Abstract
Cancer survivorship has increased considerably, but common cancer treatments may threaten female reproductive health and fertility. In females, standard fertility preservation techniques include egg and embryo banking and ovarian tissue cryopreservation, but these methods are not suitable for all individuals. Emerging fertility preservation technologies include in vitro follicle growth and ovarian bioprosthetics. Although these platforms hold tremendous promise, they remain in the preclinical phase likely because of our inability to adequately phenocopy the complexity of the in vivo ovarian environment. The goal of this study was to use an established research archive of fixed human ovarian tissue established through the Oncofertility Consortium to better understand the dynamics and milieu of growing follicles within the human ovary. We performed a histological analysis of the immediate surroundings of primary and secondary stage follicles. We evaluated oocyte and follicle diameters of these growing follicles, analyzed their growth trajectories, and mapped their precise relationships to other stage follicles within a defined area. We also stratified our findings according to participant age and previous treatment history. Our results serve as in vivo benchmarks for follicles grown in vitro and provide insight into how follicles should be seeded spatially within bioprosthetic ovaries, potentially improving the efficacy and clinical translation of these emerging technologies.
Impact statement
Life-preserving cancer treatments have greatly increased survivorship. However, treatments often have off-target health consequences that threaten female reproductive health and fertility. Although several standard fertility preservation options exist, there is a constant need to explore and expand options for all populations. In vitro follicle growth and ovarian bioprosthetics are new experimental procedures, which are currently limited to proof of concept. In this study, we analyzed human ovarian tissue from a deidentified biospecimen repository to characterize the growing follicle landscape with the ultimate goal of informing bioengineering practices. This spatial analysis pinpoints the geometry of growing follicles within the human ovary and provides a framework for paralleling this environment in ex vivo platforms.
Keywords: ovary, follicle, fertility preservation, human, ovarian tissue cryopreservation
Introduction
Cancer survivorship has increased considerably due to improvements in early detection and life-saving treatments. In the United States alone, the number of cancer survivors reached 14.5 million in 2014, with this number expected to increase to 19 million by 2024.1 However, cancer treatments can have off-target and unintended consequences on organ systems, including the female reproductive system. It is well known that the hypothalamic–pituitary–gonadal axis, the ovary, and the reproductive tract are highly susceptible to chemotherapy and radiation-induced damage, which can contribute to infertility and premature menopause.2–7
Standard fertility preservation methods such as egg and embryo banking require hormonal stimulation and thus are not suitable for prepubertal girls, those with ethical concerns about gamete preservation, or those with aggressive cancers who cannot delay treatment to undergo assisted reproductive technology (ART) procedures.8 One alternative to traditional ART procedures is ovarian tissue cryopreservation (OTC). In OTC, ovarian tissue is removed before cancer treatment, cryopreserved, and later transplanted post-treatment back into the patient to restore endocrine function and fertility. This procedure has resulted in over 130 live births,9–12 but is contraindicated in patients with ovarian cancer, blood-borne malignancies, or other cancers where there is risk of reintroducing cancerous cells upon transplantation.13–16
The need to develop new fertility preservation options has driven technological advances. These include the ability to grow early stage human follicles in various culture systems, generating mature eggs that could be cryopreserved or fertilized.17–21 Additionally, bioengineering breakthroughs, including decellularization and three-dimensional (3D) printing, have been used to generate bioprosthetic ovaries and soft tissue transplants, which support endocrine function and fertility in mouse models.22,23 Decellularized ovarian scaffolds and decellularized ovarian tissue papers have also been used to successfully support the survival of human preantral stage follicles.24,25 These technologies, although holding tremendous potential, remain at the preclinical phase and are primarily proof of concept. One possible explanation for why these methods have not advanced further is a reliance on primarily reductionist approaches, whereas an effective ovarian bioprosthetic must authentically recapitulate the complexity of the in vivo ovarian environment.26
Existing 3D printed ovaries have mimicked the ovary using gelatin scaffolds containing follicles, while decellularized models have consisted of just a protein matrix and a population of follicles.8,22,23,25,27–32 However, the ovary has many different cellular components that are essential for proper function, including theca cells, immune cells, endothelial cells, fibroblasts, and extracellular matrix components.33–35 Additionally, the architecture of the ovary consists of structurally distinct regions. Whereas the outer cortex of the ovary is collagen dense and primarily contains primordial follicles, the inner medulla is less collagen dense and supports growth of later stage follicles.36,37
Moreover, how different stage follicles are organized with respect to each other is critical as spatial relationships influence biological functions. For example, spatial analysis of follicles in the mouse ovary demonstrates that interfollicular distances are reflective of potential sources of regulatory signals that either inhibit activation or promote growth.38,39 In addition, in vitro studies have shown that growing follicles produce and secrete paracrine factors that are essential to support follicle growth and gamete quality.36,40 Therefore, understanding spatial relationships between follicles and other cellular components in the ovary is of critical importance, but has been historically lacking for the human ovary due to the limited material available for research purposes.
The goal of this study was to use an existing biospecimen repository of fixed human ovarian tissue established through the Oncofertility Consortium to define the landscape of growing follicles in the human ovary and, in doing so, better inform engineering efforts to generate organotypic bioprosthetic ovaries. The majority of histological analyses in human ovarian tissue have focused on primordial follicles, which dictate the ovarian reserve, but considerably less is known about follicles once they have started to grow.41–44
Primary and secondary follicles represent a critical developmental period of oogenesis and folliculogenesis and thus a better understanding of the follicle environment at these stages is warranted. We quantified growing follicles at the primary, secondary, and early antral stages, analyzed their growth trajectories, and mapped their spatial distribution relative to other follicles to articulate the growing follicle landscape. Additionally, we characterized the growing follicle pool in the context of age and previous treatment history as follicle geography may be impacted by these parameters. These data develop a spatial framework for the human ovary and can be used as benchmarks for in vitro follicle growth (IVFG) systems and as roadmaps for ovarian bioprosthetics to better mimic in vivo physiology.
Materials and Methods
Human ovarian tissue and participant data acquisition
The Oncofertility Consortium is a global network committed to providing patients with fertility preservation options.45 Between 2007 and 2017, clinical sites within a network known as the Oncofertility Consortium's National Physicians Cooperative (OC-NPC) performed OTC for females in need under Institutional Review Board (IRB) approval. These IRB protocols partnered clinical medicine and basic research as 80% of ovarian tissue from participants was cryopreserved for future clinical use to restore endocrine function and/or fertility and 20% of the ovarian tissue could be donated to basic research focused on fertility preservation.46–48 Over this period, more than 400 females underwent OTC at an OC-NPC site. Ten percent of the 20% donated ovarian tissue was formalin fixed and paraffin embedded (FFPE) and used to generate a histological, ovarian tissue biospecimen repository.46,47 At the time of study enrollment, demographic and health information for each participant was obtained, including age, race, ethnicity, clinical diagnosis, and previous treatment history. All participant information and ovarian tissues were deidentified before research use.
Ovarian tissue fixation and histological processing
For patient cases in the Chicago area, ovarian tissue designated for research was brought to the basic science laboratory within 2–4 h of the OTC procedure. For all other cases, ovarian tissue designated for research was transported to Northwestern University in SAGE OTC Holding Media (CooperSurgical, Trumbull, CT) at 4°C for up to 24 h. These transport conditions do not compromise the reproductive potential of the tissue and help maintain tissue integrity.47,49,50
Ovarian tissue was fixed in 10% neutral buffered formalin overnight at 4°C, processed using an automated tissue processor (Leica Biosystems, Buffalo Grove, IL), and embedded in paraffin wax. The tissue was sectioned at 5-μm thickness and mounted upon slides and stained with hematoxylin and eosin (H&E) using an Autostainer XL (Leica Biosystems) according to standard protocols. These slides were then filed into the tissue repository.
An initial screening of the repository was performed to identify participant tissues that contained growing follicles (primary, secondary, and antral follicles), resulting in the subset of 37 participant samples used for this study. Demographics and clinical parameters of these participants are reported in Table 1. For each participant, between 15 and 104 tissue sections were evaluated. The variability in the number of sections analyzed was based on the number of slides and tissue sections that were readily available for each participant in the tissue repository. In total, there were 482 follicles of interest that were analyzed, which included 296 primary follicles, 184 secondary follicles, and 2 antral follicles. Slides were visualized using an EVOS FL Cell Imaging system (Thermo Fisher Scientific, Waltham, MA). To note, the pieces of tissue designated for research were random with respect to ovarian architecture. However, based on morphology, the majority of tissues appeared to be cortical tissue.
Table 1.
Participant Characteristics
| Age | Race | Ethnicity | Diagnosis | Previous Cancer Treatment |
|---|---|---|---|---|
| Solid Tumor | ||||
| 22 months | White | Non-Hispanic | Rhabdomyosarcoma (right chest wall: liver) | None |
| 4 | More than 1 | Non-Hispanic | Alveolar rhabdomyosarcoma | Irinotecan (250mg/m2) vincristine (4/5mg/m2) |
| 4 | White | Non-Hispanic | Wilms Tumor, Bilateral multifocal | EE4A and other chemotherapy including cyclophrophamide (8-10g/m2) |
| 4 | Asian | Non-Hispanic | Stage IV embryonal rhabdomyosarcoma, abdominal primary tumor with peritoneal carcinomatosis and nodal disease throughout her abdomen and pelvis | ARST 0431 with VCR, Irinotecan, Doxorubicin, Cytoxan, Ifosfamide, VP-16 through week 16 |
| 5 | Black or African American | Non-Hispanic | Pelvic rhabdomyosarcoma | None |
| 7 | White | Non-Hispanic | Ewing Sarcoma, Right clavicle | N/A |
| 9.6 | White | Non-Hispanic | Rhabdomyosarcoma, vagina | Vincristine, actinomycin, cyclophosphamide, ifosfamide (Yes, CTX 32.13 g/m2 + Ifsosfamide 9 g/m2) |
| 7 | White | Hispanic | Parameningeal Rhabdomyosarcoma | ARST0531 (Vincristine, Actinomycin, Cyclophosphamide 1.2 gr/m2, Irinotecan) |
| 13 | N/A | Non-Hispanic | Ewings; Sarcoma | None |
| 16 | White | Non-Hispanic | Ewings Sarcoma of pelvis | N/A |
| 16 | White | Non-Hispanic | Low grade midline glioneuronal | None |
| 16.3 | White | Non-Hispanic | Ewings Sarcoma in the Breast | None |
| 16.6 | Black or African American | Non-Hispanic | Penioblastoma | None |
| 18.8 | White | Non-Hispanic | Intracranial germinoma - pituitary | None |
| 20 | White | Non-Hispanic | Ewing Sarcoma of the left humerus | AEWS0031 Reg B: Doxorubicin (37.5 mg/m2), cyclophosphamide (1200 mg/m2), vincristine (1.25 mg/m2) |
| 21.5 | Osteosarcoma | None | ||
| 27 | White | Non-Hispanic | High grade sarcoma | N/A |
| Hemoglobinopathy | ||||
| 2 | White | Non-Hispanic | Beta Thalassemia Intermedia | Hydroxyurea |
| 2 | Black or African American | Non-Hispanic | Sickle cell anemia | N/A |
| 6 | Black or African American | Non-Hispanic | Sickle cell disease | Chemotherapy received and doses: Alemtuzumeb (48 mg/m2) |
| 11 | Black or African American | Non-Hispanic | Sickle cell disease | N/A |
| 12 | Unknown or Not Reported | Non- Hispanic | Beta Thalassemia Major | N/A |
| 14.6 | White | Non-Hispanic | Myelodysplastic syndrome, go for transplantation | No chemo/radio |
| 18 | Black or African American | Non-Hispanic | Aplastic anemia | None |
| Hematological Malignancy | ||||
| 15 months | White | Non-Hispanic | B-Cell ALL | AALL0631 for BMT prep |
| 4 | White | Non-Hispanic | CML | N/A |
| 10 | White | Non-Hispanic | Refractory T/Myeloid Leukemia | None |
| 13 | Unknown or Not reported | Non-Hispanic | Stage IVB Hodgkin's (Diagnosed in May 2015) | Treated with AHOD1331 with brentuximab, PET negative after cycle 2 and completed chemo in September 2015. Completed radiation to bulk mediastinal disease in late October 2015 (2100 Gy). Bv-AVE-PC doxorubicin, cytoxan (6 g/m2), etoposide (1868 mg/m2), prednisone, vincristine. |
| 15 | White | Non-Hispanic | Acute Myloid Leukemia | AAML 1031 (3 cycles: Induction I and II, Intesification I) Total doses: ARAC IV-157mg ARAC Intrathercal-70mg High dose cytarabine-7500mg Daunorubicin-78.5mg Mitoxantrone-72mg Bortezomib-14mg Etoposide-382mg (alkylating) |
| 16 | Black or African American | Non-Hispanic | AML, monocytic acute leukemia, diagnosed in June 2014. CNS disease at diagnosis. AML relapsed in Feb 2015. | Initial Therapy: AAML 1031/Arm B: ARA-C (IT), Daunorubicin, Dexrazoxane, Etoposide, Bortezomib, Mitoxantrone; no radiation |
| Relapse Therapy: FLAG x 2 cycles: ARA-C (IV), Fludarabine, Triple Intrathecals (ARA-C, Hydrocortisone, MTX); no radiation | ||||
| 26.3 | Recurrent Hodgkin's | ABVDx6 | ||
| Gynecological | ||||
| 13 | White | Non-Hispanic | Small cell carcinoma of ovary | None |
| 26 | White | Non-Hispanic | Neoplasm of borderline malignancy | N/A |
| 28 | White | Non-Hispanic | Carcinoma of the uterus | N/A |
| 34.9 | Atypical complex hyperplasia | None | ||
| Benign Tumor | ||||
| 28.6 | White | Non-Hispanic | Endometriosis | None |
| 31 | White | Non-Hispanic | Left ovarian cyst | N/A |
ABVD, adriamycin, bleomycin, vinblastine.
Morphological criteria for growing follicles and follicle counting
Follicles were classified and counted within each H&E-stained ovarian tissue section using previously defined morphological criteria.51 Of note, this analysis was limited to morphologically normal follicles. Primordial follicles were defined as oocytes surrounded by a single or incomplete layer of squamous granulosa cells; primary follicles were defined as oocytes surrounded by a single complete layer of cuboidal granulosa cells; secondary follicles were defined as oocytes surrounded by two or more layers of granulosa cells; and antral follicles were defined as oocytes surrounded by multiple layers of granulosa cells and the presence of an antral space (Fig. 1A–F). Follicles from the primary stage and beyond were considered growing follicles. Only growing follicles with the oocyte nucleus visible in the tissue section were counted and analyzed to ensure proximity to the mid-section of each follicle and to avoid double counting.
FIG. 1.
Histological evaluation of follicle morphology and interfollicular distances. H&E-stained, human ovarian tissue sections depicting follicles. Follicle classes were defined according to morphological criteria. (A) Representative image of a primordial follicle identified as an oocyte surrounded by a single or incomplete layer of squamous granulosa cells. The asterisk indicates the primordial follicle. (B) Representative image of a primary follicle, defined as an oocyte surrounded by a single complete layer of cuboidal granulosa cells. Early secondary (C) and secondary (D) follicles were defined as oocytes surrounded by two or more layers of cuboidal granulosa cells. The insets in (A–C) magnify the follicle of interest to more clearly show the distinguishing features used to classify follicles. (C) The white arrow highlights two layers of cuboidal granulosa cells characteristic of secondary follicles. (E) Representative image of an antral follicle, the largest follicle class, defined by multiple layers of granulosa cells and a fluid-filled space. (F) Image of a growing, multilayer secondary follicle and all neighboring follicles within the defined area (910 × 685 μm field of view). White lines indicate the distance of the follicle of interest to primordial follicles, red lines to primary follicles, blue lines to secondary follicles, and the yellow line to an antral follicle. The scale bar in (A–E) is 200 μm and the scale bar in (F) is 100 μm. H&E, hematoxylin and eosin. Color images are available online.
Measurement of oocyte and follicle diameters and interfollicular distances
Each growing follicle identified in ovarian tissue sections was imaged with the EVOS FL Cell Imaging system (Thermo Fisher Scientific) using a 40 × objective. Software tools provided with the system were used to measure oocyte and follicle diameters. Follicle and oocyte diameter measurements were made by averaging two perpendicular measurements from basement membrane to basement membrane and plasma membrane to plasma membrane of the structures, respectively.
To measure the distance of growing follicles relative to other follicles, individual follicles were centered in a 910 × 685 μm field of view and imaged on the EVOS FL Cell Imaging system (Thermo Fisher Scientific) using a 20 × objective. All neighboring follicles within the defined field of view were identified and their distances from the growing follicle of interest were measured with the EVOS FL Cell Imaging system software (Thermo Fisher Scientific; Fig. 1F).
Distances to a total of 10,259 surrounding follicles were measured. In specific, distances of 7593 neighboring follicles to primary follicles of interest were measured (7159 primordial, 305 primary, 126 secondary, and 3 antral follicles) and distances of 2666 neighboring follicles to secondary follicles of interest were measured (2362 primordial, 194 primary, 107 secondary, and 3 antral follicles). Follicles directly adjacent to one another were assigned distances of 0 μm.
Statistics
All results were analyzed and graphed using GraphPad Prism software, version 8.0.0 (La Jolla, California), and Microsoft Excel. For all analyses, p-values <0.05 were considered statistically significant. Unpaired Mann–Whitney tests, Student's t-test, linear regression modeling, one-way analysis of variance (ANOVA), and two-way ANOVA with Fisher's least significant difference post hoc analysis were used.
Results
Samples from the human tissue archive comprised a diverse participant cohort
To examine growing follicles, we identified a subset of 37 participants from our repository of fixed, human ovarian tissue samples in which primary, secondary, and early antral follicles were readily visible within H&E-stained tissue sections (Fig. 1B–E). The ages of these participants spanned 1.25 to 34.9 years, with a mean age of 14.1 ± 1.51 years and a median age of 13.0 years (Fig. 2A, Table 1). Participants were from different racial backgrounds, including White/Caucasian (59.5%, n = 22), Black/African American (18.9%, n = 7), and Asian (2.7%, n = 1). There were participants who reported more than one race (2.7%, n = 1) as well as those where race information was not available (16.2%, n = 6; Fig. 2B, Table 1).
FIG. 2.
Cohort population demographics. (A) Histogram depicting the age distribution of participants who underwent OTC at an OC-NPC site and whose ovarian tissue had growing follicles present. (B) The races of participants with ovarian tissue that contained growing follicles preserved for basic research. (C) The distribution of clinical diagnoses (malignant and nonmalignant) of participants who underwent OTC and whose tissues were used in these analyses. (D) The treatment status of females before ovarian tissue removal and OTC (chemotherapy, radiation, disease-modifying agent, or a combination of these). n = 37 participants. More detailed information about each participant can be found in Table 1. OC-NPC, Oncofertility Consortium's National Physicians Cooperative; OTC, ovarian tissue cryopreservation. Color images are available online.
These participants had diverse diagnoses that required OTC for fertility preservation. The majority of participants had solid tumors (46.0%, n = 17), but other reported conditions included hemoglobinopathy (18.9%, n = 7), hematological malignancy (18.9%, n = 7), gynecological cancer (10.8%, n = 4), and benign fertility-threatening conditions (5.4%, n = 2; Fig. 2C, Table 1). Although it is ideal to undergo OTC before chemotherapy, radiation, or other disease-modifying agents, this is not always possible. Additionally, there may be cases where OTC is performed post-treatment due to a relapse in cancer. Of the participants whose tissues were used for analysis, 35.1% (n = 13) had received such treatments before OTC, while 64.9% (n = 24) had not received previous treatment (Fig. 2D, Table 1).
History of previous treatment does not affect the number of growing follicles present within ovarian tissue
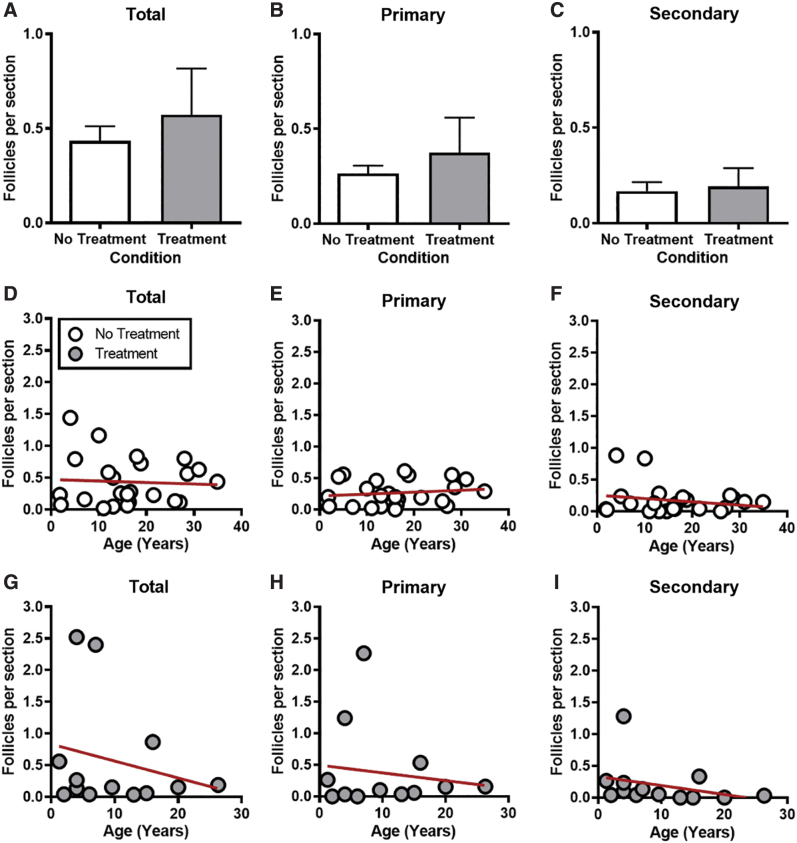
To determine the number of growing follicles in the ovarian tissue, H&E-stained tissue sections were evaluated and follicles in each class were classified and counted. These data were then stratified by previous treatment history and age to determine whether these parameters impacted the number of growing follicles present in the ovarian tissue. The total number of growing follicles was similar across participants, irrespective of whether they had received previous treatment or not; 0.6 ± 0.24 and 0.4 ± 0.08 follicles per section, respectively (p > 0.05; Fig. 3A). When further analyzing the data according to follicle stage, we found that there were, on average, more primary follicles in the tissue compared to secondary follicles (0.30 ± 0.069 and 0.18 ± 0.045, respectively), and previous treatment history did not impact the numbers of either class (p > 0.05; Fig. 3B, C). Of note, only two antral follicles in total were observed (data not shown). The low number of antral follicles was expected as the tissue examined comprised primarily the ovarian cortex.
FIG. 3.
Growing follicle numbers are similar regardless of prior treatment or reproductive age. (A) Graph showing the total number of growing follicles per tissue section for all participants with no prior treatment and with prior treatment. The total number of primary follicles (B) and secondary follicles (C) for all participants with and without prior treatment. Data are represented as mean ± SEM. (D) Scatterplot with best-fit slope depicting the total number of growing follicles per tissue section for participants with no prior treatment history. Scatterplots with best-fit slopes depicting the total number of primary follicles (E) and secondary follicles (F) for participants without prior treatment. (G) Scatterplot with best-fit slope depicting the total number of growing follicles per tissue section for participants with prior treatment history. Scatterplots with best-fit slopes depicting the total number of primary follicles (H) and secondary follicles (I) for participants with prior treatment history. Each data point represents one participant. n = 37 participants. Color images are available online.
We next examined whether there was a correlation between the number of growing follicles in ovarian tissue and reproductive age and whether this differed based on previous treatment history. There was no significant relationship between age and the number of growing follicles when analyzed in aggregate or separated into primary and secondary follicles (p > 0.05; Fig. 3D–I). However, there was a tendency of a negative slope between follicle number and age in participants with prior treatment history (Fig. 3G–I).
Follicle growth dynamics are altered in participants with a history of prior treatment
Follicle and oocyte growth is tightly coordinated such that as the follicle grows, the oocyte grows.52 To investigate growth trajectories of growing follicles, we measured follicle and oocyte diameters for each individual follicle. Within human ovarian tissue, the average oocyte diameter increased from 29.9 ± 0.35 μm at the primary follicle stage to 34.8 ± 0.92 μm at the secondary stage. The average follicle diameter also increased from 40.6 ± 0.40 μm at the primary follicle stage to 56.3 ± 2.02 μm at the secondary stage (Fig. 4A, B).
FIG. 4.
Primary oocyte and follicle diameters are significantly reduced in participants with prior treatment history. (A) Graph showing the average oocyte diameter for each primary and secondary follicle and (B) graph showing the average follicle diameter for each primary and secondary follicle, both stratified by treatment history. t-Tests were performed for both parameters; asterisks indicate p < 0.0001. Each data point represents one follicle. Color images are available online.
The data were then further analyzed based on treatment history. Individuals without previous treatment had larger oocytes at the primary follicle stage than those who had prior treatment; 31.0 ± 0.47 μm and 27.7 ± 0.41 μm, respectively (p < 0.0001; Fig. 4A). The follicle diameter was also significantly larger in primary follicles from individuals without previous treatment relative to those with prior treatment; 41.9 ± 0.53 μm and 38.1 ± 0.49 μm, respectively (p < 0.0001; Fig. 4B). This reduction in oocyte and follicle diameters associated with previous treatment history was unique to the primary follicle stage as it was not observed in secondary follicles.
Oocyte diameters were similar for secondary follicles in participants with and without prior treatment; 33.3 ± 1.05 μm and 35.7 ± 1.29 μm, respectively, as were follicle diameters; 54.0 ± 2.69 μm and 57.5 ± 2.73 μm, respectively (p > 0.05; Fig. 4A, B). Conclusions from antral follicles could not be drawn due to the limited number of follicles at this particular stage within the cortical tissue.
Because oocyte and follicle diameters from each follicle were measured, we were able to examine the relationship between oocyte and follicle size stratified by previous treatment history (Fig. 5A–D). There was a strong positive linear correlation between oocyte and follicle diameters for both participant groups. However, the overall growth trajectories differed significantly between follicles from individuals who had received previous treatment compared to those who did not, indicating disrupted coordinated growth associated with previous treatment (p = 0.0005; Fig. 5D).
FIG. 5.
Follicle growth trajectories are altered in participants with prior medical treatment. (A) A representative, H&E-stained, human ovarian tissue section illustrating how oocyte and follicle diameter measurements were made. Green and blue lines and values indicate oocyte diameter measurements used to calculate the average oocyte diameter. Orange and pink lines and values indicate follicle diameter measurements used to calculate the average follicle diameter measurement. Scale bar is 100 μm. Scatterplots showing follicle diameter versus oocyte diameter for each growing follicle identified in participants with no prior treatment history (B) and with prior treatment history (C), separated by follicle class. (D) Graph depicting best-fit slopes of coordinated follicle and oocyte growth trajectories for participants with and without previous treatment. Asterisk indicates a significant difference between trajectories (p = 0.0005). Color images are available online.
Growing follicles are primarily surrounded by primordial follicles
To characterize the follicle landscape of growing follicles within the human ovary, each primary and secondary stage follicle was centered in a defined field of view and neighboring follicle classes were documented. We first examined the number of surrounding follicles for each primary follicle. Primary follicles had an average of 13.0 ± 4.69 follicle neighbors per section across participants. This total comprised an average of 12.2 ± 4.56 primordial follicles, 0.7 ± 0.23 primary follicles, 0.4 ± 0.13 secondary follicles, and 0.2 ± 0.00 neighboring antral follicles (Fig. 6A). Secondary follicles had an average of 10.7 ± 2.72 follicle neighbors per section across participants. This total comprised an average of 10.3 ± 2.64 primordial follicles, 0.8 ± 0.26 primary follicles, 0.5 ± 0.20 secondary follicles, and 0.1 ± 0.00 neighboring antral follicles (Fig. 6B). Primary and secondary follicles had significantly more primordial follicle neighbors compared with primary and secondary follicle neighbors (p = 0.02 and p < 0.005, respectively; Fig. 6A, B).
FIG. 6.
Primary and secondary follicles are primarily surrounded by primordial follicles. Graphs showing the average numbers of primordial, primary, secondary, and antral follicle neighbors for primary follicles (A) and secondary follicles (B) of interest. One-way ANOVAs (with Fisher's LSD post hoc analysis for multiple comparisons) were performed. Graphs showing the average numbers of primordial, primary, secondary, and antral follicle neighbors for primary follicles (C) and secondary follicles (D) of interest, stratified by treatment history. Two-way ANOVAs (with Fisher's LSD post hoc analysis for multiple comparisons of all row and column means) were performed. Asterisks indicate significant differences (p < 0.05). Data are represented as mean ± SEM. ANOVA, analysis of variance; LSD, least significant difference.
Total neighbor follicle counts were then further analyzed with regard to previous treatment history. For participants without prior treatment, this total comprised an average of 5.9 ± 1.12 primordial follicles, 0.4 ± 0.09 primary follicles, 0.3 ± 0.07 secondary follicles, and 0.2 ± 0.00 antral follicles (Fig. 6C). For participants with treatment history, this total comprised an average of 26.2 ± 13.85 primordial follicles, 1.4 ± 0.72 primary follicles, 0.8 ± 0.57 secondary follicles, and 0 antral follicles (Fig. 6C). Primary follicles in tissues from participants with previous treatment were surrounded by significantly more primordial follicles compared with participants who had not received treatment (p = 0.0014; Fig. 6C).
This same analysis was then done for each secondary follicle. For those participants who had no prior treatment history, this total comprised an average of 7.0 ± 1.82 primordial neighbors, 0.7 ± 0.17 primary neighbors, 0.3 ± 0.05 secondary neighbors, and 0.1 ± 0.00 antral neighbors (Fig. 6D). For those participants who had prior treatment history, this total comprised an average of 19.5 ± 8.04 primordial neighbors, 1.3 ± 0.95 primary neighbors, 1.0 ± 0.71 secondary neighbors, and 0 antral neighbors (Fig. 6D). Similarly to primary follicles, secondary follicles were also surrounded by more primordial follicle neighbors in tissues from participants who had received previous treatment compared to those who had not (p = 0.0021, Fig. 6D).
Distances between growing follicles and immediate surrounding follicles are similar across classes
In addition to the number and types of follicles surrounding follicles as they grow, their position relative to each other is an important parameter to consider when attempting to phenocopy the in vivo ovarian environment (Fig. 1). Therefore, we measured the interfollicular distances between each growing follicle in the human ovarian tissue and their respective neighboring follicles. In tissues from participants with no prior treatment, the average distance between primary follicles and primordial follicles was 217.6 ± 16.37 μm and the distance between primary follicles and other primary follicles was larger compared with participants without previous treatment; 286.6 ± 44.44 μm and 171.9 ± 27.41 μm, respectively (p = 0.04; Fig. 7A). Although the distance from primary follicles to secondary follicles appeared to be greater in tissues from participants with prior treatment, this was not significant (243.8 ± 10.52 μm and 178.9 ± 25.51 μm, respectively; Fig. 7A).
FIG. 7.
Primary and secondary follicles are separated from other follicles by ∼200–300 μm. (A) Graph showing the average distances of primordial, primary, and secondary follicles to each primary follicle of interest per participant, stratified by prior treatment history. Each data point indicates one follicle. t-Tests were performed. (B) Graph showing the average distances of primordial, primary, and secondary follicles to each secondary follicle of interest per participant, stratified by prior treatment history. t-Tests were performed; asterisks indicate significant differences (p < 0.05). Color images are available online.
This same analysis was then done for each secondary follicle. The distance between secondary follicles and primordial follicles was similar between participant groups and, on average, 205.4 ± 16.62 μm in participants without previous treatment and 219.0 ± 31.17 μm in participants with prior treatment. The distances between secondary and primary follicles tended to be greater in participants with prior treatment relative to those without, although this was not significant (239.4 ± 32.40 μm and 171.8 ± 17.26 μm, respectively; Fig. 7B). However, the interfollicular distance from secondary follicles to other secondary follicles was significantly different between participants, 314.1 ± 47.37 μm in those with previous treatment and 126.9 ± 24.42 μm in those without (p = 0.01; Fig. 7B). Thus, despite some differences related to previous treatment history and follicle stage, the average distance between growing follicles and surrounding follicles ranged between ∼170 and 290 μm.
Discussion
In this study, we performed a histological analysis of the growing follicle landscape in the human ovary. This analysis was performed using an existing deidentified biospecimen repository of FFPE human ovarian tissue. We first investigated the quantity of growing follicles present within the tissue and, as expected, the number of these follicles tended to decline with reproductive age. Interestingly, the number of growing follicles was similar between study participants who had received prior treatment and those who had not. Chemotherapy and radiation are known to contribute to premature ovarian failure and accelerate reproductive aging, primarily by targeting primordial follicles.53–55 Although chemotherapy can target growing follicles, it is important to recognize that the growing follicles we characterized in participants who had received prior treatment would likely not have been exposed to chemotherapy, but rather were derived from primordial follicles that survived the exposure.
Although the presence of growing follicles in tissues from participants with previous treatment is encouraging, the quality of these follicles remains unknown. Follicles from participants with a history of prior treatment had smaller oocyte and follicle diameters at the primary follicle stage. These size differences translated into dysregulated follicle growth trajectories, which may be indicative of poorer quality follicles. These findings are remarkably similar to the altered follicle growth trajectories observed in a mouse model of physiologic reproductive aging where there is disrupted bidirectional communication between the oocyte and its surrounding granulosa cells.51,56
However, why these growth trajectories are altered remains to be fully elucidated. One possibility is that following chemotherapy, damaged oocytes or granulosa cells are not eliminated effectively and thus the persisting follicles may have compromised quality, which is reflected in their dysregulated follicle growth trajectories. Another possibility is that the follicles in participants who have been exposed to previous treatment may be growing in an altered ovarian environment. For example, late effects of radiation and chemotherapy include increased stromal fibrosis and vascular damage, which could impact follicle growth and quality.57 Exposure of human ovarian stromal tissue to chemotherapy in vitro resulted in increased apoptosis and decreased proliferation.58 In addition, following chemotherapy and/or radiation, accelerated activation of primordial follicles as well as atresia of growing follicles occurs in humans and mice.59–61 Cellular debris resulting from excessive follicle atresia could generate inflammatory signaling cascades through damage-associated molecular patterns or danger signals produced by dying cells.62,63
The surrounding follicle landscape may also change following exposure to treatment. Surprisingly, we observed that growing follicles in tissues from participants who had been exposed to prior treatment were found in proximity to significantly more primordial follicles compared with participants who had not received prior treatment. This may indicate that follicles are growing in regions of treated tissue where they would not normally in naïve tissues. These results are similar to what were observed in adult human ovaries following chemotherapeutic regimens of adriamycin, bleomycin, vinblastine, and dacarbazine, in which the ovaries contained higher densities of nongrowing follicles compared with participants who did not receive this treatment.64 However, the mechanism behind this phenomenon has not been determined.
In addition to the observed trends in follicle number and growth trajectories, the information elucidated in this study regarding neighboring follicle and interfollicular distances could be used to optimize IVFG systems and inform the development of ovarian bioprosthetics. Our analysis establishes important benchmarks for the in vivo transition of follicles from the primary to secondary stage. Specifically, as benchmarks for IVFG, the oocyte diameter should increase from ∼30 to 35 μm and follicle diameter should increase from ∼40 to 55 μm from the primary to secondary follicle stage to closely mimic in vivo follicle growth dynamics. These data can also be used to inform the number and positioning of follicles within ovarian bioprosthetics. Based on our results, for example, a primary follicle should be surrounded by ∼15 follicles, mainly primordial follicles, spaced as close as ∼100 μm, but within 300 μm.
Although this study has provided important insight into the organization of growing follicles within the human ovary, there are several limitations to our approach. First, our analyses were performed using two-dimensional images of histological sections, but the ovary is a 3D organ. Stereology, a method that uses geometry and statistical principles to generate 3D structures from a two-dimensional image, could be useful.65 However, this methodology can be costly and may be difficult to apply due to the rare availability of human tissue. Additionally, we examined growing follicles within ovarian tissue at a single static time point, but the ovary is a highly dynamic organ that undergoes extensive remodeling throughout the menstrual cycle. Thus, we cannot exclude the possibility that the relative positions of the follicles may change depending on the cycle stage. In addition, follicles do not exist in isolation, but instead reside in a heterogeneous stroma. Therefore, in the future, it will be critical to identify where to position follicles relative to other stromal cells and extracellular matrix components. To better understand the composition of the ovarian microenvironment, decellularization and matrisome analyses are ongoing and will be informative.22,66,67
By positioning the diverse cell types of the ovary in the correct spatial orientation, more physiologic artificial ovaries can be generated. Ultimately, these data are a step forward in being able to do so, potentially improving our ability to engineer ex vivo ovarian platforms and advancing fertility preservation options.
Acknowledgments
The authors would like to acknowledge all participants who donated ovarian tissue for this research and the personnel of the National Physicians Cooperative who facilitated these studies, specifically Brigid Martz Smith and Kristin Smith. The authors also thank Megan Romero and Keisha Barreto from the Histology Core at Northwestern University for their technical expertise. Furthermore, the authors would like to thank the Master of Science of the Biotechnology program at the McCormick School of Engineering at Northwestern University and the Master of Science of Reproductive Science and Medicine at the Feinberg School of Medicine at Northwestern University.
Authors' Contributions
F.E.D. and M.E.P. were involved in conceptualization, project administration, funding, and resources; F.E.D. was involved in supervision; N.Q. and A.R.G. were involved in the methodology, investigation, and formal analysis; J.N.M., N.Q., A.R.G., and F.E.D. were involved in writing; and all authors gave the final approval.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Center for Reproductive Health After Disease (P50 HD076188) from the National Centers for Translational Research in Reproduction and Infertility (NCTRI).
References
- 1. DeSantis C.E., Lin C.C., Mariotto A.B., et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64, 252, 2014 [DOI] [PubMed] [Google Scholar]
- 2. Blumenfeld Z. Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol 26, 379, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Critchley H.O., Bath L.E., and Wallace W.H.. Radiation damage to the uterus—review of the effects of treatment of childhood cancer. Hum Fertil (Camb) 5, 61, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Gracia C.R., Sammel M.D., Freeman E., et al. Impact of cancer therapies on ovarian reserve. Fertil Steril 97, 134, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnston R.J., and Wallace W.H.. Normal ovarian function and assessment of ovarian reserve in the survivor of childhood cancer. Pediatr Blood Cancer 53, 296, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Meirow D., Biederman H., Anderson R.A., and Wallace W.H.. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 53, 727, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Sklar C.A., Mertens A.C., Mitby P., et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 98, 890, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Kim S.Y., Kim S.K., Lee J.R., and Woodruff T.K.. Toward precision medicine for preserving fertility in cancer patients: existing and emerging fertility preservation options for women. J Gynecol Oncol 27, e22, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amorim C.A., Leonel E.C.R., Afifi Y., Coomarasamy A., and Fishel S.. Cryostorage and retransplantation of ovarian tissue as an infertility treatment. Best Pract Res Clin Endocrinol Metab 33, 89, 2019 [DOI] [PubMed] [Google Scholar]
- 10. Donnez J., and Dolmans M.M.. The ovary: from conception to death. Fertil Steril 108, 594, 2017 [DOI] [PubMed] [Google Scholar]
- 11. Gellert S.E., Pors S.E., Kristensen S.G., et al. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet 35, 561, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoekman E.J., Louwe L.A., Rooijers M., et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand 99, 213, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bastings L., Beerendonk C.C., Westphal J.R., et al. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update 19, 483, 2013 [DOI] [PubMed] [Google Scholar]
- 14. Bockstaele L., Tsepelidis S., Dechene J., Englert Y., and Demeestere I.. Safety of ovarian tissue autotransplantation for cancer patients. Obstet Gynecol Int 2012, 495142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dolmans M.M., Luyckx V., Donnez J., Andersen C.Y., and Greve T.. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril 99, 1514, 2013 [DOI] [PubMed] [Google Scholar]
- 16. Rosendahl M., Andersen M.T., Ralfkiaer E., Kjeldsen L., Andersen M.K., and Andersen C.Y.. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil Steril 94, 2186, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Laronda M.M., Duncan F.E., Hornick J.E., et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet 31, 1013, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Telfer E.E., and Zelinski M.B.. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 99, 1523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang T.R., Yan J., Lu C.L., et al. Human single follicle growth in vitro from cryopreserved ovarian tissue after slow freezing or vitrification. Hum Reprod 31, 763, 2016 [DOI] [PubMed] [Google Scholar]
- 20. Xiao S., Zhang J., Romero M.M., Smith K.N., Shea L.D., and Woodruff T.K.. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep 5, 17323, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu M., Barrett S.L., West-Farrell E., et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 24, 2531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laronda M.M., Jakus A.E., Whelan K.A., Wertheim J.A., Shah R.N., and Woodruff T.K.. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 50, 20, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laronda M.M., Rutz A.L., Xiao S., et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun 8, 15261, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jakus A.E., Laronda M.M., Rashedi A.S., et al. “Tissue Papers” from organ-specific decellularized extracellular matrices. Adv Funct Mater 27, 1700992, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pors S.E., Ramlose M., Nikiforov D., et al. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod 34, 1523, 2019 [DOI] [PubMed] [Google Scholar]
- 26. Dolmans M.M., and Amorim C.A.. Construction and use of artificial ovaries. Reproduction 158, F15, 2019 [DOI] [PubMed] [Google Scholar]
- 27. Chiti M., Viswanath A., Vanacker J., et al. Hydrogel from bovine decellularized ovarian extracellular matrix supports mouse follicle survival in vitro. Front Bioeng Biotechnol 14, 273, 2016 [Google Scholar]
- 28. Hassanpour A., Talaei-Khozani T., Kargar-Abarghouei E., Razban V., and Vojdani Z.. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther 9, 252, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu W.Y., Lin S.G., Zhuo R.Y., et al. Xenogeneic decellularized scaffold: a novel platform for ovary regeneration. Tissue Eng Part C Methods 23, 61, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mendez U., Zhou H., and Shikanov A.. Synthetic PEG hydrogel for engineering the environment of ovarian follicles. Methods Mol Biol 1758, 115, 2018 [DOI] [PubMed] [Google Scholar]
- 31. Rios P.D., Kniazeva E., Lee H.C., et al. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol Bioeng 115, 2075, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vanacker J., Luyckx V., Dolmans M.M., et al. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 33, 6079, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Dath C., Dethy A., Van Langendonckt A., et al. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Hum Reprod 26, 1431, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Soares M., Sahrari K., Chiti M., et al. The best source of isolated stromal cells for the artificial ovary: medulla or cortex, cryopreserved or fresh? Hum Reprod 30, 1589, 2015 [DOI] [PubMed] [Google Scholar]
- 35. Woodruff T.K., and Shea L.D.. The role of the extracellular matrix in ovarian follicle development. Reprod Sci 14, 6, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hornick J.E., Duncan F.E., Shea L.D., and Woodruff T.K.. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction 145, 19, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Woodruff T.K., and Shea L.D.. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet 28, 3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Da Silva-Buttkus P., Marcelli G., Franks S., Stark J., and Hardy K.. Inferring biological mechanisms from spatial analysis: prediction of a local inhibitor in the ovary. Proc Natl Acad Sci U S A 106, 456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaytan F., Morales C., Leon S., Garcia-Galiano D., Roa J., and Tena-Sempere M.. Crowding and follicular fate: spatial determinants of follicular reserve and activation of follicular growth in the mammalian ovary. PLoS One 10, e0144099, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou H., Decker J.T., Lemke M.M., et al. Synergy of paracrine signaling during early-stage mouse ovarian follicle development in vitro. Cell Mol Bioeng 11, 435, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hansen K.R., Hodnett G.M., Knowlton N., and Craig L.B.. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 95, 170, 2011 [DOI] [PubMed] [Google Scholar]
- 42. Kerr J.B., Myers M., and Anderson R.A.. The dynamics of the primordial follicle reserve. Reproduction 146, R205, 2013 [DOI] [PubMed] [Google Scholar]
- 43. Takae S., Tsukada K., Maeda I., et al. Preliminary human application of optical coherence tomography for quantification and localization of primordial follicles aimed at effective ovarian tissue transplantation. J Assist Reprod Genet 35, 627, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Westergaard C.G., Byskov A.G., and Andersen C.Y.. Morphometric characteristics of the primordial to primary follicle transition in the human ovary in relation to age. Hum Reprod 22, 2225, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Smith B.M., Duncan F.E., Ataman L., et al. The National Physicians Cooperative: transforming fertility management in the cancer setting and beyond. Future Oncol 14, 3059, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Armstrong A.G., Kimler B.F., Smith B.M., Woodruff T.K., Pavone M.E., and Duncan F.E.. Ovarian tissue cryopreservation in young females through the Oncofertility Consortium's National Physicians Cooperative. Future Oncol 14, 363, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duncan F.E., Pavone M.E., Gunn A.H., et al. Pediatric and teen ovarian tissue removed for cryopreservation contains follicles irrespective of age, disease diagnosis, treatment history, and specimen processing methods. J Adolesc Young Adult Oncol 4, 174, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woodruff T.K. The oncofertility consortium—addressing fertility in young people with cancer. Nat Rev Clin Oncol 7, 466, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dittrich R., Lotz L., Keck G., et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril 97, 387, 2012 [DOI] [PubMed] [Google Scholar]
- 50. Duncan F.E., Zelinski M., Gunn A.H., et al. Ovarian tissue transport to expand access to fertility preservation: from animals to clinical practice. Reproduction 152, R201, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duncan F.E., Jasti S., Paulson A., Kelsh J.M., Fegley B., and Gerton J.L.. Age-associated dysregulation of protein metabolism in the mammalian oocyte. Aging Cell 16, 1381, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eppig J.J., Wigglesworth K., and Pendola F.L.. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A 99, 2890, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Green D.M., Sklar C.A., Boice JD, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the childhood cancer survivor study. J Clin Oncol 27, 2374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kalich-Philosoph L., Roness H., Carmely A., et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 5, 185ra62, 2013 [DOI] [PubMed] [Google Scholar]
- 55. Morgan S., Anderson R.A., Gourley C., Wallace W.H., and Spears N.. How do chemotherapeutic agents damage the ovary? Hum Reprod Update 18, 525, 2012 [DOI] [PubMed] [Google Scholar]
- 56. El-Hayek S., Yang Q., Abbassi L., FitzHarris G., and Clarke H.J.. Mammalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Curr Biol 28, 1124, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spears N., Lopes F., Stefansdottir A., et al. Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 25, 673, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lopes F., Liu J., Morgan S., et al. Single and combined effects of cisplatin and doxorubicin on the human and mouse ovary in vitro. Reproduction 159, 193, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kimler B.F., Briley S.M., Johnson B.W., Armstrong A.G., Jasti S., and Duncan F.E.. Radiation-induced ovarian follicle loss occurs without overt stromal changes. Reproduction 155, 553, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luo Q., Yin N., Zhang L., et al. Role of SDF-1/CXCR4 and cytokines in the development of ovary injury in chemotherapy drug induced premature ovarian failure mice. Life Sci 179, 103, 2017 [DOI] [PubMed] [Google Scholar]
- 61. Winship A.L., Carpenter M., Griffiths M., and Hutt K.J.. Vincristine chemotherapy induces atresia of growing ovarian follicles in mice. Toxicol Sci 169, 43, 2019 [DOI] [PubMed] [Google Scholar]
- 62. Feldman N., Rotter-Maskowitz A., and Okun E.. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev 24, 29, 2015 [DOI] [PubMed] [Google Scholar]
- 63. Lotze M.T., Deisseroth A., and Rubartelli A.. Damage associated molecular pattern molecules. Clin Immunol 124, 1, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McLaughlin M., Kelsey T.W., Wallace W.H., Anderson R.A., and Telfer E.E.. Non-growing follicle density is increased following adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy in the adult human ovary. Hum Reprod 32, 165, 2017 [DOI] [PubMed] [Google Scholar]
- 65. Brown D.L. Practical stereology applications for the pathologist. Vet Pathol 54, 358, 2017 [DOI] [PubMed] [Google Scholar]
- 66. Henning N.F., LeDuc R.D., Even K.A., and Laronda M.M.. Proteomic analyses of decellularized porcine ovaries identified new matrisome proteins and spatial differences across and within ovarian compartments. Sci Rep 9, 20001, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ouni E., Vertommen D., Chiti M.C., Dolmans M.M., and Amorim C.A.. A draft map of the human ovarian proteome for tissue engineering and clinical applications. Mol Cell Proteomics 18, S159, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]