Brains can be viewed as vast ensembles of highly diverse and dynamic synapses that shape and store information as it travels through the networks of neurons that generate and interconnect those synapses. There are more than 100 trillion synapses per human neocortex (1), and each synapse is itself a highly complex entity, comprising thousands of diverse and cooperative signal-transduction proteins (2). Growing knowledge of these very large and heterogeneous populations of synapses, or “synaptomes,” thus offers perhaps the most compelling glimpse to date of brains as machines deep and wide enough to support the richness and subtlety of the human mind. On page 270 of this issue, Cizeron et al. (3) describe the use of a mouse model to begin the task of systematically mapping synaptomes brain-wide, from birth to old age.
Cizeron et al. explored brains of transgenic mice fixed at 10 time points from birth through late adulthood. These mice expressed transgenes encoding two postsynaptic scaffold proteins of the PDZ domain–containing family—synapse-associated protein 102 (SAP102) and postsynaptic density protein 95 (PSD-95)—each genetically tagged with a different fluorophore. At least one of these proteins normally occurs in the postsynaptic density (PSD), a signaling complex common to all glutamatergic synapses (see the figure). Because the majority of central nervous system (CNS) synapses are glutamatergic, labeling SAP102 and PSD-95 provides an opportunity to detect most individual synapses. By imaging these two PDZ proteins and using machine-learning image-analysis tools (4), Cizeron et al. generated data representing intensities and morphologies of two vast sets of fluorescent puncta. On the basis of such data, this laboratory previously developed a classification scheme imputing individual puncta to three major synapse types, divided into a total of 37 subtypes (4). They now provide a view of synapse diversity as it unfolds from birth to late adulthood, across a fine-scaled anatomic parcellation.
Census challenges: Synapse complexity and diversity.
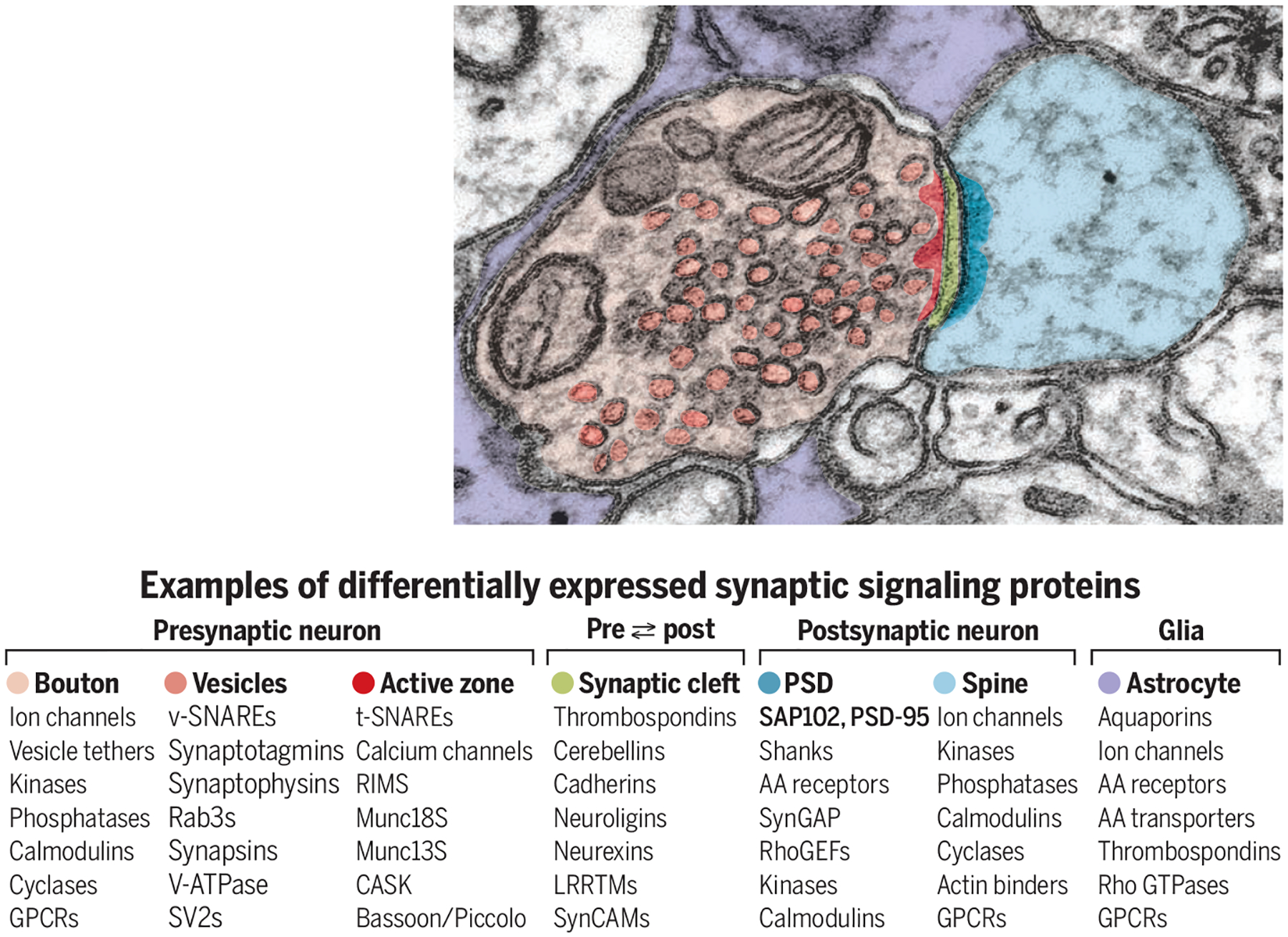
Glutamatergic synapses comprise hundreds of different proteins from both presynaptic and postsynaptic neurons. Synapse diversity reflects the various neurons as well as other influences, including electrical activity, neuromodulators, and glial cells.
AA, amino acid; CASK, calcium/calmodulin-dependent serine protein kinase; GPCR, G protein–coupled receptor; GTPase, guanosine triphosphatase; LRRTMs, leucine-rich repeat transmembrane proteins; MUNC, mammalian homolog of Unc; PSD-95, postsynaptic density protein 95; RhoGEFs, Rho guanine nucleotide exchange factors; RIMS, regulating synaptic membrane exocytosis protein; SAP102, synapse-associated protein 102; SV2, synaptic vesicle protein 2; SynCAM, synaptic cell adhesion molecule; SynGAP, synaptic Ras GTPase-activating protein; t-SNARE, target SNAP receptor; v-SNARE, vesicle SNARE; V-ATPase, vacuolar-type H+–adenosine triphosphatase.
SAP102 and PSD-95 play partially overlapping roles in organizing the PSD. Both of these PDZ proteins interact with postsynaptic glutamate receptors of the N-methyl-D-aspartate (NMDA) type to establish the core of a glutamatergic synapse. SAP102 and PSD-95 are expressed with different timing during mouse brain development, however, and appear to interact differentially with two receptor subunit isoforms (5). SAP102 interacts with the NMDA receptor subtype 2B (NR2B), which is characteristic of immature synapses, and PSD-95 interacts with the NR2A subtype associated with mature synapses. A progressive replacement of SAP102 and NR2B with PSD-95 and NR2A may underlie the transition from a highly plastic early stage in synaptogenesis to a more stable mature synapse.
Cizeron et al. argue that differing developmental trajectories of synaptic SAP102 and PSD-95 in functionally distinct brain regions provide a possible mechanistic basis for behavioral changes during a lifespan. Moreover, their work establishes a powerful and versatile template for further exploration of synaptomes in development and aging. Such explorations might probe larger subsets of the hundreds of other protein components of glutamatergic synapses, and proteins of the inhibitory synapses that use γ-aminobutyric acid (GABA) as their primary neurotransmitter. GABAergic synapses, the second-largest broad class of CNS synapses, are likely just as diverse and dynamic as glutamatergic synapses (6, 7). Thus, extending the Cizeron et al. synapse census template from SAP102 and PSD-95 to other diversely expressed synaptic proteins might greatly deepen our knowledge of circuit development, homeostasis, modulation, and plasticity (2).
The same basic template for brain-wide developmental histology should also be adaptable to other protein-tagging and imaging modalities. Electron microscopy or super-resolution fluorescence microscopy would enable more reliable detection and mapping of the numerous tiny glutamatergic synapses that are critical to early synaptic network development and some forms of synaptic plasticity (8).
Because functional properties of individual synapses are also highly diverse and must somehow reflect diverse molecular properties (2), the Cizeron et al. histological results bring a need for parallel physiological studies of single synapses into the limelight. A recent in vivo calcium imaging study (9) provides an example of an approach that might fit this need. Perhaps the most promising avenue toward mapping functional synaptomes at scale would be to develop a “codebook” capable of predicting the difficult-to-measure function of an individual synapse from more readily accessible molecular and/or morphological measurements (2, 10). The potential payoffs could be enormous, enhancing the much richer understanding of the brain’s synaptic network architectures that are now beginning to emerge from electron microscopy connectomics (11) and viral trans-synaptic tracing methods (12).
The Cizeron et al. results on the intriguing patterning of CNS synapse diversity through development and aging also highlight the need to address more mechanistic questions about the underlying cell biology. The diverse phenotypes of individual synapses must somehow “inherit” their diversity largely from the proteomes of their two parent neurons. Such inheritance certainly involves trans-synaptic signaling interactions beyond mere juxtaposition of a stereotyped presynaptic part from one neuron with a stereotyped postsynaptic part from a second (6, 13). There must also be experience-dependent environmental influences, including patterned electrical activity, paracrine neuromodulatory ligands, and adhesive interactions with extracellular matrix and glial cells (14). Brain-wide developmental synaptomes like those developed by Cizeron et al. may also be combined in the near future with transcriptomic neuron taxonomies (15) to reveal the origins of programmed synapse diversity in cell-type differentiation that must be fundamental to brain development, function, and plasticity.
ACKNOWLEDGMENTS
S. JS. is grateful to Allen Institute founder P. G. Allen for his vision, encouragement, and support. The work was also supported by NIH grants R01 MH111768, R01 NS094499 (K.D.M.), R01NS039444 (R.J.W.), and 1U24NS109113 (S.JS.).
REFERENCES AND NOTES
- 1.Tang Y, Nyengaard JR, De Groot DM, Gundersen HJ, Synapse 41, 258 (2001). [DOI] [PubMed] [Google Scholar]
- 2.O’Rourke NA, Weiler NC, Micheva KD, J Smith S, Nat. Rev. Neurosci 13, 365 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cizeron M et al. , Science 369, 270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu F et al. , Neuron 99, 781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Won S, Levy JM, Nicoll RA, Roche KW, Curr. Opin. Neurobiol 43, 94 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Südhof TC, Cell 171, 745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang ZJ, Paul A, Nat. Rev. Neurosci 20, 563 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burette A, Collman F, Micheva KD, J Smith S, Weinberg RJ, Front. Neuroanat 9, 100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu R et al. , Nat. Methods 17, 291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groc L, Choquet D, Science 368, eaay4631 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Kubota Y, Front. Neural Circuits 13, 64 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saleeba C, Dempsey B, Le S, Goodchild A, McMullan S, Front. Neurosci 13, 897 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang S, Lee H, Kim E, Curr. Opin. Neurobiol 45, 45 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Privitera L et al. , J. Neurosci 40, 4644 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasic B et al. , Nature 563, 72 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


