Abstract
Epithelia lining the respiratory tract represent a major portal of entry for microorganisms and allergens and are equipped with innate and adaptive immune signaling receptors for host protection. These include Toll-like receptors (TLRs) that recognize microbial components and evoke diverse responses in cells of the respiratory system. TLR stimulation by microorganism-derived molecules activates antigen presenting cells, control T helper (Th) 1, Th2, and Th17 immune cell differentiation, cytokine production by mast cells, and activation of eosinophils. It is clear that TLR are involved in the pathophysiology of allergic airway diseases such as asthma. Dendritic cells (DCs), a kind of antigen presenting cells, which play a key role in the induction of allergic airway inflammation, are privileged targets for pathogen associated molecular patterns (PAMPs). During the allergic responses, engagement of TLRs on DCs determines the Th2 polarization of the T cells. TLR signaling in mast cells increases the release of IL-5, and TLR activation of airway epithelial cells forces the generation of proallergic Th2 type of cytokines. Although these responses aim to protect the host, they may also result in inflammatory tissue damage in the airway. Under certain conditions, stimulation of TLRs, in particular, TLR9, may reduce Th2-dependent allergic inflammation by induction of Th1 responses. Therefore, understanding the complex regulatory roles of TLRs in the pathogenesis of allergic airway inflammation should facilitate the development of preventive and therapeutic measures for asthmatic patients.
Keywords: Allergic airway inflammation, Host response, TLRs
1. Introduction
Toll-like receptors (TLRs) are pattern recognition receptors crucial for innate and adaptive immune responses to pathogens [1]. In the airway, TLRs are expressed in a wide variety of cells including epithelial cells, macrophages, mast cells, eosinophils and dendritic cells (DCs). Pathogens entering the airway encounter these cells, and then are recognized by TLRs. The activation of the signaling cascades coupled to TLRs in DCs induces costimulatory molecule expression and cytokine secretion by these cells and also initiate the development of specific immune responses aimed at eliminating the pathogens [2]. The essence of the hygiene hypothesis is that the increased incidence of allergic diseases in developed countries may have been caused by decreased incidence of contacting pathogens and their components during childhood [3,4]. The exposure to pathogens during bacterial and viral infections normally drives the maturation of the immune system in infancy and childhood toward a T helper (Th) 1 phenotype; thus deviating away from a Th2 phenotype, which is associated with allergic diseases. It has been reported that polymorphisms in CD14, a TLR4 associated protein, and in TLR2 are correlated with dysfunctional increased risk of allergic diseases [3–5]. In addition, bacterial and viral infections in the airway provide diverse ligands for TLRs and thereby exacerbate airway inflammation in asthmatic patients. Therefore, the cells expressing TLRs not only detect invading pathogens, but also play an important role in the development of allergic diseases in human. The aim of this review is to summarize the current knowledge about the role of TLRs in allergic airway inflammation.
2. Overview of TLRs
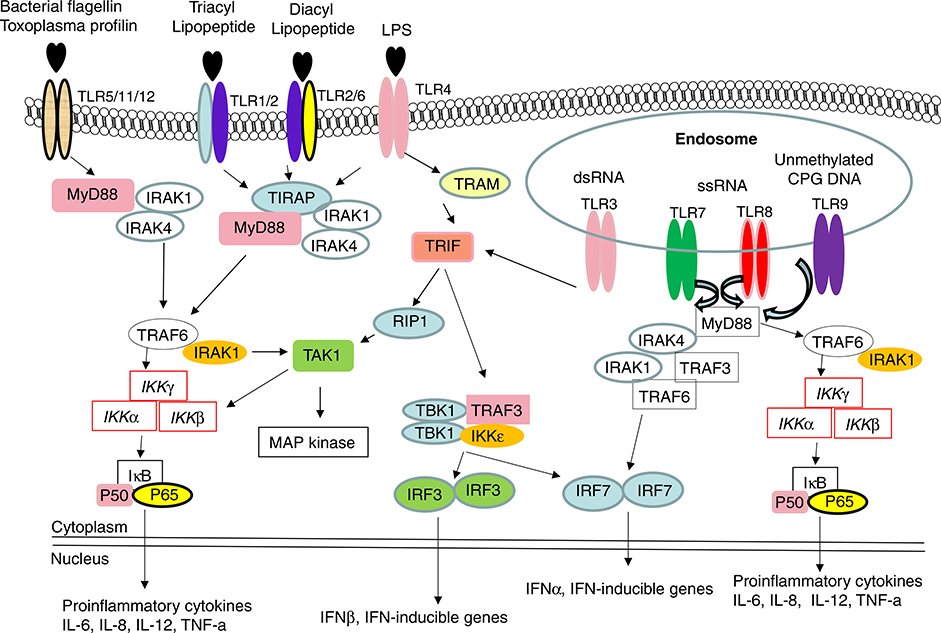
Human and mouse TLRs consist of a large family with at least 11 members. TLRs 1–9 are observed in human and mouse, while TLR10 is functional only in human. In mouse the C-terminal half of Tlr10 gene is substituted by a non-related sequence, therefore mouse TLR10 is non-functional. In contrast, mouse TLR11 is functional and can be activated by uropathogenic bacteria, whereas the presence of a stop codon in human TLR11 gene, results in lack of its translation [6]. TLRs, TLR ligands, and TLR signaling pathways were shown in Fig. 1.
Fig. 1.
The TLR signaling pathway and downstream effector molecules. TLRs localized to different subcellular compartments according to the molecular nature of the relevant ligands. TLR3, TLR7/TLR8 and TLR9 are located at endolysosomal compartment and recognized dsRNA or ssRNA or unmethylated CpG DNA, respectively. In contrast, TLR2, TLR4, TLR5, and TLR11 are mainly displayed on plasma membrane and recognize indicated ligands. TLR2/1 heterodimers respond to triacyl lipopeptide, while TLR2/6 heterodimers recognize diacyl lipopeptide. Following ligation with their respective ligands, TLRs recruit downstream adaptor molecules, such as MyD88 (all TLRs except TLR3) and TRIF (TLR3 and TLR4), to activate IRAK4, TRAF6 and IKKε/TBK1. MyD88: Myeloid differentiation primary-response protein 88, IRAK: IL-1R-associated kinase, TRAM: TRIF-related adaptor molecules, TIRAP: TIR domain containing adaptor protein, TRAF: TNF receptor-associated factor, TAK: TGF-activated kinase, IKK: inhibitor of kappa light polypeptide gene enhancer in B-cells kinase.
TLR2 is a major receptor to recognize Gram-positive cell-wall components, including peptidoglycan and lipoteichoic acid, as well as mycobacterial cell-wall components such as lipoarabinomannan and mycolylarabinogalactan, and yeast cell-wall zymosan. In vitro, TLR2-deficient macrophages produced reduced tumor necrosis factor and interleukin-6 in response to Staphylococcus aureus when compared to wild-type macrophages [7,8].
TLR4 is crucial for effective host cell responses to Gram-negative bacterial lipopolysaccharide (LPS). Delivery of LPS to TLR4 requires the accessory proteins LBP (LPS-binding protein found in serum), CD14 and MD-2 (the latter two proteins either exist in soluble form or bound to cell membrane or to TLR itself). There are additional cell-surface molecules, such as the integrin CD11b/CD18 that may facilitate cellular responses to LPS. TLR4 is also involved in host responses to pneumolysin, a major virulence factor of Streptococcus pneumoniae, and proteins derived from respiratory syncytial virus [6].
There are four TLRs that are intracellularly situated and can be stimulated by nucleic acids during viral infections to initiate antiviral immunity, including interferon production. TLR3 recognize double-stranded RNA and West Nile virus, TLR7 and TLR8 can be stimulated by antiviral derivatives such as imidazoquinoline and ixoribine, and guanosine- and uridine-rich single-stranded RNA oligonucleotides derived from human immunodeficiency virus-1. DNA from bacteria has stimulatory effects on mammalian immune cells, which depend on the presence of unmethylated CpG dinucleotides in the bacterial DNA. In contrast, mammalian DNA has a very low frequency of CpG dinucleotides, and these are mostly methylated. CpG DNA induces a strong Th1-like inflammatory response, while mammalian DNA does not have immunostimulatory activity. The cellular response to CpG DNA is mediated by TLR9 [9].
TLR11 is expressed by bladder epithelial cells and mediates resistance to infection by uropathogenic bacteria in mouse [10]. It also reported that a profilin-like molecule from the protozoan parasite Toxoplasma gondii activate DCs through TLR11 and to generate a potent interleukin-12 (IL-12) response in mice [11].
In addition to detecting pathogen-derived ligands, TLRs interact with host molecules. There are about four groups of putative endogenous ligands for TLR reported [9]. 1). Several inflammatory proteins or peptides were reported to signal through TLRs. For example, heatshock protein (Hsp)60 family chaperones were suggested as ligands for TLR2 and/or TLR4 in macrophages and B cells. High mobility group [dummy_chk]box 1 (HMGB1) is released extracellularly during acute inflammatory responses. It was reported that stimulation of neutrophils, monocytes, or macrophages by HMGB1 required both TLR2 and TLR4 resulting in increased nuclear translocation of NF-kB and enhanced expression of proinflammatory cytokines. Murine b-defensin 2 acted directly on immature dendritic cells as an endogenous ligand for TLR4, inducing up-regulation of costimulatory molecules and dendritic cell maturation. 2). Some collectin molecules may signal through TLRs. For instance, the collectin surfactant protein-A is involved in innate host defense and the regulation of inflammatory processes in the lung. Surfactant protein-A-induced activation of the NF-kB signaling pathway and up-regulation of cytokine synthesis were reported to be critically dependent on the TLR4 functional complex. 3). Nucleic acids of mammalian origin may act as endogenous ligands for TLRs and may promote systemic lupus erythematosus. Such as mammalian DNA and RNA, when complexed with autoantibodies, are potent self-antigens for TLR9 and TLR7, respectively, and induce IFN-a production by plasmacytoid predendritic cells. 4). The breakdown species of glycosaminoglycans. Oligosaccharides of hyaluronan (HA) activated dendritic cells and endothelial cells via TLR4 [12].
It has been realized that TLR signaling pathways consist, at least, of a MyD88-dependent pathway and a MyD88-independent pathway that is selective to TLR3 and TLR4 [13]. Upon ligand binding to TLR, the adaptor molecule MyD88 is recruited to TLR complex as a dimer. Binding of MyD88 promotes association with interleukin-1 receptor associated kinase 4 (IRAK4) and IRAK-1. Tumor necrosis factor-associated factor 6 (TRAF6) is recruited to IRAK-1. The complex IRAK-4/IRAK-1/TRAF6 dissociates from the receptor and then interacts with another complex consisting of transforming growth factor-b-activated kinase (TAK1), TAK1-binding protein 1 (TAB1), and TAB2. TAK1 is subsequently activated in the cytoplasm, leading to the activation of IkB kinase kinases (IKKs). IKK activation leads to phosphorylation and degradation of IkB, and consequent release of NF-kB. Once translocated into the nucleus, NF-kB activates the expression of inflammatory chemokines and cytokines. TRIF-dependent pathway needs TIRAP (TIR-domain containing adaptor protein), TRIF (TIR-domain containing adaptor protein inducing interferon-b), and TRAM (TRIF-related adaptor molecule) to mediate MyD88-independent induction of interferon-β, which in turn activates the expression of interferon-inducible genes such as CXCL10 [9,14].
3. The role for TLRs on DCs in determining the outcome of allergen encounter in the lung
Asthma is a disease of chronic airway inflammation characterized by reversible obstruction, airway hyperresponsiveness (AHR), infiltration of eosinophils and T-helper type 2 (Th2) cells into the submucosal region, mucus hypersecretion, and remodeling of the airway. It has been well established that DCs are crucial in determining the outcome of allergen encounter in the lung. Studies of DCs in the lung and their surface receptors have provided a framework for the discovery of novel anti-allergic compounds.
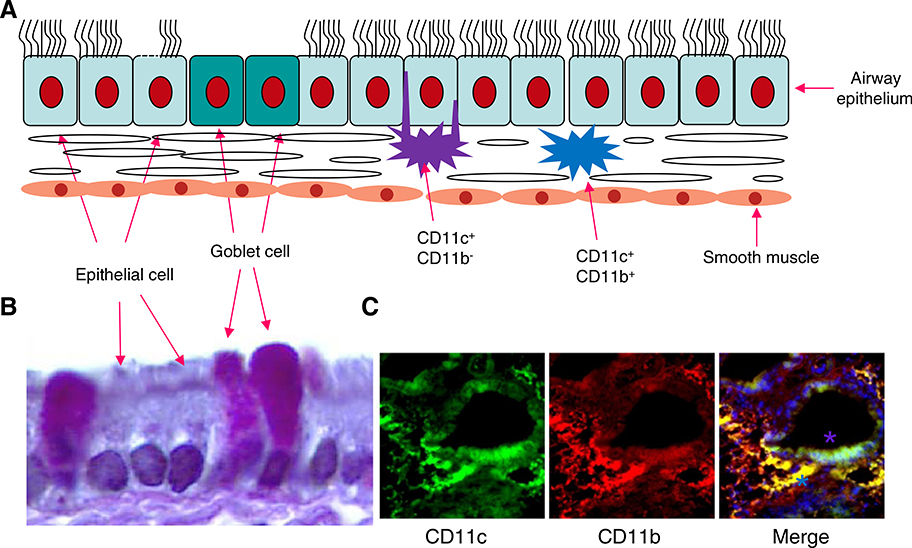
Immature (i)DCs are distributed throughout the lung and are at the focal control point determining the induction of pulmonary immunity or tolerance. Airway DCs are located immediately above and beneath the basement membrane of respiratory epithelium [15,16] (Fig. 2). They constitute a dense network in the lung ideally placed to sample inhaled antigens, by forming tight junctions with airway epithelial cells and extending their dendrites into the airway lumen. Antigens inhaled into the upper airways deposit on the epithelial linings of the trachea and bronchi and even in the deep alveolar compartment, where, under normal conditions, they are cleared by mucociliary transport or phagocytosed by alveolar macrophages that neutralize antigens, despite their inherent pathogenic properties. Inhaled pathogens trigger pattern recognition receptors of the innate immune system such as TLRs on epithelial cells and macrophages that subsequently produce chemokines to recruit neutrophils into the airway to eliminate inhaled pathogens, thus localizing infection. Inhaled allergens are also recognized by iDCs beneath the airway epithelial cell layer. These iDCs have the potential to take up, process and present antigens in the cleft of major histocompatibility complex (MHC) I and MHCII molecules to T cell receptors. When an antigen is encountered, the simultaneous triggering of pattern recognition receptors on the surface or within the vacuoles (endosomes) of DCs leads to their increased expression of chemokine receptors such as CCR7 that direct the trafficking of DCs into the T-cell zone of the regional draining lymph nodes in responses to chemokine ligands. In the T-cell zone, mature (m)DCs present antigenic peptides of the inhaled allergens to naive CD4 T cells and induce Th1- or Th2-cell differentiation.
Fig. 2.
Distribution of DCs in the lung. A: CD11c+CD11b− lung DCs have the propensity of extending dendrites into the airway lumen through formation of tight junctions with bronchial epithelial cells. Immediately below, the lamina propria of the conducting airways contains CD11c+CD11b+ DCs which are a rich source of proinflammatory chemokines that induce immune cell accumulation. B: Mouse airway epithelial cells and increased number of goblet cells during OV-induced allergic responses. C: CD11c+CD11b+ DCs in the lamina propria and CD11c+CD11b− DCs in the junction with bronchial epithelial cells (Green: CD11c+; Red: CD11b+; Blue: DAPI staining to show nuclei).
It is now clear that in mice at least five different subsets of DCs can be found in the lung (Table 1). The mouse lung is grossly divided into large conducting airways and interstitium containing alveolar septa and capillaries where gas exchange takes place. In steady-state conditions, the conducting airways of all species studied are lined with an intraepithelial highly dendritic network of MHCIIhiCD11chi cells that are mostly CD11b− and at least in the mouse and rat express langerin and the mucosal integrin CD103 (aEb7). In addition they have the propensity of extending dendrites into the airway lumen through formation of tight junctions with bronchial epithelial cells. Immediately below the epithelium, the lamina propria of the conducting airways contain MHCIIhiCD11chi cells that highly express CD11b and are a rich source of proinflammatory chemokines. The CD11b+CD103− subset also expresses the SIRPα molecule, a binding partner of CD47 involved in DC migration. Lung interstitial DCs can be further divided into CD11b+ and CD11b− subsets. Since both CD11b+ and CD11b− subsets express high levels of CD11c, they can best be recognized as myeloid DCs, to distinguish this DC type from another population of CD11cint plasmacytoid DCs (pDCs) that express Siglec-H and the bone-marrow stromal antigen-1 as well as some markers shared with granulocytes and B cells. The exact anatomical location of lung pDCs is unclear although the cells are found to line alveolar septa in situ and have been recovered from digests of large conducting airways. Under inflammatory conditions, such as viral infection, allergen challenge, or LPS administration, there is recruitment of additional subsets of CD11b+ monocyte-derived DCs with rapidly upregulated CD11c and retention of Ly6C as a remnant of their monocytic descent, and thus they can be easily confused with resident CD11b+ myeloid DCs [17]. In normal human lung parenchyma, two subsets of mDCs (CD11c+/BDCA-1+ and CD11c+/BDCA-3+) and one pDC subset (CD11c−/BDCA-2+) have been identified [18].
Table 1.
| Resident DC | CD11b+CD11c+SIRPα+ |
| CD11b−CD11c+Langerin+CD103+ | |
| Interferon-producing, killer DC | CD11CdimB220+SiglecH+CD19−CD3−NK 1.1+ |
| Plasmacytoid DC | CD11b−CD11cdimL-selectin+Ly6C+SiglecH+ |
| Inflammatory DC | CD11b+CD11c+Ly6C+SIRPα+ |
DCs express different TLRs depending on their subsets [19] (Table 2). iDCs in the peripheral tissues recognize pathogens through TLRs, followed by increased expression of cell-surface costimulatory molecules (CD80 and CD86) and major histocompatibility class (MHC) II molecules. Concomitantly, captured pathogens or allergens are processed and presented to T cells as antigen-MHC class II complexes. The induction of CD80/86 on DCs by activation of TLRs results in the efficient activation of T cells specific for pathogens or allergens.
Table 2.
TLR expression by human DC subsets [8].
| Freshly isolated myeloid DCs | Freshly isolated plasmacytoid DCs | Monocyte-derived DC (in vitro differentiated with IL-4 + GM-CSF) | |
|---|---|---|---|
| TLR1 | ++ | + | ++ |
| TLR2 | ++ | − | ++ |
| TLR3 | ++ | − | ++ |
| TLR4 | − | − | ++ |
| TLR5 | + | − | +/− |
| TLR6 | ++ | ++ | ++ |
| TLR7 | +/− | ++ | − |
| TLR8 | ++ | − | ++ |
| TLR9 | − | ++ | − |
| TLR10 | + | + | ND |
| TLR11 | ND | ND | ND |
ND = not detected.
TLR stimulation also induces the expression of cytokines, such as IL-12, chemokines, and their receptors in DCs. Depending on their cytokine-secreting profile, DCs are able to skew the immune responses. IL-12p70 producing DCs drive T cells towards a Th 1 type response [20]. However, different TLRs or even different ligands for the same TLR can differ substantially in their ability to induce the production of heterodimeric IL-12 p70. For example, CpG is a particularly potent IL-12 p70 inducer for TLR9-expressing DCs, whereas Escherichia coli LPS is a much weaker inducer [21]. Atypical LPS from Porphyromonas gingivalis, a ligand for TLR2, does not induce IL-12 p70 production by mouse DCs [22]. Similarly, peptidoglycan (PGN), another TLR2 ligand, was able to induce IL-12 p40 but not IL-12 p70 in human monocyte-derived DCs (Mo-DCs) [23]. However, TLR2 ligands contained in mycobacterial fractions induce high levels of IL-12 p70 production by DCs when combined with CD40 ligand [24]. Human DCs matured with Poly I:C, a ligand for TLR3 or R848, a ligand for TLR7/8, are able to produce high levels of IL-12 p70, but with a reduced migratory capacity. The addition of prostaglandin E2 (PGE2) improved the migratory capacity of TLR-ligand matured Mo-DCs while maintaining their IL-12 p70 production [25]. Interestingly, LPS plays a dual role in skewing DC responses: in a mouse model of airway sensitization, low levels of inhaled LPS that stimulate TLR4 are necessary to induce Th2 responses to the antigen. Furthermore, DCs are involved in TLR4-induced Th2 response since DC maturation and their migration into the draining lymph nodes are diminished in TLR4 deficient mice. In contrast, inhalation of high levels of LPS with antigen results in a Th1 response, suggesting that the level of LPS exposure determines the type of allergic inflammation. Consistent with these observations, bone marrow-derived DCs produce IL-12 in response to high, but not low, doses of LPS in vitro [26]. Therefore, TLR signaling in DCs is critical for determining the Th1/Th2 balance. The role of TLRs in the polarization of DCs toward Th1 or Th2 is shown in Table 3.
Table 3.
| Th1 |
Th2 |
||
|---|---|---|---|
| TLRs | Ligands | TLRs | Ligands |
| TLR3 | dsRNA | TLR2 | LPS from P. gengivalis |
| TLR4 | LPS from E. coli | TLR2/6 | PGN |
| TLR5 | Flagellin | TLR2/6 | Zymosan |
| TLR7 | R848 | TLR2/1 | Pam3Cys |
| TLR9 | CPG | ||
Effector CD4 T cells are divided into several functional subpopulations including Th1, Th2, and T regulatory cells (Treg) cells. In addition, Th17 cells belong to a distinct proinflammatory T-cell lineage, which produces IL-17A, a cytokine that induces the production of a chemokine CXCL8 (IL-8) for recruiting neutrophils to inflamed sites [27–29]. Transforming growth factor (TGF)-β combined with IL-6 and RORγt expression induce Th17 differentiation from naive CD4 T cells [30]. In addition to TGF-β, Th17 cell differentiation also requires a soluble DC factor elicited by TLR4 and MyD88-dependent signals [31]. Furthermore, TLR3 and TLR9 activations also effectively induce DC factors (soluble, MyD88 dependent, and induced by TLR ligation) that act in concert with TGF-β to differentiate Th17 cells [31]. An analysis of the DC-derived factors led to the identification of IL-6 as a critical co-factor. IL-6- and TGF-β-induced Th17 cells are critically involved in the pathogenesis of autoimmune diseases [32]. Since IL-17A mRNA level in the sputum is significantly elevated in asthmatic patients, it is possible that a Th17 response may also enhance the development of allergic airway inflammation [33]. However, in another observation, exogenous IL-17 reduces the severity of established airway inflammation in ovalbumin (OVA)-sensitized mice [34]. It appears that IL-17 activates DCs and promotes antigen uptake, leading to reduced T-cell activation and reduced production of IL-4, IL-5, and IL-13 in the airway, thus preventing Th2 allergic response.
4. The contribution of TLRs expressed by epithelial cells, eosinophils and mast cells to airway inflammation
In addition to DCs, airway epithelial cells, eosinophils and mast cells also express a variety of TLRs and contribute to airway inflammation including asthma. TLRs expressed by these cell types are shown in Table 4.
Table 4.
TLR expression by human airway cell populations [2].
| Airway epithelial cells | Eosinophils | Mast cells | |
|---|---|---|---|
| TLR1 | + | + | + |
| TLR2 | ++ | ++ | + |
| TLR3 | ++ | − | + |
| TLR4 | ++ | + | + |
| TLR5 | ++ | + | + |
| TLR6 | ++ | + | + |
| TLR7 | − | + | + |
| TLR8 | − | − | − |
| TLR9 | + | + | + |
| TLR10 | − | − | + |
4.1. The role of TLRs expressed in respiratory epithelial cells
Airway epithelial cells lie at the interface between the host and the environment and are the first line of defense against dust, microorganisms, gases, and other allergens. Recent studies have revealed that TLR1 through TLR6 and TLR9 are expressed on human airway epithelial cells (Table 4). It has also been reported that all TLRs with the exception of TLR8 were found in a human airway epithelial cell line, BEAS-2B, and all ten TLRs were found to be expressed in primary bronchial epithelial cells (PBEC). However, the levels of the expression of each TLR are different in the airway epithelial cells. For instance, TLR8 and TLR10 mRNA were present, but at low levels. The most highly expressed TLRs were TLR2–5, with TLR7, TLR8 and TLR10 at the lowest level [35].
Viral infection is known to cause exacerbation of asthma in patients suffering from chronic airway inflammation or airway hyperreactivity. Among the viruses that infect the airway, respiratory syncytial virus (RSV), influenza virus, and parainfluenza virus are pathogens that exacerbate asthma. These are RNA viruses and synthesize double stranded (ds)RNA during replication in infected cells. For example, rhinoviruses (RV) are the major cause of the common cold and also cause acute exacerbation of asthma and chronic obstructive pulmonary disease. RV replication increases the expression of both TLR3 mRNA and protein in the BEAS-2B airway epithelia cell line. Blocking TLR3 results in a decrease in the production of IL-6, CXCL8 (IL-8), and CCL5 by the cells in response to poly(IC), while these cytokines are increased when the cells are infected by RV, suggesting that TLR3 in airway epithelial cells mediates host response against live virus infection [36]. Recently, it has been demonstrated that TLR3 and melanoma differentiation-associated gene (MDA)-5, but not RIG-I, are required for maximal sensing of RV dsRNA and that TLR3 and MDA-5 signal through a common downstream signaling intermediate, IRF3 [37].
Thymic stromal lymphoprotein (TSLP) is an IL-7-like cytokine and is expressed mainly in epithelial cells [38,39]. Activation of TLR3 or Th2 cytokines induce TSLP production by airway epithelial cells. [40]. TSLP activates iDCs to produce chemokines CCL24/26 (eotaxin-2/3) and CCL17 (TARC) that attract eosinophils and Th2 T cells, respectively. TSLP-activated DCs also express increased levels of OX40L, which preferentially triggers the differentiation of allergen specific naive CD4 T cells into Th2 cells [40]. These observations indicate that while TLR3 plays a crucial role in the immune responses against viral infection in the airway, TLR3 activation also contributes through TSLP to the exacerbation of inflammation.
4.2. The contribution of TLRs expressed in eosinophils
Eosinophils are the major effector cells in allergic inflammation. Significant levels of expression of TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, and TLR9 have been detected in human peripheral blood eosinophils [41] (Table 4). TLR ligands PGN (TLR2/6/1), flagellin (TLR5), Imiquimod R837 and single-stranded RNA (ssRNA) (TLR7), LPS (TLR4), and CpG (TLR9) all induce the release of cytokines and chemokines IL-1β, IL-6, CXCL8 (IL-8), and CXCL1 (GRO-α) from eosinophils [2,41].
Eosinophils generate a respiratory burst in response to activation of TLR7 by ssRNA that may contribute to immunity against ssRNA viruses. During respiratory syncytial virus (RSV) infection, eosinophils are recruited to the lungs. It has been demonstrated that mouse eosinophils express TLR7 and become activated and degranulate after ssRNA stimulation via the TLR7-MyD88 pathway. RSV clearance in the lung was significantly delayed in eosinophil lineage-deficient mouse strain DdblGATA. In contrast, IL-5 transgenic mice, which are hypereosinophilic, eliminated RSV in the lung more rapidly. Similarly, prior adoptive transfer of eosinophils to the lungs of mice inoculated with RSV accelerated viral clearance, an effect that was lost when the donor eosinophils were MyD88-deficient. The importance of TLR7-MyD88 pathway in eosinophil mediated anti-RSV infection was further confirmed by the observation that enhanced protection against RS shown in IL-5 transgenic mice was abolished in the absence of MyD88 [42].
One of the prominent pathological features of asthma is an increased infiltration of eosinophils in the bronchial mucosa. Eosinophils play a crucial role in the innate immune responses against infecting bacteria and viruses but also in the initiation of allergic inflammation by releasing cytokines, chemokines, superoxides, and toxic granular proteins. It has been reported that TLR stimulation enhances the accumulation of activated eosinophils [43,44] which exacerbates allergic inflammation.
4.3. The role of TLRs expressed by mast cells
Mast cells are tissue-dwelling cells that reside predominantly in mucosal tissues including the bronchial mucosa. Therefore, these cells have been implicated in immune surveillance and host defense. Mast cell numbers are increased in the bronchial mucosa of asthmatic subjects, and of particular interest, they accumulate in the smooth muscle bundles in the thickened airway wall [45]. In the context of allergic asthma, mast cells are key effectors that bind IgE on the surface through the high- affinity Fc receptor and release inflammatory mediators such as histamine, prostaglandins, and leukotrienes. In addition, mast cells regulate the level of allergic airway inflammation by producing a number of cytokines (e.g., IL-4,IL-5,IL-6,IL-10,IL-13, and TNF-α), which are important in the pathogenesis of allergic reactions [2].
Mast cells express most known TLRs [45,46] (Table 4). But human and murine mast cells show some difference in the expression pattern. For instance, murine mast cells express TLR1, TLR2, TLR6, and TLR8, but not TLR5 [47], which is expressed in human cells. Because mast cell numbers are very low in most tissue compartments, many in vitro studies have been performed using mast cells derived from bone marrow (BM). BM-derived mast cells release a variety of cytokines in response to stimulation by TLR2 and TLR4 ligands, as shown in Table 5. Similarly, activation of TLR2 or TLR4 on human cord blood-derived mast cells also promotes the release of IL-13 and stimulation with the TLR2 ligand PGN induced the release of histamine, which is crucial for the allergic responses and airway constriction [48].
Table 5.
Cytokine production by mouse BM-derived mast cells.
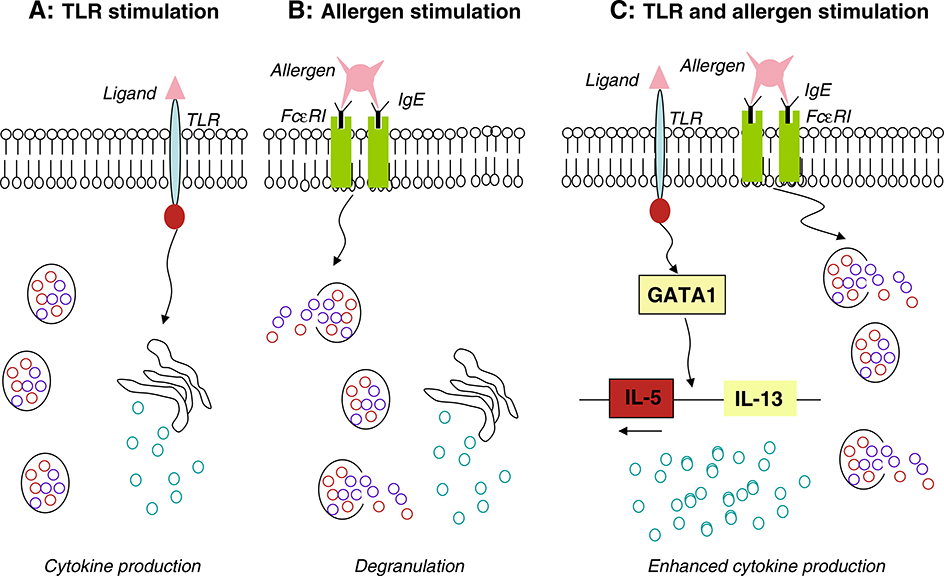
Activation of TLR4 on mast cells may exacerbate the allergic airway inflammation [49]. OVA-induced eosinophilic allergic airway inflammation in the lung was markedly increased by intranasal administration of low-dose LPS (1 μg) in wild-type mice, but no such increase was observed in either mast cell-deficient or TLR4-deficient mice. However, adoptive transfer of BM-derived mast cells (BMMCs) from wild-type, but not from TLR4-deficient mice, restores the eosinophilic inflammation in mast cell-deficient mice. Wild-type BMMCs pretreated with LPS in vitro also reconstitute the eosinophilic inflammation. Moreover, treatment of BMMCs with LPS results in NF-κB activation, a sustained upregulation of GATA1 and GATA2 expression, and an increased capability of the cells to produce the Th2 cytokines, IL-5 and IL-13. Marked increases in the expression of IL-5 and IL-13 are detected in LPS-treated BMMCs after costimulation with IgE and antigen. In addition, overexpression of GATA1, but not GATA2, in mast cell lines results in increased transcription of IL-4 and IL-5. Furthermore, the transcription of Th2 cytokines in BMMC is decreased by the introduction of small interfering RNA of GATA1. Based on these observations, mast cells appear to control allergic airway inflammation through TLR4-mediated induction of GATA1 and subsequent increase in Th2 cytokine production. It has also been reported that TLR3, 7, 9 ligands fail to induce degranulation and IL-13 production in mast cells [50,51]. Therefore, studies thus far indicate that TLR stimulation enhances cytokine production by mast cells, but fails to induce degranulation (Fig. 3). In the experiments to determine the contributions of human mast cells to antiviral immunity, human mast cells (HCMCs) derived from peripheral blood stimulated with TLR3 ligand or RSV were found to produce IFN-α, an antiviral cytokine not previously shown to be released by mast cells [46]. Although these findings provide evidence that mast cells may participate in host defense against viral infection, they do not necessarily reflect mast cell function in situ or within a pathologic environment in the lung. In this context, it will be important to determine whether RV and RSV infections, the most common triggers of asthma exacerbation, activate TLRs expressed by bronchial mucosal mast cells in vivo, and whether such interaction promotes mast cell degranulation and airway contraction.
Fig. 3.
The role of TLRs and allergen on mast cell activation. A: Stimulation of mast cells with TLRs induces cytokine production, but does not release chemical mediators (degranulation); B: Allergen/IgE-mediated activation of mast cells induces release of cytokines and degranulation via FcεRI, a high-affinity IgE receptor; C: Allergen and TLR costimulation of mast cells enhances Th2 cytokine production with increased expression of GATA1, but does not enhance degranulation.
5. Therapeutic potential of TLR9 in allergic airway inflammation
Since the allergic airway inflammation is in general a consequence of Th2 immune responses, it is logically conceived that skewing the responses to Th1 direction may reverse the course of the disease and provide therapeutic potential. One of the promising target molecules among TLRs that may mediate Th1 dominant airway responses is TLR9. TLR9 is widely expressed in airway cells including mast cells, eosinophils, epithelial cells, and plasmacytoid DCs (Tables 1, 3). The TLR9 ligand CpG DNA has been consistently shown to downregulate OVA-induced allergic inflammation in murine models [52,53]. A large number of animal studies have demonstrated the activation of TLR-9 by CpG rich unmethylated DNA segments commonly found in bacteria. These are also known as immunostimulatory DNA sequences (ISS), which are able to skew immune responses towards Th1 phenotype [54]. The mechanisms for the therapeutic effects of CpG motifs are thought to be based on activation of DCs to produce IL-12 and subsequent IFN-γ release by Th1 cells. Also, coadministration of CpG motif together with an allergen is more effective in inhibiting allergic airway inflammation than the administration of the CpG motif alone. The reason for the enhanced effect of coadministration was that CpG-ODN alone stimulates TLR9 expressing innate immune cells, which coadministration of an allergen may induce allergen-specific Th1 cells more efficiently, resulting in the inhibition of antigen-specific Th2 cells. TLR9-L is an ISS-ODN containing CpG-ODN. Systemic administration of TLR9-L significantly inhibited the development of AHR, eosinophil infiltration, mucus production, and most importantly, airway remodeling which narrows the airway. In addition, TLR9-L significantly reduced the level of the profibrotic cytokine, TGF-β, in the bronchoalveolar lavage fluid (BALF) and the lung. Thus, administration of TLR9-L prevents not only Th2-mediated airway inflammation in response to acute allergen challenge, but also the subsequent airway remodeling associated with chronic allergen exposure [52].
CPG-ODN has also been shown to interfere with mast cell function in allergic reaction. In mast cells, CpG-ODNs inhibit IL-4 secretion and degranulation induced by IgE cross-linking [52]. CpG-ODNs also inhibit IgE production by upregulating T-bet expression in B cells [52]. Allergens conjugated with CpG-ODN bind to allergen-specific B cells and may selectively inhibit the production of allergen-specific antibodies. Based on its therapeutic potential, CpG-ODN is currently being tested in allergy patients in clinical trials [55]. Amb a 1–immunostimulatory phosphorothioate oligonucleotide conjugate (AIC) is a novel therapeutic compound that consists of purified Amb a 1 obtained from short ragweed proteins linked to an immunostimulatory phosphorothioate oligodeoxyribonuleotide. Subcutaneous administration of AIC to ragweed-sensitive patients before the ragweed season decreases nasal inflammatory responses [56]. IL-4 production by ragweed-specific T cells in peripheral blood mononuclear cells also was decreased after AIC administration [57]. Therefore, CPG-ODN and its allergen conjugates have shown promising preventive and therapeutic effects on allergic inflammatory responses of the respiratory system by targeting TLR9 bearing immune cells in the airway and periphery.
6. Conclusions
Allergic airway inflammation is one of the most common diseases in humans and incurs huge social and economic damages all year around. Significant progress has been achieved in the prevention and therapy of the disease including the use of steroid hormones and airway dilators that may break down the mucus for better airway passage. However, due to the complexity of the cause of the disease and variable host responses, a better grasp of the mechanisms of the disease is clearable to develop more effective therapeutics. The emerging picture of the role of TLRs in mediating the initiation and progression of allergic airway inflammation provides a promising revenue for the prevention and therapy of the disease. While many of TLRs on different airway cells have been shown to be critical for the development of the host responses, in most cases they may exacerbate the pathogenesis of airway inflammation, TLR9, an excellent inducer of Th1 immune responses, has been implicated as a therapeutic target and TLR9 agonists have been used in clinical trials in patients with allergic asthma. It is anticipated that further elucidation of the involvement of TLRs will greatly benefit the understanding and therapy of allergic airway diseases.
Acknowledgments
The authors thank Dr. J. J. Oppenheim for critically reviewing the manuscript, Ms. C. Lamb and Ms. S. Sheriff for secretarial assistance.
This project has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The research was also supported in part by the Intramural Research Program of the NCI, NIH.
References
- [1].Chen K, Le Y, Liu Y, Gong W, Ying G, Huang J, et al. A critical role for the g protein-coupled receptor mFPR2 in airway inflammation and immune responses. J Immunol 184:3331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Iwamura C, Nakayama T. Toll-like receptors in the respiratory system: their roles in inflammation. Curr Allergy Asthma Rep 2008;8:7–13. [DOI] [PubMed] [Google Scholar]
- [3].Marks GB. Environmental factors and gene-environment interactions in the aetiology of asthma. Clin Exp Pharmacol Physiol 2006;33:285–9. [DOI] [PubMed] [Google Scholar]
- [4].Schroder NW, Maurer M. The role of innate immunity in asthma: leads and lessons from mouse models. Allergy 2007;62:579–90. [DOI] [PubMed] [Google Scholar]
- [5].Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, Nowak D, Martinez FD. Toll-like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol 2004;113:482–8. [DOI] [PubMed] [Google Scholar]
- [6].Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. Int Immunopharmacol 2007;7:1271–85. [DOI] [PubMed] [Google Scholar]
- [7].Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and grampositive bacterial cell wall components. Immunity 1999;11:443–51. [DOI] [PubMed] [Google Scholar]
- [8].Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 1999;401:811–5. [DOI] [PubMed] [Google Scholar]
- [9].Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res 2006;16:693–701. [DOI] [PubMed] [Google Scholar]
- [10].Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 2004;303: 1522–6. [DOI] [PubMed] [Google Scholar]
- [11].Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 2005;308:1626–9. [DOI] [PubMed] [Google Scholar]
- [12].Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 2005;11:1173–9. [DOI] [PubMed] [Google Scholar]
- [13].So EY, and Ouchi T The application of Toll like receptors for cancer therapy. Int J Biol Sci 6:675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen K, Huang J, Liu Y, Gong W, Cui Y, Wang JM. Synergy of TRIF-dependent TLR3 and MyD88-dependent TLR7 in up-regulating expression of mouse FPR2, a promiscuous G-protein-coupled receptor, in microglial cells. J Neuroimmunol 2009;213:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II major histocompatibility complex antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med 1991;173:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lambrecht BN , Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol 1998;160:4090–7. [PubMed] [Google Scholar]
- [17].Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity 2009;31:412–24. [DOI] [PubMed] [Google Scholar]
- [18].Demedts IK, Brusselle GG, Vermaelen KY, Pauwels RA. Identification and characterization of human pulmonary dendritic cells. Am J Respir Cell Mol Biol 2005;32:177–84. [DOI] [PubMed] [Google Scholar]
- [19].Iwasaki A Mucosal dendritic cells. Annu Rev Immunol 2007;25:381–418. [DOI] [PubMed] [Google Scholar]
- [20].Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol 2001;31:3388–93. [DOI] [PubMed] [Google Scholar]
- [21].Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 2002;20:709–60. [DOI] [PubMed] [Google Scholar]
- [22].Pulendran B, Kumar P, Cutler CW, Mohamadzadeh M, Van Dyke T, Banchereau J. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J Immunol 2001;167:5067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem 2001;276:37692–9. [DOI] [PubMed] [Google Scholar]
- [24].Edwards AD, Manickasingham SP, Sporri R, Diebold SS, Schulz O, Sher A, Kaisho T, Akira S, Reis e Sousa C. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol 2002;169:3652–60. [DOI] [PubMed] [Google Scholar]
- [25].Boullart AC, Aarntzen EH, Verdijk P, Jacobs JF, Schuurhuis DH, Benitez-Ribas D, Schreibelt G, van de Rakt MW, Scharenborg NM, de Boer A, Kramer M, Figdor CG, Punt CJ, Adema GJ, de Vries IJ. Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration. Cancer Immunol Immun- other 2008;57:1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Duez C, Gosset P, Tonnel AB. Dendritic cells and toll-like receptors in allergy and asthma. Eur J Dermatol 2006;16:12–6. [PubMed] [Google Scholar]
- [27].Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005;6:1123–32. [DOI] [PubMed] [Google Scholar]
- [28].Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol 2000;165:6107–15. [DOI] [PubMed] [Google Scholar]
- [29].Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006;126:1121–33. [DOI] [PubMed] [Google Scholar]
- [31].Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006;24:179–89. [DOI] [PubMed] [Google Scholar]
- [32].Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 2006;24:677–88. [DOI] [PubMed] [Google Scholar]
- [33].Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med 2006;203:2715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol 2004;31:358–64. [DOI] [PubMed] [Google Scholar]
- [36].Hewson CA, Jardine A, Edwards MR, Laza-Stanca V, Johnston SL. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J Virol 2005;79:12273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang Q, Nagarkar DR, Bowman ER, Schneider D, Gosangi B, Lei J, Zhao Y, McHenry CL, Burgens RV, Miller DJ, Sajjan U, Hershenson MB. Role of double-stranded RnA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J Immunol 2009;183:6989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med 2006;203:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol 2006;7:709–14. [DOI] [PubMed] [Google Scholar]
- [40].Kato A, Favoreto S Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol 2007;179:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol 2007;37:85–96. [DOI] [PubMed] [Google Scholar]
- [42].Phipps S, Lam CE, Mahalingam S, Newhouse M, Ramirez R, Rosenberg HF, Foster PS, Matthaei KI. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 2007;110:1578–86. [DOI] [PubMed] [Google Scholar]
- [43].Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol 2004;172:2739–43. [DOI] [PubMed] [Google Scholar]
- [44].Didierlaurent A, Ferrero I, Otten LA, Dubois B, Reinhardt M, Carlsen H, Blomhoff R, Akira S, Kraehenbuhl JP, Sirard JC. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J Immunol 2004;172:6922–30. [DOI] [PubMed] [Google Scholar]
- [45].Phipps S, Lam CE, Foster PS, Matthaei KI. The contribution of toll-like receptors to the pathogenesis of asthma. Immunol Cell Biol 2007;85:463–70. [DOI] [PubMed] [Google Scholar]
- [46].Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol 2004;114:174–82. [DOI] [PubMed] [Google Scholar]
- [47].Marshall JS, McCurdy JD, Olynych T. Toll-like receptor-mediated activation of mast cells: implications for allergic disease? Int Arch Allergy Immunol 2003;132:87–97. [DOI] [PubMed] [Google Scholar]
- [48].Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol 2004;4:787–99. [DOI] [PubMed] [Google Scholar]
- [49].Nigo YI, Yamashita M, Hirahara K, Shinnakasu R, Inami M, Kimura M, Hasegawa A, Kohno Y, Nakayama T. Regulation of allergic airway inflammation through Toll-like receptor 4-mediated modification of mast cell function. Proc Natl Acad Sci USA 2006;103:2286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol 2004;173:531–41. [DOI] [PubMed] [Google Scholar]
- [51].Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcepsilonR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood 2006;107:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hayashi T, Raz E. TLR9-based immunotherapy for allergic disease. Am J Med 2006;119(897):e891–6. [DOI] [PubMed] [Google Scholar]
- [53].Hessel EM, Chu M, Lizcano JO, Chang B, Herman N, Kell SA, Wills-Karp M, Coffman RL. Immunostimulatory oligonucleotides block allergic airway inflammation by inhibiting Th2 cell activation and IgE-mediated cytokine induction. J Exp Med 2005;202:1563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Long AA. Monoclonal antibodies and other biologic agents in the treatment of asthma. MAbs 2009;1:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med 2007;13: 552–9. [DOI] [PubMed] [Google Scholar]
- [56].Tulic MK, Fiset PO, Christodoulopoulos P, Vaillancourt P, Desrosiers M, Lavigne F, Eiden J, Hamid Q. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol 2004;113:235–41. [DOI] [PubMed] [Google Scholar]
- [57].Simons FE, Shikishima Y, Van Nest G, Eiden JJ, HayGlass KT. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J Allergy Clin Immunol 2004;113:1144–51. [DOI] [PubMed] [Google Scholar]
- [58].GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol 2008;1:442–50. [DOI] [PubMed] [Google Scholar]
- [59].Hirschfeld M, Weis JJ, Toshchakov V, Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM, Vogel SN. Signaling by toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect Immun 2001;69: 1477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mazzoni A, Segal DM. Controlling the Toll road to dendritic cell polarization. J Leukoc Biol 2004;75:721–30. [DOI] [PubMed] [Google Scholar]