Abstract
Purpose
Breast cancer during pregnancy (BC-P) or the first year post-partum (BC-PP) is rare and whether it differs from breast cancer (BC) in young women not associated with pregnancy is uncertain.
Methods
We queried our institutional database for BC-P and BC-PP cases and matched controls with BC not associated with pregnancy diagnosed between January 1, 1985 and December 31, 2013. We performed two parallel retrospective cohort studies evaluating clinico-pathologic features, treatment and outcomes for BC-P and BC-PP cases compared to their controls.
Results
In our population of 65 BC-P cases, 135 controls for BC-P cases, 75 BC-PP cases and 145 controls for BC-PP cases, high grade and estrogen receptor-negativity were more frequent in both case groups than their controls. Among those with stage I–III BC, patterns of local therapy were similar for both case groups and their controls, with the majority undergoing surgery and radiation. Over three-fourths of those with stage I–III BC received chemotherapy. BC-P cases tolerated chemotherapy well, with the majority receiving doxorubicin/cyclophosphamide every 3 weeks. On multivariate analyses of those with stage I–III BC, BC-P cases had non-significantly higher hazards of recurrence and death compared to their controls, while BC-PP cases had non-significantly lower hazards of recurrence and death compared to their controls.
Conclusion
BC-P and BC-PP were associated with adverse clinic-pathologic features in our population. However, we did not observe inferior outcomes for BC-P or BC-PP compared to controls, likely due to receipt of aggressive multi-modality therapy.
Keywords: Pregnancy, Post-partum, Breast cancer, Young women, Prognosis
Introduction
Pregnancy-associated breast cancer (PABC) is commonly defined as breast cancer (BC) during pregnancy (BC-P) or the first year post-partum (BC-PP) [1, 2]. Although rare, the incidence of PABC has risen and up to 15% of BC cases under age 35 are pregnancy-associated [3–6]. PABC is often characterized by adverse features, including estrogen receptor (ER)-negativity, high grade, large size and nodal involvement; however, it is uncertain whether these features differ from BC in young women not associated with pregnancy [7–14]. In addition, whether PABC carries a less favorable prognosis than non-PABC is uncertain [1, 2, 5, 9, 11, 12, 15–19]. Separating BC-P and BC-PP is important as BC-PP may carry a poorer prognosis [1, 2, 9, 13, 15, 19, 20].
Guidelines recommend that BC-P treatment adhere as closely as possible to standard BC treatment with chemotherapy considered safe after the first trimester. Cancer treatment and antenatal care must be coordinated with the goal of term delivery, individualizing sequencing according to oncologic needs and gestational age [21, 22]. Although doxorubicin and cyclophosphamide (AC) is commonly used for BC-P, few publications have described the clinical experience associated with this regimen during pregnancy [21, 23–28].
We present two parallel retrospective matched cohort analyses evaluating BC-P and BC-PP cases separately, each compared to matched controls with non-PABC. We describe treatment patterns of BC-P, especially the use of AC and the sequencing of cancer treatment and delivery.
Methods
Participant selection
After obtaining approval from the Johns Hopkins (JH) institutional review board (IRB), we identified cases and controls from the JH Integrated BC Research Database. Informed consent was not required for this retrospective study. Candidate participants were females diagnosed with ductal carcinoma in situ (DCIS) or invasive BC between January 1, 1985 and December 31, 2013 who attended at least one medical oncology appointment at JH and who had pathology reviewed at JH. We created two separate case groups (BC-P and BC-PP) and two separate control groups (controls for BC-P cases and controls for BC-PP cases). BC-P cases were pregnant at diagnosis and BC-PP cases were diagnosed ≤ 365 days after delivery. To identify cases, we queried the database for candidate participants diagnosed with BC at age ≤ 45 years using the following search terms: pregnant, pregnancy, delivery, post-partum, partum, gestation, infant, baby, breastfeeding and lactation. After forming the case groups, we aimed to identify two controls for each case by randomly selecting from the candidate participants with non-PABC (defined as not being pregnant at diagnosis and having no delivery within 365 days before diagnosis). Each control was matched to a case according to age (± 5 years), extent of disease (DCIS, node-negative invasive BC, node-positive invasive BC or metastatic BC) and time period of diagnosis (1985–1999, 2000–2004 or 2005–2013). The categories for time period of diagnosis were selected based upon when major changes in breast cancer therapy occurred, such as the introduction of the sentinel node procedure in approximately 2000 and the incorporation of taxanes into adjuvant chemotherapy regimens in approximately 2004 [29, 30].
Information regarding demographics, tumor characteristics, treatment, family history, reproductive history, disease status (no evidence of recurrence, loco-regional recurrence or distant recurrence) and vital status was obtained by chart review. For BC-P cases, we reviewed available records regarding gestation at diagnosis, obstetric outcomes and sequencing of BC therapy and delivery. We considered women with a previous delivery as parous. The data that support the findings of this study are not publicly available as they contain information that could compromise individual patient privacy, however, they are available upon reasonable request from the corresponding author (KLS).
Statistical analysis
We used descriptive statistics to summarize patient, tumor and treatment characteristics. Each case group was compared to its control group using the Welch two-sample t test for continuous variables and the Pearson’s Chi-square test for categorical variables. Exploratory analysis comparing the two case groups was performed. Comparisons of treatments received were limited to those with stage I–III BC. Statistical significance was based on a two-sided type-I error rate of 0.05.
We calculated recurrence free survival (RFS) from the time of diagnosis to the time of loco-regional or distant recurrence or death. We censored patients who remained alive without recurrence at the time of last known follow-up. We calculated overall survival (OS) from the time of diagnosis to the time of death from any cause. We censored those who remained alive at the last date they were known to be alive. Survival distributions were described using the Kaplan–Meier method. Stratified cox proportional hazards models stratified for our matching variables were used to estimate the hazard ratios (HR) with 95% confidence intervals (CI) for recurrence and death for each case group compared to its control group. Univariate and multivariate analyses were performed to evaluate other factors associated with recurrence and death, including ER status, receipt of chemotherapy, grade, margin status and stage. Analyses of RFS and OS were limited to patients with stage I–III BC.
Results
Study population
After excluding candidate participants who did not meet eligibility criteria, our study population included 140 cases (65 with BC-P and 75 with BC-PP) plus 280 controls (135 for BC-P cases and 145 for BC-PP cases) (Fig. 1). Due to limitations in the number of young candidate participants in our database, we could not identify two controls for each case.
Fig. 1.
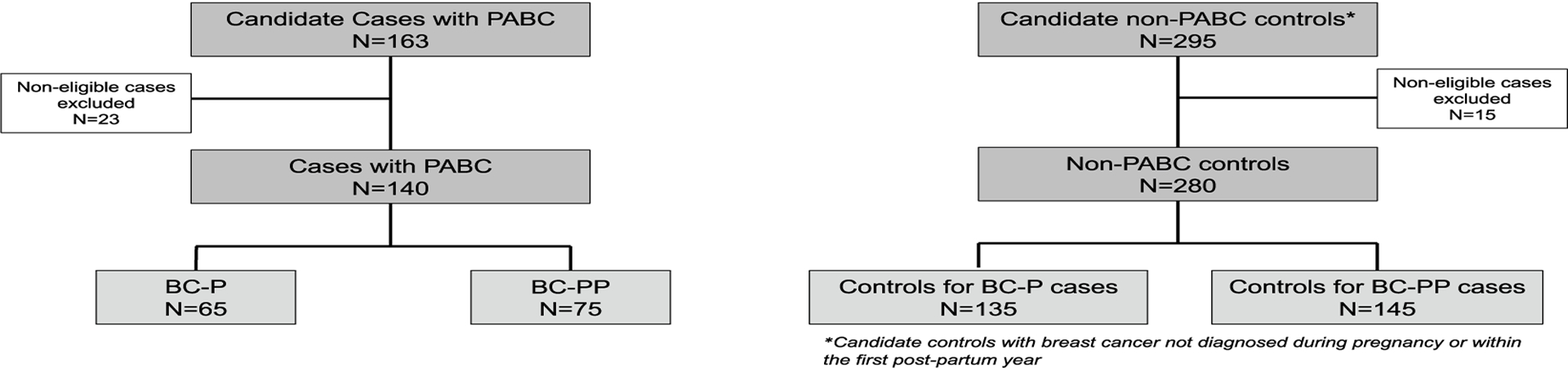
Consolidated standards of reporting trials (CONSORT) diagram of study population
Clinico-pathologic features
Over 70% of each group were stage II or higher. High grade and ER-negativity were more common in both case groups than their controls. Both case groups were less likely to receive all treatment at JH and had shorter follow-up than their controls. Approximately two thirds of each control group was parous. Despite matching, mean age was younger in both case groups than their controls. Tumor characteristics did not differ between the two case groups (Table 1).
Table 1.
Patient and tumor characteristics
| Characteristic | BC-P cases N = 65 | Controls for BC-P cases N = 135 | BC-PP cases N = 75 | Controls for BC-PP cases N = 145 | P value for comparison of BC-P cases to their controlsb | P value for comparison of BC-PP cases to their controlsb | P value for comparison of BC-P cases to BC-PP casesb |
|---|---|---|---|---|---|---|---|
| Mean age at diagnosis in years (SD) | 34.5 (5.0) | 36.7 (5.3) | 34.7 (3.8) | 37.2 (4.7) | 0.007 | < 0.001 | 0.83 |
| Treatment location (N, %) | |||||||
| Johns Hopkins | 9 (14%)a | 25 (19%) | 7 (9%) | 35 (24%) | < 0.001 | < 0.001 | 0.65 |
| Another institution | 30 (46%) | 24 (18%) | 34 (45%) | 25 (17%) | |||
| Both | 26 (40%) | 82 (63%) | 34 (45%) | 84 (58%) | |||
| Year of diagnosis (N, %) | |||||||
| 1985–1989 | 1 (2%) | 4 (3%) | 1 (1%) | 3 (2%) | 0.83 | 0.28 | 0.99 |
| 1990–1994 | 2 (3%) | 7 (5%) | 3 (4%) | 13 (9%) | |||
| 1995–1999 | 9 (14%) | 17 (13%) | 9 (12%) | 11 (8%) | |||
| 2000–2004 | 26 (40%) | 49 (37%) | 3 (40%) | 47 (33%) | |||
| 2005–2009 | 13 (20%) | 20 (15%) | 13 (17%) | 40 (28%) | |||
| 2010–2014 | 14 (22%) | 36 (27%) | 19 (25%) | 30 (21%) | |||
| Race (N, %) | |||||||
| White | 42 (67%) | 97 (73%) | 59 (79%) | 97 (67%) | 0.52 | 0.43 | 0.35 |
| Black | 15 (24%) | 28 (21%) | 11 (15%) | 34 (24%) | |||
| Asian | 1 (2%) | 3 (2%) | 2 (3%) | 3 (2%) | |||
| Other | 5 (8%) | 4 (3%) | 3 (4%) | 9 (6%) | |||
| Unknown | 0 (0%) | 1 (1%) | 0 (0%) | 1 (1%) | |||
| Stage (N, %) | |||||||
| 0 (DCIS) | 3 (5%) | 4 (3%) | 3 (4%) | 4 (3%) | 0.81 | 0.68 | 0.30 |
| I | 10 (15%) | 27 (20%) | 11 (15%) | 34 (23%) | |||
| II | 26 (40%) | 53 (39%) | 25 (33%) | 46 (32%) | |||
| III | 14 (22%) | 33 (24%) | 16 (21%) | 33 (23%) | |||
| IV | 7 (11%) | 10 (7%) | 20 (17%) | 25 (17%) | |||
| Unknown | 5 (8%) | 8 (6%) | 5 (7%) | 3 (2%) | |||
| Grade (N, %) | |||||||
| 1 | 1 (2%) | 11 (9%) | 2(3%) | 8 (6%) | 0.017 | 0.008 | 0.60 |
| 2 | 14 (22%) | 41 (34%) | 11 (16%) | 46 (34%) | |||
| 3 | 49 (77%) | 69 (57%) | 57 (81%) | 81 (60%) | |||
| ER-positive (N, %) | 29 (51%) | 94 (72%) | 42 (63%) | 111 (80%) | 0.004 | 0.006 | 0.19 |
| HER2-positive (N, %) | 13 (25%) | 27 (27%) | 19 (33%) | 25 (23%) | 0.77 | 0.14 | 0.31 |
| Family history of breast or ovarian cancer (N, %) | 28 (47%) | 54 (46%) | 32 (47%) | 60 (44%) | 0.91 | 0.69 | 0.97 |
| Age at Menarche in years (N, %) | |||||||
| ≤10 | 2 (8%) | 4 (6%) | 4 (9%) | 5 (6%) | 0.25 | 0.39 | 0.68 |
| 11 | 2 (8%) | 6 (9%) | 8 (19%) | 14 (18%) | |||
| 12 | 10 (38%) | 13 (20%) | 16 (37%) | 19 (24%) | |||
| 13 | 7 (27%) | 14 (22%) | 7 (16%) | 29 (23%) | |||
| ≥14 | 5 (19%) | 27 (42%) | 8 (19%) | 18 (23%) | |||
| Parity at diagnosis (N, %) | |||||||
| 0 | 21 (34%) | 41 (33%) | 0 (0%) | 44 (33%) | 0.51 | < 0.001 | < 0.001 |
| 1–3 | 38 (61%) | 79 (63%) | 65 (92%) | 80 (60%) | |||
| 4–5 | 1 (2%) | 5 (4%) | 5 (7%) | 6 (4%) | |||
| ≥6 | 2 (3%) | 1 (1%) | 1 (1%) | 4 (3%) | |||
| Mean duration of follow-up in months (SD) | 50.9 (51.9) | 78.4 (66.0) | 55.8 (55.6) | 72.8 (61.1) | 0.002 | 0.04 | 0.59 |
All percentages based on non-missing values
P values for t tests and Chi-squared tests performed on non-missing proportions
Treatment of cases and controls with stage I–III breast cancer
There were no significant differences in the frequency of surgery, radiation, and receipt of chemotherapy between each case group and its control group. Over 75% of each case and control group received chemotherapy. Reconstruction and receipt of HER2-targeted therapy were less frequent among BC-P cases with stage I–III disease than their controls (Table 2). When limited to the HER2-positive subset of BC-P cases and their controls with stage I–III disease, we also observed less receipt of HER2-targeted therapy among cases. Three of ten (30%) of BC-P cases with Stage I–III HER2-positive breast cancer received HER2-targeted therapy compared to 13 of 23 (57%) controls for BC-P cases with stage I–III HER2-positive breast cancer. Receipt of endocrine therapy (ET) was less frequent among both case groups with stage I–III disease compared to their controls (Table 2). When limited to cases and controls with stage I–III ER-positive disease, we also observed less receipt of ET among cases. Of 20 BC-P cases with stage I–III ER-positive breast cancer, 8 (40%) received ET compared to 56 of 81 (60%) controls for BC-P cases with stage I–III ER-positive breast cancer. Similarly, of 27 BC-PP cases with stage I-III ER-positive breast cancer, 13 (48%) received ET compared to 67 of 89 (75%) of controls for BC-PP cases with stage I–III ER-positive breast cancer. We observed no differences in breast conservation rates, margins and use of sentinel node procedure across groups.
Table 2.
Treatment characteristics of cases and controls with stage I-III breast cancer
| Characteristic | BC-P cases N = 50 | Controls for BC-P cases N = 113 | BC-PP cases N = 52 | Controls for BC‒PP cases N = 113 | P value for comparison of BC‒P cases and their controlsb | P value for comparison of BC‒PP cases and their controlsb | P value for comparison of BC‒P cases to BC‒PP casesb |
|---|---|---|---|---|---|---|---|
| Breast surgery(N, %) | |||||||
| Mastectomy | 33 (67%)a | 81 (74%) | 32 (65%) | 65 (58%) | 0.42 | 0.39 | 0.83 |
| Breast conservation | 16 (33%) | 29 (26%) | 17 (35%) | 47 (42%) | |||
| Margins (N, %) | |||||||
| Positive | 4 (9%) | 5 (4%) | 2 (4%) | 4 (4%) | 0.21 | 0.78 | 0.17 |
| Negative | 34 (74%) | 96 (86%) | 41 (89%) | 96 (86%) | |||
| Close | 8 (17%) | 11 (10%) | 3 (7%) | 11 (10%) | |||
| Nodal evaluation (N, %) | |||||||
| None | 0 (0%) | 1 (1%) | 0 (0%) | 0 (0%) | 0.18 | 0.67 | 0.20 |
| Sentinel node biopsy | 8 (19%) | 35 (32%) | 14 (30%) | 37 (34%) | |||
| Axillary node dissection | 35 (81%) | 72 (67%) | 32 (70%) | 72 (66%) | |||
| Reconstruction (N, %) | |||||||
| None | 30 (75%) | 47 (47%) | 21 (51%) | 62 (58%) | 0.01 | 0.73 | 0.08 |
| Implant | 5 (12%) | 28 (28%) | 11 (27%) | 23 (21%) | |||
| Autologous | 5 (12%) | 24 (24%) | 9 (22%) | 22 (21%) | |||
| Radiation (N, %) | 28 (78%) | 64 (65%) | 30 (77%) | 66 (70%) | 0.17 | 0.43 | 0.93 |
| (Neo)adjuvant chemotherapy (N, %) | 40 (80%) | 89 (83%) | 40 (78%) | 84 (86%) | 0.63 | 0.26 | 0.85 |
| Endocrine therapy (N, %) | 14 (48%) | 61 (86%) | 21 (75%) | 72 (92%) | < 0.001 | 0.02 | 0.04 |
| HER2-targeted therapy (N, %) | 5 (22%) | 14 (54%) | 7 (33%) | 8 (36%) | 0.02 | 0.84 | 0.39 |
All percentages based on non-missing values
P values for Chi-squared tests performed on non-missing proportions
Multi-disciplinary management of BC-P cases
Obstetric outcome was known for 43% of BC-P cases. Of 17 known live births, 6 were via vaginal delivery and 11 via cesarean sections. Labor was induced during the third trimester in 11 BC-P cases and 9 births were pre-term. Sequencing of BC treatment and delivery was variable, with 20 women undergoing surgery and 20 receiving chemotherapy while pregnant. Of those treated with chemotherapy during pregnancy, 15 received AC every 3 weeks. No BC-P cases received dose dense AC during pregnancy. One BC-P case with metastatic BC received a taxane during pregnancy and two received myeloid growth factors during pregnancy. Complications associated with receipt of AC during pregnancy were uncommon and included one maternal infection and one neonate with cytopenias (Table 3).
Table 3.
Multi-disciplinary management of 65 cases with breast cancer diagnosed during pregnancy
| Characteristic | N (%) |
|---|---|
| Trimester at diagnosis | |
| 1 | 26 (40%) |
| 2 | 13 (20%) |
| 3 | 17 (26%) |
| Missing | 9 (14%) |
| Obstetric outcome | |
| Elective termination | 7 (11%) |
| Spontaneous abortion | 4 (6%)b |
| Pre-term delivery | 9 (14%) |
| Term delivery | 7 (11%) |
| Post-dates delivery | 1 (2%) |
| Missing | 37 (57%) |
| BCa therapy during pregnancy | |
| Chemotherapy | 20 (31%)c |
| Endocrine therapy | 0 (0%) |
| Radiation | 0 (0%) |
| HER2‒targeted therapy | 0 (0%) |
| Surgery | 20 (31%) |
| Sequencing of BC treatment and delivery | |
| Delivery → Cancer therapy | 10 (15%) |
| Breast cancer surgery → Delivery ± → Adjuvant systemic therapy | 8 (12%) |
| Neoadjuvant chemotherapy → Delivery ± → Further breast cancer therapy | 8 (12%) |
| Breast cancer surgery → Adjuvant chemotherapy → Delivery ± → Further adjuvant systemic therapy | 12 (18%) |
| Missing | 27 (42%) |
| Maternal complications | |
| Infection | 2 (3%)c,d |
| Pre-eclampsia | 3 (5%) |
| Hemorrhage | 1 (2%) |
| Fetal/neonatal complications | |
| Intrauterine growth retardation | 1 (2%)c |
| Malpresentation | 2 (3%) |
| Cytopenias at birth | 1 (2%)e |
BC breast cancer
One spontaneous abortion occurred during AC
Only the percentage of BC-P cases who received each type of treatment during pregnancy or in whom each type of complication occurred is reported
One maternal infection occurred during AC
The neonate with cytopenias at birth was born to a mother receiving AC
Recurrence and survival of cases and controls with stage I–III breast cancer
Median follow-up exceeded 4 years for all participants, but was shorter for cases than their controls (Table 1). RFS and OS decreased over time with no statistically significant differences observed between each case group and its controls (Fig. 2). Five-year RFS was 51% among BC-P cases, 60% among controls for BC-P cases, 63% among BC-PP cases and 74% among controls for BC-PP cases. In univariate analysis, there were slightly higher hazards of recurrence for BC-P cases than their controls (HR 1.43, 95% CI 0.70–2.93, Table 4) and for BC-PP cases than their controls (HR 1.43, 95% CI 0.69–3.00, Table 5) although these did not reach statistical significance. Distant recurrences were more common than loco-regional recurrences, representing 58% and 60% in BC-P cases and their controls, respectively; and 78% and 57% in BC-PP cases and their controls, respectively. Five-year OS was 75% among BC-P cases, 81% among controls for BC-P cases, 81% among BC-PP cases and 87% among controls for BC-PP cases. In univariate analysis, the hazard of death was almost twice as high for BC-P cases than their controls (HR 1.91, 95% CI 0.70–5.21, Table 4) and was slightly higher for BC-PP cases than their controls (HR 1.39, 95% CI 0.56–3.47, Table 5), however, these differences did not reach statistical significance.
Fig. 2.
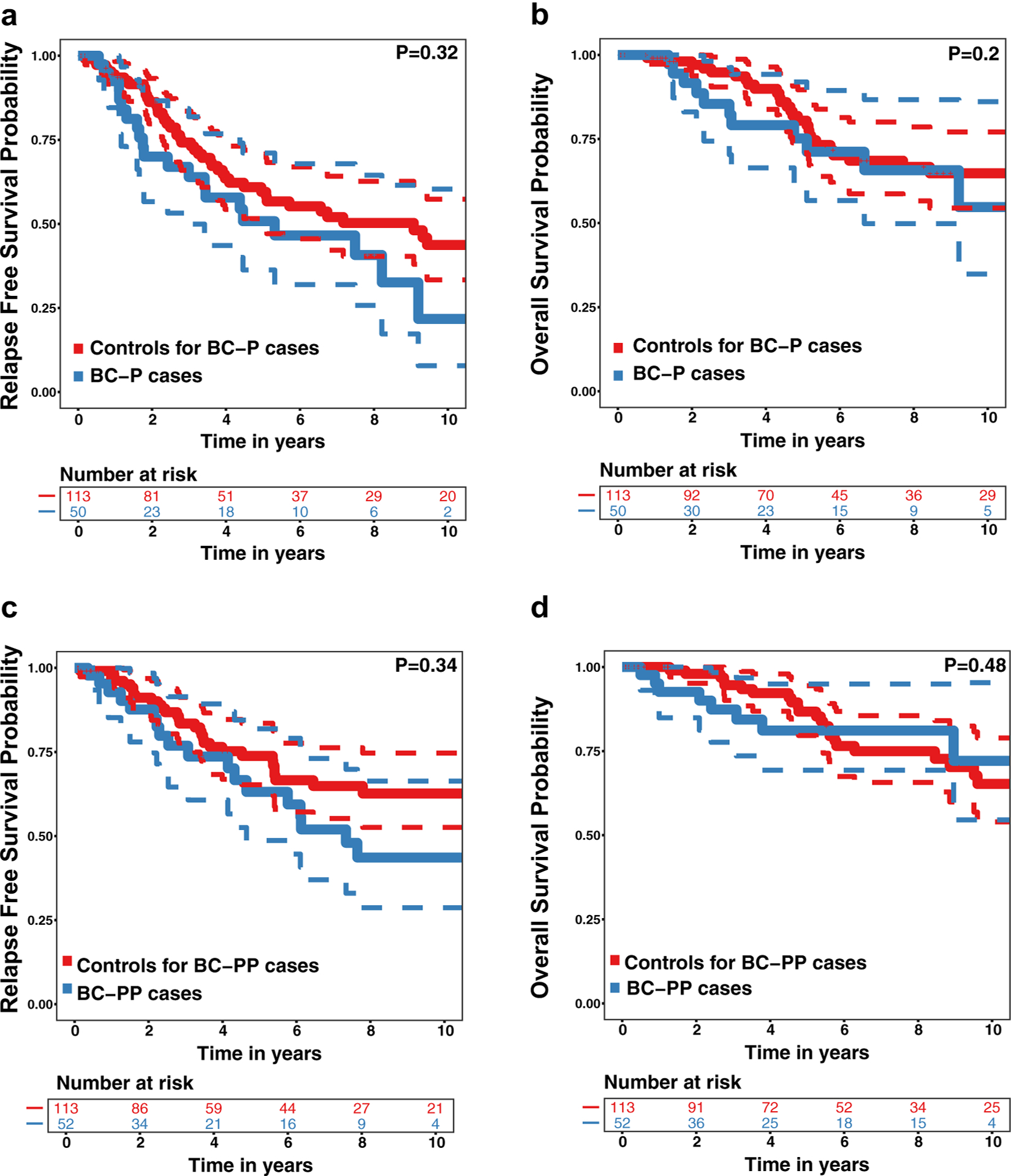
Recurrence Free Survival (RFS) and Overall Survival (OS) (with 95% confidence intervals) for Cases with Stage I–III Pregnancy-Associated Breast Cancer and Their Controls. a RFS for women with breast cancer diagnosed during pregnancy (BC-P cases) and their controls, b OS for BC-P cases and their controls, c RFS for women with breast cancer diagnosed during the first year post-partum (BC-PP cases) and their controls, d OS for BC-PP cases and their controls
Table 4.
Factors associated with recurrence and death among cases with stage i–iii breast cancer diagnosed during pregnancy and their controls
| Recurrence | Death | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HRa | 95% CIb | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Case versus control | 1.43 | 0.70–2.93 | 0.32 | 1.51 | 0.56–4.06 | 0.41 | 1.91 | 0.70–5.21 | 0.20 | 2.85 | 0.57–14.29 | 0.20 |
| Stage | 1.34 | 0.69–2.61 | 0.38 | 2.14 | 0.83–5.51 | 0.11 | 1.33 | 0.55–3.24 | 0.53 | 2.21 | 0.53–9.21 | 0.28 |
| Grade | 0.89 | 0.49–1.61 | 0.71 | 1.10 | 0.50–2.45 | 0.81 | 1.39 | 0.61–3.16 | 0.44 | 1.24 | 0.41–3.74 | 0.71 |
| ER-positive | 1.37 | 0.55–3.41 | 0.50 | 1.16 | 0.41–3.32 | 0.78 | 0.51 | 0.13–1.94 | 0.32 | 0.38 | 0.08–1.84 | 0.23 |
| Negative margins | 0.99 | 0.38–2.62 | 0.99 | 1.11 | 0.30–1.08 | 0.88 | 1.15 | 0.31–4.41 | 0.84 | 0.93 | 0.15–5.59 | 0.94 |
| Receipt of chemotherapy | 0.67 | 0.23–1.98 | 0.47 | 0.50 | 0.10–2.53 | 0.40 | 1.50 | 0.31–7.20 | 0.61 | 1.08 | 0.11–10.45 | 0.95 |
HR hazard ratio
CI confidence interval
Table 5.
Factors associated with recurrence and death in cases with stage i-iii breast cancer diagnosed during the first year post-partum and their controls
| Recurrence | Death | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HRa | 95% CIb | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Case versus control | 1.43 | 0.69–3.00 | 0.34 | 0.81 | 0.26–2.52 | 0.72 | 1.39 | 0.56–3.47 | 0.48 | 0.57 | 0.13–2.58 | 0.47 |
| Stage | 1.74 | 0.83–3.65 | 0.14 | 1.17 | 0.39–3.49 | 0.77 | 3.27 | 1.05–10.13 | 0.04 | 5.33 | 0.60–47.54 | 0.13 |
| Grade | 3.00 | 1.12–8.04 | 0.03 | 3.30 | 0.86–12.65 | 0.08 | 1.53 | 0.46–5.12 | 0.49 | 3.13 | 0.32–30.44 | 0.33 |
| ER-positive | 0.45 | 0.17–1.17 | 0.10 | 0.55 | 0.14–2.11 | 0.38 | 0.27 | 0.07–1.03 | 0.06 | 0.59 | 0.08–4.27 | 0.60 |
| Negative margins | 0.58 | 0.13–2.50 | 0.46 | 1.11 | 0.21–5.76 | 0.90 | 0.43 | 0.07–2.46 | 0.34 | 0.34 | 0.02–6.76 | 0.48 |
| Receipt of chemotherapy | 1.17 | 0.31–4.35 | 0.82 | 0.53 | 0.06–4.84 | 0.57 | 0.58 | 0.08–4.30 | 0.59 | 0.15 | 0.003–6.86 | 0.33 |
HR hazard ratio
CI confidence interval
After adjusting for the pre-specified covariates, there were non-significant but higher hazards of recurrence and death for BC-P cases than their controls (multivariate HR for recurrence 1.51, 95% CI 0.56–4.06; multivariate HR for death 2.85, 95% CI 0.57–14.29). Other covariates were not significantly associated with recurrence or death for BC-P cases and their controls on multivariate or univariate analysis (Table 4). After adjusting for the pre-specified covariates, there were non-significant but lower hazards of recurrence and death for BC-PP cases than their controls (multivariate HR for recurrence 0.81, 95% CI 0.26–2.52; multivariate HR for death 0.57, 95% CI 0.13–2.58). In univariate analyses among BC-PP cases and their controls, higher grade was associated with threefold higher risk of recurrence and higher stage was associated with threefold higher risk of death, but these associations were not statistically significant on multivariate analyses (Table 5).
Discussion
BC in young women is associated with adverse features [10]. Whether PABC differs from non-PABC in young women and whether BC-P and BC-PP differ from one another has been unclear, potentially due to limitations of prior studies, including small sample size, inconsistent control populations, variable definitions of PABC, lack of consideration of the effects of treatment on outcomes, and grouping BC-P and BC-PP together [1, 2, 5, 7–13, 15–19]. We report here results of a large retrospective study with long follow-up and comprehensive treatment information in which we considered BC-P and BC-PP separately. We observed that both BC-P and BC-PP are frequently ER-negative and high grade. However, these differences did not translate into statistically significantly inferior outcomes for BC-P or BC-PP cases compared to matched controls with non-PABC. We did, however, observe non-significant but higher hazards of recurrence and death for BC-P cases than their controls.
In a meta-analysis of 30 studies, Azim et al. concluded that PABC carries a higher risk of recurrence and death than non-PABC, with especially poor outcomes for BC-PP [1] An updated meta-analysis of 41 studies by Hartman et al. reached a similar conclusion [2]. Some have suggested that the negative impact of a post-partum diagnosis on prognosis extends to 5 or even 10 years after delivery [2, 9, 13, 15, 19, 31]. Changes in the post-partum breast microenvironment associated with breast involution, such as immune cell influx, extracellular matrix remodeling, and lymphangiogenesis may explain these unfavorable outcomes [9, 20]. Given the apparent differences between BC-P and BC-PP, we considered them separately in this study, however, we did not confirm the finding that PABC, especially BC-PP, is associated with significantly inferior outcomes compared to non-PABC. Almost all participants with stage I–III BC in our study underwent surgery and over 75% received chemotherapy, suggesting that aggressive multi-modality treatment may overcome the adverse biologic features of PABC.
Women were eligible to be controls in our study if they had not had a delivery within one year of diagnosis. Approximately two thirds of our controls were parous, thus, it is conceivable that some had “late post-partum” BC diagnosed more than one year after delivery. If these women carried poor prognosis based on late post-partum diagnosis, their inclusion in our control groups may have masked our ability to detect differences in outcomes between our cases and controls [2, 9, 13, 15]. To avoid this limitation, we suggest that future PABC studies limit eligibility for controls to nulliparous women or those with remote prior deliveries.
In keeping with previous reports, we observed that PABC cases were less likely to receive ET than controls [11, 12, 21, 22, 32]. It is possible this finding reflects missing data due to delayed initiation of ET in women with PABC. We do not think this finding is attributable to lower rates of ER-positive disease among women with PABC as rates of receipt of ET were lower among BC-P and BC-PP cases than their controls even when the analysis was limited to the stage I-III ER-positive subpopulation. Ensuring women with ER-positive PABC receive ET is important as the majority of young women with BC have ER-positive disease and outcomes are the least favorable among this subset [33]. We also observed that BC-P cases were less likely to receive HER2-targeted therapy and reconstruction. These findings may also reflect incomplete ascertainment of delayed therapies. In addition, low rates of receipt of HER2-targeted therapy in our study population may be partly explained by the fact that some cases and controls were diagnosed prior to publication of the adjuvant trastuzumab trials [34–36]. Other than avoiding chemotherapy during the first trimester and delaying initiation of ET, HER2-targeted therapy and radiation until after delivery, treatment of BC-P should adhere to standard BC therapy [21, 22] and efforts to ensure delivery of ET and HER2-targeted therapy are of paramount importance.
Consistent with guidelines supporting administration of chemotherapy after the first trimester, we report the safety of AC during pregnancy [21, 22]. Our findings add to the available literature regarding chemotherapy for BC-P, the majority of which focuses on use of 5-fluorouracil-based regimens [23–25]. At this time, data remain limited regarding taxanes and dose dense (every 2 weeks) therapy with growth factor support during pregnancy, although recent guidelines support consideration of these therapies [21, 26–28]. It is notable that the majority of BC-P cases in our study population received AC every 3 weeks. We do not know if this schedule was selected due to lack of comfort using growth factors during pregnancy or based on the need to spread chemotherapy out over a longer period of time since many BC-P cases were diagnosed early in pregnancy. In general, data indicates superior outcomes with the use of dose dense therapy compared to every 3 week therapy, so it is possible this regimen negatively impacted outcomes [37].
Sequencing of BC care and delivery were variable in our population, emphasizing the need to individualize management based on gestational age and oncologic considerations [21, 22]. Induction of labor, pre-term delivery and cesarean section was not uncommon among our BC-P cases. Prenatal exposure to chemotherapy is associated with increased premature rupture of membranes, premature labor and pre-term delivery, although, as we observed, pre-term delivery in women with BC-P is often iatrogenic [21, 23, 38–40]. Children exposed to chemotherapy in utero generally experience normal cognitive development adjusted for gestational age at birth. However, prematurity negatively impacts cognitive development, thus, it is optimal to deliver as close to term as possible [16, 21, 25, 39, 41]. Some literature suggests delays in diagnosis, initiation of therapy and interruptions of therapy in women with BC-P may contribute to more advanced stage at diagnosis and to poorer outcomes [32, 42]. Unfortunately, we were unable to capture delays in diagnosis and treatment in our study population.
It is now well accepted that breast cancer surgery can be performed in pregnant women, even during the first trimester. Mastectomy should not be recommended solely because of pregnancy and breast-conserving surgery should be considered if feasible. Similarly, axillary lymph node dissection is not required solely because of pregnancy as sentinel node biopsy, preferably with radioactive tracer, can be performed during pregnancy [21, 43]. In line with published guidelines on the surgical management of breast cancer during pregnancy, we observed no differences in breast conservation rates, margins and use of the sentinel node procedure across groups. Notably, immediate reconstruction was less frequent among BC-P cases than their controls. This, too, is in keeping with published guidelines that discourage immediate reconstruction during pregnancy, although there is some data regarding immediate expander placement [21, 43]. It is noteworthy that our data did not capture reconstruction after delivery and delayed implant placement is recommended for women diagnosed with breast cancer during pregnancy [43].
Weaknesses of our study include inclusion of parous controls, retrospective single institution design, incomplete data (especially regarding obstetric outcomes, receipt of adjuvant ET, receipt of adjuvant HER2-targeted therapy, delays and gaps in treatment), differences in mean age between case and control groups despite matching, shorter follow-up for cases than controls, differences in treatment location and possible incomplete ascertainment of cases from our database. In addition, we were unable to evaluate the association of race and socioeconomic status with recurrence and death in our study population. Cancer mortality rates are higher among blacks and among individuals of lower socioeconomic status [44]. Our case and control groups were well-balanced with regard to race but, owing to limitations in patient numbers, we were unable to formally test the association of race with outcomes in our multivariate models. Unfortunately, information about socioeconomic status for our study population was not available, thus, we could not evaluate the association of this important variable with outcomes. Similarly, we were unable to evaluate the association of adherence to adjuvant ET to outcomes in our cases and controls as this information was not available. Non-adherence is more common among young breast cancer patients and is associated with increased breast mortality, thus, lack of consideration of the impact of adherence on outcomes is a limitation of our study [45, 46]. Notably, 27% of our study population received care exclusively at other institutions, likely contributing to missing data regarding adjuvant therapies and long term outcomes and potentially also contributing to differences in outcomes if patterns of care differed across treatment locations. However, we feel that our population is representative of the general United States cancer population for whom much of cancer care is delivered in community settings [47].
In conclusion, we found that, despite adverse characteristics, outcomes were not statistically significantly inferior for PABC compared to non-PABC in our population; a finding that we think is likely a reflection of aggressive multi-modality treatment. However, BC recurrence and death were not uncommon in our population, consistent with previous data demonstrating unfavorable outcomes for young women with BC [10]. In addition, there were non-significant but higher hazards of recurrence and death for BC-P cases than their controls, thus, it is possible that with more power, we may have detected a significant difference in outcomes between our PABC cases and their controls. In particular, suboptimal treatment due to delay or incomplete administration of adjuvant therapies may negatively impact outcomes in women with PABC. Moving forward, larger multi-institutional efforts to study outcomes and efforts to ensure optimal treatment delivery are important for women with PABC. The interaction between reproductive factors and BC is complex and expanded efforts to collect reproductive histories and to explore the biology of BC in young women are needed. Further study of the pregnant and post-partum breast microenvironment may define pathways implicated in tumorigenesis and metastasis, facilitating development of agents for prevention and treatment [48, 49].
Funding
This work was supported by funding from the Charles Evans Foundation, Centers for Disease Control and Prevention (U58DP005410) and the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center Support Grant (P30CA006973).
Footnotes
Conflict of interest Ciara C. O’Sullivan has received research support from Lilly. Gary L. Rosner owns stock in Johnson & Johnson and Novartis. Gary L. Rosner is a member of an independent data monitoring committee for Novartis. Vered Stearns has received institutional research support from Abbvie, Biocept, Pfizer, Novartis, Medimmune, and Puma Biotechnology. Vered Stearns has served as a consultant for Iridium Therapeutics, Inc. Vered Stearns is a member of a data safety monitoring board for Immunomedics, Inc. Karen Lisa Smith’s spouse with stock ownership in ABT Labs and Abbvie. Karen Lisa Smith has received research support from CancerIncite and Pfizer. The following authors declare that they have no conflicts of interest: Sheeba Irshad, Zheyu Wang, Zhuojun Tang, Christopher Umbricht and Mindy S. Christianson.
Research involving human participants and/or animals All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We obtained approval from the Johns Hopkins (JH) institutional review board (IRB) to conduct this retrospective study.
References
- 1.Azim HA Jr, Santoro L, Russell-Edu W, Pentheroudakis G, Pavlidis N, Peccatori FA (2012) Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev 38(7):834–842. 10.1016/j.ctrv.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 2.Hartman EK, Eslick GD (2016) The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Cancer Res Treat 160(2):347–360. 10.1007/s10549-016-3989-3 [DOI] [PubMed] [Google Scholar]
- 3.Andersson TM, Johansson AL, Hsieh CC, Cnattingius S, Lambe M (2009) Increasing incidence of pregnancy-associated breast cancer in Sweden. Obstet Gynecol 114(3):568–572. 10.1097/AOG.0b013e3181b19154 [DOI] [PubMed] [Google Scholar]
- 4.Moreira WB, Brandao EC, Soares AN, Lucena CE, Antunes CM (2010) Prognosis for patients diagnosed with pregnancy-associated breast cancer: a paired case-control study. Sao Paulo Med J 128(3):119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beadle BM, Woodward WA, Middleton LP, Tereffe W, Strom EA, Litton JK, Meric-Bernstam F, Theriault RL, Buchholz TA, Perkins GH (2009) The impact of pregnancy on breast cancer outcomes in women %3c or=35 years. Cancer 115(6):1174–1184. 10.1002/cncr.24165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cottreau CM, Dashevsky I, Andrade SE, Li DK, Nekhlyudov L, Raebel MA, Ritzwoller DP, Partridge AH, Pawloski PA, Toh S (2019) Pregnancy-associated cancer: A US population-based study. Womens Health 28(2):250–257. 10.1089/jwh.2018.6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton LP, Amin M, Gwyn K, Theriault R, Sahin A (2003) Breast carcinoma in pregnant women: assessment of clinico-pathologic and immunohistochemical features. Cancer 98(5):1055–1060. 10.1002/cncr.11614 [DOI] [PubMed] [Google Scholar]
- 8.Amant F, von Minckwitz G, Han SN, Bontenbal M, Ring AE, Giermek J, Wildiers H, Fehm T, Linn SC, Schlehe B, Neven P, Westenend PJ, Muller V, Van Calsteren K, Rack B, Nekljudova V, Harbeck N, Untch M, Witteveen PO, Schwedler K, Thomssen C, Van Calster B, Loibl S (2013) Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol 31(20):2532–2539. 10.1200/JCO.2012.45.6335 [DOI] [PubMed] [Google Scholar]
- 9.Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, Urquhart A, Schedin P, Borges VF (2013) Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat 138(2):549–559. 10.1007/s10549-013-2437-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azim HA Jr, Partridge AH (2014) Biology of breast cancer in young women. Breast Cancer Res 16(4):427 10.1186/s13058-014-0427-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae SY, Jung SP, Jung ES, Park SM, Lee SK, Yu JH, Lee JE, Kim SW, Nam SJ (2018) Clinical characteristics and prognosis of pregnancy-associated breast cancer: poor survival of luminal B subtype. Oncology 95(3):163–169. 10.1159/000488944 [DOI] [PubMed] [Google Scholar]
- 12.Iqbal J, Amir E, Rochon PA, Giannakeas V, Sun P, Narod SA (2017) Association of the timing of pregnancy with survival in women with breast cancer. JAMA Oncol 3(5):659–665. 10.1001/jamaoncol.2017.0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goddard ET, Bassale S, Schedin T, Jindal S, Johnston J, Cabral E, Latour E, Lyons TR, Mori M, Schedin PJ, Borges VF (2019) Association between postpartum breast cancer diagnosis and metastasis and the clinical features underlying risk. JAMA Netw Open 2(1):e186997 10.1001/jamanetworkopen.2018.6997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labrosse J, Abdennebi I, Thibault L, Laas E, Merckelbagh H, Morel C, Lam T, Lae M, Reyal F, Hamy AS (2018) Chemosensitivity, tumor infiltrating lymphocytes (TILs), and survival of postpartum PABC patients treated by neoadjuvant chemotherapy. Breast 42:61–67. 10.1016/j.breast.2018.08.103 [DOI] [PubMed] [Google Scholar]
- 15.Lee GE, Mayer EL, Partridge A (2017) Prognosis of pregnancy-associated breast cancer. Breast Cancer Res Treat 163(3):417–421. 10.1007/s10549-017-4224-6 [DOI] [PubMed] [Google Scholar]
- 16.Amant F, Loibl S, Neven P, Van Calsteren K (2012) Breast cancer in pregnancy. Lancet 379(9815):570–579. 10.1016/s0140-6736(11)61092-1 [DOI] [PubMed] [Google Scholar]
- 17.Litton JK, Warneke CL, Hahn KM, Palla SL, Kuerer HM, Perkins GH, Mittendorf EA, Barnett C, Gonzalez-Angulo AM, Hortobagyi GN, Theriault RL (2013) Case control study of women treated with chemotherapy for breast cancer during pregnancy as compared with nonpregnant patients with breast cancer. Oncologist 18(4):369–376. 10.1634/theoncologist.2012-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson AL, Andersson TM, Hsieh CC, Jirstrom K, Dickman P, Cnattingius S, Lambe M (2013) Stage at diagnosis and mortality in women with pregnancy-associated breast cancer (PABC). Breast Cancer Res Treat 139(1):183–192. 10.1007/s10549-013-2522-1 [DOI] [PubMed] [Google Scholar]
- 19.Johansson AL, Andersson TM, Hsieh CC, Cnattingius S, Lambe M (2011) Increased mortality in women with breast cancer detected during pregnancy and different periods postpartum. Cancer Epidemiol Biomark Prev 20(9):1865–1872. 10.1158/1055-9965.EPI-11-0515 [DOI] [PubMed] [Google Scholar]
- 20.Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, Marusyk A, Tan AC, Schedin P (2011) Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med 17(9):1109–1115. 10.1038/nm.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loibl S, Schmidt A, Gentilini O, Kaufman B, Kuhl C, Denkert C, von Minckwitz G, Parokonnaya A, Stensheim H, Thomssen C, van Calsteren K, Poortmans P, Berveiller P, Markert UR, Amant F (2015) Breast cancer diagnosed during pregnancy: adapting recent advances in breast cancer care for pregnant patients. JAMA Oncol 1(8):1145–1153. 10.1001/jamaoncol.2015.2413 [DOI] [PubMed] [Google Scholar]
- 22.Shachar SS, Gallagher K, McGuire K, Zagar TM, Faso A, Muss HB, Sweeting R, Anders CK (2017) Multidisciplinary management of breast cancer during pregnancy. Oncologist 22(3):324–334. 10.1634/theoncologist.2016-0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loibl S, Han SN, von Minckwitz G, Bontenbal M, Ring A, Giermek J, Fehm T, Van Calsteren K, Linn SC, Schlehe B, Gziri MM, Westenend PJ, Muller V, Heyns L, Rack B, Van Calster B, Harbeck N, Lenhard M, Halaska MJ, Kaufmann M, Nekljudova V, Amant F (2012) Treatment of breast cancer during pregnancy: an observational study. Lancet Oncol 13(9):887–896. 10.1016/S1470-2045(12)70261-9 [DOI] [PubMed] [Google Scholar]
- 24.Berry DL, Theriault RL, Holmes FA, Parisi VM, Booser DJ, Singletary SE, Buzdar AU, Hortobagyi GN (1999) Management of breast cancer during pregnancy using a standardized protocol. J Clin Oncol 17(3):855–861. 10.1200/JCO.1999.17.3.855 [DOI] [PubMed] [Google Scholar]
- 25.Hahn KM, Johnson PH, Gordon N, Kuerer H, Middleton L, Ramirez M, Yang W, Perkins G, Hortobagyi GN, Theriault RL (2006) Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer 107(6):1219–1226. 10.1002/cncr.22081 [DOI] [PubMed] [Google Scholar]
- 26.Cardonick E, Gilmandyar D, Somer RA (2012) Maternal and neonatal outcomes of dose-dense chemotherapy for breast cancer in pregnancy. Obstet Gynecol 120(6):1267–1272. 10.1097/AOG.0b013e31826c32d9 [DOI] [PubMed] [Google Scholar]
- 27.Cardonick E, Bhat A, Gilmandyar D, Somer R (2012) Maternal and fetal outcomes of taxane chemotherapy in breast and ovarian cancer during pregnancy: case series and review of the literature. Ann Oncol 23(12):3016–3023. 10.1093/annonc/mds170 [DOI] [PubMed] [Google Scholar]
- 28.Mir O, Berveiller P, Goffinet F, Treluyer JM, Serreau R, Gold-wasser F, Rouzier R (2010) Taxanes for breast cancer during pregnancy: a systematic review. Ann Oncol 21(2):425–426. 10.1093/annonc/mdp517 [DOI] [PubMed] [Google Scholar]
- 29.Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, Wickerham DL, Yothers G, Soran A, Wolmark N (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23(16):3686–3696. 10.1200/JCO.2005.10.517 [DOI] [PubMed] [Google Scholar]
- 30.Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, Intra M, Veronesi P, Robertson C, Maisonneuve P, Renne G, De Cicco C, De Lucia F, Gennari R (2003) A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349(6):546–553. 10.1056/NEJMoa012782 [DOI] [PubMed] [Google Scholar]
- 31.Strasser-Weippl K, Ramchandani R, Fan L, Li J, Hurlbert M, Finkelstein D, Shao ZM, Goss PE (2015) Pregnancy-associated breast cancer in women from Shanghai: risk and prognosis. Breast Cancer Res Treat 149(1):255–261. 10.1007/s10549-014-3219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson ALV, Weibull CE, Fredriksson I, Lambe M (2018) Diagnostic pathways and management in women with pregnancy-associated breast cancer (PABC): no evidence of treatment delays following a first healthcare contact. Breast Cancer Res Treat. 10.1007/s10549-018-05083-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Tamimi RM (2016) Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 34(27):3308–3314. 10.1200/JCO.2015.65.8013 [DOI] [PubMed] [Google Scholar]
- 34.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684. 10.1056/NEJMoa052122 [DOI] [PubMed] [Google Scholar]
- 35.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant Trial Study T (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672. 10.1056/NEJMoa052306 [DOI] [PubMed] [Google Scholar]
- 36.Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, Winer EP, Colon-Otero G, Davidson NE, Mamounas E, Zujewski JA, Wolmark N (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32(33):3744–3752. 10.1200/JCO.2014.55.5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Grad-ishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L (2003) Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 21(8):1431–1439. 10.1200/JCO.2003.09.081 [DOI] [PubMed] [Google Scholar]
- 38.Shechter Maor G, Czuzoj-Shulman N, Spence AR, Abenhaim HA (2018) Neonatal outcomes of pregnancy-associated breast cancer: population-based study on 11 million births. Breast J 10.1111/tbj.13156 [DOI] [PubMed] [Google Scholar]
- 39.Amant F, Vandenbroucke T, Verheecke M, Fumagalli M, Halaska MJ, Boere I, Han S, Gziri MM, Peccatori F, Rob L, Lok C, Witteveen P, Voigt JU, Naulaers G, Vallaeys L, Van den Heuvel F, Lagae L, Mertens L, Claes L, Van Calsteren K, International Network on Cancer I, Pregnancy (2015) Pediatric outcome after maternal cancer diagnosed during pregnancy. N Engl J Med 373(19):1824–1834. 10.1056/NEJMoa1508913 [DOI] [PubMed] [Google Scholar]
- 40.de Haan J, Verheecke M, Van Calsteren K, Van Calster B, Shmakov RG, Mhallem Gziri M, Halaska MJ, Fruscio R, Lok CAR, Boere IA, Zola P, Ottevanger PB, de Groot CJM, Peccatori FA, Dahl Steffensen K, Cardonick EH, Polushkina E, Rob L, Ceppi L, Sukhikh GT, Han SN, Amant F, International Network on C, Infertility P (2018) Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol 19(3):337–346. 10.1016/S1470-2045(18)30059-7 [DOI] [PubMed] [Google Scholar]
- 41.Amant F, Van Calsteren K, Halaska MJ, Gziri MM, Hui W, Lagae L, Willemsen MA, Kapusta L, Van Calster B, Wouters H, Heyns L, Han SN, Tomek V, Mertens L, Ottevanger PB (2012) Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: an observational study. Lancet Oncol 13(3):256–264. 10.1016/S1470-2045(11)70363-1 [DOI] [PubMed] [Google Scholar]
- 42.Basaran D, Turgal M, Beksac K, Ozyuncu O, Aran O, Beksac MS (2014) Pregnancy-associated breast cancer: clinico-pathological characteristics of 20 cases with a focus on identifiable causes of diagnostic delay. Breast Care 9(5):355–359. 10.1159/000366436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toesca A, Gentilini O, Peccatori F, Azim HA Jr, Amant F (2014) Locoregional treatment of breast cancer during pregnancy. Gynecol Surg 11(4):279–284. 10.1007/s10397-014-0860-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61(4):212–236. 10.3322/caac.20121 [DOI] [PubMed] [Google Scholar]
- 45.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI (2010) Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 28(27):4120–4128. 10.1200/JCO.2009.25.9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI (2011) Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat 126(2):529–537. 10.1007/s10549-010-1132-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.American Society of Clinical O (2017) The state of cancer care in America, 2017: a report by the American Society of Clinical Oncology. J Oncol Pract 13(4):e353–e394. 10.1200/JOP.2016.020743 [DOI] [PubMed] [Google Scholar]
- 48.Azim HA Jr, Peccatori FA, Brohee S, Branstetter D, Loi S, Viale G, Piccart M, Dougall WC, Pruneri G, Sotiriou C (2015) RANK-ligand (RANKL) expression in young breast cancer patients and during pregnancy. Breast Cancer Res 17:24 10.1186/s13058-015-0538-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Brien J, Hansen K, Barkan D, Green J, Schedin P, O’Brien J, Hansen K, Barkan D, Green J, Schedin P (2011) Non-steroidal anti-inflammatory drugs target the pro-tumorigenic extracellular matrix of the postpartum mammary gland. Int J Dev Biol 55(7–9):745–755. 10.1387/ijdb.113379jo [DOI] [PubMed] [Google Scholar]


