Abstract
Data suggest that nutrient order during a meal significantly impacts postprandial glucose and insulin excursions in type 2 diabetes, while its’ effects in prediabetes have not been reported. Fifteen participants with prediabetes consumed the same meal on 3 days in random order: carbohydrate first, followed 10 minutes later by protein and vegetables (CF); protein and vegetables first, followed 10 minutes later by carbohydrate (PVF); or vegetables first followed by protein and carbohydrate (VF). Blood was sampled for glucose and insulin measurements at 0, 30, 60, 90, 120,150 and 180 min. Incremental glucose peaks were similarly attenuated by >40% in the PVF and VF meal conditions compared with CF. The incremental area under the curve for glucose (iAUC0–180) was 38.8% lower following the PVF meal order, compared with CF, and postprandial insulin excursions were significantly lower in the VF meal condition compared to CF. The CF meal pattern demonstrated marked glycemic variability whereas glucose levels were stable in the PVF and VF meal conditions. Food order presents a novel, simple behavioral strategy to reduce glycemic excursions in prediabetes.
Introduction
Concordant with the increasing prevalence of overweight and obesity, prediabetes is a growing epidemic in the US and globally. Approximately 37% of adults in the United States over the age of 20, and 51% of those over 65, had prediabetes between 2009 to 2012, as determined by fasting glucose or A1c (1). The estimated worldwide prevalence of impaired glucose tolerance was 280 million in 2011, with projections of 398 million by 2030 (2). Data suggest prediabetes itself increases the risk for both microvascular and macrovascular complications, independent of progression to type 2 diabetes (3–7).
Intensive lifestyle interventions including diet modification and increased physical activity have been shown to reduce the risk of progression to T2DM in several randomized controlled trials in a wide variety of populations (8–10). Key components of standard nutritional counseling include reducing calorie intake and glycemic load. Sequential nutrient ingestion is a novel strategy to attenuate the glycemic effect of a meal researched by our group and others in patients with type 2 diabetes (11–14). In a previous crossover study of metformin-treated subjects with T2DM, we demonstrated that ingestion of protein plus vegetables before carbohydrate led to lower postprandial glucose excursions over 180 min, compared to eating the same foods in the reverse order (13). Furthermore, we showed that the carbohydrate-last meal pattern stimulated a lower insulin response compared to consuming carbohydrate first. In this study, we assessed the generalizability of these findings to individuals with prediabetes and to a meal with a different macronutrient composition. In addition, we sought to determine the glycemic effect of a third real-world meal pattern of simply consuming a salad first, followed by protein and carbohydrate together. We postulated that: 1) eating vegetables and protein together as the first course (PVF food order), followed by carbohydrate will generate lower post-meal glucose excursions compared to the carbohydrate-first meal pattern (CF food order); and 2) that the meal order of vegetables first followed by protein and carbohydrate together (VF food order) will result in glycemic effects intermediate between PVF and CF.
Methods
Participants
Subjects were recruited from our institutional research database following review of electronic medical records and through flyers posted within and around the institution. Potential subjects were pre-screened via a phone interview, and all interested subjects who met preliminary eligibility criteria were formally screened. Male and female participants between 30 and 65 years of age, body mass index 25–40 kg/m2, and with prediabetes (HbA1c 5.7-6.4%) were included in the study. Patients taking corticosteroids, antidiabetic medication, and patients with chronic renal or hepatic disease or history of prior bariatric surgery and pertinent food allergies were excluded. The study was approved by the Weill Cornell Medical College Institutional Review Board (IRB#1612017822) and registered retrospectively in Clinicaltrials. gov (NCT03536364). All participants gave written informed consent.
Study Protocol
We used a within-subjects crossover design in which all participants consumed isocaloric meals (Table 1) with exactly the same composition, on three separate days, 1 week apart, after a 12 hour overnight fast. Participants were counseled to maintain their usual level of physical activity and diet throughout the study period and in particular the day prior to each study visit. All meals were prepared in the metabolic kitchen of the Clinical and Translational Science Center at Weill Cornell Medical College under the supervision of a Registered Dietitian. Each food item was measured and weighed, and the ESHA Food Processer was used to assess the nutrient composition of the meal. Each meal was consumed in 30 min, under the following experimental conditions that were randomly assigned using research randomizer:
Carbohydrate first (CF) (ciabatta bread) over 10 min, a 10 min rest interval, and then protein (skinless grilled chicken breast) and vegetables (lettuce, tomatoes, bell peppers, red cabbage, with balsamic vinegar and olive oil) over 10 min.
Protein and vegetables first (PVF) over 10 min, a 10 min rest interval, and then carbohydrate over 10 min.
Vegetables first (VF) over 10 min, a 10 min rest interval, and then protein and carbohydrate together over 10 min.
Table 1:
Test Meal Composition
| Ciabatta bread – “au bon pain” | 90 g | ||
| Chicken breast (skinless, flame grilled) | 100 g | ||
| Lettuce (romaine) | 35 g | ||
| Peppers (sweet, bell, red) | 110 g | ||
| Tomatoes (red) | 110 g | ||
| Cabbage (red) | 30 g | ||
| Oil (olive) | 15 g | ||
| Vinegar (balsamic) | 8 g | ||
| Herb (oregano) | 0.1 g | ||
| Herb (thyme) | 0.1 g | ||
| Spice (powdered garlic) | 0.1 | ||
| Calories (kJ) | Protein (g) | Fat (g) | Carbohydrate (g) |
| 2417.64 | 41.05 | 20.06 | 58.28 |
Participants were closely monitored to ensure that the test meals were consumed in their entirety within the allotted time. Blood samples were drawn from an in-dwelling venous cannula at baseline (just before meal ingestion) and at 30, 60, 90, 120, 150 and 180 min after the start of the meal. Glucose concentrations were assessed in whole blood using a quantitative enzymatic photometry cassette from Alere (San Diego, California, USA). The intra-assay and inter-assay coefficients of variation were ≤6.2% and ≤5.0%, respectively. The plasma concentrations of insulin were determined using quantitative immunoradiometric assay kits from Millipore (St. Charles, Missouri, USA). The intra-assay and inter-assay coefficients of variation were ≤4.4% and ≤6.0%. The measurement range was 21.72–1390.0 pmol/L for insulin.
Sample Size and Statistical Analysis
Our previous crossover study of 16 subjects with type 2 diabetes showed a 54% reduction in incremental glucose peak (IGP) when protein and vegetables were consumed first vs carbohydrate consumed first. In this study, we estimated an IGP of about 2.8 mmol/L when eating carbohydrates first. We predicted that the IGP for the meal pattern of vegetables first followed by protein and carbohydrate together would be 30-40% lower compared to carbohydrate first. Therefore, with 15 subjects enrolled, we would have 80% power to detect a mean of paired differences of 0.84 mmol/L in IGP (30% of 2.8) at an alpha level of 0.05, assuming a standard deviation of differences of 1.1 mmol/L.
Demographics for participants were described as mean ± SD. Glucose and insulin concentrations, and their respective incremental areas under the curves (iAUCs) at 180 min, and IGP were described as mean ± SEM. Linear mixed effects models accounting for correlation within the same participant were implemented for each outcome of interest to compare the three groups. Post-hoc pairwise comparisons were performed by Tukey’s method with Bonferroni adjustment. P values were two-sided with statistical significance evaluated at the 0.05 alpha level or the Bonferroni-corrected 0.05 alpha level, where applicable. Analyses were performed in R V.3.4.0 (Vienna, Austria).
Results
Twenty-five subjects were screened, and fifteen (eleven females and four males) were included in the study. The average age and BMI were 52.4±3.4 years and 34.2±1.1 kg/m2, respectively. The mean HbA1c was 6.0%±0.06%. One participant did not have VF data due to an intercurrent illness that precluded participation in the third test session; however, this participant was not lost in analyses because mixed effects models handle missing values by maximum likelihood estimation and are robust to missing random data. Furthermore, on performing sensitivity analyses by excluding this participant, the significance of the results did not change.Baseline fasting glucose concentrations were similar in the three meal conditions. Postprandial glucose levels were significantly decreased at 30 and 60 min (Figure 1), respectively, and the iAUC0–180 was 38.8% lower (114.9±22.87 vs 187.8±30.79 mmol/L×180 min, p=0.008) following the PVF meal order, compared with CF, the reverse meal order. The VF meal pattern also showed reduced postprandial glucose levels at 30 and 60 min compared with CF and a decrease in iAUC0–180 of 23.4%(143.8±34.5 vs 187.8± 30.79 mmol/L×180 min, p=0.205). Mean glucose concentrations were significantly lower in the CF meal condition compared to both PVF and VF at 120, 150 and 180 mins. IGP was 45.8% lower for the PVF meal order compared with CF (1.56±0.24 vs 2.88±0.31 mmol/L, p<0.001) and 43.1% lower for VF compared with CF (1.64±0.32 vs 2.88±0.31 mmol/L, p<0.001). IGP and iAUC0–180 were similar between the PVF and VF meal conditions.
Fig.1.
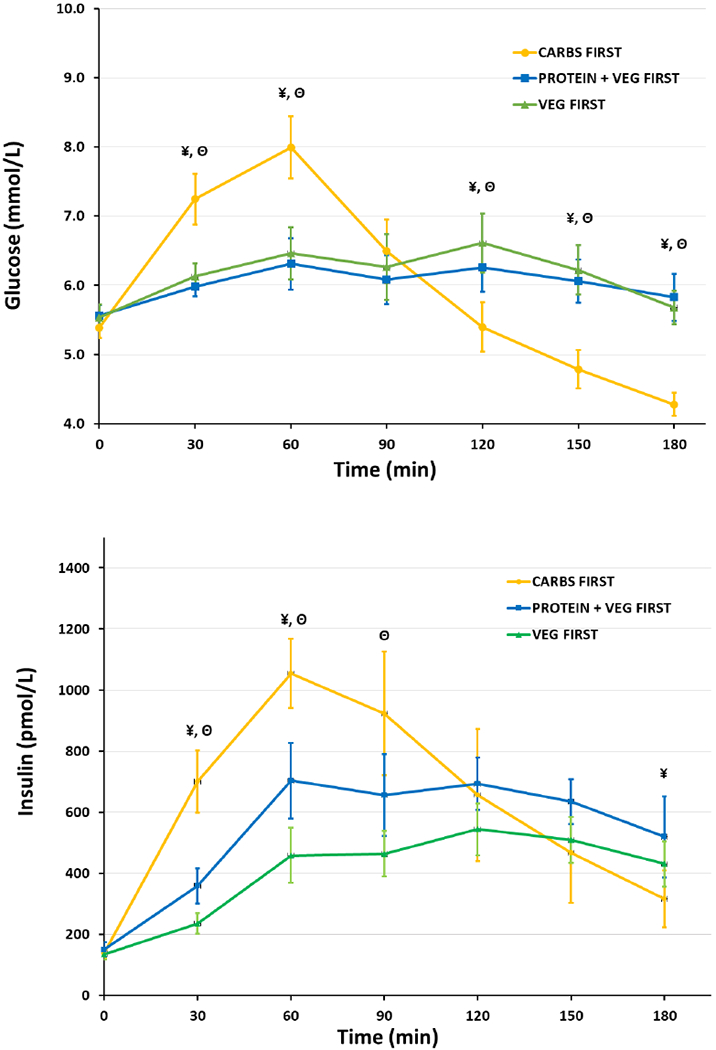
Postprandial glucose and insulin levels following, carbohydrate-first, protein and vegetables-first and vegetables-first meal orders. Values are mean ± SEM. ¥. Statistically significant differences (p<0.05) between: Carbs-First vs. Protein and Vegetables-FirstΘ. Statistically significant differences (p<0.05) between: Carbs-First vs. Vegetables-First
The VF meal resulted in lower insulin excursions; iAUC0–180 was 43.8% lower compared with CF (54,116.7±7310.6 vs 96,382.1±21,909.8 pmol/L×180 min, p=0.03). The iAUC0–180 in the PVF meal condition was lower, albeit not significantly different compared with CF (74,428.5±10,739.3 vs 96,382.1±21,909.8 pmol/L ×180 min, p=0.44).
Conclusions
In this study, we demonstrate that controlling for calorie intake and macronutrient composition, manipulation of nutrient order during a meal significantly impacts postprandial glucose and insulin excursions in individuals with prediabetes. Compared with the CF meal order, both VF and PVF meal orders attenuated postprandial glucose peaks similarly, with the VF food order requiring the least insulin to dispose the same amount of carbohydrate. Overall, the CF meal pattern demonstrated marked glycemic variability with a peak at 60 min and a nadir at 180 min, whereas glucose levels were stable in other meal conditions. In fact, the CF meal order provoked symptoms of hypoglycemia in one participant, an adverse event that was not observed in the other meal conditions. Commonly prescribed nutritional strategies to ameliorate prediabetes (as well as prevent reactive hypoglycemia) include carbohydrate restriction and avoidance of high-glycemic index foods. While these measures are effective, adherence to a low-carbohydrate diet can be challenging for patients. Food order is an alternative strategy that could mitigate the metabolic effects of carbohydrate (15).
Similar to our previously reported findings in patients with T2DM (12,13), we found that consuming carbohydrate at the end of the meal after protein and vegetables reduced iAUC and IGP compared with the reverse order in individuals with prediabetes. However, the insulin response, albeit lower, was not significantly different between these meal conditions, likely due to limitations of sample size and greater heterogeneity in insulin secretory patterns in individuals with prediabetes. Notably, the VF meal order stimulated the least insulin response among the three meal conditions, while the reductions in IGP and glucose iAUC (0–180) vs the CF meal condition were similar to the PVF meal order. These effects are plausibly mediated by slowed carbohydrate absorption due to the effects of fiber and fat in the VF meal order, although more work is needed to clearly delineate these mechanisms. This intervention has practical relevance since the idea of having a salad with a dressing before the main course can be easily implemented within diverse cultural settings and meal patterns.
Our study has limitations including the small sample and unclear generalizability to postprandial meal conditions. We used HbA1c as the diagnostic criteria for inclusion in the study, therefore the effects of food order in individuals with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) cannot be distinguished. The effects are predicted to be more robust in individuals with IGT and modest in those with only IFG. In designing the meal we used spices, herbs and vinegar in addition to macronutrients to improve palatability and replicate a meal that people regularly consume. Although the amounts used were small, they may have effects on postprandial glucose and insulin, albeit minor. The stringent study design and the use of meals and meal interventions that simulate real world eating behavior are strengths of our study.
In conclusion, food order presents a novel, simple behavioral strategy to attenuate glycemic excursions in prediabetes. Further prospective studies are needed to assess its feasibility and effectiveness as a diabetes prevention tool.
Acknowledgement
The study was supported by the Clinical and Translational Science Center at Weill Cornell Medicine(Grant UL1 TR000457).
Financial Support/Conflict of Interest: None
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- 1.National diabetes statistics report: estimates of diabetes and its burden in the United States. Centers for Disease Control and Prevention national diabetes statistics report. Atlanta (GA): U.S: Department of Health and Human Services; 2014. Available at: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. [Google Scholar]
- 2.Aguirre F, Brown A, Cho NH, et al. International Diabetes Federation. IDF Diabetes Atlas, 6th edition Brussels (Belgium): International Diabetes Federation; 2013. [Google Scholar]
- 3.Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med 2007;24:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JS, Yang YC, Lin TS, et al. Epidemiological evidence of altered cardiac autonomic function in subjects with impaired glucose tolerance but not isolated impaired fasting glucose. J Clin Endocrinol Metab 2007;92:3885–9. [DOI] [PubMed] [Google Scholar]
- 5.Putz Z, Tabak AG, Toth N, et al. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care 2009;32:181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner EJ, Shipley MJ, Witte DR, et al. Relation between blood glucose and coronary mortality over 33 years in the Whitehall Study. Diabetes Care 2006;29: 26–31. [DOI] [PubMed] [Google Scholar]
- 7.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (Aus- Diab). Circulation 2007;116:151–7. [DOI] [PubMed] [Google Scholar]
- 8.Knowler William C., Barrett-Connor Elizabeth, Fowler Sarah E., Hamman Richard F., Lachin John M., Walker Elizabeth A., Nathan David M., and Diabetes Prevention Program Research Group. 2002. “Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin.” The New England Journal of Medicine 346 (6): 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 10.Lindström J, Ilanne-Parikka P, Peltonen M, et al. ; Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 2006;368:1673–1679 [DOI] [PubMed] [Google Scholar]
- 11.Imai S, Fukui M, and Kajiyama S. “Effect of Eating Vegetables before Carbohydrates on Glucose Excursions in Patients with Type 2 Diabetes.” Journal of Clinical Biochemistry and Nutrition 541 (2014): 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla AP, Iliescu RG, Thomas CE, Aronne LJ. Food Order has a Significant Impact on Postprandial Glucose and Insulin Levels. Diabetes Care July 2015. vol. 38 no. 7 e98–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shukla AP, Andono J, Touhamy SH, Casper A, Iliescu RG, Mauer E, Shan Zhu Y, Ludwig DS, Aronne LJ. Carbohydrate-last meal pattern lowers postprandial glucose and insulin excursions in type 2 diabetes. BMJ Open Diabetes Res Care. 2017. September 14;5(1):e000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tricò D, Filice E, Trifirò S, Natali A. Manipulating the sequence of food ingestion improves glycemic control in type 2 diabetic patients under free-living conditions. Nutr Diabetes. 2016. August 22;6(8):e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orloff JN, Aronne LJ, Shukla AP. The challenge of meeting prescribed carbohydrate intake goals in low-carbohydrate diet studies. Am J Clin Nutr. 2018. April 1;107(4):673–675 [DOI] [PubMed] [Google Scholar]


