13 μM (30). These adequate in vitro results on chloroquine urged scientists to move this drug toward human clinical trials. Due to its low EC50 and higher cytotoxic concentration CC50, chloroquine was introduced into human clinical trials conducted at more than 10 hospitals in China. The results were found to be promising in terms of lowering the viral load and disease duration as well as preventing the exacerbation of COVID-19 pneumonia (38). The probable mechanism by which chloroquine exhibits anti-COVID-19 effects is its ability to inhibit the fusion and entry of the virus into the host cell (39). However, due to the toxicity-mediated limitations of chloroquine, it has not been introduced into clinical trials by the NIH.
Hydroxychloroquine, a suitable alternative shown to be considerably less toxic (app. 40%) and having comparable anti-COVID-19 activity at its parent drug, is currently undergoing randomised controlled phase III clinical trials (NCT04261517) (40,41). The underlying anti-viral mechanisms of hydroxychloroquine are an increase in endosomal pH and the prevention virus entry into the target cell (42). Unfortunately, no convincing evidence was obtained during the recent trials to support the curing use of chloroquine and hydroxychloroquine in COVID-19 (43).
Antibiotics
An open-label randomised control trial was performed in China comparing post-treatment virus load in three randomised groups treated with hydroxychloroquine and azithromycin, hydroxychloroquine alone and standard care. The results of this study clearly identified the superiority (100%) of combined treatment over hydroxychloroquine monotherapy (57.1%) and standard care (2020-000890-25) (44). However, the results of non-randomised, observational study recently carried out in Michigan, USA, suggesting the combined use of hydroxychloroquine and azithromycin in COVID-19 patients is associated with significant reduction in viral load and mortality rate. However, results are not much practical due to numerous limitations of the study and negating current NIH recommendations, suggesting to avoid their use in COVID-19 (45). According to recent updates, carrimycin, another macrolide, is undergoing a phase IV clinical trial under the direction of Ronghua Jin at Beijing YouAn Hospital (NCT04286503) (46).
Anti-Parasitics
Nitazoxanide is an agent, previously indicated as anti-parasitic drug, that also exhibits prominent COVID-19 inhibitory activity, but with effects inferior to those of remdesivir and chloroquine (30). Recently, Australian researchers have demonstrated the pronounced in vitro anti-SARS-CoV-2 activity of ivermectin, which is also active against human immunodeficiency virus (HIV) and dengue virus. This anti-parasitic agent has been found to significantly reduce viral RNA levels, identified using RT-PCR, at a concentration of approximately 2 μM, possibly owing to the inhibition of IMPα/β1, a viral protein carrier involved in replication (47,48).
Novel Small Molecule Anti-COVID-19 Drugs—Blocking Cellular Entry
The need of the hour is to develop novel and effective anti-COVID-19 agents because of the expanding spectrum of COVID-19 infection throughout the world. Camostat mesylate, a potent protease inhibitor previously approved in Japan for various indications such as pancreatitis and flu, possesses considerable blocking activity with regarding to viral entry into the host cell via the lysis of TMPRSS2, a serine protease that interacts with the COVID-19 spike (S) protein (49). Unfortunately, there is a lack of data, studies and trials on this innovative drug against COVID-19. Another entity, Arbidol (umifenovir), is believed to inhibit COVID-19 endocytosis into the host cell. This is why Arbidol is currently enrolled in several clinical trials against COVID-19 (50). The Chinese clinical trial agencies along with the NIH are both conducting trials on the efficacy of Arbidol, either alone or in combination with the protease inhibitor favipiravir (NCT04260594, ChiCTR2000030254) (36).
Soluble ACE2 parenteral administration results in the binding of SARS-CoV-2 spike protein, followed by inhibition of cellular endocytosis (51); this, in turn, would result in pronounced alleviation of lung cellular injury. On the basis of these optimistic findings, a human recombinant ACE2 (APN01) was designed and shown to lead to a prominent reduction in AN-II-mediated lung injury and IL-6 levels. This evidence was sufficient to support a RhACE2 clinical trial. Currently, Apeiron Biologics are sponsoring clinical trials on the efficacy and safety of APN01, which are currently in phase II (NCT04335136) (52).
Host-Targeting Strategy—Immunity Modifiers
Natural Killer Cells
It has been hypothesised that there is high death rate among the geriatric population owing to their inadequate immune response. Thus, simply stabilising the patient’s immune profile could be helpful toward an earlier resolution of COVID-19 symptoms. Chen et al. claimed that CD4+ T cells, CD8+ T cells and antibodies were found to reduce the symptoms associated with SARS-COV (53). Thus, natural killer (NK) cells may be a reasonable candidate for clinical trials currently in phase I (safety and efficacy assessment) at present (NCT04280224) (54).
Monoclonal Antibodies
Monoclonal antibodies (mAbs) usually alter the immune system response of the host organism, i.e. a reduction in the IL-6 plasma level, which is often elevated in COVID-19 patients on mechanical ventilation (55). However, the exact mechanism by which mAbs work against COVID-19 is unclear. Tocilizumab, a monoclonal antibody clinically approved for several autoimmune disorders, has been evaluated in terms of efficacy and response either alone or in combination with favipiravir. Several randomised studies are being carried out in China and the USA (still ongoing) (NCT04315480) (56). Moreover, Regeneron Pharmaceuticals, in collaboration with Sanofi, are evaluating the safety and efficacy of sarilumab among hospitalised COVID-19 patients, now in phase II (NCT04315298) (57).
mAbs also have clinical significance in myriad malignancies, i.e. advanced colorectal cancer, non-small cell carcinoma of the lung, renal cell carcinoma and breast cancer. For example, bevacizumab impairs angiogenesis by antagonising vascular endothelial growth factor (VEGF), which is considered to be responsible for pulmonary oedema in acute respiratory distress (58,59). This opportunistic strategy to curb the symptoms of severe COVID-19 is currently undergoing phase II clinical trials (NCT04275414) by the NIH and in Wuhan, China (60).
Interferon-α and Corticosteroids
Due to the structural similarities between SARS-CoV-1 and SARS-CoV-2, interferon-α (INF-α) may boost SARS-CoV-2 patient innate immunity. In order to verify this, China has launched clinical trials to treat SARS-CoV-2 patients using combination therapy with INF-α and ribavirin (ChiCTR2000029387) (61). Use of the corticosteroid therapy for treatment and/or reducing the duration of COVID-19 infection was previously questionable due to a lack of clinical evidence (62). However, the results of the recent studies, a pre-test post-test quasi experiment carried out in Michigan and a study conducted in Greece, transparently suggested the beneficial impacts of corticosteroid use in critically ill COVID-19 patients having hyperactive immune system also termed as “cytokine syndrome” (63,64). The recommendations about the use and dosing of dexamethasone for COVID-19 patients are provided by NIH. These recommendations, however, suggested the initiation of steroids in COVID-19 patients on supplemental oxygen and artificial ventilation only (65). Zongji Hospital has sponsored a clinical trial registered with the NIH to assess the safety of INF-α2β (NCT04293887) and is currently in early phase I (66).
Miscellaneous Agents
Thalidomide, an immunomodulatory, anti-fibrotic and anti-inflammatory drug, has previously been indicated by the US FDA for the treatment of erythema nodosum leprosum, systemic lupus erythematous, inflammatory bowel disease, cystic fibrosis, heart failure and several malignant anomalies (67). The anti-viral activity of thalidomide has been reported, leading to a surge in cellular immunity, i.e. CD8+ T cells against cytomegalovirus (CMV) (68). Due to the enhancement of cellular immunity and significant reduction in TNF-α levels, it is presently undergoing a randomised control clinical trial (NCT04273529) in China (69,70). Fingolimod is an oral sphingosine 1-phosphate receptor activator, currently being utilised for the treatment of multiple sclerosis. It has immune system modifying potential and leads to a significant reduction in peripheral lymphocytes (71). Informed by these characteristics, a clinical trial to assess the efficacy and safety of fingolimod is in progress (NCT04280588) (72) (Table III).
Table III.
Promising Anti-COVID-19 Repurposing Treatment Options—Completed and Undergoing Clinical Trials
| Anti-COVID-19 repurposing options | In vitro anti-2019-nCOV activity determined (+) or not (−) | Trial registration no. | Current phase of trial | Regimen using/used in clinical trial/s | Reference |
|---|---|---|---|---|---|
| Lopinavir + ritonavir | + | ChiCTR2000029539 | Completed (rejected) |
Lopinavir: 400 mg PO Ritonavir: 100 mg PO BD for 14 days |
(28) |
| Favipiravir | + | ChiCTR2000030254 | Completed (recommended for use in China and Japan) | 1600 mg/per dose PO bid for day 1, followed by 600 mg/dose PO bid until the end of the trial | (73) |
| Remdesivir | + | NCT04292899 | Phase III | 200 mg/dose OD IV for 1 day followed by 100 mg/dose OD IV for the next 4–9 days | (32) |
| Arbidol | − | NCT04260594 | Phase IV | Two tablets PO TID for 10–14 days | (35) |
| Chloroquine phosphate | + | ChiCTR2000029542 |
- Phase IV |
500 mg/dose PO BID for not more than 10 days | (74) |
| Hydroxychloroquine | + |
ChiCTR2000029559 |
Completed | 200 mg/dose TID PO for 5 days | (41) |
| Carrimycin | − | NCT04286503 | Phase IV | - | (46) |
| Hydroxychloroquine-azithromycin | − | French national agency for drug safety: 2020-000890-25 | Completed |
Hydroxychloroquine: 200 mg PO TID for 10 days Azithromycin: 500 mg PO for day 1 followed by 250 mg daily for next 4 days |
(44) |
| Tocilizumab | − |
ChiCTR2000029765 |
Completed Phase II |
4–8 mg/kg IV diluted in NS (single dose) | (56,75) |
| Sarilumab | − | NCT04315298 | Phase II | Single dose IV | (57) |
| Bevacizumab | − | NCT04275414 | Phase II | 500 mg in 100 ml NS IV rip | (60) |
| Favipiravir + tocilizumab | − | ChiCTR2000030894 | - |
Favipiravir: 1600 mg/dose BD for 2 days followed by 600/dose BD for 7 days Tocilizumab: 4–8 mg/IV |
(76) |
|
INF-α + ribavirin, INF-α + ribavirin + LPR/RTR |
− | ChiCTR2000029387 | Not completed | INF-α: atomised inhalation, 5 million U/50 μg per dose, BID for 14 days | (61) |
| INF-α2β | − | NCT04293887 | Phase I | 10 μg BD in a nebulised solution | (66) |
| RhACE2 (APN01) | − | NCT04335136 | Phase II | - | (52) |
| NK cells | − | NCT04280224 | Phase I | 0.1–0.2 × 107 cells/kg of body weight twice weekly | (54) |
| Thalidomide | − | NCT04273529 | Phase II | 100 mg PO every night for 14 days | (69) |
| Fingolimod | − | NCT04280588 | Phase II | 0.5 mg PO OD for 3 consecutive days | (72) |
| Dexamethasone* | − | NCT04395105 | Phase III | 16 mg IV for first 5 days followed by 8 mg IV for next 5 days | (77) |
| Enoxaparin** | − | NCT04359277 | Phase III | 1 mg/kg every 12 h SC | (78) |
PO- Oral administration; BD- Twice daily; TID- Thrice daily ; IV- Intravenous ; NS- Normal Saline; SC- Subcutaneously; DIC- Disseminated intravascular coagulation; VTE- Venous thromboembolism; INFα- interferon-α; RhACE2- Recombinant human angiotensin-converting enzyme-2; NK cells- Natural killer cells ; LPR- Lopinavir; RTR- Ritonavir
*Recommended to use in patient with hyperactive immune system—cytokine syndrome
**Recommended to use in patients with evidence of DIC/VTE
Symptomatic Treatment Strategies
Mild Infection
Mild symptomatic COVID-19 patients (with fever > 37.5°C, fatigue but no dyspnoea), suspected or confirmed cases, should be isolated in quarantine. Moreover, symptomatic relief is recommended. The fever is usually treated with paracetamol or non-steroidal anti-inflammatory drugs, and no specific treatment is suggested at this stage. Additionally, Chinese health workers and doctor also recommend the use of Chinese traditional medicine for the management of patient symptoms (79). European guidelines, however, have supported the use of hydroxychloroquine (HCQ) in patients with confirmed COVID-19. The details are shown in Table IV (81).
Table IV.
COVID-19 Treatment Guidelines
| Clinical stage of COVID-19 | Chinese recommendations (2) | European recommendations (80) | Italian recommendations (81) |
|---|---|---|---|
| Suspected patients or confirmed patient with mild symptoms (fever > 37.5°C, fatigue, no dyspnoea) |
Suspected patient must be isolated. However, confirmed cases can be confined or treated in the same room. For fever, ibuprofen is recommended (if above 38.5°C). For symptomatic relief, use traditional Chinese medicine. |
Isolation is recommended. For fever, paracetamol is recommended. No use of anti-virals is recommended for suspected COVID-19 patients. |
Isolation is recommended. No anti-virals. Only symptomatic treatment is recommended. |
| Confirmed patients with mild-moderate symptoms (fever with persistent cough, no requirement for O2) | For fever, ibuprofen is recommended. For symptomatic relief, use traditional Chinese medicine. | Symptomatic treatment as above. Treatment with hydroxychloroquine. |
Symptomatic treatment with adequate hydration. Can be treated with lopinavir/ritonavir or hydroxychloroquine or chloroquine. |
| Severe confirmed cases accompanied with increased respiratory rate and pneumonia |
Supportive care in the ICU. For fever, ibuprofen is recommended. For symptomatic relief, use of traditional Chinese medicine. Use of anti-viral agents. |
Supportive care in the ICU, use of appropriate antibiotics to prevent opportunistic infection. Treat with hydroxychloroquine chloroquine; if hydroxychloroquine is not available, use lopinavir/ritonavir. |
Supportive care in the ICU, oral hydration, use of appropriate antibiotics to prevent opportunistic infection, adequate peripheral oxygenation. First line: use of remdesivir**; if not available, use lopinavir/ritonavir + hydroxychloroquine or use chloroquine + tocilizumab* |
| Critical COVID-19 cases (acute respiratory distress syndrome) |
Non-invasive/invasive mechanical ventilation. If not responding, extra-corporeal life support is given. Use of vasoactive drugs to improve circulation, empirical antibiotic therapy, corticosteroids (not used unnecessary), anti-virals. In case of septic shock, crystalloids will be given through the IV route. |
Supportive care in ICU, mechanical ventilation, broad-spectrum antibiotics. First line: use of remdesivir**; if not available, use HCQ + tocilizumab + steroids |
Life support by mechanical ventilation, use of broad-spectrum antibiotics to prevent opportunistic infections. Use of systemic steroids (methylprednisolone/dexamethasone). Use of ECMO if refractory hypoxemia occurs. First line: use of remdesivir**; if not available, use lopinavir/ritonavir + HCQ or use CQ + tocilizumab* |
ICU- Intensive care unit; IV- Intravenous; CQ- Chloroquine; HCQ- Hydroxychloroquine; ECMO- Extra corporeal membrane oxygenationl; ARDS- Acute respiratory distress syndrome
*Steroids are commonly used only in combination with tocilizumab or in ARDS patients to improve pulmonary function
**Compassionate use of remdesivir
COVID-19 Pneumonia
For patients experiencing not only cough and fever but also increased respiratory rate and pulmonary infiltrates, aggressive symptomatic management is required. Such cases should be given broad-spectrum antibiotics to prevent opportunistic pulmonary infections along with adequate oral hydration. For the purpose of reducing pulmonary secretions, Chinese studies have recommended the use of selective M3 blockers as well. Moreover, anti-COVID-19 specific treatment is also recommended at this stage with the use of either lopinavir or ritonavir, if anti-malarials are contraindicated (2,81).
COVID-19 ARDS and Septic Shock
Patients with severe pulmonary involvement could progress to the development of acute respiratory distress syndrome (ARDS) and septic shock. These patients should be aggressively shifted to medical emergency/ICU for vital sign monitoring and life support. In addition, respiratory depression should be managed by artificial ventilation either using non-invasive ventilation continuous positive airway pressure (CPAP)/bilevel positive airway pressure (BiPAP) or invasive mechanical ventilation to prevent hypoxemia. Patients with refractory hypoxemia are candidates for extra-corporeal membrane oxygenation. In addition to life support and stabilisation of vitals, cases should be treated with broad-spectrum antibiotics as well as glucocorticoids (to increase pulmonary function). COVID-19 septic shock patients should be administered intravenous crystalloids and vasoactive agents (2,80,81).
Recent Advancements in Symptomatic Treatment—Antithrombic Therapy
With a more in-depth consideration of COVID-19 management, several researchers have observed the manifestation of disseminated intravascular coagulation (DIC) and venous thromboembolism (VTE) in COVID-19 patients with severe pulmonary involvement, evidenced by elevated levels of D-dimer (82). This coagulopathy may result in pulmonary thrombosis as well (83). To cope with COVID-19-induced coagulopathy, several treatment modalities, i.e. conventional DIC treatment options, have been tried. A case series has been reported utilising tissue plasminogen activators (TPA), but among three subjects, only one patient demonstrated a significant improvement in ARDS (84). Other treatment options for DIC may also be employed to address the development of secondary diseases, most probably cardiac dysfunction. Aspirin, with its proven cardioprotective effects, is being tested in an NIH clinical trial (NCT04365309) at Xijing Hospital, China (85). As entered in phase III clinical trials, enoxaparin is also showing better anti-coagulation among COVID-19 patients experiencing DIC or VTE (86).
Convalescent Plasma/Immunoglobulin Utilisation
The plasma of recovered patients contains immunoglobulins that can be used to treat patients with active viral disease. In 2014, clinical trials were launched by the NIH for the assessment of convalescent plasma therapy against MERS-COV-induced ARDS. However, the results were not satisfactory, and the clinical trial was stopped at phase II (NCT02190799) (87). This mode of treatment has also been evaluated regarding African Ebola virus. Moreover, based on knowledge of immunoglobulin responses obtained from trials organised against emerging viral respiratory infections in the last two decades, the FDA has recently given approval for the initiation of clinical trials for the purpose of assessing the efficacy and safety of this mode of therapy in the current pandemic. Recently, a randomized clinical trial was carried out to evaluate the benefits of convalescent plasma in critically ill COVID-19 patients, and they appeared with results showing little or no improvement. Moreover, the duration of disease also not appeared to be shortened by its addition in standard treatment of COVID-19 (88). However, there are risks associated with the administration of immunoglobulins, which is why it should be used only for severe or life-threatening COVID-19, according to the FDA advisory board (89).
IMMUNIZATION: PIPELINE VACCINES
The high rate of person-to-person spread makes this virus extremely contagious. The two most effective ways to alleviate the spread of a pandemic are protective precautionary measures and the use of a vaccine. Following the major concern of our review, vaccination is the mainstay for the prevention of any viral pandemic disease. Most essentially, the vaccine should be required to eliminate the high spread ability of COVID-19, which if not controlled will continue to drive the current pandemic. In order to develop a COVID-19 vaccine, the most commonly opted target is the spike (S) protein anchored around on the membrane of COVID-19 (50). Multiple platforms are currently working on vaccine development against COVID-19. Globally, important COVID-19 pipeline vaccination projects are described in Table V. Currently, the NIH has registered six vaccination trials, conducting in USA, UK and China.
Table V.
Emerging COVID-19 (Pipeline) Vaccines
| Nature of vaccine | Target of vaccine | Principal developer of vaccine | Country | Clinical trial status | Reference |
|---|---|---|---|---|---|
| DNA Vaccine (INO-4800) | Spike (S) Protein | Inovio Pharmaceuticals | USA |
Phase I |
(90) |
| Non-replicating virus | Spike (S) protein | University of Oxford | UK |
Phase I/II trial |
(91) |
| Shenzhen Geno-Immune Medical Institute | China |
Phase I/II |
(92) | ||
| Inactivated vaccine | Entire virus | Sinovac Research and Development Co. Ltd. | China and Brazil |
Phase II |
(93) |
| Wuhan institute of Biological Sciences | China |
Phase I ChiCTR2000031809 |
(94) | ||
| mRNA vaccine | Spike (S) Protein | Moderna, USA | USA |
Phase I |
(95) |
| Recombinant vaccine (adenovirus type-5 vector) | Spike (S) Protein | CanSino Biologics | China |
Phase I clinical trial Completed |
(96,97) |
| Attenuated live vaccine | Entire virus | Serum Institute of India in collaboration with Codagenix | India and USA | Pre-clinical/animal studies | (98) |
DNA- Deoxyribonucleic acid; mRNA- Messenger-Ribonucleic acid; S- spike proteins
CONCLUDING REMARKS
The alarming WHO statistics about active COVID-19 cases and deaths have already been discussed. Rapid diagnosis and timely management of this deadly infection are the necessary steps required to eradicate the COVID-19 pandemic. The current standard diagnostic approach still has some limitations regarding a lack of robustness and lengthy procedures. Alternate nucleic acid detection techniques including reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) and CRISPR as well as immunoassay-based devices are currently undergoing through approval phase. In addition, there is an urgent requirement for the development of effective drugs as well as vaccines in order to control this deadly pandemic, which is dangerous to the entire global population. The high death rate and unleashed spread of COVID-19 require effective drugs and vaccines as essential tools to limit the explosive spread of this disease. Many entities with already approved indications (repurposing) are being evaluated for COVID-19 treatment. Although inadequate progress has been made in this regard, remdesivir and dexamethasone treatment have led to a cautiously promising response. Moreover, a number of drugs are currently being investigated in clinical trials. In addition, six vaccines are currently being evaluated in clinical trials, but it will require almost a year or more to approve and market the safe and immunogenic vaccine.
Compliance with Ethical Standards
Ethics Approval
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Han Gon Choi, Email: hangon@hanyang.ac.kr.
Gul Majid Khan, Email: gmkhan@qau.edu.pk.
Fakhar ud Din, Email: fudin@qau.edu.pk.
References
- 1.Schünemann HJ, Zhang Y, Oxman AD. Distinguishing opinion from evidence in guidelines. BMJ. 2019;366:l4606. doi: 10.1136/bmj.l4606. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y-H, Cai L, Cheng Z-S, Cheng H, Deng T, Fan Y-P, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. Coronavirus disease 2019 (COVID-19) Situation Report – 51. http://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 (2020). Accessed 04 April 2020.
- 4.Organization WH. Coronavirus disease (COVID-19) Situation Dashboard. http://covid19.who.int/ (2020). Accessed 08 July 2020.
- 5.Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC, et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 7.Vashist SK. In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Multidisciplinary Digital Publishing Institute; 2020. [DOI] [PMC free article] [PubMed]
- 8.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vashist SK. In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics. 2020;10:202. doi: 10.3390/diagnostics10040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim XF, Lee CB, Pascoe SM, How CB, Chan S, Tan JH, et al. Detection and characterization of a novel bat-borne coronavirus in Singapore using multiple molecular approaches. J Gen Virol. 2019;100:1363–1374. doi: 10.1099/jgv.0.001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed]
- 12.Administration USFD. Xpert® Xpress SARS-CoV-2. http://www.fda.gov/media/136314/download (2020). Accessed 4 April 2020.
- 13.Wang X, Zhong M, Liu Y, Ma P, Dang L, Meng Q, et al. Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a-based detection with naked eye readout, CRISPR/Cas12a-NER. Sci Bull. 2020. 10.1016/j.scib.2020.04.041. [DOI] [PMC free article] [PubMed]
- 14.Zhang Y, Odiwuor N, Xiong J, Sun L, Nyaruaba RO, Wei H, et al. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP. MedRxiv. 2020. 10.1101/2020.02.26.20028373.
- 15.Administration USFD. ID NOW COVID-19 for use under an Emergency Use Authorization (EUA) only. https://www.fda.gov/media/136525/download (2020). Accessed.
- 16.Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, et al. Assay techniques and test development for COVID-19 diagnosis. ACS Publications; 2020. [DOI] [PMC free article] [PubMed]
- 17.Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem. 2016;60:111–120. doi: 10.1042/EBC20150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.BioMedomics. COVID-19 IgM/IgG Rapid Test. http://www.biomedomics.com/products/infectious-disease/covid-19-rt/ (2020). Accessed 4 April 2020.
- 19.Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020. 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed]
- 20.Diazyme. Diazyme DZ-Lite SARS CoV-2 IgM and IgG CLIA Kits. http://www.diazyme.com/dz-lite-sars-cov-2 (2020). Accessed 27 May 2020.
- 21.Diagnostics S. The world’s first 2019-nCoV (SARS-CoV-2) CLIA Kits received CE mark. http://www.snibe.com/zh_en/en_newsView.aspx?id=576 (2020). Accessed 27 May 2020.
- 22.Deng C-X. The global battle against SARS-CoV-2 and COVID-19. Int J Biol Sci. 2020;16:1676–1677. doi: 10.7150/ijbs.45587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, et al. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020 200230. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed]
- 25.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through post-mortem core biopsies. Mod Pathol. 2020. 10.20944/preprints202003.0311.v1. [DOI] [PMC free article] [PubMed]
- 27.Xu X-W, Wu X-X, Jiang X-G, Xu K-J, Ying L-J, Ma C-L, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. Bmj. 2020;368:m606. doi: 10.20944/preprints202003.0001.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Registry CCT. A randomized, open-label study to evaluate the efficacy and safety of lopinavir-ritonavir in patients with mild novel coronavirus pneumonia (COVID-19). http://www.chictr.org.cn/showprojen.aspx?proj=48991 (2020). Accessed 4 April 2020.
- 29.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulangu S, Dodd LE, Davey RT, Jr, Tshiani Mbaya O, Proschan M, Mukadi D, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NIH. Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with severe coronavirus disease (COVID-19). http://clinicaltrials.gov/ct2/show/NCT04292899 (2020). Accessed 4 April 2020.
- 33.Reuters. Pakistan’s Ferozsons to begin producing COVID-19 drug remdesivir. http://www.reuters.com/article/us-health-coronavirus-pakistan-remdesivi/pakistans-ferozsons-to-begin-producing-covid-19-drug-remdesivir-idUSKBN22R208 (2020). Accessed 27 May 2020.
- 34.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—preliminary report. New England Journal of Medicine. 2020. http://dpi.org/10.1056/NEJMoa2007764. Accessed 8 July 2020. [DOI] [PubMed]
- 35.NIH. Clinical study of Arbidol hydrochloride tablets in the treatment of pneumonia caused by novel coronavirus. http://clinicaltrials.gov/ct2/show/NCT04260594 (2020). Accessed 4 April 2020.
- 36.Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, et al. Favipiravir versus Arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020. 10.1101/2020.03.17.20037432.
- 37.Sheahan TP, Sims AC, Zhou S, Hill C, Leist SR, Schaefer A, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 and multiple endemic, epidemic and bat coronavirus. bioRxiv. 2020. 10.1126/scitranslmed.abb5883.
- 38.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Bioscience Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 39.Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9:231. doi: 10.13140/RG.2.2.35430.37443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020. 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed]
- 41.NIH. Efficacy and Safety of hydroxychloroquine for treatment of pneumonia caused by 2019-nCoV ( HC-nCoV ). http://clinicaltrials.gov/ct2/show/NCT04261517 (2020). Accessed 4 April 2020.
- 42.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. Jama. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 44.Register ECT. Treatment of coronavirus SARS-Cov2 respiratory infections with hydroxychloroquine. http://www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-000890-25 (2020). Accessed 4 April 2020.
- 45.Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, Brar I, Alangaden GJ, Ramesh MS, McKinnon JE, O’Neill W, Zervos M, Nauriyal V, Hamed AA, Nadeem O, Swiderek J, Godfrey A, Jennings J, Gardner-Gray J, Ackerman AM, Lezotte J, Ruhala J, Fadel R, Vahia A, Gudipati S, Parraga T, Shallal A, Maki G, Tariq Z, Suleyman G, Yared N, Herc E, Williams J, Lanfranco OA, Bhargava P, Reyes K. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NIH. The clinical study of carrimycin on treatment patients with COVID-19. http://clinicaltrials.gov/ct2/show/NCT04286503 (2020). Accessed 4 April 2020.
- 47.Caly L D, Druce G, Catton M A, Jans D M, Wagstaff K. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Research. 2020;In press, pre-proof. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed]
- 48.Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Publications; 2020. [DOI] [PMC free article] [PubMed]
- 51.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.NIH. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis SARS CoV-2 infection. http://clinicaltrials.gov/ct2/show/NCT04283461 (2020). Accessed 4 April 2020.
- 53.Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289–1301. doi: 10.1128/jvi.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.NIH. NK Cells Treatment for COVID-19. http://clinicaltrials.gov/ct2/show/NCT04280224 (2020). Accessed 27 May 2020.
- 55.Herold T, Jurinovic V, Arnreich C, Hellmuth JC, von Bergwelt-Baildon M, Klein M, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medRxiv. 2020. 10.1101/2020.04.01.20047381.
- 56.NIH. Tocilizumab for SARS-CoV2 severe pneumonitis. http://clinicaltrials.gov/ct2/show/NCT04315480 (2020). Accessed 4 April 2020.
- 57.NIH. Evaluation of the efficacy and safety of sarilumab in hospitalized patients With COVID-19. http://clinicaltrials.gov/ct2/show/NCT04315298 (2020). Accessed 27 May 2020.
- 58.Keating GM. Bevacizumab: a review of its use in advanced cancer. Drugs. 2014;74:1891–1925. doi: 10.1007/s40265-014-0302-9. [DOI] [PubMed] [Google Scholar]
- 59.Medford ARL, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621–626. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.NIH. Bevacizumab in severe or critical patients with COVID-19 pneumonia (BEST-CP). http://clinicaltrials.gov/ct2/show/NCT04275414 (2020). Accessed 27 May 2020.
- 61.Registry CCT. Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha and ribavirin plus lopinavir/ritonavir plus interferon-alphain in patients with mild to moderate novel coronavirus pneumonia. http://www.chictr.org.cn/showprojen.aspx?proj=48782 (2020). Accessed 4 April 2020.
- 62.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolilekas L, Loverdos K, Giannakaki S, Vlassi L, Levounets A, Zervas E, et al. Can steroids reverse the severe COVID-19 induced ‘cytokine storm’? J Med Virol. 2020. 10.1002/jmv.26165. [DOI] [PMC free article] [PubMed]
- 64.Fadel R, Morrison A, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, et al. Early short course corticosteroids in hospitalized patients with COVID-19. medRxiv. 2020. 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed]
- 65.NIH. The National Institutes of Health COVID-19 Treatment Guidelines Panel provides recommendations for dexamethasone in patients with COVID-19. http://www.covid19treatmentguidelines.nih.gov/dexamethasone/ (2020). Accessed 8 July 2020.
- 66.NIH. Efficacy and safety of IFN-α2β in the treatment of novel coronavirus patients. http://clinicaltrials.gov/ct2/show/NCT04293887 (2020). Accessed 27 May 2020.
- 67.Liu T, Guo F, Zhu X, He X, Xie L. Thalidomide and its analogues: a review of the potential for immunomodulation of fibrosis diseases and opthalmopathy. Exp Ther Med. 2017;14:5251–5257. doi: 10.3892/etm.2017.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haslett PAJ, Hanekom WA, Muller G, Kaplan G. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J Infect Dis. 2003;187:946–955. doi: 10.1086/368126. [DOI] [PubMed] [Google Scholar]
- 69.NIH. The efficacy and safety of thalidomide in the adjuvant treatment of moderate new coronavirus (COVID-19) pneumonia. http://clinicaltrials.gov/ct2/show/NCT04273529 (2020). Accessed 27 May 2020.
- 70.Dastan F, Tabarsi P, Marjani M, Moniri A, Hashemian SM, Tavakoli-Ardakani M, et al. Thalidomide against coronavirus disease 2019 (COVID-19): a medicine with a thousand faces. Iran J Pharm Res. 2020;19:1–2. doi: 10.22037/IJPR.2020.113369.14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCormack PL. Natalizumab: a review of its use in the management of relapsing-remitting multiple sclerosis. Drugs. 2013;73:1463–1481. doi: 10.1007/s40265-013-0102-7. [DOI] [PubMed] [Google Scholar]
- 72.NIH. Fingolimod in COVID-19. http://clinicaltrials.gov/ct2/show/NCT04280588 (2020). Accessed 27 May 2020.
- 73.Registry CCT. The efficacy and safety of favipiravir for novel coronavirus–infected pneumonia: a multicenter, randomized, open, positive, parallel-controlled clinical study. http://www.chictr.org.cn/showprojen.aspx?proj=50137 (2020). Accessed 4 April 2020.
- 74.Registry CCT. Study for the efficacy of chloroquine in patients with novel coronavirus pneumonia (COVID-19). http://www.chictr.org.cn/showprojen.aspx?proj=48968 (2020). Accessed 4 April 2020.
- 75.Registry CCT. A multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID-19). http://www.chictr.org.cn/showprojen.aspx?proj=49409 (2020). Accessed 4 April 2020.
- 76.Registry CCT. Favipiravir combined with tocilizumab in the treatment of novel coronavirus pneumonia (COVID-19)-a multicenter, randomized, controlled trial. http://www.chictr.org.cn/showprojen.aspx?proj=51126 (2020). Accessed 4 april 2020.
- 77.NIH. Dexamethasone for COVID-19 related ARDS: a multicenter, randomized clinical trial. https://clinicaltrials.gov/ct2/show/NCT04395105 (2020). Accessed 8 july 2020.
- 78.NIH. A randomized trial of anticoagulation strategies in COVID-19. https://clinicaltrials.gov/ct2/show/NCT04359277 (2020). Accessed 8 July 2020.
- 79.Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int J Biol Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium. http://epidemio.wiv-isp.be/ID/Documents/Covid19/COVID-19_InterimGuidelines_Treatment_ENG.pdf (2020). Accessed 4 April 2020.
- 81.Nicastri E, Petrosillo N, Bartoli TA, Lepore L, Mondi A, Palmieri F, et al. National Institute for the Infectious Diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Disease Rep. 2020;12:8543. doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marongiu F, Grandone E, Barcellona D. Pulmonary thrombosis in 2019-nCoV pneumonia? J Thromb Haemost. 2020;18:1511–1513. doi: 10.1111/jth.14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tpa) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18:1752–1755. doi: 10.1111/jth.14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.NIH. Protective effect of aspirin on COVID-19 patients (PEAC). http://clinicaltrials.gov/ct2/show/NCT04365309 (2020). Accessed 27 May 2020.
- 86.Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020 1. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed]
- 87.NIH. Anti-MERS-CoV convalescent plasma therapy. http://clinicaltrials.gov/ct2/show/NCT02190799 (2020). Accessed 4 April 2020.
- 88.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020. 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed]
- 89.Administration USFD. Investigational COVID-19 convalescent plasma - emergency INDs. http://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds (2020). Accessed 4 April 2020.
- 90.NIH. Safety, tolerability and immunogenicity of INO-4800 for COVID-19 in healthy volunteers. https://clinicaltrials.gov/ct2/show/NCT04336410 (2020). Accessed 8 July 2020.
- 91.NIH. A study of a candidate COVID-19 vaccine (COV001). http://clinicaltrials.gov/ct2/show/NCT04324606 (2020). Accessed 27 May 2020.
- 92.NIH. Immunity and safety of Covid-19 synthetic minigene vaccine. https://clinicaltrials.gov/ct2/show/NCT04276896 (2020). Accessed 8 July 2020.
- 93.NIH. Safety and immunogenicity study of inactivated vaccine for prophylaxis of SARS CoV-2 infection (COVID-19). https://clinicaltrials.gov/ct2/show/NCT04352608 (2020). Accessed 8 July 2020.
- 94.Registry CCT. A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated novel coronavirus pneumonia vaccine (Vero cells). http://www.chictr.org.cn/showprojen.aspx?proj=52227 (2020). Accessed 8 July 2020.
- 95.NIH. Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) for prophylaxis of SARS-CoV-2 infection (COVID-19). https://clinicaltrials.gov/ct2/show/NCT04283461 (2020). Accessed 8 July 2020.
- 96.NIH. Phase I clinical trial of a COVID-19 vaccine in 18-60 healthy adults (CTCOVID-19). https://clinicaltrials.gov/ct2/show/NCT04313127 (2020). Accessed 8 July 2020.
- 97.Zhu F-C, Li Y-H, Guan X-H, Hou L-H, Wang W-J, Li J-X, Wu SP, Wang BS, Wang Z, Wang L, Jia SY, Jiang HD, Wang L, Jiang T, Hu Y, Gou JB, Xu SB, Xu JJ, Wang XW, Wang W, Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.AReNA CT. Serum Institute of India brings Covid-19 vaccine into animal testing. http://www.clinicaltrialsarena.com/news/serum-institute-india-covid-19-vaccine/ (2020). Accessed 4 April 2020.