Abstract
It is well known that during ovarian cancer progression, the omentum transforms from a thin lacy organ to a thick tougher tissue. However, the mechanisms regulating this transformation and the implications of the altered microenvironment on ovarian cancer progression remain unclear. To address these questions, the global and local concentrations of collagen I were determined for normal and metastatic human omentum. Collagen I was increased 5.3-fold in omenta from ovarian cancer patients and localized to areas of activated fibroblasts rather than regions with a high density of cancer cells. Transforming growth factor beta 1 (TGFβ1) was detected in ascites from ovarian cancer patients (4 ng/mL), suggesting a potential role for TGFβ1 in the observed increase in collagen. Treatment with TGFβ1 induced fibroblast activation, proliferation, and collagen deposition in mouse omental explants and an in vitro model with human omental fibroblasts. Finally, the impact of increased collagen I on ovarian cancer cells was determined by examining proliferation on collagen I gels formulated to mimic normal and cancerous omenta. While collagen density alone had no impact on proliferation, a synergistic effect was observed with collagen density and heparin-binding epidermal growth factor treatment. These results suggest that TGFβ1 induces collagen deposition from the resident fibroblasts in the omentum and that this altered microenvironment impacts cancer cell response to growth factors found in ascites.
Impact statement
Using quantitative analysis of patient samples, in vitro models of the metastatic ovarian cancer microenvironment were designed with pathologically relevant collagen densities and growth factor concentrations. Studies in these models support a mechanism where transforming growth factor β1 in the ascites fluid induces omental fibroblast proliferation, activation, and deposition of collagen I, which then impacts tumor cell proliferation in response to additional ascites growth factors such as heparin-binding epidermal growth factor. This approach can be used to dissect mechanisms involved in microenvironmental modeling in multiple disease applications.
Keywords: collagen, ovarian cancer, microenvironment, fibroblast, TGFbeta, omentum
Color images are avaliable online
Introduction
High-grade serous ovarian carcinoma (HGSOC) is the most lethal gynecological cancer, with a 5-year survival rate of less than 30% for patients with metastatic disease.1 HGSOC metastasizes when individual cells or spheroids detach from the primary tumor and implant throughout the peritoneal cavity on organs such as the omentum, a fatty tissue that covers the bowel and abdominal cavity.2 The omentum is thought to be an early metastatic site,3 and omental metastases are found in nearly 80% of women with HGSOC.4
Recently, in vitro systems resembling the healthy omenta have been developed to analyze attachment and spreading of tumor cells.5–8 However, with cancer progression, the thin lacy omenta transforms to a thick tougher tissue. Therefore, understanding the mechanisms responsible for changes in the omentum and determining the impact of these biochemical and biophysical changes on the tumor cells could identify therapeutic targets to delay or prevent further metastasis.
A recent characterization of the matrisome of the human omentum during HGSOC progression demonstrated changes in the levels of many extracellular matrix (ECM) proteins, including increases in collagen I (Col19). Col1 is a structural protein whose upregulation is associated with increased tissue stiffness10; relative quantitation of the omental matrisome demonstrated that in normal and diseased omenta, Col1 was the most abundant ECM component.9 Furthermore, Col1 fiber density and alignment in the omentum is significantly altered as ovarian cancer progresses.11
While these observations suggest that Col1 may play a role in tumor progression, the quantitative levels needed for accurate tissue engineered models have not been determined, and the mechanism responsible for the increased Col1 deposition and the impact of increased Col1 on tumor cells has not been examined as rigorously in HGSOC as in other tumor types.12,13 While ovarian cancer cells produce ECM,14 cancer-associated fibroblasts (CAFs) display an activated phenotype and produce and remodel many ECM proteins, including Col1.15 The omentum contains a small population of resident fibroblasts interior to the outer mesothelial layer,5 but the role of fibroblasts in omental remodeling remains unclear.
Concurrent with omental transformation, many patients accumulate peritoneal ascites rich in growth factors.16 Transforming growth factor beta (TGFβ) is the canonical factor that transdifferentiates fibroblasts into activated fibroblasts17 and is produced by ovarian cancer cells, immune cells, and activated fibroblasts themselves, all of which contribute secreted factors to ascites. Thus, we hypothesized that fibroblasts in the omentum are activated by TGFβ in the ascites to produce Col1 and that this omental remodeling impacts further HGSOC progression.
We investigated tissue Col1 levels, fibroblast coverage and activation, as well as TGFβ concentrations in samples from ovarian cancer patients and women without a history of ovarian cancer. Using these clinical data in combination with tissue engineering approaches, we evaluated the role of fibroblast-mediated omental remodeling and the influence of omental remodeling on ovarian cancer proliferation.
Materials and Methods
Cell lines and reagents
Unless stated, all reagents were purchased from Thermo Fisher Scientific (Waltham, MA). Primary human omental fibroblasts were generously provided by Dr. Hilary Kenny (Department of Obstetrics and Gynecology, University of Chicago) and isolated under a protocol approved by the University of Chicago Institutional Review Board (IRB).5 Omental fibroblasts were cultured in DMEM/F-12 with 1% penicillin–streptomycin and 10% fetal bovine serum (FBS). Ovarian cancer cell lines OVCAR3 and OV90 were purchased from ATCC, and OVCA433 and OVCAR8 were obtained from the NCI 60 panel (NIH, Bethesda, MD). Ovarian cancer cells were maintained in a 1:1 (v/v) ratio of MCDB105:Medium199 (Corning, Corning, NY) with 1% penicillin–streptomycin and 15% heat-inactivated FBS.
Col1 quantitative dot blot
Three formalin-fixed, paraffin-embedded samples from women who had undergone surgical debulking for HGSOC were obtained from archived pathology samples through a protocol approved by the University of Wisconsin (UW)-Madison IRB. Additionally, four omenta from women without a history of ovarian cancer were procured from autopsies performed at the UW Hospital (Supplementary Table S1). A dot blot was performed as described.18 Briefly, paraffin-embedded sections (20 μm) of tissue samples or Col1 gels of increasing concentrations (Advanced BioMatrix, San Diego, CA; protocol below) were placed in a 1.5-mL tube, and deparaffinization was performed by incubating with 1 mL of SafeClear II Xylene Substitute for 10 min at room temperature. The tissue and any undissolved paraffin were pelleted by centrifuging for 3 min at 16,000 g. SafeClear washing and centrifuging were repeated two additional times until all of the paraffin was dissolved and only tissue remained.
The tissue pellet was rehydrated using serial dilutions of ethanol (100%, 70%, and 50%). The pellet was resuspended in 200 μL of protein extraction buffer containing 20 mM Tris–hydrochloride (Promega, Fitchburg, WI), 2% sodium dodecyl sulfate (SDS; Boston BioProducts, Ashland, MA), pH 8.0. Non-protein material was removed from the solution by centrifuging at 16,000 g for 20 min at 4°C. Collagen I levels were quantified by dot blot.19 Briefly, a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA) was soaked in methanol for 1 min and then rinsed in water for 2 min.
The membrane was then allowed to dry, and 1 μL of standard and samples were pipetted onto the membrane. The membrane was incubated at 37°C for 5 min to set the protein into the membrane, rinsed three times with Tris-buffered saline (TBS; Boston BioProducts), and blocked in TBS with 0.1% Tween-20 (TBST; Dot Scientific, Burton, MI) and 1% normal goat serum for 1 h at room temperature. Antibodies (anti-collagen I; ab34710; Abcam, Cambridge, United Kingdom) at 1:1000 were diluted in blocking solution and incubated with the blots overnight at 4°C with agitation. The membrane was rinsed three times in TBST and incubated in goat anti-rabbit IgG H&L (HRP) secondary antibody (ab6721; Abcam) at 1:2000 in blocking buffer for 1 h. The membrane was then washed three times with TBST and three times with TBS. The membrane was imaged using Clarity Western ECL Substrate (Bio-Rad) and an Odyssey Fc Imaging System (LI-COR, Lincoln, NE) and analyzed using Image Studio (LI-COR).
Ex vivo mouse omental explant
All animal protocols were approved by the UW School of Medicine and Public Health Institutional Animal Care and Use Committee (IACUC). Female BALB/cJ mice (6–12 weeks) were purchased from Jackson Laboratory (Bar Harbor, ME). Omenta were collected from euthanized mice and rinsed twice in phosphate-buffered saline (PBS) with 1% penicillin–streptomycin. The corners of each omentum were pinned to a 2-mm-thick polydimethylsiloxane substrate (Dow Corning, Midland, MI) in the bottom of a six-well plate and cultured in serum-free DMEM/F-12 with 1% penicillin–streptomycin ±10 ng/mL recombinant human TGFβ1 (PeproTech, Rocky Hill, NJ). Cultures were maintained at 37°C and 5% CO2 for 24 h, at which point they were fixed in 10% neutral buffered formalin and embedded in paraffin.
Immunostaining
Five-micron sections of the human omenta and mouse omenta from explant cultures were cut and processed as described7 using the antibodies outlined in Supplementary Table S2. In addition to the omenta evaluated via dot blot, an additional 5 non-HGSOC omenta and 12 HGSOC omenta from the pathology archives were examined by immunostaining (Supplementary Table S1). Sections were imaged using a Zeiss Axio Observer Z1, an Axiocam 506 mono camera, Plan-Apochromat 5 × 1.6 numerical aperture (NA) objective, and Plan-Apochromat 20 × 0.8 NA air objective. Image analysis was performed using the FIJI ImageJ software (NIH).
Proximity ligation in situ hybridization
Probes were designed (Supplementary Table S3; IDT, Coralville, IA) and hybridization procedure was carried out as described.20 Sections were hybridized with Alexa Fluor 555 fluorescently labeled detection oligos (IDT), followed by immunohistochemistry for FSP1 and CK7 as above.
Image analysis
Image analysis was performed using the FIJI ImageJ software. To quantify cellularity or cell type coverage in images, the 4′,6-diamidino-2-phenylindole (DAPI)+ or marker+ area was divided by the total area. To quantify Col1 and Col1A1 levels in specific regions, image masks were generated in FIJI from the region marker (e.g., CK7, FSP1) and the median fluorescent intensity (MFI) was determined. Correlation analysis was conducted using the Just Another Colocalization Plug-In (JACoP) to find the overlap coefficient, which ranges from 0 to 1.21
Spatial Col1 concentration was calculated as described.18 The total area of each omentum section was measured in FIJI, and the tissue section volume was calculated. The global Col1 concentration was calculated from the Col1 content determined by dot blot divided by the tissue volume. The local Col1 concentration was calculated from the global concentration multiplied by the ratio of the MFI (region of interest) to the MFI (tissue section). Multiple images were examined for each section.
Characterization of ascites fluid
Informed written consent was obtained from patients recruited under a study approved by the UW-Madison IRB. Studies were conducted in accordance with recognized ethical guidelines (e.g., Declaration of Helsinki, CIOMS, Belmont Report, U.S. Common Rule). Ascites was collected from patients with HGSOC (Stage IIIC-IVa; n = 20) or benign conditions (n = 4, Supplementary Table S4). Samples were assayed for TGFβ1, TGFβ2, TGFβ3, and heparin-binding epidermal growth factor (HB-EGF) using a Bio-Plex Pro Immunoassay according to the manufacturer's instructions.7
Col1 hydrogel fabrication
Col1 hydrogels were prepared in clear-bottom, black-walled 96-well plates (μ-Plate Angiogenesis 96 Well; IBIDI, Fitchburg, WI) by combining high-concentration bovine Col1 (Advanced BioMatrix), 0.1 N NaOH, and either 1 × or 10 × PBS for a final concentration of 1, 2.5, or 6.5 mg/mL Col1, 1 × PBS, at pH 7.4. The hydrogels were allowed to polymerize overnight in the incubator and then rinsed with PBS.
In vitro omental fibroblast activation assay
Primary human omental fibroblasts were seeded at 31,250 cells/cm2 (10,000 cells per well) on 1 mg/mL Col1 hydrogels in DMEM/F-12 with 1% penicillin–streptomycin and 10% FBS and allowed to attach overnight. The media was then changed to DMEM/F-12 with 1% penicillin–streptomycin ±10 ng/mL recombinant human TGFβ1. Cultures were maintained at 37°C and 5% CO2 for 24 h, at which point they were fixed in 4% paraformaldehyde.
To visualize Col1 deposited by omental fibroblasts, samples were incubated with 25 μM enhanced green fluorescent protein (EGFP)-tagged collagen-binding adhesion protein 35 (CNA35-EGFP) in 5% bovine serum albumin overnight at 37°C, followed by frequent PBS rinses.18 The pET28a-EGFP-CNA35 was a gift from Maarten Merkx (Addgene plasmid #61603; http://n2t.net/addgene:61603; RRID:Addgene_61603).22 Cell proliferation was detected by Click-iT Edu7 and fibroblast activation by immunostaining (Supplementary Table S2). Samples were imaged on a Nikon A1R confocal laser microscope with PLAN-APO-VC 5 × 0.75 NA and PLAN-APO-VC 20 × 0.75 NA objectives. Image analysis was performed using NIS-Elements (Nikon, Melville, NY).
In vitro ovarian cancer proliferation assay
Ovarian cancer cell lines were transduced with pLenti-CMV-Puro-LUC (w168–1), a gift from Eric Campeau and Paul Kaufman (Addgene plasmid #17477; http://n2t.net/addgene:17477; RRID:Addgene_17477).23 Plasmid DNA was isolated using a QIAprep Spin Miniprep Kit (Qiagen, Germantown, MD), and lentivirus was produced by adding 1 μg plasmid DNA to 3 μL FuGENE HD (Promega), 1 μg Lenti-vpak packaging plasmids (OriGene, Rockville, MD), and 100 μL Opti-MEM1 Reduced Serum Media. The mix was added dropwise to HEK293T cells seeded in a poly-D-lysine-coated six-well plate at 750,000 cells per well. After 24 h, the media was replaced with DMEM plus 10% FBS. Forty-eight hours later, media was collected, filtered through a 0.4-μm syringe filter, and added to OVCAR3, OVCAR8, OVCA433, or OV90 cells seeded at 50,000 cells per well in a 24-well plate. Transduced cells were selected with 4 μg/mL puromycin. Luciferase expression was confirmed with D-luciferin (150 μg/mL) and measurement of bioluminescent signal using an Infinite M1000 plate reader (Tecan, San Jose, CA).
Luciferase-positive ovarian cancer cells were seeded at 100,000 cells/cm2 (32,000 cells per well) in ovarian cancer media with 1% penicillin–streptomycin and 10% FBS on hydrogels composed of 1, 2.5, or 6.5 mg/mL Col1 and allowed to attach overnight. Initial cell density was measured using D-luciferin, and media was then changed to serum-free media with 1% penicillin–streptomycin ±20 ng/mL HB-EGF. Cultures were maintained at 37°C and 5% CO2 for 48 h, and then, luminescence was measured as above. The proliferation index was calculated as the luminescence at 48 h divided by the luminescence at 0 h.
Statistical analysis
Data are presented as mean ± standard deviation. Statistical analysis (one-way analysis of variance [ANOVA] with Tukey, two-way ANOVA with interaction term analysis and Bonferroni correction, or t-tests) was performed in Prism 7 software (GraphPad, San Diego, CA).
Results
Omental Col1 density is significantly increased in high-grade serous ovarian carcinoma
The distribution of Col1 and cells in omenta from HGSOC patients (OC-omenta) and patients with no history of HGSOC (nOC-omenta) was visualized using immunohistochemistry (Fig. 1A; higher magnification images are shown in Supplementary Fig. S1). Globally, Col1 was increased throughout OC-omenta compared with nOC-omenta, with increased density at the exterior as well as large dense regions of Col1 in the tissue center. The concentration of Col1 in each section was measured via dot blot (Fig. 1B) and found to be significantly increased in OC-omenta versus nOC-omenta (1.67 ± 0.46 vs. 0.31 ± 0.16 mg/mL). Tissue cellularity (percent area covered by DAPI) was also significantly increased in OC-omenta (Fig. 1C). Increased cellularity would be expected with tumor cells; however, it may also indicate increases in stromal cells.
FIG. 1.
Col1 density in the omentum is significantly increased in ovarian cancer patients. (A) Immunofluorescent detection of Col1 in OC-omenta and nOC-omenta. Scale bar = 5 mm. (B) Quantification of Col I in four nOC-omenta and three OC-omenta. (C) Quantification of cellularity in omentum from four nOC-omenta and three OC-omenta. Data are expressed as average ± SD; *p < 0.05 by t-test.
Col1 is increased in the fibroblastic region of ovarian carcinoma-omenta
Therefore, to determine which cell types were increased in the omentum and to determine the types that are potentially responsible for the Col1 deposits in metastatic tissue, the distribution of Col1, cytokeratin 7 (CK7, ovarian cancer cell marker), and FSP1 (fibroblast marker) was evaluated (Fig. 2A). Visually, increased Col1 deposition was not observed in regions with dense tumor cells. Instead, Col1 levels were elevated in areas near the tumor cells, where fibroblast density appeared to be elevated. Additionally, the overall level of FSP1-positive cells appeared to be increased in OC-omenta compared with nOC-omenta. To confirm these observations, FSP1 coverage was quantified and OC-omenta were found to have a 7.5-fold increase in FSP1-positive cells (Fig. 2B).
FIG. 2.
Col1 is increased in fibroblastic region of HGSOC omentum. (A) Immunofluorescent detection of Col1, CK7, FSP1, and DAPI in nOC-omenta and OC-omenta. Scale bar = 200 μm. (B) Percent area covered by FSP1 in omentum from nOC-omenta and OC-omenta. *p < 0.05 by t-test. (C) MFI of Col1 in omentum from nOC-omenta, the CK7+ region in OC-omenta, and the FSP1+ regions in OC-omenta. *p < 0.05 versus nOC-omenta and ^p < 0.05 versus OC-omenta CK7+ by one-way ANOVA with Tukey's post-test. (D) Overlap coefficient for Col1 in OC-omenta within CK7+ and FSP1+ regions. *p < 0.05 by t-test. (E) Immunofluorescent detection of Col1, PCK, αSMA, and DAPI in nOC-omenta and OC-omenta. Scale bar = 200 μm. (F) Percent area covered by αSMA in omentum from nOC-omenta and OC-omenta. *p < 0.05 by t-test. (G) MFI of Col1 in nOC-omenta, the PCK+ region in OC-omenta, and the αSMA+ regions in OC-omenta. *p < 0.05 versus nOC-omenta and ^p < 0.05 versus PCK+ OC-omenta by one-way ANOVA with Tukey's post-test. (H) Overlap coefficient for Col1 in OC-omenta within CK7+ and αSMA+ regions. *p < 0.05 by t-test. In all panels, n = 8 nOC-omenta, 16 OC-omenta; data are presented as individual points for each patient sample, bars indicate average ± SD. HGSOC, high-grade serous ovarian carcinoma; MFI, median fluorescent intensity; αSMA, α-smooth muscle actin; PCK, pan-cytokeratin; CK7, cytokeratin 7; FSP1, fibroblast surface protein 1; ANOVA, analysis of variance. Color images are available online.
Next, the intensity of the Col1 signal in the nOC-omenta was quantified and compared with the CK7+ and FSP1+ regions of the OC-omenta (Fig. 2C; given the low levels of FSP1 and lack of CK7, global intensity was quantified in nOC-omenta). Consistent with our initial observations, Col1 was the highest in the FSP1-positive regions of OC-omenta. Finally, we examined the overlap between Col1 signal and either FSP1 or CK7 in the OC-omenta (Fig. 2D); this metric demonstrated that in addition to the elevated levels in the FSP1+ region, there was a stronger colocalization of Col1 with FSP+ cells versus CK7+ cells.
Given the increase in fibroblasts in OC-omenta, we sought to determine if these fibroblasts demonstrated a CAF phenotype, which is associated with α-smooth muscle actin (αSMA) expression and increased Col1 deposition.24 OC-omenta and nOC-omenta were immunostained for αSMA, pan-cytokeratin (PCK, ovarian cancer cell marker; due to antibody hosts PCK was used vs. CK7), Col1, and DAPI (Fig. 2E). Similar to the findings with FSP1, OC-omenta had elevated levels of the activated fibroblast marker αSMA, and Col1 appeared to be excluded from the PCK+ region, with enrichment in the αSMA+ regions. OC-omenta exhibited a 280-fold increase in αSMA coverage (Fig. 2F). Additionally, the αSMA+ region in OC-omenta exhibited a 75% increase in Col1 compared with the PCK-positive region (Fig. 2E), even larger than the difference noted for all fibroblasts (FSP+) versus tumor cells (30% increase). As with the more general fibroblast marker (FSP1), there was a significant increase in overlap between the αSMA marker and Col1 signal relative to CK7 and Col1 (Fig. 2F).
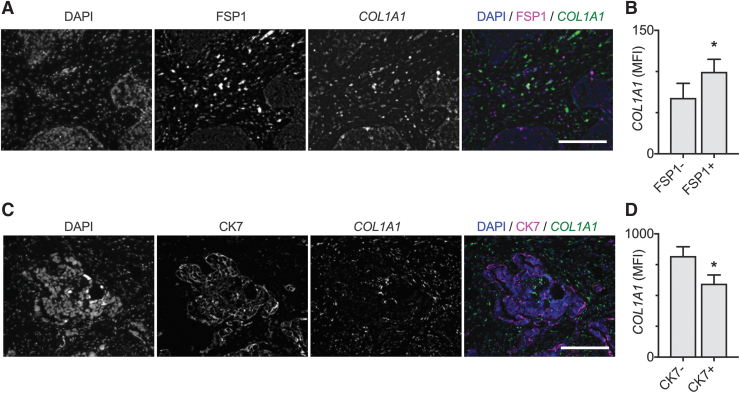
Taken together, these results suggest that as ovarian cancer progresses, resident omental fibroblasts increase in number and activate, leading to increased Col1 deposition. To more definitively evaluate the production of Col1 by tumor cells and fibroblasts in the tumor microenvironment, proximity ligation in situ hybridization for COL1A1 was performed concurrently with immunostaining for FSP1 or CK7. Similar to the immunostaining results, the expression of COL1A1 was significantly elevated in FSP1+ versus FSP1− cells (Fig. 3A, B). Furthermore, COL1A1 expression was significantly reduced in CK7+ versus CK7− cells (Fig. 3C, D). These data confirm that fibroblasts in the tumor microenvironment are responsible for the increased Col1 present in OC-omenta.
FIG. 3.
PLISH demonstrates that COL1A1 is upregulated in fibroblasts in OC-omenta. (A) Simultaneous labeling of COL1A1 RNA transcripts and FSP1 in OC-omenta. Scale bar = 200 μm. (B) MFI of COL1A1 in the FSP1− and FSP1+ regions in OC-omenta. Data are expressed as average ± SD, n = 3 OC-omenta; *p < 0.05 by paired t-test. (C) Simultaneous labeling of COL1A1 RNA expression and CK7 in OC-omenta. Scale bar = 200 μm. (D) MFI of COL1A1 in the CK7− and CK7+ regions in OC-omenta. Data are expressed as average ± SD, n = 3 OC-omenta; *p < 0.05 by paired t-test. PLISH, proximity ligation in situ hybridization. Color images are available online.
TGFβ1 activates omental fibroblasts and increases Col1 deposition
Since our data support a mechanism where resident fibroblasts are activated to a CAF-like phenotype, we next sought to examine potential sources of this activation. In vitro studies have demonstrated that HGSOC tumor cells produce TGFβ1 that induced fibroblast proliferation and αSMA production25 and TGFβ2 that induced normal fibroblasts to a CAF-like phenotype.26 TGFβ is the canonical activator of fibroblasts and has been shown to be produced by ovarian cancer cells, immune cells, and activated fibroblasts, all of which are present in the ovarian cancer tumor microenvironment.17
Since the secreted factors from these cells and others accumulate in ascites, we quantified TGFβ isoforms in ascites from HGSOC (OC-ascites) and non-HGSOC (nOC-ascites) patients. TGFβ1, TGFβ2, and TGFβ3 levels were detectable in both groups, with TGFβ1 increased fivefold in OC-ascites compared with nOC-ascites (Fig. 4A). TGFβ2 was elevated 8.5-fold but was not statistically significant, whereas TGFβ3 was elevated 3.7-fold and was statistically significant (Supplementary Fig. S2).
FIG. 4.
TGFβ1 activates human omental fibroblasts and increases deposition of Col1. (A) OC-ascites had elevated TGFβ1 compared with nOC-ascites. Data are expressed as average ± SD, n = 4 nOC-ascites, n = 20 OC-ascites; *p < 0.05 by t-test. (B) Confocal Z-stack reconstruction of CNA35-EGFP (green) labeled fibrillar Col1 from human omental fibroblasts treated ±10 ng/mL TGFβ1. Samples were counterstained with DAPI (blue). Scale bar = 200 μm. (C) MFI of CNA35-EGFP signal from human omental fibroblasts ±10 ng/mL TGFβ1. Data are expressed as average ± SD, n = 3; *p < 0.05 by t-test. (D) Percentage of human omental fibroblasts ±10 ng/mL TGFβ1 that were EdU-positive. Data are expressed as average ± SD, n = 4; *p < 0.05 versus vehicle control (Veh) at 72 h by two-way ANOVA with Bonferroni correction. (E) Confocal projection of αSMA-positive human omental fibroblasts ±10 ng/mL TGFβ1 after 72 h of treatment. Scale bar = 100 μm. (F) Percentage of human omental fibroblasts ±10 ng/mL TGFβ1 that were αSMA-positive. Data are expressed as average ± SD, n = 4; *p < 0.05 versus vehicle control (Veh) at 72 h, ^p < 0.05 versus TGFβ at 24 h by Tukey's post-test. TGFβ1, transforming growth factor beta 1. Color images are available online.
To determine if this elevated TGFβ could lead to fibroblast activation and Col1 deposition in the omentum, we tested the effect on human omental fibroblasts. Since TGFβ1 and TGFβ3 activate TGFβR1 and TGFβR2,27 we elected to use TGFβ1 due to its higher levels in ascites. The concentration range in OC-ascites (1–14 ng/mL) is consistent with doses of TGFβ1 used in vitro28,29; therefore, human omental fibroblasts were seeded on 1 mg/mL bovine Col1 hydrogels and treated with 10 ng/mL TGFβ1. Samples were stained with CNA35-EGFP (which binds to fibrillar collagen and therefore indicative of newly deposited Col1 rather than the initial bovine Col1) and counterstained with DAPI (Fig. 4B).
When treated with TGFβ1, human omental fibroblasts deposited a continuous sheet of fibrillar Col1, whereas those treated with the vehicle control deposited a diffuse smaller amount of fibrillar Col1. The intensity of the fibrillar Col1 was significantly elevated in omental fibroblasts treated with TGFβ1, increasing nearly sevenfold (Fig. 4C). We next examined fibroblast proliferation and activation status. TGFβ1 treatment significantly increased the percentage of proliferating fibroblasts (Fig. 4D). Fibroblast activation followed a similar pattern, with significantly increased αSMA coverage following TGFβ1 treatment (Fig. 4E, F).
To evaluate the role of TGFβ1 in omental remodeling in intact tissue, omenta were isolated from mice and treated with 10 ng/mL TGFβ1. Similar to the human OC-omenta, mouse omenta treated with TGFβ1 had increased cellularity (Fig. 5A, B). To investigate changes in Col1 and omental fibroblasts, sections were immunostained for Col1, FSP1, and αSMA (Fig. 5A). TGFβ1 treatment resulted in a twofold increase in Col1 in FSP-positive regions and a twofold increase in FSP1 coverage (Fig. 5C, D). Similarly, TGFβ1 treatment induced a 1.7-fold increase in Col1 in αSMA-positive regions and increased αSMA coverage by 4.5-fold (Fig. 5E, F). Overall, these data indicate that TGFβ1 increased omental fibroblast proliferation and activation, leading to increased Col1 density.
FIG. 5.
TGFβ1 increases fibroblast proliferation and deposition of Col1 in ex vivo mouse omental explant. Omenta from female BALC/cJ mice were excised, cultured ex vivo, and treated with 10 ng/mL TGFβ1 for 24 h. (A) Immunofluorescent detection of FSP1 (magenta) or αSMA (magenta) in mouse omentum explants ± TGFβ1. Tissue was counterstained for Col1 (green) and DAPI (blue). Scale bar = 200 μm. (B) Quantification of cellularity in omentum explants ± TGFβ1. (C) MFI of Col1 in the FSP+ regions of mouse omentum explants ± TGFβ1. (D) Percent area covered by FSP1 in mouse omentum explants ± TGFβ. (E) MFI of Col1 in the αSMA+ regions of mouse omentum explants ± TGFβ1. (F) Percent area covered by αSMA in mouse omentum explants ± TGFβ1. All data are expressed as average ± SD, n = 4; *p < 0.05 by t-test. Color images are available online.
Ovarian cancer cell proliferation in response to heparin-binding epidermal growth factor is increased as Col1 density increases
Given the dramatic increase in Col1 laid down by omental fibroblasts, we next sought to examine its impact on the neighboring tumor cells. Using the Col1 immunostaining and dot blot data (Fig. 1), we quantified local Col1 concentrations on the surface of nOC-omenta and OC-omenta, where tumor cells will initially reside (Fig. 6A, B). The edges of nOC-omenta had a relatively uniform, thin band of Col1 with an average concentration of ∼1 mg/mL. The edges of OC-omenta displayed two distinct phenotypes: a slightly thicker band covering the remaining adipose-rich regions (tumor distal, 2.5 mg/mL Col1) and a distinctly thicker region close to the tumor (tumor proximal, 6.5 mg/mL Col1).
FIG. 6.
In vitro model of omental edges. (A) Immunofluorescent detection of Col1 and DAPI in omental tissue from nOC-omenta, OC-omenta distal, and OC-omenta proximal. Scale bar = 100 μm. (B) Quantification of Col1 in four nOC-omenta and three OC-omenta. Data are expressed as average ± SD, *p < 0.05 versus nOC-omenta by one-way ANOVA with Tukey's post-test. (C) OC-ascites had elevated HB-EGF compared with nOC-ascites. Data are expressed as average ± SD, n = 4 nOC-ascites, n = 20 OC-ascites; *p < 0.05 versus nOC-ascites by t-test. (D) Luciferase-transfected HGSOC cell lines (OV90, OVCAR8, OVCAR3, OVCA433) were cultured on Col1 hydrogels of 1, 2.5, or 6.5 mg/mL Col1 ± 20 ng/mL HB-EGF for 48 h. Data are expressed as average ± SD, n = 4; *p < 0.05 versus vehicle control (Veh) of the same Col1 concentration by two-way ANOVA with Bonferroni multiple comparison correction. HB-EGF, heparin-binding epidermal growth factor. Color images are available online.
ECM density has been shown to impact cellular behaviors such as proliferation and migration30; therefore, we sought to examine how the increased Col1 density would influence tumor cell response to stimuli found in the metastatic microenvironment. Our laboratory has previously shown that tumor cells proliferate in response to macrophage-secreted heparin-binding EGF-like growth factor (HB-EGF),31 but that only some tumor cell lines are sensitive to HB-EGF when cultured on tissue culture plastic.32 Our prior studies demonstrated that immune cells within OC-ascites produce HB-EGF31; when we quantified the concentration of HB-EGF levels in ascites fluid from nOC-ascites and OC-ascites, HB-EGF was increased 3.8-fold in OC-ascites (Fig. 6C).
To evaluate if changes in omental Col1 levels impacted tumor cell response to HB-EGF, luciferase-positive HGSOC cells were seeded on Col1 hydrogels matching the density of different omental edges (nOC-omenta, distal OC-omenta, and proximal OC-omenta) and cell number was quantified after 48 h (Fig. 6D). As expected, the increase in Col1 density resulted in an increase in the modulus of these gels (Supplementary Fig. S3); however, the increase in stiffness to ∼1 kPa remains substantially less than the stiffness of tumors.33
Increases in Col1 density alone did not impact cell number in any of the four lines in the absence of HB-EGF. In the presence of HB-EGF, ovarian cancer proliferation was increased for many of the Col I densities. For OVCAR3 and OVCA433, a significant increase in proliferation in response to HB-EGF was observed, but the increase in collagen density did not have a significant interaction with HB-EGF. In contrast, significant interaction between the effects of Col1 density and HB-EGF was observed in OV90 and OVCAR8 (p = 0.0036 and 0.0011, respectively). Taken together, these data indicate that the increased Col1 density and HB-EGF levels observed in HGSOC patients can have synergistic effects on ovarian cancer proliferation.
Discussion
By coupling pathological and biochemical analysis of clinical samples with tissue engineering approaches, we identified a path by which the omentum is transformed from a thin lacy organ into a thick tougher tissue and examined effects this transformation has on ovarian cancer progression. Specifically, our results suggest that TGFβ1 induces proliferation, activation, and Col1 deposition from resident omental fibroblasts, which, when combined with additional factors in the ascites leads to increased tumor cell proliferation. Overall, these data indicate that pathways such as TGFβ1 and Col1 synthesis could potentially be therapeutic targets in ovarian cancer.
The omentum is the most common site of distal metastasis, leading to peritoneal organ adhesion and bowel obstruction.34,35 When Pearce et al. compared omenta across different stages of ovarian cancer, they observed a global increase in collagen fibers as well as an increase in Col1, specifically.9 Our work builds upon this description by providing the first quantification of the global and local levels of Col1. One important difference between our study and prior matrisome characterizations36 is that we directly compared omenta from HGSOC patients with patients without tumor involvement, rather than to HGSOC patients diagnosed before omental metastasis. In particular, we noted that surfaces of OC-omenta without detectable cancer cells still showed alterations in matrix density. This suggests that changes may occur before metastatic colonization, which could have profound effects on the behaviors of tumor cells when they encounter this microenvironment. These data can be used to develop further in vitro models of the early- and late-stage OC-omenta to better understand the role of omental ECM in the metastatic cascade.
Fibroblasts within the tumor stroma, often referred to as CAFs, play an important role in the progression of many cancers15 but are understudied in the omentum. This may be due to the fact that healthy omentum is primarily adipose tissue—we observed less than 3% fibroblast coverage and less than 1% activated fibroblast coverage in nOC-omenta. However, fibroblast numbers as well as activated fibroblast coverage were significantly increased in OC-omenta. Furthermore, using a mouse explant model, we observed TGFβ1 treatment activated fibroblasts in the interior of the tissue.
These results are consistent with a mouse in vivo study by Cai et al., where intraperitoneally injected ovarian cancer cells activated resident omental fibroblasts to express αSMA.25 Consistent with our findings, they observed that inhibiting the TGFβ1 receptor in their mouse model reduced activated fibroblast coverage as well as tumor burden. While we examined the impacts of this activation process on Col1 production, they observed that activated CAFs secreted potent growth factors including HGF and metalloproteinases such as MMP-2, which were capable of inducing ovarian cancer attachment and invasion in an in vitro co-culture system.25
Another study demonstrated that fibroblast-secreted TGFα promoted ovarian cancer peritoneal metastasis in an orthotopic ovarian cancer xenograft model.37 CAFs have also been shown to produce IL-6, CXCL10, and CCL5, which initiated metabolism of glycogen stores in the cancer cells and increased tumor cell proliferation, invasion, and metastasis.38 Combined, these results demonstrate that fibroblast activation can have multiple downstream effects and inhibiting fibroblasts may slow disease progression. Recently, the metabolic regulator N-methyltransferase (NNMT) was shown to control the CAF phenotype in HGOSC, as NNMT inhibition lowered αSMA levels in CAFs from ovarian cancer patients.39
Our in vitro results support that TGFβ1 stimulated Col1 production in fibroblasts, and analysis of human tumor samples confirmed that COL1A1 expression was elevated in fibroblasts. These findings complement a prior study that identified a 10 ECM gene signature in primary HGSOC tumors predictive of overall survival.40 While this signature did not include COL1A1 or COL1A2, pathway analysis suggested a role for TGFβ1 signaling and treatment of ovarian stromal cells with TGFβ1 increased expression of all 10 genes, suggesting that TGFβ1 regulation of ECM occurs across primary and metastatic stages. An increase in Col1 would be expected to increase tissue stiffness,41 and tissue stiffness has been correlated with disease progression in HGSOC.9
Other studies have demonstrated that increased matrix density/stiffness can induce tumor cell proliferation,42 a critical step for further progression. Tissue culture plastic coated with Col1 has been shown to induce tumor cell proliferation through β1 integrins43; however, different levels of Col1 density/stiffness were not examined. Interestingly, our results demonstrated that increasing Col1 density (with only small increases in stiffness) did not impact tumor cell proliferation. However, during ovarian cancer progression, many aspects of the tumor microenvironment change concurrently. For example, as the omentum is remodeling, many patients accumulate ascites,44 leading to an increase in the levels of growth factors and cytokines.45
Previous studies have demonstrated that HGSOC tumor cells proliferate in response to EGF family growth factors,46,47 and our analysis confirmed that HGSOC patients have elevated levels of HB-EGF in particular. Interestingly, only one cell line proliferated in response to HB-EGF when cultured on Col1 gels that mimicked the matrix concentration of the normal omentum. However, all the cell lines tested here became sensitive to HB-EGF as the Col1 microenvironment progressed to a denser matrix comparable to the distal and proximal regions of diseased omenta.
There are several potential mechanisms that could explain the interaction between increased Col1 density and HB-EGF to induce tumor cell proliferation. First, it is possible that the tumor cells are acting in response to the Col1 in a manner that sensitizes them to the HB-EGF. For example, prior studies have demonstrated that increased matrix density/substrate stiffness impacts EGFR expression,48 causing cells to be more sensitive to EGFR ligands.49 Another potential explanation for our observed synergy between HB-EGF and Col1 density is that both stiffness and EGFR have been shown to regulate Yes-associated protein-1 (YAP1),50 and inhibition of YAP1 decreased ovarian cancer proliferation in a xenograft mouse model.51 However, we note that our changes in stiffness were fairly small in this system (Supplementary Fig. S3); to reach the range of stiffnesses observed in human tumors, it would be necessary to use an alternative approach, such as a semi-interpenetrating network of methacrylated gelatin and Col1.30 Alternatively, the impact of Col1 on tumor cell proliferation may result from entrapment and concentration of the HB-EGF, as many growth factors interact with the ECM.52 Results from our laboratory have demonstrated that physical immobilization of growth factors can alter cell behavior due to the ability for the EGF receptor to cluster.53
Our results support that identifying and mimicking changes in the ECM during HGSOC metastasis will help to clarify the role of other tumor microenvironment alterations associated with disease progression. In particular, our results support further investigation into TGFβ1, Col1, and HB-EGF as a dynamic interaction that regulates tumor cell proliferation.
Supplementary Material
Acknowledgments
We thank the University of Wisconsin Carbone Cancer Center Optical Imaging Core, Translational Research Initiatives in Pathology Laboratory, and Microtechnology Core supported by NIH 5P30CA014520. We are grateful for the support from Dr. Erin Brooks, University of Wisconsin Department of Pathology for procuring whole omenta for these studies.
Disclosure Statement
P.K. Kreeger has a financial arrangement with Novartis International AG related to research that is not included in this study. All other authors declare that no competing financial interests exist.
Funding Information
Funding was provided by the American Cancer Society (RSG-13-026-01-CSM and Midwest Division supplement to P.K. Kreeger), NIH (1DP2CA195766, 1R01CA232517, and R21CA227922 to P.K. Kreeger, R01HL144086 to D.M. Wellik), and the Marsha Rivkin Center for Ovarian Cancer Research Scientific Scholar Fellowship to K.C. Fogg).
Supplementary Material
References
- 1. Siegel R., Ma J., Zou Z., and Jemal A.. Cancer statistics, 2014. CA Cancer J Clin 64, 9, 2014 [DOI] [PubMed] [Google Scholar]
- 2. Sodek K.L., Murphy K.J., Brown T.J., and Ringuette M.J.. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev 31, 397, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 177, 1053, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nieman K.M., Kenny H.A., Penicka C.V., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med 17, 1498, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kenny H.A., Krausz T., Yamada S.D., and Lengyel E.. Use of a novel 3D culture model to elucidate the role of mesothelial cells, fibroblasts and extra-cellular matrices on adhesion and invasion of ovarian cancer cells to the omentum. Int J Cancer 121, 1463, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Carroll M.J., Fogg K.C., Patel H.A., et al. Alternatively activated macrophages upregulate mesothelial expression of P-selectin to enhance adhesion of ovarian cancer cells. Cancer Res 78, 3560, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fogg K.C., Olson W.R., Miller J.N., et al. Alternatively activated macrophage-derived secretome stimulates ovarian cancer spheroid spreading through a JAK2/STAT3 pathway. Cancer Lett 458, 92, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooks E.A., Gencoglu M.F., Corbett D.C., Stevens K.R., and Peyton S.R.. An omentum-inspired 3D PEG hydrogel for identifying ECM-drivers of drug resistant ovarian cancer. APL Bioeng 3, 026106, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pearce O.M.T., Delaine-Smith R.M., Maniati E., et al. Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers. Cancer Discov 8, 304, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gimenez A., Duch P., Puig M., Gabasa M., Xaubet A., and Alcaraz J.. Dysregulated collagen homeostasis by matrix stiffening and TGF-beta1 in fibroblasts from idiopathic pulmonary fibrosis patients: role of FAK/Akt. Int J Mol Sci 18, E2431, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campbell K.R., and Campagnola P.J.. Assessing local stromal alterations in human ovarian cancer subtypes via second harmonic generation microscopy and analysis. J Biomed Opt 22, 1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conklin M.W., Eickhoff J.C., Riching K.M., et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol 178, 1221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Provenzano P.P., Inman D.R., Eliceiri K.W., et al. Collagen density promotes mammary tumor initiation and progression. BMC Med 6, 11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sherman-Baust C.A., Weeraratna A.T., Rangel L.B., et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell 3, 377, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Kalluri R., and Zeisberg M.. Fibroblasts in cancer. Nat Rev Cancer 6, 392, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Mikula-Pietrasik J., Uruski P., Szubert S., et al. Biochemical composition of malignant ascites determines high aggressiveness of undifferentiated ovarian tumors. Med Oncol 33, 94, 2016 [DOI] [PubMed] [Google Scholar]
- 17. Ronnov-Jessen L., and Petersen O.W.. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 68, 696, 1993 [PubMed] [Google Scholar]
- 18. Fleszar A.J., Walker A., Porubsky V.L., et al. The extracellular matrix of ovarian cortical inclusion cysts modulates invasion of fallopian tube epithelial cells. APL Bioeng 2, 031902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hutson H.N., Marohl T., Anderson M., Eliceiri K., Campagnola P., and Masters K.S.. Calcific aortic valve disease is associated with layer-specific alterations in collagen architecture. PLoS One 11, e0163858, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagendran M., Riordan D.P., Harbury P.B., and Desai T.J.. Automated cell-type classification in intact tissues by single-cell molecular profiling. Elife 7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bolte S., and Cordelieres F.P.. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224, 213, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Aper S.J., van Spreeuwel A.C., van Turnhout M.C., et al. Colorful protein-based fluorescent probes for collagen imaging. PLoS One 9, e114983, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campeau E., Ruhl V.E., Rodier F., et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One 4, e6529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuzet S.E., and Gaggioli C.. Fibroblast activation in cancer: when seed fertilizes soil. Cell and tissue research 365, 607, 2016 [DOI] [PubMed] [Google Scholar]
- 25. Cai J., Tang H., Xu L., et al. Fibroblasts in omentum activated by tumor cells promote ovarian cancer growth, adhesion and invasiveness. Carcinogenesis 33, 20, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Ko S.Y., Barengo N., Ladanyi A., et al. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J Clin Invest 122, 3603, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vander Ark A., Cao J., and Li X.. TGF-beta receptors: in and beyond TGF-beta signaling. Cell Signal 52, 112, 2018 [DOI] [PubMed] [Google Scholar]
- 28. Midgley A.C., Rogers M., Hallett M.B., et al. Transforming growth factor-beta1 (TGF-beta1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J Biol Chem 288, 14824, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abnaof K., Mallela N., Walenda G., et al. TGF-beta stimulation in human and murine cells reveals commonly affected biological processes and pathways at transcription level. BMC Syst Biol 8, 55, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berger A.J., Linsmeier K.M., Kreeger P.K., and Masters K.S.. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials 141, 125, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carroll M.J., Kapur A., Felder M., Patankar M.S., and Kreeger P.K.. M2 macrophages induce ovarian cancer cell proliferation via a heparin binding epidermal growth factor/matrix metalloproteinase 9 intercellular feedback loop. Oncotarget 7, 86608, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bourgeois D.L., Kabarowski K.A., Porubsky V.L., and Kreeger P.K.. High-grade serous ovarian cancer cell lines exhibit heterogeneous responses to growth factor stimulation. Cancer Cell Int 15, 112, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Acerbi I., Cassereau L., Dean I., et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr Biol (Camb) 7, 1120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kucukmetin A., Naik R., Galaal K., Bryant A., and Dickinson H.O.. Palliative surgery versus medical management for bowel obstruction in ovarian cancer. Cochrane Database Syst Rev 7, CD007792, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tran E., Spiceland C., Sandhu N.P., and Jatoi A.. Malignant bowel obstruction in patients with recurrent ovarian cancer. Am J Hosp Palliat Care 33, 272, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naba A., Pearce O.M.T., Del Rosario A., et al. Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J Proteome Res 16, 3083, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lau T.S., Chan L.K., Wong E.C., et al. A loop of cancer-stroma-cancer interaction promotes peritoneal metastasis of ovarian cancer via TNFalpha-TGFalpha-EGFR. Oncogene 36, 3576, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Curtis M., Kenny H.A., Ashcroft B., et al. Fibroblasts mobilize tumor cell glycogen to promote proliferation and metastasis. Cell Metab 29, 141, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eckert M.A., Coscia F., Chryplewicz A., et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 569, 723, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheon D.J., Tong Y., Sim M.S., et al. A collagen-remodeling gene signature regulated by TGF-beta signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin Cancer Res 20, 711, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cox T.R., and Erler J.T.. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 4, 165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tilghman R.W., Cowan C.R., Mih J.D., et al. Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One 5, e12905, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmed N., Riley C., Rice G., and Quinn M.. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin Exp Metastasis 22, 391, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Nougaret S., Addley H.C., Colombo P.E., et al. Ovarian carcinomatosis: how the radiologist can help plan the surgical approach. Radiographics 32, 1775, 2012 [DOI] [PubMed] [Google Scholar]
- 45. Matte I., Lane D., Laplante C., Rancourt C., and Piche A.. Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res 2, 566, 2012 [PMC free article] [PubMed] [Google Scholar]
- 46. Yagi H., Miyamoto S., Tanaka Y., et al. Clinical significance of heparin-binding epidermal growth factor-like growth factor in peritoneal fluid of ovarian cancer. Br J Cancer 92, 1737, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khabele D., Kabir S.M., Dong Y., Lee E., Rice V.M., and Son D.S.. Preferential effect of akt2-dependent signaling on the cellular viability of ovarian cancer cells in response to EGF. J Cancer 5, 670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grasset E.M., Bertero T., Bozec A., et al. Matrix stiffening and EGFR cooperate to promote the collective invasion of cancer cells. Cancer Res 78, 5229, 2018 [DOI] [PubMed] [Google Scholar]
- 49. Singh B., Carpenter G., and Coffey R.J.. EGF receptor ligands: recent advances. F1000Res 5, F1000, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xia H., Dai X., Yu H., et al. EGFR-PI3K-PDK1 pathway regulates YAP signaling in hepatocellular carcinoma: the mechanism and its implications in targeted therapy. Cell Death Dis 9, 269, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Feng J., Gou J., Jia J., Yi T., Cui T., and Li Z.. Verteporfin, a suppressor of YAP-TEAD complex, presents promising antitumor properties on ovarian cancer. Onco Targets Ther 9, 5371, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Naba A., Clauser K.R., Ding H., Whittaker C.A., Carr S.A., and Hynes R.O.. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol 49, 10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim C.S., Yang X., Jacobsen S., Masters K.S., and Kreeger P.K.. Leader cell PLCgamma1 activation during keratinocyte collective migration is induced by EGFR localization and clustering. Bioeng Transl Med 4, e10138, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.