Abstract
Objective
Leptospirosis is a zoonotic disease caused by pathogenic spirochetes of Leptospira spp., and peridomiciliary rodents are the most important reservoir animals for human infection. Dogs are known to be the reservoir animal of L. interrogans serovar Canicola, but the importance of dogs in zoonotic transmission of other Leptospira serotypes/genotypes remains unclear. This study reports the isolation of L. interrogans serogroup Autumnalis from two human patients in Japan and describes the genetic comparison between canine and mouse isolates using multiple-locus variable-number tandem repeat analysis (MLVA).
Results
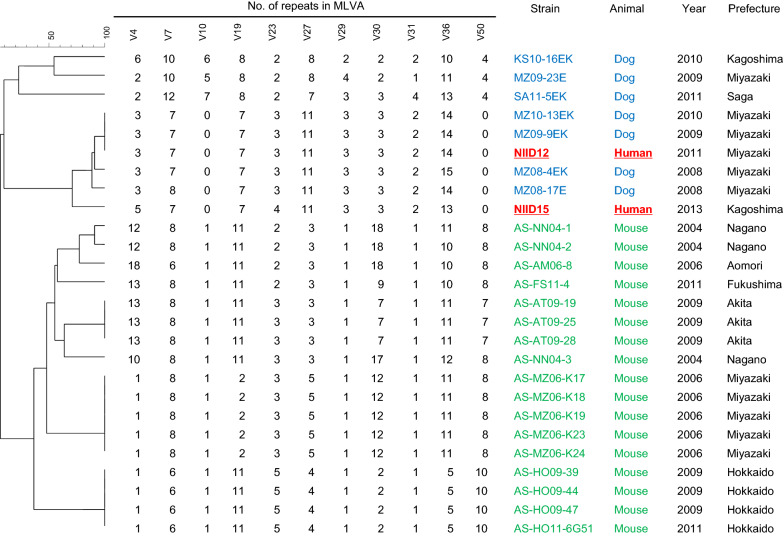
MLVA revealed that 8 out of the 11 loci compared were identical between the two human isolates. The human isolates clustered with the dog but not the mouse isolates. Moreover, the profile of one of the human isolates was identical to that of one of the dog isolates.
Keywords: Dog, Leptospira interrogans, Leptospirosis, Maintenance host, MLVA, Mouse
Introduction
Leptospirosis is a zoonotic disease caused by infection with the pathogenic spirochetes, Leptospira spp. [1, 2]. Human leptospirosis is an acute febrile illness with an extremely broad clinical spectrum ranging from influenza-like illness to severe disease forms characterized by jaundice, bleeding, renal failure, and death [1, 2]. Leptospires colonize the proximal renal tubules of maintenance hosts and are excreted in urine [2, 3]. Leptospirosis in humans is mainly contracted by exposure to water or soil contaminated with the urine of infected animals. Peridomiciliary rodents are important maintenance hosts for human infection [1, 2]. However, for Leptospira interrogans serovar Canicola, dogs serve as the primary maintenance host and pose potential zoonotic transmission to humans [4].
Historically, clinical Leptospira isolates were characterized via serology. There are more than 300 serovars in the genus Leptospira, and antigenically related serovars are classified into serogroups [1]. However, serovar identification is currently extremely difficult, therefore most clinical isolates are characterized at the serogroup level. Therefore, molecular typing has become the main method for the characterization of Leptospira isolates [5]. Multi-locus sequence typing is a highly reliable and reproducible method for the molecular typing of pathogenic Leptospira species [6]. Multiple-locus variable-number tandem repeat analysis (MLVA) has an excellent discrimination power in L. interrogans and its results are concordant with those from serotyping [7, 8]. In our previous studies, we isolated L. interrogans serogroup Autumnalis from large Japanese field mice (Apodemus speciosus) and dogs in Miyazaki, Kagoshima, Saga, Hokkaido, Aomori, Akita, Fukushima, and Nagano Prefectures, Japan [9, 10]. MLVA performed on the mouse and dog isolates demonstrated that they are of different genotypes (MLVA type) [7, 8].
In the present study, we isolated L. interrogans serogroup Autumnalis from two human patients and conducted a genetic comparison between canine and mouse isolates using MLVA.
Main text
Leptospires were isolated from two human patients using blood culture in liquid Korthof’s medium in 2011 (case 1) and 2013 (case 2). The patient in case 1 was a female in her 60 s. She engaged in rice-harvesting in Miyazaki Prefecture 3 weeks before the onset of the disease. At the time of hospitalization, she presented with fever (40.1 °C), vomiting, diarrhea, conjunctival suffusion, and jaundice. Her laboratory tests revealed thrombocytopenia (7.0 × 104/μL) and elevated total bilirubin (1.3 mg/dL) and serum creatinine (0.9 mg/dL) levels. Piperacillin sodium was administered to the patient for treatment. Blood was inoculated into liquid Korthof’s medium 2 days after the disease onset and Leptospira sp. was isolated 20 days after the inoculation. The patient in case 2 was a schoolboy in his 10 s. He had undertaken a recreational activity in a river in Kagoshima Prefecture adjoining west of Miyazaki Prefecture 10 days prior to the disease onset. He presented with fever (39.9 °C), headache, and myalgia at the time of hospitalization. His laboratory tests revealed proteinuria and thrombocytopenia (9.5 × 104/μL). Ceftriaxone was administered. Heparinized blood collected 3 days after the disease onset was transferred to our institute, where the blood was inoculated into liquid Korthof’s medium. Leptospira sp. was isolated 17 days after the inoculation.
Leptospiral DNA was extracted using DNeasy Blood & Tissue Kits (Qiagen, Hilden, Germany) and subjected to PCR targeting the flagellar gene flaB of pathogenic leptospires, followed by DNA sequencing for species identification [9]. The flaB sequences of the isolates, NIID12 for case 1 and NIID15 for case 2, were identical to each other and the isolates were identified as L. interrogans (DDBJ accession numbers: LC521310 for NIID12 and LC521311 for NIID15). The serogroup of both isolates was identified as Autumnalis using the microscopic agglutination test and a panel of 18 antisera [9]. To investigate the genetic relatedness among human, murine, and canine isolates of L. interrogans serogroup Autumnalis, MLVA was performed for 11 loci [7] on the two human isolates, and their profiles were compared with those of the murine and canine isolates. The sizes of the amplicons were converted to repeat copy numbers for analysis using a categorical multi-state coefficient and unweighted pair group method with arithmetic averages (UPGMA) as a clustering algorithm with BioNumerics software version 7.6 (Applied Maths, Belgium). MLVA showed that 8 out of the 11 loci were identical between the two isolates. Interestingly, the human isolates clustered with the dog but not mouse isolates. Moreover, the profile of NIID12 was identical to that of one of the dog isolates (Fig. 1). The genetic diversity among Leptospira serogroups is variable, and the host animal is suggested to be an important factor in Leptospira diversification [8, 11]. Genetic diversity in L. interrogans serogroup Autumnalis was observed among canine isolates, which could explain the genetic difference observed between the human isolates. The medical research ethics committee of the National Institute of Infectious Diseases for the use of human subjects exempts their reviews for the characterization of leptospiral isolates obtained by laboratory diagnosis when requested formally by prefectural governments.
Fig. 1.
A dendrogram based on multiple-locus variable-number tandem repeat analysis (MLVA) using 11 loci showing relationships among human, dog, and mouse isolates of L. interrogans serogroup Autumnalis. Profiles of the copy numbers of the repeats of the 11 loci were analyzed using a categorical multi-state coefficient and unweighted pair group method with arithmetic averages (UPGMA) as a clustering algorithm with BioNumerics software version 7.6 (Applied Maths, Belgium)
This study revealed that human isolates are genetically identical or similar to canine but not mouse isolates (Fig. 1). Although peridomiciliary rodents are the most important reservoir animals for human infection and we had thought they were the most possible infection source of L. interrogans serogroup Autumnalis for humans in Japan, this study demonstrated that other animals than mouse may constitute the reservoir animal for this genotype of L. interrogans serogroup Autumnalis. L. interrogans has been isolated from brown rats and raccoons in Japan, but this genotype strain has never been isolated from these animals [8, 12]. L. interrogans flaB sequences have been detected in wild boars throughout Japan [13]. One of the flaB sequences (691 bp) detected in wild boar was identical to those of L. interrogans serogroup Autumnalis human isolates in this study, although that particular flaB sequence has been detected in different serogroups of L. interrogans.
Dogs are known to be the reservoir animal of L. interrogans serovar Canicola [4]. There are several reports of possible dog-to-human transmission of L. interrogans serovar Canicola, indicating the important role of dogs in zoonotic transmission of leptospirosis [14–18]. However, the widespread use of bivalent vaccines containing serovar Canicola is likely to decrease the circulation of serovar Canicola among dogs in the U.S., Europe, and Japan [7, 19, 20]. Other Leptospira species and serovars have been detected in asymptomatic dogs, but it remains unclear whether these dogs serve as the maintenance host of other genotypes/serotypes and facilitate zoonotic transmission to humans [14, 15]. It is possible that both humans and dogs are incidental hosts, and other animals, such as wild boars, maintain this genotype of L. interrogans serogroup Autumnalis. However, the identical or similar MLVA profile in human and canine isolates may suggest that dogs are the reservoir animal of this genotype of L. interrogans serogroup Autumnalis and a source for human infection. The canine isolates described in this study were obtained from symptomatic dogs [9]. L. interrogans serovar Canicola causes acute and subacute hepatic and renal failure in dogs, and the dogs become asymptomatic carriers of this serovar strain after recovering from acute infection [21]. Although we have not investigated the carrier status of this genotype of L. interrogans serogroup Autumnalis among asymptomatic dogs, as with serovar Canicola infection, infected dogs would be chronic carriers of this genotype strain after recovery.
Limitations
The study was conducted on only two human isolates. We did not investigate the carrier status of this genotype of L. interrogans serogroup Autumnalis among asymptomatic dogs. We did not obtain L. interrogans isolate from wild boars and its serological and detailed genetic characteristics remain unknown. Therefore, we cannot conclude on which animal(s) is the reservoir animal of this genotype of L. interrogans serogroup Autumnalis.
Acknowledgements
None.
Abbreviations
- MLVA
Multiple-locus variable-number tandem repeat analysis
- UPGMA
Unweighted pair group method using arithmetic averages
Authors’ contributions
Conceptualization: NK; data curation: NK and HI; funding acquisition: NK; investigation: NK; methodology: NK and HI; resources: NK; supervision: MO; visualization: NK and HI; writing—original draft: NK; writing—review and editing: NK, HI and MO. All authors read and approved the final manuscript.
Funding
Funding was provided by the Research Program on Emerging and Re-emerging Infectious Diseases (19fk0108049j1903 and 20fk0108139j0701) from the Japan Agency for Medical Research and development, AMED.
Availability of data and materials
The flaB sequences have been deposited in a public database (DDBJ accession numbers LC521310 and LC521311).
Ethics approval and consent to participate
The medical research ethics committee of the National Institute of Infectious Diseases for the use of human subjects exempts their reviews for the characterization of leptospiral isolates obtained by laboratory diagnosis when requested formally by prefectural governments and information that has already been anonymized and cannot identify individuals. We used MLVA data from previously published papers to create Fig. 1.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nobuo Koizumi, Email: nkoizumi@niid.go.jp.
Hidemasa Izumiya, Email: izumiya@niid.go.jp.
Makoto Ohnishi, Email: ohnishi7@niid.go.jp.
References
- 1.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;12:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. 2. Melbourne: MediSci; 1999. [Google Scholar]
- 4.Sykes JE, Hartmann K, Lunn KF, Moore GE, Stoddard RA, et al. 2010 ACVIM small animal consensus statement on leptospirosis: diagnosis, epidemiology, treatment, and prevention. J Vet Intern Med. 2011;25:1–13. doi: 10.1111/j.1939-1676.2010.0654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerqueira GM, Picardeau M. A century of Leptospira strain typing. Infect Gen Evol. 2009;9:760–768. doi: 10.1016/j.meegid.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Boonsilp S, Thaipadungpanit J, Amornchai P, Wuthiekanun V, Bailey MS, et al. A single multilocus sequence typing (MLST) scheme for seven pathogenic Leptospira species. PLoS Negl Trop Dis. 2013;7:e1954. doi: 10.1371/journal.pntd.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koizumi N, Muto MM, Izumiya H, Suzuki M, Ohnishi M. Multiple-locus variable-number tandem repeat analysis and clinical characterization of Leptospira interrogans canine isolates. J Med Microbiol. 2015;64:288–294. doi: 10.1099/jmm.0.000027. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi N, Izumiya H, Mu JJ, Arent Z, Okano S, et al. Multiple-locus variable-number tandem repeat analysis of Leptospira interrogans and Leptospira borgpetersenii isolated from small feral and wild mammals in East Asia. Infect Gen Evol. 2015;36:434–440. doi: 10.1016/j.meegid.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi N, Muto M, Yamamoto S, Baba Y, Kudo M, et al. Investigation of reservoir animals of Leptospira in the northern part of Miyazaki Prefecture. Jpn J Infect Dis. 2008;61:465–468. [PubMed] [Google Scholar]
- 10.Koizumi N, Muto MM, Akachi S, Okano S, Yamamoto S, et al. Molecular and serological investigation of Leptospira and leptospirosis in dogs in Japan. J Med Microbiol. 2013;62:630–636. doi: 10.1099/jmm.0.050039-0. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich M, Wilkinson DA, Soarimalala V, Goodman SM, Dellagi K, et al. Diversification of an emerging pathogen in a biodiversity hotspot: Leptospira in endemic small mammals of Madagascar. Mol Ecol. 2014;23:2783–2796. doi: 10.1111/mec.12777. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi N, Uchida M, Makino T, Taguri T, Kuroki T, Muto M, Kato Y, Watanabe H. Isolation and characterization of Leptospira spp. from raccoons in Japan. J Vet Med Sci. 2009;2009(71):425–429. doi: 10.1292/jvms.71.425. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi N, Muto M, Yamada A, Watanabe H. Prevalence of Leptospira spp. in the kidneys of wild boars and deer in Japan. J Vet Med Sci. 2009;2009(71):797–799. doi: 10.1292/jvms.71.797. [DOI] [PubMed] [Google Scholar]
- 14.Suepaul SM, Carrington CVF, Campbell M, Borde G, Adesiyun AA. Serovars of Leptospira isolated from dogs and rodents. Epidemiol Infect. 2010;138:1059–1070. doi: 10.1017/S0950268809990902. [DOI] [PubMed] [Google Scholar]
- 15.Llewellyn JR, Krupka-Dyachenko I, Rettinger AL, Dyachenko V, Stamm I, et al. Urinary shedding of leptospires and presence of Leptospira antibodies in healthy dogs from Upper Bavaria. Berl Munch Tierarztl Wochenschr. 2016;129:251–257. [PubMed] [Google Scholar]
- 16.Misao T, Hiroyoshi S, Katsuta K, Nishihara Y, Kobayashi Y, et al. Canicola fever in Japan. Am J Hyg. 1956;63:294–307. doi: 10.1093/oxfordjournals.aje.a119813. [DOI] [PubMed] [Google Scholar]
- 17.Ward MK, McDaniel MB, Tatum HW, Starr LE, Williams HR. An epidemic of canicola fever in man with the demonstration of Leptospira canicola infection in dogs, swine and cattle. II. Laboratory studies. Am J Hyg. 1956;64:59–69. doi: 10.1093/oxfordjournals.aje.a119824. [DOI] [PubMed] [Google Scholar]
- 18.Barkin RM, Glosser JW. Leptospirosis–an epidemic in children. Am J Epidemiol. 1973;98:184–191. doi: 10.1093/oxfordjournals.aje.a121547. [DOI] [PubMed] [Google Scholar]
- 19.Ellis WA. Control of canine leptospirosis in Europe: time for a change? Vet Rec. 2010;167:602–605. doi: 10.1136/vr.c4965. [DOI] [PubMed] [Google Scholar]
- 20.Gautam R, Wu C, Guptill LF, Potter A, George E, et al. Detection of antibodies against Leptospira serovars via microscopic agglutination tests in dogs in the United States, 2000–2007. J Am Vet Med Assoc. 2010;237:293–298. doi: 10.2460/javma.237.3.293. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein RE. Canine leptospirosis. Vet Clin North Am Small Anim Pract. 2010;40:1091–1101. doi: 10.1016/j.cvsm.2010.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The flaB sequences have been deposited in a public database (DDBJ accession numbers LC521310 and LC521311).