Abstract
Since the beginning of clinical medicine, the human uterus has held the fascination of clinicians and researchers, given its critical role in the reproduction of our species. The endometrial lining provides residence for the embryo; however, this symbiotic interaction can be disrupted if the timing is not correct and the endometrium is not receptive. Diseases associated with the endometrium interfere with the reproductive process and cause a life-altering burden of pain and even death. With the advancement of technologies and new insights into the biology of the endometrium, much has been uncovered about the dynamic and essential changes that need to occur for normal endometrial function, as well as aberrations that lead to endometrial diseases. As expected, the more that is uncovered, the more the complexity of the endometrium is made evident. In this study, we bring together three areas of scientific advancement that remain in their infancy, but which together have the potential to mirror this complexity and enable understanding. Studies on induced pluripotent stem cells, three-dimensional tissue mimics, and microfluidic culture platforms will be reviewed with a focus on the endometrium. These unconventional approaches will provide new perspectives and appreciation for the elegance and complexity of the endometrium.
Impact statement
The ability of the human endometrium to regenerate on a monthly basis for ∼4 decades of reproductive years exemplifies its complexity as well as its susceptibility to disease. Restrictions on the types of research that can be done in the human endometrium motivate the development of new technologies and model systems. The three areas of technological advancement reviewed here—induced pluripotent stem cells, three-dimensional model systems, and microfluidic culture systems—will highlight some of the tools that can be applied to studying the human endometrium in ways that have not been done before.
Keywords: endometrium, organoids, iPSC, microfluidics, stem cells
Introduction
There has been a keen interest in the uterus from the beginning of medical practice given its essential role in human reproduction. The writings of Plato and Hippocrates described the uterus as being an “animal within an animal” that wanders inside a woman's body, also known as the “wandering womb,” subjecting women to some unusual medical practices.1,2 Early gynecologic theories assumed that all disease in women originated from the uterus. With advancements in science and medicine, it is known that the uterus does not wander and is kept in place with ligaments. Since then, much has been discovered concerning the anatomy and physiology of the uterus and there is still more biology to understand.
The human endometrium, which is the inner lining of the uterus, is a complex, dynamic, regenerative tissue that is essential for human reproduction. The normal function of the endometrium is housing a developing fetus, but this tissue is also subject to a multitude of diseases that range from excessive, uncontrolled bleeding to cancer, most of which require better treatment strategies. The essential function of the uterus in reproduction limits the types of studies that can be done on the endometrium. However, increased knowledge and advancement of technologies make it possible to study the endometrium outside of a woman's body in a manner that resembles the in vivo situation.
Three key advances that provide a microphysiological model of the endometrium will be reviewed in this study: induced pluripotent stem cells (iPSCs) to regenerate endometrial tissue, organoid models, and microfluidic culture technologies. The iPSCs provide a source of stem cells that retain the genetic makeup; organoids provide a three-dimensional (3D) culture model that responds to external cues in a physiological manner, some of which do not occur in two-dimensional culture; and microfluidic culture platforms provide an in vitro system through which culture medium flows to promote interaction between cells and/or tissues, replenishment of new media, and the mechanical influences of flow. While these research areas are still in their infancy, a review of studies will aid in the appropriate design of experiments and use of the new technologies.
Endometrial Stem Cells
Stem cell populations in the human endometrium
The hypothesis of the existence of stem cells in the human endometrium due to its extraordinary and incessant regenerative capability during the female reproductive life was suggested years ago by Prianishnikov and Padykula et al.3,4; since then, many efforts have been made in support of this possibility. Although studies have described different types of stem cells in the human endometrium, no consensus has been reached as to the localization or established markers of this population.5,6 Nevertheless, due to the complexity of this tissue, a possible explanation for the conflicting reports could be the existence of diverse stem cell populations, as described in skin, hair, and cardiac tissues.7–9 The identification and characterization of these stem cells, along with the study of the mechanisms controlling their regeneration, are expected to improve our understanding of the physiology and pathophysiology of the female reproductive tract.
In 1954, the first evidence of a multipotent endometrial resident stem-like cell population located in the endometrial-myometrial junction came from observations in malignant mixed Müllerian tumors.10,11 Most of the tumors arose from the stroma, which under the appropriate neoplastic stimulus gave rise to all the histological elements seen in these tumors: proof of endometrial multipotent differentiation potential. Other studies subsequently described the existence of a resident stem-like cell population in the human endometrium, present in the endometrial-myometrial junction, with highly regenerative capabilities (Table 1).3,4,12,13
Table 1.
Summary of Stem Cell Populations Described in the Human Endometrium
| Description | Stem cell type | Niche | Differentiation | Properties | Markers | Ref. |
|---|---|---|---|---|---|---|
| Endometrial-myometrial junction stem cells | Resident stem cells | Endometrial-myometrial junction and endometrial basalis | Myometrial smooth muscle cells Endometrial cells (epithelium, stroma, microvasculature) |
Proliferation is hormone independent | Tritiated thymidine slow cycling cells. | 3,4,10,11 |
| Side population endometrial stem cells | Mesenchymal stem cells Epithelial stem cells Endothelial progenitor cells |
Endometrial basalis and functionalis, and vessel locations | Endometrial cells (epithelium and stroma) Decidualization-in vitro mesodermal differentiation In vivo tissue reconstitution. |
Telomerase activity Telomere length Cloning efficiency Quiescent cell population and side population not from bone marrow origin |
CD105, CD146, CD31, CD34, CD144, CD90, BCRP1, MDR1, c-kit, and Oct-4. | 13,21–24,51 |
| Endometrial stromal/mesenchymal stem cells | Mesenchymal stem cells Endothelial progenitor cells |
Endometrial perivascular located in the basalis and functionalis |
In vitro mesodermal differentiation In vivo tissue reconstitution. |
Colony-forming assays Angiogenic properties Immunoregulatory properties |
CD146, PDGFRB, W5C5-SUSD2, CD31, CD29, CD44, CD73, CD90. and CD105. | 12–15 |
| Endometrial epithelial stem cells | Epithelial stem cells | Glands in the basalis | Gland-like structures | Methylation patterns and quiescent cell population | SSEA-1, Nanog, Oct-4, SOX9, N-cadherin and Lgr5. | 16–19 |
| Menstrual blood stem cells | Mesenchymal stem cells Epithelial stem cells Endothelial progenitor cells |
Endometrial functionalis | Multilineage differentiation (mesodermal, neuronal, and cardiac lineage) | High proliferative capacity and cloning efficiency | CD44, CD90, Oct-4, Nanog, SOX2, SSEA-4, c-kit, CD29, CD73, CD34 and CD105. | 25,92–94 |
Distinct stem cell populations have been described as regenerating the cells of endometrial lineages, located in different niches, theoretically suggesting a spatiotemporal contribution to the regeneration of the endometrium. The first endometrial stem cell population isolated was the mesenchymal stem-like cell population, consisting of pericytes or endothelial-like cells in the basalis and functionalis layers with mesenchymal stem cell characteristics.14–17 This population enabled stromal and vascular renewal during each menstrual cycle.
Epithelial replacement is carried out by the less-characterized endometrial epithelial stem-like cells, described in the literature with various markers that most likely identify different states of stem cell proliferation,18–21 rather than one single stem cell population. These distinct endometrial stem cell populations were isolated using flow cytometry and the Hoechst methodology of isolating side population stem cells.15,22–25 As a proof of concept, these cells were represented in the menstrual blood stem cell population.26 More recently, in vivo lineage tracing in mice demonstrated the existence of endometrial epithelial progenitors, which self-renewed during development, growth, and regeneration, with distinct activity from stromal cell renewal.27
All the aforementioned populations of progenitor/stem cells highlight the complexity of endometrial regeneration and growth. As stated by Padykula,28 the resident stem-like cell population migrates and gives rise to a group of progenitor cells that become committed to specific types of cell differentiation, for example, epithelial, stromal, and vascular cells, within certain microenvironments.29 Studies of the various endometrial progenitor/stem cells and their markers have been reviewed by others5,30–32 and are outlined in Table 1. Endometrial stem cells have been described as a powerful tool for clinical and therapeutic applications. Endometrial mesenchymal stem cells are considered a good source for stem cell therapy in several gynecological diseases, especially those related to tissue damage, based on in vitro and in vivo models.33
Induced pluripotent stem cells for building the human endometrium
The use of iPSCs as a progenitor pool for generating endometrial cells has gained attention in recent years, given the increased interest in personalized medicine and in understanding the genetics of reproductive diseases. The generation of human iPSCs from dermal fibroblasts was a breakthrough that provided an alternative to embryonic stem cell (ESC) research.34 Induction of four transcription factors referred as the Yamanaka factors (OCT3/4, SOX2, c-MYC, and KLF4) enabled the reprogramming of differentiated somatic cells into an embryonic/undifferentiated-like state.35 These iPSCs have the ability to self-renew indefinitely and can differentiate into all three germ layers, similar to ESCs. Dermal fibroblasts, adipocytes, peripheral blood cells, nasal epithelial cells, keratinocytes from hair, and renal tubular epithelial cells found in urine are all examples of somatic cell sources that have been used to generate iPSCs.
Since the introduction of human iPSCs in 2007, they have been utilized for drug development, disease modeling, and regenerative medicine. Due to their differentiation and proliferation capacities, iPSCs are a promising platform for cell-based therapies. Clinical trials based on iPSCs have been initiated for the treatment of macular degeneration, diabetes mellitus, and spinal cord injury in humans (reviewed in Guhr et al.36).
Endometrial lineages have been generated from human ESCs,37–39 with only one study confirming the expression of Müllerian Duct markers during differentiation from mesoderm.38 Moreover, only one study has demonstrated the generation of endometrial stromal cells from iPSCs.40 This was accomplished by understanding the developmental process of the uterus during embryogenesis. The human female reproductive tract develops from the intermediate mesoderm (IM).41–43 Shortly after gastrulation, the IM differentiates into the urogenital tract with primary kidneys that transiently form, as well as the Wolffian (mesonephric) and Müllerian (paramesonephric) ducts. In females, the mesonephric ducts degenerate as the paramesonephric ducts elongate and differentiate into the uterus, cervix, and upper vagina. While still in utero, at 16 weeks of age, the endometrium is lined with a single layer of columnar epithelial cells, and by week 18, the glands elongate and branch throughout the stroma.42,43 At birth, the uterine corpus remains underdeveloped, reaching functional and anatomical maturity at puberty.43,44 By puberty, the endometrium comprises a basal region (basalis) from which the endometrium regenerates, and an upper region that is shed (functionalis).
Miyazaki et al. differentiated iPSCs into endometrial stromal cells.40 The treatment regimen consisted of stimulating pathways that were previously demonstrated to differentiate cells from the IM into the Müllerian duct. Over the course of 4 days, iPSCs cultured as embryoid bodies were treated with a potent GSK3B inhibitor/CTNNB1 pathway agonist (CHIR99021) to create the day 2.5 primitive streak,45 followed by treatment with retinoic acid46,47 and fibroblast growth factor 2.48 To differentiate IM to coelomic epithelium, the cells were treated with a cocktail of CHIR, NOGGIN, and PDGF-BB over an additional 4 days.39,40,49 Finally, to generate endometrial stromal fibroblasts, D8 embryoid bodies were cultured for 6 days with 5′-aza-2′-deoxycytidine,50 CHIR99021, 17b-estradiol, FGF9,51 and PDGF-BB. Day-14 embryoid bodies showed endometrial stromal fibroblast lineage characteristics and markers supporting endometrial stromal identity, and did not contain any epithelial gland.
Thus, a stepwise differentiation protocol using customized cocktails was used to generate endometrial stromal fibroblasts from human iPSCs for the first time.40 Furthermore, the decidualization capacity of these endometrial stromal fibroblasts was confirmed after treatment with estradiol, medroxyprogesterone acetate, and 8-bromoadenosine 3′–5′-cyclic monophosphate. Generation of endometrial epithelial cells has yet to be done.
The generation of endometrial cells from iPSCs can ultimately be applied to clinical medicine. The genetic origins of reproductive diseases, including endometriosis and polycystic ovarian syndrome (PCOS), can be studied with this approach, as iPSCs can be generated on a patient-to-patient basis. As the pathogenesis of endometriosis and PCOS involve multiple tissues and organs, iPSCs can be used to understand whether the defects occur in nonreproductive tissues as well. The possibility of regenerating the endometrium from iPSCs in cases like Asherman's syndrome, where fibrosis of the endometrium occurs following endometrial trauma, could also be investigated.52
Human Endometrial Organoids: Recapitulating Native Tissues with Tissue Mimics
Organs function in three dimensions and are comprised of multiple cell types. Recapitulating the architecture and composition of organs ex vivo would permit scientists to understand their physiology. Historically, in vitro studies of the human endometrium have primarily been done using monolayer cultures, which preserve some characteristics of tissue function, for example, decidualization of the stromal fibroblasts. However, the architecture and cell-to-cell contacts that occur in three dimensions are lost in monolayer culture, resulting in suboptimal function, including a loss of hormone responsiveness.53,54 In particular, endometrial epithelial cells grown in monolayer cultures are short-lived, lose their columnar phenotype and polarity, and are prone to senescence.53 Furthermore, when endometrial epithelial and stromal cells are combined, the stromal cells quickly overtake the culture, making it difficult to study the important interactions between epithelial and stromal cells.
The emergence of organoid systems has enabled biologists to study cells in a more physiologic context. Although different definitions exist,55 organoids can be generally described as 3D models that reproduce the architecture, histology, and functions of an organ or tissue.5,54,56–59 Organoids may consist of a single cell type, often epithelial cells, or may include other types of cells.54,56,59 They can be generated from either somatic or stem cells, including ESCs, pluripotent stem cells, or adult stem cells.57,59 Many organoids contain both differentiated cells and tissue-specific stem or progenitor cells.5,56 Organoids self-organize to mimic in vivo tissue physiology and are often embedded in a matrix.5,57,59 They can be propagated and expanded, and are genetically stable.5,54,56–59
Organoids are appropriate for studying tissue functions in diverse contexts using various experimental techniques.57 Importantly, organoids are also suitable for conducting high-throughput screens, drug testing, and biobanking.57,59 Some limitations include the inability to reproduce some important aspects of the tissue microenvironment, such as circulating immune cells, and for many organoid systems, the dependence on artificial extracellular matrix components.57
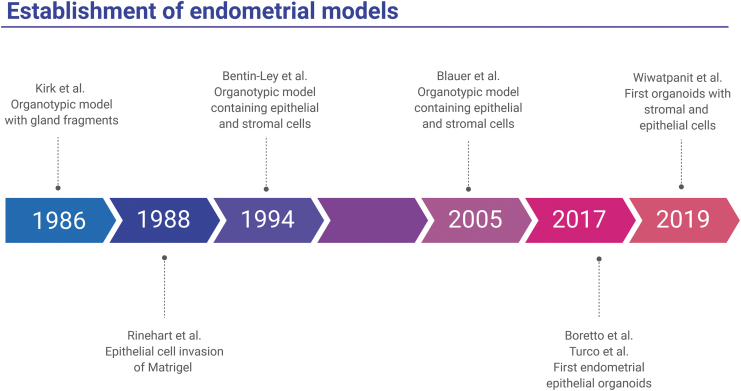
The first approach to an organotypic model of the endometrium was undertaken in 1986 by Kirk and Alvarez (Fig. 1).58 Endometrial gland fragments were isolated and grown in low-attachment conditions. These glandular structures consisted of a single, polarized layer of epithelial cells, and could respond to hormone treatments.58 A 1988 study was the first to show that endometrial epithelial cells could invade a Matrigel layer to form large glandular structures, consisting of polarized columnar epithelial cells surrounding a lumen.53 These epithelial cells contained secretory vesicles, reflecting their in vivo function. Furthermore, these structures could be passaged for up to 6 months.
FIG. 1.
Timeline of the development of three-dimensional organotypic models of the endometrium. Six studies from 1986 to 2019 that demonstrate novel three-dimensional models of the endometrium are highlighted: (1) a complex, free-floating endometrial epithelial structure (Kirk and Alvarez58), (2) an endometrial epithelial structure that invaded a Matrigel matrix (Rinehart et al.53), (3) polarized endometrial epithelial cells overlaid endometrial stromal cells embedded in collagen gel (Bentin-Ley et al.60), (4) endometrial organoids cultured in Matrigel in a tissue culture insert on top of endometrial stromal cells grown on plastic (Bläuer et al.61), (5) endometrial epithelial organoids embedded in Matrigel (Turco et al.63, and Boretto et al.54), (6) matrix-free endometrial organoids in which endometrial epithelial cells surround an inner cluster of endometrial stromal cells (Wiwatpanit et al.64). Images are created with BioRender.
Later, Bentin-Ley et al. established the first endometrial culture system to combine endometrial epithelial and stromal cells.60 Stromal cells were plated in a collagen matrix and overlaid with Matrigel, and the endometrial cells were cultured in a monolayer on top of the Matrigel, creating a physiologic model. The epithelial cells had a polarized, columnar epithelial phenotype, and displayed functional characteristics, including glycogen production. Another organotypic model of the endometrium was developed in 2005, in which endometrial glands were grown in Matrigel inside a tissue culture insert, while stromal cells were grown in 2D on the plate beneath the insert.61 These epithelial cells responded to hormone treatments by proliferating. This model was also used to test the effects of tamoxifen treatment on the endometrium.62
However, what were considered the first two true benign endometrial organoid models were published independently in May 2017 by Boretto et al.54 and Turco et al.63 Both models were derived from isolated endometrial epithelial cells embedded in Matrigel, resulting in organoids comprising polarized columnar epithelial cells surrounding a lumen. Both organoid models recapitulated important endometrial characteristics, including glycogen production and response to hormone treatment. In both cases, the organoids could be passaged long term, for more than 4–6 months. In addition, endometrial epithelial organoids were genetically stable and could be frozen and thawed, which would be important for tissue biobanking.63
Recently, a multicellular endometrial organoid model that contains both endometrial epithelial and stromal cells was established.64 These organoids were scaffold free and consisted of polarized epithelial cells on the outer surface, surrounding stromal cells in the center. The organoids displayed functional characteristics such as secretion of collagen and mucins. Because it includes both epithelial and stromal cells, this model allows for the investigation of paracrine actions between these two different cell types. A timeline of these studies is shown in Figure 1.
In addition to benign endometrial organoid models, organoids have also been derived from endometrial cancer tissues, and used for drug testing.65–68 These endometrial cancer organoids recapitulated genetic and phenotypic characteristics of endometrial tumors, and could be an efficient way of testing therapies.66–68 Organoids have also been derived from the endometrium of patients with other diseases, including endometrial hyperplasia, Lynch syndrome, and endometriosis.65 Endometrial organoids have provided an alternate physiologic model of the endometrium that can be adapted to biobanking, drug screenings, and investigation of endometrial physiology and pathology.
Dynamic Microfluidics: Interactions Between Reproductive Tissues
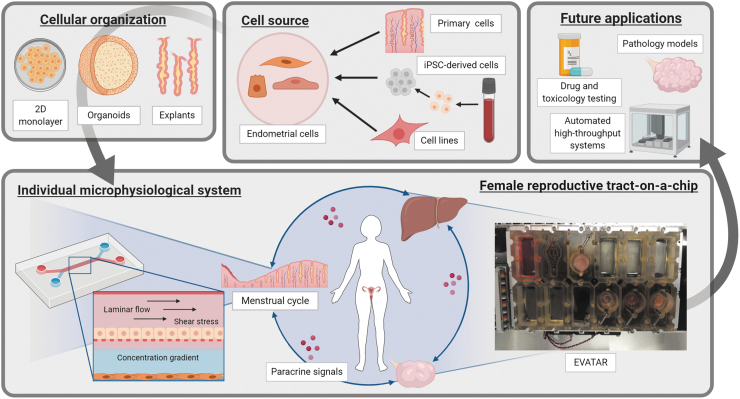
To successfully recreate the cyclical reorganization and functional changes of the endometrium under influence of female sex hormones, another technology has shown much promise, namely microfluidics and by extension organs-on-chips, also referred to as microphysiological systems (MPS).69–72 Microfluidics encompass technologies where small amounts of fluid are controlled with great precision, commonly using channels with diameters ranging from 1 mm to tens of micrometers.73 The main advantage of microfluidic systems is that physiological environments at tissue or organ level are recapitulated by accurately controlling fluid shear force, concentration gradients, dynamic mechanical shear stress, and cell patterning.69,74 The role of microfluidics in investigations in reproductive medicine is illustrated in Figure 2.
FIG. 2.
Microfluidic systems for reproductive biology. In vitro modeling of reproductive tissues and organs, require several design aspects. Cell source: cell sources, including cell lines, tissue biopsies, and iPSCs are valid options, each with their own pros and cons (reproducibility, availability, ease of use, costs, etc.) with different ideal use cases. Cellular organization: once isolated, these cells are cultured as monolayers or three-dimensional organoids, or maintained as tissue explants. Microphysiological systems: within a microfluidic device, cells are subjected to mechanical stresses, concentration gradients, and, depending on the design, paracrine factors between tissue types. On the left is a system conceptually similar to the one used by Gnecco et al.76,77 On the right is EVATAR, the Quintet-Microfluidic Platform, combining five modules to recreate the endocrine signaling in the female reproductive tract. Future applications: when combined with high-throughput technologies enabling parallelization, standardization, and automation, microfluidics can possibly become a powerful tool to analyze in vitro pathological models, drug testing, and toxicological testing. Images are created with BioRender. iPSC, induced pluripotent stem cell.
The complexity of these microfluidic environments can vary69: from simple microfluidic chambers with one cell type,75 to multicellular setups that recapitulate parenchymal-vascular interactions,76,77 to entire organ systems.72,78 Reproductive MPS are of particular interest because of their potential to recapitulate the complex hormonal feedback and feedforward signals between reproductive tissues and organs.70,71 As a result, MPS of different reproductive organs, including the endometrium,75–77 placenta,79–81 oviduct,82,83 amnion and fetal membrane,84–86 as well as a multiorgan microfluidic framework of the female reproductive tract,78 have been built.
Gnecco et al. created a microfluidic system to investigate cellular interactions in the perivascular endometrial stroma. The design uses two orthogonal microfluidic chambers separated by a semipermeable membrane.75–77 Human primary umbilical vein endothelial cells were subjected to a dynamic flow and cocultured with a static culture of human primary endometrial stromal cells. The importance of shear stress was demonstrated by the polarization of the endothelial cells. Furthermore, the model permitted decidualization of stromal cells after treatment with cAMP. This endothelial-stromal crosstalk and the importance of laminar shear stress were further investigated in a following publication.77
Dynamic culture conditions enhanced stromal decidualization, measured by higher levels of prolactin and insulin-like growth factor binding protein 1. This mechanosensing-dependent modulation was the result of increased production of prostaglandin E2 and prostacyclin from endothelial cells.77 A single-chamber version of this model was also used to recapitulate host-microbial interactions in vitro and elucidate the role of the decidual stromal cells on macrophages.75
The main advantage of these microfluidic systems is their ability to reproduce physiologic conditions, such as dynamic flow, shear stress, and cell-to-cell communication. However, these models are limited because they contain only certain cell types and cell lines cultured in two dimensions, and they do not account for other important organs that can influence the endometrium.
Currently, the only model combining multiple reproductive tissues and microfluidic technology was published by Xiao et al.,78 allowing the investigation of organ-organ interactions of the female reproductive tract during the human menstrual cycle. In this study, the female reproductive tract tissues (murine ovary, human fallopian tube, endometrium, and ectocervix) and liver were integrated in a new platform called EVATAR. Specifically, endometrial cells were grown in decellularized uterine scaffolds and were subjected to a 28-day menstrual cycle simulated by microfluidic cultures. Endometrial stromal cells expressed estrogen and progesterone receptors as well as markers of proliferation and decidualization at the end of the 28-day culture, indicative of active cell proliferation and endometrial cell function. Overall, this investigation demonstrated as proof of concept that interactive cultures of multiple organ systems can be developed for basic and translational research in reproductive medicine (Fig. 2).87
Microfluidic systems could change the design of experiments and allow the query of new research questions. Primary human cells in 3D tissue constructs can share paracrine signals with other tissue types in a system that provides continuous replenishment of nutrients, prolonged culture periods, and mechanical flow. These systems are especially useful in reproductive science where recapitulating the unique biology of sex hormone action over a period of a month of fluctuating levels of hormones in vitro or in animal models is extremely challenging. These systems can also be implemented in a scalable high-throughput MPS setup, facilitating the discovery of new drugs, toxicology screenings, and the study of complex multiorgan pathologies in a reproducible manner with high biological relevance.88
Conclusions and Perspectives
New advances in technology have changed the way research is done, introducing endless possibilities for investigating the biology of the endometrium and its associated diseases. The ability to generate stem cells from somatic cells, while retaining their genetic makeup, gives clues to the biology of differentiated cells in a personalized manner and within a particular disease. For example, can inherent defects in the genetic makeup of a woman with endometriosis promote a phenotype in endometrial stromal, immune, endothelial, or other cells that arises from iPSCs that is different from a woman without endometriosis? As clinical trials are underway for testing iPSCs to treat spinal cord injury, Parkinson's disease, diabetes, and others,89 the possibility of regenerating an injured endometrium from iPSCs can also be considered.
The hormone response of the human endometrium can be studied ex vivo using 3D mimics that closely resemble the tissue in vivo. Organoids can be manipulated and insulted with an experimental stimulus to study the direct influence of risk factors for reproductive cancers and other diseases. Increasing the complexity of organoids to include other cell types, including blood vessels and immune cells, would be an exciting direction to pursue and would provide a model centered around these new cell types.
Microfluidic platforms allow cells or tissues to mimic physiology with regard to flow, conditions for long-term viability, and interactions with other tissues, which increases the complexity of the experimental system, but in a controlled manner. A number of microfluidic systems have been and continue to be developed, each with its unique features and designs,90–95 diversifying the types of research that can be done.
Throughout history, the types of discoveries made were limited to the tools that were available. Imagine what Plato and Hippocrates could have done with microfluidics, organoids, and iPSCs. Because of breakthrough technologies like these, we are poised on the cusp of a new set of profound discoveries that will once again reshape our story of the uterus.
Disclosure Statement
No competing financial interests exist.
Funding Information
We would like to acknowledge support from grants NIEHS/NIH/NCATS UG3 (ES029073; T.W., J.J.K.), NIH/NCI R01CA243249-01 (J.J.K.), and ISCIII (PI17/01039 and CP19/00149; I.C.) and Generalitat Valenciana (PROMETEO/2018/137; I.C.).
References
- 1. Dinsdale N.L., and Crespi B.J.. Revisiting the wandering womb: oxytocin in endometriosis and bipolar disorder. Horm Behav 96, 69, 2017 [DOI] [PubMed] [Google Scholar]
- 2. Faraone C.A. Magical and medical approaches to the wandering womb in the ancient Greek world. Class Antiq 30, 1, 2011 [Google Scholar]
- 3. Padykula H.A., Coles L.G., Okulicz W.C., et al. The basalis of the primate endometrium: a bifunctional germinal compartment. Biol Reprod 40, 681, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Prianishnikov V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 18, 213, 1978 [DOI] [PubMed] [Google Scholar]
- 5. Tempest N., Maclean A., and Hapangama D.K.. Endometrial stem cell markers: current concepts and unresolved questions. Int J Mol Sci 19, 3240, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maruyama T. Endometrial stem/progenitor cells. J Obstet Gynaecol Res 40, 2015, 2014 [DOI] [PubMed] [Google Scholar]
- 7. Levy V., Lindon C., Harfe B.D., and Morgan B.A.. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 9, 855, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Lush M.E., Diaz D.C., Koenecke N., et al. scRNA-Seq reveals distinct stem cell populations that drive hair cell regeneration after loss of Fgf and Notch signaling. Elife 8, e4431, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monsanto M.M., White K.S., Kim T., et al. Concurrent isolation of 3 distinct cardiac stem cell populations from a single human heart biopsy. Circ Res 121, 113, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bird C.C., and Willis R.A.. The production of smooth muscle by the endometrial stroma of the adult human uterus. J Pathol Bacteriol 90, 75, 1965 [DOI] [PubMed] [Google Scholar]
- 11. Sternberg W.H., Clark W.H., and Smith R.C.. Malignant mixed mullerian tumor (mixed mesodermal tumor of the uterus); a study of twenty-one cases. Cancer 7, 704, 1954 [DOI] [PubMed] [Google Scholar]
- 12. Fujii S., Konishi I., and Mori T.. Smooth muscle differentiation at endometrio-myometrial junction. An ultrastructural study. Virchows Arch A Pathol Anat Histopathol 414, 105, 1989 [DOI] [PubMed] [Google Scholar]
- 13. Chan R.W., Schwab K.E., and Gargett C.E.. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 70, 1738, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Gurung S., Deane J.A., Darzi S., Werkmeister J.A., and Gargett C.E.. In vivo survival of human endometrial mesenchymal stem cells transplanted under the kidney capsule of immunocompromised mice. Stem Cells Dev 27, 35, 2018 [DOI] [PubMed] [Google Scholar]
- 15. Masuda H., Anwar S.S., Buhring H.J., Rao J.R., and Gargett C.E.. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant 21, 2201, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Schwab K.E., and Gargett C.E.. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 22, 2903, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Spitzer T.L., Rojas A., Zelenko Z., et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod 86, 58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim J.Y., Tavare S., and Shibata D.. Counting human somatic cell replications: methylation mirrors endometrial stem cell divisions. Proc Natl Acad Sci U S A 102, 17739, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valentijn A.J., Palial K., Al-Lamee H., et al. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod 28, 2695, 2013 [DOI] [PubMed] [Google Scholar]
- 20. Cervello I., Gil-Sanchis C., Santamaria X., et al. Leucine-rich repeat-containing G-protein-coupled receptor 5-positive cells in the endometrial stem cell niche. Fertil Steril 107, 510, 2017 [DOI] [PubMed] [Google Scholar]
- 21. Nguyen H.P.T., Xiao L., Deane J.A., et al. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum Reprod 32, 2254, 2017 [DOI] [PubMed] [Google Scholar]
- 22. Cervello I., Gil-Sanchis C., Mas A., et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One 5, e10964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cervello I., Gil-Sanchis C., Mas A., et al. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One 7, e30260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato K., Yoshimoto M., Kato K., et al. Characterization of side-population cells in human normal endometrium. Hum Reprod 22, 1214, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Tsuji S., Yoshimoto M., Takahashi K., Noda Y., Nakahata T., and Heike T.. Side population cells contribute to the genesis of human endometrium. Fertil Steril 90, 1528, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Patel A.N., Park E., Kuzman M., Benetti F., Silva F.J., and Allickson J.G.. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant 17, 303, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Syed S.M., Kumar M., Ghosh A., et al. Endometrial Axin2(+) cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell 26, 64, 2020 [DOI] [PubMed] [Google Scholar]
- 28. Padykula H.A. Regeneration in the primate uterus: the role of stem cells. Ann N Y Acad Sci 622, 47, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Hall A.K. Stem cell is a stem cell is a stem cell. Cell 33, 11, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Gargett C.E., Schwab K.E., and Deane J.A.. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update 22, 137, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teixeira J., Rueda B.R., and Pru J.K.. Uterine Stem Cells. Cambridge, MA: StemBook, 2008 [PubMed] [Google Scholar]
- 32. Zhu X., Peault B., Yan G., Sun H., Hu Y., and Ding L.. Stem cells and endometrial regeneration: from basic research to clinical trial. Curr Stem Cell Res Ther 14, 293, 2019 [DOI] [PubMed] [Google Scholar]
- 33. Zuo W., Xie B., Li C., et al. The clinical applications of endometrial mesenchymal stem cells. Biopreserv Biobank 16, 158, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shi Y., Inoue H., Wu J.C., and Yamanaka S.. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 16, 115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takahashi K., and Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Guhr A., Kobold S., Seltmann S., Seiler Wulczyn A.E.M., Kurtz A., and Loser P.. Recent trends in research with human pluripotent stem cells: impact of research and use of cell lines in experimental research and clinical trials. Stem Cell Reports 11, 485, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song T., Zhao X., Sun H., et al. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng Part A 21, 353, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ye L., Mayberry R., Lo C.Y., et al. Generation of human female reproductive tract epithelium from human embryonic stem cells. PLoS One 6, e21136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu W.Z., Chen X.M., Niu W.B., Wang F., Sun B., and Sun Y.P.. Role of Wnt5a in the differentiation of human embryonic stem cells into endometrium-like cells. Int J Clin Exp Pathol 8, 5478, 2015 [PMC free article] [PubMed] [Google Scholar]
- 40. Miyazaki K., Dyson M.T., Coon V.J., et al. Generation of progesterone-responsive endometrial stromal fibroblasts from human induced pluripotent stem cells: role of the WNT/CTNNB1 pathway. Stem Cell Reports 11, 1136, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mullen R.D., and Behringer R.R.. Molecular genetics of Mullerian duct formation, regression and differentiation. Sex Dev 8, 281, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cunha G.R., Robboy S.J., Kurita T., et al. Development of the human female reproductive tract. Differentiation 103, 46, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robboy S.J., Kurita T., Baskin L., and Cunha G.R.. New insights into human female reproductive tract development. Differentiation 97, 9, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cooke P.S., Spencer T.E., Bartol F.F., and Hayashi K.. Uterine glands: development, function and experimental model systems. Mol Hum Reprod 19, 547, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lam A.Q., Freedman B.S., Morizane R., Lerou P.H., Valerius M.T., and Bonventre J.V.. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol 25, 1211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nakajima T.I., Iguchi T., and Sato T.. Retinoic acid signaling determines the fate of uterine stroma in the mouse Müllerian duct. Proc Natl Acad Sci USA 113, 14354, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kobayashi A., and Behringer R.R.. Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 4, 969, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Filant J., DeMayo F.J., Pru J.K., Lydon J.P., and Spencer T.E.. Fibroblast growth factor receptor two (FGFR2) regulates uterine epithelial integrity and fertility in mice. Biol Reprod 90, 7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Little M., Georgas K., Pennisi D., and Wilkinson L.. Kidney development: two tales of tubulogenesis. Curr Top Dev Biol 90, 193, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Banerjee S., and Bacanamwo M.. DNA methyltransferase inhibition induces mouse embryonic stem cell differentiation into endothelial cells. Exp Cell Res 316, 172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsai S.J., Wu M.H., Chen H.M., Chuang P.C., and Wing L.Y.. Fibroblast growth factor-9 is an endometrial stromal growth factor. Endocrinology 143, 2715, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Miyazaki K., Maruyama T., Masuda H., et al. Stem cell-like differentiation potentials of endometrial side population cells as revealed by a newly developed in vivo endometrial stem cell assay. PLoS One 7, e50749, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rinehart C.A., Lyn-Cook B.D., and Kaufman D.G.. Gland formation from human endometrial epithelial cells in vitro. In Vitro Cell Dev Biol 24, 1037, 1988 [DOI] [PubMed] [Google Scholar]
- 54. Boretto M., Cox B., Noben M., et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 144, 1775, 2017 [DOI] [PubMed] [Google Scholar]
- 55. Simian M., and Bissell M.J.. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol 216, 31, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deane J.A., Cousins F.L., and Gargett C.E.. Endometrial organoids: in vitro models for endometrial research and personalized medicine. Biol Reprod 97, 781, 2017 [DOI] [PubMed] [Google Scholar]
- 57. Fatehullah A., Tan S.H., and Barker N.. Organoids as an in vitro model of human development and disease. Nat Cell Biol 18, 246, 2016 [DOI] [PubMed] [Google Scholar]
- 58. Kirk D., and Alvarez R.B.. Morphologically stable epithelial vesicles cultured from normal human endometrium in defined media. In Vitro Cell Dev Biol 22, 604, 1986 [DOI] [PubMed] [Google Scholar]
- 59. Li M., and Izpisua Belmonte J.C.. Organoids—preclinical models of human disease. N Engl J Med 380, 569, 2019 [DOI] [PubMed] [Google Scholar]
- 60. Bentin-Ley U., Pedersen B., Lindenberg S., Larsen J.F., Hamberger L., and Horn T.. Isolation and culture of human endometrial cells in a three-dimensional culture system. J Reprod Fertil 101, 327, 1994 [DOI] [PubMed] [Google Scholar]
- 61. Bläuer M., Heinonen P.K., Martikainen P.M., Tomás E., and Ylikomi T.. A novel organotypic culture model for normal human endometrium: regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum Reprod 20, 864, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Bläuer M., Heinonen P.K., Rovio P., and Ylikomi T.. Effects of tamoxifen and raloxifene on normal human endometrial cells in an organotypic in vitro model. Eur J Pharmacol 592, 13, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Turco M.Y., Gardner L., Hughes J., et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol 19, 568, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wiwatpanit T., Murphy A.R., Lu Z., et al. Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. J Clin Endocrinol Metab 105, dgz100, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Boretto M., Maenhoudt N., Luo X., et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol 21, 1041, 2019 [DOI] [PubMed] [Google Scholar]
- 66. Girda E., Huang E.C., Leiserowitz G.S., and Smith L.H.. The use of endometrial cancer patient–derived organoid culture for drug sensitivity testing is feasible. Int J Gynecol Cancer 27, 1701, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Maru Y., Tanaka N., Itami M., and Hippo Y.. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol Oncol 154, 189, 2019 [DOI] [PubMed] [Google Scholar]
- 68. Tamura H., Higa A., Hoshi H., et al. Evaluation of anticancer agents using patient-derived tumor organoids characteristically similar to source tissues. Oncol Rep 40, 635, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bhatia S.N., and Ingber D.E.. Microfluidic organs-on-chips. Nat Biotechnol 32, 760, 2014 [DOI] [PubMed] [Google Scholar]
- 70. Laronda M.M., Burdette J.E., Kim J., and Woodruff T.K.. Recreating the female reproductive tract in vitro using iPSC technology in a linked microfluidics environment. Stem Cell Res Ther 4(Suppl 1), S13, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eddie S.L., Kim J.J., Woodruff T.K., and Burdette J.E.. Microphysiological modeling of the reproductive tract: a fertile endeavor. Exp Biol Med (Maywood) 239, 1192, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Edington C.D., Chen W.L.K., Geishecker E., et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci Rep 8, 4530, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Whitesides G.M. The origins and the future of microfluidics. Nature 442, 368, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Wu Q., Liu J., Wang X., et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomed Eng Online 19, 9, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rogers L.M., Anders A.P., Doster R.S., et al. Decidual stromal cell-derived PGE2 regulates macrophage responses to microbial threat. Am J Reprod Immunol 80, e13032, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gnecco J.S., Pensabene V., Li D.J., et al. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann Biomed Eng 45, 1758, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gnecco J.S., Ding T., Smith C., Lu J., Bruner-Tran K.L., and Osteen K.G.. Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin E2 and prostacyclin in a microfluidic model of the human endometrium. Hum Reprod 34, 702, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Xiao S., Coppeta J.R., Rogers H.B., et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 8, 14584, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pemathilaka R.L., Reynolds D.E., and Hashemi N.N.. Drug transport across the human placenta: review of placenta-on-a-chip and previous approaches. Interface Focus 9, 20190031, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee J.S., Romero R., Han Y.M., et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med 29, 1046, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Blundell C., Tess E.R., Schanzer A.S., et al. A microphysiological model of the human placental barrier. Lab Chip 16, 3065, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ferraz M., Rho H.S., Hemerich D., et al. An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming. Nat Commun 9, 4934, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ferraz M., Henning H.H.W., Costa P.F., et al. Improved bovine embryo production in an oviduct-on-a-chip system: prevention of poly-spermic fertilization and parthenogenic activation. Lab Chip 17, 905, 2017 [DOI] [PubMed] [Google Scholar]
- 84. Richardson L., Jeong S., Kim S., Han A., and Menon R.. Amnion membrane organ-on-chip: an innovative approach to study cellular interactions. FASEB J 33, 8945, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gnecco J.S., Anders A.P., Cliffel D., et al. Instrumenting a fetal membrane on a chip as emerging technology for preterm birth research. Curr Pharm Des 23, 6115, 2017 [DOI] [PubMed] [Google Scholar]
- 86. Richardson L., Gnecco J., Ding T., et al. Fetal membrane organ-on-chip: an innovative approach to study cellular interactions. Reprod Sci 2019. [Epub ahead of print]; DOI: 10.1177/1933719119828084 [DOI] [PubMed]
- 87. Nawroth J., Rogal J., Weiss M., Brucker S.Y., and Loskill P. Organ-on-a-chip systems for women's health applications. Adv Healthc Mater 7, 2018. DOI: 10.1002/adhm.201700550 [DOI] [PubMed] [Google Scholar]
- 88. Probst C., Schneider S., and Loskill P.. High-throughput organ-on-a-chip systems: current status and remaining challenges. Curr Opin Biomed Eng 6, 33, 2018 [Google Scholar]
- 89. Trounson A., and DeWitt N.D.. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol 17, 194, 2016 [DOI] [PubMed] [Google Scholar]
- 90. Beckwitt C.H., Clark A.M., Wheeler S., et al. Liver ‘organ on a chip’. Exp Cell Res 363, 15, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hu C., Chen Y., Tan M.J.A., Ren K., and Wu H.. Microfluidic technologies for vasculature biomimicry. Analyst 144, 4461, 2019 [DOI] [PubMed] [Google Scholar]
- 92. Mofazzal Jahromi M.A., Abdoli A., Rahmanian M., et al. Microfluidic brain-on-a-chip: perspectives for mimicking neural system disorders. Mol Neurobiol 56, 8489, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ruzycka M., Cimpan M.R., Rios-Mondragon I., and Grudzinski I.P.. Microfluidics for studying metastatic patterns of lung cancer. J Nanobiotechnology 17, 71, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bein A., Shin W., Jalili-Firoozinezhad S., et al. Microfluidic organ-on-a-chip models of human intestine. Cell Mol Gastroenterol Hepatol 5, 659, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sontheimer-Phelps A., Hassell B.A., and Ingber D.E.. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer 19, 65, 2019 [DOI] [PubMed] [Google Scholar]