Abstract
Background: While a sex effect on outcomes following anterior cruciate ligament (ACL) reconstruction surgery has been previously documented, less is known following bridge-enhanced ACL repair (BEAR). We hypothesized that female sex would have significantly worse early functional outcomes and higher retear rates following primary repair of the ACL enhanced with a tissue-engineered scaffold.
Methods: Sixty-five patients (28 males and 37 females), age 14–35 with a complete ACL tear underwent primary repair of the ACL enhanced with a tissue-engineered scaffold (bridge-enhanced ACL repair) within 45 days of injury. International Knee Documentation Committee (IKDC) and Knee Injury and Osteoarthritis Outcome (KOOS) scores, as well as instrumented anteroposterior (AP) laxity through KT-1000 testing and functional outcome measures were obtained at time points up to 2 years postoperatively and compared between males and females using mixed model repeated measures analyses and chi square tests.
Results: There was no significant sex difference on the postoperative IKDC Subjective Score at 3, 6, 12, or 24 months or any of the five KOOS scores at 12 and 24 months. Instrumented AP laxity testing demonstrated mean (standard deviation) side-to-side differences that were similar in the two sexes at 2 years; 1.7 (2.7) mm and 1.5 (3.7) mm in females and males, respectively, p = 0.72. At 6 months postoperatively, males had a larger deficit in hamstring strength on the operated leg (14.0% vs. 1.7%; p = 0.03) and a larger deficit in quadriceps strength on the operated leg (11.3% vs. 2.0%; p = 0.004); however, no sex difference was noted at 12 or 24 months. Females demonstrated superior single leg hop testing at 6 and 12 months ([91.3% vs. 78.1%, p = 0.001], [96.9% vs. 87.0%, p = 0.01] respectively). There were no significant sex differences on ipsilateral (males; 14.3% vs. females; 13.9%, p = 1.00) or contralateral (males; 3.6% vs. females; 2.8%, p = 1.00) ACL reinjury rates.
Conclusions: Female subjects had better hamstring and quadriceps strength indices at 6 months than males as well as better hop test results at the 6 and 12-month time period. Despite this, there was no significant sex difference on patient-reported outcomes and objective AP laxity testing at time points up to 2 years postoperatively.
Impact statement
This is the first study comparing sex specific outcomes following the bridge-enhanced ACL repair technique (BEAR). The results of this study suggest that females have earlier recovery of both muscle strength and functional outcomes compared to their male counterparts. This is an important finding when considering future modifications to postoperative care and rehabilitation in females and males following this tissue-engineered BEAR technique.
Keywords: anterior cruciate ligament, human, ACL repair, sex, bridge-enhanced ACL repair, BEAR
Introduction
While there is a well-established higher risk profile for females with regard to ACL injury and reinjury rates following anterior cruciate ligament (ACL) surgery, evidence relating to the effect of sex on outcomes following ACL surgery is often contradictory.1–10
In preclinical studies, sex-specific differences for postoperative outcomes have been found following ACL reconstruction; namely, females had lower graft yield loads and linear stiffness, significantly greater side-to-side difference in knee laxity, lower graft vascular density, and significantly larger areas of cartilage damage in relevant models.11
In clinical studies there have been mixed results regarding sex-related differences in patient-reported outcomes (PROs) following ACL reconstruction surgery. Sex has not been found to significantly affect the International Knee Documentation Committee (IKDC) subjective score at time points between 1 and 5 years after ACL reconstruction.3,7,12–14 In comparison, while some have shown that the Knee Injury and Osteoarthritis Score (KOOS) subscales do not differ between sexes after an ACL reconstruction with hamstring autograft,1 others have shown significant differences in the KOOS quality-of-life subscore between the two sexes at 1 year following ACL reconstruction with hamstring autograft tendon; females having lower scores than their male counterparts.6
There have also been mixed results of sex-related differences in outcomes of ACL reconstruction in relation to physical examination measures, including subjective and instrument-measured anteroposterior (AP) laxity as well as functional outcomes, such as muscle strength recovery and hop testing. Ahlden et al.2 and Ferrari et al.5 both found that females treated with hamstring and bone–patellar tendon–bone autograft reconstruction, respectively, had significantly greater AP laxity on instrumented testing at 2 years postoperatively compared with males. This was also shown to be true in a separate study at longer-term follow-up following hamstring tendon autograft.15 Other studies have demonstrated no differences in AP laxity between sexes at the 2 to 3-year postoperative time point for patients undergoing single or double bundle hamstring and bone–patellar tendon–bone autograft.3,9,10
Muscle strength testing following ACL reconstruction, which has been used to guide rehabilitation as well as determine readiness for return to sport, has previously been shown to have variable results when comparing sexes. While many studies have shown no significant effect of sex on muscle recovery after surgery for postoperative time points between 1 and 2 years postoperatively,8,16,17 when differences have been observed, it has been consistently the females who have had deficits in both hamstring and quadriceps strength recovery after autograft ACL reconstruction.4,18,19
The bridge-enhanced ACL repair (BEAR) technique is being evaluated in clinical trials as an alternative to ACL reconstruction.20,21 The procedure involves suture repair of the ACL combined with a specific extracellular matrix scaffold (the BEAR implant), which is placed in the space between the two torn ends of the ACL and activated with the patient's blood. Sex-specific outcomes utilizing BEAR repair have also been identified in preclinical models,22 where transected female ACLs treated with this technique had lower linear stiffness, yield, and maximum load than males treated with this technique when absorbable sutures were used.22 Interestingly, if nonabsorbable sutures were used, there was no significant difference between the sexes in the mechanical properties of the repair. The differences in outcomes between the sexes for this new technique in clinical studies have not been reported to date.
The purpose of this study was to determine if there was a significant effect of sex on outcomes after BEAR, including subjective questionnaires (IKDC, KOOS) and objective outcomes (muscle strength, hop testing, AP laxity, and retear rates). We hypothesized that female sex would have significantly worse subjective outcome scores, AP laxity, and muscle strength recovery following the BEAR surgery.
Methods
IRB and FDA approvals were obtained before the start of the BEAR II Trial, and the trial was registered on Clinical Trials.gov (FDA IDE G150268, IRB P00021470, NCT 02664545). All patients granted their informed consent before participation in the study. One hundred patients, ages 13 to 35, who presented with a complete ACL tear, were less than 45 days from injury, had closed physes, and had at least 50% of the length of the ACL attached to the tibia (as determined from a preoperative MR image) were randomized in an approximate 2:1 ratio to undergo either the bridge-enhanced ACL repair procedure (Figure 1) (BEAR group, 65 patients), or autograft ACL reconstruction (ACLR group, 35 patients). Patients were excluded from enrollment if they had a history of prior ipsilateral knee surgery, history of prior knee infection, or had risk factors that could adversely affect ligament healing (nicotine/tobacco use, corticosteroids in the past 6 months, chemotherapy, diabetes, inflammatory arthritis). Patients were also excluded if they had a displaced bucket handle tear of the medial meniscus requiring repair. All other meniscal injuries were included. Patients were also excluded if they had a full-thickness chondral injury, a grade III MCL injury, a concurrent complete patellar dislocation, or an operative posterolateral corner injury.
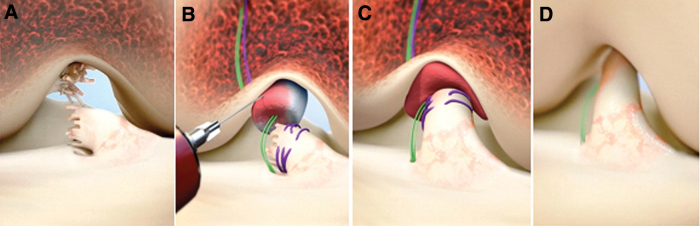
FIG. 1.
Stepwise demonstration of the “Bridge-Enhanced ACL Repair” technique using the implant. In this technique, the torn ACL tissue is preserved (A). A whip stitch of #2 absorbable suture (purple) is placed into the tibial stump of the ACL. Small tunnels (4 mm) are drilled in the femur and tibia and a cortical button with two #2 nonabsorbable sutures (green sutures) and the #2 absorbable ACL sutures attached to it is passed through the femoral tunnel and engaged on the proximal femoral cortex. The nonabsorbable sutures are threaded through the BEAR® implant and tibial tunnel and secured in place with an extracortical button. The implant is then saturated with 5 to 10 mL of the patient's blood (B), and the tibial stump pulled up into the saturated implant (C). The ends of the torn ACL then grow into the implant, which is gradually replaced by healing ligament tissue (D) (From Murray et al.20). ACL, anterior cruciate ligament. Color images are available online.
The results of the outcomes of BEAR procedure versus autograft reconstruction have been previously reported.23 For the purposes of this analysis, only the 65 patients randomized to the BEAR procedure were included, to address sex-based comparisons within this particular cohort. Preinjury sports participation based on the amount of cutting/pivoting activities24,25 were also recorded, with level 1, including football, soccer, basketball, field hockey, rugby, lacrosse, volleyball, and ultimate frisbee; level 2, including skiing/snowboarding, tennis, softball/baseball, gymnastics, cheerleading, and ice hockey; and level 3, including running, swimming, cross country skiing, weight lifting and biking. All surgeons were fellowship trained in sports medicine and experienced at ACL reconstruction surgery.
Outcome measures
IKDC subjective score
The IKDC Subjective score was used per the published instructions26–28 and collected at 3, 6, 12, and 24 months postoperatively.
Knee Injury and Osteoarthritis Score (KOOS)
All five domains of the KOOS scores were collected as per the published instructions29,30 at 12 and 24 months postoperatively.
Physical examination (IKDC objective score)
The IKDC Objective Score was calculated for all patients as per the IKDC instructions.31 As previously described,21,23 knee effusion, range of motion, and clinical knee stability measures (Lachman test and pivot shift test) were evaluated for both knees preoperatively and 2 years after surgery. An independent examiner performed the tests and knee sleeves were used to cover both knees. The examiner was blinded to the surgical side and study group assignment when performing the physical examination, until the end, when effusion was assessed after removal of the sleeves.
Instrumented anteroposterior laxity testing of the knee
Consistent with previously described methods,21,23 arthrometer testing (KT-1000; MEDMetric, San Diego, CA) was used to measure the AP laxity of the knee, specifically the anterior displacement of the tibia with respect to the femur under 130N of applied anterior force as recommended by the manufacturer.32 AP laxity testing was performed in duplicate on each leg and both values recorded. The results were reported as a side-to-side difference between limbs (average on the surgical knee minus the average on the contralateral knee).
Functional outcomes
Measurement technique was consistent with previous studies.21,23 Hamstring, quadriceps and hip abductor muscle isometric strengths were measured using a hand-held dynamometer (HHD) (Microfet 2; Hoggan Scientific, LLC, Salt Lake City, UT) that has specifically been validated as a reliable HHD in multiple studies.33–36 The hamstring strength was measured with the patient prone and the knee in 90° of flexion. The dynamometer was placed on the posterior surface of the lower leg proximal to the ankle. The hip abductor strength was tested with the patient lying on their side with the knee extended, placing the dynamometer over the mid-lateral thigh. The quadriceps strength was measured with the knee at 90° of flexion with the dynamometer positioned at the distal tibia. Patients also performed a single hop, triple hop, 6-m timed hop, and crossover hop test (in an ACL brace) as previously described.37 All measures were performed in duplicate on each side and the duplicate measurements averaged for further analysis. Results were normalized by expressing the injured knee result as a percentage of the uninjured contralateral knee result for all strength and hop testing measures and the percentage reported as the index measurement.
Additional knee surgery
All incidences of ACL reinjury requiring a second ipsilateral ACL procedure were recorded for each sex, as well as any occurrences of a contralateral ACL injury requiring surgery.
Statistical methods
Subject characteristics at baseline were compared between males and females using t-tests for continuous variables and Fisher's Exact test for categorical measures. Mixed model repeated measures analyses were used to evaluate objective and subjective outcomes assessed across postoperative follow-up time points. Simple effects (i.e., sex differences within time point) were assessed using Fisher's least significant difference procedure. Means presented are least square mean, which account for missing data due to incomplete follow-up. Additionally, males and females were compared on categorical outcomes using Fisher's Exact tests and on ordinal outcomes (i.e., IKDC Objective scores) using Cochran–Armitage test for trend. Due to the finding that mean age and body mass index (BMI) were significantly different between males and females, separate analyses of covariance were performed to determine if observed sex differences in functional outcomes remained significant after adjusting for age and BMI as potential confounders. All analyses were performed using SAS Statistical Software Version 9.4 (SAS Institute, Cary, NC). Statistical significance was based on p < 0.05.
Results
Patient demographics
Sixty-five patients, 28 males and 37 females, with an average age of 19.4 years were enrolled (Table 1). Females in the study were significantly younger than males (17.4 vs. 22.0, p < 0.01) and had significantly lower BMIs (23.6 vs. 26.1, p = 0.01). There were no significant differences between sexes in posterior tibial slope (females; 6.3 vs. males; 6.6, p = 0.68), or the percentage of subjects that were Caucasian (females; 79% white vs. males; 89% white, p = 0.31). There was no mechanism of injury more specific to one sex, with the majority of patients in both sexes injured in sport; only one female patient had a nonsport-related injury. The majority of sports in which the injuries occurred were Level 1 sports (>75% for both sexes, p = 0.95).
Table 1.
Baseline Demographics of the Patients of Each Sex
| Total (N = 65) | Male (n = 28) | Female (n = 37) | p | |
|---|---|---|---|---|
| Age at surgery (mean, SD) | 19.4 (5.1) | 22.0 (6.0) | 17.4 (3.1) | <0.01 |
| BMI (mean, SD) | 24.7 (3.8) | 26.1 (4.5) | 23.6 (2.8) | 0.01 |
| Posterior tibial slope (mean, SD) | 6.4 (3.1) | 6.6 (3.5) | 6.3 (2.7) | 0.68 |
| White, non-Hispanic (n, %) | 55 (85) | 22 (79) | 33 (89) | 0.31 |
| Knee side, left (n, %) | 33 (50.1) | 14 (50.0) | 19 (51.4) | 1.00 |
| Mechanism of injury, sports (n, %) | 64 (98.5) | 28 (100.0) | 36 (97.3) | 1.00 |
| Injury by contact | 17 (26.2) | 6 (21.4) | 11 (29.7) | 0.57 |
| Sports breakdowna (n, %) | ||||
| Basketball | 7 (10.9) | 1 (3.6) | 6 (16.7) | |
| Bike polo | 1 (1.6) | 1 (3.6) | 0 (0) | |
| Cheerleading | 1 (1.6) | 0 (0) | 1 (2.8) | |
| Field hockey | 2 (3.1) | 0 (0) | 2 (5.6) | |
| Football | 6 (9.4) | 6 (21.4) | 0 (0) | |
| Frisbee | 1 (1.6) | 1 (3.6) | 0 (0) | |
| Hockey | 1 (1.6) | 0 (0) | 1 (2.8) | |
| Lacrosse | 6 (9.4) | 4 (14.3) | 2 (5.6) | |
| Powderpuff football | 1 (1.6) | 0 (0) | 1 (2.8) | |
| Rugby | 2 (3.1) | 2 (7.1) | 0 (0) | |
| Skiing | 11 (17.2) | 5 (17.9) | 6 (16.7) | |
| Soccer | 23 (35.9) | 7 (25.0) | 16 (44.4) | |
| Softball | 1 (1.6) | 0 (0) | 1 (2.8) | |
| Volleyball | 1 (1.6) | 1 (3.6) | 0 (0) | |
| Level of sport (n, %) | ||||
| 1 | 50 (76.9) | 22 (78.6) | 28 (75.7) | 0.95 |
| 2 | 14 (21.5) | 5 (17.9) | 9 (24.3) | |
| 3 | 1 (1.5) | 1 (3.6) | 0 (0.0) | |
One patient did not indicate a sports injury.
BMI, body mass index; SD, standard deviation.
Patient-reported outcomes
No significant sex differences were observed for any of the PROs after BEAR at any time point. There were no significant differences between IKDC Subjective Scores or all five subcomponents of the KOOS subjective questionnaires between sexes at baseline (p > 0.1, data not shown). IKDC Subjective Scores completed at 3, 6, 12, and 24 months were not significantly different by sex (Table 2, p > 0.16 for all comparisons). All five subcomponents of the KOOS subjective questionnaires completed by subjects at 12 and 24 months were also not significantly different between the two sexes (Table 2, p > 0.11 for all comparisons).
Table 2.
International Knee Documentation Committee Subjective Score and Knee Injury and Osteoarthritis Outcome Scores
| Parameter | n | Male | N | Female | p |
|---|---|---|---|---|---|
| IKDC | |||||
| 3 months | 26 | 66.0 (12.4) | 37 | 69.4 (8.4) | 0.29 |
| 6 months | 27 | 83.1 (14.4) | 37 | 87.7 (8.4) | 0.16 |
| 12 months | 27 | 87.6 (9.3) | 37 | 86.7 (12.2) | 0.78 |
| 24 months | 27 | 88.7 (14.3) | 35 | 88.7 (12.6) | 0.98 |
| KOOS | |||||
| Pain | |||||
| 12 months | 27 | 95.2 (6.9) | 37 | 93.8 (6.4) | 0.49 |
| 24 months | 27 | 92.8 (9.7) | 34 | 93.7 (10.5) | 0.68 |
| Symptoms | |||||
| 12 months | 27 | 88.4 (8.9) | 37 | 88.3 (9.6) | 0.97 |
| 24 months | 27 | 89.2 (10.7) | 34 | 89.1 (13.1) | 0.98 |
| ADLs | |||||
| 12 months | 27 | 99.2 (1.1) | 37 | 98.3 (2.9) | 0.35 |
| 24 months | 27 | 98.6 (5.1) | 34 | 98.8 (4.1) | 0.88 |
| QoL | |||||
| 12 months | 27 | 74.1 (16.3) | 37 | 66.0 (21.4) | 0.11 |
| 24 months | 27 | 78.2 (23.0) | 34 | 74.6 (17.1) | 0.47 |
| Sports | |||||
| 12 months | 27 | 85.8 (11.6) | 37 | 85.8 (18.1) | 0.99 |
| 24 months | 27 | 88.0 (18.7) | 34 | 89.1 (18.4) | 0.80 |
ADLs, activities of daily living; QoL, quality of life; IKDC, International Knee Documentation Committee.
Physical examination outcomes
There were no sex differences in the IKDC Objective Score at 2 years from surgery (p = 0.46). Eighty-five percent of males and 90% of females achieved either an A or B IKDC Objective Score at the 2-year visit (Table 3). Ninety-six percent of males and 91% of females achieved an IKDC grade A Lachman (p = 0.37), whereas 90% of males and 73% of females achieved an IKDC grade A pivot exam at 2 years postoperatively (p = 0.13); differences that were not statistically significant.
Table 3.
Physical Examination Outcomes at 2 Years Postoperatively
| Parameter | n | Male | n | Female | p |
|---|---|---|---|---|---|
| IKDC Effusion | 24 | 33 | 0.47 | ||
| A | 23 (96%) | 30 (91%) | |||
| B | 1 (4%) | 3 (9%) | |||
| C | 0 (0%) | 0 (0%) | |||
| D | 0 (0%) | 0 (0%) | |||
| IKDC ROM | 26 | 34 | 0.51 | ||
| A | 12 (46%) | 20 (59%) | |||
| B | 10 (38%) | 10 (29%) | |||
| C | 3 (12%) | 2 (6%) | |||
| D | 1 (4%) | 2 (6%) | |||
| IKDC Lachman | 24 | 32 | 0.37 | ||
| A | 23 (96%) | 29 (91%) | |||
| B | 1 (4%) | 2 (6%) | |||
| C | 0 (0%) | 1 (3%) | |||
| D | 0 (0%) | 0 (0%) | |||
| IKDC Pivota | 21 | 30 | 0.13 | ||
| A | 19 (90%) | 22 (73%) | |||
| B | 2 (10%) | 8 (27%) | |||
| C | 0 (0%) | 0 (0%) | |||
| D | 0 (0%) | 0 (0%) | |||
| IKDC Overallb | 20 | 30 | 0.46 | ||
| A | 7 (35%) | 12 (40%) | |||
| B | 10 (50%) | 15 (50%) | |||
| C | 2 (10%) | 3 (10%) | |||
| D | 1 (5%) | 0 (0%) |
At 2 years, three BEAR patients had had a second ACL surgery less than 6 months prior and a pivot shift examination was not performed as per the study protocol.
IKDC Overall computed for patients with complete data for components, ligament based on worst of Lachman and Pivot Shift Examination.
ACL, anterior cruciate ligament; BEAR, bridge-enhanced ACL repair.
Instrumented AP laxity
There was no significant difference in the side-to-side difference in instrumented AP laxity between the sexes at any time point (Table 4, p > 0.70 for all comparisons), and both sexes had a mean side-to-side difference at 2 years that was <2 mm.
Table 4.
Instrumented Anteroposterior Laxity
| Parameter | n | Male | n | Female | p |
|---|---|---|---|---|---|
| KT-1000 | |||||
| 6 months | 27 | 2.9 (2.8) | 37 | 2.6 (3.0) | 0.72 |
| 12 months | 27 | 2.4 (2.8) | 32 | 2.5 (2.7) | 0.90 |
| 24 months | 26 | 1.5 (3.7) | 32 | 1.7 (2.7) | 0.72 |
Functional outcomes
There were significant differences between males and females on the quadriceps, hamstring and hip abductor indices at 6 months after surgery with females having better indices than males (Table 5, p < 0.03 for all three comparisons). There was no significant effect of sex on the side-to-side differences in muscle strength at the 1 or 2-year time points (p > 0.27 for all time points, Table 5).
Table 5.
Functional Measures: Muscle strength and Hop Testing
| Parameter | n | Male | n | Female | p |
|---|---|---|---|---|---|
| Quad strength | |||||
| 6 months | 27 | 88.7 (19.0) | 37 | 98.0 (11.9) | 0.004 |
| 12 months | 27 | 95.6 (9.4) | 35 | 97.0 (8.1) | 0.66 |
| 24 months | 27 | 98.2 (14.0) | 32 | 101.8 (10.5) | 0.27 |
| Ham strength | |||||
| 6 months | 27 | 86.0 (18.4) | 37 | 98.3 (25.8) | 0.03 |
| 12 months | 27 | 98.8 (11.7) | 35 | 94.5 (19.7) | 0.45 |
| 24 months | 27 | 101.1 (31.2) | 32 | 94.6 (22.0) | 0.27 |
| Hip Abd Thigh | |||||
| 6 months | 27 | 97.1 (14.6) | 36 | 106.5 (15.7) | 0.02 |
| 12 months | 27 | 104.8 (18.0) | 34 | 102.9 (15.1) | 0.63 |
| 24 months | 25 | 106.3 (17.8) | 31 | 104.5 (13.2) | 0.66 |
| Hop test–single | |||||
| 6 months | 22 | 78.1 (20.6) | 30 | 91.3 (11.9) | 0.001 |
| 12 months | 23 | 87.0 (16.7) | 29 | 96.9 (10.1) | 0.01 |
| 24 months | 18 | 93.0 (16.0) | 24 | 95.0 (10.6) | 0.48 |
| Hop test–triple | |||||
| 6 months | 19 | 87.9 (6.4) | 28 | 92.4 (8.2) | 0.10 |
| 12 months | 23 | 88.9 (13.3) | 29 | 95.1 (7.7) | 0.02 |
| 24 months | 18 | 92.6 (12.1) | 23 | 95.9 (7.4) | 0.27 |
| Hop test–6M timed | |||||
| 6 months | 20 | 114.3 (24.1) | 30 | 103.6 (8.6) | 0.01 |
| 12 months | 23 | 109.2 (23.1) | 29 | 102.1 (7.6) | 0.09 |
| 24 months | 18 | 104.6 (12.8) | 22 | 104.4 (8.6) | 0.97 |
| Hop test–Xover | |||||
| 6 months | 16 | 90.1 (7.0) | 28 | 93.2 (8.1) | 0.36 |
| 12 months | 23 | 90.5 (16.0) | 28 | 97.7 (10.9) | 0.02 |
| 24 months | 17 | 96.1 (8.0) | 22 | 96.7 (11.2) | 0.87 |
For hop testing, females had a greater single hop test index than males at 6 months (91% vs. 78%, p < 0.001, Table 5), and at 12 months (97% vs. 87%, p < 0.01). Females also performed significantly better on the triple hop test (95.1% vs. 88.9%, p = 0.02) and crossover hop test (97.7% vs. 90.5%, p = 0.02) at 12 months. By 2 years, there were no significant differences between sexes on any of the hop testing (p > 0.27 for all comparisons).
Analyses of covariance were performed to examine the potential contribution of age and BMI on observed sex differences in functional measures, and produced similar results. Age was a significant predictor of quadriceps strength (p = 0.008). However, sex differences at 6 months remained significant (p = 0.042) with adjusted means of 90.2 (19.0) versus 96.8 (11.9) for males and females, respectively. Age was not a significant predictor of any other functional outcomes (p > 0.21 for all outcomes). BMI was a significant predictor of hip abductor strength (p = 0.018), which resulted in a greater adjusted 6-month mean difference between males and females [96.1 (14.6) vs. 107.3 (15.7), p = 0.006)]. Additionally, BMI was marginally predictive of quadriceps strength (p = 0.06) with sex differences remaining significant (p = 0.014). BMI was not a predictor of any other functional outcomes (p > 0.44 for all outcomes).
Anterior cruciate ligament reinjury rates
There were no significant sex differences on the incidence of ipsilateral ACL reinjury (p = 1.00) or contralateral ACL injury (p = 1.00, Table 6). At 2 years postoperatively, five female subjects (14.3%) and four male subjects (13.9%) had had an ipsilateral ACL injury requiring surgical intervention, while one female (2.8%) and one male (3.6%) subject each had a contralateral ACL tear requiring surgery (Table 6).
Table 6.
Secondary Anterior Cruciate Ligament Injury Rates in the First 2 Years
| Parameter | Male (n = 28), N (%) | Female (n = 36), N (%) | p |
|---|---|---|---|
| Ipsilateral revision ACL reconstruction within 2 years | 4 (14.3) | 5 (13.9) | 1.00 |
| Contralateral ACL tear within 2 years | 1 (3.6) | 1 (2.8) | 1.00 |
Discussion
In this cohort, females had improved strength indices at 6 months and better hop testing indices at 6 months and 1 year; however, there was no significant effect of sex on these indices at the 2-year time point. In addition, there were no significant effects of sex on PROs, physical exam measures, instrumented knee laxity, or need for revision surgery after BEAR. This is the first report of sex-specific outcomes following the BEAR procedure, which has recently been shown to be noninferior to ACL reconstruction utilizing this same cohort.23 Our study is the first to demonstrate similar outcomes in PROs and knee stability among the two sexes following the BEAR procedure for acute ACL injuries.
One major difference that we did observe was the early delay in functional muscle recovery of male participants. Patients within this trial were followed systematically and longitudinally up until 2 years, allowing analysis of progression over time. We observed that males were slower than females to recover both their hamstring and quadriceps strength, having on average a 12% reduced hamstring recovery and 9% reduced quadriceps strength recovery at 6 months postoperatively when compared with females. Male patients also had significantly worse single-leg hop testing at this early time point, which has previously been established to correlate with quadriceps deficits.37 By the 12-month time point, the deficit in strength indices noted in the males was ameliorated, and no significant differences were seen between the sexes at either 1- or 2 years postoperatively.
Previous literature has been scarce with regard to sex analysis on functional testing at early follow-up after ACL reconstruction using both autograft and allograft tendons. Keays et al.18 analyzed a cohort of 31 subjects after ACL reconstruction and found that at 6 months, women had larger deficits in both quadriceps and hamstring recovery (22% vs. 10% and 12% vs. 9%, respectively; statistical analysis was not reported). In contrast, Yasuda et al.19 found no difference in hamstring recovery between the sexes at 3 months after an ACL reconstruction using ipsilateral patellar tendon or quadriceps tendon autograft (43% in males vs. 38% in females), while females had a greater quadriceps deficit of 67% versus 49% in males at the same time point, a difference which persisted at 12 months and at long-term follow-up (between 3 and 7 years). Yasuda et al. utilized either patellar or quadriceps tendon autograft, which likely accounts for the greater absolute quadriceps deficit values as compared with our study or studies utilizing hamstring autografts. The results of our study, in combination with that of the ACLR literature, demonstrates the necessity to include similar numbers of male and females in clinical studies assessing early postoperative muscle recovery, as sex may influence these outcomes. More specifically, current BEAR rehabilitation protocols may require adjustments for male subjects if adequate lower extremity muscle recovery is desired at the 6-month time point. Alternatively, the delayed return of muscle strength and inferior hop test results in males undergoing the BEAR procedure should be considered in planning return to sport in this group.
Symmetrical isokinetic quadriceps and hamstring strength as well as single-leg hop test performance is regarded as a key factor before clearance for return to sport after ACL reconstruction.38 Therefore, one clinically significant outcome from this finding is in keeping with the growing trend for a more delayed return to sport following ACL surgery. Historically, at our institution, patients were cleared to return to play at 6 months if there were no obvious contraindications. General consensus among the sports orthopedic community in recent times, however, has extended the recommended guidelines for return to play to between 9 months and 1 year.39–41 Given the early delay in functional recovery in our male cohort, this seems fitting, at least for males who undergo the BEAR procedure. Given that many females were able to achieve 90% results in the hop test at 6 months after the BEAR procedure, one may consider an earlier return to sports in this group based on muscle strength testing alone. However, muscle strength testing is commonly only one component of the decision-making process surrounding timing of return to sport, with increasing evidence to suggest psychological readiness, age, level of sport being returned to, and functional testing such as hop testing also playing major roles.40,42,43 It does however, continue to lend weight to the argument that rehabilitation protocols following ACL surgery may need to be tailored to sex, given the observed differences.
Recently, specific MRI outcomes of a healing ACL ligament following the BEAR procedure were documented within this same cohort; a lower signal intensity (higher tissue quality) and larger cross-sectional area of the repaired ligament was associated with a greater side-to-side difference in quadriceps strength at early time points.44 This lends evidence to the fact that lower quadriceps recovery in the early postoperative period may indicate relative protection of the healing ligament and may be beneficial for a repair procedure such as this. While we found no further evidence of sex differences in outcome measures to suggest females had less robust healed ligaments, future analysis on different physical therapy protocols following the BEAR procedure will need to be done to address this topic further.
In contradiction to some previous studies comparing sex outcomes after ACL reconstruction,3,9,10 we found no difference in overall AP laxity through clinical exam between males and females at all time points during the trial, as well as instrumented AP laxity side-to-side difference testing (Tables 3 and 4). Salmon et al.15 compared outcomes between sexes following hamstring autograft reconstruction, and found that increased AP laxity was present in females for both instrumented testing and clinical examination tests at 1, 2, and 7 years postoperatively. At 1 and 2 years, there were significantly more female than males with a side-to-side manual maximum difference of more than 3 mm. At 7 years, the average side-to-side instrumented AP laxity was 1.3 mm for males and 1.9 mm for females. Ahlden et al.2 found similar differences between sexes in their cohort of patients undergoing hamstring autograft reconstruction, whereby at 2 years postoperatively, females averaged 2.9 mm AP laxity, whereas males were 2.2 mm. In contradiction, Aldrian et al.3 conducted a study comparing outcomes of single-bundle versus double-bundle ACL reconstruction and found no differences in AP laxity between sexes for either technique at 2 years. While the studies done by Salmon et al. and Ahlden et al. showed statistical significance, whether the findings were clinically significant remain questionable given the small overall differences noted. In support of this, a meta-analysis by Tan et al.45 for all clinical examination testing, including anterior drawer test, Lachman tests, and pivot shift tests demonstrated no significant differences between sexes. Another interesting discovery in the study done by Tan et al. was the finding that the date of publication of the studies included within the meta-analysis was a significant moderator of both subjective and objective parameters. More recent publications have found comparable objective parameters such as extension loss and quadriceps testing when comparing sexes; in contrast to earlier publications where females were reported to have greater incidences of extension loss and deficits in quadriceps testing compared with males. The BEAR procedure therefore is comparable to this more recent literature, displaying no significant differences in knee laxity between sexes at 2 years postoperatively.
In addition, while differences between sexes in postoperative knee laxity and graft retear for certain ACL graft types has led to sex-specific recommendations for ACL graft selection–that is, the recommendation to consider bone–patellar tendon–bone autograft over hamstring tendon autograft in females46,47–our finding that knee laxity was not significantly different between males and females suggests that sex of the patient should not currently be a weighting factor when evaluating BEAR as a surgical option for patients with an ACL injury.
Subjective PRO scores are becoming more important in both assessing clinical status as well as predicting future functional outcomes following ACL surgery. In our study, we found no significant differences in either the subjective IKDC or the five components of the subjective KOOS questionnaire between sexes undergoing the BEAR procedure. Again this has been a topic of continued debate with conflicting published results following ACL reconstruction. Various subjective PRO questionnaires have been utilized in the literature, which may lend itself to variability in results across difference studies. Tan et al.45 assessed each individual PRO tool and found that both the IKDC and KOOS PRO tools did not demonstrate statistically significant differences between sexes after ACL reconstruction. Muller et al.48 recently established threshold values for the achievement of a patient acceptable symptom state on the IKDC subjective knee form and the five individual subset components of the KOOS from 1 to 5 years after primary ACL reconstruction. Both male and female patients undergoing the BEAR procedure within this trial achieved the threshold for IKDC and each individual subcomponent of the KOOS (except for the KOOS-activities of daily living, which had a threshold of 100, but a mean of 97.4, which was achieved by both sexes in our cohort).
Limitations
There are several limitations to our study. While the observed results were statistically significant, the study was constructed primarily for the analysis of noninferiority comparing the BEAR technique with ACL reconstruction, including adequate enrollment to facilitate power to detect the occurrence of any adverse events. Thus, we acknowledge the possibility that this study may be underpowered for the purposes of a sex comparison for all included measures, and larger studies targeting sex analysis as a primary outcome will be of interest. In addition, the female group was significantly younger and had a lower BMI than their male counterparts. However, results of analyses of covariance, which examined age and BMI as explanatory variables indicated that observed sex differences in functional outcomes remained significant even after accounting for sex differences on these potential confounders. We elected not to perform a separate sex comparison within the ACLR arm of this clinical trial given the smaller sample size of this cohort. Finally, the complete clinical significance of the differences in muscle strength and hop test results that were identified are unclear.
Conclusion
ACL repair with the BEAR implant produced similar outcomes in both sexes 2 years postoperatively, in both patient-reported outcomes as well as objective functional measures. On average, we found that the male cohort had greater deficits in hamstring and quadriceps strength recovery as well as inferior hop test results at 6 months postoperatively compared with females, and that these differences were rectified over time. The results suggest that despite early differences, males and females both had overall similarly positive outcomes following the BEAR procedure. Further research into the correlation of early muscle strength and hop test deficits in males may provide more depth and insight into sex-based differences in outcomes following this procedure.
Acknowledgments
The authors would like to acknowledge the significant contributions of the clinical trial team, including Bethany Trainor, Andrea Hale, Elizabeth Carew, Brett Flutie, Laura Thurber, Shanshan Liu, and Shanika Coney. They would also like to acknowledge the contributions of their medical safety monitoring team of Joseph DeAngelis, Peter Nigrovic, and Carolyn Hettrich, our data monitors, Maggie Malsch, Megan Fitzgerald, and Erica Denhoff, as well as the clinical care team for the trial patients, including Kathryn Ackerman, Alyssa Aguiar, Judd Allen, Michael Beasley, Jennifer Beck, Dennis Borg, Nicole Bottino, Jeff Brodeur, Stephanie Burgess, Melissa Christino, Andrea Cianci, Sarah Collins, Gianmichel Corrado, Sara Cline, Corey Dawkins, Pierre D'Hemecourt, Peter Fabricant, Jon Ferguson, Michele Flannery, Joseph Founds, Casey Gavin, Ellen Geminiani, Stacey Gigante, George Georgoudis, Christine Gonzalez, Annie Griffin, Emily Hanson, Elspeth Hart, Jackie Hastings, Pamela Horne-Goffigan, Leslie Kalish, Meghan Keating, Elizabeth KillKelley, Elizabeth Kramer, Pamela Lang, Hayley Lough, Kathleen Maguire, Chaimae Martin, Steven Mathew, Michael McClincy, William Meehan, Ariana Moccia, Jen Morse, Mariah Mullen, Stacey Murphy, Emily Niu, Michael O'Brien, Nikolas Paschos, Katrina Plavetsky, Bridget Quinn, Brianna Quintiliani, Lauren Redler, Nicholas Sant, Shannon Savage, Edward Schleyer, Benjamin Shore, Cynthia Stein, Andrea Stracciolini, Dai Sugimoto, Dylan Taylor, Ashleigh Thorogood, Jessica Travers, Natasha Trentacosta, Patrick Vavken, Lisa Vopat, Kevin Wenner, Cecily Whitehead, and Lenise Young. They would also like to thank the perioperative and operating room staff and the members of the Department of Anesthesia who were extremely helpful in developing the perioperative and intraoperative protocols. They would also like to acknowledge the efforts of the scaffold manufacturing team, including Gabe Perrone, Gordon Roberts, Doris Peterkin and Jakob Sieker. The authors are also grateful for the study design guidance provided by the Division of Orthopedic Devices at the Center for Devices and Radiological Health at the U.S. Food and Drug Administration under the guidance of Laurence Coyne and Mark Melkerson, particularly the efforts of Casey Hanley, Peter Hudson, Jemin Dedania, Pooja Panigrahi, and Neil Barkin. They are also especially grateful to the patients and their families who participated in this study, their willingness to participate in research that may help others in the future inspires all of the authors.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Medical School, Harvard University or its affiliated academic health care centers, the National Football League Players Association, Boston Children's Hospital, Rhode Island Hospital, or the National Institutes of Health.
Disclosure Statement
M.M.M. and Boston Children's Hospital have equity interests in MIACH Orthopedics, a company that has licensed the BEAR implant technology from Boston Children's Hospital. M.M.M. also is a consultant for MIACH, and receives royalties from Springer Publishing and research grants from the NIH, Department of Defense, and NFLPA through the Harvard Football Players Health Study. B.C.F. is a cofounder of MIACH Orthopedics, is an associate editor for The American Journal of Sports Medicine, and receives royalties from Springer Publishing. A.M.K. receives research grants from the NIH and NFLPA through the Harvard Catalyst's Football Players Health Study. B.L.P. has equity interests in and is a consultant for MIACH Orthopedics. D.E.K. has received educational support and hospitality payments from Kairos Surgical. Y.-M.Y. has received educational funding from Kairos Surgical and hospitality payments from Smith & Nephew and Kairos Surgical.
Funding Information
Funding was received from the Translational Research Program at Boston Children's Hospital, the Boston Children's Hospital Orthopedic Surgery Foundation, the Boston Children's Hospital Sports Medicine Foundation, and the NFLPA through the Harvard Catalyst's Football Players Health Study, as well as the NIH and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR065462 and R01-AR056834). This research was also conducted with support from the Football Players Health Study at Harvard University. The Football Players Health Study is funded by a grant from the National Football League Players Association.
References
- 1. Ageberg E., Forssblad M., Herbertsson P., and Roos E.M.. Sex differences in patient-reported outcomes after anterior cruciate ligament reconstruction: data from the Swedish knee ligament register. Am J Sports Med 38, 1334, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Ahlden M., Sernert N., Karlsson J., and Kartus J.. Outcome of anterior cruciate ligament reconstruction with emphasis on sex-related differences. Scand J Med Sci Sports 22, 618, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Aldrian S., Valentin P., Wondrasch B., et al. Gender differences following computer-navigated single- and double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 22, 2145, 2014 [DOI] [PubMed] [Google Scholar]
- 4. Barber-Westin S.D., Noyes F.R., and Andrews M.. A Rigorous Comparison Between the Sexes of Results and Complications After Anterior Cruciate Ligament Reconstruction. Am J Sports Med 25, 514, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Ferrari J.D., Bach B.R. Jr., Bush-Joseph C.A., Wang T., and Bojchuk J.. Anterior cruciate ligament reconstruction in men and women: an outcome analysis comparing gender. Arthroscopy 17, 588, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Lindstrom M., Strandberg S., Wredmark T., Fellander-Tsai L., and Henriksson M.. Functional and muscle morphometric effects of ACL reconstruction. A prospective CT study with 1 year follow-up. Scand J Med Sci Sports 23, 431, 2013 [DOI] [PubMed] [Google Scholar]
- 7. Shelbourne K.D., Barnes A.F., and Gray T.. Correlation of a single assessment numeric evaluation (SANE) rating with modified Cincinnati knee rating system and IKDC subjective total scores for patients after ACL reconstruction or knee arthroscopy. Am J Sports Med 40, 2487, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Tohyama H., Kondo E., Hayashi R., Kitamura N., and Yasuda K.. Gender-based differences in outcome after anatomic double-bundle anterior cruciate ligament reconstruction with hamstring tendon autografts. Am J Sports Med 39, 1849, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Wiger P., Brandsson S., Kartus J., Eriksson B.I., and Karlsson J.. A comparison of results after arthroscopic anterior cruciate ligament reconstruction in female and male competitive athletes: a two- to five-year follow-up of 429 patients. Scand J Med Sci Sports 9, 290, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Noojin F.K., Barrett G.R., Hartzog C.W., and Nash C.R.. Clinical comparison of intraarticular anterior cruciate ligament reconstruction using autogenous semitendinosus and gracilis tendons in men versus women. Am J Sports Med 28, 783, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Kiapour A.M., Fleming B.C., Proffen B.L., and Murray M.M.. Sex influences the biomechanical outcomes of anterior cruciate ligament reconstruction in a preclinical large animal model. Am J Sports Med 43, 1623, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn W.R., Spindler K.P., and Consortium M.. Predictors of activity level 2 years after anterior cruciate ligament reconstruction (ACLR): a Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. Am J Sports Med 38, 2040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harreld K., Nyland J., Cottrell B., and Caborn D.N.. Self-reported patient outcomes after ACL reconstruction with allograft tissue. Med Sci Sports Exerc 38, 2058, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Reinke E.K., Spindler K.P., Lorring D., et al. Hop tests correlate with IKDC and KOOS at minimum of 2 years after primary ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 19, 1806, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salmon L.J., Refshauge K.M., Russell V.J., Roe J.P., Linklater J., and Pinczewski L.A.. Gender differences in outcome after anterior cruciate ligament reconstruction with hamstring tendon autograft. Am J Sports Med 34, 621, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Bizzini M., Gorelick M., Munzinger U., and Drobny T.. Joint laxity and isokinetic thigh muscle strength characteristics after anterior cruciate ligament reconstruction: bone patellar tendon bone versus quadrupled hamstring autografts. Clin J Sport Med 16, 4, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Segawa H., Omori G., Koga Y., Kameo T., Iida S., and Tanaka M.. Rotational muscle strength of the limb after anterior cruciate ligament reconstruction using semitendinosus and gracilis tendon. Arthroscopy 18, 177, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Keays S.L., Bullock-Saxton J.E., Newcombe P., and Keays A.C.. The relationship between knee strength and functional stability before and after anterior cruciate ligament reconstruction. J Orthop Res 21, 231, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Yasuda K., Ohkoshi Y., Tanabe Y., and Kaneda K.. Quantitative evaluation of knee instability and muscle strength after anterior cruciate ligament reconstruction using patellar and quadriceps tendon. Am J Sports Med 20, 471, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Murray M.M., Flutie B.M., Kalish L.A., et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med 4, 2325967116672176, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray M.M., Kalish L.A., Fleming B.C., et al. Bridge-enhanced anterior cruciate ligament repair: two-year results of a first-in-human study. Orthop J Sports Med 7, 232596711882435, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiapour A.M., Fleming B.C., and Murray M.M.. Biomechanical outcomes of bridge-enhanced anterior cruciate ligament repair are influenced by sex in a preclinical model. Clin Orthop Relat Res 473, 2599, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murray M.M., Fleming B.C., Badger G.J., et al. Bridge-enhanced ACL repair is non-inferior to autograft ACL reconstruction at 2-years: results of a prospective randomized clinical trial. Am J Sports Med 48, 1305, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hefti F., Müller W., Jakob R.P., and Stäubli H.-U.. Evaluation of knee ligament injuries with the IDKC form. Knee Surg Sports Traumatol Arthroscopy 1, 226, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Daniel D.M., Lou Stone M., Dobson B.E., Fithian D.C., Rossman D.J., and Kaufman K.R.. Fate of the ACL-injured patient: a prospective outcome study. Am J Sports Med 22, 632, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Irrgang J.J., Anderson A.F., Boland A.L., et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med 29, 600, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Irrgang J.J., Anderson A.F., Boland A.L., et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med 34, 1567, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Rossi M.J., Lubowitz J.H., and Guttmann D.. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med 30, 152, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Paradowski P.T., Bergman S., Sunden-Lundius A., Lohmander L.S., and Roos E.M.. Knee complaints vary with age and gender in the adult population. Population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskelet Disord 7, 38, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roos E.M., and Lohmander L.S.. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 1, 64, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson A.F., Irrgang J.J., Kocher M.S., Mann B.J., Harrast J.J., and International Knee Documentation Committee.. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med 34, 128, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Daniel D.M. Reference, Maintenance and User's Guide for the Knee Ligament Arthrometer. San Diego, CA: MEDmetric Corporation, 1993 [Google Scholar]
- 33. Bohannon R. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch Phys Med Rehabil 78, 26, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Kelln B., McKeon P., Gontkof L., and Hertel J.. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil 17, 160, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Mentiplay B.F., Perraton L.G., Bower K.J., et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS One 10, e0140822, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reurink G., Goudswaard G.J., Moen M.H., Tol J.L., Verhaar J.A., and Weir A.. Strength measurements in acute hamstring injuries: intertester reliability and prognostic value of handheld dynamometry. J Orthop Sports Phys Ther 46, 689, 2016 [DOI] [PubMed] [Google Scholar]
- 37. Noyes F.R., Barber S.D., and Mangine R.E.. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med 19, 513, 1991 [DOI] [PubMed] [Google Scholar]
- 38. Cristiani R., Mikkelsen C., Edman G., Forssblad M., Engstrom B., and Stalman A.. Age, gender, quadriceps strength and hop test performance are the most important factors affecting the achievement of a patient-acceptable symptom state after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 28, 369, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. International Olympic Committee Pediatric ACLICG, Ardern C.L., Ekas G., et al. 2018 International Olympic Committee consensus statement on prevention, diagnosis, and management of pediatric anterior cruciate ligament injuries. Orthop J Sports Med 6, 2325967118759953, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feller J.A., and Webster K.E.. Where are we with return-to-sport testing following ACL reconstruction? Orthop Traumatol Surg Res 105, 1037, 2019 [DOI] [PubMed] [Google Scholar]
- 41. Grindem H., Snyder-Mackler L., Moksnes H., Engebretsen L., and Risberg M.A.. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med 50, 804, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Webster K.E., and Feller J.A.. Return to level I sports after anterior cruciate ligament reconstruction: evaluation of age, sex, and readiness to return criteria. Orthop J Sports Med 6, 2325967118788045, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davies G.J., McCarty E., Provencher M., and Manske R.C.. ACL return to sport guidelines and criteria. Curr Rev Musculoskelet Med 10, 307, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murray M.M., Kiapour A.M., Kalish L.A., et al. Predictors of healing ligament size and magnetic resonance signal intensity at 6 months after bridge-enhanced anterior cruciate ligament repair. Am J Sports Med 47, 1361, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tan S.H., Lau B.P., Khin L.W., Lingaraj K.. The importance of patient sex in the outcomes of anterior cruciate ligament reconstructions: A systematic review and meta-analysis. Am J Sports Med 44, 242, 2016 [DOI] [PubMed] [Google Scholar]
- 46. Salem H.S., Varzhapetyan V., Patel N., Dodson C.C., Tjoumakaris F.P., and Freedman K.B.. Anterior cruciate ligament reconstruction in young female athletes: patellar versus hamstring tendon autografts. Am J Sports Med 47, 2086, 2019 [DOI] [PubMed] [Google Scholar]
- 47. Shakked R., Weinberg M., Capo J., Jazrawi L., and Strauss E.. Autograft choice in young female patients: patella tendon versus hamstring. J Knee Surg 30, 258, 2017 [DOI] [PubMed] [Google Scholar]
- 48. Muller B., Yabroudi M.A., Lynch A., et al. Defining thresholds for the patient acceptable symptom state for the IKDC Subjective Knee Form and KOOS for patients who underwent ACL reconstruction. Am J Sports Med 44, 2820, 2016 [DOI] [PubMed] [Google Scholar]