Abstract
Transcription factors are attractive therapeutic targets that are considered non-druggable because they do not have binding sites for small drug-like ligands. We established a cell-free high-throughput screening assay to search for small molecule inhibitors of DNA binding by transcription factors. A screen was performed using p53 as a target, resulting in the identification of NSC194598 that inhibits p53 sequence-specific DNA binding in vitro (IC50 = 180 nM) and in vivo. NSC194598 selectively inhibited DNA binding by p53 and homologs p63/p73, but did not affect E2F1, TCF1, and c-Myc. Treatment of cells with NSC194598 alone paradoxically led to p53 accumulation and modest increase of transcriptional output owing to disruption of the MDM2-negative feedback loop. When p53 was stabilized and activated by irradiation or chemotherapy drug treatment, NSC194598 inhibited p53 DNA binding and induction of target genes. A single dose of NSC194598 increased the survival of mice after irradiation. The results suggest DNA binding by p53 can be targeted using small molecules to reduce acute toxicity to normal tissues by radiation and chemotherapy.
Introduction
Small molecule compounds have been successfully used to inhibit oncogenic kinases and hormone receptors owing to the presence of well-defined binding pockets, but targeting these upstream signals may only produce partial effects due to network redundancy. Many important disease-related pathways utilize transcription factors that specifically bind DNA (e.g., c-Myc, HIF-1, TCF1, p53) as key nodes or endpoints in complex signaling networks. In such cases the transcription factor itself is often the most attractive target. However, drugging transcription factors is challenging owing to an absence of small ligand binding sites in their DNA-binding domain and the presence of a highly charged DNA-binding surface [1].
Despite these challenges, different strategies have been used to target important transcription factors, each taking advantage of unique and well-understood features important for their function. Formation of c-Myc and Max heterodimer is important for sequence-specific DNA binding by c-Myc and thus was targeted by small molecule disruptors [2]. Canonical Wnt signaling results in the accumulation and binding of β-catenin to DNA-binding partner TCF1. Further interaction between β-catenin and coactivator Bcl9 is important for transcriptional output and has been targeted using small molecule alpha-helical mimics to disrupt protein-protein interactions [3]. The oncogenic STAT3 transcription factor is activated by JAK kinase phosphorylation of a tyrosine residue, followed by dimerization through intermolecular SH2-pTyr interaction. Small molecules that mimic the binding mode of the pTyr-containing peptide disrupt SH2 domain-mediated dimerization and inhibit DNA binding by STAT3 [4].
Structural information of most transcription factors is often fragmentary, missing important domains or intrinsically disordered regions that mediate signaling and regulation. Despite extensive studies, the 3D structure of p53 is available only for the DNA-binding domain and oligomerization domain in isolation, but not the full-length protein. Given the evidence that N and C termini of p53 are both involved in physical interactions with the DNA-binding domain and regulate DNA binding, understanding of the p53 structure is still incomplete and likely lacks features useful for rational drug design [5, 6]. Static crystal structures may also obscure the presence of transient cavities [7]. An unbiased high-throughput screening (HTS) approach using full-length proteins may overcome these challenges and identify useful chemical probes without complete knowledge of the target protein structure.
P53 is a tumor suppressor that induces cell cycle arrest and cell death by sequence-specific DNA binding and activation of target genes [8]. Therapeutic targeting of p53 for cancer therapy has focused mostly on disrupting p53–MDM2 interaction, which activates p53 and induces apoptosis [9]. This approach is applicable to tumors without p53 mutations. Counter-intuitively, p53 activation in normal organs during chemotherapy or radiation is implicated in causing tissue damage and dose-limiting toxicity [10, 11]. P53 activation in mice causes hematopoietic injury, which is responsible for the death of the animals after 10 Gy irradiation [11, 12]. Mouse experiments also showed that p53 mediates cisplatin-induced nephrotoxicity [13, 14]. P53 activation and induction of G1 arrest in tumor cells may interfere with chemotherapy drugs that target mitotic cells (such as taxol) [15]. Many tumors have p53 mutations that abrogate response to DNA-damaging chemotherapy, but normal tissues express wild-type p53 and remain sensitive to damage from treatment. For these patients, transient inhibition of p53 in normal tissues may protect vital organs and increase tolerance to aggressive therapy.
P53 activation is also implicated in ischemia/oxidative stress-related tissue damage and neuro degenerative diseases such as Alzheimer’s and Parkinson’s [10, 16]. Deletion of p53 reduces brain damage after stroke [17]. Therefore, inhibiting p53 also has clinical potential against acute or chronic tissue damage. A previous study used cell-based screen to search for compounds that inhibit the expression of p53-inducible lacZ reporter. The work identified Pifithrin-α (PFTα) that was reported to inhibit p53 activity and protect mice from the toxicities of radiation, chemotherapy drugs, and stroke [10, 18]. However, the mechanism of PFTα biological activity is complex and not well defined. Later studies showed that it has p53-independent activities and non-specifically inhibit many other proteins [10, 19]. Furthermore, whether PFTα truly inhibits p53 activity was questioned by some reports [20, 21]. Therefore, there is a clear need to develop potent and specific p53 inhibitors.
In this study, we established a cell-free reaction suitable for HTS search of inhibitors of DNA-binding proteins, and optimized the assay for p53. The assay uses full-length transcription factors without epitope tags or chemical labels and produces increased signal in the presence of an inhibitor (positive readout). A screen of ~70,000 compounds identified a p53 DNA-binding inhibitor NSC194598 with in vitro IC50 of 180 nM. The compound apparently acts by binding to the DNA-binding domain of p53, and showed specificity against several control transcription factors. Furthermore, NSC194598 suppressed p53 transcriptional output after DNA damage in culture and increased the survival of mice after irradiation.
Results
Design of a p53 DNA-binding competition assay
An HTS-compatible assay was established for identifying inhibitors of p53 DNA binding in a cell-free reaction based on luciferase fragment complementation [22]. When the N and C domains of luciferase are fused to other proteins, dimerization of the proteins brings the luciferase domains together to restore enzyme activity [22]. This system was often used for live cell analysis, and its performance in a cell-free assay is unknown. We constructed ZF1-Nluc and ZF2-Cluc fusion proteins for expression in Escherichia coli (Fig. 1a). In ZF1-Nluc, luciferase residue 1–437 was fused to the C terminus of a designed 6-finger zinc finger protein that binds to a target sequence with high affinity (ATGTAGGGAAAAGCCCGG, Kd = 50 pM) [23]. In ZF2-Cluc, luciferase 398–550 was fused to the C terminus of a designed 5-finger zinc finger protein that recognizes the first 15 bp of a 20 bp p53 consensus binding site bound by a p53 tetramer (GAACATGTCCCAACATGTTG) [24]. Mixing ZF1-Nluc, ZF2-Cluc, and DNA containing ZF1- and p53-binding sites produced luciferase activity (Fig. 1b). Addition of p53 competed with ZF2-Cluc for the overlapping DNA-binding site and suppressed Nluc/Cluc complementation signal (Fig. 1b). Inhibitors of p53 DNA binding should restore ZF2-Cluc binding to DNA and increase luciferase activity (Fig. 1b).
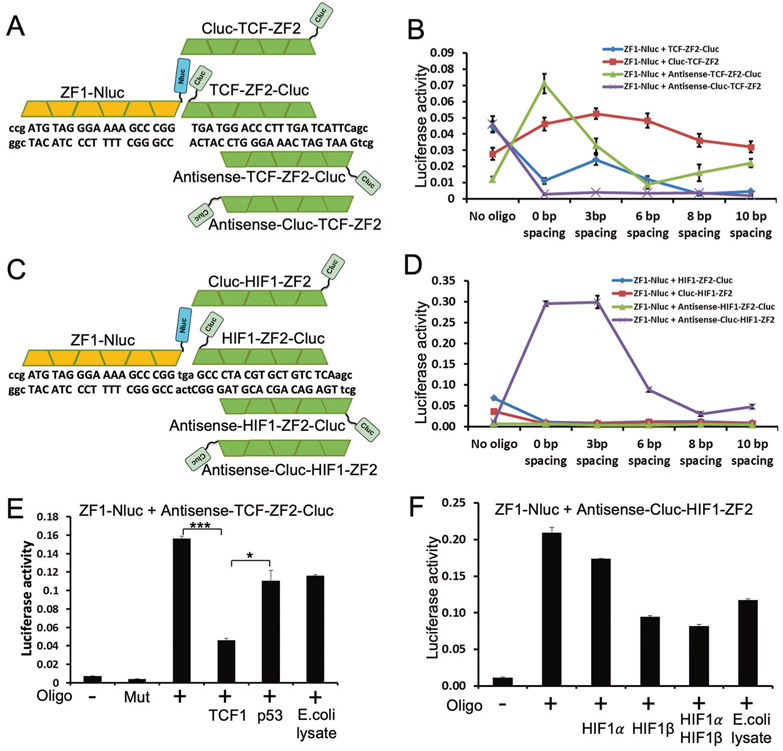
Fig. 1. A positive readout assay for p53 DNA-binding inhibitors.
a Firefly luciferase N and C terminal domains were fused to zinc finger proteins that recognize specific sequences. Incubation with DNA containing both binding sites brings the luciferase domains to close proximity to restore enzyme activity. Each triplet in the sequence is recognized by one zinc finger. Each arrow represents a quarter site of the p53-binding consensus sequence. b P53 binding to DNA displaces ZF2-Cluc and reduces luciferase activity, p53 inhibitors restore ZF2-Cluc binding and stimulate luciferase activity, producing a positive readout. c Effects of spacing between ZF1 and p53-binding sites on complementation efficiency. d Negligible luciferase activity was produced without DNA or with DNA containing mutated p53 site. ZF1 and ZF2 complementation was inhibited by wt p53 but not the R175H mutant defective for DNA binding. e P63 and p73 also bound to p53 consensus site and inhibit ZF1/ZF2 complementation. The results are average of three experiments (mean ± SD). ***p <0.001 (Student’s t test).
The spacing between ZF1 and p53-binding sites determines the distance and relative rotation of the bound proteins, thus affects complementation efficiency. Analysis of DNA oligos with 0–16 bp spacing identified 3 bp as the optimal distance for ZF1-Nluc and ZF2-Cluc complementation (Fig. 1c). Next, the specificity of the assay was validated using several controls. In the absence of DNA, ZF1-Nluc and ZF2-Cluc showed negligible complementation. Addition of a control oligo containing mutated p53-binding site did not produce signal, whereas the optimized oligo produced luciferase activity at 46-fold above background (Fig. 1d). Adding E. coli extract containing wt p53 inhibited the signal by sevenfold. Control extract containing DNA binding-defective p53-R175H mutant or without p53 reduced the activity by ~40%, presumably owing to non-specific competition from bacteria proteins and nucleic acids (Fig. 1d). As expected, the p53 homologs p63γ and p73β also bound to the p53 target sequence and suppressed the complementation signal in this reaction (Fig. 1e). Therefore, the assay was able to detect the DNA binding activity of p53.
Adapting the assay to other transcription factors
The assay described above should be applicable to other transcription factors by replacing the p53-binding site with a new sequence and designing ZF2 to bind the new site. We converted the assay for TCF1 (TGATGGACCCTTTGATCATTC, TCF1-binding site underlined.) and HIF-1 (GCCCTACGTGCTGTCTCA) [25, 26]. The results showed that in order to establish a robust complementation assay for a new sequence, several ZF2-Cluc fusions should be tested, including Cluc fusion to N and C terminus of ZF2, and designing ZF2 that binds to sense or antisense sequence of non-palindromic binding sites. For TCF1, the optimal complementation system consisted of ZF1-Nluc, antisense-ZF2-Cluc, and 0 bp spacing (Fig. 2a, b). The optimal readout system for HIF-1 consisted of ZF1-Nluc, Cluc-antisense-ZF2, and 0–3 bp spacing (Fig. 2c, d). Expression of TCF1 in E. coli produced sufficient DNA-binding activity that specifically inhibited readout of the reporter system (Fig. 2e). Specificity of the TCF1 assay was demonstrated by its insensitivity to p53 (Fig. 2e). HIF-1 is a heterodimeric transcription factor composed of HIF-1α and HIF-1β [27]. Coexpression of HIF-1α and HIF-β in E. coli did not result in active protein capable of inhibiting the reporter (Fig. 2f).
Fig. 2. Adaptation of binding assay to other transcriptional factors.
a Design of competitive binding assay for TCF1. P53-binding site was replaced with TCF1-binding site. ZF2 proteins were designed to bind the sense or antisense sequence of TCF1 site, and fused to Cluc through N or C terminus. b Effects of ZF1 and TCF1-binding site spacing on complementation efficiency of different protein combinations. c, d Design adaptation to HIF-1 using same strategy as a and b. e The TCF1-specific complementation reaction was inhibited by TCF1 but not p53. f Recombinant HIF-1 proteins did not provide sufficient DNA binding to inhibit HIF-1-specific complementation. The results are average of three experiments (mean ± SD). *p < 0.05, ***p < 0.001 (Student’s t test).
The assay depends on competition between ZF2 and transcription factor for the same DNA sequence. The affinity of ZF2 to DNA affects assay sensitivity and specificity, and the amount of transcription factor needed to displace ZF2 from DNA. We found that shortening ZF2 from five fingers to four fingers reduced complementation signal, and did not produce sufficient benefit in terms of reducing the amount of transcription factor needed to achieve strong competition (Fig. S1A). Therefore, five-finger ZF2 appears to provide optimal performance in this assay.
Identification of NSC194598 as an inhibitor of p53 DNA binding
The p53 assay was used to screen ~70,000 compounds, resulting in the identification of NSC194598 (Fig. 3a). NSC194598 inhibited p53 (Fig. 3b), p63γ, and p73β (Fig. 3c) DNA binding at low micromolar concentrations, without effect on TCF1 (Fig. 3d). In an in vitro oligonucleotide pull down assay, NSC194598 inhibited p53 DNA binding, whereas PFTα had no effect (Fig. 3e). In a similar pull down assay, NSC194598 did not inhibit DNA binding by TCF1 (Fig. 3f). In the absence of p53, NSC194598 did not stimulate readout produced by ZF1-Nluc and ZF2-Cluc complementation (Fig. S1B), indicating it had no effect on zinc finger proteins. PFTα unexpectedly inhibited ZF1-Nluc/ZF2-Cluc complementation and also the activity of full-length luciferase (Fig. S1C, D), suggesting it is a potent luciferase inhibitor. Therefore, NSC194598 showed specificity for p53, p63, p73, and did not inhibit TCF1 and zinc finger proteins in vitro.
Fig. 3. NSC194598 inhibits p53 DNA binding in vitro.
a Structure of NSC194598. b NSC194598 inhibits the ability of p53 to compete with zinc finger protein for DNA binding. c NSC194598 inhibits the ability of p63 and p73 to compete with zinc finger protein for DNA binding. d NSC194598 does not inhibit TCF1 competition with zinc finger protein. e P53 binding to DNA in the presence of compounds was determined by biotinylated oligonucleotide pull down and p53 western blot. f FLAG-TCF1 was incubated with biotinylated oligonucleotide containing TCF1-binding site in a pull down assay. The amount of TCF1 bound to DNA was detected by FLAG western blot. g Diagram of luciferase fragment complementation assay that detects the DNA binding of p53-Cluc fusion protein. h IC50 of NSC194598 for inhibiting DNA binding by p53-Cluc fusion protein. The results are average of three experiments (mean ± SD). ***p < 0.001 (Student’s t test).
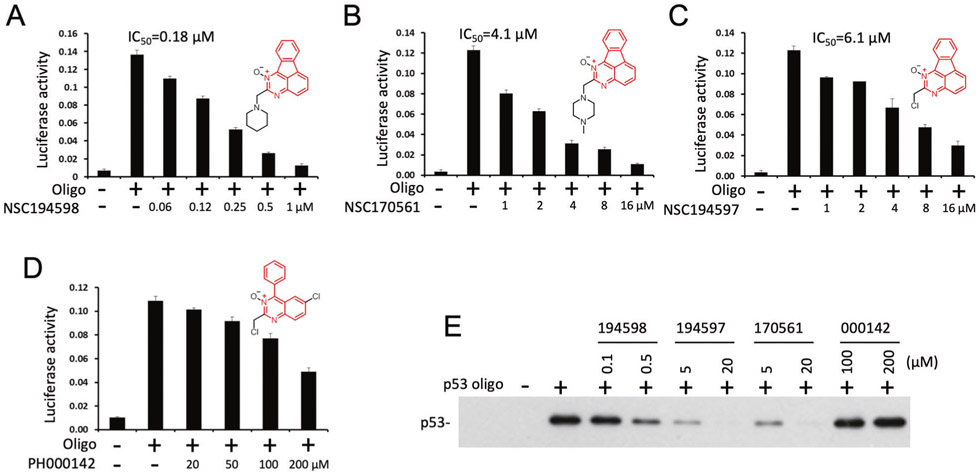
The competition assay used for HTS was not suitable for measuring IC50. For this purpose, ZF2-Cluc was replaced with p53-Cluc fusion protein that bound to DNA and complemented with ZF1-Nluc to generate signal (Fig. 3g) [5]. In this assay, NSC194598 inhibited p53-Cluc DNA binding with an IC50 of 0.18 μm (Fig. 3h). Interestingly, a p53 quadruple mutant (p53-M133L/V203A/N239Y/N268D) with a thermo-stable DNA-binding domain was more sensitive to inhibition by NSC194598 (IC50 = 0.06 μm, Fig. S1E), suggesting the compound preferentially interacts with p53 in the native conformation. In the same assay, the compound was also more potent in inhibiting p63γ-Cluc DNA binding (IC50 = 0.07 μm, Fig. S1E), which is a thermo-stable homolog of p53 [28].
Structure and function of NSC194598
To determine the structure moieties of NSC194598 critical for inhibiting p53 DNA binding, several compounds with similar chemical structures were tested. The piperidine of NSC194598 was replaced by chlorine in NSC194597, and replaced by piperazine in NSC170561. PH000142 shared only the quinazolone oxide moiety with the other compounds. The analysis showed that NSC194597 (IC50 = 6.1 μm) and NSC170561 (IC50 = 4.1 μm) inhibited p53 DNA binding in vitro, but with significantly reduced potency compared to NSC194598 (IC50 = 0.18 μm), suggesting the piperidine ring contributed to its activity and replacement of this ring system leads to significant loss of activity (Fig. 4a-c). PH000142 was inactive against p53 (Fig. 4d). Analysis using oligonucleotide pull down assay also confirmed the different potencies of these compounds in inhibiting p53 DNA binding (Fig. 4e). Overall, our data suggest the piperidine moiety was important for improving the potency, whereas the indeno quinazoline core was critical for the activity of NSC194598.
Fig. 4. Structure and activity of NSC194598 analogs.
a–d The compounds were compared for the ability to inhibit DNA binding by p53-Cluc in the in vitro complementation assay. e Biotinylated oligonucleotide containing p53-binding site was incubated with E. coli extract containing p53 in the presence of inhibitors. The protein–DNA complex was captured and analyzed by western blot for the amount of p53 bound to DNA.
NSC194598 inhibits p53 DNA binding in vivo
Chromatin immunoprecipitation (ChIP) analysis showed that NSC194598 reduced p53 occupancy at p21 and MDM2 promoters in irradiated U2OS cells, suggesting the compound inhibited p53 DNA binding in vivo (Fig. 5a, Fig. S2A). Western blot and RT-PCR analyses confirmed the inhibition of p21, MDM2, PUMA protein and mRNA expression by NSC194598 after irradiation (Fig. 5b, Fig. S2B, S2C, S2C). NSC194598 also inhibited p21 induction after p53 activation by MDM2 inhibitor Nutlin and DNA-damaging drug doxorubicin (Fig. S2F).
Fig. 5. NSC194598 inhibits p53 DNA binding in cell culture.

a U2OS cells were treated with NSC194598 alone or in combination with IR for 4 hrs. P53 binding to the p21 promoter was determined by ChIP. b U2OS cells were treated with NSC194598 and IR for 4 h and analyzed by western blot. c U2OS with or without 4 h NSC194598 treatment were analyzed by sample titration and western blot to compare MDM2/p53 ratio. d H1299 cells stably transfected with p53-A138V temperature-sensitive mutant were maintained at 39 °C or activated at 32 °C in the presence of p53 inhibitor for 8 h. P53 level and target gene induction were analyzed by western blot. e SJSA cells were treated with MDM2 inhibitor Nutlin and p53 inhibitor for 32 h. P53 level and target gene induction were analyzed by western blot. The results are average of three experiments (mean ± SD). **p < 0.01, ***p < 0.001 (Student’s t test).
Unexpectedly, treatment with the compound alone increased p53 occupancy at the p21 promoter at certain concentrations (10, 20 μm, Fig. 5a). Western blot revealed NSC194598 caused p53 accumulation and upregulation of target genes p21 and MDM2 at these concentrations (Fig. 5b), whereas 40 μm started to cause decline in p53 target gene expression despite high p53 level. Similar effects were also observed in HCT116 (Fig. S3A) and A549 cells (data not shown). The compound did not induce p21 and MDM2 in HCT116-p53−/− cells, indicating its induction of these genes were owing to activation of p53 (Fig. S3A). The rise in p53 level was likely owing to reduced turnover as there was no increase in p53 mRNA level (Fig. S2E). There was no increase of γH2AX or p53 Ser15 phosphorylation (Fig. S2B), ruling out induction of DNA damage as a cause of p53 accumulation. NSC194598 also had no effect on MDM2-mediated ubiquitination of p53 (Fig. S3C).
Certain kinases under complex negative feedback regulation in vivo (e.g., AMPK, Raf) were paradoxically activated when cells were treated with inhibitors developed to function in vitro [29-31]. P53 is constantly degraded by the MDM2-negative feedback loop [32, 33]. Partial inhibition of p53 DNA binding may disrupt the p53/MDM2 balance, resulting in p53 accumulation and increase of transcriptional output. Consistent with this hypothesis, quantitation showed MDM2/p53 ratio decreased ~50% in U2OS cells after treatment with NSC194598 (Fig. 5c, compare lane 1–5 and 6), which should reduce p53 degradation. In H1299-p53-A138V cells expressing temperature-sensitive mutant p53 that turnover slowly despite activation at 32 °C (apparently resistant to degradation by MDM2), NSC194598 inhibited p21 induction without changing p53 level (Fig. 5d). In SJSA, cells overexpressing MDM2 owing to gene amplification, NSC194598 at 5–20 μm induced much weaker p53 accumulation and p21 expression than in U2OS (Fig. 5e). Therefore, the p53-stabilizing effect of NSC194598 was most significant in cells with a delicate MDM2-p53 balance. In irradiated cells with inactivated MDM2 and stabilized p53 owing to DNA damage signaling, the compound showed robust p53-inhibitory effect.
The specificity of NSC194598 in vivo was tested by ChIP using several transcription factors. Consistent with in vitro results, the compound inhibited in vivo DNA binding by p63γ-A167P and p73β-A156I temperature-sensitive mutants at 32 °C (Fig. S4A, B) [34]. NSC194598 did not inhibit DNA binding of E2F1 expressed in the form of tamoxifen-regulated E2F1-ER fusion protein (Fig. S4C), or transiently expressed c-Myc (Fig. S4D). In further tests, NSC194598 inhibited the ability of p53 to activate expression from a p53-responsive luciferase reporter in transfected cells (Fig. S4E), but did not inhibit the ability of Foxo1 to activate its luciferase reporter (Fig. S4F). Therefore, NSC194598 showed specificity for p53 and family members but not for other transcription factors tested.
NSC194598 interacts with p53 DNA-binding domain
NSC194598 treatment of p53 did not change sensitivity to trypsin digestion (Fig. S3D), suggesting it did not induce significant conformational change. Chemical crosslinking of p53 in the presence of NSC194598 detected changes in the banding pattern of dimer and tetramer species for wt p53 (Fig. 6a, compare lanes 2 and 5), suggesting that it induced changes in certain intra or intermolecular contacts in p53 oligomers. However, there was no reduction in the total amount of oligomeric p53, suggesting that the compound did not block oligomerization. NSC194598 did not affect the crosslinking pattern of the misfolded R175H conformational mutant, suggesting the compound specifically interacts with wt p53 conformation (Fig. 6a, compare lanes 8 and 11). In a different assay, NSC194598 did not inhibit oligomer formation after mixing HA-p53 and FLAG-p53 in vitro, further supporting the notion that it does not act by blocking p53 oligomerization (Fig. S5A).
Fig. 6. NSC194598 interacts with p53 DNA-binding domain.
a H1299 extract containing wt and mutant p53 were treated with glutaraldehyde in the presence of 10 μm NSC194598. The crosslinking of p53 monomer into dimer and tetramer were determined by western blot. b Diagram of p53 domains and deletion mutants. c P53 mutants expressed in E. coli were analyzed for inhibition by 2.5 μm NSC194598 using oligonucleotide pull down assay. d FLAG-tagged p53–94–312 DNA-binding domain was expressed in E. coli. The purified protein was analyzed by DSF assay in the presence of 100 μm NSC194598. e TAF1 bromodomain 2 was analyzed by DSF assay in the presence of 100 μm NSC194598. f FLAG-p53 immobilized on beads were incubated with 10 μm NSC194598 for 30 min, washed with DNA-binding buffer for 3 min or 3 hr, eluted with FLAG peptide, and tested for DNA binding. NSC194598 (2 μm) was added to the oligonucleotide binding reaction where indicated. The p53 captured by DNA was detected by western blot.
To identify the p53 domain targeted by NSC194598, p53 deletion mutants (Fig. 6b, Fig. S5B) were tested in oligonucleotide pull down assay. The results showed 1–360 and 90–393 were strongly inhibited by the compound, indicating the DNA-binding domain and oligomerization domain were targets of the compound (Fig. 6c). After further removal of the oligomerization domain, the DNA-binding domain (90–312) had very weak DNA-binding activity as expected, but still showed inhibition by NSC194598 (Fig. 6c). The results suggest NSC194598 targets the DNA-binding domain of p53.
To test whether NSC194598 binds to p53, differential scanning fluorimetry (DSF) was used to detect changes in p53 melting temperature. The presence of NSC194598 caused a modest increase (0.5 °C) in the melting temperature of FLAG-94-312 (Fig. 6d). As specificity control, NSC194598 did not affect the melting temperature of TAF1 bromodomain 2 (aa 1501–1635) (Fig. 6e). The results suggest that NSC194598 directly interacts with the DNA binding domain of p53.
To further test whether NSC194598 interaction with p53 alone was sufficient to inhibit DNA binding, FLAG-p53 was immobilized on beads, incubated with the compound, and washed to remove free compound. The treated p53 was eluted from beads and tested for DNA-binding activity in the oligonucleotide pull down assay. The results showed that NSC194598-treated p53 did not bind DNA if the washing was short (3 min). After extensive washing (3 h), the treated p53 regained DNA-binding activity (Fig. 6f, compare lane 2 and 5). The washed p53 remained sensitive to re-inhibition if NSC194598 was added back to the DNA-binding reaction (Fig. 6f, compare lane 5 and 6). The results suggest that p53-NSC194598 complex had no DNA-binding activity, whereas the complex dissociated after the long wash and p53 DNA-binding function was restored.
NSC194598 increases the survival of mice after irradiation
Primary mouse thymocytes were used as a model to test whether NSC194598 protects normal cells from DNA damage-induced apoptosis. It is well established that gamma radiation induces apoptosis of thymocyte in a p53-dependent manner [35]. Treatment with 0.5–10 μm NSC194598 blocked PARP and Caspase 3 cleavage after IR treatment in a dose-dependent manner (Fig. 7a). Similar to human cell lines, high concentrations of NSC194598 also caused p53 accumulation in mouse thymocytes and appearance of cleaved PARP (Fig. 7a). However, despite PARP cleavage, trypan blue exclusion test suggested thymocytes did not lose viability in the presence of NSC194598, significant cell death only occurred after IR treatment (Fig. 7b). Furthermore, NSC194598 increased thymocyte viability 20 hours after IR (Fig. 7b, Fig. S6). Unlike human cell lines, NSC194598 treatment (1 μm or higher) of irradiated thymocytes reduced p53 level through unknown mechanism (Fig. 7a). The results suggest NSC194598 may be useful for protecting normal cells from acute radiation toxicity.
Fig. 7. NSC194598 protects mice from radiation toxicity.
a Primary mouse thymocytes were treated with NSC194598 alone or in combination with gamma radiation. PARP and Caspase 3 cleavage were analyzed after 6 h. b Thymocyte viability was determined by trypan blue exclusion 20 h after treating with 5 Gy IR and 5 μm NSC194598 (blue=dead cells). c C57BL/6 mice were given a single i.p. injection of NSC194598, immediately followed by 8 Gy gamma radiation. Viability was monitored for 40 days. d, e Body weight of a subset of mice in c were monitored after irradiation and individually plotted. Lines terminating with X represent animals that have reached experimental endpoint.
To test whether NSC194598 provides protection against radiation toxicity, mice were given a single i.p. injection of the compound immediately before 8 Gy gamma radiation treatment. Owing to solubility limit, the amount of compound administered was not expected to reach levels used in cell culture (<5 μm). Western blot analysis of animals treated with the compound did not reveal increase of p53 or PARP cleavage in the thymus (data not shown). Therefore, the in vivo level and inhibition of p53 by the compound were likely to be weaker than cell culture conditions. However, administration of the compound resulted in a statistically significant improvement in survival in the 40-day observation period after irradiation (Fig. 7c). The compound did not prevent body weight loss for most animals after irradiation, but increased the probability of weight recovery after the initial decrease (Fig. 7d, e). A previous study showed altering p53 activity by 20–30% had significant impact on viability after irradiation [36]. It remains to be determined whether NSC194598 increased radiation resistance by subtle reduction of p53 activity in key cell types such as the hematopoietic compartment.
Discussion
Transcription factors are central nodes in signaling networks, making them attractive targets in many diseases. Inhibiting the DNA-binding activity of a transcription factor is an effective approach to disrupt a pathway. Such inhibitors in principle may act by a variety of mechanisms such as disrupting intramolecular or intermolecular interactions critical for DNA binding, stabilizing the protein in an auto-inhibited conformation, allosterically altering the DNA-binding domain, or directly blocking the DNA-binding site. In the absence of complete structural information, developing robust assays for DNA-binding inhibitors will enable unbiased search for chemical probes and lead compounds.
We established an HTS-compatible DNA-binding assay that is adaptable to different transcription factors. The positive readout assay requires specific DNA binding by zinc finger proteins and increased luciferase activity to indicate a hit. Therefore, undesirable compounds such as metal chelators, thio-reactive compounds and luciferase inhibitors are counter-selected in the screen. The assay uses non-purified recombinant proteins in E. coli extracts and does not require special chemical labeling or proprietary reagents. As it is possible to design zinc fingers that specifically bind to nearly any consecutive 15 bp DNA sequence, the assay can be customized to other transcription factors [24].
As demonstrated in our experiments, ZF1-Nluc/ZF2-Cluc/DNA readout systems specific for p53, TCF1, and HIF-1 were successfully designed, suggesting the strategy is adaptable to other DNA-binding proteins. A hurdle unique to each target is the production of active protein capable of competing with ZF2 for DNA binding. This was readily achievable for homo-oligomeric p53/p63/p73 proteins and monomeric TCF1, but unsuccessful for the heterodimeric HIF-1. Producing functional heterodimeric protein is inherently challenging and may require more-sophisticated approach. Assay performance may be improved using mutants that mimic PTMs such as acetylation and phosphorylation, mutants that are constitutivel y active, and mutants that increase the thermodynamic stability of the target.
Most chemical libraries contain compounds developed to inhibit enzymes; therefore, the rate of identifying DNA-binding inhibitors was unclear. Our screen using p53 as target identified 1 hit from ~70,000 compounds, suggesting that small molecules with such activity are present in the libraries at very low frequency. Large-scale HTS campaigns will be needed to identify multiple drug-like leads for further development. NSC194598 exhibited sub-micromolar potency and specificity against p53 family proteins, but did not inhibit TCF1, E2F1, c-Myc, and Foxo1 in vitro or in vivo. To our knowledge NSC194598 is the first inhibitor of p53 DNA binding, although caution is warranted regarding its degree of specificity. A recent report showed this compound binds G-quadruplex DNA structures and inhibits the transcription of RET oncogene [37]. NSC194598 in its present form has sufficient potency and specificity in vitro and may be useful as a chemical probe to investigate specific mechanism of p53 inhibition. The p53 screen suggests the strategy may be used to identify inhibitors of other transcription factors.
Similar to the paradoxical in vivo activation of auto-regulated kinases by inhibitors, NSC194598 caused p53 accumulation and modest induction of target genes at certain concentrations. Puzzling at first this is in fact an expected effect given that p53 is regulated by the MDM2-negative feedback loop. Mutant p53 with inactivating point mutations in the DNA-binding domain accumulate partly owing to failure to induce MDM2 expression [38, 39]. SV40 T antigen interaction with the p53 DNA-binding surface also causes p53 accumulation [40]. Therefore, non-saturating levels of p53 DNA-binding inhibitors are expected to cause p53 stabilization. The accumulated p53 is not fully inactivated by the compound, thus resulting in partial induction of target genes. Our results suggest that other p53 DNA-binding inhibitors will also have similar paradoxical weak activating effects in vivo.
Clinical trials of MDM2 inhibitors showed repeated administration of potent p53 activators causes toxicity [41]. An important question that needs to be addressed is whether the paradoxical activation of p53 by DNA-binding inhibitors is detrimental to their use for radiation and chemo protection. Cell culture analyses showed that NSC194598 was less potent in inducing p53 target genes compared with irradiation, as the accumulated p53 was not fully functional in DNA binding. Therefore, it is unlikely that p53 inhibitors will cause similar level of toxicity as MDM2 inhibitors. Our animal test showed NSC194598 provided radiation protection benefits. More definitive answers will require the ability to test the compound at higher levels in vivo.
In summary, our HTS assay led to the identification of a sub-micromolar p53 DNA-binding inhibitor, providing proof of principle on the therapeutic potential of such molecules. Analysis of the compound also provided unexpected insight on p53 dynamic response to DNA-binding inhibition. Further structural analysis of the inhibitor interaction with p53 should provide valuable details on the mechanism of inhibition and facilitate the design of improved compounds.
Materials and methods
Cell lines, proteins, and compounds
H1299 (lung cancer, p53-null), A549 (lung cancer, wt p53), U2OS (osteosarcoma, wt p53), HCT116 (colon cancer, wt p53) cells were maintained in Dulbecco modified Eagle medium with 10% fetal bovine serum. All cell lines used in this study were obtained from the ATCC and authenticated and tested negative for mycoplasma contamination before use. NSC194598, NSC194597, NSC170561 were kindly provided by the National Cancer Institute Developmental Therapeutics Program. PH000142 was purchased from Sigma. Molecular weight of NSC194598 was confirmed by HPLC-MS analysis and purity was determined to be ~90% (Fig. S7). Zinc finger proteins were designed using the Zinc Finger Tools website (https://www.scripps.edu/barbas/zfdesign/zfdesignhome.php) [24]. The ORF sequences of zinc finger-luciferase fusion proteins were synthesized (GenScript, Inc.) and cloned into the NcoI site of pET-28b vector to prevent the addition of epitope tags. TCF1, p53, and p53-R175H were also cloned into pET-28b vector at the NcoI site. The proteins were expressed in E. coli strain BL21 DE3. Transformed cells were grown in LB containing Kanamycin at 37 °C for 18 h, diluted 1:50 in 2xYT containing Kanamycin, and cultured to OD = 0.6 at 600 nm. Isopropyl thiβ-d-galactoside (1 mm) and ZnCl2 (0.1 mm) were added and further cultured at 18 °C for 18 h. Cells were harvested (5000 × g, 10 min at 4 °C) and resuspended in lysis buffer (100 mm potassium phosphate pH7.8, 1 mm ZnCl2, 1 mm DTT). The cells were lysed by sonication and centrifuged at 20,000 × g for 20 min at 4°C. Cell extract (5 ml prepared from 180 ml culture) was stored at −80 °C with the addition of 5% glycerol.
Amino acid sequences of zinc finger fusion proteins
ZF1-Nluc (Uppercase: ZF1. Lowercase: Nluc. Binds to 5’ATGTAGGGAAAAGCCCGG):
MGISEFGSSSSVAQAALEPGEKPYACPECGKSFSRS DHLAEHQRTHTGEKPYKCPECGKSFSDKKDLTRHQR THTGEKPYKCPECGKSFSQRANLRAHQRTHTGEKP YACPECGKSFSQLAHLRAHQRTHTGEKPYKCPECGKS FSREDNLHTHQRTHTGEKPYKCPECGKSFSRRDALNV HQRTHTGKKTSGGGGSGGGGSmedaknikkgpapfypledgta geqlhkamkryalvpgtiaftdahievnityaeyfemsvrlaeamkryglntnhriv vcsenslqffmpvlgalfigvavapandiynerellnsmmsqptvvfvskkglqki lnvqkklpiiqkiiimdsktdyqgfqsmytfvtshlppgfneydfvpesfdrdktial imnssgstglpkgvalphrtacvrfshardpifgnqiipdtailsvvpfhhgfgmfttl gylicgfrvvlmyrfeeelflrslqdykiqsallvptlfsffakstlidkydlsnlheiasg Gaplskevgeavakrfhlpgirqgygltettsailitpegddkpgavgkvvpffeakv vdldtgktlgvnqrgelcvrgpmimsgyvnnpeatnalidkdgwlhsgdlaywd edehffivgr*
ZF2-Cluc (Uppercase: ZF2. Lowercase: Cluc. Binds to 5’GAACATGTCCCAACA of p53-binding site 5’GAACATGTCCCAACATGTTG.):
MGGGGSLEPGEKPYKCPECGKSFSSPADLTRHQRT HTGEKPYKCPECGKSFSTSHSLTEHQRTHTGEKPYKC PECGKSFSDPGALVRHQRTHTGEKPYKCPECGKSFST SGNLTEHQRTHTGEKPYKCPECGKSFSQSSNLVRHQR THTGKKTSGGGGSGGGGSsgyvnnpeatnalidkdgwlhsgdlay wdedehffivgrlkslikykgyqvapaelesillqhpnifdagvaglpdddagelp-aavvvlehgktmtekeivdyvasqvttakklrggvvfvdevpkgltgkrdarkire ilikakkggkskl*
Antisense-TCF-ZF2-Cluc (Binds to 5’ GAATGATCAAAGGGTCCATCA, antisense strand of TCF1 site 5’TGATGGACCCTTTGATCATTC.):
MGLEPGEKPYKCPECGKSFSDPGALVRHQRTHT-GEKPYKCPECGKSFSRSDHLTNHQRTHTGEKPYKCP-ECGKSFSQSGNLTEHQRTHTGEKPYKCPECGKSFST-SGNLVRHQRTHTGEKPYKCPECGKSFSTTGNLTVH-QRTHTGKKTSGGGGSGGGGSGGGGSsgyvnnpeatnalid-kdgwlhsgdlaywdedehffivgrlkslikykgyqvapaelesillqhpnifda-gvaglpdddagelpaavvvlehgktmtekeivdyvasqvttakklrggvvfv-devpkgltgkrdarkireilikakkggkskldykddddk*
Antisense-Cluc-HIF-1-ZF2 (Binds to 5’TGAGACAGCACGTAGGGC, antisense strand of HIF-1 site 5’GCCCTACGTGCTGTCTCA.):
Mdykddddksgyvnnpeatnalidkdgwlhsgdlaywdedehffivgrl kslikykgyqvapaelesillqhpnifdagvaglpdddagelpaavvvlehgktm tekeivdyvasqvttakklrggvvfvdevpkgltgkrdarkireilikakkggkskl GGGGSGGGGSGGGGSLEPGEKPYKCPECGKSFSRED-NLHTHQRTHTGEKPYKCPECGKSFSRTDTLRDHQRT-HTGEKPYKCPECGKSFSERSHLREHQRTHTGEKPYKC PECGKSFSDPGNLVRHQRTHTGEKPYKCPECGKSFSQ AGHLASHQRTHTGKKTS*
Luciferase complementation assay
The 40 μl p53 competition reaction for high-throughput screen and compound analysis contained crude E. coli extracts (0.2 μl ZF1-Nluc, 0.2 μl ZF2-Cluc, 3 μl p53), 30 ng annealed oligonucleotides, and compounds in luciferase buffer (100 mm potassium phosphate pH7.8, 1 mm ZnCl2, 5% glycerol, 1 mm DTT). The alternative p53 DNA-binding assay for IC50 determination replaced ZF2-Cluc and p53 extracts with 0.5 μl p53-Cluc extract and 50 ng ZPBS12 plasmid, which is a pUC57 vector containing a 560 bp synthesized DNA insert containing 12 copies of ZF1 and p53-binding sites with various spacing (0, 2, 4, 6, 8, 10 bp). The mixture was incubated for 35 min at 32 °C. Luciferase substrate A (25 mm glycylglycin pH7.8, 15 mM potassium phosphate pH7.8, 15 mm MgSO4, 4 mm EGTA, 2 mm ATP, 1 mm DTT) was combined with equal volume of luciferase substrate B (0.4 mg/ml D-luciferin, 25 mm glycylglycin pH7.8, 2 mm DTT), 30 μl was added to the reaction mixture. After incubating for 10 min at 23 °C, luciferase signal was measured using a microplate luminometer. Compound screening was carried out by the University of Illinois High-throughput Screening Facility.
Oligonucleotide pull down assay
The DNA-binding reaction contained 100 ng p53 (~0.1 μl E. coli lysate), 2 μg poly(dI-dC) and 100 ng double-stranded biotinylated oligonucleotide DNA containing p53-binding site (Biotin-5’TCGAGAGGCATGTCTAGGCATGTCTC annealed to 5’GAGACATGCCTAGACATGCCTCTCGA) in 200 μl DNA-binding buffer (150 mm NaCl, 20 mm Tris-HCl pH7.2, 1 mm EDTA, 0.1% Triton X-100, 5 mm DTT, 4% glycerol), incubated for 30 min at 4 °C. Magnetic streptavidin beads (50 μl packed volume, Promega) were added to capture the DNA/protein complexes for 30 min at 4 °C. The beads were collected using a magnet, washed three times with DNA binding buffer, and eluted by boiling in Laemmli sample buffer. P53 was detected by western blot.
Western blot
Cells were lysed in lysis buffer (50 mm Tris-HCl pH8.0, 5mm EDTA, 150 mm NaCl, 0.5% Nonidet P-40, protease inhibitors), centrifuged at 14,000 × g for 10 min, and the cell debris was discarded. The samples were boiled in Laemmli sample buffer, fractionated by SDS-PAGE, and transferred to Immobilon-P filters (Millipore). The filters were blocked, incubated with primary and secondary antibodies in PBS containing 5% nonfat dried milk and 0.1% Tween 20, and developed using SuperSignal reagent (Thermo Scientific). The following antibodies were used: FL393 (Santa Cruz, sc-6243) and DO-1 (BD Pharmingen, 554293) for p53. FLAG antibody (Sigma, F7425). P21 antibody (BD Pharmingen, 556430). Monoclonal antibody 2A9 for MDM2 (produced in house). β-actin antibody (Sigma, A5441). P53 pSer15 and PUMA antibody (Cell Signaling, 16G8, 4976 S). PARP antibody (BD Pharmingen, 611039), Cleaved caspase 3 antibody (Cell Signaling, 9661), γH2AX (EMD Millipore, 05–636).
Chromatin immunoprecipitation
Chromatin immunoprecipitation was carried out using standard procedure. The samples were subjected to SYBR Green real-time PCR analysis using the following primers: p21 promoter (AGGAAGGGGATGGTAGGAGA and ACACAAGCACACATGCATCA). MDM2 promoter (GGGCTATTTAAACCATGCATTTTC and GTCCGTGCCCACAGGTCTA). ARF promoter (CCCTCGTGCTGATGCTACTG and ACCTGGTCTTCTAGGAAGCGG). PUMA promoter (CTGTGGCCTTGTGTCTGTGAGTAC and CC TAGCCCAAGGCAAGGAGGAC). MXD3 promoter (CATTCGCACATGGCTTCTCA and CCAGCCAATAGGGCATCAGA). The following antibodies were used: DOI for p53, C29F4 HA tag antibody (Cell Signaling) for p63, p73, and c-MYC, KH95 for E2F1.
Glutaraldehyde crosslinking
H1299 cells were transiently transfected with pcDNA3 vector expressing FLAG-tagged wild type or mutant p53. Cells (~2×106 from a 10 cm plate) were lysed in 1 ml HEPES buffer (50 mm HEPES, pH8.0, 5 mm EDTA, 150 mm NaCl, 0.5% Nonidet P-40, protease inhibitors) and centrifuged at 14,000 × g for 10 min to remove cell debris. Cell lysate was incubated with 10 μm NSC194598 for 20 min at 23 °C. Glutaraldehyde was added (0.01–0.1%) and incubated for 5 min at 23 °C. Tris-HCl pH8.0 was added to 100 mm to terminate the reaction. P53 was detected by western blot using FL393 antibody.
Luciferase reporter assay
H1299 cells (50,000/well) were seeded in 24-well plate and transfected with 20 ng p53-responsive BP100-luciferase reporter plasmid, 5 ng CMV-lacZ plasmid, and 0.2 ng CMV-p53 plasmid. Alternatively, 20 ng Foxo1-responsive 3IRS-luciferase reporter plasmid, 5 ng CMV-lacZ and 10 ng CMV-Foxo1 were transfected. Transfection was achieved using Lipofectamine 3000 (Invitrogen) for 24 h. Cells were treated with NSC194598 for additional 6 h and analyzed for luciferase and beta-galactosidase activity. The transcriptional activity of p53 and Foxo1 was indicated by the ratio of luciferase/beta-galactosidase activity.
In vivo ubiquitination assay
H1299 cells were seeded in 10 cm plate and transfected with 2 μg His6-ubiquitin, 0.2 μg p53, and 2 μg MDM2 expression plasmids using the PEI method for 36 h. The cells were treated with NSC194598 for additional 5 h. Cells were lysed in buffer A (6 m guanidinium-HCl, 0.1 m Na2HPO4/NaH2PO4, 0.01 m Tris-HCl pH8.0, 5 mm imidazole, 10 mm β-mercaptoethanol) and incubated with Ni2+-NTA beads (Qiagen) for 16 h at 23 °C. The beads were washed with buffer A, buffer B (8 m urea, 0.1 m Na2PO4–NaH2PO4, 0.01 m Tris-HCl pH8.0, 10 mm β-mercaptoethanol), buffer C plus (8 m urea, 0.1 m Na2PO4-NaH2PO4, 0.01 m Tris-HCl pH6.3, 10 mm β-mercaptoethanol, 0.2% Triton X-100), and buffer C (8 m urea, 0.1 m Na2PO4-NaH2PO4, 0.01m Tris-HCl pH6.3, 10 mm β-mercaptoethanol) for 5min each. The beads were incubated with 50 μl elution buffer (200 mm imidazole, 0.15 m Tris-HCl pH6.7, 30% glycerol, 0.72 m β-mercaptoethanol, 5% SDS) for 20 min at 23 °C with shaking at 800 rpm. The samples were boiled in Laemmli buffer and analyzed by western blotting using DO-1 antibody.
Animal experiment
Animal experiments were reviewed and approved by the University of South Florida IACUC. Thymocytes were isolated from mice at age 4–7 weeks. Each thymus was cut into small fragments, digested with 3 ml PBS containing 0.5 mg/ml collagenase D and 20 U/ml DNase I for 30 min at 37 °C, neutralized with 10 ml RPMI-1640 containing 10% fetal bovine serum (FBS), filtered by 70 μm cell strainer, and centrifuged at 500 × g for 5 min at 23 °C. The cells were cultured in RPMI-1640 containing 10% FBS and used immediately for radiation and drug treatments (3 × 106 cells for western blot). To test NSC194598 effect on radiation toxicity, C57BL/6 mice (~2 month old, males and females, ramdomized) were given i.p injection of 80 μg NSC194598 dissolved in 0.1 ml 40% (2-Hydroxypropyl)-β-cyclodextrin (Sigma H107), immediately followed by 8 Gy gamma irradiation. Sample size was chosen based on the assumption that the treatment caused 50% change in survival with a 50% deviation, 12 mice will have >98% power to detect the decrease in a one tail test with 95% confidence. The mice were killed when appearing moribund and were considered to have reached experimental endpoint.
DSF analysis
FLAG-p53–94–312 was expressed in E. coli using pET-28b vector, purified using M2 beads (Sigma), and eluted using FLAG peptide. The expression plasmid for TAF1 (Uniprot ID P21675) 2nd bromodomain (TAF1–2, residues 1501–1635) was obtained from Addgene (plasmid 39117). TAF1–2 protein was purified using Ni-NTA column and size exclusion column. DSF experiments were carried out in Applied Biosystem StepOnePlus real-time PCR system (Thermo Fisher Scientific) using sealed 96-well format plates, assayed in quadruplicate. In brief, 4.5 μm of each protein was mixed with 100 μm compound in the presence of 5× fluorescence dye SYPRO Orange (Invitrogen, Thermo Fisher Scientific) to the final volume of 20 μl in assay buffer (50 mm HEPES pH7.4, 150 mm NaCl, 2 mm DTT, 2% DMSO). Reaction mixtures were heated from 25 °C to 95 °C at 1 °C/min with fluorescence signal recorded every 0.5 °C at 610 nm. The observed thermal shift (ΔTm) was calculated as the difference between the Tm of sample and DMSO reference wells.
Supplementary Material
Acknowledgements
The authors wish to thank the National Cancer Institute Developmental Therapeutics Program for providing key compounds. This work is supported in part by grants from the National Institutes of Health (CA141244, CA186917, CA208363 to J.C. GM115556 to D.G. R50CA211447 to H. R. L.). H. Lee Moffitt Cancer Center & Research Institute is an NCI designated Comprehensive Cancer Center (P30-CA076292).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information The online version of this article (https://doi.org/10.1038/s41388-020-1344-y) contains supplementary material, which is available to authorized users.
References
- 1.Bushweller JH. Targeting transcription factors in cancer - from undruggable to reality. Nat Rev Cancer. 2019;19:611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg T Small-molecule modulators of c-Myc/Max and Max/Max interactions. Curr Top Microbiol Immunol. 2011;348:139–49. [DOI] [PubMed] [Google Scholar]
- 3.Hoggard LR, Zhang Y, Zhang M, Panic V, Wisniewski JA, Ji H. Rational design of selective small-molecule inhibitors for beta-catenin/B-cell lymphoma 9 protein-protein interactions. J Am Chem Soc. 2015;137:12249–60. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Yue P, Page BD, Li T, Zhao W, Namanja AT, et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci USA. 2012;109:9623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He F, Borcherds W, Song T, Wei X, Das M, Chen L, et al. Interaction between p53 N terminus and core domain regulates specific and nonspecific DNA binding. Proc Natl Acad Sci USA. 2019;116:8859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retzlaff M, Rohrberg J, Kupper NJ, Lagleder S, Bepperling A, Manzenrieder F, et al. The regulatory domain stabilizes the p53 tetramer by intersubunit contacts with the DNA binding domain. J Mol Biol. 2013;425:144–55. [DOI] [PubMed] [Google Scholar]
- 7.Wassman CD, Baronio R, Demir O, Wallentine BD, Chen CK, Hall LV, et al. Computational identification of a transiently open L1/S3 pocket for reactivation of mutant p53. Nat Commun. 2013;4:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. [DOI] [PubMed] [Google Scholar]
- 9.Khoo KH, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov. 2014;13:217–36. [DOI] [PubMed] [Google Scholar]
- 10.Gudkov AV, Komarova EA. Prospective therapeutic applications of p53 inhibitors. Biochem Biophys Res Commun. 2005;331: 726–36. [DOI] [PubMed] [Google Scholar]
- 11.Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, et al. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23:3265–71. [DOI] [PubMed] [Google Scholar]
- 12.Westphal CH, Hoyes KP, Canman CE, Huang X, Kastan MB, Hendry JH, et al. Loss of atm radiosensitizes multiple p53 null tissues. Cancer Res. 1998;58:5637–9. [PubMed] [Google Scholar]
- 13.Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, et al. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25:4056–66. [DOI] [PubMed] [Google Scholar]
- 14.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Ren Physiol. 2007;293:F1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvajal D, Tovar C, Yang H, Vu BT, Heimbrook DC, Vassilev LT. Activation of p53 by MDM2 antagonists can protect proliferating cells from mitotic inhibitors. Cancer Res. 2005;65:1918–24. [DOI] [PubMed] [Google Scholar]
- 16.Checler F, Alves, da Costa C. p53 in neurodegenerative diseases and brain cancers. Pharmacol Ther. 2014;142:99–113. [DOI] [PubMed] [Google Scholar]
- 17.Crumrine RC, Thomas AL, Morgan PF. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab. 1994;14:887–91. [DOI] [PubMed] [Google Scholar]
- 18.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–7. [DOI] [PubMed] [Google Scholar]
- 19.Komarova EA, Neznanov N, Komarov PG, Chernov MV, Wang K, Gudkov AV. p53 inhibitor pifithrin alpha can suppress heat shock and glucocorticoid signaling pathways. J Biol Chem. 2003;278:15465–8. [DOI] [PubMed] [Google Scholar]
- 20.Walton MI, Wilson SC, Hardcastle IR, Mirza AR, Workman P. An evaluation of the ability of pifithrin-alpha and -beta to inhibit p53 function in two wild-type p53 human tumor cell lines. Mol Cancer Ther. 2005;4:1369–77. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Singh M, Selivanova G, Peuget S. Pifithrin-alpha alters p53 post-translational modifications pattern and differentially inhibits p53 target genes. Sci Rep. 2020;10:1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci USA. 2004;101:12288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal DJ, Crotty JW, Bhakta MS, Barbas CF 3rd, Horton NC. Structure of Aart, a designed six-finger zinc finger peptide, bound to DNA. J Mol Biol. 2006;363:405–21. [DOI] [PubMed] [Google Scholar]
- 24.Mandell JG, Barbas CF 3rd. Zinc Finger Tools: custom DNA-binding domains for transcription factors and nucleases. Nucleic Acids Res. 2006;34:W516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–8. [DOI] [PubMed] [Google Scholar]
- 26.van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–99. [DOI] [PubMed] [Google Scholar]
- 27.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoo KH, Andreeva A, Fersht AR. Adaptive evolution of p53 thermodynamic stability. J Mol Biol. 2009;393:161–75. [DOI] [PubMed] [Google Scholar]
- 29.Hall-Jackson CA, Eyers PA, Cohen P, Goedert M, Boyle FT, Hewitt N, et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chem Biol. 1999;6:559–68. [DOI] [PubMed] [Google Scholar]
- 30.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross FA, Hawley SA, Auciello FR, Gowans GJ, Atrih A, Lamont DJ, et al. Mechanisms of paradoxical activation of AMPK by the kinase inhibitors SU6656 and sorafenib. Cell Chem Biol. 2017;24:813–24. e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 auto-regulatory feedback loop. Genes Dev. 1993;7:1126–32. [DOI] [PubMed] [Google Scholar]
- 34.Pochampally R, Li C, Lu W, Chen L, Luftig R, Lin J, et al. Temperature-sensitive mutants of p53 homologs. Biochem Biophys Res Commun. 2000;279:1001–10. [DOI] [PubMed] [Google Scholar]
- 35.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–9. [DOI] [PubMed] [Google Scholar]
- 36.Pant V, Xiong S, Jackson JG, Post SM, Abbas HA, Quintas-Cardama A, et al. The p53-Mdm2 feedback loop protects against DNA damage by inhibiting p53 activity but is dispensable for p53 stability, development, and longevity. Genes Dev. 2013;27:1857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin YJ, Kumarasamy V, Camacho D, Sun D. Involvement of G-quadruplex structures in regulation of human RET gene expression by small molecules in human medullary thyroid carcinoma TT cells. Oncogene. 2015;34:1292–9. [DOI] [PubMed] [Google Scholar]
- 38.Goh AM, Xue Y, Leushacke M, Li L, Wong JS, Chiam PC, et al. Mutant p53 accumulates in cycling and proliferating cells in the normal tissues of p53 R172H mutant mice. Oncotarget. 2015;6:17968–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Midgley CA, Lane DP. p53 protein stability in tumour cells is not determined by mutation but is dependent on Mdm2 binding. Oncogene. 1997;15:1179–89. [DOI] [PubMed] [Google Scholar]
- 40.Lilyestrom W, Klein MG, Zhang R, Joachimiak A, Chen XS. Crystal structure of SV40 large T-antigen bound to p53: interplay between a viral oncoprotein and a cellular tumor suppressor. Genes Dev. 2006;20:2373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tisato V, Voltan R, Gonelli A, Secchiero P, Zauli G. MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer. J Hematol Oncol. 2017;10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.