Abstract
Two HIV virologic control advances are in various stages of development, including long-acting antiretroviral therapy (ART) formulations and strategies aimed at sustained ART-free HIV control. Perceptions of risks and benefits toward HIV virologic control strategies may be different based on an individual's age due to differing experiences of the impacts of the domestic HIV epidemic, altruistic attitudes toward research participation, and general levels of engagement in health care. We examined preferences of HIV virologic control strategies by age groups. In 2018, we conducted a nationwide, online cross-sectional survey to examine differences in HIV virologic control strategies among a sample of people living with HIV who were <50 and ≥50 years of age. From a total of 281 participants, 3 findings were noteworthy: (1) Participants <50 years of age were more likely to be demotivated by perceived social risks (e.g., stigma, discrimination, HIV disclosure, and fear of transmitting HIV during a treatment interruption), compared with those ≥50 years; (2) participants ≥50 years of age were more motivated by altruistic notions compared with those <50 years; and (3) we noted greater desirability of longer long-acting ART and new HIV cure-related strategies among participants <50 years versus those ≥50 years. Our analysis provides a deeper understanding of differences in perceptions among various age groups regarding desirable future ART characteristics, and motivations and barriers to participating in HIV cure-related strategies. Our findings can help inform community engagement and education, and assist researchers in tailoring study design and recruitment efforts to major age groups.
Keywords: HIV cure-related research, HIV virologic control strategies, age categories, long-acting antiretroviral therapy, perceptions and attitudes, socio-behavioral research
Introduction
Presently, over 30 antiretroviral medications have been approved by the U.S. Food and Drug Administration (FDA) for treating HIV. These medications, mostly administered as once per day single-pill oral regimens with high potency and minimal toxicity, have resulted in near-normal life expectancy for those in their 20s.1 HIV medications have also averted nearly 9.5 million deaths due to AIDS and 7.9 million new HIV infections worldwide since the start of the epidemic.2 Despite the life-saving benefits of antiretroviral therapy (ART) and the availability of simplified regimens, ART adherence remains suboptimal, adherence barriers persist (such as substance use, pill fatigue, stigma, etc.), and sustaining viral suppression remains a challenge.3
Two therapeutic advances that are in various stages of development include long-acting ART formulations (including injectables and implants).4,5 and strategies aimed at sustained ART-free HIV control (such as immune-based strategies, stem cell transplantations, gene editing approaches, latency reversing agents, and various combinations).6 Long-acting formulations will simplify dosing, remove the need for daily adherence, and address pill fatigue; however, the drawbacks include the need for additional adherence to clinic visits and sustained virologic suppression using oral ART before initiating a long-acting formulation. There are currently no known direct clinical benefits of participating in HIV cure-related research and participants may experience adverse effects.7 Therefore, unlike the early days of the HIV epidemic when people living with HIV (PLWH) joined trials in the hopes of their survival, otherwise healthy PLWH are currently being asked to take risks to advance HIV cure-related research without any anticipation of direct clinical benefits.7–10
Importantly, there are numerous potential risks and increasing number of HIV therapeutic options, which makes it more important than ever to ascertain patient perspectives regarding their willingness, motivations, barriers, and perceptions of HIV virologic control strategies (including therapeutic and cure-related strategies). We previously published data on patient preferences and willingness to participate in research regarding HIV virologic control strategies and the risks they are willing to take.7,11,12 We published results on sex/gender differences with regard to preferences12; however, there are limited data on preferences for HIV virologic control strategies among various age groups. Perceptions of potential risks and benefits toward HIV virologic control strategies may be vastly different among age groups due to differing experiences of the impacts of the domestic HIV epidemic, altruistic attitudes toward participation, and general levels of engagement in health care. Therefore, our objective was to understand how preferences, attitudes, barriers, and willingness to participate may vary by age groups; an understanding that can inform community engagement and assist researchers in their study designs and recruitment efforts to advance the field of HIV therapeutics.
Methods
Between May and August 2018, we conducted a nationwide, online cross-sectional survey via Qualtrics (Provo, UT) to examine differences in preferences, motivators, and demotivators to participation in research regarding HIV cure-related strategies among a sample of PLWH who were <50 and ≥50 years of age. Additionally, we examined scenarios and tradeoffs of choosing a new HIV virologic control strategy over standard daily ART. The U.S. Department of Human and Health Services defines older adults living with HIV as 50 years or older.13 We chose the age cutoff of 50 given growing data regarding the aging population of PLWH having differing physical, mental, and social needs.14,15 For enhanced ease of interpretation of results, we refer to those <50 as “younger” and those ≥50 as “older” age groups.
Survey items were developed in collaboration with community members and extensively piloted as further detailed in a prior publication.12 Individuals who were ≥18 years of age, living with HIV, willing to provide their opinion on HIV virologic control research strategies, and living in the United States or its territories were included. Participants were recruited via a convenience sample of PLWH who had subscribed to HIV treatment and cure listservs [such as immune-based therapy, the Martin Delaney Collaboratories Toward an HIV Cure Community Advisory Boards, the AIDS Clinical Trials Group (ACTG), AIDS Treatment Activists Coalition (ATAC), Body, POZ, Forum for Collaborative Research, Well Project, and Positive Women's Network-USA]. One in 10 participants was randomly chosen to receive a $20 USD Visa® gift card. The survey was approved by The University of North Carolina at Chapel Hill Non-Biomedical IRB.
Measures
Participant characteristics
These characteristics included demographics (age, sex/gender, race/ethnicity, U.S. region of residence, highest education completed, yearly household income, and marital status) and history with and interest in participation in research regarding HIV virologic control strategies.
Motivators and barriers to participating in HIV cure-related research
Participants chose the extent to which various factors would prevent them from participating or motivate them to participate in HIV cure-related research. Responses were categorized to three options, including (1) “not at all or some extent/degree,” (2) “moderate extent/degree,” or (3) “great or very great extent/degree.” Barriers to participation in HIV cure-related research included 18 potential personal health risks and burdens (such as dementia, pain, drug resistance, etc.) and 7 potential social risks (such as financial risks, stigma or discrimination, loss of confidentiality, etc.). Motivators included 12 potential social, psychological, and emotional factors (such as feeling good contributing to HIV cure-related research, feeling good helping future PLWH, etc.) and 4 social factors (such as financial compensation, support from family and friends, etc.).
Perceived improvements over current ART
Participants rated 12 different potential outcomes of HIV cure-related strategies over their current daily ART. Responses were categorized to three options, including (1) “no or small improvement,” (2) “moderate improvement,” or (3) “large or life-changing improvement” over current ART.
Scenario choices
Participants were asked about their likelihood of choosing an HIV cure-related strategy over their current daily ART under seven different hypothetical scenarios. They included a possibility of adverse effects, requiring treatment interruption, need for increased laboratory monitoring, and others. Response options were categorized to three options, including (1) “not at all likely or somewhat unlikely,” (2) “neither likely nor unlikely,” or (3) “moderately or very likely” to choose an HIV cure-related scenario over their current daily ART.
Acceptable tradeoffs
Participants were asked about how bothersome five hypothetical tradeoffs of a new HIV cure-related strategy would be compared to their current daily ART. These tradeoffs included impact on mental health status, changes to appearance, experience of mild to moderate pain, etc. Responses were categorized to three options, including (1) “not or somewhat bothered,” (2) “moderately bothered,” or (3) “very bothered or unacceptable.”
Choice between current daily ART, long-acting antiretroviral medications, and HIV cure-related strategies
Participants were asked to choose between either (1) daily ART; (2) injectable or implantable antiretroviral medications that last 1 month, (3) injectable or implantable antiretroviral medications that last 2 months, (4) injectable or implantable antiretroviral medications that last 6 months; or (5) long-lasting cure-related strategy as their preferred hypothetical virologic control strategy.
Analysis
All measures were compared between those <50 and ≥50 years of age. The proportions of the two age groups were broken down by their responses to the categorical measures. The differences in the proportions of the age groups that responded to categorical measures with their lowest values (e.g., “not at all or some extent/degree,” “no or small improvement,” “not at all likely or somewhat unlikely,” etc.) were tested for statistical significance. Likewise, the differences in the proportions of the age groups that responded to categorical measures with their highest values (e.g., “great or very great extent/degree,” “large or life-changing improvement,” “moderately or very likely,” etc.) were tested for statistical significance. Statistical significance (all p < .05) was determined through chi-square tests. Based on the sample size and exploratory nature of these analyses, we also examined variables with p-value <.1 to identify and assess these variables in future research. All analyses were conducted using Stata (version 15; StataCorp, College Station, TX).
Results
Participant characteristics
A total of 281 participants completed the survey and identified their age (1 person did not provide their age and was excluded from this analysis). The majority of participants were white (65%), cisgender men (64%), from the South of the United States (45%), and with a 4-year college degree or higher (50%). Participants were nearly evenly split between the 2 age groups: 147 (52%) were in the younger age group and 134 (48%) were in the older age group (Table 1). The mean age among the younger age group was 37 years [standard deviation (SD) = 7.9] and 58 (SD = 5.4) among those in the older age group. Participants' distribution between the two groups was balanced with regard to sex/gender, ethnicity, U.S. region of residence, highest level of formal education completed, and household income. However, among the younger age group, more participants were African American (31%), more were single (56%), and fewer were widowed (0%) compared with the older age group (16%, 32%, and 10%, respectively) (Table 1).
Table 1.
Characteristics of Study Participants
| Age ≥50 years | Age <50 years | |
|---|---|---|
| N = 134 | N = 147 | |
| Age, years | ||
| Mean (SD) | 58 (5.4) | 37 (7.9) |
| Median (IQR) | 56 (54–62) | 37 (30–44) |
| Age groups, years, n (%) | ||
| 19–29 | 0 (0) | 33 (23) |
| 30–39 | 0 (0) | 52 (35) |
| 40–49 | 0 (0) | 62 (42) |
| 50–59 | 90 (67) | 0 (0) |
| 60–72 | 44 (33) | 0 (0) |
| N = 133 | N = 146 | |
|---|---|---|
| Sex assigned at birth, n (%) | ||
| Female |
45 (34) |
51 (35) |
| Male | 88 (66) | 95 (65) |
| N = 134 | N = 147 | |
|---|---|---|
| Gender, n (%) |
|
|
| Cisgender woman |
48 (36) |
50 (34) |
| Cisgender man |
85 (63) |
94 (64) |
| Other | 1 (1) | 3 (2) |
| N = 133 | N = 145 | |
|---|---|---|
| Race, n (%) |
|
|
| White or Caucasian |
98 (74) |
84 (58) |
| Black or African American |
21 (16) |
45 (31) |
| Other | 14 (10) | 16 (11) |
| N = 130 | N = 141 | |
|---|---|---|
| Ethnicity, n (%) | ||
| Hispanic or Latinx |
13 (10) |
19 (13) |
| Not Hispanic or Latinx |
112 (86) |
114 (81) |
| Not sure/prefer not to answer | 5 (4) | 8 (6) |
| N = 134 | N = 137 | |
|---|---|---|
| Region of residence, n (%) | ||
| Northeast |
24 (18) |
18 (13) |
| Midwest |
18 (13) |
14 (10) |
| South |
51 (38) |
72 (53) |
| West | 41 (31) | 33 (24) |
| N = 134 | N = 146 | |
|---|---|---|
| Highest level of formal education completed, n (%) | ||
| Some high school, but no diploma |
4 (3) |
5 (3) |
| High school diploma or GED |
12 (9) |
18 (12) |
| Some college, but no diploma |
46 (34) |
38 (26) |
| 2- or 4-year college degree |
44 (33) |
49 (34) |
| Master's/Professional degree/Doctorate degree or equivalent | 28 (21) | 36 (25) |
| N = 103 | N = 117 | |
|---|---|---|
| Yearly household income, n (%) | ||
| Less than $15,000 |
21 (20) |
20 (17) |
| $15,000 to $25,000 |
15 (15) |
13 (11) |
| $25,001 to $50,000 |
24 (23) |
32 (27) |
| $50,001 to $75,000 |
11 (11) |
13 (11) |
| More than $75,000 |
31 (30) |
30 (26) |
| Prefer not to answer | 1 (1) | 9 (8) |
| N = 103 | N = 117 | |
|---|---|---|
| Marital status, n (%) | ||
| Single or never married |
33 (32) |
65 (56) |
| Married or living with partner |
36 (35) |
31 (26) |
| Separated or divorced |
21 (20) |
17 (15) |
| Widowed |
10 (10) |
0 (0) |
| Other | 3 (3) | 4 (3) |
| N = 133 | N = 144 | |
|---|---|---|
| Ever volunteered for a study to test safety or efficacy of an ART drug or related drug, n (%) | ||
| Yes |
36 (27) |
21 (15) |
| No |
94 (71) |
121 (84) |
| Don't know | 3 (2) | 2 (1) |
| N = 133 | N = 144 | |
|---|---|---|
| Ever volunteered for a medical study respondent believed to be an HIV cure study, n (%) | ||
| Yes |
20 (15) |
8 (6) |
| No |
110 (83) |
133 (92) |
| Don't know | 3 (2) | 3 (2) |
| N = 110 | N = 132 | |
|---|---|---|
| Reasons why the respondents did not participate in HIV cure studies, n (%) | ||
| Did not know about them |
69 (63) |
104 (79) |
| Study site too far away/did not compensate for travel costs |
22 (20) |
18 (14) |
| Did not qualify for them |
20 (18) |
18 (14) |
| Frightened because it would stop HIV medications for some period of time |
17 (15) |
16 (12) |
| Frightened of side effects or negative health effects |
12 (11) |
12 (9) |
| Could not get away from work |
7 (6) |
9 (7) |
| Required too much time away from regular routine |
8 (7) |
4 (3) |
| Regular health provider recommended that I not participate |
6 (5) |
5 (4) |
| Friend or family member said that I shouldn't participate |
2 (2) |
2 (2) |
| Study did not cover childcare/family care costs involved in participation |
0 (0) |
1 (1) |
| Other | 12 (11) | 10 (8) |
| N = 133 | N = 144 | |
|---|---|---|
| Currently in a study respondent believes to be an HIV cure study, n (%) | ||
| Yes |
3 (2) |
5 (4) |
| No |
129 (97) |
137 (95) |
| Don't know | 1 (1) | 2 (1) |
ART, antiretroviral therapy; GED, General Educational Development; IQR, interquartile range; SD, standard deviation.
Fewer participants in the younger age group had previously participated in HIV treatment and HIV cure-related research versus those in the older age group (15% and 27%, respectively) (Table 1). In both groups, the main reasons for not participating in HIV cure-related research was not knowing about these studies, followed by transportation barriers of study distance and travel costs.
Motivators and barriers to participating in HIV cure-related research
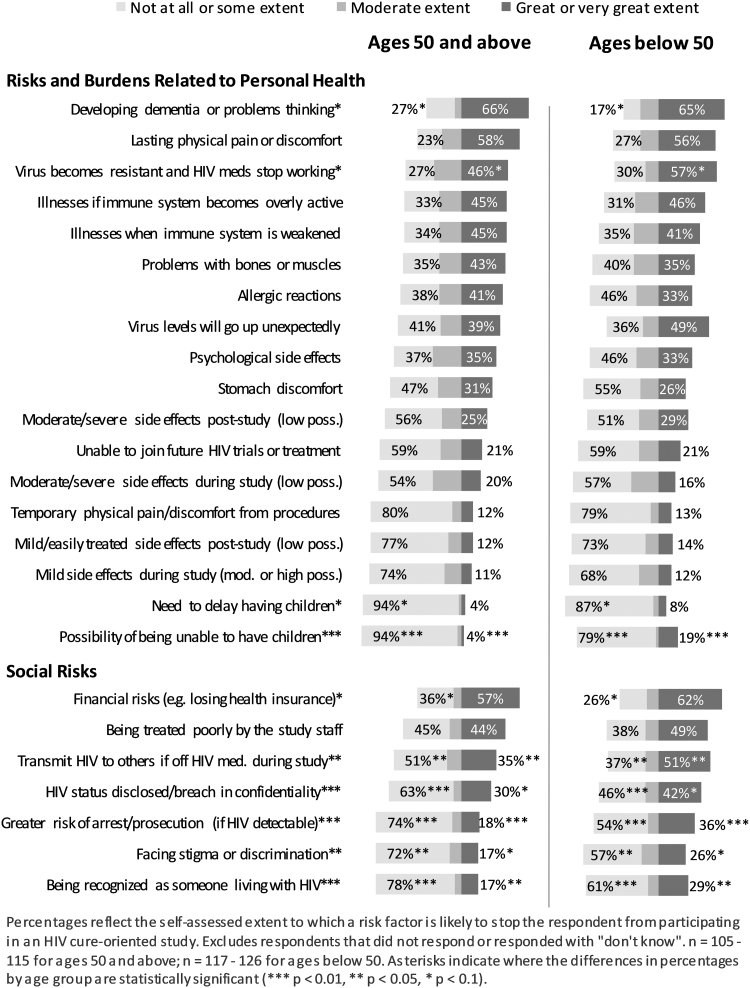
The top 3 reasons that would deter respondents from participating in HIV cure-related research, in both age groups, were developing dementia or problems thinking, lasting physical pain or discomfort, and financial risks (Fig. 1). In general, perceptions of social risks were more significantly different between the two age groups when compared to risks related to personal health, with those younger being more demotivated by several social risks versus older participants. Among the barriers to personal health, the possibility of not being able to have children was a greater demotivator for those in the younger compared with those in the older age groups (p < .01). Among social risks, younger participants were more demotivated from participating in HIV cure-related research due to the fear of transmitting HIV to others when being off their ART during the study (p < .05), greater risk of arrest or prosecution (p < .01), and being recognized as someone living with HIV (p < .05). Other possible deterring risks that were marginally statistically significant (p < .1) were the virus becoming resistant to ART, the possibility of having their HIV serostatus disclosed or breach in confidentiality, and facing stigma or discrimination. More of the older participants were not as deterred by financial risks (e.g., losing health insurance) (p < .1), developing dementia or problems thinking, and need to delay having children as people in the younger age group (Fig. 1).
FIG. 1.
Extent to which risk factors are likely to stop respondent from participating in an HIV cure-related study, by age group.
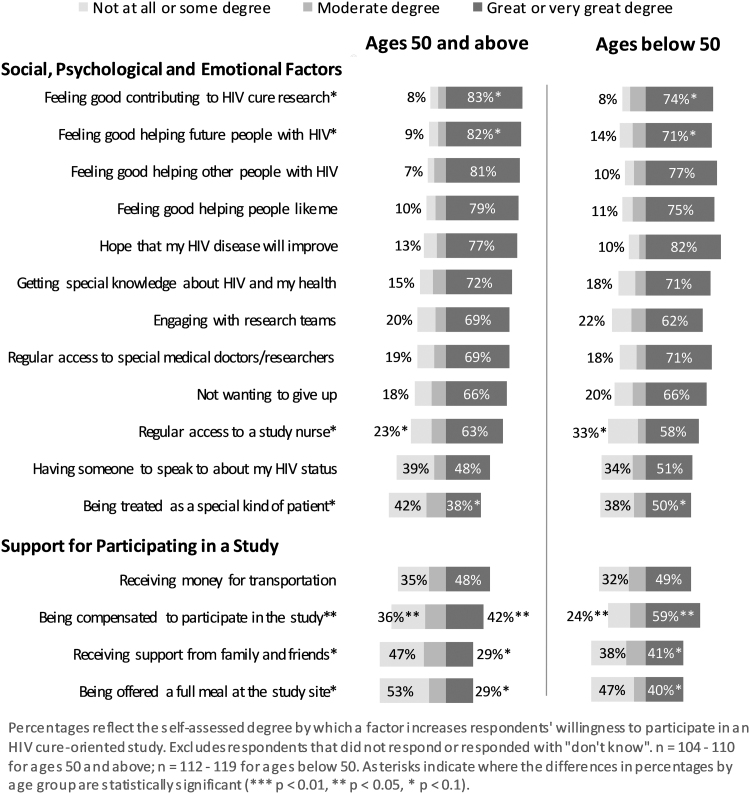
Altruistic reasons, such as feeling good contributing to HIV cure-related research and helping future people with HIV were marginally statistically significantly greater motivators for being willing to participate in HIV cure-related research for those in the older compared with those in the younger age groups (p < .1). Being treated as a special kind of patient (i.e., receiving more attention from clinical staff) was a marginally statistically significantly greater motivator for the younger age group than the older age group (p < .1). The older age group was marginally statistically significantly less motivated by having regular access to a study nurse than the younger age group (p < .1) (Fig. 2). Being compensated for participating in HIV cure-related research was a significantly greater motivator for younger versus older age groups (p < .05). Additionally, receiving support from family and friends, and being offered a full meal at the study site, were marginally statistically significantly greater motivators for those younger compared with older age groups (p < .1).
FIG. 2.
Degree by which factors increase respondents' willingness to participate in an HIV cure-related study, by age group.
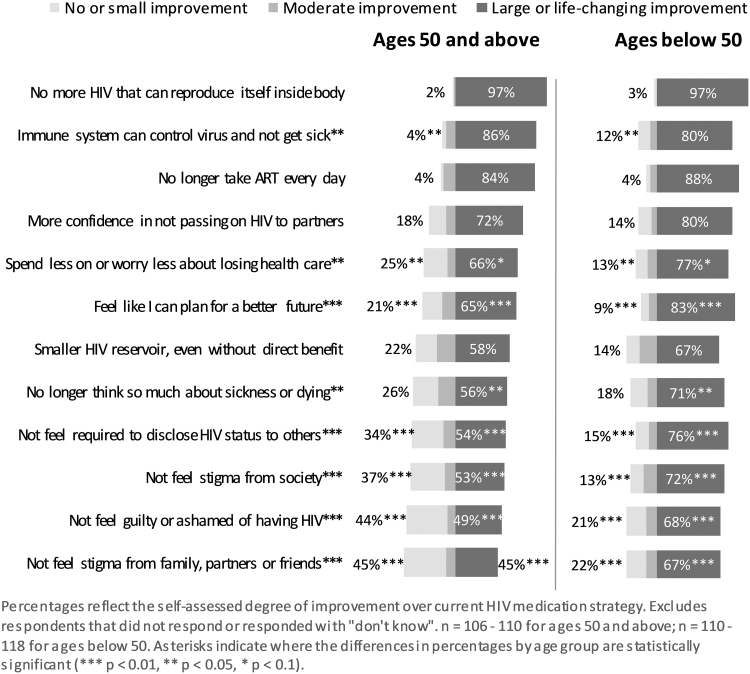
Perceived improvements over current ART
When asked about perceived improvements that a future HIV cure-related strategy may have over current ART (Fig. 3), the younger age group noted feeling that they could plan for a better future, not feeling required to disclose their HIV serostatus to others, not feeling stigma from society, not feeling guilty or ashamed of having HIV, not feeling stigma from family, partner, or friends (all p < .01), and no longer thinking so much about sickness or dying (p < .05) as large or life-changing improvements statistically significantly more than those in the older age group. More of those in the younger age group considered potential benefits related to their immune system controlling the virus to prevent sickness to be of little significance (p < .05) compared with those in the older age group. More of those in the older age group considered spending less on health care or worrying less about losing health care as not substantial (p < .05) than those in the younger age group (Fig. 3).
FIG. 3.
Improvement over current daily ART strategy offered by a promising future HIV cure-related strategy, by age group. ART, antiretroviral therapy.
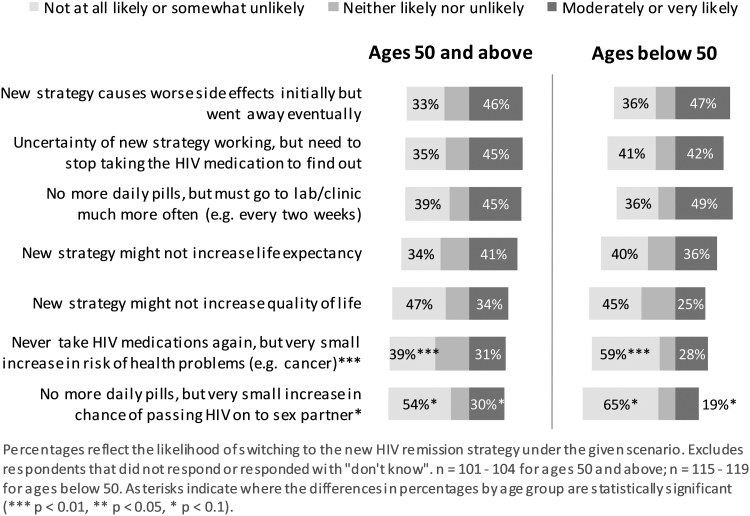
Scenario choices
Among the seven scenarios under which participants would choose a new HIV cure-related strategy over standard daily ART (Fig. 4), compared with those in the older age group, participants in the younger age group were statistically significantly more unlikely or somewhat unlikely to choose an HIV cure-related strategy if it meant an increase in risk of health problems, such as cancer, despite never having to take daily ART again (p < .01). A very small increase in the chance of passing HIV on to a sexual partner was also marginally associated with being less likely to choose a new HIV cure-related strategy among those in the younger age group compared with those in the older age group (p < .1) (Fig. 4).
FIG. 4.
Likelihood of choosing a new HIV cure-related strategy over standard daily ART under different scenarios, by age group.
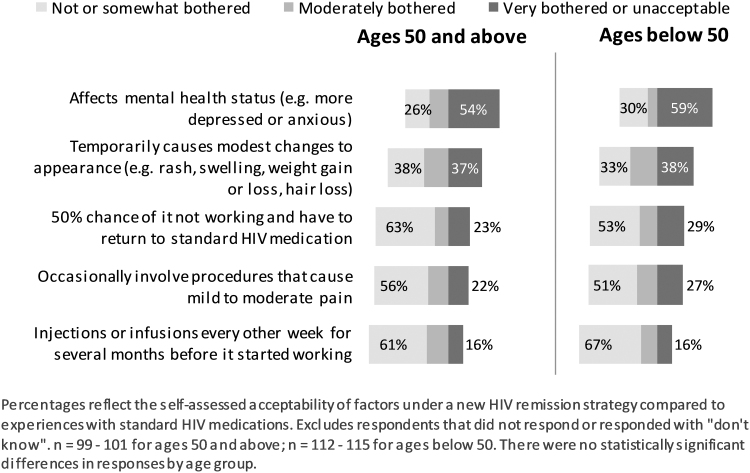
Acceptable tradeoffs
When asked about the level of bother related to various hypothetical tradeoffs of a new HIV cure-related strategy over their current oral daily ART (Fig. 5), participants in both age groups chose impacts on mental health status and modest temporary changes to appearance as the top factors that they would be most bothered by or that would be unacceptable to them. There were no statistically significant differences between the younger and the older age groups in their perceptions of tradeoffs (Fig. 5).
FIG. 5.
Acceptability of factors under a new HIV cure-related strategy compared to experiences with standard daily ART, by age group.
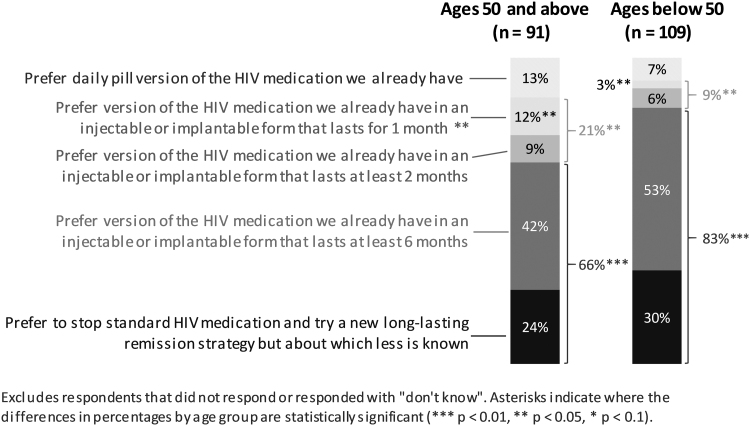
Choice between current daily ART, long-acting antiretroviral medications, and HIV cure-related strategies
When asked about choosing between their current oral daily ART (Fig. 6), a long-acting antiretroviral option, or a new experimental HIV cure-related strategy, those in the older age group were significantly more likely to prefer a version of a currently available ART in an injectable or implantable form that would last 1 or 2 months compared with the younger age group (p < .05). Inversely, participants in the younger age group were statistically significantly more likely to choose an injectable or implantable form of antiretroviral medications that would last at least 6 months or try a new cure-related strategy about which is less is known in comparison with those in the older age group (p < .01) (Fig. 6).
FIG. 6.
Choice between current standard daily ART versus long-acting antiretroviral medications versus new experimental HIV cure-related strategy, by age group.
Discussion
This analysis provides a deeper understanding of differences in perceptions among those in the younger age group (i.e., <50 years) compared with those in the older age group (i.e., ≥50 years) regarding motivators and barriers to participating in HIV cure-related research, improvements of future cure-related strategies over oral daily ART, and desirable future product characteristics for HIV virologic control strategies. Our results extend the HIV virologic control social science knowledge base and provide information to researchers to better align product development with end user perspectives.16,17 We describe three central findings here.
First, younger participants were more likely to be demotivated by perceived social risks (e.g., stigma, discrimination, and HIV disclosure), compared with older participants. This is not necessarily surprising as older PLWH may have developed stigma mitigating strategies over their lifespans, and had more opportunities to disclose their HIV status, compared with younger PLWH. Future HIV therapeutic and cure-related research must be attentive to concerns of younger participants who may continue to be faced with social risks associated with living with HIV.
Second, older participants were marginally more motivated by altruistic notions compared with those in the younger age group, who were more motivated by compensation, and support from family and friends or being treated as a special kind of patient. Altruism is defined as unselfish concern for the welfare of others,18 and has been shown to not be the sole factor but an important factor in a person's decision-making process.19 Given the history of HIV over the past 40 years, older participants may be more familiar with the activism surrounding expanding new HIV treatment options, whereas those who were younger may have different lived experiences with HIV, family support, or desires regarding special treatment. Recruitment and engagement of younger participants in HIV research may require more investment as they may not have had the chance to develop HIV-specific altruism, have competing professional or life needs that are different from those in the older age group. Given that HIV-specific altruism has been central to the development of many advancements in the field of HIV therapeutics and has been described as a reason to participate in HIV virologic control research,7,20–22 it is critical for researchers to galvanize support from the next generation of research participants in ways that best fit their needs while catering to their altruistic intentions.
Lastly, there was a greater desirability among younger participants for longer long-acting antiretroviral regimens (which would last at least 6 months) or new cure-related strategies compared with the older age group. These data are consistent with a previous cross-sectional survey conducted among 303 youth living with HIV in the United States showing high enthusiasm for emerging long-acting treatment strategies over daily oral ART.23 Our data show that PLWH in the younger age group, versus those in the older age group, were more likely to choose a treatment that is less familiar, and perhaps, riskier over daily ART. Given unknown adverse effects of these long-acting antiretroviral regimens, older PLWH may be making more personal decisions regarding the amount of risk they are willing to tolerate with regard to HIV treatment regimens, despite their willingness to participate in new HIV cure-related research in general. Much like research on contraceptives, what is clear is that a variety of HIV treatment options will be needed to meet the preferences, needs, and desires of diverse PLWH.
There were also exploratory results worth noting; although more robust research is needed to more carefully expand upon these results. First, participants in the younger age group were more concerned with any reproductive risks or delaying reproduction. However, there were more younger participants who did not consider a strengthened immune system controlling the virus under a cure-related strategy to be a significant improvement over their current ART compared with older participants. This may be due to the fact that they have benefited from newer ART and been diagnosed earlier after seroconversion due to expansion of HIV testing programs compared with older participants. Overall, new paradigms may be needed to engage younger participants in HIV virologic control research, as this is a research priority of the National Institutes of Health (NIH), pharmaceutical industries, and private foundations.
Our study has several notable limitations. Our relatively small sample of participants were English-speaking and had access to various HIV treatment and cure listservs; therefore, our results may not be generalizable to all PLWH. Additionally, owing to our relatively small sample size, we were unable to examine our findings in smaller subdivisions of participants (e.g., based on deciles of age, racial/ethnic groups, or other demographic variables). The survey used hypothetical questions and relied on self-report; therefore, it is prone to hypothetical and recall biases. Additionally, the survey items have been used in other studies; however, have not been validated. Therefore, in future research, we will examine these items in larger and more diverse populations to examine the external validity of our findings. Similar research will also be needed in resource-limited settings worldwide where the need for virologic control strategies may be greatest. Despite these limitations, we believe that our study can help inform community engagement and education, and assist researchers in tailoring study design, recruitment, and retention efforts to various age groups.
Conclusion
Prior studies have examined the perceptions of key stakeholders on the risks and benefits of HIV virologic control research24–26; however, few have explored differences in willingness to participate in this research among different age groups. While biomedical ethicists recognize the validity of differing motivations for participation in clinical research, attention is increasingly being focused on altruistic attitudes as a justification for increased risk. Given the difference in expressed altruism by those in the older age group, researchers should consider how these individuals may differ from younger individuals in their assessment of acceptable risks and correspondingly adapt materials and processes to ensure their informed and ethical participation.
As this field of research continues to grow and innovate27 and as PLWH continue to age28 and become ineligible for participation in virologic control studies (which usually exclude people >65–70 years of age), younger individuals will be at the forefront of decision making on HIV virologic control research. Therefore, it is imperative to examine differences of attitudes of various age groups who may have had differing experiences of the impacts of the HIV epidemic, altruistic attitudes toward participation, and general levels of engagement in health care, and who may perceive clinical and social risks and benefits of this emerging field of research differently.
Acknowledgments
The authors thank the study participants and the community members who reviewed the survey. The data used in this article were supported by grant funding from Gilead Sciences, Inc. Gilead Sciences, Inc. had no input in the development or content of these materials. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. David Evans participated in unpaid activities that are not part of this study for Gilead Sciences, Inc. and has received honoraria for participating on the Gilead Sciences community advisory board since the completion of this survey. Other authors do not have a commercial or other association that might pose a conflict of interest.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Research reported in this publication was supported by the National Institute of Mental Health under award numbers R21 MH108414-01A1 (co-PI's Parya Saberi and Karine Dubé) and R21 MH118120 (PI Karine Dubé).
References
- 1. Marcus JL, Chao CR, Leyden WA, et al. : Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016;73:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forsythe SS, McGreevey W, Whiteside A, et al. : Twenty years of antiretroviral therapy for people living with HIV: Global costs, health achievements, economic benefits. Health Aff (Millwood) 2019;38:1163–1172 [DOI] [PubMed] [Google Scholar]
- 3. Masters MC, Krueger KM, Williams JL, Morrison L, Cohn SE: Beyond one pill, once daily: Current challenges of antiretroviral therapy management in the United States. Expert Rev Clin Pharmacol 2019;12:1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. D'Amico R, Margolis DA: Long-acting injectable therapy: An emerging paradigm for the treatment of HIV infection. Curr Opin HIV AIDS 2020;15:13–18 [DOI] [PubMed] [Google Scholar]
- 5. Weld ED, Flexner C: Long-acting implants to treat and prevent HIV infection. Curr Opin HIV AIDS 2020;15:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deeks SG, Lewin SR, Ross AL, et al. : International AIDS Society global scientific strategy: Towards an HIV cure 2016. Nat Med 2016;22:839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubé K, Taylor J, Sylla L, et al. : ‘Well It's the Risk of the Unknown.. Right?': A qualitative study of perceived risks and benefits of HIV cure research in the United States. PLoS One 2017;12:e0170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubé K, Henderson GE, Margolis DM: Framing expectations in early HIV cure research. Trends Microbiol 2014;22:547–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubé K, Dee L, Evans D, et al. : Perceptions of equipoise, risk-benefit ratios, and “Otherwise Healthy Volunteers” in the context of early-phase HIV cure research in the United States: A qualitative inquiry. J Empir Res Hum Res Ethics 2018;13:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Power J, Westle A, Dowsett GW, et al. : Perceptions of HIV cure research among people living with HIV in Australia. PLoS One 2018;13:e0202647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dubé K, Evans D, Sylla L, et al. : Willingness to participate and take risks in HIV cure research: Survey results from 400 people living with HIV in the US. J Virus Erad 2017;3:40–50.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubé K, Eskaf S, Evans D, et al. : The dose response: Perceptions of people living with HIV in the United States on alternatives to oral daily antiretroviral therapy. AIDS Res Hum Retroviruses 2020;36:324–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. U.S. Department of Health and Human Services: HIV and Specific Populations: HIV and Older People. Understanding HIV/AIDS 2020. Available at https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/25/80/hiv-and-older-adults accessed April6, 2020
- 14. Greene M, Justice AC, Lampiris HW, Valcour V: Management of human immunodeficiency virus infection in advanced age. JAMA 2013;309:1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Negredo E, Back D, Blanco JR, et al. : Aging in HIV-infected subjects: A new scenario and a new view. Biomed Res Int 2017;2017:5897298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grossman CI, Ross AL, Auerbach JD, et al. : Towards multidisciplinary HIV-cure research: Integrating social science with biomedical research. Trends Microbiol 2016;24:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dubé K, Sylla L, Dee L, et al. : Research on HIV cure: Mapping the ethics landscape. PLoS Med 2017;14:e1002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee DY, Lee JY, Kang CH: Development and validation of an altruism scale for adults. Psychol Rep 2003;92:555–561 [DOI] [PubMed] [Google Scholar]
- 19. Bidad N, MacDonald L, Winters ZE, et al. : How informed is declared altruism in clinical trials? A qualitative interview study of patient decision-making about the QUEST trials (Quality of Life after Mastectomy and Breast Reconstruction). Trials 2016;17:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kall M, Simmons R, Collins S, et al. : Altruism and medical advice are key factors in decision-making about participating in HIV cure research: Results from a UK-wide survey of people living with HIV. HIV Med 2015;16:62–6324919923 [Google Scholar]
- 21. Balfour L, Corace K, Tasca GA, Tremblay C, Routy JP, Angel JB: Altruism motivates participation in a therapeutic HIV vaccine trial (CTN 173). AIDS Care 2010;22:1403–1409 [DOI] [PubMed] [Google Scholar]
- 22. Evans D: An activist's argument that participant values should guide risk-benefit ratio calculations in HIV cure research. J Med Ethics 2017;43:100–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weld ED, Rana MS, Dallas RH, et al. : Interest of youth living with HIV in long-acting antiretrovirals. J Acquir Immune Defic Syndr 2019;80:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arnold MP, Evans D, Vergel N: Recruitment and ethical considerations in HIV cure trials requiring treatment interruption. J Virus Erad 2015;1:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McMahon JH, Elliott JH, Roney J, Hagenauer M, Lewin SR: Experiences and expectations of participants completing an HIV cure focused clinical trial. AIDS 2015;29:248–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dubé K, Evans D, Dee L, et al. : “We Need to Deploy Them Very Thoughtfully and Carefully”: Perceptions of analytical treatment interruptions in HIV cure research in the United States: A qualitative inquiry. AIDS Res Hum Retroviruses 2018;34:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jefferys R: Research Toward a Cure Trials. Treatment Action Group 2019. Available at www.treatmentactiongroup.org/cure/trials accessed March1, 2019
- 28. Wing EJ: HIV and aging. Int J Infect Dis 2016;53:61–68 [DOI] [PubMed] [Google Scholar]