Abstract
Proof-of-principle for large-scale engineering of edible muscle tissue, in vitro, was established with the product’s introduction in 2013. Subsequent research and commentary on the potential for cell-based meat to be a viable food option and potential alternative to conventional meat have been significant. While some of this has focused on the biology and engineering required to optimize the manufacturing process, a majority of debate has focused on cultural, environmental, and regulatory considerations. Animal scientists and others with expertise in muscle and cell biology, physiology, and meat science have contributed to the knowledge base that has made cell-based meat possible and will continue to have a role in the future of the new product. Importantly, the successful introduction of cell-based meat that looks and tastes like conventional meat at a comparable price has the potential to displace and/or complement conventional meat in the marketplace.
Keywords: cell-based meat, cultured meat, cultured protein, lab-grown meat
Introduction
Meat has long served human cultures as a dense source of essential nutrients and will continue to do so in the future. To date, the acquisition of meat has required the killing of an animal, whether obtained through hunting or husbandry efforts. The concept of producing meat from cell culture systems dates back to the 1930s when Winston Churchill predicted: “Fifty years hence we shall escape the absurdity of growing a whole chicken in order to eat the breast or wing by growing these parts separately under a suitable medium” (Churchill, 1932). The recent evolution of cell culture techniques that facilitate the growth of edible animal tissue in vitro represents an example of potential disruptive technology (van der Weele et al., 2019) with many interesting aspects to consider (Stephens et al., 2018, 2019).
The application of scientific principles and prior discoveries to advance research and answer questions of interest has long been a focus of the Journal of Animal Science. Biological science provides a means to address possibilities and demonstrate the fundamental potential to realize a goal. Social science research demonstrates the probabilities to which scientific potential is likely to be adopted; it considers values as much as facts of nature. In her best-selling book, Gulp, Roach (2013) captured this critical concept when she noted her initial hesitancy to taste Inuit-prepared muktuk by writing, “…to a far greater extent than most of us realize, culture writes the menu. And culture doesn’t take kindly to substitutions.” Neophobia associated with foods is an obstacle associated with the negative connotations of new or nontraditional foods and is not uncommon given that many consumers tend to be conventional with their food purchases. A number of meat alternatives have been developed and continue to emerge in the marketplace (van der Weele et al., 2019; Kuhn, 2020); their acceptance depends on various cultural considerations and this will be especially true for cell-based meat. Our goal was to provide a holistic assessment of cell-based meat as food and highlight the challenges and opportunities associated with this novel food product.
Cell-Based Meat
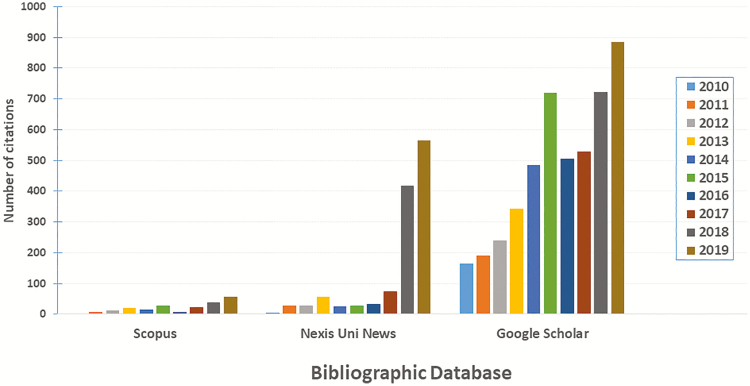
Cell-based meat has been identified by many other names, including, artificial meat, cell-cultured meat, cellular meat, clean meat, cultured meat, engineered meat, factory-grown meat, fake meat, in vitro meat, lab-grown meat, laboratory-grown meat, and synthetic meat. Regardless of the identifier used, recent interest in the topic has been significant. The number of articles published in the last 10 years that have focused on this topic has grown dramatically with 885 recorded for 2019 in the Google Scholar bibliographic database (Figure 1). For the purposes of this review, we have chosen to adopt the term “cell-based meat”; this moniker was endorsed by both the North American Meat Institute and Memphis Meats, a producer of an animal-free product and universally accepted as official terminology among several companies involved with its production (Watson, 2018). The latter recently formed the Alliance for Meat, Poultry and Seafood Innovation, as a single point of contact for regulatory and other discussions (Bottemiller Evich, 2019).
Figure 1.
Cell-based meat in published articles. Citations included one or more of the following phrases in their titles: cell-based meat, cellular meat, clean meat, cultured meat, engineered meat, artificial meat, in vitro meat, lab-grown meat, laboratory-grown meat, cell-cultured meat, and synthetic meat.
Cell-based meat, at its simplest, is muscle grown without the involvement of an animal and its physiological processes. Unlike plant-based analogs or other meat alternatives (van der Weele et al., 2019), it is derived from muscle cells and much more closely approximates postmortem skeletal muscle from livestock species (i.e., mammals, birds, fish historically raised for their edible tissues; Figure 2). To be clear, cultured muscle tissue is not technically meat (Hocquette, 2016) as the latter is also a product of postmortem biochemistry. Thorrez and Vandenburgh (2019) have highlighted several challenges that remain with the functional engineering of meat including whether or not cell-based meat can provide essential minerals, creatine, carnosine, and B and D vitamins to the same extent as conventional meat.
Figure 2.
General approach for the production of cell-based meat.
One of the earliest U.S. patents related to the tissue engineering of meat was awarded to Jon Vein in 2004 (Vein, 2004). However, it was not until August 2013 that Dutch researchers, led by Dr Mark Post, established proof-of-principle for the manufacture of cell-based meat when they presented the first cell-cultured hamburger to the public (Fountain, 2013). It was produced at a cost of US$325,000 $325,000 and required 2 years to produce. There are now more than 34 companies worldwide involved with efforts to produce cell-based meat (Cell Based Tech, 2020), and their goal is to produce this food at a cost and scale sufficient to compete with conventional meat.
Specific processes/inputs associated with current cell-based meat efforts are largely proprietary. The general cell culture and related needs for growing a meat-like product have been reviewed with different emphases considered (e.g., Edelman et al., 2005; Post, 2012; Kadim et al., 2015; Mattick et al., 2015; Sajid Arshad et al., 2017; Bodiou et al., 2020; Boler et al., 2020). Ben-Arye and Levenberg (2019) recently published a highly detailed and extensively referenced summary of the issues critical to tissue engineering of cell-based meat.
The starting materials for producing cell-based meats are myoblasts (satellite cells), which are difficult to proliferate in vitro, but easily differentiate into myotubes (immature muscle cells) and myofibrils under the appropriate conditions. To facilitate replication of skeletal muscle satellite cells in vitro, cells are attached to an immobile substratum such as a scaffold or microbead that may be coated with protein (e.g., laminin, collagen, or chitosan) to mimic the natural tissue. The scaffold may be edible, biodegradable during the culture process, or it may be made of a material that can be reused to save resources (Stephens et al., 2018). Satellite cells are grown in a nutrient-rich medium, unique to the proliferation phase and the differentiation phase, as well as antibiotics, anti-fungal agents, or other chemicals to prevent contamination. Historically, a small amount of fetal bovine serum (e.g., 5% to10%) in culture media was used to optimize growth and differentiation of satellite cells in vitro, although, some laboratories have had success with commercially available, chemically defined and serum-free culture media (Edelman et al., 2005). However, commercially available serum-free media are very expensive and the composition is proprietary.
The majority of protein content and quality in these cultured muscle cells consists of contractile proteins; however, tissue engineering could allow expression of other proteins important for texture, color, and taste of cell-cultured food products. For example, myoglobin (an iron-carrying protein) is partially responsible for the pink or red color of meat and may enhance the taste of meat. The transcriptional regulation of myoglobin is reasonably well understood (Kanatous and Mammen, 2010), and it seems plausible that myoglobin synthesis could be stimulated before the harvest of muscle cells to enhance the flavor of cell-cultured food products. Similarly, bioengineered removal of the carbohydrate, galactose-α-1,3-galactose (α-gal), would reduce allergies to conventional meats due to the lone star tick (Kuehn, 2018).
Scaling up muscle cell culture in large bioreactors on an industrial scale also presents significant challenges. It has been estimated that approximately 8 trillion muscle cells will be required to produce 1 kg of protein from a 5,000-liters traditional bioreactor (Stephens et al., 2018). When cultured muscle cells reach more than 200-µm thick, oxygen and nutrients cannot penetrate to the inner layer of cells and those cells begin to die (Jones, 2010). At this point, strips of muscle are harvested from the bioreactor, processed, and various compounds are added to enhance nutritional value, flavor, color, and texture (e.g., vitamins, iron, fat, seasonings, beet juice or heme for color, and bread crumbs or other binding agents to hold the patties together). Production of a specific cut of meat (e.g., steaks, chops, or roasts) requires additional technology to organize the muscle cells into the correct shape and structure. For example, formation of blood vessels or channels in the cultured muscle cells would be needed to transport oxygen and nutrients into the structured muscle cells to prevent the death of the interior cells.
Why Does Cell-Based Meat Have Traction?
Between 1961 and 2011, global meat consumption on a per capita basis increased from 61 to 80g/d and was highly correlated with growth in per capita annual gross domestic product (Sans and Combris, 2015). In 2006, a Food and Agriculture Organization (FAO) report predicted that total global meat consumption would double from 1999/2001 to 2050 (465 million tons; Steinfeld et al., 2006). This prediction is based on increasing population size and improved standards of living that generally lead to increased consumption of animal protein. Post (2014) noted the relative caloric inefficiency of procuring meat through raising livestock. Potential concerns for increased production of meat included the concomitant need for more land, water, and energy to support greater numbers of animals which, in turn, will lead to increased greenhouse gas emissions and an increased carbon footprint for food-producing livestock over what currently exists (Nijdam et al., 2012; Post, 2012; Mattick et al., 2015; Hocquette, 2016; Poore and Nemecek, 2018). Capper (2011, 2014), Mattick et al. (2015), and Lynch and Pierrehumbert (2019) have assessed the relative climate impacts of livestock and beef cattle.
Their results support a lesser environmental impact for meat obtained through cell-based processes than by conventional means. It appears that the energy requirements for cell-based beef culture could be significant and the associated climate impacts could approximate or exceed those for cattle husbandry (Lynch and Pierrehumbert, 2019). However, a well-defined process for the manufacture of cell-based meat has yet to be finalized and as such, it is not possible to accurately predict the climate impacts of cell-based meat (Mattick et al., 2015; Thorrez and Vandenburgh, 2019).
Additional concerns expressed for the anticipated increase in conventional meat production stem from issues related to the ethics of raising animals for food, animal welfare, and the perception that conventional meat may be detrimental to the human diet (Post, 2012; Stephens et al., 2018). Wilks et al. (2019) reported that the positive perceptions of consumers for cell-cultured meat arise from reduced waste, reduced environmental impacts of farmed meat, and improved animal welfare. In some cases, even the removal of an animal from the meat production process may fail to satisfy animal welfare concerns that motivate nonmeat eaters toward their dietary choice. Alvaro (2019) argued that cell-based meat should not be supported because it originates from “unvirtuous motivations,” a desire to satisfy a taste for meat despite the concomitant alienation from a nature that accompanies cell-based processes.
Sociocultural Acceptability of Cell-Based Meat
Culture is critical to the acceptance of a new or nontraditional food, and how cell-cultured meat is framed in research studies (Siegrist et al., 2018) and in the popular press (Goodwin and Shoulders, 2013) has a substantial impact on study results and consumer acceptance, respectively. Hocquette (2016) cited evidence that culturally based opposition to cell-based meat may be underrepresented in the media. Bryant and Dillard (2019) noted that consumer attitudes toward cell-based meat trend more negative when the food product is presented from a strong technology perspective, and there may be important parallels between genetically modified organisms foods and the introduction of cell-cultured meat that require careful consideration of sociocultural perspectives (Mohorčich and Reese, 2019). Several cultural issues have been featured in the literature and these include the connection between meat and animals, perceptions of naturalness, sustainability, religious perspectives, and affordability.
The connectivity between animals and their flesh/organs figured significantly into how Chinese, Ethiopians, and Dutch consumers defined meat (Bekker et al., 2017), and Stephens et al. (2019) raised an interesting point regarding the relative cultural value placed on animal husbandry/livestock in different global cultures. They noted that in the Global South (Asia/Africa/Latin America/Caribbean) there is a relatively greater value placed on the connection of food to its source and they cautioned about assumptions made relative to the degree to which those cultures would accept a move away from conventional to cell-based meat (particularly if encouraged to do so by the Global North).
Psychological predictors of cell-based meat acceptance were investigated by Wilks et al. (2019). They reported that the most significant predictors of opposition to cell-based meat were “food and hygiene disgust sensitivity, food neophobia and conspiratorial ideation.” Verbeke et al. (2015) surveyed consumer reactions to the concept of cell-based meat in Belgium, Portugal, and the United Kingdom. They reported that consumers demonstrated initial reactions to the idea that fostered perceptions of disgust and unnaturalness. While those surveyed expressed an understanding of potential global benefit for cell-based meats to replace conventional meat, they failed to identify much in the way of personal benefit (largely due to unknowns related to price and taste). Similar results related to unnaturalness and/or realization of potential environmental benefits were reported for surveys of Italian (Mancini and Antonioli, 2019), Swiss (Siegrist and Sütterlin, 2017), and German (Weinrich et al., 2020) consumers.
Hartmann and Siegrist (2017) reviewed 38 different studies concerned with the general concept of sustainable protein consumption. They reported that consumers appeared reluctant to reduce their meat consumption and were not generally aware of environmental challenges associated with procuring conventional meat. Bryant and Barnett (2018) similarly reviewed 14 studies of consumer acceptance of cultured meat conducted in at least eight different countries and reported that the greatest perceived benefit of cell-based meat over conventional meat was related to animal welfare. At the same time, the authors noted that results to date suggest that animal welfare or environmental issues are not central to consumer purchase decisions.
Wilks and Phillips (2017) surveyed potential consumers of cell-based meat in the United States and found that approximately one-third of respondents would definitely/probably be willing to eat the new product as a replacement for conventional meat. Respondents also noted a perceived preference for cell-based meat over soy analogs. A majority of respondents favored conventional meat over a cell-based option derived from traditional meat-producing species (fish, poultry, pork, and beef); however, 5.3% and 3.1% of respondents reported a statistically significant and greater willingness to try cell-based products from horses and dog/cat, respectively, than if these were available as farmed meats. The authors noted that this finding was not completely unexpected given the Western definition of what does and does not constitute a food-producing animal.
Islamic and Orthodox Jewish communities follow specific dietary laws based on their religions. The extent to which cell-based meat would be seen as compliant with these laws and acceptable for consumption by observant consumers has been considered (Chriki and Hocquette, 2020). Hamdan et al. (2018) suggested that for cell-based meat to be considered halal and appropriate for consumption, stem cells for production of the product would have to be obtained from an animal slaughtered according to Islamic law. Additionally, no blood or serum would be permissible in the process of growing cell-based meat. The former would be relatively easy to accomplish but the latter much less so. Parallel concerns have been raised regarding whether or not cell-based meats could be considered Kosher. Kenigsberg and Zivotofsky (2020) noted that the source of cells and culturing method would determine the suitability of cell-based meat for Kosher designation. They maintained that cells secured from the ritualistic slaughter of a Kosher species should be acceptable but were reluctant to express an opinion on the role of culturing method/process without greater transparency of how a given process is undertaken. The nature of growth media and specific manufacturing processes used by different cell-based meat companies will likely be highly proprietary, at least initially, and so a decision regarding Halal or Kosher status may come later rather than sooner.
In addition to cultural considerations, taste (Wilks and Phillips, 2017; Kuhn, 2020) and affordability (Frankl-Duval, 2019) of cell-based meat will strongly influence near- and long-term success of this nontraditional food. When cell-based meat becomes publicly available, it is expected to carry an initial price premium over conventional meat, which would deter some consumers from engaging with the product (Wilks and Phillips, 2017). Johnson et al. (2018) undertook a survey of consumer willingness to try cell-based meat and reported that high-income earners (> US$75,000 $75,000/year) and younger respondents (18 to 29 year olds) reported the greatest intention to purchase. A survey of 394 consumers in Mumbai, India, found that they would be willing to pay a premium of US$0.81/kg over the price of conventional meat (Arora, 2019). Stephens et al. (2018) noted that a high cost product could result in a “nonmeat eating elite.” Studies have addressed markets/market share for cell-based meats (Mouat and Prince, 2018; Slade, 2018) but extensive economic analyses have not appeared in the public literature; this is not unexpected given that manufacturing processes are still being refined.
When Is Meat, Meat?
Considerable energy has been expended around debates of what cell-based meat should be called. Each sector that has a vested interest in the outcome of this new technology has a preferred term that is consistent with advancing their respective perspective. Proponents of cell-based meat have favored a name that does not alienate consumers; advocates for conventional meat want their product identity to remain as it has been without concern for consumer confusion (Bryant and Barnett, 2019; Chriki and Hocquette, 2020; Ong et al., 2020). In the conversations focused on naming, a logical starting point has been to consider the definition of meat. The American Meat Science Association addressed this topic recently (Boler and Woerner, 2017; Seman et al., 2018) and noted that meat is “the portions of the animal consumed as food.” This includes skeletal and cardiac muscle as well as organ/variety meats. Boler and Woerner (2017) maintained that cell-based meat can be considered “meat” if it is sourced from an animal cell, inspected for safety, and at a minimum provides nutritional and sensory properties comparable with conventional meat. This is helpful, particularly for standardizing communication among scientists, but the marketplace and/or courts will likely prevail on the final term. Unlike milk, meat lacks a federal standard of identity. The U.S. Department of Agriculture (USDA) defines meat products as “any product … made wholly or in part from any meat or other portion of the carcass” and Food and Drug Administration (FDA) defines meat as “part of the muscle of any cattle, sheep, swine or goats which is skeletal” (Ong et al., 2020). FDA and USDA have preferred the term “cell-cultured product” or “cell-cultured food product” (Stephens et al., 2019). In each of their press releases, USDA and FDA referred to cell-based meat as a cell-cultured product and appear to have intentionally avoided using the term “meat” (USDA, 2019). Livestock producers clearly have a vested interest in protecting how the term “meat” is applied to food products as do the U.S. states with a significant portion of their economy derived from animal agriculture. In late 2019, legislation was introduced in the U.S. Senate entitled the Real MEAT (Marketing Edible Artificials Truthfully) Act of 2019 (National Cattlemen’s Beef Association, 2019). In brief, the bill attempts to protect the use of the words meat and beef to refer only to edible tissue from livestock. Missouri was the first U.S. state to prevent the use of the word “meat” if the food product did not originate from livestock or poultry. Fourteen states passed meat labeling laws in 2019, and nine states were considering such legislation in 2020 (Poinski, 2020). These laws are not focused only on differentiating conventional meat from cell-based meat but more broadly from all meat substitutes including plant- and insect-based sources. Lawsuits have been initiated challenging these laws on the grounds they violate First Amendment rights provided by the U.S. Constitution (Good Food Institute, 2018; Sullivan, 2018; Judkis, 2019), and decisions by courts appear destined to determine what foods may/may not be identified as meat.
Regulation as a Food
The National Academies of Sciences, Engineering, and Medicine (2017) recently reported that cell-based meat products will be among a larger and varied group of biotechnology products that have the potential to overwhelm the U.S. regulatory system. Safety of new food substances/products is of paramount concern and safety assessment applies to both the nature of the substances themselves and the processes used to produce them (Fasano, 2018).
Penn (2018) noted that rennet is a cellular agricultural product that is regulated by the FDA as a food additive. As such, it required premarket approval. However, rennet is an enzyme ingredient while cultured meat would be an end product and thus require inspection/certification for safety. She further noted that Courts have established that substances used for their effect(s) on another substance classify them as food additives. This would not generally be the case for cell-based meat intended for use by itself and as such would be identified as a food (Penn, 2018) although it is not clear how the inclusion of chemical substances necessary for tissue engineering would be regulated.
In March 2019, USDA and FDA reached agreement on the responsibilities of each agency for regulating cell-based meat. In brief, FDA will oversee cell collection and development of cells to harvest. USDA will be responsible for regulating manufacture and labeling of food products derived from the cells (USDA, 2019). Michael and Fasano (2020) recently summarized the essential components of that agreement and noted that it only “applies to cells extracted from animals already under USDA-FSIS jurisdiction – livestock, poultry and fish of the order Siluriformes.” Game meat is overseen only by FDA and presumably, cells obtained from these species would only be subject to that agency’s regulatory authority. Additional legislation is not anticipated to be required for accommodating the introduction of cell-based meats into the U.S. food system by either agency (Michael and Fasano, 2020). The agreement between USDA and FDA (USDA, 2019) currently calls for the latter to “Conduct premarket consultation processes to evaluate production materials/processes and manufacturing controls, to include oversight of tissue collection, cell lines and banks, and all components and inputs.” This would appear to parallel the process used for new bioengineered plant varieties (Watson, 2019). As such, the differentiation of cell-based meat as a food additive or generally recognized as safe (GRAS) substance as previously hypothesized (Liu and Gasteratos, 2019), and the expected controversy that would surround such a debate (Faustman et al., 2020) does not appear likely to occur. More specific details of how each agency will apply its regulatory authority within their scope of responsibility for cell-based meat manufacture and processing remain to be communicated but there is likely to be some controversy that develops (Johnson, 2019; Sachs and Kettenmann, 2019; Watson, 2019).
Labeling will be the responsibility of USDA and conditions under which cell-based meat could be identified as organic or natural remain to be determined. At present, and based on public information available to date, cell-based meat would not be considered a genetically engineered substance and so the definition for bioengineered foods by USDA, and required labeling would not apply. However, one potential future advantage of cell-based meats would be the opportunity for designing products with specific nutritional characteristics not normally possible with animal feeding approaches.
Summary
Animal and meat scientists have contributed significantly to the understanding of animal tissues as food. Their discoveries have, ironically, facilitated the development of meat without slaughter of an animal. There has been significant investment in the science dedicated to the development of meat alternatives and cultured meat has been projected to grow rapidly such that by 2040, it would capture 35% of the value of meat sold (Kuhn, 2020). However, this would require a substantive change to how various cultures view the role of conventional meat in a diet and the degree to which they would accept cell-cultured meat as a substitute. A recent Canadian study that employed a hypothetical choice approach demonstrated a strong preference for conventional beef burger over plant-based or cell-based meat products (Slade, 2018). If and when cell-based meat becomes publicly available, it is not anticipated to eliminate the need for meat-producing animals. It is even possible that the total consumption of meat (cell-cultured plus conventional) will be increased because: 1) cell-cultured food products from livestock and poultry provide alternatives that satisfy the concerns of some people regarding the perceived animal welfare and environmental issues of conventional livestock or poultry production and 2) the increased demand for animal-sourced foods from a growing global population with increased incomes may exceed the global supply of conventionally produced meat (Stephens et al, 2018). Hybrid products might also be developed. At present, there are several blended products that combine plant-based alternatives and conventional meat into a single food item (Kuhn, 2020) and hybrid products of conventional and cell-based meat could bring opportunities for future designer foods (Rajasekaran and Kalaivani, 2013).
The challenges of providing conventional meat to a nation have been highlighted by the COVID-19 pandemic, and the potential advantages of a cell-based meat industry cannot be dismissed. The livestock/meat industry will be challenged to address the competition, perceived or real, provided by cell-based meat. We are confident that the livestock/meat industry will continue to improve its approach and communication regarding the nutritional benefits of conventional meat and the continued development and implementation of sustainable approaches to the production of conventional meat.
Acknowledgments
We thank Carolyn Mills, UConn Libraries, for assistance with obtaining data used in Figure 1, and Jodi Boles and the American Society of Animal Science for the development of Figure 2.
Glossary
Abbreviations
- FDA
Food and Drug Administration
- USDA
U.S. Department of Agriculture
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Alvaro C. 2019. Lab-grown meat and veganism: a virtue-oriented perspective. J. Agric. Environ. Ethics. 32:127–141. doi: 10.1007/s10806-019-09759-2 [DOI] [Google Scholar]
- Arora R. 2019. Assessing the viability of meat alternatives to mitigate the societal concerns associated with animal agriculture in India [M.S. thesis]. State College (PA): The Pennsylvania State University. [Google Scholar]
- Bekker G. A., Tobi H., and Fischer A. R. H.. . 2017. Meet meat: an explorative study on meat and cultured meat as seen by Chinese, Ethiopians and Dutch. Appetite 114:82–92. doi: 10.1016/j.appet.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Ben-Arye T., and Levenberg S.. . 2019. Tissue engineering for clean meat production. Front. Sustain. Food Syst. 3:1–19. doi: 10.3389/fsufs.2019.00046 [DOI] [Google Scholar]
- Bodiou V., Moutsatsou P., and Post M. J.. . 2020. Microcarriers for upscaling cultured meat production. Front. Nutr. 7:10. doi: 10.3389/fnut.2020.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boler D., Kim M. K., Krieger J., Martin J., Milkowski A., Mozdziak P. and Sylvester B. P.. . 2020. Producing food products from cultured animal tissues [cited April 2020]. CAST Commentary Available from https://www.cast-science.org/wp-content/uploads/2020/04/QTA2020-1-Cultured-Tissues-1.pdf [accessed May 5, 2020]. [Google Scholar]
- Boler D. D., and Woerner D. R.. . 2017. What is meat? a perspective from the American Meat Science Association. Anim. Front. 7:8–11. doi: 10.2527/af.2017.0436 [DOI] [Google Scholar]
- Bottemiller Evich H. 2019. Cell-based meat companies join forces [cited August 29, 2019]. Politico Available from https://www.politico.com/story/2019/08/29/cell-based-meat-companies-join-1689710 [accessed May 11, 2020]. [Google Scholar]
- Bryant C., and Barnett J.. . 2018. Consumer acceptance of cultured meat: a systematic review. Meat Sci. 143:8–17. doi: 10.1016/j.meatsci.2018.04.008 [DOI] [PubMed] [Google Scholar]
- Bryant C. J., and Barnett J. C.. . 2019. What’s in a name? Consumer perceptions of in vitro meat under different names. Appetite 137:104–113. doi: 10.1016/j.appet.2019.02.021 [DOI] [PubMed] [Google Scholar]
- Bryant C., and Dillard C.. . 2019. The impact of framing on acceptance of cultured meat. Front. Nutr. 6:103. doi: 10.3389/fnut.2019.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capper J. L. 2011. The environmental impact of beef production in the United States: 1977 compared with 2007. J. Anim. Sci. 89:4249–4261. doi: 10.2527/jas.2010-3784 [DOI] [PubMed] [Google Scholar]
- Capper J. L. 2014. Replacing rose-tinted spectacles with a high-powered microscope: the historical versus modern carbon footprint of animal agriculture. Anim. Front. 1:26–32. doi: 10.2527/af.2011-0009 [DOI] [Google Scholar]
- Cell Based Tech. 2020. Lab grown meat companies. Available from https://cellbasedtech.com/lab-grown-meat-companies [accessed June 19, 2020].
- Chriki S., and Hocquette J. F.. . 2020. The myth of cultured meat: a review. Front. Nutr. 7:7. doi: 10.3389/fnut.2020.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill W. 1932. Fifty years hence. In J. W. Muller, editor: Thoughts and adventures. London: Thornton Butterworth; p. 24–27. [Google Scholar]
- Edelman P. D., McFarland D. C., Mironov V. A., and Matheny J. G.. . 2005. Commentary: In vitro-cultured meat production. Tissue Eng. 11:659–662. doi: 10.1089/ten.2005.11.659 [DOI] [PubMed] [Google Scholar]
- Fasano J. 2018. Foods produced using animal cell culture technology. FDA Public Meeting; July 12, 2018; College Park, MD. Docket No.: FDA-2018-N-2155. Baltimore (MD): Capital Reporting Co.; pp. 34–52. [Google Scholar]
- Faustman C., Aaron D., Negowetti N., and Broad Leib. E.. 2020. Ten years post-GAO assessment, FDA remains uninformed of potentially harmful GRAS substances in foods. Crit. Rev. Food Sci. Nutr. (in press). doi: 10.1080/10408398.2020.1756217 [DOI] [PubMed] [Google Scholar]
- Fountain M. 2013. A lab-grown burger gets a taste test [cited August 5, 2013]. The New York Times Available from https://www.nytimes.com/2013/08/06/science/a-lab-grown-burger-gets-a-taste-test.html [accessed April 1, 2020].
- Frankl-Duval M. 2019. Lab-grown meat is coming, but the price is hard to stomach [cited May 2, 2019]. The Wall Street Journal Available from https://www.wsj.com/articles/lab-grown-meat-is-coming-but-the-price-is-hard-to-stomach-11556805600 [accessed April 15, 2019].
- Good Food Institute 2018. Producers’ first amendment right to use clear labels on food Available from https://www.gfi.org/images/uploads/2018/05/FirstAmendmentFactsGFI.pdf [accessed April 15, 2020].
- Goodwin J. N., and Shoulders C. W.. . 2013. The future of meat: a qualitative analysis of cultured meat media coverage. Meat Sci. 95:445–450. doi: 10.1016/j.meatsci.2013.05.027 [DOI] [PubMed] [Google Scholar]
- Hamdan M. N., Post M. J., Ramli M. A., and Mustafa A. R.. . 2018. Cultured meat in Islamic perspective. J. Relig. Health 57:2193–2206. doi: 10.1007/s10943-017-0403-3 [DOI] [PubMed] [Google Scholar]
- Hartmann C., and Siegrist M.. . 2017. Consumer perception and behavior regarding sustainable protein consumption: a systematic review. Trends Food Sci. Technol. 61:11–25. doi: 10.1016/j.jpgs.2016.12.006 [DOI] [Google Scholar]
- Hocquette J. F. 2016. Is in vitro meat the solution for the future? Meat Sci. 120:167–176. doi: 10.1016/j.meatsci.2016.04.036 [DOI] [PubMed] [Google Scholar]
- Johnson W. G. 2019. Conflict over cell-based meat: who should coordinate agencies in U.S. biotechnology regulation. Food Drug Law J. 74:478–500. [Google Scholar]
- Johnson W., Maynard A. and Kirshenbaum S.. . 2018. Would you eat ‘meat’ from a lab? Consumers aren’t necessarily sold on ‘cultured meat’. [cited August 23, 2018]. The Conversation Available from https://theconversation.com/would-you-eat-meat-from-a-lab-consumers-arent-necessarily-sold-on-cultured-meat-100933 [accessed April 14, 2020].
- Jones N. 2010. Food: a taste of things to come? Nature 468:752–753. doi: 10.1038/468752a [DOI] [PubMed] [Google Scholar]
- Judkis M. 2019. Tofurky takes Arkansas to court over the word ‘meat’. [cited July 23, 2019]. Washington Post Available from https://www.washingtonpost.com/news/voraciously/wp/2019/07/23/tofurky-takes-arkansas-to-court-over-the-word-meat/ [accessed April 15, 2020].
- Kadim I. T., Mahgoub O., Baqir S., Faye B. and Purchas R.. . 2015. Cultured meat from muscle stem cells: a review of challenges and prospects. J. Integr. Agric. 14:222–233. doi: 10.1016/S2095-3119(14)60881-9 [DOI] [Google Scholar]
- Kanatous S. B., and Mammen P. P.. . 2010. Regulation of myoglobin expression. J. Exp. Biol. 213(Pt 16):2741–2747. doi: 10.1242/jeb.041442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenigsberg J. A., and Zivotofsky A. Z.. . 2020. A Jewish religious perspective on cellular agriculture. Front. Sustain Food Syst. 3:1–6. doi: 10.3389/fsufs.2019.00128 [DOI] [Google Scholar]
- Kuehn B. M. 2018. Rise in fall-related deaths. JAMA. 319:2471. doi: 10.1001/jama.2018.7978 [DOI] [PubMed] [Google Scholar]
- Kuhn M. E. 2020. Meet the next generation of plant-based meat. Food Technol. 74(3):25–34. [Google Scholar]
- Liu S. L., and Gasteratos K.. . 2019. Assessing cell-based animal proteins. Science 363:826. doi: 10.1126/science.aau3905 [DOI] [PubMed] [Google Scholar]
- Lynch J., and Pierrehumbert R.. . 2019. Climate impacts of cultured meat and beef cattle. Front. Sustain Food Syst. 3:1–11. doi: 10.3389/fsufs.2019.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M. C., and Antonioli F.. . 2019. Exploring consumers’ attitude towards cultured meat in Italy. Meat Sci. 150:101–110. doi: 10.1016/j.meatsci.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Mattick C. S., Landis A. E., Allenby B. R., and Genovese N. J.. . 2015. Anticipatory life cycle analysis of in vitro biomass cultivation for cultured meat production in the United States. Environ. Sci. Technol. 49:11941–11949. doi: 10.1021/acs.est.5b01614 [DOI] [PubMed] [Google Scholar]
- Michael M., and Fasano J.. . 2020. Animal cell-culture food technology: a new regulatory frontier. [cited February/March 2020]. Food Safety Magazine Available from https://www.foodsafetymagazine.com/magazine-archive1/februarymarch-2020/animal-cell-culture-food-technology-a-new-regulatory-frontier/ [accessed April 17, 2020].
- Mohorčich J., and Reese J.. . 2019. Cell-cultured meat: lessons from GMO adoption and resistance. Appetite 143:104408. doi: 10.1016/j.appet.2019.104408 [DOI] [PubMed] [Google Scholar]
- Mouat M. J., and Prince R. 2018. Cultured meat and cowless milk: on making markets for animal-free food. J. Cult. Econ. 11:315–329. doi: 10.1080/17530350.2018.1452277 [DOI] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine 2017. Preparing for future products of biotechnology. Washington (DC): The National Academies Press. [PubMed] [Google Scholar]
- National Cattlemen’s Beef Association 2019. NCBA applauds U.S. Senate introduction of Real MEAT Act [cited December 11, 2019]. Available from https://www.ncba.org/newsreleases.aspx?NewsID=7105 [accessed April 15, 2020].
- Nijdam D., Rood T. and Westhoek H.. . 2012. The price of protein: review of land use and carbon footprints from life cycle assessments of animal food products and their substitutions. Food Policy 37:760–770. doi: 10.1016/j.foodpol.2012.08.002 [DOI] [Google Scholar]
- Ong S., Choudhury D. and Naing M. W.. . 2020. Cell-based meat: current ambiguities with nomenclature. Trends Food Sci. Technol. doi: 10.106/j.jpgs.2020.02.010 [DOI] [Google Scholar]
- Penn J. 2018. “Cultured meat”: lab-grown beef and regulating the future meat market. UCLA J. Environ. Law Policy 36L:104–126. [Google Scholar]
- Poinski M. 2020 Cell-based meat products are years away, so why are states making so many laws about them? [cited February 12, 2020]. Available from https://www.fooddive.com/news/cell-based-meat-products-are-years-away-so-why-are-states-making-so-many-l/571071/ [accessed April 15, 2020].
- Poore J., and Nemecek T.. . 2018. Reducing food’s environmental impacts through producers and consumers. Science 360:987–992. doi: 10.1126/science.aaq0216 [DOI] [PubMed] [Google Scholar]
- Post M. J. 2012. Cultured meat from stem cells: challenges and prospects. Meat Sci. 92:297–301. doi: 10.1016/j.meatsci.2012.04.008 [DOI] [PubMed] [Google Scholar]
- Post M. J. 2014. An alternative animal protein source: cultured beef. Ann. N. Y. Acad. Sci. 1328:29–33. doi: 10.1111/nyas.12569 [DOI] [PubMed] [Google Scholar]
- Rajasekaran A., and Kalaivani M.. . 2013. Designer foods and their benefits: a review. J. Food Sci. Technol. 50:1–16. doi: 10.1007/s13197-012-0726-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach M. 2013. Liver and opinions. Why we eat what we eat and despise the rest. Ch. 3. In: Gulp. New York (NY): W.W. Norton and Co; p. 65. [Google Scholar]
- Sachs A., and Kettenmann S.. . 2019. A burger by any other name: regulatory challenges and opportunities for cell-cultured meat SciTech Lawyer 15:18–23. Available from https://www.americanbar.org/groups/science_technology/publications/scitech_lawyer/2019/winter/a-burger-any-other-name/ [accessed June 19,2020]. [Google Scholar]
- Sajid Arshad M., Javed J., Sohaib M., Saeed F., Imran A. and Amjad Z.. . 2017. Tissue engineering approaches to develop cultured meat from cells: a mini review. Cogent Food Agric. 3:1. doi: 10.1080/23311932.2017.1320814 [DOI] [Google Scholar]
- Sans P., and Combris P.. . 2015. World meat consumption patterns: an overview of the last fifty years (1961-2011). Meat Sci. 109:106–111. doi: 10.1016/j.meatsci.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Seman D. L., Boler D. D., Carr C. C., Dikeman M. E., Owens C. M., Keeton J. T., Pringle T. D., Sindelar J. J., Woerner D. R., deMello A. S.. et al. 2018. Meat science lexicon. Meat Muscle Biol. 2:1–15. doi: 10.22175/mmb2017.12.0059 [DOI] [Google Scholar]
- Siegrist M., and Sütterlin B.. . 2017. Importance of perceived naturalness for acceptance of food additives and cultured meat. Appetite 113:320–326. doi: 10.1016/j.appet.2017.03.019 [DOI] [PubMed] [Google Scholar]
- Siegrist M., Sutterlin B. and Hartmann C.. . 2018. Perceived naturalness and evoked disgust influence acceptance of cultured meat. Meat Sci. 13:213–219. doi: 10.1016/j.meatsci.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Slade P. 2018. If you build it, will they eat it? Consumer preferences for plant-based and cultured meat burgers. Appetite 125:428–437. doi: 10.1016/j.appet.2018.02.030 [DOI] [PubMed] [Google Scholar]
- Steinfeld H., Gerber P., Wassenaar T., Castel V., Rosales M. and de Haan C.. . 2006. Livestock’s long shadow. Environmental issues and options. FAO Report. Rome (Italy): FAO; Available from http://www.fao.org/3/a0701e/a0701e00.pdf [accessed April 20, 2020]. [Google Scholar]
- Stephens N., Di Silvio L., Dunsford I., Ellis M., Glencross A., and Sexton A.. . 2018. Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. Technol. 78:155–166. doi: 10.1016/j.jpgs.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens N., Sexton A. E. and Driessen C.. . 2019. Making sense of making meat: key moments in the first 20 years of tissue engineering muscle to make food. Front. Sustain Food Syst. 3:1–16. doi: 10.3389/fsufs.2019.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E. 2018. What’s meat, anyway? Missouri Label Law says it comes from an animal; some disagree [cited August 29, 2018]. Available from https://www.npr.org/2018/08/29/642937901/whats-meat-anyway-missouri-label-law-says-it-comes-from-an-animal-some-disagree [accessed April 15, 2020].
- Thorrez L., and Vandenburgh H.. . 2019. Challenges in the quest for ‘clean meat’. Nat. Biotechnol. 37:215–216. doi: 10.1038/s41587-019-0043-0 [DOI] [PubMed] [Google Scholar]
- USDA 2019. USDA and FDA announce a formal agreement to regulate cell-cultured food products from cell lines of livestock and poultry [cited March 7, 2019]. Available from https://www.usda.gov/media/press-releases/2019/03/07/usda-and-fda-announce-formal-agreement-regulate-cell-cultured-food [accessed April 14, 2020].
- Vein J. 2004. Method for producing tissue engineered meat for consumption. United States Patent and Trademark Office; US 6,835,390 B1. December 28, 2004. [Google Scholar]
- Verbeke W., Marcu A., Rutsaert P., Gaspar R., Seibt B., Fletcher D., and Barnett J.. . 2015. ‘Would you eat cultured meat?’: consumers’ reactions and attitude formation in Belgium, Portugal and the United Kingdom. Meat Sci. 102:49–58. doi: 10.1016/j.meatsci.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Watson E. 2018. Memphis Meats, NAMI: FDA and USDA both have roles to play in regulating cell-based meat and poultry [cited August 23, 2018]. Available from https://www.foodnavigator-usa.com/article/2018/08/23/memphis-meats-nami-fda-and-usda-both-have-roles-to-play-in-regulating-cell-based-meat-and-poultry [accessed April 13, 2020].
- Watson E. 2019. So the FDA and USDA will share oversight for cell-based meat …but what will this mean in practice? [cited July 30, 2019]. Food Navigator Available from https://www.foodnavigator-usa.com/Article/2019/01/03/So-the-FDA-and-USDA-will-share-oversight-for-cell-based-meat-but-what-will-this-mean-in-practice [accessed April 22, 2020].
- van der Weele C., Feindt P., van der Goot A. J., van Mierlo B., and van Boekel M.. . 2019. Meat alternatives: an integrative comparison. Trends Food Sci. Technol. 88:505–512. doi: 10.1016/j.jpgs.2019.04.018 [DOI] [Google Scholar]
- Weinrich R., Strack M., and Neugebauer F.. . 2020. Consumer acceptance of cultured meat in Germany. Meat Sci. 162:107924. doi: 10.1016/j.meatsci.2019.107924 [DOI] [PubMed] [Google Scholar]
- Wilks M., and Phillips C. J.. . 2017. Attitudes to in vitro meat: a survey of potential consumers in the United States. PLoS One. 12:e0171904. doi: 10.1371/journal.pone.0171904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks M., Phillips C. J. C., Fielding K. and Hornsey M. J.. . 2019. Testing potential psychological predictors of attitudes towards cultured meat. Appetite 136:137–145. doi: 10.1016/j.appet.2019.01.027 [DOI] [PubMed] [Google Scholar]