Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the current global pandemic of COVID-19, which is associated with significant morbidity and mortality.1 As of 26 April 2020, it has infected over three million people worldwide and caused more than 200 000 deaths.2 Chronic liver diseases from HCV, HBV, alcoholism or non-alcoholic fatty liver disease (NAFLD) represent a major disease burden in the world. Around 1.5 billion people have chronic liver diseases worldwide, and it causes around two million deaths per year. While self-resolving elevations of transaminases are reported in 15%–54% of patients with COVID-19, those with more severe disease experience worse liver injury.3–5 An open international registry, SECURE-Cirrhosis, is reporting a mortality rate of 40% among the 118 patients with cirrhosis.6 Thus, patients with chronic liver disease represent a vulnerable population who are at higher risk of acquiring COVID-19 and suffering from its complications.7 8
International societies, including the American Association for the Study of Liver Diseases (AASLD),9 the European Association for the Study of the Liver (EASL),10 the International Liver Cancer Association (ILCA),11 the Gastroenterological Society of Australia, The Transplantation Society (TTS),12 the American Society of Transplantation Surgeons,13 and the Liver Transplant Society of India,14 have released guidance to aid physicians taking care of patients with chronic liver diseases and liver transplantation. Most of these recommendations are based on expert consensus, as rigorous data are not yet available. We compare these major international recommendations and discuss a consolidated approach to managing liver disease in the setting of COVID-19. We also share our views on the path towards the eventual transition back to normality.
Recommendations for inpatient care of chronic liver disease during COVID-19
Patients with liver diseases continue to require hospitalisation during the pandemic both for COVID-related and liver-related indications, and attempts should be made to minimise their risk of contracting COVID-19.15 Both AASLD and EASL recommend infection prevention and control measures that include the cohorting of inpatients with COVID-19 from other non-infected patients and maximising the use of telemedicine to reduce contact between patients and healthcare workers 9 10 (figure 1). EASL recommends considering early admission for patients with COVID-19 with advanced liver disease, especially in the presence of other risk factors. Patients with COVID-19 and elevated liver biochemistries should be evaluated for coexisting viral hepatitis and complications, including myositis, ischaemia and cytokine release syndrome.10 Additionally, liver biochemistries should be regularly monitored, especially for those treated with novel antiviral drugs like remdesivir or tocilizumab. Diagnostic imaging should only be performed in patients with strong clinical suspicion of deep venous thrombosis or biliary obstruction. Both EASL 10 and AASLD suggest that liver biopsies should be deferred in most patients, but do acknowledge that biopsies may be needed in some cases to rule out acute rejection or diagnose acute autoimmune hepatitis. In the coming weeks, as the number of COVID-19 cases decrease, we expect a second surge of patients with hepatic decompensation and other liver-related complications to require hospitalisation. Some of the steps that hospitals could take now to handle this surge include maximising hospital bed capacity, optimising personal protective equipment (PPE) availability and mobilising healthcare workers.
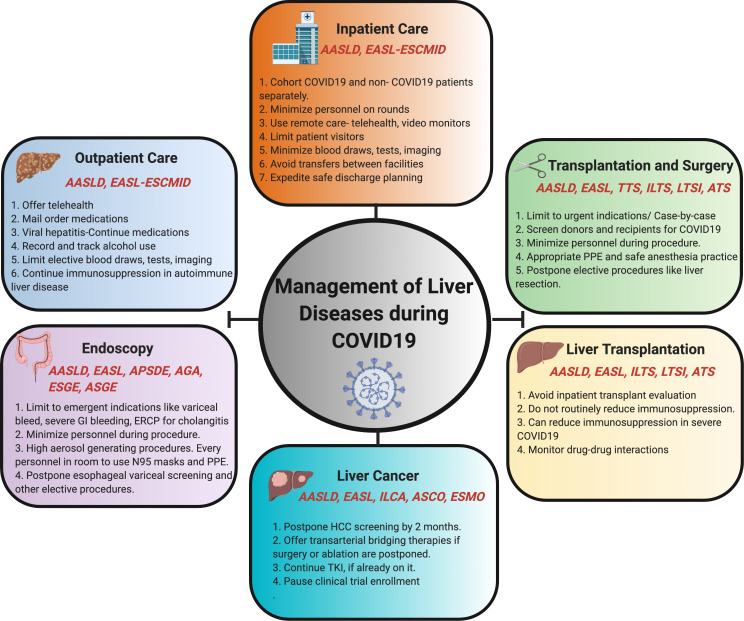
Figure 1.
Schematic showing the recommendations from major international guidelines for management of specific classes of patients with liver disease during COVID-19. AASLD, American Association for the Study of Liver Diseases; AGA, American Gastroenterology Association; APSDE, Asian Pacific Society for Digestive Endoscopy; ASCO American Society of Clinical Oncology; ASGE, American Society of Gastrointestinal Endoscopy; ASTS, American Society of Transplant Surgeons; EASL, European Association for the Study of Liver Disease; ERCP, endoscopic retrograde cholangio-pancreatography; ESCMID, European Society of Clinical Microbiology & Infectious Diseases; ESGE, European Society of Gastrointestinal Endoscopy; ESMO, European Society of Medical Oncology; HCC, hepatocellular carcinoma; ILCA, International Liver Cancer Association; ILTS, International Liver Transplantation Society; LTSI, Liver Transplant Society of India; PPE, personal protective equipment; TKI, tyrosine kinase inhibitor; TTS, The Transplantation Society.
Recommendations for outpatient care of chronic liver disease during COVID-19
The major international recommendations outline basic mitigation principles which generally apply to all patients with chronic liver disease being managed in the outpatient setting.9 10 16 They emphasise the need to limit in-person outpatient visits and recommend phone visits or telemedicine as an alternative wherever possible. Recommendations suggest that medications should preferentially be delivered using mail services, and lab tests should be performed at local laboratories instead of hospitals. AASLD recommends in-person new patient visits only for those with significant liver disease, such as jaundice, elevated transaminases of >500 U/L or recent decompensation. During such in-person visits, appropriate PPE should be used. AASLD also specifically states that patients with COVID-19 symptoms or known exposure should not be evaluated in the hepatology clinic but rather in designated COVID-19 clinics.
Recommendations for specific liver diseases
For patients with viral hepatitis B or C, both AASLD and EASL suggest continuing ongoing antiviral treatment. For patients with HCV who need to be initiated on therapy, EASL specifically recommends pursuing non-invasive evaluation of fibrosis instead of biopsy. AASLD takes a slightly different approach and suggests considering delaying the initiation of direct-acting antiviral (DAA) therapy when possible, likely to avoid the need for repeated clinic and lab visits. Patients with alcoholic liver disease may be particularly vulnerable to relapse during the pandemic, since in-person support groups such as Alcoholics Anonymous (AA) are not available. AASLD recommends offering these patients either telephone or online resources. Patients with NAFLD usually suffer from comorbid conditions such as diabetes, hypertension or obesity,10 putting them at an increased risk of progression to severe COVID-1917; hence, mitigation efforts are particularly crucial in this population. Patients with autoimmune hepatitis who are on immunosuppressants should generally continue treatment except in cases of severe COVID-19, lymphopaenia or superinfection, where reduction of immunosuppression might be necessary. To summarise, the current recommendations for patients with chronic compensated liver disease are quite similar: to stay calm, stay home and continue their current regimen. However, the larger questions about reopening care for these patients still remain: When is it a good time to initiate DAA for HCV if it had been postponed? When should we resume elective procedures like transient elastography? When do we restart clinical trial enrolment for non-alcoholic fatty liver disease (NAFLD) and other chronic liver diseases? How can we help patients get back to support services like AA? We hope there will be specific guidance to answer these questions in the near future.
Liver transplantation and COVID-19
COVID-19 adds another layer of complexity to the care of patients who are either awaiting or have already undergone liver transplantation. While making decisions regarding transplantation, one has to now consider resource prudence not only in the context of organ use but also in terms of availability of intensie care unit (ICU) beds, transplant staff and blood products (online supplementary table 1). Some societies like TTS recommend completely halting deceased donor liver transplantation programmes in regions of widespread COVID-19 prevalence, particularly in low-resource settings,12 while most other societies have advocated for limiting transplantation to patients with acute liver failure, high MELD (20 or above) or HCC close to the limits of Milan criteria.9 10 14 Although AASLD recommends stopping almost all living donor liver transplantation (LDLT), EASL as well as societies from India and China recommend continuing LDLT on a case-by-case basis.9 10 14 18 There will be long-term and profound implications of completely suspending transplant activity; hence, blanket guidelines should pave the way for more granular recommendations based on the principles of safety and priority.
gutjnl-2020-321553supp001.pdf (75.7KB, pdf)
Given that the risk of transmission from donors is not well known, most societies recommend avoiding organs from COVID-19-positive donors.10 19 Though there are no reported cases of donor-derived coronavirus infection, SARS-CoV-2 viraemia is found in approximately 15% of cases20; hence, most societies recommend screening all donors for possible exposure and/or symptoms.9 The Canadian and Japanese Society of Transplantation give specific recommendations to employ plans to risk stratify or isolate donors for a long-enough duration to minimise donor-related transmission of SARS-CoV-2.21 22 AASLD recommends against transplanting COVID-19-positive recipients.9 Hence, patients on top of the list should be screened for COVID-19 and the possibility of false negative tests should be considered before proceeding with the transplant.
Whether post-transplant patients are at increased risk of COVID-19 is not known. In a recent report from Italy, deaths were noted in patients who had a remote history of transplant and were on minimal immunosuppression, while the immediate post-transplant patients fared better.23 This is in contrast to data from an international registry, SECURE-Cirrhosis, which shows that all four deaths in their cohort occurred in patients who had undergone transplant within the past 2 years.24 These contradictory reports clearly highlight the need for more robust data. For now, all major societies have recommended against routine reduction of immunosuppression in post-transplant patients.9 10 14 18 If a patient tests positive for COVID-19, the dose of steroids should be decreased to a minimum to avoid adrenal insufficiency.9 10 18 In patients who are 6 months or more post-transplant and develop fever, lymphopaenia and/or worsening pneumonia, it is recommended to reduce or stop azathioprine and mycophenolate and to consider reducing, but not stopping, calcineurin inhibitors (CNI).9 10 18 Another important issue to watch out for are drug–drug interactions in patients infected with COVID-19 who will undergo various treatments. Drug levels of CNI and mammalian Target Of Rapamycin (mTOR) inhibitors should be closely monitored when they are administered together with drugs such as hydroxychloroquine or azithromycin.25
More than ever, the decision to transplant or not requires a multidisciplinary approach with input from local health officials. The dilemma between allocating resources for transplant versus saving PPE and ICU beds for patients with COVID-19 is a major one and is best answered locally based on available resources and prevalence of disease. Overall, while the direct impact of COVID-19 on transplant patients is not known, we expect it to be indirectly detrimental by leading to decrease in organ availability and increase in waitlist mortality.26
Endoscopy in patients with liver disease during COVID-19 pandemic
All GI and endoscopy societies suggest deferring non-urgent endoscopies.27–30 Only urgent procedures for life-threatening conditions such as acute GI bleeding like variceal haemorrhage, oesophageal food impaction and endoscopic retrograde cholangio-pancreatography (ERCP) for acute cholangitis should be undertaken. For variceal screening, EASL recommends performing risk assessments, such as applying the Baveno VI criteria, whereas AASLD goes even further to suggest primary prophylaxis with beta blockers instead of screening endoscopy in patients with clinically significant portal hypertension or high risk of decompensation. EASL suggests that ERCP for dilatation or stent replacement in postliver transplantation or primary sclerosing cholangitis should be performed after careful risk–benefit considerations. In addition, upper GI endoscopy is considered to be an aerosol-generating procedure requiring adequate levels of PPE, including N95 respirators, goggles, face shield, hairnet and long-sleeved gowns. We believe that each centre has to develop its own strategies to ensure that deferred endoscopies are not missed once normal care resumes, especially for patients at risk of variceal bleeding.
Hepatocellular carcinoma (HCC) and COVID-19
Overall, patients with cancer have worse outcomes with COVID-19.31 32 This likely applies to patients with hepatocellular carcinoma (HCC) as well since they are generally older, frailer and require multiple visits to healthcare facilities. All the societies uniformly recommend maintaining continuity of care for patients with HCC by offering in-person visits when needed, performing follow-up scans in a timely manner and avoiding treatment interruptions.9–11 33 AASLD guidelines suggest it is reasonable to delay HCC surveillance by 2–3 months, since HCC is generally felt to be a slow-growing tumour. However, given the heterogenous growth patterns in HCC, we recommend physicians weigh the decision to postpone HCC surveillance on a case-by-case basis and to err on the side of caution.34 We suggest that multidisciplinary tumour boards continue to function remotely and provide treatment recommendations. ILCA made specific recommendations for management of the various stages of HCC.11 For patients with early-stage HCC whose curative resection or ablation has been cancelled, they recommend transarterial therapies as a bridge to definitive treatment. Patients with more advanced HCC who are receiving tyrosine kinase inhibitors (TKIs) should be able to continue therapy uninterrupted. EASL recommendations suggest that immunotherapy might have to be temporarily suspended to avoid exposure to COVID-19 at the infusion centre. Enrolment in clinical trials has generally been halted during the pandemic, and these patients are recommended to be treated with TKIs, if eligible. We believe that patients with HCC are particularly prone to suffer collateral damage during COVID-19, and hence they should be followed up closely. We recommend using one of the published risk scores for HCC to prioritise patients for restarting surveillance in the post-COVID-19 era.35 36
Strategic reopening of care for patients with chronic liver diseases
We acknowledge that the COVID-19 pandemic is far from over. However, rebuilding is an essential part of disaster management. While hospitals hope to resume ‘near-normal’ operations soon, there are several factors to consider: the persistent need for social distancing, inadequate hospital capacities, possible impaired access to healthcare due to financial difficulties and healthcare avoidance due to fears of contracting coronavirus.37 Moreover, we expect a second surge of patients whose care has now been deferred, and we need to strategically plan ahead to manage this. A scoring system based on medical necessity, tailored to liver patients, would be ideal to prioritise patients who need to be seen earlier.38 Though less palatable, evening and weekend slots for clinic visits, as well as screening and surveillance imaging, may need to be initiated. Additionally, a multipronged communication system needs to be set up to ensure all patients have access to care. Particularly vulnerable patient populations include those who relapsed into alcoholism, decompensated liver disease or those with HCC. Specifically, for patients with HCC, we have to expeditiously reschedule the imaging scans that have been postponed, safely resume elective procedures like surgical resections and ablative therapies, and ramp up clinical trial enrolment. Lastly, we believe this is the right time for all hospitals and clinics to be investing in organisational preparedness and capacity planning to handle the second surge.
Conclusions
The response of the international liver community during the COVID-19 pandemic has been swift and thoughtful. Several major international societies have undertaken extensive efforts to provide recommendations on how to manage patients with liver disease during COVID-19. Our comparative review clearly highlights the fact that despite differences in healthcare resources and disease prevalence, the major concepts outlined in the various international recommendations are relatively similar but for minor differences that we highlight. Broad principles of infection control, mitigation, risk stratification and supportive management remain universal. While our knowledge of COVID-19 is still rapidly evolving, in this comprehensive overview, we provide a global perspective on the management of liver disease during COVID-19. We call on policymakers and governments to craft a comprehensive plan now, rather than later, in anticipation of the ‘second surge’ of patients with liver diseases.
Footnotes
Twitter: @stevenbollipo, @kapuriamd, @RashidLui, @renumathyd
Contributors: All authors have contributed to this article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement: There are no data in this work.
References
- 1. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med 2020. 10.1056/NEJMoa2004500. [Epub ahead of print: 30 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johns Hopkins Coronavirus Resource Center Johns Hopkins coronavirus resource center. Available: https://coronavirus.jhu.edu/map.html [Accessed 21 Apr 2020].
- 3. Wong SH, RNS L, Sung JJY. Covid‐19 and the digestive system. J Gastroenterol Hepatol 2020;26:37. [DOI] [PubMed] [Google Scholar]
- 4. Fan Z, Chen L, Li J, et al. Clinical features of COVID-19-Related liver damage. Clin Gastroenterol Hepatol 2020. 10.1016/j.cgh.2020.04.002. [Epub ahead of print: 10 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng SC, Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut 2020;69:973–4. 10.1136/gutjnl-2020-321195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Updates and data | secure cirrhosis registry. Available: https://covidcirrhosis.web.unc.edu/updates-and-data/ [Accessed 22 Apr 2020].
- 7. Mao R, Liang J, Shen J, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol 2020;5:425–7. 10.1016/S2468-1253(20)30076-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guan W-J, Z-Y N, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology 2020. 10.1002/hep.31281. [Epub ahead of print: 16 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep 2020;2:100113. 10.1016/j.jhepr.2020.100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tim Meyer Stephen Chan Management of HCC during COVID-19 pandemic: ILCA guidance, 2020. Available: https://ilca-online.org/covid19andlivercancer/ [Accessed 16 Apr 2020].
- 12. Colarusso R. An update and guidance on 2019 novel coronavirus (2019-nCov) for transplant ID clinicians. Available: https://tts.org/tid-about/tid-presidents-message/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians [Accessed 22 Apr 2020].
- 13. ASTS COVID-19 strike force initial guidance. Available: https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/asts-covid-19-strike-force-initial-guidance#.XqDYi1NKhBw [Accessed 22 Apr 2020].
- 14. Saigal S, Gupta S, Sudhindran S, et al. Liver transplantation and COVID-19 (coronavirus) infection: guidelines of the liver transplant Society of India (LTSI). Hepatol Int 2020. 10.1007/s12072-020-10041-1. [Epub ahead of print: 08 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ong J, Young BE, Ong S. COVID-19 in gastroenterology: a clinical perspective. Gut 2020;69:1144–5. 10.1136/gutjnl-2020-321051 [DOI] [PubMed] [Google Scholar]
- 16. Danese S, Ran ZH, Repici A, et al. Gastroenterology department operational reorganisation at the time of covid-19 outbreak: an Italian and Chinese experience. Gut 2020;69:981–3. 10.1136/gutjnl-2020-321143 [DOI] [PubMed] [Google Scholar]
- 17. Ji D, Qin E, Xu J, et al. Implication of non-alcoholic fatty liver diseases (NAFLD) in patients with COVID-19: a preliminary analysis. J Hepatol (Published Online First: 8 April 2020). [Google Scholar]
- 18. Liu H, He X, Wang Y, et al. Management of COVID-19 in patients after liver transplantation: Beijing Working Party for liver transplantation. Hepatol Int 2020. 10.1007/s12072-020-10043-z. [Epub ahead of print: 10 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. American Society of Transplantation 2019-nCoV (coronavirus): FAQs for organ donation and transplantation 2020.
- 20. Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev 2020. 10.1016/j.tmrv.2020.02.003. [Epub ahead of print: 21 Feb 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar D, Manuel O, Natori Y, et al. COVID-19: a global transplant perspective on successfully navigating a pandemic. Am J Transplant 2020. 10.1111/ajt.15876. [Epub ahead of print: 23 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the diamond Princess cruise SHIP, Yokohama, Japan, 2020. Euro Surveill 2020;25. 10.2807/1560-7917.ES.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhoori S, Rossi RE, Citterio D, et al. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol 2020. 10.1016/S2468-1253(20)30116-3. [Epub ahead of print: 09 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Webb GJ, Moon AM, Barnes E, et al. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol 2020. 10.1016/S2468-1253(20)30125-4. [Epub ahead of print: 24 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. COVID-19 drug interactions, 2020. Available: http://www.covid19-druginteractions.org
- 26. Boyarsky BJ, Po-Yu Chiang T, Werbel WA, et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant 2020. 10.1111/ajt.15915. [Epub ahead of print: 13 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lui RN, Wong SH, Sánchez-Luna SA, et al. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol 2020;35:749–59. 10.1111/jgh.15053 [DOI] [PubMed] [Google Scholar]
- 28. Sultan S, Lim JK, Altayar O, et al. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID-19 pandemic. Gastroenterology 2020. 10.1053/j.gastro.2020.03.072. [Epub ahead of print: 31 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gralnek IM, Hassan C, Beilenhoff U, et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy 2020. 10.1055/a-1155-6229. [Epub ahead of print: 17 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiu PWY, Ng SC, Inoue H, et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for digestive endoscopy (APSDE-COVID statements). Gut 2020;69:991–6. 10.1136/gutjnl-2020-321185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020. 10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. ESMO management and treatment adapted recommendations in the Covid-19 era: hepatocellular carcinoma (HCC). Available: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/gastrointestinal-cancers-hepatocellular-carcinoma-hcc-in-the-covid-19-era [Accessed 16 Apr 2020].
- 34. Rich NE, John BV, Parikh ND, et al. Hepatocellular carcinoma demonstrates heterogeneous growth patterns in a multi‐center cohort of patients with cirrhosis. Hepatology 2020. 10.1002/hep.31159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson PJ, Pirrie SJ, Cox TF, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev 2014;23:144–53. 10.1158/1055-9965.EPI-13-0870 [DOI] [PubMed] [Google Scholar]
- 36. Hcc risk calculator. Available: http://www.hccrisk.com/ [Accessed 26 Apr 2020].
- 37. Ferguson N, Laydon D, Nedjati Gilani G, et al. Report 9: impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand 2020. [DOI] [PMC free article] [PubMed]
- 38. Prachand VN, Milner R, Angelos P, et al. Medically necessary, Time-Sensitive procedures: scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic. J Am Coll Surg 2020. 10.1016/j.jamcollsurg.2020.04.011. [Epub ahead of print: 09 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-321553supp001.pdf (75.7KB, pdf)