Abstract
Background:
This study aimed to examine the agreement between sexually transmitted infection (STI) screening using self-collected specimens and physician-collected specimens, and to investigate the acceptability of self-collection for screening in an 18-month study of female sex workers in a high-risk, low-resource setting.
Methods:
A total of 350 female sex workers in Nairobi, Kenya, participated in a prospective study from 2009 to 2011. Women self-collected a cervicovaginal specimen. Next, a physician conducted a pelvic examination to obtain a cervical specimen. Physician- and self-collected specimens were tested for Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium (MG) using Aptima nucleic acid amplification assays (Hologic). Specimens were collected at 3-month intervals over 18-month follow-up. κ Statistics measured agreement of positivity between self-collection and physician collection.
Results:
Baseline STI prevalence was 2.9% for N. gonorrhoeae, 5.2% for C. trachomatis, 9.2% for T. vaginalis, and 20.1% for MG in self-collected samples, and 2.3%, 3.7%, 7.2%, and 12.9%, respectively, in physician-collected samples. κ Agreement was consistently strong (range, 0.66–1.00) for all STIs over the 18-month study period, except for MG, which had moderate agreement (range, 0.50–0.75). Most participants found self-collection easy (94%) and comfortable (89%) at baseline, with responses becoming modestly more favorable over time.
Conclusions:
Self-collected specimen screening results showed strong agreement to clinical-collected specimens, except for MG, which was consistently detected more commonly in self-collected than in physician-collected specimens. Acceptability of the self-collection procedure was high at baseline and increased modestly over time. In high-risk, low-resource settings, STI screening with self-collected specimens provides a reliable and acceptable alternative to screening with physician-collected specimens.
Untreated sexually transmitted infections (STIs) such as Chlamydia trachomatis (CT), Neisseria gonorrhoeae (GC), Trichomonas vaginalis (TV), and Mycoplasma genitalium (MG) may increase the risk of HIV acquisition, pelvic inflammatory disease, or preterm birth.1–8 The diagnosis and control of STIs is an important global public health issue relating to overall well-being of women and children who are disproportionately affected by long-term consequences of untreated STIs.9 In low-resource settings, the World Health Organization strategy for STI management involves identification and treatment of easily recognized signs of STIs, including vaginal discharge.10,11 However, such syndromes are often a poor proxy for vaginal and endocervical infections.11,12
Morbidity and Mortality Weekly Report Laboratory 2014 guidelines for CT/NG recommend nucleic acid amplification techniques as the test of choice for CT and NG testing.13 Specimens obtained with a vaginal swab are the preferred specimen type given that vaginal swab specimens are as or more sensitive than cervical swab specimens for STI detection, and result in no difference in specificity for STI detection of CTand NG infections.13 In low-resource settings, specimen collection is often complicated by clinic-based difficulties such as staff shortages, space limitations, and cultural norms that may prevent women from being screened for STIs.11,12 Self-collected specimens for STI screening could potentially increase a woman’s access to STI screening and treatment. In the United States, self-collected specimens have been recommended for the screening of CT and GC, and are also being considered for the screening of TV and MG.14 In addition, longitudinal assessment of self-collection performance in comparison to the criterion standard physician collection approach will provide evidence of assay performance over time.
Self-collected specimens are a potentially viable alternative to physician-collected specimens, if self-collected specimens perform comparably with physician-collected specimens for the detection of multiple common STIs. However, studies which have tested agreement between physician and self-collected specimens in STI detection, have rarely examined MG in conjunction with multiple STIs in a global clinic setting.15–20 Furthermore, to our knowledge, no previous studies have examined the longitudinal agreement between physician- and self-collected specimens for screening of multiple STIs in a high-risk, low-resource setting.
Here, we examine the agreement of STI (CT, GC, TV, and MG) screening using self- versus physician-collected specimens, as well as the acceptability of the self-collection procedure in a population of female sex workers (FSWs) in Nairobi, Kenya, over a follow-up of 18 months.
MATERIALS AND METHODS
Study Population
The study was conducted between August 2009 and March 2011, in Korogocho, Nairobi, Kenya, and the population has been described in detail previously.21 Briefly, female study participants were FSWs 18 to 49 years of age attending an STI clinic; not currently in the second trimester of pregnancy or later; and with an intact cervix. A total of 350 FSWs were enrolled and followed up at 3-month intervals for 18 months. At baseline, participants were administered a questionnaire to collect sociodemographic and sexual behavior data. To account for a population of low literacy, questionnaires were verbally given by a trained nurse with extensive interview experience.
Specimen Collection
Each woman self-collected a cervicovaginal sample using the Aptima Cervical Specimen Collection and Transport cytobrush (Hologic Corporation, Marlborough, MA) using pictorial instructions (Appendix 1, http://links.lww.com/OLQ/A231). Participants were instructed to stand, sit, or lie down in a comfortable position and gently push the brush into their vagina until they felt resistance. Participants were then instructed to turn the brush 5 times while inside their vagina and slowly remove the brush. The final steps were inserting the brush into the tube, white side down, and then releasing the brush head into the tube before capping the lid. The self-collection cytobrush was then swirled in the Aptima specimen transport medium and then discarded. During a pelvic examination, the physician collected 1 cervical sample from each woman using a Cervex-Brush (Rovers Medical Devices, Oss, the Netherlands), which was then swirled in the PreservCyt medium (Hologic Corporation) and then discarded. The physician then collected a second cervical sample for conventional Papanicolaou test.
Screening for STIs
The physician- and self-collected specimens were tested for CT and GC using the Aptima Combo 2 assay; for TV, the Aptima TV assay; and for MG, the Aptima research-use-only assay. The Aptima assays comprise 3 main steps, namely, target capture, transcription-mediated amplification of the target, and finally target detection by hybridization with complementary probes linked to chemiluminescent labels.21 A cutoff of 100k relative light units for the AMG assay was used, given that cutoffs between 30k and 100k gave nearly identical sensitivity and specificity results.22 All assays were performed in San Diego, California, at the Hologic testing laboratory, according to the manufacturer’s instructions, with technicians blinded to Papanicolaou test or other study results.
Of a total of 350 FSWs screened at baseline, viable STI results were available for 348 for GC, 347 physician-collected and 348 self-collected for CT, 348 for TV, and 348 for MG after excluding inconclusive laboratory results. All women with abnormal cytology results were referred to secondary colposcopy and treatment per standard clinical practice. Because MG was a research-only test at the time of the study, we did not treat individuals with this condition. All other laboratory-detected STIs were treated immediately for symptomatic STIs and with results for the asymptomatic STIs.
Statistical Analyses
We measured κ score percent agreement of positivity comparing self-collection with physician collection methods. The given κ statistic takes into account whether interrater agreement occurs due to chance.23 Given data that strongly suggest that STI infections are more often identified in cervicovaginal than cervical samples, assigned cervical results as being “true positive” would result in a subsequent reduction of associated specificity estimates.
To assess the longitudinal performance of STI screening based on the self-collected specimens relative to physician-collected specimens, plots of the prevalence percentage of participants having positive test results for each mode of specimen collection and STI were produced.
The secondary goal of the study was to assess feasibility and acceptability of the self-collection process over time. We used longitudinal analyses to determine if the overall trends of women’s reported acceptability were maintained over time. At each time point, participants were asked to describe the difficulty level of the specimen collection procedure with possible responses: very easy, easy, difficult, or very difficult. Participants were also asked to describe whether or not the specimen collection process was comfortable with possible responses: very comfortable, comfortable, uncomfortable, or very uncomfortable.
Plots of the proportion of participants reporting easy or very easy collection, and of the proportion of participants reporting comfortable or very comfortable collection at each time point were produced. These proportions provide a valid estimate of time trends in self-collection acceptability under the assumption that missing data at later time points, due to dropout or other reasons, are unrelated to the acceptability of the sample collection process.
Sensitivity regression analyses were performed to investigate the impact of missing data on the observed trends. In this analysis, we fit generalized linear-mixed logistic regression models, which assume that the missing data are nonignorable missing (NMAR).24 The NMAR methods account for the fact that a participant who finds the specimen collection process burdensome at one time point may not perform self-collection at the next time point (i.e., dropout). We used a logistic model for the missing data mechanism as proposed by Ibrahim and Chen.25 In this sensitivity analysis, the probability of a response being missing at a given time point was allowed to depend on the response value at the previous time point. To fit these models, we used a Bayesian approach with flat priors. The choice to use a Bayesian approach for model fitting was based largely on availability of standard software to implement regression analysis under an NMAR missing data mechanism.
RESULTS
Participant Characteristics
There were no statistically significant demographic or sexual differences between women who tested positive for one or more STIs and those who tested negative at baseline (data not shown). Participants had a median age of 28.0 years (range, 18–48 years), and most (76%) had no secondary education (Table 1). Almost all women were unmarried, with 45% self-reporting being single and 55% being widowed, divorced, or separated. Most women (73.5%) recorded using a condom with their sexual clients most of the time or always, 20.8% for sometimes or half the time, and 5.8% admitted to never or rarely using them.
TABLE 1.
Summary of Study Characteristics Among 348 Female Sexual Workers in Nairobi, Kenya
| Enrollment Characteristic | n | (Range) or % |
|---|---|---|
| Age, y | ||
| Median | 28.0 | (18–48) |
| 18–24 | 89 | 25.6 |
| 25–29 | 108 | 31.0 |
| 30–34 | 72 | 20.7 |
| 35+ | 79 | 22.7 |
| Physician-collected specimens | ||
| Chlamydia trachomatis | 13 | 3.7 |
| Neisseria gonorrhoeae | 8 | 2.3 |
| Trichomonas vaginalis | 25 | 7.2 |
| Mycoplasma genitalium | 45 | 12.9 |
| Self-collected specimens | ||
| Chlamydia trachomatis | 18 | 5.2 |
| Neisseria gonorrhoeae | 10 | 2.9 |
| Trichomonas vaginalis | 32 | 9.2 |
| Mycoplasma genitalium | 70 | 20.1 |
| Education | ||
| Primary or less | 265 | 76.0 |
| Secondary or more | 83 | 24.0 |
| Marital status | ||
| Single | 147 | 45.0 |
| Widowed, divorced, or separated | 191 | 55.0 |
| Condom use with sexual clients | ||
| Most of the time/always | 255 | 73.5 |
| Sometimes/half the time | 72 | 20.8 |
| Never/rarely | 20 | 5.8 |
| HIV infection status | ||
| Seronegative | 265 | 76.0 |
| Seropositive | 82 | 24.0 |
Utility of STI Screening With Self-Collected Specimens
Baseline STI test results for each mode of specimen collection provided in Table 1 demonstrate positivity rates across all pathogens, as well as the individual prevalence of each pathogen. N. gonorrhoeae was the least prevalent (2.3%), appearing lower than CT (3.7%), TV (7.2%), or MG (12.9%) from clinician-collected brushes. Prevalence results for physician collection decreased from 2% (baseline visit) to 1% (18 months) for GC, from 4% to 1% for CT, from 7% to 4% for TV, and from 13% to 11% for MG (Fig. 1). Overall, trends in prevalence over time were similar for self-collected samples but with generally lower prevalence percentages compared with clinician-collected samples. M. genitalium had the highest prevalence percentage for both specimen collection methods over time.
Figure 1.
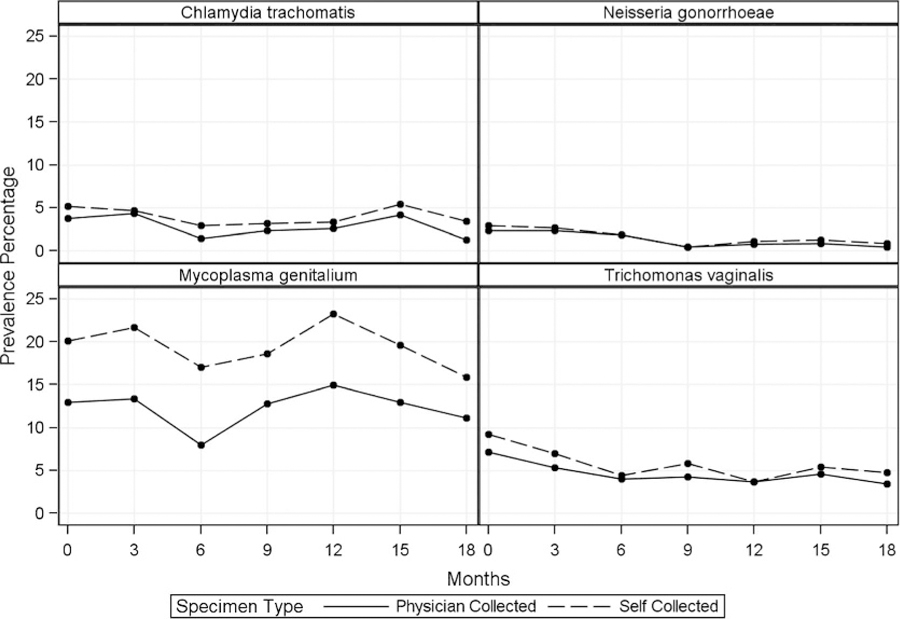
Prevalence percentage of sexually transmitted infections detection over time by collection method.
Overall κ statistics indicate moderate to strong agreement between self-collected and physician-collected specimen results with 0.77 (95% confidence interval, 0.69–0.85) for CT, 0.86 (0.76–0.96) for GC, 0.85 (0.79–0.90) for TV, and 0.66 (0.62–0.71) for MG. κ Statistics for GC (range, 0.66–1.00, over 18-month follow-up) and TV (range, 0.77–1.00) met the criteria for substantial (cutoff, 0.61) or almost perfect (cutoff, 0.81) agreement (Table 2).26 Only 2 κ values (CT at month 18: 0.54 [range, 0.54–0.96]; MG at month 6: 0.50 [range, 0.50–0.75]) met the criteria for moderate agreement.
TABLE 2.
Agreement of the Detection of Sexually Transmitted Infections, by Collection Method, Overall, and by Visit
| Physician-Collected |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlamydia trachomatis |
Neisseria gonorrhoeae |
Trichomonas vaginas |
Mycoplasma genitalium |
||||||||||
| Visit | Self-Collected | Negative | Positive | κ (95% CI) | Negative | Positive | κ (95% CI) | Negative | Positive | κ (95% CI) | Negative | Positive | κ (95% CI) |
| Overall | Negative | 1838 | 4 | 0.77 | 1888 | 1 | 0.86 | 1808 | 4 | 0.85 | 1534 | 18 | 0.66 |
| Positive | 25 | 52 | (0.69–0.85) | 7 | 25 | (0.76–0.96) | 26 | 88 | (0.79–0.90) | 158 | 220 | (0.62–0.71) | |
| Baseline | Negative | 329 | 1 | 0.79 | 338 | 0 | 0.89 | 315 | 1 | 0.83 | 274 | 4 | 0.66 |
| Positive | 5 | 12 | (0.63–0.95) | 2 | 8 | (0.73–1.00) | 8 | 24 | (0.72–0.94) | 29 | 41 | (0.55–0.76) | |
| Month 3 | Negative | 286 | 0 | 0.96 | 292 | 0 | 0.93 | 279 | 0 | 0.86 | 230 | 5 | 0.60 |
| Positive | 1 | 13 | (0.89–1.00) | 1 | 7 | (0.80–1.00) | 5 | 16 | (0.73–0.98) | 30 | 35 | (0.48–0.72) | |
| Month 6 | Negative | 266 | 0 | 0.66 | 267 | 1 | 0.80 | 261 | 2 | 0.77 | 226 | 3 | 0.50 |
| Positive | 4 | 4 | (0.35–0.97) | 1 | 4 | (0.52–1.00) | 3 | 9 | (0.58–0.97) | 28 | 19 | (0.35–0.64) | |
| Month 9 | Negative | 246 | 1 | 0.71 | 254 | 0 | 1.00 | 242 | 0 | 0.84 | 209 | 1 | 0.75 |
| Positive | 3 | 5 | (0.43–0.98) | 0 | 1 | (1.00–1.00) | 4 | 11 | (0.68–0.99) | 16 | 32 | (0.64–0.86) | |
| Month 12 | Negative | 262 | 1 | 0.74 | 270 | 0 | 0.80 | 265 | 0 | 1.00 | 209 | 2 | 0.69 |
| Positive | 3 | 6 | (0.50–0.99) | 1 | 2 | (0.41–1.00) | 0 | 10 | (1.00–1.00) | 25 | 39 | (0.58–0.79) | |
| Month 15 | Negative | 225 | 1 | 0.77 | 236 | 0 | 0.80 | 226 | 1 | 0.82 | 192 | 1 | 0.73 |
| Positive | 4 | 9 | (0.58–0.97) | 1 | 2 | (0.41–1.00) | 3 | 10 | (0.66–0.99) | 17 | 30 | (0.61–0.84) | |
| Month 18 | Negative | 224 | 0 | 0.54 | 231 | 0 | 0.66 | 220 | 0 | 0.84 | 194 | 2 | 0.73 |
| Positive | 5 | 3 | (0.18–0.89) | 1 | 1 | (0.05–1.00) | 3 | 8 | (0.65–1.00) | 13 | 24 | (0.60–0.86) | |
CI indicates confidence interval
Feasibility of Self-Collection
Overall, more than 99% of self-collected samples were collected and sufficient for testing. No appreciable differences in demographic characteristics were found between women who reported the self-collection as easy or comfortable, the 5.5% of women who found the process difficult, or the 10.5% who found self-collection uncomfortable at baseline (data not shown).
Acceptability of Self-Collection
Figure 2 presents the results of the sensitivity regression analysis estimates overlaid on the sample estimates of participants who reported easy or very easy specimen collection, and easy or very easy comfortable specimen collection at each time point to facilitate comparison. The sample estimates and the sensitivity regression estimates should not be compared directly because they represent slightly different concepts quantities (population averaged vs. subject specific results estimates). However, the nature of the acceptability time trend is comparable. If the observed acceptability upward time trend was manifested by nonresponse among those subjects who found the collection process burdensome, the estimates from the sensitivity analysis should be less positively biased than the crude sample estimates. There is little difference in the estimated acceptability trends for either ease or comfort. Both analyses support the conclusion that the acceptability of specimen self-collection modestly increased over time.
Figure 2.
Sensitivity analysis for positive time trend. probability of patients reporting comfortable/very comfortable collection. *NMAR is not missing at random analysis.
DISCUSSION
Similar diagnostic detection from and acceptability of self- and physician-collected specimens was found among a population of approximately 350 FSWs at baseline and over the course of a prospective 18-month study. Overall, self-collected specimens provided an effective means to screen for STIs, with comparable detection prevalence to physician-collected sampling. Furthermore, we demonstrate that self-collection can be reliably implemented in a clinic-based, less-developed country setting. Reported acceptability of self-collection was largely favorable and improved over study follow-up, suggesting that participants became more confident with the self-collection process over time. The sensitivity analysis also suggests that dropout is not the cause of the increased acceptability of specimen collection over time.
Our findings of high comparability for infection detection between self-collection and physician collection are consistent with previous research confirming the utility of self-collection for STI screening in low-resource settings of rural Australia, Brazil, and South Africa.15,16,19,20 Previous studies screened for CT, GC, and TV, which our study expands to include screening for MG as well. The South African study reported relatively low sensitivity for TV, whereas our study protocol of self-collection using cytobrushes and testing with Aptima TV assay reported greater sensitivity (96%) for TV as compared with the previous study protocol, which used vaginal swabs and tampons (54.5%, 28.0%) tested by monocyte-derived macrophages.20 More recently, one study of 189 women in Canada found high crude agreement between self-sampling and clinician sampling for both chlamydia (94.7%) and gonorrhea (98.4%) testing using Aptima Combo 2 assay.17 Another recent study of 708 women in Canada also showed high agreement between self-sample and clinician cervical samples for chlamydia (κ = 0.89–0.93) and trichomonas (κ = 0.78–0.97) testing using Aptima assays.18 Simultaneous screening for 4 common STIs in our study using cytobrushes and Aptima assays builds on previous research using other screening types with similarly strong agreements to physician collection.16–20
Our longitudinal analysis of the acceptability of the self-collection procedure demonstrates that most participants found self-collection both easy and comfortable, and that women’s acceptability of the process increased modestly over time. A 2015 systematic review of 36 studies assessing patients’ experiences of self-sampling found that self-sampling was a highly acceptable method in 85% of patients.27 Twenty-eight of these studies reported on ease of self-sampling, and most patients (88%) found self-sampling to be an “easy” procedure.27 The high acceptability reported by participants is consistent with previous research and highlights the feasibility of self-collection as an effective alternative in scenarios in which physician collection may be unavailable due to limited resources or staff constraints.
The study is novel because it prospectively evaluates self-sampling with clinician sampling, as well as acceptability in a resource-limited country. It also includes the emerging MG, of which there are limited data in the self-collection literature. Our study has several advantages that improved the reliability of the comparison of STI detection between self-collected and physician-collected specimens. Our study participants reported relatively higher number of sexual partners, and higher baseline and longitudinal STI prevalence compared with previous reports from STI clinics in Australia, Brazil, and South Africa.15,16,19,20 Physician-collected and self-collected specimens were collected on the same day and analyzed at the same facility, enabling the direct comparison of STI detection. The longitudinal study period allowed for greater number of patient encounters and allowed for the monitoring of changes in STI detection rates and acceptability. Permitting the screening of 4 STIs with one self-specimen collection could allow low-resource clinics to enhance service provision and broaden treatment efforts.
One study disadvantage is small to moderate absolute numbers of true positives for the STIs being investigated. Particularly, only 8 (2.3%) participants tested positive for GC at baseline among physician-collected specimens resulting in imprecise estimates. A second limitation is that we were unable to compare any potential influence of our specimen collection procedures and devices, sampled anatomical sites, or transportation processes to those used in other studies. Another limitation is that we were unable to compare the reliability of using Aptima transport media and PreservCyt media before performing our collection comparisons. Therefore, we are unable to account for the effect of using 2 different transport media on the collection comparison results. However, Aptima testing has been approved for both Aptima and PreservCyt transport media for clinical use.
The lower agreement for MG between self-collection and physician collection as compared with the other pathogens could be initially attributed to relatively lower MG loads within cervical cells in infected women.28 Additional reasons for lower agreement could also be due to vaginal specimen collection during self-collection as compared with physician collection that obtains a higher proportion of cervical cells or to differences in the dilution of the sample types. Other studies have found that first-void urine samples may be a superior sample method to cervical swabs for the detection of MG.29 There is currently no standard detection assay or mode for MG to use as reference standard,28 and the current recommendation is to combine screening modalities of urine and vaginal samples in women to optimize MG detection.28 Follow-up studies that include vaginal self-collected, cervical physician-collected specimens or first-void urine samples for a more direct comparison of MG infections may elucidate the optimal screening modality and anatomic origin of infection. Given the difficulty of culturing MG, nucleic acid amplification techniques are currently the best method available for MG screening,28,30 and our study adds to the few studies of self-collection research into MG as an emerging sexually transmitted pathogen.29
Our assessment of acceptability was limited by the unavailability of follow-up questions for the minority of participants who reported self-collection to be difficult or uncomfortable. Additional questions evaluating the ease and comfort of physician collection would allow for better comparison between the 2 collection methods and more comprehensively establish patient preference. Our STI results were based on testing a high-risk FSW population in a resource-poor setting and are not necessarily generalizable to low-risk populations. Although STI detection is an essential first step, successful treatment and follow-up is essential for STI prevention. Women who tested positive for an STI were contacted and provided antibiotic treatment free of charge, a necessary follow-up measure that poses separate challenges to low-resource clinics.
Sexually transmitted infection testing of self-collected specimens may not be a suitable alternative to testing of physician-collected specimens in every setting. However, consistent with other studies, our findings show that Aptima assays used in self-collection for STI testing have excellent performance and high comparability to physician-collected specimens without the need for an initial gynecologic examination, and may be preferable to optimize MG infection detection. Increased use of self-collection could provide an alternative to symptom-based treatment that underestimates STI prevalence and may contribute to antibiotic resistance.30
Sub-Saharan Africa bears a disproportionate burden of STI prevalence worldwide, an inequity further exacerbated by poor health infrastructures generally unable to deliver regular screening to low-resource women who need it most.9 Our data support the use of self-collection to regularly screen asymptomatic, high-risk women. The high longitudinal agreement and upward trend in acceptability of self-collection build on findings from previous studies and demonstrate how technological advances in STI screening assays can allow for improved access to care.
Supplementary Material
Acknowledgments
Sources of Funding: None declared
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
REFERENCES
- 1.Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect 2013; 89:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotch MF, Patorek JG 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis 1997; 24: 353–360. [DOI] [PubMed] [Google Scholar]
- 3.Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis 2002; 34:519–522. [DOI] [PubMed] [Google Scholar]
- 4.Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary Chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol 2000; 183:662–668. [DOI] [PubMed] [Google Scholar]
- 5.Alger L, Lovchik J, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol 1988; 159:397–404. [DOI] [PubMed] [Google Scholar]
- 6.Oakeschott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for Chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (Prevention Of Pelvic Infection) trial. BMJ 2010; 340:c1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirkcaldy RD, Harvey A, Papp JR, et al. Neisseria gonorrhoeae antimicrobial susceptibility surveillance—The Gonococcal Isolate Surveillance Project, 27 sites, United States, 2014. MMWR Surveill Summ 2016; 65:1–19. [DOI] [PubMed] [Google Scholar]
- 8.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin Infect Dis 2015; 61:418–426. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MS. HIV and sexually transmitted diseases: Lethal synergy. Top HIV Med 2004; 12:104–107. [PubMed] [Google Scholar]
- 10.Mayaud P, Mabey D. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sex Transm Infect 2004; 80:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Global strategy for the prevention and control of sexually transmitted infections: 2006–2015. Available at: http://www.who.int/reproductivehealth/publications/rtis/9789241563475/en/index.html. Accessed September 3, 2013.
- 12.Weinstein SA, Stiles BG. A review of the epidemiology, diagnosis and evidence-based management of Mycoplasma genitalium. Sex Health 2011; 8:143–158. [DOI] [PubMed] [Google Scholar]
- 13.Papp J, Schafter J, Gaydos C, et al. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014; 63:1–19. [PMC free article] [PubMed] [Google Scholar]
- 14.Hobbs MM, van der Pol B, Totten P, et al. From the NIH: Proceedings of a workshop on the importance of self-obtained vaginal specimens for detection of sexually transmitted infections. Sex Transm Dis 2008; 35:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrow SC, Smith DW, Harnett GB. The diagnosis of chlamydia, gonorrhoea, and trichomonas infections by self obtained low vaginal swabs, in remote northern Australian clinical practice. Sex Transm Infect 2002; 78:278–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippman SA, Jones HE, Luppi CG, et al. Home-based self-sampling and self-testing for sexually transmitted infections: acceptable and feasible alternatives to provider-based screening in low-income women in São Paulo, Brazil. Sex Transm Dis 2007; 34:421–428. [DOI] [PubMed] [Google Scholar]
- 17.Arias M, Jang D, Gilchrist J, et al. Ease, comfort, and performance of the HerSwab vaginal self-sampling device for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Dis 2016; 43:125–129. [DOI] [PubMed] [Google Scholar]
- 18.Chernesky M, Jang D, Gilchrist J, et al. Ease and comfort of cervical and vaginal sampling for Chlamydia trachomatis and Trichomonas vaginalis with a new Aptima specimen collection and transportation kit. J Clin Microbiol 2014; 52:668–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Wijgert J, Altini L, Jones H, et al. Two methods of self-sampling compared to clinician sampling to detect reproductive tract infections in Gugulethu, South Africa. Sex Transm Dis 2006; 33:516–523. [DOI] [PubMed] [Google Scholar]
- 20.Jones HE, Altini L, de Kock A, et al. Home-based versus clinic-based self-sampling and testing for sexually transmitted infections in Gugulethu, South Africa: Randomised controlled trial. Sex Transm Infect 2007; 83: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ting J, Mugo N, Kwatampora J, et al. High-risk human papillomavirus messenger RNA testing in physician- and self-collected specimens for cervical lesion detection in high-risk women, Kenya. Sex Transm Dis 2013; 40:584–589. [DOI] [PubMed] [Google Scholar]
- 22.Getman D, Jiang A, O’Donnell M, et al. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study cohort in the United States. J Clin Microbiol 2016; 54:2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J A coefficient of agreement for nominal scales. Educ Psychol Meas 1960; 20:37–46. [Google Scholar]
- 24.Little RJ, Rubin DB. Statistical Analysis with Missing Data. Brisbane: John Wiley & Sons, 1987. [Google Scholar]
- 25.Ibrahim JG, Chen MH. Missing responses in generalised linear mixed models when the missing data mechanism is nonignorable. Biometrika 2001; 88:551–564. [Google Scholar]
- 26.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 27.Paudyal P, Llewellyn C, Lau J, et al. Obtaining self-samples to diagnose curable sexually transmitted infections: a systematic review of patients’ experiences. PLoS One 2015; 10:e0124310 Available at: 10.1371/journal.pone.0124310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross JD, Jensen JS. Mycoplasma genitalium as a sexually transmitted infection: Implications for screening, testing, and treatment. Sex Transm Infect 2006; 82:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cazanave C, Manhart LE, Bebear C. Mycoplasma genitalium, an emerging sexually transmitted pathogen. Med Mal Infect 2012; 42:381–392. [DOI] [PubMed] [Google Scholar]
- 30.Lewis DA, Lukehart SA. Antimicrobial resistance in Neisseria gonorrhoeae and Treponema pallidum: Evolution, therapeutic challenges, and the need to strengthen global surveillance. Sex Transm Infect 2011; 87:ii39–ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


