Abstract
Objective
To determine the prognostic value of atrial fibrillation (AF) in patients with heart failure (HF) and preserved, mid-range or reduced ejection fraction (EF).
Methods
Patients hospitalised for acute HF were enrolled in the Korean Acute Heart Failure registry, a prospective, observational, multicentre cohort study, between March 2011 and February 2014. HF types were defined as reduced EF (HFrEF, LVEF <40%), mid-range EF (HFmrEF, LVEF 40%–49%) or preserved EF (HFpEF, LVEF ≥50%).
Results
Of 5414 patients enrolled, HFrEF, HFmrEF and HFpEF were seen in 3182 (58.8%), 875 (16.2%) and 1357 (25.1%) patients, respectively. The prevalence of AF significantly increased with increasing EF (HFrEF 28.9%, HFmrEF 39.8%, HFpEF 45.2%; p for trend <0.001). During follow-up (median, 4.03 years; IQR, 1.39–5.58 years), 2806 (51.8%) patients died. The adjusted HR of AF for all-cause death was 1.06 (0.93–1.21) in the HFrEF, 1.10 (0.87–1.39) in the HFmrEF and 1.22 (1.02–1.46) in the HFpEF groups. The HR for the composite of all-cause death or readmission was 0.97 (0.87–1.07), 1.14 (0.93–1.38) and 1.03 (0.88–1.19) in the HFrEF, HFmrEF and HFpEF groups, respectively, and the HR for stroke was 1.53 (1.03–2.29), 1.04 (0.57–1.91) and 1.90 (1.13–3.20), respectively. Similar results were observed after propensity score matching analysis.
Conclusions
AF was more common with increasing EF. AF was seen to be associated with increased mortality only in patients with HFpEF and was associated with an increased risk of stroke in patients with HFrEF or HFpEF.
Trial registration number
Keywords: atrial fibrillation, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, epidemiology, stroke
Introduction
Heart failure (HF) and atrial fibrillation (AF) are leading causes of mortality and ischaemic stroke.1–5 The prevalence of AF in patients with HF and the mortality rate in these patients vary according to ethnicity.6 Their prevalence is also expected to increase due to a growing burden of risk factors, such as an ageing population, hypertension, obesity, diabetes mellitus and ischaemic heart disease.6
Because AF and HF share common risk factors, they frequently coexist, and patients with both AF and HF have a worse prognosis than those with either of these conditions alone.7–9 However, the prognostic implications of AF in patients with HF remain controversial. The majority of current data suggest that AF is associated with increased mortality in patients with HF and preserved ejection fraction and in those with reduced ejection fraction.10–17 By contrast, the HF long-term registry of the European Society of Cardiology showed that AF was not associated with poor outcomes in patients with HFrEF.18
There are similarities and differences in HF across different ethnicities.19 The Korean Acute Heart Failure (KorAHF) registry is the largest prospective registry in Korea and includes all patients hospitalised for HF. Here, this database has been used to investigate the clinical characteristics and prognostic impact of AF in Korean patients according to the HF subtypes defined by ejection fraction (EF).
Methods
Data source
The KorAHF registry, supported by the Korea National Institute of Health, is a prospective, observational, multicentre cohort registry that enrolled 5625 patients hospitalised with acute HF at 10 tertiary university hospitals across the country from March 2011 to February 2014.19 20 Patients with signs or symptoms of HF and either lung congestion, objective findings of left ventricular systolic dysfunction or structural heart disease were eligible for inclusion in the study and were scheduled for follow-up until the end of 2018. The study design, data validation and an interim analysis were reported previously.19 20 All patients were followed up for at least 3 years, and data, including cause of death or readmission and various clinical measurements, were collected until December 2018. Mortality data for patients lost to follow-up were obtained from national death records.
Study variables
Left ventricular EF (LVEF) was measured using the Simpson’s biplane method at the acute HF hospitalisation. If the Simpson’s method was not possible, then LVEF was assessed using M-mode or visual estimation. Patients with HF were categorised as reduced EF (HFrEF) (EF <40%), mid-range EF (HFmrEF) (EF 40%–49%) or preserved EF (HFpEF) (EF ≥50%). The presence of AF was confirmed by ECG during the index admission. The primary outcomes were all-cause mortality, the composite of all-cause mortality and readmission for HF, cardiovascular (CV) mortality, and stroke during the follow-up period.
Most hospitals participating in this registry routinely collected data on either brain natriuretic peptide (BNP) or N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels. A composite variable was created by combining the two individual variables as follows: (1) BNP >900 or NT-proBNP >5000 pg/mL and (2) other.
Statistical analysis
Baseline characteristics according to AF and HF type at the time of index admission were captured. Continuous variables were expressed as mean±SD and compared using t-tests; categorical variables were expressed as frequency (percentage) and compared using χ2 tests. The prevalence of AF in patients with HFrEF, HFmrEF and HFpEF, according to age and sex, was calculated, and p for trend was obtained using the Cochran-Armitage method.
The event rates of outcomes (all-cause mortality, the composite of all-cause mortality or readmission, CV mortality, and stroke) were reported as the number of patients per 1000 person-years. Kaplan-Meier curves were created for time to outcomes according to the presence of AF in the EF group; survival distributions were compared using the log-rank test when proportional assumption was met, otherwise using the Breslow’s (the generalised Wilcoxon) test.21 The proportional hazards assumption was tested using the scaled Schoenfeld residuals.22
The association between AF and every outcome in each EF group was determined by the Cox proportional hazard regression model with adjustment for potential confounders, including sex, age, smoking status, alcohol consumption, medical history (New York Heart Association functional class, hypertension, diabetes mellitus, ischaemic heart disease, renal failure, cancer, prior stroke), heart rate, systolic blood pressure, diastolic blood pressure, body mass index, laboratory examination (white cell count, glycated haemoglobin, potassium, sodium, haemoglobin, blood urea nitrogen, creatinine, BNP or NT-proBNP), and medications before or at admission or at discharge (use of ACE inhibitor or angiotensin receptor blockers, beta-blockers, aldosterone antagonist, nitrate, diuretic, anticoagulant, antiplatelet and statin). Considering the high mortality rate of patients with acute HF, we also performed the competing risk regression analysis using the Fine and Gray model.23
To reduce the possibility of biased effect estimates in this study, propensity score matching analyses were performed. A propensity score according to AF status was estimated using variables known to be related to both the group assignments and the outcome variables: demographics, medical history, examinations, laboratory examination and medications.
All statistical tests were two-tailed and p values <0.05 were considered statistically significant. All statistical analyses were performed using SAS V.9.4 software and R V.3.5.3 (R Foundation, Vienna, Austria). R packages (http://cran.r-project.org) of ‘survival’, ‘MatchIt’ and ‘cmprsk’ were used to conduct the survival analysis, construct the matched cohort and conduct competing risk regression analysis, respectively.
Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
Baseline characteristics
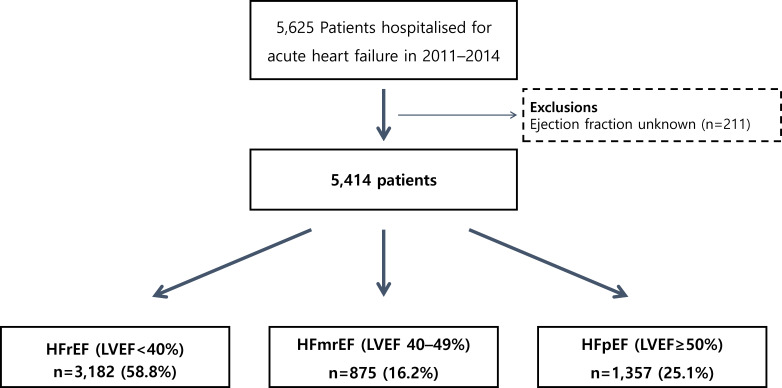
Of 5625 patients enrolled in the KorAHF registry, 211 patients with missing EF information were excluded. Of the remaining 5414 patients, 1883 (34.8%) exhibited AF, as confirmed by ECG at admission. According to the EF, 3182 (58.8%), 875 (16.2%) and 1357 (25.1%) patients were classified as having HFrEF, HFmrEF and HFpEF, respectively (figure 1).
Figure 1.
Study population flow chart. HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction.
The baseline characteristics of patients with and without AF differed according to the HF type. In general, patients with AF were older and less likely to be diabetic or current smokers than those without AF. Regarding HF type, the age and proportion of the female patients increased with EF (table 1).
Table 1.
Baseline characteristics of the study population according to AF status in patients with HFrEF, HFmrEF and HFpEF (unmatched cohort)
| HFrEF (EF <40%) | HFmrEF (EF 40%–49%) | HFpEF (EF ≥50%) | |||||||
| Without AF (n=2261; 71.1%) | With AF (n=921; 28.9%) |
P value | Without AF (n=527; 60.2%) | With AF (n=348; 39.8%) |
P value | Without AF (n=743; 54.8%) | With AF (n=614; 45.2%) |
P value | |
| Characteristics | |||||||||
| Age, years | 65.6±15.5 | 67.9±12.9 | <0.001 | 69.5±14.2 | 73.0±10.9 | <0.001 | 71.2±14.6 | 73.2±11.8 | 0.004 |
| Age group (years) | 0.006 | 0.002 | 0.003 | ||||||
| <60 | 690 (30.5) | 227 (24.6) | 109 (20.7) | 38 (10.9) | 144 (19.4) | 74 (12.1) | |||
| 60–69 | 484 (21.4) | 208 (22.6) | 98 (18.6) | 69 (19.8) | 109 (14.7) | 107 (17.4) | |||
| 70–79 | 686 (30.3) | 320 (34.7) | 193 (36.6) | 144 (41.4) | 264 (35.5) | 241 (39.3) | |||
| ≥80 | 401 (17.7) | 166 (18.0) | 127 (24.1) | 97 (27.9) | 226 (30.4) | 192 (31.3) | |||
| Sex | 0.048 | 0.030 | 0.359 | ||||||
| Male | 1361 (60.2) | 589 (64.0) | 265 (50.3) | 149 (42.8) | 294 (39.6) | 228 (37.1) | |||
| Female | 900 (39.8) | 332 (36.0) | 262 (49.7) | 199 (57.2) | 449 (60.4) | 386 (62.9) | |||
| Smoking status | 0.021 | 0.003 | <0.001 | ||||||
| Current | 514 (22.7) | 172 (18.7) | 83 (15.7) | 31 (8.9) | 114 (15.3) | 50 (8.1) | |||
| Ex | 504 (22.3) | 233 (25.3) | 117 (22.2) | 66 (19.0) | 112 (15.1) | 112 (18.2) | |||
| Never | 1243 (55.0) | 516 (56.0) | 327 (62.0) | 251 (72.1) | 517 (69.6) | 452 (73.6) | |||
| Alcohol consumption | <0.001 | 0.114 | 0.655 | ||||||
| Heavy | 147 (6.5) | 96 (10.4) | 26 (4.9) | 24 (6.9) | 36 (4.8) | 36 (5.9) | |||
| Social | 790 (34.9) | 343 (37.2) | 159 (30.2) | 85 (24.4) | 194 (26.1) | 153 (24.9) | |||
| Never | 1324 (58.6) | 482 (52.3) | 342 (64.9) | 239 (68.7) | 513 (69.0) | 425 (69.2) | |||
| Medical history | |||||||||
| Location | <0.001 | <0.001 | <0.001 | ||||||
| De novo HF | 1222 (54.0) | 409 (44.4) | 328 (62.2) | 164 (47.1) | 474 (63.8) | 270 (44.0) | |||
| Acute decompensated HF | 1039 (46.0) | 512 (55.6) | 199 (37.8) | 184 (52.9) | 269 (36.2) | 344 (56.0) | |||
| NYHA functional class | 0.005 | 0.021 | 0.515 | ||||||
| II | 310 (13.7) | 113 (12.3) | 87 (16.5) | 55 (15.8) | 134 (18.0) | 115 (18.7) | |||
| III | 795 (35.2) | 380 (41.3) | 165 (31.3) | 140 (40.2) | 269 (36.2) | 237 (38.6) | |||
| IV | 1156 (51.1) | 428 (46.5) | 275 (52.2) | 153 (44.0) | 340 (45.8) | 262 (42.7) | |||
| Hypertension | 1340 (59.3) | 531 (57.7) | 0.402 | 362 (68.7) | 226 (64.9) | 0.248 | 501 (67.4) | 407 (66.3) | 0.656 |
| Diabetes mellitus | 1021 (45.2) | 327 (35.5) | <0.001 | 238 (45.2) | 103 (29.6) | <0.001 | 269 (36.2) | 190 (30.9) | 0.042 |
| Ischaemic heart disease | 1164 (51.5) | 286 (31.1) | <0.001 | 340 (64.5) | 103 (29.6) | <0.001 | 291 (39.2) | 125 (20.4) | <0.001 |
| Renal failure | 654 (28.9) | 244 (26.5) | 0.167 | 182 (34.5) | 69 (19.8) | <0.001 | 201 (27.1) | 110 (17.9) | <0.001 |
| Cancer | 190 (8.4) | 83 (9.0) | 0.578 | 44 (8.3) | 32 (9.2) | 0.664 | 57 (7.7) | 40 (6.5) | 0.410 |
| Prior stroke | 290 (12.8) | 165 (17.9) | <0.001 | 70 (13.3) | 71 (20.5) | 0.005 | 99 (13.3) | 117 (19.1) | 0.004 |
| Examinations | |||||||||
| Heart rate, beats/min | 94.2±22.9 | 99.2±30.0 | <0.001 | 89.0±23.3 | 97.4±30.0 | <0.001 | 82.3±22.1 | 91.0±28.7 | <0.001 |
| Systolic blood pressure, mm Hg | 129.3±30.0 | 125.1±25.9 | <0.001 | 138.2±34.4 | 135.2±28.0 | 0.161 | 137.6±32.3 | 133.4±27.9 | 0.010 |
| Diastolic blood pressure, mm Hg | 78.9±18.7 | 80.2±18.3 | 0.066 | 78.6±20.1 | 80.7±18.6 | 0.127 | 75.7±17.9 | 78.8±18.5 | 0.002 |
| Body mass index, kg/m2 | 23.1±3.9 | 23.4±3.8 | 0.046 | 23.4±3.6 | 23.4±3.8 | 0.823 | 23.9±4 | 23.5±4.1 | 0.059 |
| Laboratory examinations | |||||||||
| White cell count, 109/L | 9.1±4.5 | 8.1±3.5 | <0.001 | 9.6±4.6 | 7.9±3.5 | <0.001 | 8.7±3.7 | 7.5±3.0 | <0.001 |
| HbA1c, % | 2.9±3.5 | 2.3±3.3 | <0.001 | 2.6±3.4 | 1.9±3.0 | 0.003 | 2.3±3.2 | 1.9±3.0 | 0.011 |
| Potassium, mmol/L | 4.4±0.7 | 4.4±0.7 | 0.197 | 4.4±0.8 | 4.3±0.6 | 0.016 | 4.3±0.7 | 4.3±0.6 | 0.255 |
| Sodium ≥135 mmol/L | 1786 (79.0) | 705 (76.6) | 0.137 | 426 (80.8) | 290 (83.3) | 0.348 | 593 (79.9) | 478 (77.9) | 0.352 |
| Haemoglobin ≥120 g/L | 1350 (59.8) | 671 (72.9) | <0.001 | 228 (43.3) | 203 (58.3) | <0.001 | 354 (47.8) | 328 (53.4) | 0.038 |
| Blood urea nitrogen ≥26 mg/dL | 827 (36.6) | 368 (40.0) | 0.071 | 190 (36.1) | 117 (33.6) | 0.461 | 260 (35.0) | 176 (28.7) | 0.012 |
| Creatinine ≥2 mg/dL | 351 (15.5) | 127 (13.8) | 0.217 | 116 (22.0) | 33 (9.5) | <0.001 | 111 (15.0) | 47 (7.7) | <0.001 |
| BNP >900 pg/mL or NT-proBNP >5000 pg/mL | 1212 (59.1) | 463 (55.3) | 0.059 | 257 (55.2) | 129 (41.2) | <0.001 | 233 (35.2) | 155 (28.4) | 0.012 |
| Medications and device therapies | |||||||||
| ACEI or ARB | 1863 (82.4) | 724 (78.6) | 0.013 | 382 (72.5) | 255 (73.3) | 0.797 | 472 (63.5) | 366 (59.6) | 0.139 |
| Beta-blockers | 1500 (66.3) | 634 (68.8) | 0.174 | 375 (71.2) | 219 (62.9) | 0.011 | 391 (52.6) | 344 (56.0) | 0.211 |
| Aldosterone antagonist | 1445 (63.9) | 582 (63.2) | 0.703 | 242 (45.9) | 202 (58.0) | <0.001 | 332 (44.7) | 368 (59.9) | <0.001 |
| Nitrate | 1321 (58.4) | 481 (52.2) | 0.001 | 351 (66.6) | 188 (54.0) | <0.001 | 403 (54.2) | 290 (47.2) | 0.010 |
| Diuretic | 2145 (94.9) | 887 (96.3) | 0.082 | 458 (86.9) | 326 (93.7) | 0.001 | 639 (86.0) | 596 (97.1) | <0.001 |
| Anticoagulant | 1189 (52.6) | 744 (80.8) | <0.001 | 292 (55.4) | 267 (76.7) | <0.001 | 342 (46.0) | 442 (72.0) | <0.001 |
| Antiplatelet | 1603 (70.9) | 517 (56.1) | <0.001 | 409 (77.6) | 198 (56.9) | <0.001 | 436 (58.7) | 343 (55.9) | 0.296 |
| Statin | 1206 (53.3) | 344 (37.4) | <0.001 | 312 (59.2) | 126 (36.2) | <0.001 | 327 (44.0) | 199 (32.4) | <0.001 |
Continuous variables are reported as mean±SD; categorical variables are reported as n (%).
ACEI, ACE inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blockers; BNP, brain natriuretic peptide; EF, ejection fraction; HbA1c, glycated haemoglobin; HF, heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association.
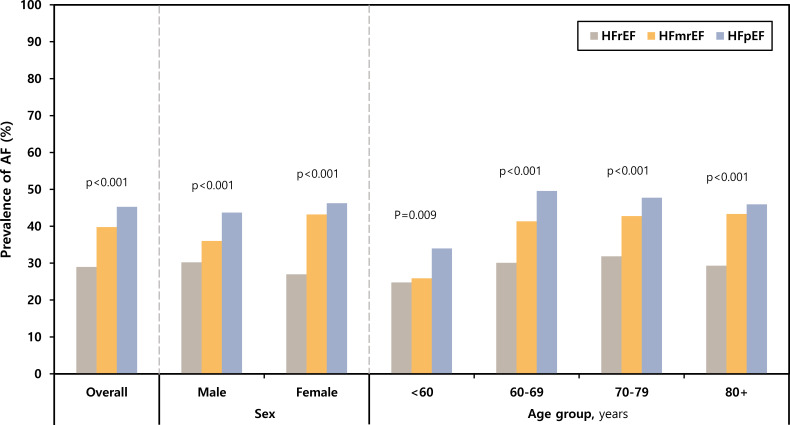
The prevalence of AF in the HFrEF, HFmrEF and HFpEF groups was 28.9%, 39.8% and 45.2%, respectively. The prevalence of AF according to age and sex significantly increased with increasing EF (figure 2).
Figure 2.
Prevalence of AF in patients with HFrEF, HFmrEF and HFpEF according to age and sex; p value for trend. AF, atrial fibrillation; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Clinical outcomes
During the follow-up period (median, 4.03 years; IQR, 1.39–5.58 years), 2806 (51.8%) patients died; the all-cause mortality did not differ between patients with AF (969 patients, 51.5%) and those without AF (1837 patients, 52.0%; p=0.692).
When stratifying the patients according to HF type, patients with AF had lower all-cause mortality in the HFrEF group (129.46 vs 145.03 events/1000 patient-years in patients with AF), but all-cause mortality was higher among patients with AF in the HFpEF group (150.42 vs 139.68 events/1000 patient-years; table 2). Similar findings were observed for CV mortality and the composite of all-cause mortality and readmission. The event rate for stroke was higher in patients with AF than in those without AF across all HF types (HFrEF: 16.60 vs 11.63 events/1000 patient-years, respectively; HFmrEF: 28.06 vs 22.00 events/1000 patient-years, respectively; HFpEF: 25.57 vs 12.89 events/1000 patient-years, respectively).
Table 2.
Event rates per 1000 person-years according to AF status and ejection fraction group
| Outcomes | Without AF | With AF | ||
| Cases/person-years | IR (95% CI) | Cases/person-years | IR (95% CI) | |
| All-cause mortality | ||||
| HFrEF | 1167/8046.49 | 145.03 (136.83 to 153.47) | 442/3414.18 | 129.46 (117.67 to 141.80) |
| HFmrEF | 282/1908.76 | 147.74 (131.00 to 165.47) | 189/1279.76 | 147.68 (127.38 to 169.47) |
| HFpEF | 388/2777.88 | 139.68 (126.12 to 153.91) | 338/2247.09 | 150.42 (134.81 to 166.87) |
| Composite of all-cause mortality and readmission | ||||
| HFrEF | 1819/3705.35 | 490.91 (468.61 to 513.73) | 713/1676.43 | 425.31 (394.66 to 457.09) |
| HFmrEF | 414/935.75 | 442.43 (400.83 to 486.05) | 289/585.46 | 493.63 (438.35 to 552.14) |
| HFpEF | 596/1237.30 | 481.69 (443.79 to 521.12) | 492/1121.42 | 438.73 (400.81 to 478.33) |
| CVD mortality | ||||
| HFrEF | 389/8046.49 | 48.34 (43.66 to 53.26) | 141/3414.18 | 41.30 (34.76 to 48.39) |
| HFmrEF | 70/1908.76 | 36.67 (28.59 to 45.75) | 45/1279.76 | 35.16 (25.65 to 46.16) |
| HFpEF | 81/2777.88 | 29.16 (23.16 to 35.84) | 80/2247.09 | 35.60 (28.23 to 43.82) |
| Stroke | ||||
| HFrEF | 91/7823.36 | 11.63 (9.37 to 14.14) | 54/3253.85 | 16.60 (12.47 to 21.31) |
| HFmrEF | 40/1818.28 | 22.00 (15.72 to 29.32) | 34/1211.74 | 28.06 (19.43 to 38.25) |
| HFpEF | 35/2715.16 | 12.89 (8.98 to 17.50) | 54/2111.44 | 25.57 (19.21 to 32.83) |
AF, atrial fibrillation; CVD, cardiovascular disease; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IR, incidence rate.
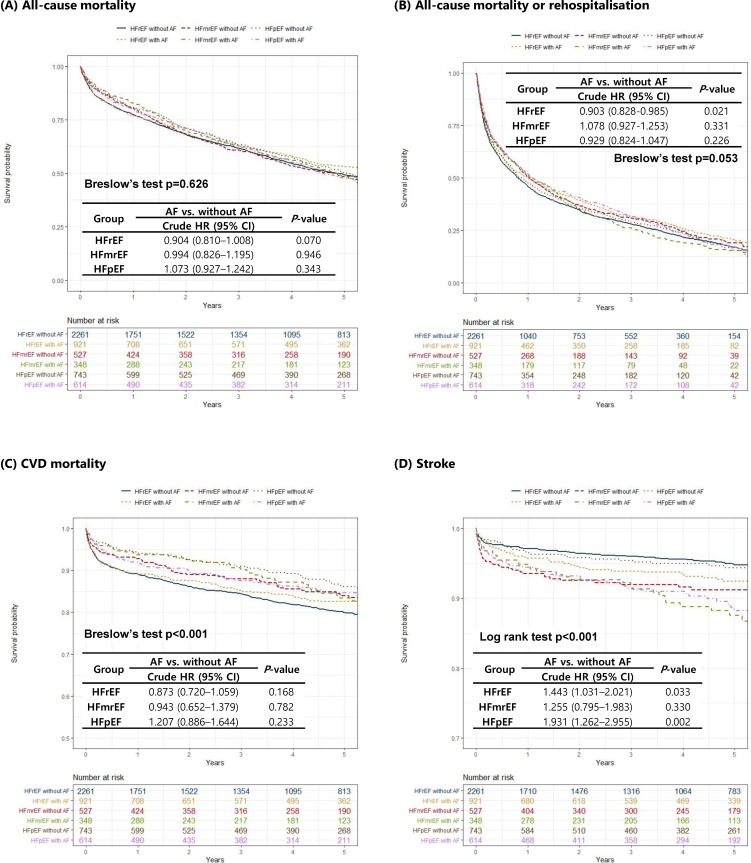
In the Kaplan-Meier survival analysis, all-cause mortality and the composite of all-cause mortality or readmission did not differ between the groups. By contrast, there was a significant difference for CV mortality and stroke events. Patients with HFrEF without AF had the highest CV mortality risk, while patients with HFpEF without AF had the lowest. Regarding stroke, patients with HFrEF without AF had the lowest stroke event risk and those with HFmrEF with AF had the highest (figure 3).
Figure 3.
Kaplan-Meier curves for unmatched cohort. Data are stratified by the three ejection fraction groups and the presence or absence of AF for (A) all-cause mortality, (B) all-cause mortality or rehospitalisation, (C) CVD mortality and (D) stroke. AF, atrial fibrillation; CVD, cardiovascular disease; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
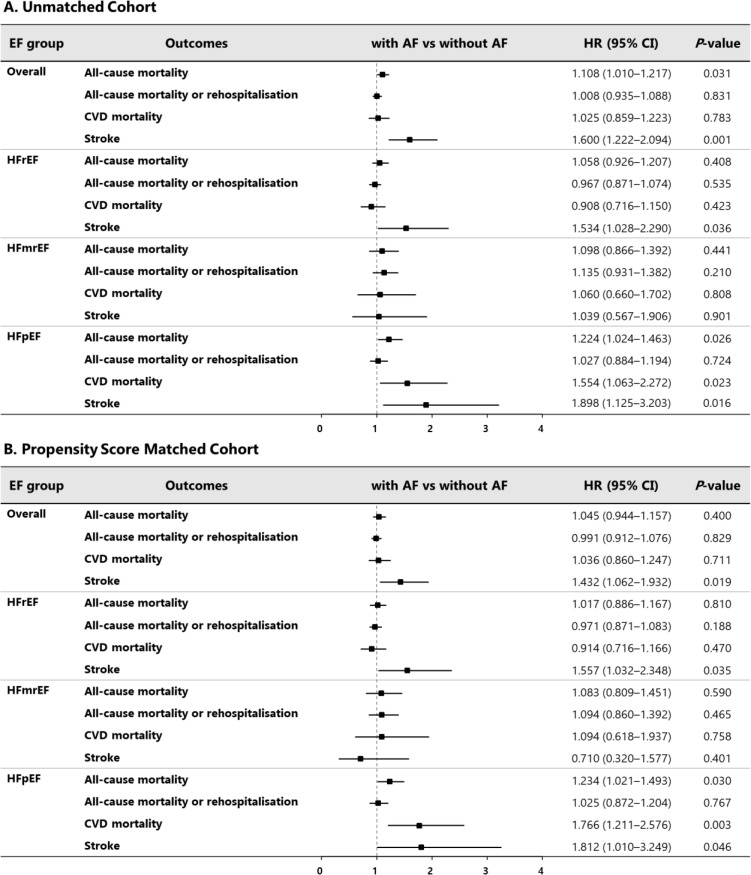
After multivariable adjustment for covariates, AF was significantly associated with increased all-cause mortality (HR, 1.11; 95% CI 1.01 to 1.22), while the statistical significance disappeared with propensity score matching (HR, 1.05; 95% CI 0.94 to 1.16; figure 4). By contrast, AF was associated with a 1.6-fold increased risk for stroke in all patients. Analysis of the data according to HF type showed that AF was associated with increased all-cause and CV mortality in patients with HFpEF (HR, 1.22, 95% CI 1.02 to 1.46 for all-cause mortality; HR, 1.55, 95% CI 1.06 to 2.27 for CV mortality) but not in those with HFrEF and HFmrEF. Regarding stroke, AF was associated with an increased risk for stroke in patients with HFrEF (HR, 1.53; 95% CI 1.03 to 2.29) and HFpEF (HR, 1.90; 95% CI 1.13 to 3.20; online supplementary files 1-1 to 1-4). Regarding stroke and CV mortality, these are similar to the results obtained from the competing risk analysis after considering all-cause death from causes other than outcomes of interest as a competing risk (table 3).
Figure 4.
Multivariable HR for adverse outcomes associated with AF, according to EF groups: A. unmatched cohort and B. propensity score matched cohort. AF, atrial fibrillation; CVD, cardiovascular disease; EF, ejection fraction; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Table 3.
Competing risk for CVD mortality and stroke according to AF in patients with HFrEF, HFmrEF and HFpEF
| HFpEF | HFmrEF | HFrEF | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Stroke | ||||||
| Model 1 | 1.900 (1.240 to 2.910) | 0.003 | 1.280 (0.812 to 2.020) | 0.290 | 1.460 (1.040 to 2.050) | 0.027 |
| Model 2 | 1.766 (1.056 to 2.950) | 0.030 | 1.120 (0.659 to 1.904) | 0.680 | 1.544 (1.040 to 2.290) | 0.031 |
| CVD mortality | ||||||
| Model 1 | 1.210 (0.892 to 1.650) | 0.220 | 0.956 (0.658 to 1.390) | 0.810 | 0.882 (0.727 to 1.070) | 0.200 |
| Model 2 | 1.507 (1.005 to 2.260) | 0.047 | 1.049 (0.634 to 1.736) | 0.850 | 0.880 (0.685 to 1.130) | 0.320 |
Model 1: unadjusted.
Model 2: adjusted for sex, age, smoking status, alcohol consumption, medical history (New York Heart Association functional class, hypertension, diabetes mellitus, ischaemic heart disease, renal failure, cancer, prior stroke), heart rate, systolic blood pressure, diastolic blood pressure, body mass index, laboratory examination (white cell count, glycated haemoglobin, potassium, sodium, haemoglobin, blood urea nitrogen, creatinine, BNP or NT-proBNP), and medications before or at admission or at discharge (use of ACE inhibitor or angiotensin receptor blockers, beta-blockers, aldosterone antagonist, nitrate, diuretic, anticoagulant, antiplatelet and statin).
AF, atrial fibrillation; BNP, brain natriuretic peptide; CVD, cardiovascular disease; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NT-proBNP, N-terminal prohormone of brain natriuretic peptide.
heartjnl-2019-316219supp001.pdf (48.5KB, pdf)
heartjnl-2019-316219supp002.pdf (59.5KB, pdf)
heartjnl-2019-316219supp003.pdf (73KB, pdf)
heartjnl-2019-316219supp004.pdf (72.8KB, pdf)
Under stratification by rhythm, in patients without AF, there was no significant difference in all-cause mortality, the composite of all-cause mortality and hospitalisation for HF, and stroke between the HF subtypes. Similar findings were observed for patients with AF (online supplementary file 2). The similar clinical outcomes of the three HF phenotypes imply that the differential effect of AF according to HF phenotypes was mainly driven by AF per se, not by HF phenotypes (p for interaction 0.174 for all-cause mortality, 0.136 for the composite of mortality or readmission for HF, 0.220 for CV mortality, and 0.382 for stroke, respectively).
Subgroup analysis
The event rates for mortality, the composite of mortality or readmission, and CV mortality were higher in patients with renal failure with and without AF in each of the EF groups (online supplementary files 3-1 to 3-3). The event rate for stroke was higher in patients with prior stroke in each of the EF groups (online supplementary file 3-4). In the subgroup analysis of CV mortality, there was a significant interaction with diabetes in the HFpEF group, and patients without diabetes appeared to be more affected by AF (p for interaction=0.033). Regarding stroke, the interaction was significant with renal failure in patients with HFpEF (p for interaction=0.019); the effect of AF on stroke was higher in patients without renal failure. By contrast, regarding mortality and stroke, there was no significant interaction of drug therapy with AF.
Propensity score matching
After matching, the baseline characteristics were well balanced between patients with and without AF in each of the three EF groups (standardised mean difference <0.25; online supplementary file 4). As seen in the unmatched cohort, there was a significant difference in stroke event and CV mortality rates between patients with and without AF across the HF types, but all-cause mortality and the composite of all-cause mortality or readmission did not differ between the groups (online supplementary file 5). AF was also significantly associated with an increased risk for all-cause and CV mortality in patients with HFpEF, and stroke in patients with HFpEF and HFrEF (figure 4). Regarding the AF types, the all-cause mortality and stroke did not differ between AF types (paroxysmal AF vs permanent or persistent AF) in the propensity score matched cohort (online supplementary file 6).
heartjnl-2019-316219supp005.pdf (33.1KB, pdf)
heartjnl-2019-316219supp006.pdf (795.7KB, pdf)
Discussion
In this study of patients with acute HF, the prevalence of AF increased with increasing EF. Surprisingly, no differences in mortality were seen between patients with and without AF. However, when patients were stratified according to HF type, AF was associated with increased mortality in patients with HFpEF but not in those with HFrEF and HFmrEF. By contrast, AF was associated with an increased risk for stroke in all HF types. Regarding the CV mortality, there was a significant interaction of diabetes in the HFpEF group, and patients without diabetes appeared to be more affected by AF. To the best of our knowledge, this study is the first to report the differential clinical impact of AF in East Asian patients with HFpEF, HFmrEF and HFrEF.
AF and HF in Korea
The prevalence of AF in patients with HF varies between different ethnic populations.18 24 25 In this study of Korean patients with acute HF, 34.8% had AF at admission, which is similar to that reported in European patients with HF (30.3%).18 The current study also demonstrated that the prevalence of AF increased with increasing EF and age, with patients in the HFpEF group aged 60–69 having the highest prevalence (49.5%). This is in agreement with previous reports from other regions, which have shown the prevalence of AF in patients with HFrEF, HFmrEF and HFpEF to be 53%, 60% and 65%, respectively, in the SwedeHF registry24; 27%, 29% and 39%, respectively, in the European Society of Cardiology-Heart Failure (ESC-HF) long-term registry18; and 26.2%, 25.6% and 31.3% in the Canddesartan in Heart failure-Assessment of moRtality and Morbidity (CHARM) trial.25
AF and HF share common risk factors, the prevalence of which is increasing due to an ageing population and Westernisation of the lifestyle in Korea. Therefore, the prevalence of both AF and HF in Korea is expected to increase.6 Indeed, data have shown that the prevalence of AF increased from 0.36% in 2003 to 0.89% in 2013,26 and that the prevalence of HF is expected to increase from 1.53% in 2013 to 3.35% by 2040.27 These results indicate that both AF and HF will represent a significant public health burden in the near future.
Impact of AF on outcomes in HF
AF has important clinical implications and can complicate HF by aggravating the condition and/or by increasing thromboembolic complications. HF is, therefore, a component of the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥75 years (double score), diabetes mellitus, previous stroke/TIA (transient ischaemic attack) (double score), vascular disease, age 65-74 years, sex class (femalie)) score.28 The coexistence of HF and AF confers a substantially increased risk for CV morbidity and mortality.10 29 Until now, the clinical implications of AF according to HF type have not been fully evaluated. The current study shows that AF had a differential effect on mortality depending on HF type, being associated with a 20% increased risk for all-cause and CV mortality only in patients with HFpEF and not in those with HFrEF and HFmrEF. The nationwide SwedeHF reported that AF was associated with similarly increased risks of death, HF hospitalisation, and stroke or TIA in all EF groups.24 By contrast, the ESC-HF long-term registry showed that AF was associated with poor outcomes in patients with HFpEF and HFmrEF, but not in those with HFrEF.18 The current study is more similar to that of the ESC-HF long-term registry and confirms a similar effect of AF on outcomes in an East Asian population for the first time. The reason for the increased mortality among patients with AF with HFpEF compared with other EF groups is not clear. Our speculation is that both AF and HFpEF share common risk factors, and although we performed propensity score matched analyses, unmeasured confounders or not-well-documented risk factors may coexist and may confer excess mortality in patients with AF and HFpEF.
The most important complication of AF is stroke. As expected, AF was associated with a 54% and 94% increased risk for stroke in the HFrEF and HFpEF groups, respectively. One intriguing finding was the negative association between stroke risk and outcomes in the HFmrEF group, although the reason for this observation is not clear. Currently, CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus and previous stroke/TIA (double score)) and CHA2DS2-VASc scores do not differentiate between HFpEF and HFrEF. Because the impact of AF on stroke may be dependent on the HF type, further studies are required to determine whether the stroke risk scoring system should be revised to accommodate different weighting for each of the HF types.
This study has several limitations. First, because the KorAHF registry enrolled patients who were hospitalised for acute HF, our results may have overestimated the event rates for poor outcomes and may not be applicable to patients with chronic HF. Additional studies including patients with stable HF are required. Second, it is possible that there are additional potential confounders that were not accounted for in our study, although we adjusted for variables including medical history, signs and symptoms of HF, laboratory examinations, medications and device therapy.
Despite these limitations, this study had several strengths. First, to our knowledge, this study is the first to investigate the prognostic impact of AF in Korean patients with acute heart failure according to the three HF types. Second, the diagnosis of AF was determined according to an ECG interpreted by a cardiologist, indicating an accurate assessment of heart rhythm.
In conclusion, in East Asian patients with acute HF, AF was more common with increasing EF and was associated with increased mortality only in patients with HFpEF. AF was associated with an increased risk of stroke in both the HFrEF and HFpEF groups. These findings suggest the importance of AF management in patients with HF to minimise the risk of mortality and stroke.
Key messages.
What is already known on this subject?
The prognostic implications of atrial fibrillation (AF) in patients with heart failure (HF) remain controversial.
The majority of current data suggest that AF is associated with increased mortality in patients with HF with preserved ejection fraction and HF with reduced ejection fraction.
By contrast, the HF long-term registry of the European Society of Cardiology showed that AF was not associated with poor outcomes in patients with HF with reduced ejection fraction.
What might this study add?
This study is the first to report the differential clinical impact of AF in East Asian patients with HF with preserved ejection fraction (HFpEF), HF with mid-range ejection fraction (HFmrEF) and HF with reduced ejection fraction (HFrEF).
Data from the Korean Acute Heart Failure registry were evaluated, showing that the prevalence of AF increased with increasing ejection fraction in patients with acute heart failure.
AF was seen to be associated with increased mortality in patients with HFpEF, but not in those with HFrEF and HFmrEF.
AF was associated with an increased risk for stroke in patients with HFrEF and HFpEF.
How might this impact on clinical practice?
The current study supports the prognostic value of evaluating AF status, when patients were stratified according to HF type, the novel aspect being the East Asian population.
These findings suggest the importance of AF management in patients with HF to minimise the risk of mortality and stroke.
heartjnl-2019-316219supp007.pdf (875.4KB, pdf)
heartjnl-2019-316219supp008.pdf (848.8KB, pdf)
heartjnl-2019-316219supp009.pdf (840.1KB, pdf)
heartjnl-2019-316219supp010.pdf (70.1KB, pdf)
heartjnl-2019-316219supp011.pdf (1.5MB, pdf)
heartjnl-2019-316219supp012.pdf (202.4KB, pdf)
Acknowledgments
The Korean Acute Heart Failure (KorAHF) registry study was conducted in 10 tertiary medical centres: Seoul National University Hospital (Byung-Hee Oh), Seoul, Korea; Sungkyunkwan University College of Medicine (Eun-Seok Jeon), Seoul, Korea; University of Ulsan College of Medicine (Jae-Joong Kim), Seoul, Korea; Chungbuk National University College of Medicine (Myeong-Chan Cho), Cheongju, Korea; Kyungpook National University College of Medicine (Shung Chull Chae), Daegu, Korea; Catholic University of Korea (Sang Hong Baek), Seoul, Korea; Yonsei University College of Medicine (Seok-Min Kang), Seoul, Korea; Yonsei University Wonju College of Medicine (Byung-Su Yoo), Wonju, Korea; Seoul National University Bundang Hospital (Dong-Ju Choi), Seongnam, Korea; and Heart Research Center of Chonnam National University (Kye Hoon Kim), Gwangju, Korea.
Footnotes
MKS and JJP contributed equally.
Contributors: MKS and JJP participated in the design of the study, performed the statistical analysis, interpreted the findings and drafted the manuscript. NKL contributed to the discussion. WHK and DJC participated in the design of the study, were involved in revising the manuscript for important intellectual content and provided final approval of the version to be published. All authors have read and approved the final manuscript.
Funding: This work was supported by the Korea National Institute of Health intramural research grant 4800-4845-302 (2017-NI63001-00).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study protocol was approved by the ethics committee at each hospital, and the requirement for written informed consent was waived by the institutional review board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Legal restrictions prohibit us from making our data set publicly available. We were allowed to use the data from a South Korean government agency, Korea Centers for Disease Control (KCDC), on requested topics, and we are not allowed to open the data to the public yet. The KorAHF registry is strictly managed by KCDC, which provides the data to researchers at the contribution of each institution to the cohort enrolment. Those who are interested in related data may contact and request the data from KCDC in South Korea (email: jhkwh@nih.go.kr). Information from the KorAHF registry can be obtained from the following site: http://clinicaltrials.gov/ct2/show/NCT01389843.
References
- 1. Kaarisalo MM, Immonen-Räihä P, Marttila RJ, et al. Atrial fibrillation and stroke. mortality and causes of death after the first acute ischemic stroke. Stroke 1997;28:311–5. 10.1161/01.str.28.2.311 [DOI] [PubMed] [Google Scholar]
- 2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N–9. 10.1016/S0002-9149(98)00583-9 [DOI] [PubMed] [Google Scholar]
- 3. Lip GYH, Rasmussen LH, Skjøth F, et al. Stroke and mortality in patients with incident heart failure: the diet, cancer and health (DCH) cohort study. BMJ Open 2012;2:e000975. 10.1136/bmjopen-2012-000975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alberts VP, Bos MJ, Koudstaal PJ, et al. Heart failure and the risk of stroke: the Rotterdam study. Eur J Epidemiol 2010;25:807–12. 10.1007/s10654-010-9520-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. 10.1161/01.STR.22.8.983 [DOI] [PubMed] [Google Scholar]
- 6. Batul SA, Gopinathannair R. Atrial fibrillation in heart failure: a therapeutic challenge of our times. Korean Circ J 2017;47:644–62. 10.4070/kcj.2017.0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham heart study. Circulation 2003;107:2920–5. 10.1161/01.CIR.0000072767.89944.6E [DOI] [PubMed] [Google Scholar]
- 8. Hu C-Y, Wang C-Y, Li J-Y, et al. Relationship between atrial fibrillation and heart failure. Eur Rev Med Pharmacol Sci 2016;20:4593–600. [PubMed] [Google Scholar]
- 9. Thihalolipavan S, Morin DP. Atrial fibrillation and heart failure: update 2015. Prog Cardiovasc Dis 2015;58:126–35. 10.1016/j.pcad.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 10. Mamas MA, Caldwell JC, Chacko S, et al. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail 2009;11:676–83. 10.1093/eurjhf/hfp085 [DOI] [PubMed] [Google Scholar]
- 11. Linssen GCM, Rienstra M, Jaarsma T, et al. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 2011;13:1111–20. 10.1093/eurjhf/hfr066 [DOI] [PubMed] [Google Scholar]
- 12. Banerjee A, Taillandier S, Olesen JB, et al. Ejection fraction and outcomes in patients with atrial fibrillation and heart failure: the Loire Valley atrial fibrillation project. Eur J Heart Fail 2012;14:295–301. 10.1093/eurjhf/hfs005 [DOI] [PubMed] [Google Scholar]
- 13. McManus DD, Hsu G, Sung SH, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc 2013;2:e005694. 10.1161/JAHA.112.005694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zakeri R, Chamberlain AM, Roger VL, et al. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation 2013;128:1085–93. 10.1161/CIRCULATIONAHA.113.001475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng M, Lu X, Huang J, et al. The prognostic significance of atrial fibrillation in heart failure with a preserved and reduced left ventricular function: insights from a meta-analysis. Eur J Heart Fail 2014;16:1317–22. 10.1002/ejhf.187 [DOI] [PubMed] [Google Scholar]
- 16. Lund LH, Donal E, Oger E, et al. Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail 2014;16:992–1001. 10.1002/ejhf.137 [DOI] [PubMed] [Google Scholar]
- 17. Li S-J, Sartipy U, Lund LH, et al. Prognostic significance of resting heart rate and use of β-blockers in atrial fibrillation and sinus rhythm in patients with heart failure and reduced ejection fraction: findings from the Swedish heart failure registry. Circ Heart Fail 2015;8:871–9. 10.1161/CIRCHEARTFAILURE.115.002285 [DOI] [PubMed] [Google Scholar]
- 18. Zafrir B, Lund LH, Laroche C, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J 2018;39:4277–84. 10.1093/eurheartj/ehy626 [DOI] [PubMed] [Google Scholar]
- 19. Lee SE, Cho H-J, Lee H-Y, et al. A multicentre cohort study of acute heart failure syndromes in Korea: rationale, design, and interim observations of the Korean acute heart failure (KorAHF) registry. Eur J Heart Fail 2014;16:700–8. 10.1002/ejhf.91 [DOI] [PubMed] [Google Scholar]
- 20. Lee SE, Lee H-Y, Cho H-J, et al. Clinical characteristics and outcome of acute heart failure in Korea: results from the Korean acute heart failure registry (KorAHF). Korean Circ J 2017;47:341–53. 10.4070/kcj.2016.0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breslow N. Covariance analysis of censored survival data. Biometrics 1974;30:89–99. 10.2307/2529620 [DOI] [PubMed] [Google Scholar]
- 22. S D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–41. 10.2307/2335876 [DOI] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 24. Sartipy U, Dahlström U, Fu M, et al. Atrial fibrillation in heart failure with preserved, Mid-Range, and reduced ejection fraction. JACC Heart Fail 2017;5:565–74. 10.1016/j.jchf.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 25. Lund LH, Claggett B, Liu J, et al. Heart failure with mid-range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018;20:1230–9. 10.1002/ejhf.1149 [DOI] [PubMed] [Google Scholar]
- 26. Son MK, Lim N-K, Park H-Y. Trend of prevalence of atrial fibrillation and use of oral anticoagulation therapy in patients with atrial fibrillation in South Korea (2002-2013). J Epidemiol 2018;28:81–7. 10.2188/jea.JE20160149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee JH, Lim N-K, Cho M-C, et al. Epidemiology of heart failure in Korea: present and future. Korean Circ J 2016;46:658–64. 10.4070/kcj.2016.46.5.658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. developed with the special contribution of the European heart rhythm association. Eur Heart J 2012;33:2719–47. 10.1093/eurheartj/ehs253 [DOI] [PubMed] [Google Scholar]
- 29. Kotecha D, Chudasama R, Lane DA, et al. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: a systematic review and meta-analysis of death and adverse outcomes. Int J Cardiol 2016;203:660–6. 10.1016/j.ijcard.2015.10.220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2019-316219supp001.pdf (48.5KB, pdf)
heartjnl-2019-316219supp002.pdf (59.5KB, pdf)
heartjnl-2019-316219supp003.pdf (73KB, pdf)
heartjnl-2019-316219supp004.pdf (72.8KB, pdf)
heartjnl-2019-316219supp005.pdf (33.1KB, pdf)
heartjnl-2019-316219supp006.pdf (795.7KB, pdf)
heartjnl-2019-316219supp007.pdf (875.4KB, pdf)
heartjnl-2019-316219supp008.pdf (848.8KB, pdf)
heartjnl-2019-316219supp009.pdf (840.1KB, pdf)
heartjnl-2019-316219supp010.pdf (70.1KB, pdf)
heartjnl-2019-316219supp011.pdf (1.5MB, pdf)
heartjnl-2019-316219supp012.pdf (202.4KB, pdf)