Abstract
Purpose/Aim:
We used magnetic resonance imaging (MRI) to investigate effects of intraocular pressure (IOP), race, and other factors on optic nerve (ON) traction in adduction, a phenomenon proposed as neuropathic in open angle glaucoma (OAG).
Materials and Methods:
Thirty-five patients with OAG (26 with maximal untreated IOP ≤21mmHg, 9 with IOP >21mmHg) and 48 controls underwent axial and quasi-coronal MRI in central gaze and large (27–33°) abduction and adduction. Some underwent MRI at smaller ductions (21–28°). Effects of presence vs. absence of OAG; within OAG whether maximum IOP level was ≤21mmHg vs. >21mmHg; adduction angle; race; age; and gender on ON path length and globe translation were analyzed using generalized estimating equations to account for possible intereye correlations of individual subjects.
Results:
Average visual field mean deviation (±standard error of mean, SEM) was −8.2±1.2 dB in OAG with normal IOP, and −6.1±1.4 in high IOP. In central gaze, ON path in OAG was significantly more redundant than in controls but in both groups the ON became significantly and almost equally straighter in small (~21°) or large (~27°) adduction than in central gaze. With progressive adduction only, globes retracted in OAG (P<0.005) but not in controls; this was only weakly related to globe size and not to IOP elevation. Globe retraction in adduction was significant only in OAG, and in that group was significantly greater in Asian than white patients (P<0.02).
Conclusions:
Although ON tethering in adduction is normal, progressive adduction is associated with abnormal globe retraction, in OAG regardless of IOP level. This phenomenon is more prominent in Asians who have OAG. Traction in adduction may cause repetitive strain injury to the ON and peripapillary sclera, thus contributing to the optic neuropathy of glaucoma independent of IOP.
Introduction
Glaucoma is the leading cause of irreversible blindness worldwide1, 2. The historical view conceived glaucoma as optic nerve (ON) damage caused by intraocular pressure (IOP)3 exceeding normal. Statistically normal IOP in a study of over 110,000 patients averaged 14.5 mmHg in the fifth decade in all ethnicities, but increases to around 16.5 mmHg by the seventh decade, with standard deviation (SD) ~4 mmHg4. The pathogenic role of IOP greatly exceeding normal is unequivocal in congenital and juvenile glaucoma5, as well as in secondary glaucomas such as angle closure6, uveitic7, and traumatic8, because in such cases the optic neuropathy is arrested or decisively attenuated when highly elevated IOP is reduced therapeutically. Moreover, IOP elevation in multiple animal models reliably produces progressive optic neuropathy9–12. Nevertheless, the general etiologic role of IOP was long ago removed from the definition of glaucoma for reasons including observations that many patients with open angle glaucoma (OAG), especially in Asia13–17, lack abnormally high IOP and sustain progressive ON damage at normal IOP3. In patients with OAG, IOP is normal in 30–39% of white18–20, 57% of black21, 70% of Chinese17, and in 92% of Japanese persons14. In more than 30,000 Chinese subjects there was no statistical relationship of IOP to prevalence of OAG17. By 1992, the Primary Open-Angle Glaucoma Preferred Practice Pattern of the American Academy of Ophthalmology acknowledged existence of a “subgroup commonly referred to as normal tension glaucoma, that pose a particular diagnostic and therapeutic challenge22.” However, even the term “normal tension glaucoma (NTG)” has been controversial23, and IOP remains the only modifiable risk factor for vision loss in OAG. The Advanced Glaucoma Intervention Study24, Collaborative Normal-Tension Glaucoma Study Group25, 26, and others27 have demonstrated that IOP reduction, even when damage has occurred at low IOP, reduces the rate of progressive visual field loss. Yet the absolute level of baseline, unmedicated IOP does not statistically distinguish which patients with OAG will ultimately suffer progressive optic neuropathy, even though patients with the greatest therapeutic IOP reduction ultimately suffer less glaucoma progression28. About 20% of such patients experience progressive optic neuropathy five years after 30% IOP reduction from normal baseline levels3, even sometimes to such deeply subnormal IOP that ocular hypotension then poses a separate risk of vision loss29. Therefore, without denying the clear pathogenic role of highly elevated IOP, there seemingly exist one or more IOP-independent mechanisms of progressive optic neuropathy that operate at normal IOP and can be unresponsive to IOP reduction30, 31.
Causes of glaucomatous ON damage independent of IOP have been suggested to include translaminar IOP gradients against intracranial pressure32–35 or vasculopathy36, but a recent review concluded no clear etiology for ON damage in at normal IOP3. For example, there is no relationship between translaminar pressure and glaucoma37, even in multiple postural orientations38. Vascular dysregulation has also been suggested as pathological, but it is not clear if vasculopathy is cause or consequence of glaucoma3.
Eye-movement related deformation of the ON has been proposed as another mechanical etiology for optic neuropathy in OAG that may operate along with IOP39–42. The contribution of ocular adduction has been emphasized, because the ON is insufficiently long to permit unhindered ocular rotation, tethering the globe in adduction39 at angles exceeding ~26°43. Optical coherence tomography (OCT) shows progressive deformation of the normal ON head and peripapillary region during horizontal eye rotations43–46, but particularly in adduction43, 44. Deformations of the ON head and Bruch’s membrane produced by eye movements exceed many-fold those resulting from extreme IOP elevation47, or the IOP-related deformations proposed pathological to the retina48.
In extreme axial high myopia, MRI has demonstrated prominent globe retraction during adduction tethering39. Sibony et al. suggested that oculorotary forces may contribute to peripapillary subretinal hemorrhage49. Nonuniformities in mechanical properties or physical dimensions of ON head and peripapillary tissues could further concentrate adduction counterforce to reach locally pathological levels, as suggested by the computational mechanical engineering technique known as finite element modeling (FEM). The patterns of mechanical strain in the posterior sclera predicted by FEM during ON tethering42 closely match the temporal peripapillary atrophy typical of NTG50–52, and resemble the peripapillary staphylomata seen in high myopia53. The FEM performed by Wang et al. also suggest that horizontal eye movements would strain the peripapillary region more in ad- than in abduction40.
Our group employed MRI to investigate adduction tethering of the ON in patients with primary OAG who had never had IOP elevated above the statistical upper limit of 21 mmHg41. While age-matched, healthy control subjects also exhibited tethering in adduction, there was significant globe retraction only in OAG. We then suggested that adduction tethering in OAG concentrates abnormally high counterforce at the disc and peripapillary region, causing repetitive strain injuries over the temporal accumulation of numerous voluntary and reflexive adduction eye movements. Since adduction tethering might also operate jointly with elevated IOP to cause optic neuropathy, the current study therefore extended the investigation to include patients with OAG who had abnormally elevated IOP prior to therapeutic reduction. It has been unclear why NTG is so much more common than high pressure glaucoma in Asian populations54, where NTG represents 52–92% of all cases of primary OAG. The comparable percentage in white populations in the United States20, the Netherlands55 and Italy19 is only 30–39%. We therefore took advantage of the opportunity to compare Asians with OAG to other races. If hypothesized ON traction were an IOP-independent pathogenic factor in optic neuropathy, we predicted that MRI would demonstrate abnormally great globe retraction in adduction in all patients with OAG, regardless of IOP.
Methods
Subjects.
This study was prospectively approved by the Institutional Review Board for Protection of Human Subjects of the University of California, Los Angeles, and conformed to the tenets of the Declaration of Helsinki. Subjects gave written informed consent prior to participation. Glaucoma was diagnosed based upon Heidelberg Cirrus or Spectralis OCT evidence of retinal nerve fiber layer loss correlating with typical glaucomatous VF defects on automated perimetry, irrespective of IOP, along with ophthalmoscopic features of glaucoma such as diffuse or localized neuroretinal rim loss and disc hemorrhages56. Medical records of subjects with glaucoma included multiple IOP measurements on different days, ON photos, ON imaging by OCT, and standard automated threshold perimetry examinations (with Humphrey programs 24–2 or 30–2 SITA standard strategy) performed within one year of recruitment. All patients were being actively treated during the study by an author who is a glaucoma specialist.
As the “OAG-low” group, 26 patients were recruited who had been treated for OAG, yet had never been observed to have IOP elevated above the statistical normal maximum of 21 mmHg with or without treatment, on multiple clinical examinations; 17 of these were reported in our earlier study41. Nine treated patients were recruited as the “OAG-high” group who had IOP elevated on at least one occasion above 21 mmHg, of whom 7 had primary OAG and two had secondary OAG due to pseudoexfoliation. Patients were excluded if they had previously undergone intraocular surgeries besides those for cataract, glaucoma, or refractive error, or if they had any other cause for optic neuropathy. No adjustment to IOP values was made for central corneal thickness (CCT) as measured according to standard clinical protocol.
A control group of 48 community subjects was recruited by advertisement, and from patients with simple refractive error. Control subjects were required to have best corrected visual acuity of 20/20 in each eye, normal IOP by applanation, and no history of ocular surgeries besides those for cataract or refractive error, no ocular trauma, and no other ocular disorder except for refractive error or lens opacity. The prior study included 31 of these controls41.
MRI Acquisition.
The first author performed high resolution MRI with a 1.5T General Electric Signa scanner and surface coils (Medical Advances, Milwaukee, WI) with T2 fast spin echo pulse sequence as57 described41,57–61,41. Eye position was controlled by monocular fixation of an illuminated target in central position, or laterally to establish abduction of the fixating eye with simultaneous adduction of its fellow since the surface coil occludes the adducting eye. Axial 2-mm thick images (10–12 cm field of view, 256×256 matrix) including both orbits were generally obtained to determine gaze direction and globe axial length (AL). Quasi-coronal sets of 17–20, 2-mm thick planes perpendicular to the long axis of each orbit were obtained separately (field of view 8×8 cm, 256×256 matrix, resolution 312 microns). Acquisitions were repeated for central gaze, and large (~30°), or both moderate (~25°) and large abduction and adduction of each eye (Fig. 1) in 54 control eyes, 34 eyes with OAG-low, and 18 eyes with OAG-high. Imaging was performed with only large ductions in an additional 38 subjects. The data set here required about 1,150 individual MRI acquisitions, for which limited results were included in our earlier publication41.
Fig. 1.
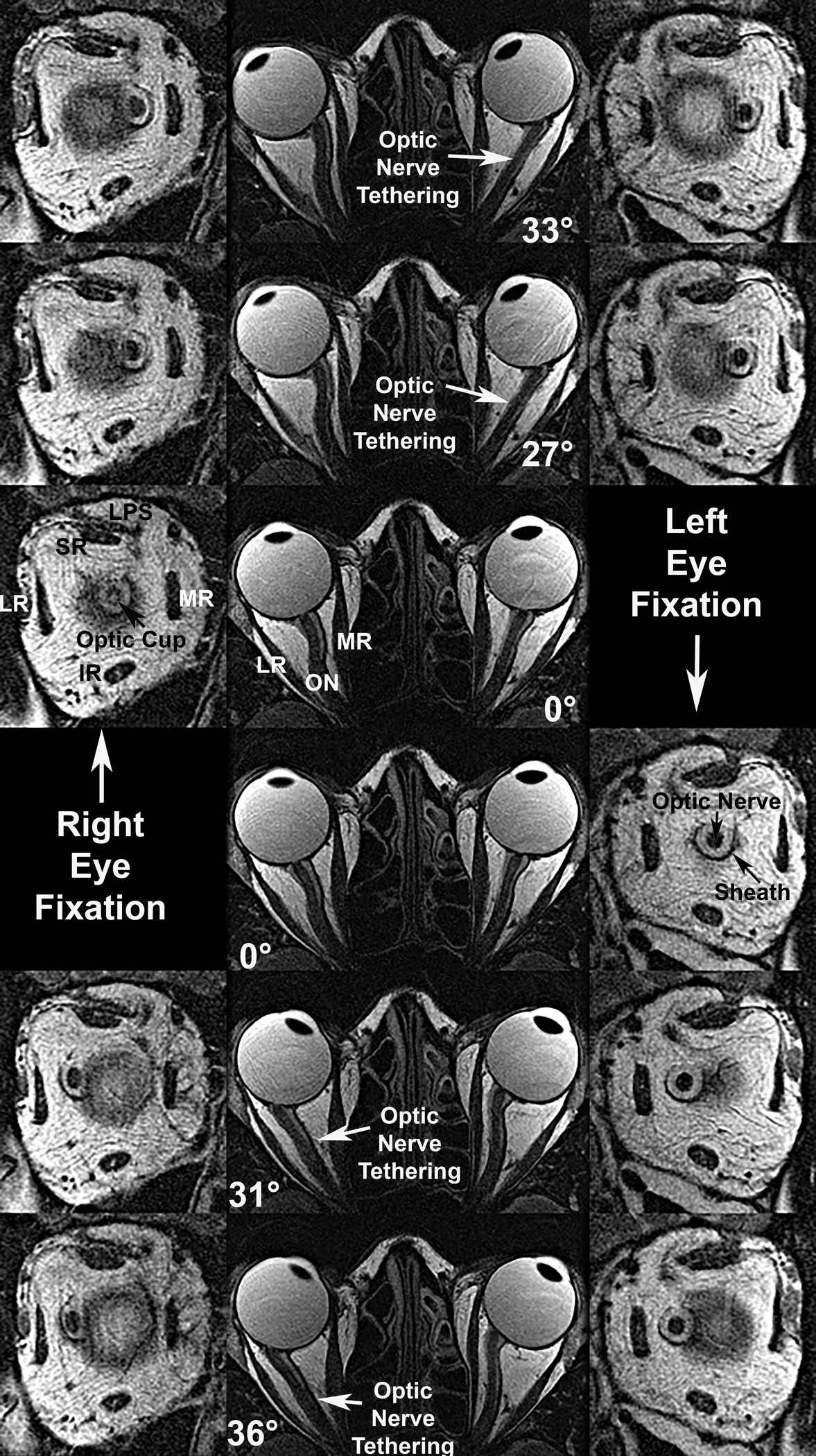
Axial (center column) and quasi-coronal (left and right right columns) MRI of both orbits in a patient with advanced OAG and maximal IOP exceeding 21 mmHg, imaged in maximal dextorversion (top row), moderate dextroversion (second row), central gaze (third and fourth rows), moderate levoversion (fifth row), and maximal levoversion (bottom row). Duction angles are listed. Each ON tethered in moderate adduction and remained so in maximal adduction, while being sinuous centrally and in abduction. Quasi-coronal just posterior to the globe-ON junction demonstrate that the ON within its sheath is surrounded by a white ring of cerebrospinal fluid. Both ON cross sections are subnormal due to glaucomatous atrophy, and an enlarged optic cup is evident in the right eye (third row, left). IR – inferior rectus muscle. LPS – levator palpebrae superioris muscle. LR – lateral rectus muscle. MR – medial rectus muscle. SR – superior rectus muscles.
MRI Analysis was performed as elsewhere described41 using ImageJ 64 and custom software. Gaze direction was computed in axial images (Fig. 1 left) as the angle of a line connecting the lens plane perpendicular to the fovea59. This approach does not account for angle kappa discrepacies between anatomic and subjective visual directions, so is only reliable for changes in gaze angle59 but not absolutely equivalent to subjective gaze angles reported for OCT studies43, 44.
Quantitative measure of ON path length and straightness were obtained by 3-D analysis of contiguous, quasi-coronal MRI images after manual outlining in each 2 mm thick image plane, with automated calculation of horizontal and vertical coordinates of the ON area centroid, and the third dimension corresponding to the image plane’s anteroposterior coordinate41. Cartesian distances between centroids were summed to compute overall path lengths. Minimum geometric distances between the globe-ON junction and the most posterior image plane in which the ON was visible were computed from Cartesian distances between points. The ratio between actual and minimum distance between the globe and the most posterior point imaged, represented in percent, was taken as a measure of straightness, with 100% being the theoretical minimum41.
Globe AL was determined as the distance from anterior corneal to retinal surface in the axial plane that included the maximum anteroposterior globe diameter for each eye, averaging measurements duplicated 3 – 6 times in different image sets for all gaze positions. Axial imaging was not obtained in some of the earliest control subjects in the study; however, axial MRI was performed in all subjects with OAG, and all controls imaged in small duction. Equatorial globe diameter was determined in all subjects at subpixel resolution from analysis of cross sections in three quasi-coronal image planes spanning the equator62. Location of the globe center was determined at sub-pixel resolution from outlines of globe cross sections in multiple quasi-coronal planes, relative to the orbit centroid62, as commonly used in MRI studies of orbital structures62–65. Analysis following manual outlining of structures in the MRI images was automated.
Validity of ON straightness determinations has been verified by independent investigators comparing axial versus quasi-coronal images for the same subjects viewing the same targets41. Robustness and validity of the quasi-coronal analyses has been confirmed by duplicate analyses by different investigators41. Such analyses have determined that gaze changes determined in prior studies from quasi-coronal image sets are significantly influenced by globe translation66, typically underestimating adduction angles. Consequently, horizontal gaze angles were measured from axial images not subject to this cofound.
Statistics.
Parametric statistical analyses were performed using GraphPad Prism software (GraphPad Software, LaJolla, CA), but confirmed using generalized estimating equations (GEE) in SPSS software (IBM Corporation) that corrects for possible confounding by possible interocular correlations between eyes of individual subjects and has type 1 error characteristics superior to t-testing67. Multiple models were evaluated to control for possible effects of diagnosis, duction angle, age, gender, race, and ocular size. Analysis of the foregoing factors was exploratory. Chi-square values were also reported for significant differences for which reported probabllility values would be rounded to zero at three decimal places by SPSS.
Results
Subject Characteristics.
Included were 50 orbits of 25 patients who had OAG without elevated IOP before or during treatment (OAG-low), and 18 orbits of 9 patients with OAG (including POAG and pseudoexfoliation) in whom IOP before treatment exceeded 21 mmHg in one or both eyes (OAG-high). These were compared with 93 orbits of 48 control subjects.
Subjects were categorized by race as summarized in Table 1. All patients with OAG in all groups had similar average ages (60 – 66 years). The average age of controls was lower, but the large overall control group included an intentional representation of ages comparable to patients with glaucoma. Gender was generally balanced, except for a predominance of women among Asian patients with OAG-low. It should be noted that myopia is highly prevalent among Asian adults68, 69, making it impractical to recruit a non-myopic Asian control group.
Table 1.
Subject Demographics
| Controls | OAG-Low | OAG-High | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Race | Age yrs | Female | Male | Age yrs | Female | Male | Age yrs | Female | Male |
| ±SD | ±SD | ||||||||
| Asian | 40±21 | 3 | 3 | 61±12 | 10 | 3 | N/A | 0 | 0 |
| Black | 62±7 | 4 | 3 | 61±2 | 2 | 0 | N/A | 0 | 0 |
| White | 44±21 | 20 | 15 | 65±9 | 6 | 5 | 66±9 | 4 | 5 |
N/A: data not available. OAG-low: subjects with primary open angle glaucoma in who maximum untreated or treated intraocular pressure never exceeded 21 mmHg. OAG-high: subjects with primary open angle or pseudoexfoliative glaucoma in whom maximum untreated intraocular pressure on at least one examination exceeded 21 mmHg. SD: standard deviation.
Intraocular Pressure.
All patients with glaucoma had undergone prior medical and surgical treatments to reduce IOP to target levels. In the OAG-low group, minimum IOP averaged 9.6±2.4 mmHg (SD), and maximum IOP averaged 13.9±2.8 mmHg. In the OAG-high group, minimum IOP averaged 10.1±2.7 mmHg, and maximum IOP (pre-treatment) averaged 23.8±7.8 mmHg. In OAG-low, reduction of IOP was accomplished by trabeculectomy in 10 eyes, selective laser trabeculoplasty (SLT) in 12 eyes, and in all but two eyes by topical ocular hypotensive medications including brimonidine, timolol, dorzolamide, latanoprost, brimatoprost, brinzolamide, and tafluprost. In OAG-high, reduction of IOP was accomplished by trabeculectomy in 8 eyes, SLT in 3 eyes, and topical ocular hypotensive medications in all except 6 eyes. Incisional glaucoma surgery was thus performed in 18 of the 68 eyes with OAG. Prostaglandin analog drops were employed in all except 15 eyes of 8 subjects with OAG.
Visual Field Loss.
Visual field mean deviation averaged over both eyes was −9.0±6.0 dB in Asians with OAG-low, −7.1±6.4 dB in whites with OAG-low, −7.0±5.6 dB in blacks with OAG-low, and −6.6±7.3 dB in (all white) patients with OAG-high. Despite aggressive IOP reduction, one white patient with OAG-low had progressed to severe glaucomatous vision precluding automated perimetry, so perimetric data was omitted for him. Mean deviation did not differ significantly between all subjects with OAG-low and OAG-high, nor between Asian subjects with OAG-low and all subjects with OAG-high.
Globe Size.
Many patients and controls had undergone cataract or refractive surgeries altering refractive error, so globe size is reported here as more relevant. Equatorial globe diameter over all patients with OAG-low averaged 25.88±1.05 mm, significantly greater than the 25.21±1.25 mm diameter in the full control group (P=0.000, chi-square >104 by GEE after controlling for age, race, prostaglandin analog use, and gender), but not significantly different from the average of 25.23±1.93 in the smaller number of patients with OAG-high, which was in turn quite similar to the control group (Table 2). Mean AL of patients with OAG-low was 25.44±1.51 mm, significantly greater than the corresponding value of 24.64±1.50 mm (P= 0.000, chi-square >104 by GEE after controlling for age, race, prostaglandin use, and gender) for all control subjects in whom it was available.
Table 2.
Globe Size
| Controls | OAG-Low | OAG-High | ||||
|---|---|---|---|---|---|---|
| Race | Axial Length mm ± SD |
Equatorial Diameter mm ± SD |
Axial Length mm ± SD |
Equatorial Diameter mm ± SD |
Axial Length mm ± SD |
Equatorial Diameter mm ± SD |
| Asian | 26.3 ± 1.7 | 26.1 ± 1.0 | 26.1 ± 1.6 | 26.2 ± 1.0 | N/A | N/A |
| Black | 24.2 ± 0.9 | 25.8 ± 0.5 | 24.3 ± 0.7 | 25.8 ± 0.4 | N/A | N/A |
| White | 24.4 ± 1.4 | 24.9 ± 1.3 | 25.0 ± 1.2 | 25.6 ± 1.0 | 23.2 ± 1.7 | 25.8 ± 0.8 |
N/A: data not available. OAG-low: subjects with primary open angle glaucoma in who maximum untreated or treated intraocular pressure never exceeded 21 mmHg. OAG-high: subjects with primary open angle or pseudoexfoliative glaucoma in whom maximum untreated intraocular pressure on at least one examination exceeded 21 mmHg. SD: standard deviation.
Optic Nerve Straightness.
In all subjects, the ON was usually redundant in abduction, but straightened in adduction (Fig. 1). A subgroup of 97 eyes (45 control, 34 OAG-low, and 18 OAG-high) underwent MRI in both small and large duction angles. While the mean angles, were similar in all groups (Fig. 2), “small” adduction averaged 20–24°, and “large” adduction averaged 27–28°, representing 5–6°average incremental adduction. The apparently larger ab- than adduction is attributable to the phenomenon of “positive angle kappa,” in which apparent anatomic globe orientation is abducted relative to subjective gaze direction by an average of ~3°70 - 5°71. Average vertical gaze position was statistically similar for all groups and did not change by more than 2° in any horizontal gaze position.
Fig. 2.
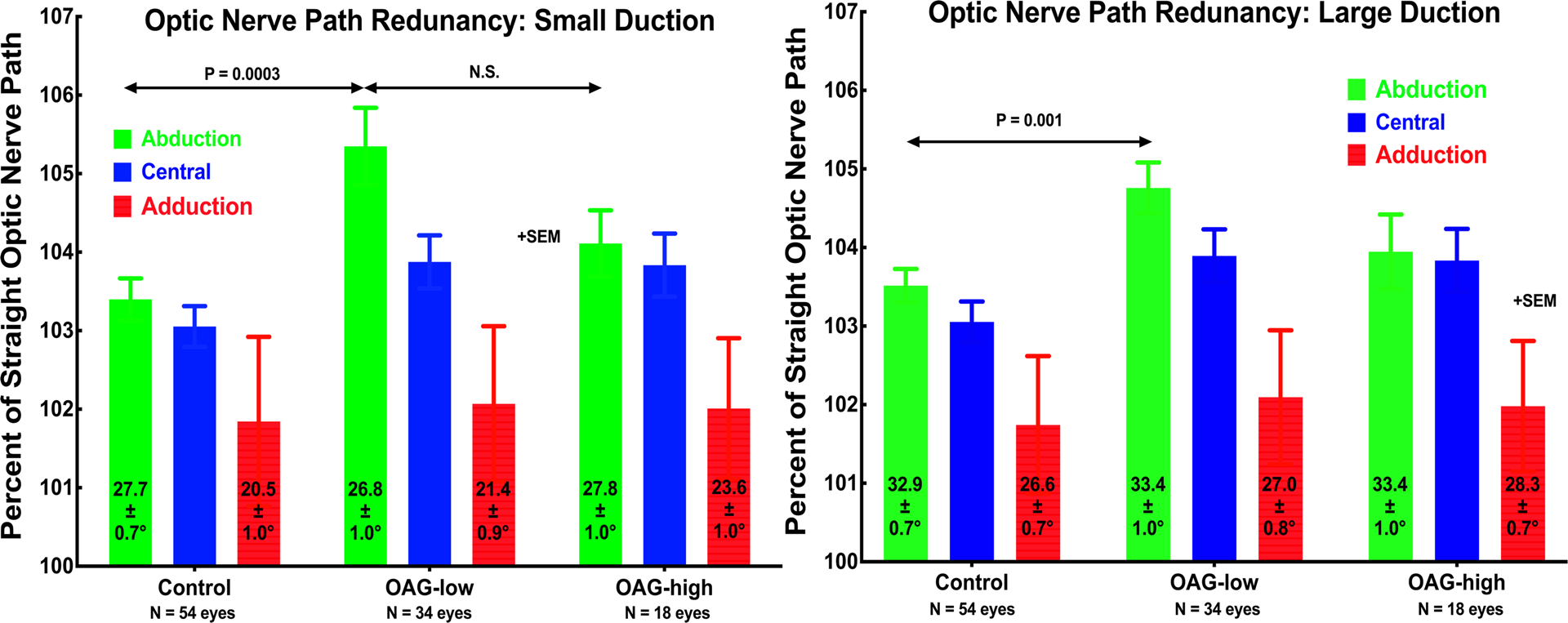
Optic nerve (ON) straightness represented as path length relative to shortest geometric distance to the globe-ON junction in central gaze, and small and large angle horizontal ductions to the average angles indicated in each column. In all groups, the ON was significantly straighter in adduction than in the other gaze positions, and its straightness in adduction did not differ significantly among groups or with duction angle (P>0.15). However, in central gaze and abduction, the ON was significantly more redundant in OAG-low than in OAG-high or the control group. For all groups, the ON was highly significantly straighter in adduction than in central gaze (P<0.0002). SEM – standard error of mean.
In central gaze and abduction, ON path redundancy did not vary with incremental duction (Fig. 2). Path redundancy was significantly greater for patients with OAG-low than controls (P 0.001 or less). Most strikingly, however, ON path redundancy significantly decreased to a consistent, minimum value of 101.5 – 102% in adduction for all subject groups for both small and large adduction (P<0.0002). Such a “floor effect” is interpreted to represent maximal straightening of the ON during adduction tethering, reflecting measurement errors among other factors.
Globe Translation.
The globe translated relative to central gaze in both ab- and adduction (Fig. 3). Mediolateral translation was greatest at up to 0.8 mm, with the globe “rolling” medially in adduction and laterally in abduction. While lateral translation in abduction was similar in control subjects and patients with OAG-high, patients with OAG-low exhibited significantly less lateral translation in abduction than the other groups (paired t-test, p<0.05, Fig. 3). All groups exhibited similar medial globe translation in adduction of 0.4–0.8 mm. Subjects with OAG exhibited highly significantly greater posterior globe translation in adduction – equivalent to globe retraction - than did controls (Fig. 3 middle). For small adduction, the ~0.5 mm posterior translation in OAG-low was significant (P<0.03), but in OAG-high the smaller posterior translation was similar to control. For large adduction, however, posterior translation in adduction increased to ~0.6 mm in OAG-low and to ~0.8 mm in OAG-high, both significantly (P<0.0002) greater than near-zero control translation. Vertical globe translation during horizontal duction was less than 0.3 mm for all groups, without major differences among groups.
Fig. 3.
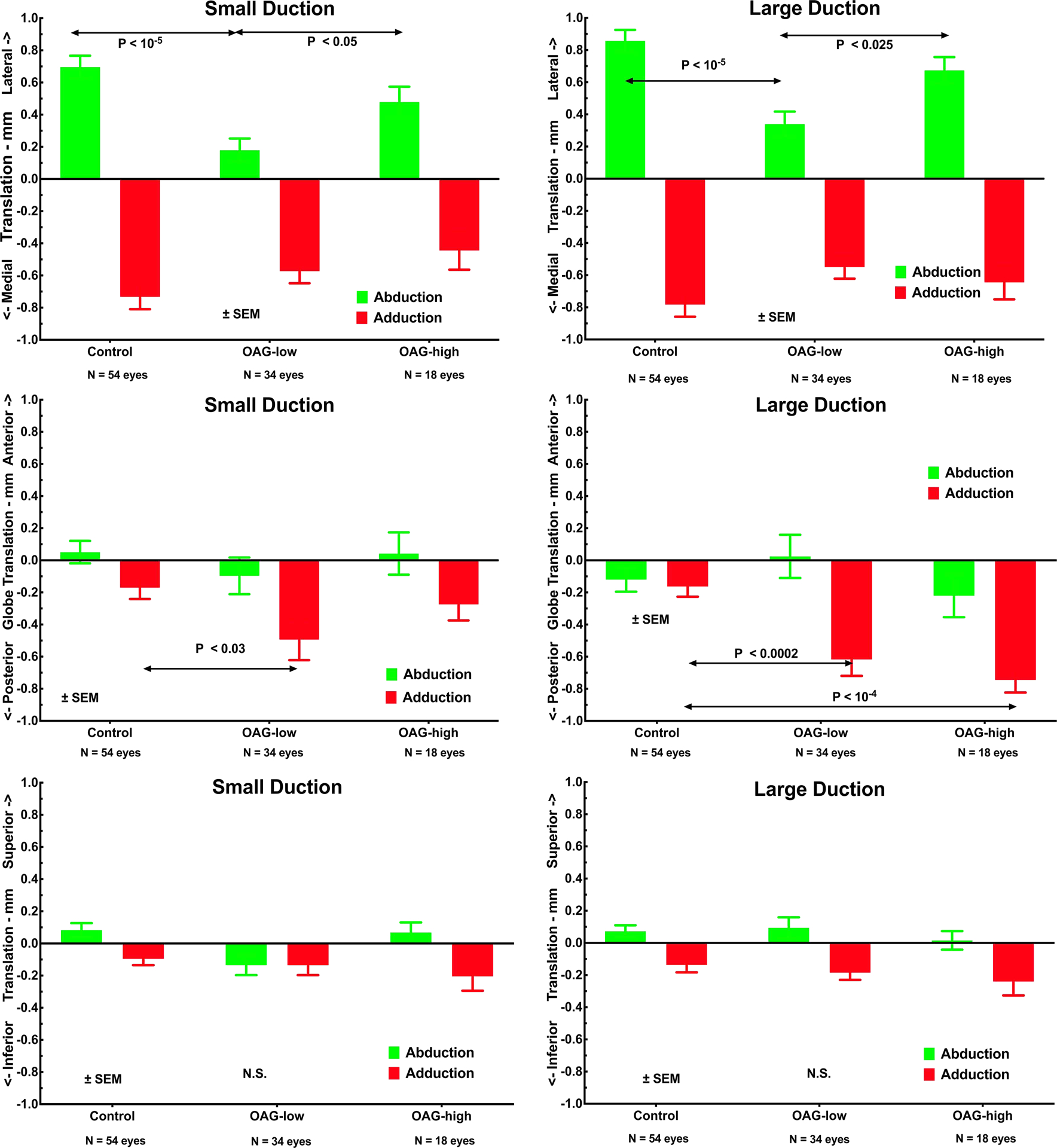
Globe translation during small and large ductions to the angles specified in Fig. 2. Upper row illustrates significantly less mediolateral translation during horizontal duction in OAG-low than in controls or OAG-high patients. Middle row shows greater posterior globe translation (retraction) than normal in OAG-low for small adduction, and for both OAG-high and OAG-low in large adduction, without significant difference in abduction. Lower row illustrates absence of significant vertical globe translation during horizontal duction in any subject group. SEM – standard error of mean. P values by t-testing.
Effects of Other Factors on Globe Retraction in Adduction.
Data on posterior globe translation in large angle adduction was pooled with data accumulated in the prior study41, permitting exploratory analysis of 12 orbits of 6 Asian control subjects, 67 orbits of 35 white control subjects, 14 orbits of 7 black control subjects, 24 orbits of 12 Asian patients with OAG-low, 22 orbits of 11 white patients with OAG-low, four orbits of two black patients with OAG-low, and 18 orbits of 9 white patients with OAG-high. This data set was subjected to multivariate analysis by GEE to explore possible predictors of posterior globe translation in ab- and adduction. Because both eyes of each subject were analyzed when available, ocular laterality was treated as a within-subject variable whose possible correlation was considered. Subject variables included gender, age, race, diagnosis (normal, OAG-low, OAG-high), duction angle, equatorial globe diameter, globe AL, path length from the orbital apex to the globe-ON junction in central gaze, and the binary variable of positive history of topical ocular prostaglandin treatment. Because the SPSS software reports significance levels only to three decimal places, resulting in significance values of “0.000” for several predictors, Table 3 reports chi-square values in lieu of P-values. From standard tables, chi-square values exceeding 3.9 for one degree of freedom (DF) and 6.0 for two DF are statistically significant at 0.05. Chi-square values exceeding 23.0 for two DF are statistically significant at 0.00001.
Table 3.
Chi-Square Values for Generalized Estimating Equation Predictors
| Measurement | Diagnosis | Race | Diagnosis×Race | Age | Axial Length |
|---|---|---|---|---|---|
| Posterior Globe Translation | |||||
| Abduction | 1.2 | 1.4 | 3.5 | 3.6 | 0.3 |
| Adduction | 35.2 | 2.6 | 9.3 | 0.5 | 0.6 |
| Globe Lateral Translation | |||||
| Abduction | 2.3 | 46.8 | 1.6 | 0.3 | 2.2 |
| Adduction | 0.5 | 0.7 | 2.2 | 3.5 | 10.2 |
| Globe Superior Translation | |||||
| Abduction | 0.5 | 8.7 | 4.1 | 1.4 | 0.1 |
| Adduction | 8.2 | 2.6 | 0.1 | 1.2 | 0.1 |
Table 3. Statistically significant values (P<0.05) are bolded. The interaction of diagnosis and race was significant for posterior translation in adduction at the 0.01 level, as was axial length for lateral translation in adduction; and race for superior translation in abduction, and diagnosis for superior translation in adduction. Chi-square values for prostaglandin treatment effect (not listed) were all less than 1.5 except for superior translation in adduction, with chi-square 6.9 giving P=0.009 (2 DF).
As seen in Table 3, posterior globe translation in adduction was predicted by diagnosis (normal, OAG-low, OAG-high), with chi-square 35.2, indicating a significance P<<0.00001. Race, and interaction of diagnosis with race, were predictive of retraction in adduction (P<0.05). AL and horizontal duction angle did not predict posterior globe translation. Lateral and superior globe translation in abduction were predicted by race (Table 3), as was superior translation in adduction, but was predicted by diagnosis for adduction.
Figure 4 illustrates effects of IOP level in OAG on posterior globe translation in large adduction, and its interaction with race in all subjects. Posterior globe translation was small, less than 0.2 mm in healthy control subjects of all racial groups, and did not vary significantly among them. It is also apparent from Fig. 4 that in both the white and Asian eyes with glaucoma, posterior globe translation in adduction was significantly larger than normal (P<0.005, t-test). There was a trend in this direction in four eyes of black patients with OAG-low, but paucity of such eyes precludes statistical conclusion. Among white patients, there was no significant difference in posterior globe translation in adduction between OAG-low and OAG-high. However, posterior globe translation in adduction was significantly greater in Asian than in white patients with OAG-low (P<0.02).
Fig. 4.
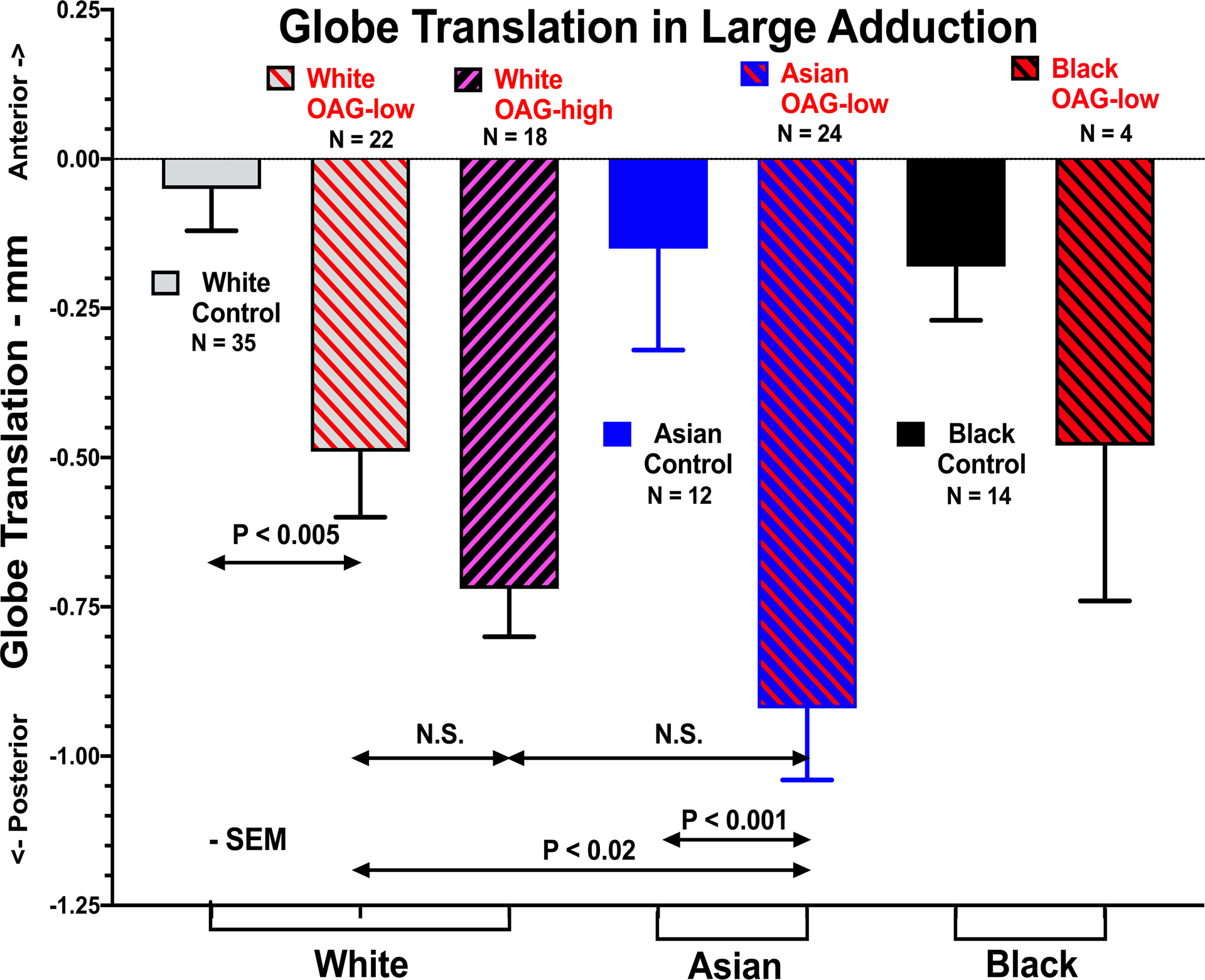
Posterior globe translation (retraction) in large adduction in healthy control subjects, patients with OAG-low, and OAG-high, separately analyzed for white, Asian, and black races. Control subjects did not differ significantly by race. N – number of eyes tested. SEM – standard error of mean. t-tests employed.
Effects of Topical Prostaglandin Drugs on Globe Retraction in Adduction.
Since all patients were under ophthalmic management to control IOP, the great majority of eyes with OAG had been treated with topical prostaglandin agents, often for many years. However, 15 eyes of 8 subjects with OAG had not received these agents. Posterior globe translation in large adduction in these eyes was 0.64±0.11mm (SEM), not significantly different by 2-tail t-test from 0.69±0.08 mm in eyes with OAG who had received topical prostaglandins. Incorporation of topical prostaglandin therapy as a factor in GEE demonstrated chi-square values less than 1.5 for all measures of globe translation during eye movement, except for superior translation in adduction, with chi-square 6.9 giving P=0.009.
Discussion
The present study confirms by augmentation of our earlier sample that progressive globe retraction in adduction is strongly associated with OAG despite absence of IOP elevation41, and extends this finding to OAG in which IOP was abnormally elevated prior to therapy. While incremental adduction past threshold of ON straightening caused little or no globe retraction in adduction in healthy subjects, such adduction caused progressive globe retraction in OAG regardless of IOP. Moreover, globe retraction in adduction was greater in Asian than in white patients with OAG, neither group having ever displayed abnormally elevated IOP. Abnormal globe retraction in adduction was not related to prior topical therapy with prostaglandin agonist agents, and thus is not likely an artifact of medical treatment.
Globe Displacement During Horizontal Duction.
As first geometrically deduced by Friedman72 and later directly demonstrated by MRI, the ON is redundant in central and abducted gazes, but becomes taut in adduction where it tethers the globe39. Beyond ~26° adduction threshold of straightening43, 73, the ON can only stretch, displace, and/or deform the globe. While ON tethering in adduction also occurs in healthy eyes, tethering has different effects in OAG. The globe normally “rolls” laterally in abduction and medially in adduction (Fig. 3 top)66; while “rolling” increased with duction angle in all subject groups, lateral rolling in abduction was significantly less in OAG-low than in OAG-high. These differences during abduction cannot be attributed to ON tethering since ON path was redundant in abduction, but instead must reflect differences in orbital anatomy or mechanical differences in tissue properties, or both.
Importantly, in OAG but not in healthy subjects, ON tethering in adduction was associated with highly statistically significant globe retraction averaging about 0.6 – 0.8 mm (Fig. 3 middle). Thus, in OAG with or without elevated IOP, the globe abnormally retracts as the ON tethers the globe in adduction.
Mechanical Strain During Adduction Tethering.
Globe translation represents mechanical strain on orbital suspensory tissues. When the healthy ON tethers in adduction, orbital and ocular structures must deform to absorb medial rectus muscle counterforce exerted against the ON. The current results indicate that this does not normally induce globe retraction, but the normal globe rolls a little farther nasally in adduction than in POAG-low (Fig. 3 top). The abnormally great globe retraction in adduction in OAG is most likely due to abnormally large force exerted by the ON, transferring the associated strain that would normally be dissipated elsewhere onto the vulnerable peripapillary junction and disc.
The foregoing interpretation is consistent with other evidence. Eye movements induce phosphenes adjacent to the cecum74, 75 even after vitreous detachment75, more with adduction and convergence than with abduction72, 74. The early model of Enoch et al. suggested that duction deforms the posterior globe75, a prediction supported by finite element biomechanical analysis (FEA)40, 42, 45. OCT has shown that the peripapillary Bruch’s membrane and optic cup of normal subjects are deformed progressively43 by horizontal duction44,49, especially in esotropia where adduction is exaggerated, but not in exotropia where adduction is less73. Sibony has reported that horizontal duction causes ONH movement directionally inverse between ab- and adduction, but augmented in papilledema49. Wang et al. demonstrated the lamina cribrosa strain by OCT during horizontal eye movement45. Li et al. imaged en face the larger epipapillary and retinal and blood vessels as fiducials to map two-dimensional strain during eye movements, reporting significantly more horizontal compression of the ON head and displacement of the peripapillary retina in ad- than in abduction76. Eye movement related deformations of the ON head and peripapillary Bruch’s membrane are much larger than the effects of severe, acute IOP elevation during angle closure47.
Frequent Occurrence of Large Adductions.
Subjects with OAG exhibited globe retraction with as little as 21° adduction, less than half of the approximately ±55° human oculomotor range77. In the laboratory, subjects touching targets made saccades up to 40–45°.78 About three saccades occur every second79, for a daily total of ~183,000 saccades80, even during sleep81. We propose that everyday eye movements most likely to produce ON tethering are associated with self-generated head movements82 and ambulation. When head and body are unrestrained, 25–45°saccades frequently occur82, including automatic ocular counter-rotations generated by the vestibulo-ocular reflex. Large gaze shifts accompanying head movement include eye movements averaging ~30˚83, 84. While adductions causing globe retraction in OAG commonly occur in active life, small eye movements during reading and similar near vision activities probably do not cause adduction tethering. Convergence in normal subjects is not associated with globe retraction, with 0.5 mm oppositely-directed proptosis occurring during 22° convergence59. Scanning saccades during reading are small and probably do not tether the ON.
Possible Factors in Pathological Adduction Tethering.
If the mechanical loading on the ON occurs during ubiquitous adduction tethering, why would optic neuropathy only result in the minority of people who develop OAG? Eye movements perhaps eventually do damage the ON in everyone, but at rates varying greatly among individuals. Some age-related thinning of the retinal nerve fiber and ganglion cell layers is considered normal,85 although only projected to be symptomatic at ages exceeding typical longevity86. An important clue is specificity of globe retraction in adduction to OAG. Figure 4 illustrates as a second clue the significantly greater globe retraction in adduction in Asian than white patients with OAG-low. In Japanese and Koreans, IOP is not abnormally elevated most cases of OAG54, 87. Lateral and superior globe translation in abduction were associated with diagnosis and race (Table 3), but this phenomenon does not cause ON tethering or retraction. Multivariate analysis in Table 3 indicates that globe retraction in adduction is significantly predicted by the interaction between the diagnosis of glaucoma, and race. The inference from these observations is that race-related factors influence adduction tethering in glaucoma, but race by itself does not cause tethering. Presumably, globe retraction due to tethering requires additional pathological factors, probably including variations in biomechanical properties of the ON, ON sheath, and sclera. Stress and strain in the ON and peripapillary sclera have been modeled by FEA42, evaluating combinations of local biomechanical properties measured in fresh post-mortem human eyes88. These properties are generally nonlinear, and interact in a non-linear manner. For adduction 6° past the point of first ON tethering, FEA suggests that realistic combinations of observed local biomechanical properties can produce large variations in the degree and distribution of stress and strain in peripapillary sclera88. In sum, there are suggestions that both race-related anatomical variations and possibly tissue biomechanical variations may influence globe retraction during adduction tethering of the ON, but that such retraction during adduction tethering is a hallmark of OAG irrespective of history of elevated IOP.
Factors Not Associated With Adduction Tethering.
Of course, the putative repetitive strain injury to the ON during natural activities would accumulate over a lifetime, paralleling the increase in glaucoma prevalence with advancing age89, 90. Absence of significant association between globe retraction in adduction and age in Table 3 does not permit causative inferences about age because of our intentional selection of an age-matched control group, but it does exclude an age effect as a potential confound of other inferences. Neither AL (Table 3) nor equatorial globe diameter were associated with posterior globe retraction in adduction, although AL was associated with lateral translation in adduction.
Topical prostaglandin analogue drugs can cause adnexal fat atrophy91, particularly in pre-aponeurotic lid fat92, and ipsilateral enophthalmos93, 94 that is reversible94. Notwithstanding, globe retraction during adduction tethering in the current study was similar in eyes not exposed to prostaglandin drugs, and multivariate analysis did not suggest an effect of prostaglandin exposure on retraction in adduction. This suggests that the globe retraction in OAG is associated with the disease, not an epiphenomenon of its treatment. This inference must be provisional since the number of patients with OAG not exposed to prostaglandin agonist agents was relatively small.
Possible Therapeutic Implications.
The current study was designed to evaluate the plausibility of the proposed contributory role of eye movements in glaucomatous optic neuropathy, and was not a therapeutic trial. Since adduction tethering of the ON is ubiquitous even in healthy people, and since precise measurements of presumably-pathological posterior globe retraction in adduction require highly specialized MRI technique, it is unlikely that the MRI measurements employed in this study would enter routine clinical practice for individual patients. However, MRI in individual patients would probably be unnecessary for therapeutic decisions in patients with OAG-low who continue to experience progressive glaucomatous optic neuropathy despite effective IOP reduction.
If adduction tethering of the ON were to be confirmed as a significant cause of OAG, then tethering could readily be therapeutically attenuated by extraocular muscle surgery limiting adduction range. Surgically-created enophthalmos would also reduce adduction tethering, and could be accomplished by surgical expansion of the bony orbit, a reduction in orbital fat volume, or a combination of approaches.
Study Limitations.
This cross-sectional comparison might have been subject to unknown confounds. While efforts were made to include a comparable representation of races and ages in the control group, imbalances were unavoidable, and analyses of these factors was exploratory; a larger and more diverse sample of subjects might demonstrate significance of additional effects. A larger sample size is desirable and might also be informative about the possible contributions of variations in anatomical factors such as orbital size and configuration, and variations in globe size and biomechanical properties of the globe, ON, and other tissues in the orbit.
Since a relatively small number of subjects with OAG-high was studied, it is remotely possible that a larger sample size might have demonstrated a significant difference in globe retraction in adduction based on IOP level. However, we calculate that MRI data would be required in over 4,200 eyes to demonstrate with 80% power the statistical significance at the 0.05 level of the observed 0.05 mm difference in globe retraction in adduction between the OAG-low and OAG-high groups. Such a study would be impractical due to its extreme cost in time and money.
Since OAG with elevated IOP is rare in Asians, we were unable to recruit any patients with this combination. Myopia is highly prevalent in the Asians69, making it impractical to recruit a non-myopic Asian control group. Even the strong demonstrated association of globe retraction in adduction with OAG does not prove causality.
Conclusion.
Tethering of the ON in adduction produces abnormal globe retraction in patients with OAG across a range of IOP within or above normal. Globe retraction in adduction, apparently specific to OAG, is more pronounced in Asian than white glaucoma patients. Greater globe retraction in adduction may in part explain the greater prevalence of OAG at lower than higher IOP in Asian than in white populations. Further investigation of pathological effects of adduction tethering may suggest future therapeutic measures to limit this cause of repetitive ON strain to the ON.
Support:
This work was supported by U.S. Public Health Service, National Eye Institute under grants EY008313 and EY000331; and by Research to Prevent Blindness under an Unrestricted Grant to the Department of Ophthalmology. J. Demer is the Arthur L. Rosenbaum Professor of Pediatric Ophthalmology.
Footnotes
Disclosure Statement: None of the authors has a financial interest in any material related to this paper.
References
- 1.Kapetanakis VV, Chan MP, Foster PJ, et al. Global variations and time trends in the prevalence of primary open angle glaucoma (POAG): A systematic review and meta-analysis. Br J Ophthalmol. 2016;100:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingman S Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004;82:887–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Killer HE, Pircher A. Normal tension glaucoma: review of current understanding and mechanisms of the pathogenesis. Eye (Lond). 2018;32:924–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan MP, Grossi CM, Khawaja AP, et al. Associations with intraocular pressure in a large cohort: Results from the UK biobank. Ophthalmology. 2016;123:771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang I, Caprioli J, Ou Y. Surgical management of pediatric glaucoma. Dev Ophthalmol. 2017;59:165–78. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Liu Y, Wang W, et al. Why does acute primary angle closure happen? Potential risk factors for acute primary angle closure. Surv Ophthalmol. 2017;62:635–47. [DOI] [PubMed] [Google Scholar]
- 7.Tan AN, Cornelissen MF, Webers CAB, et al. Outcomes of severe uveitic glaucoma treated with Baerveldt implant: can blindness be prevented? Acta Ophthalmol. 2018;96:24–30. [DOI] [PubMed] [Google Scholar]
- 8.Bai HQ, Yao L, Wang DB, et al. Causes and treatments of traumatic secondary glaucoma. Eur J Ophthalmol. 2009;19:201–6. [DOI] [PubMed] [Google Scholar]
- 9.Kwong JM, Vo N, Quan A, et al. The dark phase intraocular pressure elevation and retinal ganglion cell degeneration in a rat model of experimental glaucoma. Exp Eye Res. 2013;112:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuels BC, Siegwart JT, Zhan W, et al. A novel tree shrew (Tupaia belangeri) model of glaucoma. Invest Ophthalmol Vis Sci. 2018;59:3136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo C, Qu X, Rangaswamy N, et al. A murine glaucoma model induced by rapid in vivo photopolymerization of hyaluronic acid glycidyl methacrylate. PLoS One. 2018;13:e0196529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quigley HA. Use of animal models and techniques in glaucoma research: Introduction. Methods Mol Biol. 2018;1695:1–10. [DOI] [PubMed] [Google Scholar]
- 13.Shi D, Funayama T, Mashima Y, et al. Association of HK2 and NCK2 with normal tension glaucoma in the Japanese population. PLoS One. 2013;8:e54115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–8. [DOI] [PubMed] [Google Scholar]
- 15.Kim CS, Seong GJ, Lee NH, et al. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011;118:1024–30. [DOI] [PubMed] [Google Scholar]
- 16.Ha A, Kim YK, Jeoumg JW, et al. Association of angle width with progression of normal-tension glaucoma. A minimum 7-year follow-up study. JAMA Ophthalmol. 2019;137:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, Solano MM, Oldenburg CE, et al. Prevalence of normal-tension glaucoma in the Chinese population: A systematic review and meta-analysis. Am J Ophthalmol. 2019;199:101–10. [DOI] [PubMed] [Google Scholar]
- 18.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–7. [DOI] [PubMed] [Google Scholar]
- 19.Bonomi L, Marchini G, Marraffa M, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology. 1998;105:209–15. [DOI] [PubMed] [Google Scholar]
- 20.Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–504. [DOI] [PubMed] [Google Scholar]
- 21.Rotchford AP, Johnson GJ. Glaucoma in Zulus: A population-based cross-sectional survey in a rural district in South Africa. Arch Ophthalmol. 2002;120:471–8. [DOI] [PubMed] [Google Scholar]
- 22.Academy A Primary Open-Angle Glaucoma Preferred Practice Pattern. San Francisco: American Academy of Ophthalmology, 1992. [Google Scholar]
- 23.Mi XS, Yuan TF, So KF. The current research status of normal tension glaucoma. Clin Interv Aging. 2014;9:1563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration.The AGIS Investigators. Am J Ophthalmol. 2000;130:429–40. [DOI] [PubMed] [Google Scholar]
- 25.The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505. [DOI] [PubMed] [Google Scholar]
- 26.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:487–97. [DOI] [PubMed] [Google Scholar]
- 27.Caprioli J The treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:578–81. [DOI] [PubMed] [Google Scholar]
- 28.Seol BR, Kim SH, Kim DM, et al. Influence of intraocular pressure reduction on progression of normal-tension glaucoma with myopic tilted disc and associated risk factors. Jap J Ophthalmol. 2017;62:230–6. [DOI] [PubMed] [Google Scholar]
- 29.Tseng VL, Kim CH, Romero PT, et al. Risk factors and long-term outcomes in patients with low intraocular pressure after trabeculectomy. Ophthalmology. 2017;124:1457–65. [DOI] [PubMed] [Google Scholar]
- 30.Song BJ, Caprioli J. New directions in the treatment of normal tension glaucoma. Indian J Ophthalmol. 2014;62:529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi YJ, Kim M, Park KH, et al. The risk of newly developed visual impairment in treated normal-tension glaucoma: 10-year follow-up. Acta Ophthalmol. 2014;92:e644–9. [DOI] [PubMed] [Google Scholar]
- 32.Siaudvytyte L, Januleviciene I, Ragauskas A, et al. Update in intracranial pressure evaluation methods and translaminar pressure gradient role in glaucoma. Acta Ophthalmol Scand. 2015;93:9–15. [DOI] [PubMed] [Google Scholar]
- 33.Jonas JB, Yang D, Wang N. Intracranial pressure and glaucoma. J Glaucoma. 2013;22 Suppl 5:S13–4. [DOI] [PubMed] [Google Scholar]
- 34.Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci. 2008;49:5412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berdahl JP, Allingham RR. Intracranial pressure and glaucoma. Curr Opin Ophthalmol. 2010;21:106–11. [DOI] [PubMed] [Google Scholar]
- 36.Gramer G, Weber BH, Gramer E. Migraine and vasospasm in glaucoma: Age-related evaluation of 2027 patients with glaucoma or ocular hypertension. Invest Ophthalmol Vis Sci. 2015;56:7999–8007. [DOI] [PubMed] [Google Scholar]
- 37.Pircher A, Remonda L, Weinreb RN, Killer HE. Translaminar pressure in Caucasian normal tension glaucoma patients. Acta Ophthalmol. 2017;95:e524–e31. [DOI] [PubMed] [Google Scholar]
- 38.Linden C, Qvarlander S, Johannesson G, et al. Normal-tension glaucoma has normal intracranial pressure: A prospective study of intracranial pressure and intraocular pressure in different body positions. Ophthalmology. 2018;125:361–8. [DOI] [PubMed] [Google Scholar]
- 39.Demer JL. Optic nerve sheath as a novel mechanical load on the globe in ocular duction. Invest Ophthalmol Vis Sci. 2016;57:1826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Fisher LK, Milea D, et al. Predictions of optic nerve traction forces and peripapillary tissue stresses following horizontal eye movements. Invest Ophthalmol Vis Sci. 2017;58:2044–53. [DOI] [PubMed] [Google Scholar]
- 41.Demer JL, Clark RA, Suh SY, et al. Magnetic resonance imaging of optic nerve traction during adduction in primary open-angle glaucoma with normal intraocular pressure. Invest Ophthalmol Vis Sci. 2017;58:4114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin A, Yoo L, Park C, Demer JL. Finite element biomechanics of optic nerve sheath traction in adduction. J Biomech Eng. 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suh SY, Le A, Shin A, et al. Progressive deformation of the optic nerve head and peripapillary structures by graded horizontal duction. Invest Ophthalmol Vis Sci. 2017;58:5015–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang MY, Shin A, Park J, et al. Deformation of optic nerve head and peripapillary tissues by horizontal duction. Am J Ophthalmol. 2017;174:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Rumpel H, Lim WE, et al. Finite element analysis predicts large optic nerve strains heads during horizontal eye movements. Invest Ophthalmol Vis Sci. 2016;57:2452–62. [DOI] [PubMed] [Google Scholar]
- 46.Lee WJ, Kim YJ, Kim JH, et al. Changes in the optic nerve head induced by horizontal eye movements. PLoS One. 2018;13:e0204069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang YX, Jiang R, Wang NL, et al. Acute peripapillary retinal pigment epithelium changes associated with acute intraocular pressure elevation. Ophthalmology. 2015;122:2022–8. [DOI] [PubMed] [Google Scholar]
- 48.Fortune B Pulling and tugging on the retina: Mechanical impact of glaucoma beyond the optic nerve head. Inv Ophtalmol Vis Sci. 2019;60:26–35. [DOI] [PubMed] [Google Scholar]
- 49.Sibony PA. Gaze-evoked deformations of the peripapillary retina and papilledema and ischemic optic neuropathy. Inv Ophtalmol Vis Sci. 2016;57:4979–87. [DOI] [PubMed] [Google Scholar]
- 50.Jonas JB, Martus P, Horn FK, et al. Predictive factors of the optic nerve head for development or progression of glaucomatous visual field loss. Invest Ophthalmol Vis Sci. 2004;45:2613–8. [DOI] [PubMed] [Google Scholar]
- 51.Xu L, Wang Y, Yang H, Jonas JB. Differences in parapapillary atrophy between glaucomatous and normal eyes: the Beijing Eye Study. Am J Ophthalmol. 2007;144:541–6. [DOI] [PubMed] [Google Scholar]
- 52.Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989;30:908–18. [PubMed] [Google Scholar]
- 53.Nakazawa M, Kurotaki J, Ruike H. Longterm findings in peripapillary crescent formation in eyes with mild or moderate myopia. Acta Ophthalmol. 2008;86:626–9. [DOI] [PubMed] [Google Scholar]
- 54.Cho HK, Kee C. Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol. 2014;59:434–47. [DOI] [PubMed] [Google Scholar]
- 55.Dielemans I, Vingerling JR, Wolfs RC, et al. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology. 1994;101:1851–5. [DOI] [PubMed] [Google Scholar]
- 56.Budenz DL, Huecker JB, Gedde SJ, et al. Thirteen-year follow-up of optic disc hemorrhages in the ocular hypertension treatment study. Am J Ophthalmol. 2016;174:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demer JL, Dusyanth A. T2 fast spin echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011;15:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin GS, Demer JL, Rosenbaum AL. High resolution dynamic magnetic resonance imaging in complicated strabismus. J Pediatr Ophthalmol Strabismus. 1996;33:282–90. [DOI] [PubMed] [Google Scholar]
- 59.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–85. [DOI] [PubMed] [Google Scholar]
- 60.Demer JL, Ortube MC, Engle EC, Thacker N. High resolution magnetic resonance imaging demonstrates abnormalities of motor nerves and extraocular muscles in patients with neuropathic strabismus. J AAPOS. 2006;10:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demer JL, Clark RA. Magnetic resonance imaging demonstrates compartmental muscle mechanisms of human vertical fusional vergence. J Neurophysiol. 2015;113:2150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark RA, Miller JM, Demer JL. Three-dimensional location of human rectus pulleys by path inflections in secondary gaze positions. Invest Ophthalmol Vis Sci. 2000;41:3787–97. [PubMed] [Google Scholar]
- 63.Kono R, Clark RA, Demer JL. Active pulleys: Magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–88. [PubMed] [Google Scholar]
- 64.Clark RA, Demer JL. Magnetic resonance imaging of the effects of horizontal rectus extraocular muscle surgery on pulley and globe positions and stability. Invest Ophthalmol Vis Sci. 2006;47:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suh SY, Le A, Clark RA, Demer JL. Rectus pulley displacements without abnormal oblique contractility explain strabismus in superior oblique palsy. Ophthalmology. 2016;123:1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demer JL, Clark RA. Translation and eccentric rotation in ocular motor modeling In: Ramat S, Shaikh AG, eds. Mathematical Modeling in Motor Neuroscience: State of the Art and Translation to the Clinic. Ocular Motor Plant and Gaze Stabilization Mechanisms. Cambridge (MA): Elsevier, 2019, pp. 117–26. [Google Scholar]
- 67.Huang J, Huang JY, Chen Y, Ying GS. Evaluation of Approaches to Analyzing Continuous Correlated Eye Data When Sample Size Is Small. Ophthalmic Epidemiol. 2018;25:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verkicharla PK, Chia NE, Saw SM. What Public Policies Should Be Developed to Cope with the Myopia Epidemic? Optom Vis Sci. 2016;93:1055–7. [DOI] [PubMed] [Google Scholar]
- 69.Pan CW, Dirani M, Cheng CY, et al. The age-specific prevalence of myopia in Asia: A meta-analysis. Optom Vis Sci. 2015;92:258–66. [DOI] [PubMed] [Google Scholar]
- 70.Basmak H, Sahin A, Yildirim N, et al. The angle kappa in strabismic individuals. Strabismus. 2007;15:193–6. [DOI] [PubMed] [Google Scholar]
- 71.Gharaee H, Shafiee M, Hoseini R, et al. Angle kappa measurements: Normal values in healthy Iranian population obtained with the Orbscan II. Iran Red Crescent Med J. 2015;17:e17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friedman B Mechanics of optic nerve traction on the retina during ocular rotation with special reference to retinal detachment. Arch Ophthalmol. 1941;25:564–75. [Google Scholar]
- 73.Suh SY, Clark RA, Demer JL. Optic nerve sheath tethering in adduction occurs in esotropia and hypertropia, but not in exotropia. Invest Ophthalmol Vis Sci. 2018;59:2899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von Helmholtz H Helmholtz’s Treatise on Physiological Optics, translated from the Third German Edition.: The Optical Society of America, 1924. [Google Scholar]
- 75.Enoch JM, Choi SS, Kono M, et al. Utilization of eye-movement phosphenes to help understand transient strains at the optic disc and nerve in myopia. Ophth Physiol Opt. 2003;23:377–81. [DOI] [PubMed] [Google Scholar]
- 76.Le A, Lesgart M, Gawargious BA, et al. Horizontal duction causes age dependent deformation of the optic nerve head and peripapillary retina. ARVO Abstracts. 2019;abstract 6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guitton D, Volle M. Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. J Neurophysiol. 1987;58:427–59. [DOI] [PubMed] [Google Scholar]
- 78.Epelboim J, Steinman RM, Kowler E, et al. Gaze-shift dynamics in two kinds of sequential looking tasks. Vision Res. 1997;37:2597–607. [DOI] [PubMed] [Google Scholar]
- 79.Wu CC, Kowler E. Timing of saccadic eye movements during visual search for multiple targets. J Vis. 2013;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson DA. Control of eye movements. In: Brooks VB, ed. The nervous system, handbook of physiology. Baltimore: Williams & Wilkins, 1981; v. II, pp. 1275–320. [Google Scholar]
- 81.Leclair-Visonneau L, Oudiette D, Gaymard B, et al. Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model. Brain. 2010;133:1737–46. [DOI] [PubMed] [Google Scholar]
- 82.Anastasopoulos D, Ziavra N, Hollands M, Bronstein A. Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Exp Brain Res. 2009;193:323–36. [DOI] [PubMed] [Google Scholar]
- 83.Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. II. Interaction between saccades and the vestibuloocular reflex. J Neurophysiol. 1986;56:1558–70. [DOI] [PubMed] [Google Scholar]
- 84.Tomlinson RD, Bahra PS. Combined eye-head gaze shifts in the primate. I. Metrics. J Neurophysiol. 1986;56:1542–57. [DOI] [PubMed] [Google Scholar]
- 85.Leung CK, Ye C, Weinreb RN, et al. Impact of age-related change of retinal nerve fiber layer and macular thicknesses on evaluation of glaucoma progression. Ophthalmology. 2013;120:2485–92. [DOI] [PubMed] [Google Scholar]
- 86.Caprioli J, Zeyen T. A critical discussion of the rates of progression and causes of optic nerve damage in glaucoma. J Glaucoma. 2009;18:S1–21. [DOI] [PubMed] [Google Scholar]
- 87.Pekmezci M, Vo B, Lim AK, et al. The characteristics of glaucoma in Japanese Americans. Arch Ophthalmol. 2009;127:167–71. [DOI] [PubMed] [Google Scholar]
- 88.Park J, Giaconi JA, Nouri-Mahdavi K, et al. Finite element analysis (FEA) of anatomical factors exaggerating optic nerve (ON) strain during adduction tethering in primary open angle glaucoma (POAG) without elevated intraocular pressure (IOP). . ARVO Abstracts. 2019;6172. [Google Scholar]
- 89.Shim SH, Sung KR, Kim JM, et al. The prevalence of open-angle glaucoma by age in myopia: The Korea national health and nutrition examination survey. Curr Eye Res. 2017;42:65–71. [DOI] [PubMed] [Google Scholar]
- 90.Doucette LP, Rasnitsyn A, Seifi M, Walter MA. The interactions of genes, age, and environment in glaucoma pathogenesis. Surv Ophthalmol. 2015;60:310–26. [DOI] [PubMed] [Google Scholar]
- 91.Rabinowitz MP, Katz LJ, Moster MR, et al. Unilateral prostaglandin-associated periorbitopathy: A syndrome involving ipper eyelid retraction distinguishable from the aging sunken eyelid. Ophthal Plast Reconstr Surg. 2015;31:373–8. [DOI] [PubMed] [Google Scholar]
- 92.Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol. 2011;55:22–7. [DOI] [PubMed] [Google Scholar]
- 93.Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthal Plast Reconstr Surg. 2008;24:302–7. [DOI] [PubMed] [Google Scholar]
- 94.Jayaprakasam A, Ghazi-Nouri S. Periorbital fat atrophy - An unfamiliar side effect of prostaglandin analogues. Orbit. 2010;29:357–9. [DOI] [PubMed] [Google Scholar]


