Abstract
Purpose of review
Median survival after the diagnosis of brain metastases has historically been on the order of months. With the recent development of immune checkpoint inhibitors, intracranial activity and durable responses have been observed in brain metastases on multiple phase 2 clinical trials, which have primarily been conducted in patients with melanoma. Immune-related adverse events related to checkpoint inhibitor therapy of brain metastasis can present unique challenges for the clinician and underscore the need for a multidisciplinary team in the care of these patients. The goal of this review is to address the current knowledge, limitations of understanding, and future directions in research regarding immune therapy trials and neurologic toxicities based on retrospective, prospective, and case studies.
Recent findings
Immune therapy has the potential to exacerbate symptomatic edema and increase the risk of radiation necrosis in previously irradiated lesions. Neurologic toxicities will likely increase in prevalence as more patients with brain metastatic disease are eligible for immune therapy.
Summary
An improved understanding and heightened awareness of the unique neurologic toxicities that impact this patient group is vital for mitigating treatment-related morbidity and mortality.
Keywords: brain metastases, immunotherapy, melanoma, radiation necrosis, vasogenic edema
INTRODUCTION
As systemic therapies improve, patients are living longer, but intracranial late relapses are increasing, even among traditionally noncerebrotropic cancers. Local therapies like surgery or radiation are effective for single or oligometastases but do not address systemic disease, distant central nervous system (CNS) disease, minimize recurrence risk, or impact survival. Systemic chemotherapies for brain metastases have been limited due to concerns regarding CNS drug penetration and historical low efficacy.
Immune checkpoint inhibitors (CPIs) have revolutionized oncology by activation of host antitumor immune responses. CPIs are large mAbs theoretically incapable of crossing the blood–brain barrier (BBB). However, two main hypotheses explain intracranial CPI activity: first, antitumor T cell are primed and activated at extracerebral sites and home into the brain; and second, tumor neovessels are leaky, as indicated by postcontrast imaging enhancement, and drugs may penetrate through nonintact areas of the BBB to stimulate tumor infiltrating lymphocytes (TILs).
Food and Drug Administration-approved CPIs include the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor ipilimumab, programmed cell death protein 1 (PD-1) inhibitors nivolumab, pembrolizumab, and cemiplimab, and programmed death ligand-1 (PD-L1) inhibitors durvalumab, atezolizumab, and avelumab. CPIs are approved in multiple cancers, but initial studies excluded patients with untreated brain metastases (lesions that have not been irradiated) due to concerns of intracranial activity and exacerbation of neurologic inflammation and symptoms. In the last 5 years, early phase clinical trials began proactively enrolling these patients. This review focuses on the unique neurologic complications arising from CPI use in brain metastasis treatment and highlights critical areas needing further research.
Activity and toxicity of immune checkpoint inhibitors in brain metastases
Intracranial metastasis prognosis is worse than for extracranial disease, with a median overall survival (mOS) of 4–11 months [1,2]. Most CPI trials have required prior local brain metastasis treatment, at least 4 weeks of brain metastasis stability without new lesions, and exclude patients needing corticosteroids to control symptoms. As durable responses in extracranial disease emerged, we questioned whether CPIs were effective in untreated brain metastases.
Checkpoint inhibitor activity and toxicity
High-dose IL-2, approved in the 1990s, had significant toxicities and response rates of 5.6–14% in renal cell carcinoma (RCC) and melanoma brain metastases (MBMs) [3–5]. Expanded access ipilimumab in melanoma (NCT00495066) permitted enrollment of untreated, asymptomatic brain metastases [6]. Several prospective phase 2 trials have since evaluated CPIs for untreated brain metastases. Although most studies evaluated MBMs, a few trials include non-small cell lung cancer (NSCLC) (NCT02085070 and NCT02681549 [7,8■,9]) and RCC (NIVOREN GETUG AFU 26 [10,11]) brain metastases. Table 1 summarizes results of prospective CPI trials; Table 2 summarizes ongoing CPI trials for untreated brain metastases.
Table 1.
Completed prospective immune therapy clinical trials involving previously untreated brain metastases
| Study name or ClinicalTrials.gov identifier | Phase | Disease | Intervention | Neurologic symptoms | Steroid | Intracranial response (CR and PR) n (percentage) | Intracranial PFS (months) | Extracranial PFS (months) | Global PFS (months) | Median overall survival (months) | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Immunotherapy | |||||||||||
| CA184–042 NCT00623766 | 2 | Melanoma | Ipilimumab | Asymptomatic | Prohibited | 8 (16%) | 1.9 | 3.3 | 2.7 | 7 | Margolin et al. |
| Symptomatic | Allowed | 1 (5%) | 1.2 | 1.3 | 1.3 | 3.7 | |||||
| NIBIT-M1 NCT01654692 | 2 | Melanoma | Ipilimumab/Fotemustine | Asymptomatic | Prohibited | 8 (62%) | 3a | N.R. | 3.4a | 12.7a | Di Giacomo et al. |
| NCT02085070 | 2 | Melanoma and NSCLC | Pembrolizumab | Asymptomatic | Prohibited | 6 (26%) | 4 | 6 | 2 | 17 | Goldberg et al. and Kluger et al. |
| CheckMate-204 NCT02320058 | 2 | Melanoma | Ipilimumab/Nivolumab | Asymptomatic | Prohibited | 52 (55%) | 64.2% at 6 months; 59.5% at 9 months | 75.9% at 6 months; 70.4% at 9 months | 61.1% at 6 months; 56.6% at 9 months | 92.3% at 6 months; 82.8% at 9 months; 81.5% at 12 months | Tawbi et al. |
| ABC NCT02374242 | 2 | Melanoma | Ipilimumab/Nivolumab | Asymptomatic | Prohibited | 16 (46%) | N.R. | 13.8 | N.R. | Not Reached at median cutoff 14 months | Long et al. |
| Nivolumab | Asymptomatic | Prohibited | 5 (20%) | 2.5 | 2.6 | N.R. | 18.5 | ||||
| Nivolumab (after local therapy failure or symptoms) | 63% Symptomatic | Prohibited | 1 (6%) | 2.3 | 2.6 | N.R. | 5.1 | ||||
| Immunotherapy with radiation | |||||||||||
| NCT01703507 | 1 | Melanoma | Ipilimumab and WBRT | N.R. | N.R. | 5 Enrolled (no group-specific breakdown provided for response) | 2.53 | N.R. | 2.5 | 8 | Williams et al. |
| Ipilimumab and SRS | N.R. | N.R. | 11 Enrolled (no group-specific breakdown provided for response) | 2.45 | N.R. | 2.1 | Not Reached by median of 10.5 months | ||||
CR, complete response; N.R., not reported; NSCLC, non-small cell lung cancer; PFS, progression free survival; PR, partial response; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Includes patients with and without prior brain radiotherapy regardless of intracranial progression at enrollment. Data for the untreated brain metastasis cohort was not specifically reported.
Table 2.
Ongoing prospective immune therapy clinical trials involving previously untreated brain metastases
| Study name or ClinicalTrials.gov identifier | Phase | Disease | Intervention | Neurologic symptoms | Steroid | Enrollment study progress |
|---|---|---|---|---|---|---|
| Immunotherapy | ||||||
| NCT02681549 | 2 | Melanoma and NSCLC | Pembrolizumab/Bevacizumab | Asymptomatic | Prohibited | 53 anticipated |
| NIVOREN NCT03013335 | 2 | RCC | Nivolumab | Asymptomatic | Prohibited | 37 Enrolled with untreated BMs |
| CheckMate-204 NCT02320058 | 2 | Melanoma | Ipilimumab/Nivolumab | Symptomatic | Allowed | 20 Enrolled |
| BEAT-MBM NCT03175432 | 2 | Melanoma | Bevacizumab/Atezolizumab | Asymptomatic | Prohibited | 25 Anticipated |
| Mildly symptomatic or asymptomatic | Allowed (< 4 mg/day dex) | 15 Anticipated | ||||
| NCT03873818 | 2 | Melanoma | Ipilimumab/Pembrolizumab | Asymptomatic | Prohibited | 30 Anticipated |
| TRIDeNT NCT02910700 | 2 | Melanoma | Nivolumab/Dabrafenib/Trametinib (treated BMs) | Asymptomatic | N/A | 51 Anticipated |
| Nivolumab/Trametinib | Asymptomatic (prior PD-1) or symptomatic | Allowed (≤8 mg/day dex) | ||||
| NIBIT-M2 NCT02460068 | 3 | Melanoma | Fotemustine | Asymptomatic | Prohibited | 168 Anticipated |
| Fotemustine/Ipilimumab | Asymptomatic | |||||
| Ipilimumab/Nivolumab | Asymptomatic | |||||
| Immunotherapy with radiation | ||||||
| NCT02858869 | 1 | Melanoma and NSCLC | Pembrolizumab and 5 SRS fractions | Asymptomatic | Prohibited | 10 Anticipated |
| Pembrolizumab and 3 SRS fractions | Asymptomatic | Prohibited | 10 Anticipated | |||
| Pembrolizumab and 1 SRS fractions | Asymptomatic | Prohibited | 10 Anticipated | |||
| NCT02716948 | 1 | Melanoma | Nivolumb and SRS | N.E. | Prohibited | 90 Anticipated |
| NCT02696993 | 1/2 | NSCLC | Nivolumb and SRS | N.E. | Allowed (≤ 4 mg/day dex) | 22 anticipated |
| Nivolumb and WBRT | N.E. | Allowed (≤ 4 mg/day dex) | 22 anticipated | |||
| Ipilimumub/Nivolumb and SRS | N.E. | Allowed (≤4 mg/day dex) | 22 Anticipated | |||
| Ipilimumub/Nivolumb and WBRT | N.E. | Allowed (≤4 mg/day dex) | 22 Anticipated | |||
| GEM Study NCT02115139 | 2 | Melanoma | WBRT and Ipilimumab | Asymptomatic | Prohibited | 58 Anticipated |
| NCT02097732 | 2 | Melanoma | Ipilimumab induction prior to SRS | Asymptomatic | Prohibited | 3 Enrolled |
| SRS followed by Ipilimumab | Asymptomatic | Prohibited | 1 Enrolled | |||
| NCT02107755 | 2 | Melanoma | Ipilimumab & SRS | Asymptomatic | Prohibited | 8 Anticipated |
BM, brain metastasis; Dex, dexamethasone; N.E., not evaluated as a criterion for eligibility per available data on clinicaltrials.gov; N.R., not reported; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; RCC, renal cell carcinoma; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Intracranial complete or partial response to single agent CPI in patients not on steroids ranges from 16% with anti-CTLA-4, 26% with anti-PD-1 [8■,12], and 55% with combination anti-CTLA-4 and anti-PD-1 [13■■,14■■] in MBMs. Intracranial responses were 13.3% with anti-PD-1 in RCC based on preliminary data [11]. Neurologic adverse events increased from 24% with single agent anti-PD-1 to 36% with combined anti-CTLA-4 and anti-PD-1 therapy [13■■,14■■]. Table 3 summarizes neurologic adverse event data from CPI trials for untreated brain metastases.
Table 3.
Neurologic adverse effects reported on clinical trials involving previously untreated brain metastases
| Study Name or ClinicalTrials.gov identifier | Phase | Disease | Intervention | Neurologic symptoms | Steroid | Neurologic AEs (any grade) | Neurologic SAEs (grades 3–4) | Headache | Dizziness | Confusion | Seizure | Ataxia | Edema | Hemorrhage | RN | Citation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immunotherapy | ||||||||||||||||
| CA184–042 NCT00623766 | 2 | Melanoma | Ipilimumab | Asymptomatic | Prohibited | N.R. | N.R. | 18 (35%) | 11 (22%) | 9 (18%) | N.R. | N.R. | 3 (6%) | N.R. | N.R. | Margolin et al. |
| Symptomatic | Allowed | N.R. | N.R. | 6 (29%) | 2 (10%) | 3 (14%) | N.R. | N.R. | N.R. | N.R. | N.R. | |||||
| NIBIT-M1 NCT01654692 | 2 | Melanoma | Ipilimumab/Fotemustine | Asymptomatic | Prohibited | 5 (25%) | 2 (10%) | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | Di Giacomo et al. |
| NCT02085070 | 2 | Melanoma and NSCLC | Pembrolizumab | Asymptomatic | Prohibited | N.R. | 1 (4%) | 4 (17%) | 2 (9%) | 3 (13%) | 3 (13%) | 5 (22%) | 4 (17%) | 1 (4%) | 7 (30.4%) | Goldberg et al. and Kluger et al. |
| CheckMate-204 NCT02320058 | 2 | Melanoma | Ipilimumab/Nivolumab | Asymptomatic | Prohibited | 34 (36%) | 7 (7%) | 21 (22%) | 1 (1%) | N.R. | 2 (2%) | N.R. | 2 (2%) | 2 (2%) | N.R. | Tawbi et al. |
| ABC NCT02374242 | 2 | Melanoma | Ipilimumab/Nivolumab | Asymptomatic | Prohibited | 11 (31%) | 2 (6%) | 4 (11%) | 1 (3%) | N.R. | 1 (3%) | N.R. | N.R. | N.R. | 0 (0%) | Long et al. |
| Nivolumab | Asymptomatic | Prohibited | 6 (24%) | 0 (0%) | 5 (20%) | 1 (4%) | N.R. | 0 (0%) | N.R. | N.R. | N.R. | 0 (0%) | ||||
| Nivolumab (after local therapy failure or symptoms) | Symptomatic | Prohibited | 2 (13%) | 2 (13%) | 1 (6%) | 0 (0%) | N.R. | 0 (0%) | N.R. | N.R. | N.R. | 1 (6%) | ||||
| Immunotherapy with radiation | ||||||||||||||||
| NCT01703507 | 1 | Melanoma | Ipilimumab and WBRT | N.R. | N.R. | N.R. | 0 (0%) | 2 (40%) | 1 (20%) | N.R. | 0 (0%) | N.R. | 0 (0%) | 0 (0%) | 0 (0%) | Williams et al. |
| Ipilimumab and SRS | N.R. | N.R. | N.R. | 0 (0%) | 5 (45%) | 0 (0%) | N.R. | 1 (9%) | N.R. | 0 (0%) | 4 (36%) | 0 (0%) | ||||
AE, adverse event; N.R., not reported; NSCLC, non-small cell lung cancer; RN, radiation necrosis; SRS, stereotactic radiosurgery; WBRT, whole brain radiotherapy.
Although most trials require patients be asymptomatic from their untreated brain metastases, a handful of studies, NCT00623766 (CA184–042) [12], NCT02320058 (CheckMate-204) [13■■], NCT02374242 (ABC) [14■■], NCT03175432 (BEAT-MBM) [15], and NCT03563729 (MEMBRAINS) [16], allow smaller, symptomatic cohorts and corticosteroid use. Concurrent corticosteroids and CPIs have worse outcomes, either reflecting more aggressive disease or a dampened immune response. mOS for asymptomatic, CPI-treated patients ranges from 7 to 18.5 months; survival decreases for symptomatic patients to 3.7–5.1 months [12,14■■].
One concern with immune therapy is the prolonged time to initial response. In CheckMate-204, the median time to intracranial response was 2.3 months [13■■]. The risk of rapid disease progression in the brain for the 43–75% of patients unresponsive to CPIs is a critical concern. Furthermore, possible pseudoprogression makes early response assessments difficult [17].
Combination checkpoint inhibitor and radiation therapy activity
Multimodality therapy through combination CPI and stereotactic radiosurgery (SRS) in MBMs results in synergistic responses with decreased distant intracranial failure compared with SRS alone or SRS and targeted therapy [18]. Two retrospective series of combination SRS and ipilimumab demonstrated improved mOS compared with SRS alone (15–21.3 versus 4.9–6 months, n = 110), which was independent of the administration order (P = 0.58) [19–21], provided both were given within 4 weeks [22]. Responses were higher with combination SRS and anti-PD-1 than with anti-CTLA-4 [22]. Intracranial control rates (defined as complete, partial, or stable responses) were improved with combination CPI and SRS compared with SRS alone at 1 year (60 versus 11.5%); this was highest with combined anti-PD-1, anti-CTLA-4, and SRS [18]. A retrospective series in NSCLC failed to show improved OS with combination SRS and anti-PD-1 compared with chemotherapy, suggesting that survival improvements may not be universal across tumor types, but lesions more than 500 mm3 regressed faster, demonstrating that multimodal treatment remains best if fast responses are needed [23■].
Prospective trials are now evaluating the benefit of adding radiation to CPIs. A phase 1 trial of MBMs treated with ipilimumab and either whole brain radiotherapy (WBRT) or SRS showed intracranial progression free survival was similar at 2.53 and 2.45 months, respectively, but mOS was only 8 months with WBRT versus more than 10.5 months with SRS [24]. The optimal administration sequence of ipilimumab and SRS for MBMs is being investigated by NCT02097732 [25]. The GEM Study (NCT02115139) and NCT02107755 are, respectively, evaluating the effects of ipilimumab combined with WBRT or SRS [26,27]. Studies of nivolumab with SRS or WBRT along with combination ipilimumab and nivolumab with either SRS or WBRT are also ongoing, Table 2 [28,29].
Several studies have shown that intracranial and extracranial disease responses to CPIs were largely concordant in MBMs [7,8■,13■■]. In a retrospective series, MBM patients treated with ipilimumab and SRS had similar OS to ipilimumab-treated patients without brain metastases [30], suggesting that brain metastasis prognosis is improving. However, multimodality therapy increases risks for neurologic toxicity. As durability of responses improve, there is heightened concern regarding WBRT-induced cognitive dysfunction. SRS is the preferred method for definitive treatment of fewer brain metastases, but radiation necrosis is increasing with combined therapy [31].
Complications of immune therapy in treatment of brain metastases
CPI-related neurotoxicity reporting is variable, as many common immune-related adverse events are often not mentioned, and available data are mainly from MBM trials. Thus, evaluating the true clinical impact of neurologic adverse events is difficult. Complications can be classified as due to an excessive tumor-associated inflammatory response, autoimmune, or paraneoplastic.
Immune-related neurologic sequelae in checkpoint inhibitor-treated brain metastasis patients
An excessive inflammatory response can cause symptoms due to mass effect from vasogenic edema, radiation necrosis, or pseudoprogression. Symptoms depend on the brain area impacted. Seizures were the initial symptom in 40% of MBM patients [32] but may also be aggravated by CPIs, resulting in prophylactic antiepileptic drug use in some trials [7].
Symptomatic edema has been variably reported, with incidence ranging from 2% in CheckMate-204 [13■■] to 36% with combined ipilimumab and SRS (NCT01703507) [24] (Table 3). Baseline edema volume does not impact anti-PD-1 response in melanoma and NSCLC patients [33]. However, symptomatic edema often necessitates CPI interruption, high-dose corticosteroids, and additional local therapy with surgery or radiation. One retrospective study found 9.1% of brain metastasis patients required corticosteroids after diagnosis; response to steroids was associated with improved prognosis (4.3 versus 1.6 months when steroid unresponsive) [32]. Dexamethasone, the preferred corticosteroid due to BBB penetration and relative lack of mineralocorticoid activity, provides a cost-effective and rapid means of decreasing edema and/or dampening the CPI-stimulated immune response. Corticosteroids should not be used for imaging changes alone, at the lowest possible dose to achieve symptomatic relief, and tapered as quickly as possible to allow for subsequent therapy and to avoid adverse effects from prolonged use. Corticosteroid-sparing strategies include targeting vascular endothelial growth factor (VEGF) with bevacizumab, which has been used to treat glioma-associated edema. A small case series retrospectively evaluated 12 bevacizumab-treated MBM patients and showed bevacizumab allowed rapid steroid tapering and permitted faster CPI resumption [34]. However, bevacizumab side effects can include intracranial hemorrhage, hypertension, gastrointestinal bleeding, and delayed wound healing. There is a critical need for alternative steroid-sparing, antiedema agents.
Combination SRS and CPIs have synergistic effects presumably due to increased T-cell priming from radiation-induced tumor cell death and antigen release. However, radiation necrosis is a growing problem arising from multimodality therapy. Radiation necrosis is difficult to distinguish from tumor recurrence radiographically and often requires definitive biopsy or longitudinal imaging, as cases with growth followed by spontaneous regression are believed to be radiation necrosis. Affected areas manifest radiation-induced changes including necrosis, hyalinized vessels, and an immune infiltrate. A retrospective study of 115 CPI-treated and SRS-treated patients demonstrated increased symptomatic radiation necrosis [hazard ratio (HR) 2.56, 95% confidence interval (CI) 1.35–4.86], particularly in MBMs (HR 4.02, 95% CI 1.17–13.82) [35]. Other studies cite an incidence of 7–29% [21,36,37] with a mean time to development from SRS of 11.2–14.9 months [37,38]. Timing and sequence of SRS and CPI did not impact symptomatic radiation necrosis [39]. Higher radiation necrosis rates reported in clinical trials could be due to close radiographic monitoring and inclusion of asymptomatic cases. Other radiation necrosis risk factors include radiation dose and treated lesion size. Symptomatic radiation necrosis is treated with corticosteroids or surgery. Bevacizumab has been used based on anecdotal evidence in gliomas as a steroid-sparing alternative or for those who failed conservative management [40]. Small case series demonstrated hyperbaric oxygen can decrease steroid dependence [41]. Figure 1a and b includes representative examples of vasogenic edema and radiation necrosis.
FIGURE 1.
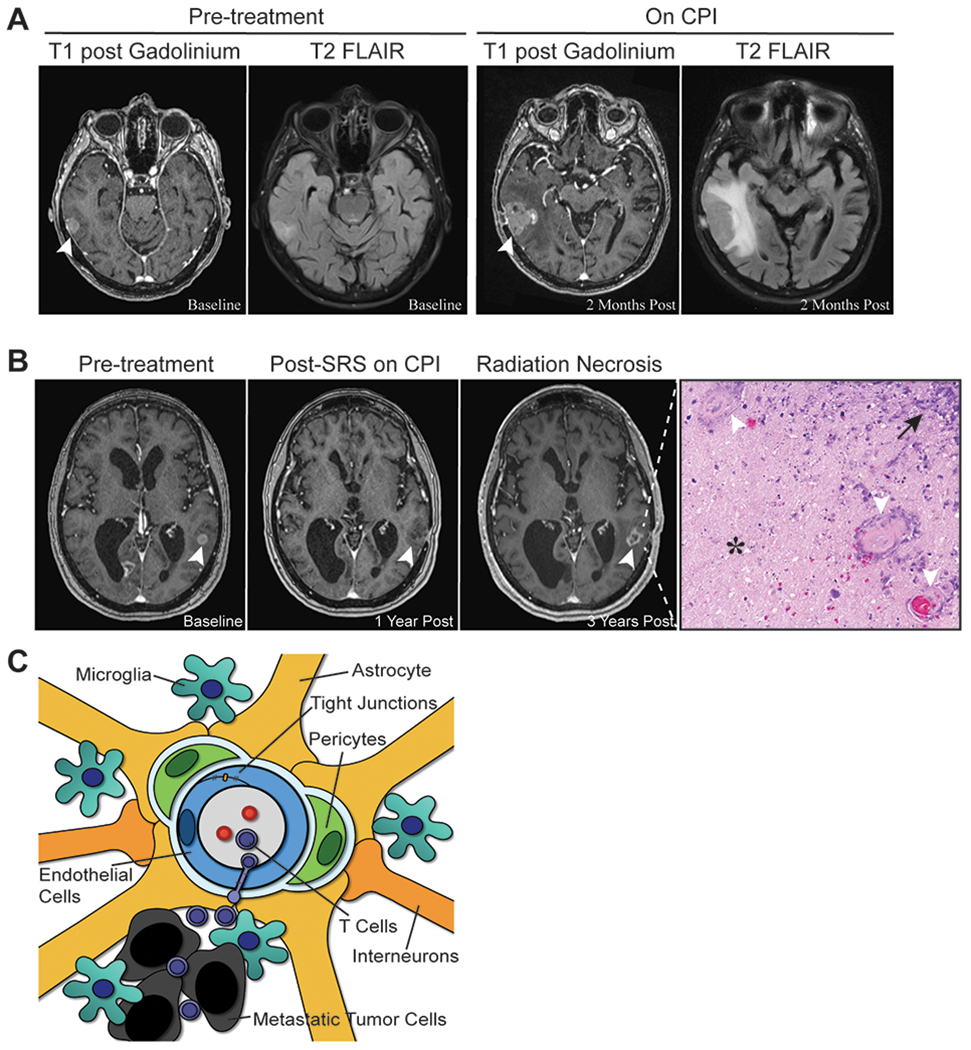
Brain metastasis-associated vasogenic edema and radiation necrosis. (a) Contrast enhanced (left) and flair (right) images depicting a lesion associated with vasogenic edema before and after initiation of checkpoint inhibitor therapy. (b) Lesion treated with stereotactic radiosurgery before (left), poststereotactic radiosurgery after initiation of pembrolizumab (middle), and after development of radiation necrosis while on pembrolizumab (right). Corresponding pathology of the growing, enhancing lesion demonstrated radiation necrosis by hematoxylin and eosin staining, with characteristic areas of paucicellular treatment-related necrosis (*), vessel hyalinization (white arrows), and immune infiltration (black arrow). (c) The blood–brain barrier in metastatic disease. The blood–brain barrier is comprised of specialized interendothelial tight junctions that limit macromolecule transport into the brain parenchyma and unique neurovascular supporting cells that play structural and immunological roles. Among these cells, pericytes, astrocytes, microglia, and interneurons contribute to maintenance of tight junctions. Cytokines and chemokines secreted by the tumor or immune cells contribute to tight junction disruption, vessel leakiness, and edema.
Pseudoprogression involves a transient enlargement of existing lesions or appearance of new lesions mimicking tumor progression, which resolves spontaneously on serial imaging. Anti-PD-1-induced pseudoprogression occurs in 7% of melanoma cases [42■]. Pseudoprogression is attributed to inflammation, including macrophages and activated microglia, reactive astrocytes, and hemorrhage [17].
Central nervous system autoimmunity in checkpoint inhibitor-treated brain metastasis patients
CNS autoimmune toxicities due to CPIs are rare but include encephalitis, aseptic meningitis, multiple sclerosis, and myasthenia gravis. These may result from an underlying autoimmune disease or occur de novo, but the incidence does not appear to be higher in patients without brain metastasis and is overall rare. No prospective CPI studies for patients with known autoimmune disease and untreated brain metastases exist.
Paraneoplastic syndromes in checkpoint inhibitor-treated brain metastasis patients
Paraneoplastic syndromes, such as cerebellar degeneration, limbic encephalitis, and encephalomyelitis, result from cross-reactivity of the antitumoral response with off-target, cancer cell-secreted proteins. There have been cases of melanoma-associated retinopathy and chronic inflammatory demyelination polyneuropathy as well. In preclinical models, anti-CTLA-4-induced paraneoplastic cerebellar degeneration can occur [43]. Case reports of limbic encephalitis [44] or cerebellar ataxia [45] resulting from anti-PD-1 therapy exists in patients without brain metastases. The incidence of paraneoplastic symptoms is not increasing due to CPIs; there are no reports of neurologic paraneoplastic syndromes in brain metastasis patients on CPI therapy [46].
Paucity of biomarkers in identifying those at risk of neurologic toxicity
No definitive biomarkers exist to identify those at risk for neurologic toxicity. The relationship between PD-L1 or TILs in brain metastases and neurologic sequelae, edema or radiation necrosis, is unknown. Biomarker development and advanced imaging techniques could potentially mitigate the need for invasive diagnostic procedures and is an area of critical unmet need.
Cellular and molecular mechanisms of edema
Unlike extracranial tumor microenvironments, the brain has specialized cells that regulate BBB permeability, Fig. 1c. The brain was once thought to be immune-privileged, as the unique BBB tightly regulated passage of molecules and cells into the brain parenchyma. CPIs are believed to activate T cells, priming them for targeting extracranial and intracranial disease. In brain metastases, cytokines released by tumor cells or the microenvironment promote brain T-cell homing. Robust brain immune responses are linked with improved survival [47].
The BBB is defined by specialized interendothelial tight junctions [48–50]. A complex neurovascular unit maintains BBB tight junction integrity and is comprised of endothelial cells, basement membrane, pericytes, astrocytes, microglia, and interneurons [51]. Dysfunction or loss of any of these cells have been shown to cause edema, wherein fluid and intravascular proteins extravasate into the cerebral parenchyma. Tumor or immune cell-secreted cytokines and chemokines, such as VEGF, basic fibroblast growth factor, and leukotrienes are implicated in increased glioma BBB permeability [52,53]. Less is known regarding BBB permeability factors in brain metastases.
It is unclear what role resident microglia or monocyte-derived macrophages play in edema or radiation necrosis, as traditional immunohistochemical markers are unable to distinguish these populations. Is it also unclear what role tumor-secreted factors used during tumor extravasation play in edema [54■]. A better understanding of the brain tumor microenvironment and how it responds to metastatic disease would potentially provide novel targets to treat neurologic toxicities.
Cellular and molecular mechanisms of radiation necrosis
SRS is an effective local therapy for treating brain metastases which spares surrounding benign brain tissue, thus limiting long-term cognitive sequelae commonly seen with WBRT. SRS local control rates range from 50.5 to 84% at 1 year [55,56]. However, in a large series of 271 SRS-treated brain metastases, the incidence of radiation necrosis was 34% at 24 months, with a median time to development of 10.8 months, and greater risk with lesions more than 1 cm [57]. Little is known regarding the pathogenesis of radiation necrosis, but it has been hypothesized to be caused by radiation-induced vascular damage leading to ischemia and subsequent necrosis and glial loss, a result of oligodendroglia damage, or autoimmune against glial antigens and other cell components released during radiation injury [58].
CPI likely exacerbates ongoing CNS inflammation at prior radiation sites and contributes to increased radiation necrosis with multimodality treatment. Our current understanding of radiation necrosis is based on late stages of inflammation. Developing an animal model to study the early steps in radiation necrosis formation is an area of critical interest, as it would permit evaluation of early inflammatory responses and provide pharmacologic targets to negate this late neurologic complication.
Metabolic profiling found increased metabolism markers in tumors, whereas radiation necrosis samples had elevations in fatty acid products and antioxidants [59]. There is a critical need to develop accurate, noninvasive imaging technology to differentiate tumor recurrence and radiation necrosis, as they can appear similar on MRI. Several ongoing trials are evaluating novel PET tracers, using differences in tumor metabolic activity or radiation necrosis-associated inflammation to distinguish the two pathologies.
CONCLUSION
Cancer patients are living longer due to better systemic treatments, but the CNS can be a common site of tumor recurrence. Patients who present with brain metastases at the time of stage IV disease diagnosis are living longer due to CPIs. Awareness and optimal treatment of CPI-induced neurologic symptoms is an emerging priority.
KEY POINTS.
The incidence of neurologic events in brain metastasis patients treated with checkpoint inhibitors (CPIs) varies by study; these adverse events require uniform reporting and should encompass all adverse event grades. Based on available data, the incidence of neurologic adverse events does not appear higher in brain metastasis patients treated with CPIs than in patients without brain metastases.
Vasogenic edema and inflammation can worsen symptoms and might affect our ability to determine early radiographic response.
Corticosteroids, a standard treatment for vasogenic edema and inflammation, likely impede antitumor immune responses and are associated with numerous toxicities. Alternative methods for controlling edema are needed that are not immune-suppressive. Combination vascular endothelial growth factor and antiprogrammed cell death protein 1 inhibitors are the subject of an ongoing trial at our institution (NCT02681549).
Radiation necrosis incidence is higher in patients treated with multimodality therapy (CPIs and stereotactic radiosurgery) compared with patients who do not receive CPIs. The mechanism of radiation necrosis is unknown and requires further research, as mediators may be pharmacologically targetable.
Biomarkers and improved imaging modalities are needed to differentiate CPI intracranial failure from radiation necrosis.
Acknowledgements
Financial support and sponsorship
The current study was funded in part by National Institute of Health grants K24CA172123, R01 CA227473, and P50 CA121974 (H.M.K., PI), National Cancer Institute grant R01CA204002 (L.J., PI), and the Research Scholar Grant (130157-RSG-16-216-01-TBG) from the American Cancer Society (L.J., PI).
Conflicts of interest
H.M.K. reports grant support from Merck, Bristol-Myers Squibb, and Apexigen along with prior personal fees from Regeneron, Alexion, Prometheus, Corvus, Nektar, Biodesix, Roche-Genentech, Pfizer, Iovance, Immunocore, Celldex, and Array Biopharma. A.O. participates in ad hoc advisory boards for Bristol-Myers Squibb and Merck. All other authors claim no relevant conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011; 117:1687–1696. [DOI] [PubMed] [Google Scholar]
- 2.D’Andrea G, Palombi L, Minniti G, et al. Brain metastases: surgical treatment and overall survival. World Neurosurg 2017; 97:169–177. [DOI] [PubMed] [Google Scholar]
- 3.Guirguis LM, Yang JC, White DE, et al. Safety and efficacy of high-dose interleukin-2 therapy in patients with brain metastases. J Immunother 2002; 25:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu MB, Fesler MJ, Armbrecht ES, et al. High-dose interleukin-2 (HD IL-2) therapy should be considered for treatment of patients with melanoma brain metastases. Chemother Res Pract 2013; 2013:726925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell S, Dudek AZ. Single-institution outcome of high-dose interleukin-2 (HD IL-2) therapy for metastatic melanoma and analysis of favorable response in brain metastases. Anticancer Res 2009; 29:4189–4193. [PubMed] [Google Scholar]
- 6.Compassionate use trial for unresectable melanoma with ipilimumab. (June 3, 2013). Retrieved April 18, 2019, from https://ClinicalTrials.gov/show/NCT00495066.
- 7.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or nonsmall-cell lung cancer and untreated brain metastases: early analysis of a nonrandomised, open-label, phase 2 trial. Lancet Oncol 2016; 17:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.■.Kluger HM, Chiang V, Mahajan A, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol 2019; 37:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study demonstrating the activity and safety of pembrolizumab in untreated melanoma brain metastses. Pembrolizumab was well tolerated with an intracranial response rate of 26%.
- 9.Pembrolizumab plus bevacizumab for treatment of brain metastases in metastatic melanoma or non-small cell lung cancer. (March 13, 2019). Retrieved April 20, 2019, from https://ClinicalTrials.gov/show/NCT02681549.
- 10.Albiges L, Negrier S, Dalban C, et al. Safety and efficacy of nivolumab in metastatic renal cell carcinoma (mRCC): final analysis from the NIVOREN GETUG AFU 26 study. J Clin Oncol 2019; 37:542–1542. [Google Scholar]
- 11.Flippot R, Dalban C, Laguerre B, et al. Brain metastases response to nivolumab in patients with renal cell carcinoma (RCC): prospective analysis from the GETUG-AFU 26 (NIVOREN) trial. Ann Oncol 2018; 29:viii303–viii331. [Google Scholar]
- 12.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 2012; 13:459–465. [DOI] [PubMed] [Google Scholar]
- 13.■■.Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 2018; 379:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]; The trial was one of two demonstrating the effectiveness and tolerability of combined ipilimumab and nivolumab for untreated melanoma brain metastases. It reported the highest intracranial response rate to date at 55%.
- 14.■■.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomized phase 2 study. Lancet Oncol 2018; 19:672–681. [DOI] [PubMed] [Google Scholar]; This was one of two demonstrating the effectiveness and tolerability of combined ipilimumab and nivolumab for untreated melanoma brain metastases. The study included patients with asymptomatic as well as symptomatic brain metastases. Symptomatic brain metastases are historically excluded; best treatment options for this group of patients is unknown.
- 15.Study of BEvacizumab in combination with ATezolizumab in patients with untreated melanoma brain metastases. (April 19, 2018). Retrieved April 20, 2019, from https://ClinicalTrials.gov/show/NCT03175432.
- 16.Melanoma metastasized to the brain and steroids. (June 20, 2018). Retrieved April 18, 2019, from https://ClinicalTrials.gov/show/NCT03563729.
- 17.Cohen JV, Alomari AK, Vortmeyer AO, et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res 2016; 4:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acharya S, Mahmood M, Mullen D, et al. Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol 2017; 2:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knisely JP, Yu JB, Flanigan J, et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012; 117:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silk AW, Bassetti MF, West BT, et al. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med 2013; 2:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaidar-Person O, Zagar TM, Deal A, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anticancer Drugs 2017; 28:669–675. [DOI] [PubMed] [Google Scholar]
- 22.Qian JM,Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016; 122:3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.■.Singh C, Qian JM, Yu JB, Chiang VL. Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in nonsmall cell lung cancer with brain metastases. J Neurosurg 2019; 1–6. [DOI] [PubMed] [Google Scholar]; This was a retrospective study evaluating the synergistic effect of combined stereotactic radiosurgery and immunotherapy. They demonstrated that NSCLC brain metastases did not gain as much benefit for combined treatment compared with melanoma brain metastases.
- 24.Williams NL, Wuthrick EJ, Kim H, et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys 2017; 99:22–30. [DOI] [PubMed] [Google Scholar]
- 25.Ipilimumab induction in patients with melanoma brain metastases receiving stereotactic radiosurgery. (February 27, 2019). Retrieved April 22, 2019, from https://ClinicalTrials.gov/show/NCT02097732.
- 26.GEM STUDY: radiation and yervoy in patients with melanoma and brain metastases. (November 27, 2018). Retrieved April 22, 2019, from https://ClinicalTrials.gov/show/NCT02115139.
- 27.Stereotactic radiation therapy and ipilimumab in treating patients with metastatic melanoma. (December 21, 2018). Retrieved April 22, 2019, from https://ClinicalTrials.gov/show/NCT02107755.
- 28.SRS and nivolumab in treating patients with newly diagnosed melanoma metastases in the brain or spine. (January 25, 2019). Retrieved April 22, 2019, from https://ClinicalTrials.gov/show/NCT02716948.
- 29.Nivolumab and radiation therapy with or without ipilimumab in treating patients with brain metastases from non-small cell lung cancer. (December 4, 2018). Retrieved April 22, 2019, from https://ClinicalTrials.gov/show/NCT02696993.
- 30.Tazi K, Hathaway A, Chiuzan C, Shirai K. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med 2015; 4:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colaco RJ, Martin P, Kluger HM, et al. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2016; 125:17–23. [DOI] [PubMed] [Google Scholar]
- 32.Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999; 43:795–803. [DOI] [PubMed] [Google Scholar]
- 33.Tran TT, Mahajan A, Chiang VL, et al. Perilesional edema in brain metastases: potential causes and implications for treatment with immune therapy. J Immunother Cancer 2019; 7:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banks PD, Lasocki A, Lau PKH, et al. Bevacizumab as asteroid-sparing agent during immunotherapy for melanoma brain metastases: A case series. Health Sci Rep 2019; 2:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol 2018; 4:1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weingarten N, Kruser TJ, Bloch O. Symptomatic radiation necrosis in brain metastasis patients treated with stereotactic radiosurgery and immunotherapy. Clin Neurol Neurosurg 2019; 179:14–18. [DOI] [PubMed] [Google Scholar]
- 37.Skrepnik T, Goldbaum D, Suszko JW, et al. Does immunotherapy influence the risk of developing radiation necrosis after radiosurgery of brain metastases? Int J Radiat Oncol Biol Phys 2017; 99:S160. [Google Scholar]
- 38.Du Four S, Janssen Y, Michotte A, et al. Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med 2018; 7:4870–4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diao K, Bian SX, Routman DM, et al. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neurooncol 2018; 139:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 2011; 79:1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel U, Patel A, Cobb C, et al. The management of brain necrosis as a result of SRS treatment for intra-cranial tumors. Transl Cancer Res 2014; 3:373–382. [Google Scholar]
- 42.■.Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol 2018; 4:717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]; The exploratory study evaluated the potential use of BRAF and NRAS mutations in circulating tumor DNA to distinguish between true disease progression and pseudoprogression due to immune therapy. It demonstrates a novel and minimally invasive technique to help differentiate the two disparate pathologies.
- 43.Yshii LM, Gebauer Cm , Pignolet B, et al. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain 2016; 139: 2923–2934. [DOI] [PubMed] [Google Scholar]
- 44.Salam S, Lavin T, Turan A. Limbic encephalitis following immunotherapy against metastatic malignant melanoma. BMJ Case Rep 2016; 2016:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valpione S, Zoccarato M, Parrozzani R, et al. Paraneoplastic cerebellar degeneration with anti-Yo antibodies associated with metastatic uveal melanoma. J Neurol Sci 2013; 335:210–212. [DOI] [PubMed] [Google Scholar]
- 46.Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS disease caused by immune checkpoint inhibitors: status and perspectives. Nat Rev Neurol 2017; 13:755–763. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton R, Krauze M, Romkes M, et al. Pathologic and gene expression features of metastatic melanomas to the brain. Cancer 2013; 119: 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 2006; 7:41–53. [DOI] [PubMed] [Google Scholar]
- 49.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 2011; 21:193–215. [DOI] [PubMed] [Google Scholar]
- 50.Lyle LT, Lockman PR, Adkins CE, et al. Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin Cancer Res 2016; 22:5287–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol 2012; 33:579–589. [DOI] [PubMed] [Google Scholar]
- 52.Black KL, Hoff JT, McGillicuddy JE, Gebarski SS. Increased leukotriene C4 and vasogenic edema surrounding brain tumors in humans. Ann Neurol 1986; 19:592–595. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi JA, Fukumoto M, Igarashi K, et al. Correlation of basic fibroblast growth factor expression levels with the degree of malignancy and vascularity in human gliomas. J Neurosurg 1992; 76:792–798. [DOI] [PubMed] [Google Scholar]
- 54.■.Glitza Oliva IC, Schvartsman G, Tawbi H. Advances in the systemic treatment of melanoma brain metastases. Ann Oncol 2018; 29:1509–1520. [DOI] [PubMed] [Google Scholar]; Well written review which includes successes of targeted therapy options in the treatment of melanoma brain metastases, along with opinions on the interactin of the blood–brain barrier in neurotropic disease dissemination.
- 55.Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget 2016; 7:12318–12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lwu S, Goetz P, Monsalves E, et al. Stereotactic radiosurgery for the treatment of melanoma and renal cell carcinoma brain metastases. Oncol Rep 2013; 29:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohutek ZA, Yamada Y, Chan TA, et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 2015; 125:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Statham P, Macpherson P, Johnston R, et al. Cerebral radiation necrosis complicating stereotactic radiosurgery for arteriovenous malformation. J Neurol Neurosurg Psychiatry 1990; 53:476–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu AY, Turban JL, Damisah EC, et al. Novel biomarker identification using metabolomic profiling to differentiate radiation necrosis and recurrent tumor following Gamma Knife radiosurgery. J Neurosurg 2017; 127:388–396. [DOI] [PubMed] [Google Scholar]


