COVID-19 infection has resulted in an unprecedented number of critical care hospitalizations and substantial mortality across the world. Emerging evidence suggests that Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), responsible for the pandemic COVID-19, causes cardiac injury reflected by elevated biomarkers, hemodynamic instability, and cardiomyopathy.1 Cardiovascular dysfunction is hypothesized to relate to microvascular dysfunction, myocarditis, myocardial ischemia, or toxicity from elevated cytokines. A condition with cross-similarities, stress (Takotsubo) cardiomyopathy is a reversible but morbid condition thought to be triggered by physical, psychological, or metabolic stress, though dysfunction of the cardiac endothelium and microvasculature have been implicated.2 Stress cardiomyopathy associated with COVID-19 has not been well described. Here, we describe and discuss the case of a patient without a history of cardiovascular disease who developed and recovered from clinical stress cardiomyopathy in the setting of severe COVID-19 infection.
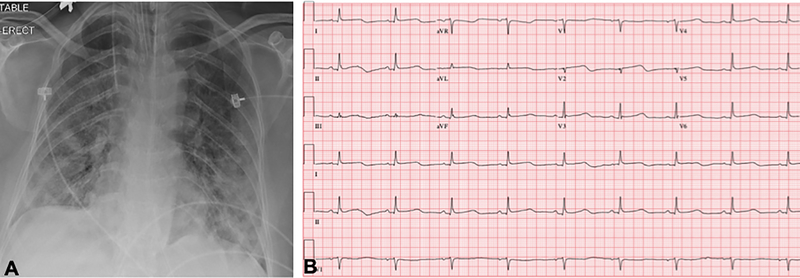
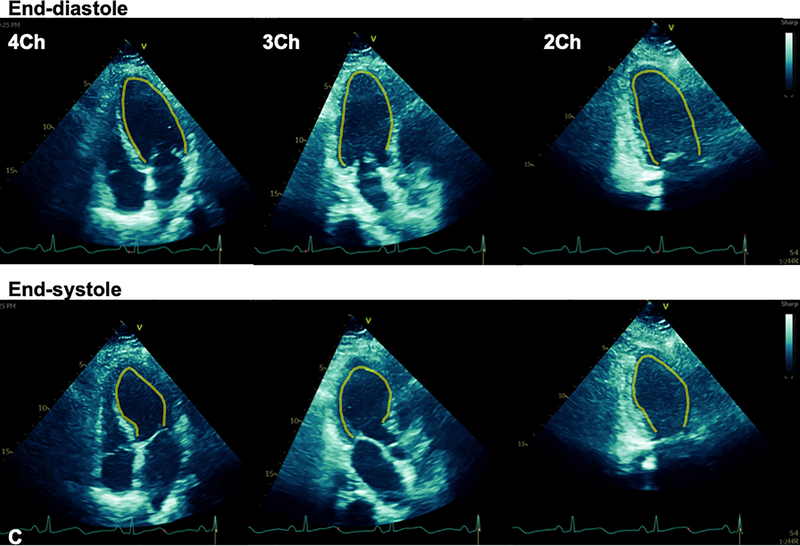
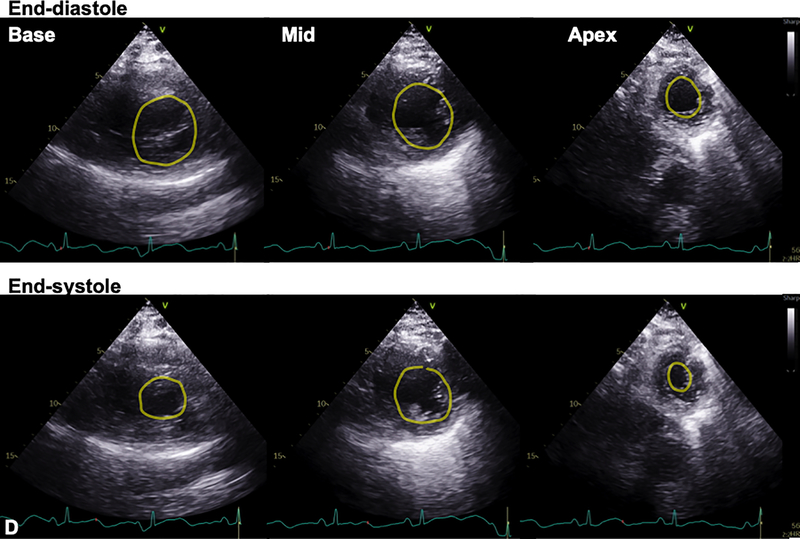
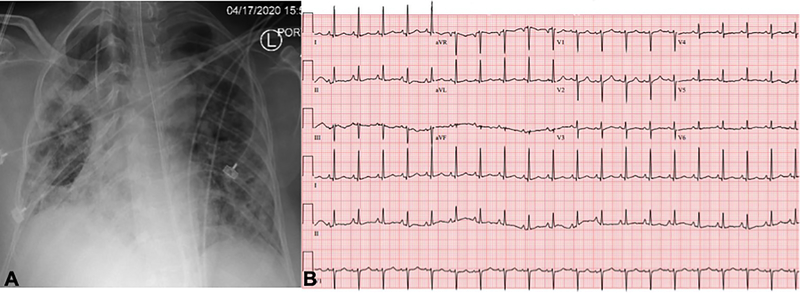
A 59-year-old woman with obesity (BMI 33 kg/m2) but otherwise healthy presented to the emergency department of our tertiary care hospital with fevers, chills, fatigue, myalgias, and cough. Within 24 hours, she had an increasing oxygen requirement, necessitating transfer to the intensive care unit (ICU) and mechanical ventilation. Chest x-ray demonstrated diffuse bilateral alveolar infiltrates without cephalization or prominent pulmonary vascular markings (Fig. 1A). SARS-CoV-2 reverse transcriptase-polymerase chain reaction test performed on her nasopharyngeal swab specimen returned positive. Inflammatory markers were elevated with C-reactive protein >300 mg/L (normal ≤5 mg/L), D-dimer 2184 ng/mL (normal ≤500 ng/mL, ferritin 2033 ng/mL (normal ≤150 ng/mL), and IL-6 724.38 pg/mL (normal <5 pg/mL). On hospital day 3, the Troponin T and CK-MB, normal on admission (<0.01 ng/mL and 2 ng/mL, respectively), rose to 1.0 ng/mL and 94 ng/mL. 12-lead electrocardiogram (ECG) demonstrated slight ST segment elevations diffusely with nonspecific T wave inversions (Fig. 1B). Transthoracic echocardiogram (TTE) was performed for evaluation of left ventricular (LV) function in the setting of elevated cardiac biomarkers and abnormal ECG (Vivid S70 echocardiograph, analyzed using EchoPAC software, ViewPoint 6.11, General Electric Healthcare, Waukesha, WI). TTE demonstrated severe hypokinesis of the mid-LV cavity, with normal to hyperdynamic contractility of basal and apical LV segments and a moderately reduced biplane ejection fraction of 36% (Figs. 1C and 1D; Videos 1–7). The patient’s hospital course was notable for profound hypoxemic respiratory failure and vasodilatory shock requiring intravenous norepinephrine and vasopressin administration. Additionally, she was noted to have multiple episodes of monomorphic ventricular tachycardia responding to lidocaine. After several days of supportive care and receipt of sarilumab, the patient’s hemodynamic and respiratory status gradually improved. She no longer required vasopressor agents and was able to be extubated after 15 days of ventilator support. During this time, her cardiac and inflammatory biomarkers peaked and declined. On the day of extubation, repeat chest x-ray showed stable to slightly decreased bilateral airspace opacities and consolidations (Fig. 2A), and ECG demonstrated resolution of diffuse ST segment and T wave abnormalities (Fig. 2B). Repeat TTE the same day, 10 days after the initial TTE, revealed resolution of the stress cardiomyopathy, with normal biventricular systolic function (Fig. 2C; Videos 8–12). She was ultimately discharged to a rehabilitation facility.
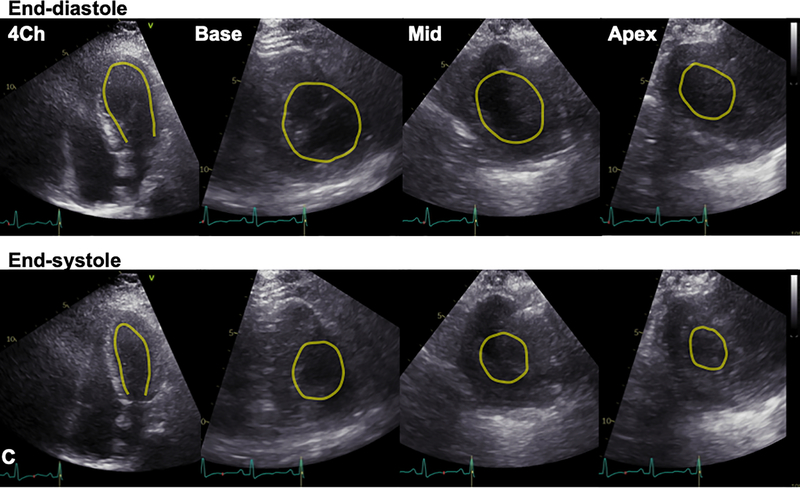
Figure 1. Stress cardiomyopathy during COVID-19 infection.
A. Chest x-ray demonstrated diffuse bilateral pulmonary infiltrates. B. 12-lead ECG acquired within the hour of echocardiogram demonstrated slight ST segment elevations. C. Transthoracic echocardiogram (TTE) demonstrated mid-wall hypokinesis of the left ventricular (LV) with normal contractility of the basal and apical segments, and normal right ventricular systolic function (L-R: 4-chamber, 3-chamber, and 2-chamber images; top panel: end-diastole; bottom: end-systole). D. Short axis TTE images demonstrate normal contractility of LV basal and apical segments and hypokinesis of the mid-ventricular segments (L-R: Basal, mid, and apical images; top panel: end-diastole; bottom: end-systole). Yellow contours in C and D outline the LV endocardial borders.
Figure 2. Recovery of stress cardiomyopathy during clinical improvement.
Panels A-C demonstrate data obtained on the day of extubation, 10 days following data in Figure 1. A. CXR continued to demonstrate pulmonary infiltrates. B. ECG abnormalities had normalized compared to prior. C. TTE demonstrated recovery of LV systolic function with resolution of regional dysfunction (L-R: 4-chamber and short-axis basal, mid, and apical images; top panel: end-diastole; bottom: end-systole). Yellow contours outline the LV endocardial borders.
The exact mechanisms of stress cardiomyopathy in COVID-19 are unknown. The cardiac tropism of the SARS-CoV-2 virus in part may be explained through viral entry via the angiotensin-converting enzyme 2 that is highly expressed in pulmonary and cardiac tissues, with associated endothelial dysfunction.1 While direct cellular infection has been evident in other viruses, thus far endomyocardial biopsy of suspected COVID-19 myocarditis has shown the presence of the virus within macrophages in the heart but not within cardiomyocytes per se, and a myocarditis-like syndrome may be distinct from that of stress cardiomyopathy. Alternatively, the etiology of cardiac toxicity in COVID-19-associated stress cardiomyopathy may be primarily the result of indirect immune-mediated injury. The medical community has widely observed the ‘cytokine storm’ of systemic inflammation and immune dysregulation that may occur with severe COVID-19 infection and multiorgan system dysfunction. This condition is marked by profound elevation of cytokines including IL-6 and inflammatory biomarkers including ferritin and d-dimer, as well as frequent modest elevations in cardiac biomarkers—all of which were notable findings in the patient described above. Supporting the immune response in myocardial injury, activation of lymphocytes resulting in cytokine release and inflammation previously has been demonstrated in non-COVID-19 viral myocarditis.3 Additionally, macrophage infiltrates and elevated cytokine levels have been demonstrated in non-infectious stress-induced cardiomyopathy.4 While the underlying trigger of non-infectious stress cardiomyopathy has centered on increased sympathetic tone, high catecholamines may in turn cause myocardial inflammation. Thus, while the pathophysiology of stress cardiomyopathy in COVID-19 remains to be defined, all of the above represent plausible etiologies that may occur in combination during the heightened stress and inflammatory milieu of severe infection. Additional assessment of the biochemical and histological profiles of the myocardium in individuals with COVID-19 associated stress cardiomyopathy may further shed light on its mechanisms. Further, in tandem with biomarkers and echocardiography, advanced cardiovascular imaging (e.g., coronary computed tomography and cardiovascular magnetic resonance) may play important roles in analyses of coronary and myocardial structure, tissue characteristics, and mechanics to understand the conjoint roles of stress, inflammation, viral toxicity, and cellular damage contributing to the pathobiology of this disorder.
In conclusion, reversible stress cardiomyopathy may occur in the setting of COVID-19-infection with elevated inflammatory and cardiac biomarkers and an abnormal ECG. The pathophysiology of COVID-19 stress cardiomyopathy may involve mechanisms shared between non-infectious stress cardiomyopathy and viral myocarditis including microvascular dysfunction, heightened sympathetic tone, and/or indirect immune-mediated injury with lymphocyte activation, cytokine release, and inflammation. Collaborative and longitudinal studies are needed to evaluate the underlying mechanisms and long-term outcomes of COVID-19-associated stress cardiomyopathy.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by research funding from the National, Heart, Lung, and Blood Institute (5R03HL145195 to CWT and 1K23HL144907 to JBS).
Footnotes
Disclosures: CWT reports consulting fees from AstraZeneca and GlaxoSmithKline and JBS reports consulting fees from Philips Healthcare unrelated to the current work. JDC and WJM report no disclosures.
REFERENCES
- 1.Hendren NS, Drazner MH, Bozkurt B and Cooper LT Jr. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation. 2020. 10.1161/circulationaha.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D and Abbate A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:1955–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsumori A, Yamada T, Suzuki H, Matoba Y and Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J. 1994;72:561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scally C, Abbas H, Ahearn T, Srinivasan J, Mezincescu A, Rudd A, Spath N, Yucel-Finn A, Yuecel R, Oldroyd K, et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation. 2019;139:1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.