Abstract
Cisplatin is a platinum(II) chemotherapy drug that can cause the side-effect of ototoxicity and hearing loss. The monofunctional platinum(II) complexes, phenanthriplatin and pyriplatin, have recently been investigated as anti-cancer agents but their side-effects are largely unknown. Here, we used the auditory hybridoma cell line, HEI-OC1, to investigate the ototoxicity of cisplatin, phenanthriplatin and pyriplatin. The effect of these compounds against cellular viability, on reactive oxygen species (ROS) production, mitochondrial membrane polarization, caspase-3/7 activity, DNA integrity and caspase-12 expression were measured using spectrophotometric, flow cytometric and blot analyses. We found that the monofunctional complexes and cisplatin decreased cellular viability. All three compounds increased ROS yield at 24 hours, but at 48 hours, ROS levels returned to normal. Also, the compounds did not depolarize the mitochondrial membrane. All three compounds reduced caspase-3/7 activity at 24 hours; cisplatin increased caspase-3/7 activity and caused apoptosis at 48 hours. Caspase-12 expression was associated with all three compounds. In summary, the monofunctional complexes may cause ototoxicity like cisplatin. Phenanthriplatin and pyriplatin may cause ototoxicity initially by inducing ROS production, but they may also signal through distinct apoptotic pathways that do not integrate caspases-3/7, or may act at different time-points in the same pathways.
Keywords: cisplatin, monofunctional platinum(II) complex, apoptosis, ototoxicity, HEI-OC1 cells
1. INTRODUCTION
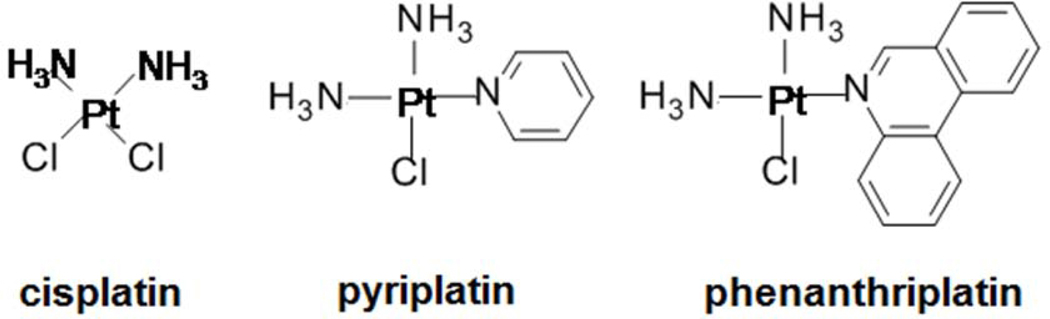
The platinum-based chemotherapy compound, cisplatin (Figure 1), is prescribed for several cancers but can also cause damage to auditory tissue and permanent hearing loss (Hill et al., 2008; Salehi et al., 2014; Sheth et al., 2017; Gentilin et al., 2019). Cisplatin is a bifunctional platinum(II) complex with two chloride leaving ligands that binds to DNA causing the double helix to bend leading to induction of apoptosis and cell death in cancer and non-cancer cells (Cepeda et al., 2007; Dasari and Tchounwou, 2014). Cisplatin can modulate several cell death mechanisms in auditory tissue including early induction of reactive oxygen species (ROS) production and mitochondrial membrane depolarization, leading to increased caspase-3/7 activity through both the extrinsic and intrinsic apoptosis pathways (Sheth et al., 2017; Gentilin et al., 2019). Additionally, cisplatin can induce stress in the endoplasmic reticulum causing increased caspase-12 production which promotes apoptosis (Zong et al., 2017). Monofunctional platinum(II) complexes, which have only one chloride leaving ligand, do not bend DNA but can reduce cancer cell viability by suppressing transcription (Park et al., 2012; Johnstone et al., 2014; Facchetti and Rimoldi, 2019). The monofunctional complexes, [cis-diammine (pyridine) chloroplatinum(II)] (pyriplatin) and [cis-diammine (phenanthridine) chloroplatinum(II)] (phenanthriplatin), have anti-cancer efficacy (Park et al., 2012), but their ototoxic mechanisms are not currently well understood.
Figure 1. Platinum(II) complexes investigated in this project.
Cisplatin is a bifunctional platinum(II) complex with anticancer activity and ototoxic effect. Pyriplatin and phenanthriplatin are heterocyclic-ligated monofunctional platinum(II) complexes that reduce cancer cell viability, but their ototoxicity has not yet been well characterized.
In this project, we used HEI-OC1 cells to identify the cell death signaling mechanisms activated by cisplatin and these monofunctional compounds. Studies conducted with HEI-OC1 cells have used flow cytometry, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), caspase-3/7, oxidative stress assays and blot analysis to elucidate the effects of cisplatin on cellular viability, apoptosis signaling, and endoplasmic reticulum stress (Kalinec et al., 2016). We found that cisplatin and the monofunctional compounds had similar effects against HEI-OC1 cellular viability at 24- and 48-hour time points. All three compounds caused increased ROS yields at 24 hours which returned to control levels at 48 hours. None of the compounds caused mitochondrial membrane depolarization. Caspase-3/7 activity was below control levels at 24 hours for all compounds, but cisplatin treatment caused increased activity at 48 hours, while the monofunctional compounds had activity equivalent to control levels at the later time point. 7-Aminoactinomycin D (7-AAD) staining suggests that cisplatin caused apoptosis at 48 hours. The three platinum compounds and controls had full length and cleaved caspase-12 expression at both time points. Our results suggest that phenanthriplatin and pyriplatin, like cisplatin, may act as ototoxins and that both monofunctional compounds may initiate apoptosis through a series of temporal stages beginning with ROS generation. However, unlike cisplatin, the monofunctional compounds may not integrate caspase-3/7 signaling and induce apoptosis until a later time point.
2. MATERIALS AND METHODS
2.1. Cell culture
The mouse auditory hybridoma cell line, HEI-OC1, was obtained from Dr. Federico Kalinec (University of California Los Angeles). Cells were cultured in Dulbecco’s Eagle’s medium (DMEM) with 10% fetal bovine serum and 50U/mL gamma interferon supplementation as described in Kalinec et al. (2003). HEI-OC1 cells were incubated at 33 °C in 10% CO2.
2.2. Cellular viability assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cellular viability assay was performed in HEI-OC1 cells using a protocol adapted from Monroe et al. (2018). Cells were seeded at 50,000 cells per well in 24-well plates and incubated at 33 °C in 10% CO2. Then, three separate experiments with replicates of three wells each were performed using a concentration series (500, 50, 5, 0.5, 0.05 μM) of cisplatin, phenanthriplatin, or pyriplatin in media solvent for 24 hours or 48 hours. A negative control (media only), positive control (Triton X-100) and series of blanks were simultaneously performed. The MTT assay was conducted for two hours, followed by rapid evacuation of all wells, and treatment with MTT solubilization solution [10% Triton X-100 in acidic (0.1N HCl) isopropanol] for 15 minutes before the plates were read using a BioTek Synergy HT (Winooski, VT) plate reader (570 nm and 690 nm absorbance wavelengths).
2.3. Reactive oxygen species assay
ROS production was measured using flow cytometry analysis. 6-well dishes were seeded with 3 × 105 HEI-OC1 cells in each well. Dishes were then incubated at 33 °C in 10% CO2 for 24 hours and then treated in triplicate with platinum compound IC50 values, media (negative control) or 100 μM H2O2 (positive control) for either 24 or 48 hours. Additionally, a no dye control was prepared for both time points. Then, media was aspirated out of each well and cells were washed with PBS in triplicate. Cells were detached by adding Accutase (Gibco, Gaithersburg, MD) to each well. Each tube was then spun for 5 minutes at 1000 rpm and the supernatant was discarded. Next, the pellet was re-suspended with 10 μM ROS indicator dye (H2DCFDA) (Invitrogen, Carlsbad, CA) in PBS except that the no-dye control tubes received 500 μL of PBS only without dye. Tubes were incubated for 30 minutes at 33 °C in 10% CO2. Next, tubes were centrifuged for 5 minutes at 1000 rpm, the supernatant was removed, and the cells washed with PBS 3X. Cells were then re- suspended in PBS. Fluorescence was measured with a Becton-Dickinson Accuri C6 flow cytometer (Franklin Lakes, NJ) set to 5000 events using the FL1 channel.
2.4. Mitochondrial membrane potential assay
Flow cytometry analysis was used to measure mitochondrial membrane potentials (MMP). 6-well dishes were seeded with 3 × 105 HEI-OC1 cells in each well. Dishes were then incubated at 33 °C in 10% CO2 for 24 hours and then treated in triplicate with platinum compound IC50 values for either 24 or 48 hours or with media (negative control). A set of positive control cells were treated with carbonyl cyanide 3-chlorophenyl hydrazine (CCCP)(Sigma-Aldrich, St. Louis, MO) for 30 minutes prior to beginning the assay. Then, media was aspirated out of each well and cells were washed with PBS in triplicate. Cells were detached by adding Accutase (Gibco, Gaithersburg, MD) to each well. Each tube was then spun for 5 minutes at 1000 rpm and the supernatant was discarded. Pellets were then washed 1X with serum-containing media. Cells were resuspended in JC-10 dye (Sigma- Aldrich, MAK #160 kit) and incubated at 33 °C for 45 minutes protected from light. Tubes were then centrifuged at 5,000 rpm for 5 minutes. The supernatant was aspirated out, and cells were resuspended in PBS. Fluorescence was measured with a Becton-Dickinson Accuri C6 flow cytometer (Franklin Lakes, NJ) set to 5000 events using both the FL1 and FL3 channels. The FL1 to FL3 ratio was converted into a percent of negative control value.
2.5. Caspase luminescence assays
A luminogenic kit (Promega, Fitchburg, WI) was used to measure caspase-3/7 activity. HEI-OC1 cells were seeded at 10,000 cells per well in replicates of six in white 96-well plates (Fisher Scientific, Waltham, MA) and then placed in an incubator for 24 hours at 33 °C in 10% CO2. Wells were then treated with 24- or 48-hour platinum compound IC50 values or with media (negative control) or 100 μM hydrogen peroxide (H2O2, positive control). A separate replicate of six was also prepared without cells and treated with media only (blank). After introduction of the luminogenic substrate, plates were kept in the dark at room temperature and then read at 0.5, 1, 2, and 3-hour timed intervals with a plate reader (BioTek, Winooski, VT) using the luminescent setting.
2.6. 7-Aminoactinomycin D assay
Flow cytometry analysis was used to measure 7-Aminoactinomycin D (7-AAD) uptake. 6-well dishes were seeded with 3 × 105 HEI-OC1 cells in each well. Dishes were then incubated at 33 °C in 10% CO2 for 24 hours and then treated in triplicate with platinum compound IC50 values, with media (negative control), or a positive control (200 μM H2O2) for either 24 or 48 hours Then, media was aspirated out of each well and cells were washed with PBS in triplicate. Cells were detached by adding Accutase (Gibco, Gaithersburg, MD) to each well. Each tube was then spun for 5 minutes at 1000 rpm and the supernatant was discarded. Cells were then centrifuged for 5 minutes at 5000 rpm followed by washing 1X with Assay Buffer HSC (Sigma Aldrich, #FCCH 100108). Cells were subsequently resuspended in Assay Buffer HSC and 7-AAD was added to each tube followed by incubation at room temperature for 5 minutes in the dark. Fluorescence was measured with a Becton-Dickinson Accuri C6 flow cytometer (Franklin Lakes, NJ) set to 5000 events. The FL4 channel setting was used.
2.7. Western blot assay
Caspase-12 expression was measured using western blot analysis. 10 cm dishes were seeded with 1.0 × 106 cells in media for 24 hours and incubated at 33 °C in 10% CO2. Next, media was aspirated out of each dish. Then, each dish was treated with one of the following preparations: 10 mL of 5 μM thapsigargin (positive control) for 24 or 48 hours, 10 mL media only (negative control) for 24 or 48 hours, 10 mL of IC50 cisplatin for 24 or 48 hours, 10 mL of IC50 phenanthriplatin for 24 or 48 hours or 10 mL of IC50 pyriplatin for 24 or 48 hours.
Media was aspirated from dishes and cells were washed with PBS and then transferred into micro-centrifuge tubes. Tubes were centrifuged at 1500 RPM for 5 minutes and the supernatant was discarded. Cells were lysed by addition of 1X sodium dodecyl sulfate (SDS) with protease/phosphatase inhibitor (Cell Signaling Technology, Danvers, MA). Tubes were sonicated, incubated on ice for 30 minutes and centrifuged for 10 minutes at 14,000 × g, at 4 °C. The supernatant was transferred to a fresh tube and determination of protein concentration was done using the Bradford assay (Thermo Scientific, Rockford, IL).
Samples containing from 2.5 to 40 μg of protein were heated to 95–100 °C for 5 minutes followed by cooling on ice. Then, samples were loaded into SDS-PAGE gel (Bio-Rad, Hercules, CA, Mini-Protean 12% TGX gel) wells, subjected to electrophoresis and then electro-transferred to a PVDF membrane using a Bio-Rad Mini-Protean Tetra Cell. Membranes were incubated with blocking buffer for one hour and then incubated with primary antibody against caspase-12 (#62484, Abcam, Cambridge, UK) or glyceraldehyde 3-phosphate dehydrogenase (#3683, Cell Signaling Technology) diluted 1:1000 in milk buffer with gentle agitation overnight at 4 °C. Next, membranes were incubated with anti-rabbit HRP-linked Antibody (Cell Signaling Technology) in blocking buffer for one hour at room temperature. Membranes were then incubated in SignalFire™ (Cell Signaling Technology) and read using an Alpha-Innotech FluorChem HD2 Gel Imaging System (San Leandro, CA).
2.8. Statistical Analysis
All statistical analyses were performed in PRISM (GraphPad, version 6, La Jolla, CA). IC50 values for cisplatin, phenanthriplatin and pyriplatin were calculated in PRISM using a sigmoidal, four parameter logistic equation. MTT assay standard deviation values were calculated using ED50 plus v1.0 online software (Mexico City, Mexico). For all other assays, a two-way ANOVA with Tukey’s multiple comparisons test comparing negative controls to the positive controls and experimental treatments was performed; p ≤ 0.05 was used as the level of significance. Values derived from the Bradford assay were used to construct a standard curve in PRISM.
3. RESULTS
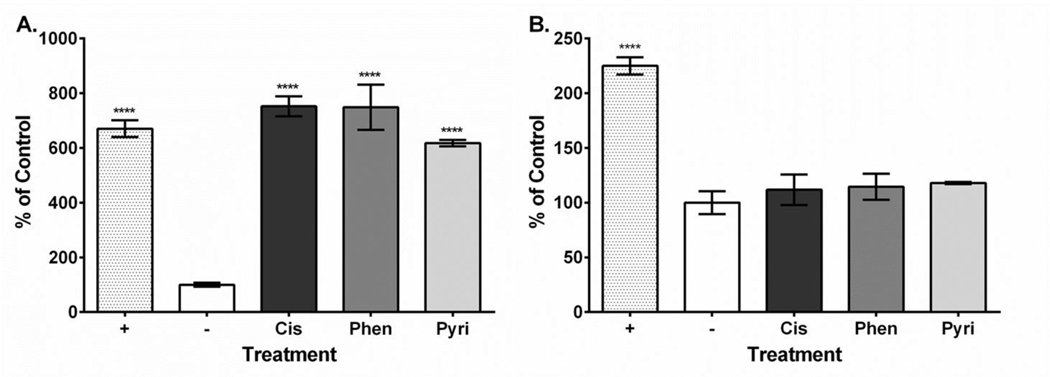
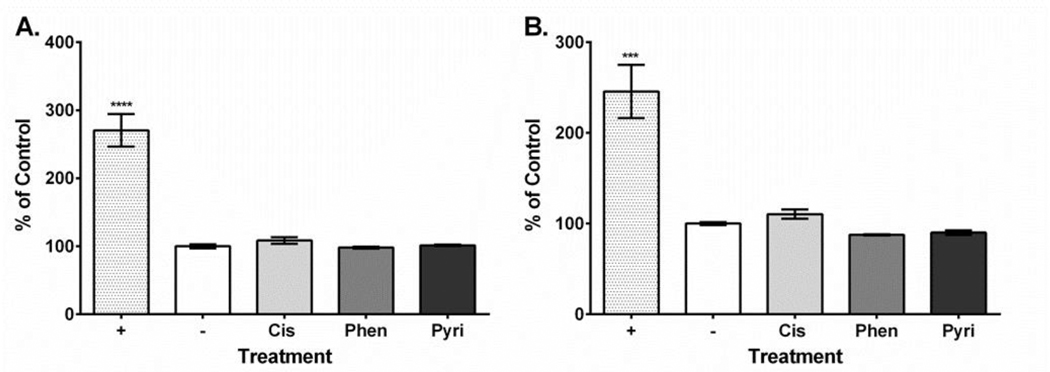
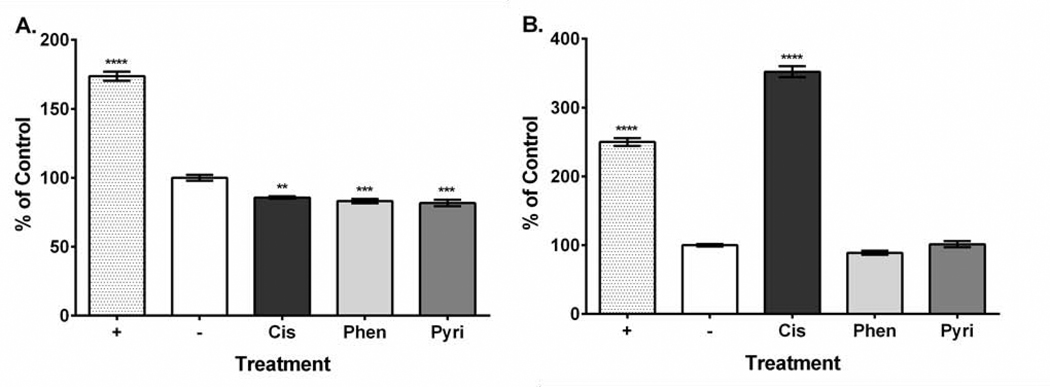
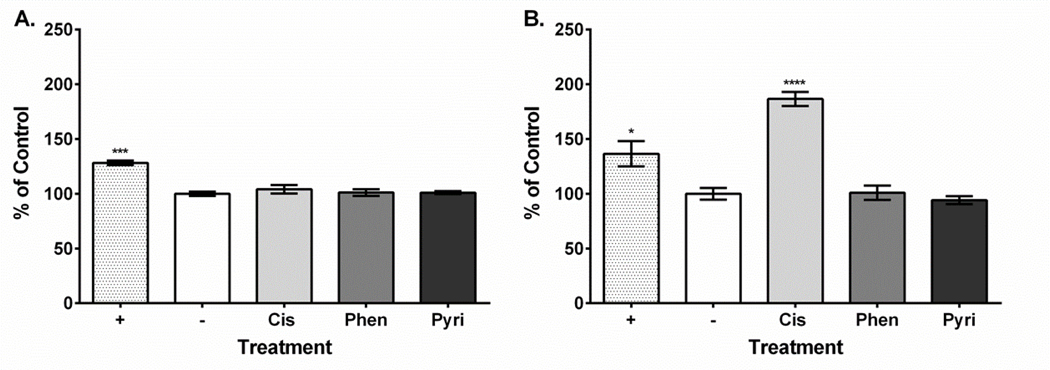
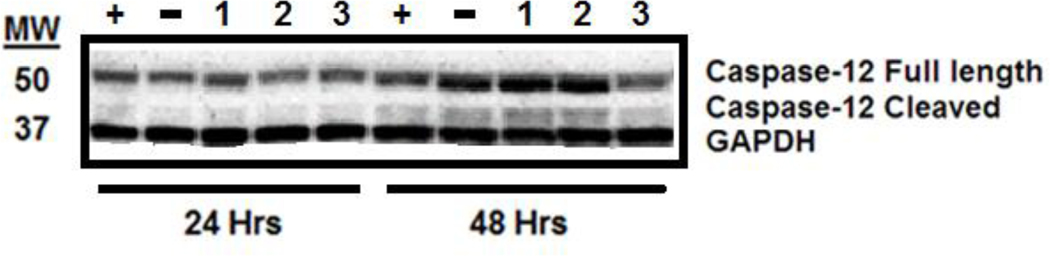
Platinum compound treatment caused similar effects on HEI-OC1 cell viability with the 24-hour values ranging from 1.64 to 11.11 μM and the 48-hour values ranging from 2.31 to 4.52 μM (Table 1). Dose-response curves showing the relationship between MTT assay formazan absorbance expressed as a percent of control versus treatment concentration expressed as a log value were also plotted for all platinum compounds at both the 24-hour and 48-hour time points (Supplementary Figures 1–3). Our ROS results showed that all three compounds increased ROS yield at 24 hours (cisplatin, 751%; phenanthriplatin, 741%; pyriplatin, 618% of negative control; Figure 2A). However, at 48 hours, the ROS levels associated with all three platinum compounds was not different from the control (Figure 2B). None of the platinum compound treatments caused mitochondrial membrane depolarization at 24 hours (cisplatin, 109%; phenanthriplatin, 98%; pyriplatin, 101% of negative control; Figure 3A). Further, none of the platinum compounds affected membrane polarization at 48 hours (cisplatin, 110%; phenanthriplatin, 88%; pyriplatin, 90% of negative control; Figure 3B). At 24 hours, the platinum compounds caused reduced caspase-3/7 activity (phenanthriplatin, 83%; pyriplatin, 82%; cisplatin, 86% of negative control; Figure 4A). Caspase activity was not different than negative control for phenanthriplatin and pyriplatin (89% and 101% of control respectively) at 48 hours, while cisplatin treatment caused increased caspase activity (352% of negative control; Figure 4B). We detected 7-AAD uptake at 24 hours for the positive control but not for any of the three platinum compounds (positive control, 128%; phenanthriplatin, 101%; pyriplatin, 101% and cisplatin, 104%; Figure 5A). However, at 48 hours, we found increased 7-AAD uptake for cisplatin and the positive control (positive control, 137%; cisplatin, 187%); whereas, the monofunctional compounds were unchanged compared to the negative control (phenanthriplatin, 101%; pyriplatin, 94%; Figure 5B). Our western blot analysis showed that full length (55 kDa) caspase-12 was present in all treatment categories at both 24 and 48 hours (Figure 6). We also found expression of the cleaved form of caspase-12 (42 kDa) at both time points in all treatment categories.
Table 1. IC50 values for cisplatin, phenanthriplatin and pyriplatin treatments in HEI-OC1 cell culture.
Standard deviation values are provided after the ± symbol for each inhibitory concentration value. Cell culture treatment times are indicated.
| Treatment | IC50 (μM) |
|---|---|
| Cisplatin (24 Hr) | 1.66 ± 0.33 |
| Cisplatin (48 Hr) | 4.52 ± 0.17 |
| Phenanthriplatin (24 Hr) | 1.64 ± 0.19 |
| Phenanthriplatin (48 Hr) | 2.31 ± 0.29 |
| Pyriplatin (24 Hr) | 11.11 ± 0.28 |
| Pyriplatin (48 Hr) | 2.52 ± 0.11 |
Figure 2. Platinum compound treatment causes increased ROS levels at 24 but not 48 hours post treatment in HEI-OC1 cells.
A. At 24 hours, cisplatin, phenanthriplatin and pyriplatin treatment caused elevated ROS production compared to the control. B. At 48 hours, ROS levels are not significantly different than control for all three platinum compounds. Labeling: Positive control (+), negative control (−), cisplatin (Cis), phenanthriplatin (Phen), pyriplatin (Pyri). Error bars represent standard error of the mean. N = 3; “****”, p < 0.0001 (p value comparison to negative control).
Figure 3. Platinum compound treatment does not induce mitochondrial membrane depolarization at either 24 or 48 hours post treatment in HEI-OC1 cells.
A. At 24 hours, only the positive control caused mitochondrial membrane depolarization. B. At 48 hours, only the positive control caused mitochondrial membrane depolarization. Labeling: Positive control (+), negative control (−), cisplatin (Cis), phenanthriplatin (Phen), pyriplatin (Pyri). Error bars represent standard error of the mean. N = 3; “***”, p < 0.001; “****”, p < 0.0001 (p value comparison to negative control).
Figure 4. Platinum compound treatment decreased caspase-3/7 activity at 24 hours, and cisplatin increased caspase-3/7 activity at 48 hours in HEI-OC1 cells.
A. At 24 hours, platinum compounds caused decreased caspase-3/7 activity, while the positive control increased caspase-3/7 activity. B. At 48 hours, cisplatin and the positive control caused increased caspase-3/7 activity, while phenanthriplatin and pyriplatin are not different than the negative control treatment. Labeling: Positive control (+), negative control (−), cisplatin (Cis), phenanthriplatin (Phen), pyriplatin (Pyri). Error bars represent standard error of the mean. N = 3; “**”, p < 0.01; “***”, p < 0.001; “****”, p < 0.0001 (p value comparison to negative control).
Figure 5. Cisplatin treatment causes late-stage apoptosis in HEI-OC1 cells.
A. At 24 hours, cisplatin and both monofunctional platinum compounds do not cause apoptosis. B. Cisplatin causes apoptosis at 48 hours post treatment. Labeling: Positive control (+), negative control (−), cisplatin (Cis), phenanthriplatin (Phen), pyriplatin (Pyri). Error bars represent standard error of the mean. N = 3; “*”, p < 0.05; “***”, p < 0.001; “****”, p < 0.0001 (p value comparison to negative control).
Figure 6: Effect of cisplatin, phenanthriplatin and pyriplatin treatment on caspase-12 expression.
Labeling: Positive control (“+”), negative control (“−“), phenanthriplatin (“1”), pyriplatin (“2”), cisplatin (“3”). Numbers on the left of the blot represent molecular weight in kilodaltons (kDa). Time course of experiments, i.e., 24 or 48 hours, indicated below treatment labeling. Protein band identification is given on the right. Western blot results show detection of the control, GAPDH, full length (55 kDa) caspase-12, and the cleaved form of caspase-12 (42 kDa) at 24 and 48 hours for all treatment categories.
4. DISCUSSION
Monofunctional platinum(II) complexes that cause cancer cell death through pathways distinct from those used by cisplatin might have reduced side-effects in non-cancer cells. Cisplatin typically forms 1,2-intrastrand cross links with guanosine residues which cause DNA kinking followed by replication arrest, transcription inhibition, cell-cycle arrest, and the recruitment of proteins that initiate signaling into apoptotic pathways (Cepeda et al., 2007; Dasari and Tchounwou, 2014). Phenanthriplatin and pyriplatin bind in a monodentate manner primarily to guanosine residues without distorting DNA and inhibit transcription while evading DNA repair mechanisms (Park et al., 2012; Riddell et al., 2016). Because these monofunctional compounds may not activate apoptotic signaling mechanisms utilized by cisplatin, they may not target mechanisms implicated in ototoxicity.
We first used the MTT assay to determine if phenanthriplatin and pyriplatin caused ototoxicity in HEI-OC1 cells and found (Table 1) that both monofunctional compounds suppressed viability to a similar degree as cisplatin. This result suggests that transcriptional blockage, the principle cell death mechanism proposed for these monofunctional compounds (Park et al., 2012; Johnstone et al., 2014), could be equally effective in causing ototoxicity as cisplatin does by acting through multiple cell death pathways. However, HEI-OC1 cells have been engineered to re-enter the cell cycle and undergo cancer cell-like proliferation (Kalinec et al., 2014; Kalinec et al., 2016). Normal auditory hair cells, which do not exhibit abnormal transcription, may not be affected as severely by monofunctional complexes that primarily target transcription than hair cells treated with cisplatin, which can target additional pathways.
Cisplatin-mediated cancer cell toxicity and ototoxicity is usually associated with elevated ROS production that acts through multiple cellular targets (Marullo et al., 2011; Karasawa and Steyger, 2015; Gentilin et al., 2019). Cisplatin can upregulate some NADPH oxidase subunits causing increased ROS production (Kim et al., 2010). The NOX3 NADPH oxidase isoform can activate signal transducer and activator of transcription-1 (STAT1) signaling causing increased tumor necrosis factor-α (TNF-α) expression which can promote the extrinsic apoptosis pathway integrating caspase-8 and then caspase-3/7 activity (Kaur et al., 2011; Karasawa and Steyger, 2015). Also, cisplatin can prevent the action of cellular antioxidant systems leading to enhanced ROS production (Dammeyer et al., 2014). Cisplatin and both monofunctional compounds caused increased ROS production at 24 hours, but ROS levels were restored to normal at 48 hours (Figure 2). Our data suggests that cisplatin, phenanthriplatin, and pyriplatin have a similar early temporal effect to increase ROS production in HEI-OC1 cells. This result could also explain the elevated caspase-3/7 activity for cisplatin at 48 hours (Figure 4), as elevated ROS at the earlier 24-hour time point could modulate the extrinsic apoptotic pathway through STAT1, TNF-α and caspase-8 to later increase caspase-3/7 activity; whereas, the monofunctional compounds may activate caspase- 3/7 at a later time point.
Cisplatin can accumulate in auditory hair cell nuclei (van Ruijven et al., 2005), and in cancer and non-cancer cells, via a DNA damage-mediated mechanism, cause the tumor suppressor, p53, to rapidly translocate to the mitochondria, where it can induce membrane depolarization, cytochrome c release, and activation of caspase-3 followed by cell death (Marchenko et al., 2000). p53 signaling is implicated in cisplatin-mediated ototoxicity (Benkafadar et al., 2017). We measured membrane depolarization at both 24 and 48 hours and found that neither cisplatin nor the monofunctional compounds caused depolarization (Figure 3). Therefore, signaling integrating depolarization of the mitochondrial membrane may not occur with these monofunctional compounds or may happen at another temporal interval.
As cisplatin can cause cell death through mechanisms that do not require induction of DNA damage (Karasawa and Steyger, 2015), we also measured the activity of caspases-3/7, which can increase as a result of cisplatin-mediated ROS causing mitochondrial membrane permeabilization and subsequent activation of the intrinsic apoptosis pathway or signaling through the extrinsic apoptosis pathway (Devarajan et al., 2002; Karasawa and Steyger, 2015; Sheth et al., 2017). We were surprised to find that at 24 hours, cisplatin and both monofunctional compounds caused decreased caspase-3/7 activity (Figure 4A), which could be a form of compensatory action triggered by an earlier effect, such as increased ROS. Although, at the later 48-hour time point, the monofunctional compounds had activity equivalent to the negative control, and cisplatin had increased caspase-3/7 activity (Figure 4B). These results suggest that cisplatin, but not the monofunctional compounds, might modulate apoptosis after an early phase of ROS production (Figure 2A) followed by activation of the extrinsic apoptosis pathway and subsequent increased caspase-3/7 activity and cell death (Figure 4B, 5B) or that the monofunctional compounds activate caspases-3/7 at a later stage.
In HEI-OC1 cells, cisplatin can cause greater uptake of 7-AAD indicating late-stage cell death (Serinan et al., 2018). Our results show that cisplatin, but not phenanthriplatin or pyriplatin, cause cell death at the later, 48-hour, time point (Figure 5). This result is correlated with our caspase-3/7 study, where cisplatin caused increased caspase-3/7 activity at 48 hours (Figure 4B). However, it is possible that the monofunctional compounds could induce apoptosis at a later stage than cisplatin.
Cisplatin treatment in HEI-OC1 cells can cause increased stress in the endoplasmic reticulum with alteration of protein and transcription factor expression (Kalinec et al., 2016; Woo et al., 2016; Lim et al., 2019). Cochlear cells treated with cisplatin can undergo endoplasmic reticulum stress and exhibit increased caspase-12 expression which is integrated into a cell death mechanism independent of the extrinsic and intrinsic apoptosis pathways (Karasawa and Steyger, 2015; Zong et al., 2017). Caspase-12 auto-cleaves during endoplasmic reticulum stress, and the cleaved form causes apoptosis in a mitochondria- independent manner (Nakagawa et al., 2000; Morishima et al., 2002). Our western blot results suggest that cisplatin and the monofunctional compounds might promote endoplasmic reticulum stress-mediated apoptosis in HEI-OC1 cells as evidenced by the detection of both the full length and cleaved form of caspase-12 for all compounds at the 24- and 48-hour time points (Figure 6). However, the detection of the cleaved form in the negative control treatment could also mean that its expression is endogenous or in response to a stress not influenced by the platinum compounds.
In conclusion, phenanthriplatin and pyriplatin may act as ototoxins with an effect comparable to cisplatin. Their mechanistic action to cause HEI-OC1 cell death may begin with elevated ROS, but unlike cisplatin, may not integrate caspase-3/7 signaling. It is possible that both monofunctional compounds could increase caspase-3/7 signaling and cell death at a later time-point. We propose that future studies using in vivo animal models would be a logical next-step to assess the ototoxicity of these monofunctional compounds.
Supplementary Material
Highlights:
Cisplatin, phenanthriplatin and pyriplatin reduced HEI-OC1 cell viability.
ROS increased in cells at 24 hours but returned to control levels at 48 hours.
The three compounds did not alter mitochondrial membrane potentials.
Only cisplatin caused increased caspase-3/7 activity and late stage apoptosis.
The compounds had associated caspase-12 expression, but this may be endogenous.
ACKNOWLEDGEMENTS
The authors would like to acknowledge National Institutes of Health awards T1 R15 CA188890– 01A1, 8 P20GM103436–12 KBRIN-IDeA and 2 P20 GM103436–14, a Western Kentucky University (WKU) Research and Creative Activities Program grant 19–8046 to MES, a WKU Faculty-Undergraduate Student Engagement grant 19-SP247 to AMJ, Naomi Rowland and the WKU Biotechnology Center for providing facilities support, and Dr. Kevin M. Williams of the WKU Chemistry Department for providing the monofunctional compounds.
Footnotes
Author Contributions
Jerry D. Monroe, Alexandra M. Johnston, Michael E. Smith: Conceptualization, Data curation, Formal analysis, Methodology, Validation. Jerry D. Monroe, Michael E. Smith: Writing - review & editing. Jerry D. Monroe, Michael E. Smith: Funding acquisition. Jerry D. Monroe, Alexandra M. Johnston: Investigation. Jerry D. Monroe, Michael E. Smith: Project administration. Michael E. Smith: Resources. Jerry D. Monroe: Software, Roles/Writing - original draft. Jerry D. Monroe, Michael E. Smith: Supervision, Visualization.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Benkafadar N, Menardo J, Bourien J, Nouvian R, François F, Decaudin D, Maiorano D, Puel JL, Wang J, 2017. Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol. Med 9, 7–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Pérez JM, 2007. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents in Medicinal Chem 7, 3–18. [DOI] [PubMed] [Google Scholar]
- Dammeyer P, Hellberg V, Wallin I, Laurell G, Shoshan M, Ehrsson H, Arnér ES, Kirkegaard M, 2014. Cisplatin and oxaliplatin are toxic to cochlear outer hair cells and both target thioredoxin reductase in organ of Corti cultures. Acta Otolaryngol 134, 448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB, 2014. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. of Pharmacol 740, 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F, 2002. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res 174, 45–54. [DOI] [PubMed] [Google Scholar]
- Facchetti G, Rimoldi I, 2019. Anticancer platinum(II) complexes bearing N-heterocycle rings. Bioorg. Med. Chem. Lett 29, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Gentilin E, Simoni E, Candito M, Cazzador D, Astolfi L, 2019. Cisplatin-Induced Ototoxicity: Updates on Molecular Targets. Trends Mol. Med S1471–4914, 30210–2. [DOI] [PubMed] [Google Scholar]
- Hill GW, Morest DK, Parham K, 2008. Cisplatin-Induced Ototoxicity: Effect of Intratympanic Dexamethasone Injections. Otol. Neurotol 29, 1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone TC, Park GY, Lippard SJ, 2014. Understanding and improving platinum anticancer drugs--phenanthriplatin. Anticancer Res 34, 471–6. [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, Kalinec F, 2003. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol. Neurootol 8, 177–89. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Thein P, Parsa A, Yorgason J, Luxford W, Urrutia R, Kalinec F, 2014. Acetaminophen and NAPQI are toxic to auditory cells via oxidative and endoplasmic reticulum stress-dependent pathways. Hearing Res 313, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec G, Thien P, Park C, Kalinec F, 2016. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hearing Res 335, 105–117. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Park C, Thein P, Kalinec F, 2016. Working with Auditory HEI-OC1 Cells. J. Vis. Exp. Sep 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Steyger PS, 2015. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett 237, 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V, 2011. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis 2:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, Park R, So HS Roles of NADPH oxidases in cisplatin- induced reactive oxygen species generation and ototoxicity. J. Neurosci 30, 3933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, Garcia P, Steinman H, Malgrange B, Ruben RJ, Rybak L, Van de Water TR, 1997. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol 18, 559–71. [PubMed] [Google Scholar]
- Lim JO, Ko JW, Shin NR, Jung TY, Moon C, Kim HC, Shin IS, Kim JC, 2019. Cisplatin-induced ototoxicity involves interaction of PRMT3 and cannabinoid system. Arch. Toxicol 93, 2335–2346. [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Zaika A, Moll UM, 2000. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J. Biol. Chem 275, 16202–12. [DOI] [PubMed] [Google Scholar]
- Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, Doetsch PW, 2013. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE 8: e81162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe JD, Hruska HL, Ruggles HK, Williams KM, Smith ME, 2018. Anti-cancer characteristics and ototoxicity of platinum(II) amine complexes with only one leaving ligand. PLoS One. 13:e0192505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y, 2002. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c- independent activation of caspase-9 by caspase-12. J. Biol. Chem 277, 34287–94. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J, 2000. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 403, 98–103. [DOI] [PubMed] [Google Scholar]
- Park GY, Wilson JJ, Song Y, Lippard SJ, 2012. Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile. P.N.A.S. (U.S.A.). 109, 11987–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell IA, Johnstone TC, Park GY, Lippard SJ, 2016. Nucleotide Binding Preference of the Monofunctional Platinum Anticancer-Agent Phenanthriplatin. ACS Chem. Biol 11, 2996–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi P, Akinpelu OV, Waissbluth S, Peleva E, Meehan B, Rak J, Daniel SJ, 2014. Attenuation of cisplatin ototoxicity by otoprotective effects of nanoencapsulated curcumin and dexamethasone in a guinea pig model. Otol. Neurotol 35, 1131–9. [DOI] [PubMed] [Google Scholar]
- Serinan E, Altun Z, Aktaş S, Çeçen E, Olgun N, 2018. Comparison of Cisplatin with Lipoplatin in Terms of Ototoxicity. J. Int. Adv. Otol 14, 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth S, Mukherjea D, Rybak LP,Ramkumar V, 2017. Mechanisms of Cisplatin- Induced Ototoxicity and Otoprotection. Front. Cell. Neurosci 11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ruijven MW, de Groot JC, Hendriksen F, Smoorenburg GF, 2005. Immunohistochemical detection of platinated DNA in the cochlea of cisplatin-treated guinea pigs. Hearing Res 203, 112–21. [DOI] [PubMed] [Google Scholar]
- Woo SM, Lee WK, Min KJ, Kim DE, Park SH, Nam SI, Kwon TK,2016. Rottlerin induces cyclooxygenase-2 upregulation through an ATF4 and reactive oxygen species-independent pathway in HEI-OC1 cells. Mol. Med. Rep 14, 845–50. [DOI] [PubMed] [Google Scholar]
- Zong S, Liu T, Wan F, Chen P, Luo P, Xiao H, 2017. Endoplasmic Reticulum Stress Is Involved in Cochlear Cell Apoptosis in a Cisplatin-Induced Ototoxicity Rat Model. Audiol. Neurootol 22, 160–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.