COVID-19 is an acute respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since the first case was identified,1 the rapid emergence of new cases, admissions to hospital, and deaths required that public health officials focus on prevention through infection control measures, clinicians focus on diagnosis and supportive care, and medical scientists focus on the development of new vaccines and therapeutics. Attention is now turning towards understanding the natural course of COVID-19 in survivors and optimising follow-up to prevent, identify, and treat any undesirable long-term sequelae.
Distinct patterns of disease progression were documented in early clinical descriptions of the first COVID-19 cases.2 Many patients with acute COVID-19 have involvement of their respiratory system, characterised by dry cough, dyspnoea, hypoxaemia, and abnormal imaging results.3 Although most patients had mild-to-moderate disease, 5–10% progress to severe or critical disease, including pneumonia and acute respiratory failure.4, 5 Severe cases can occur early in the disease course but clinical observations typically describe a two-step disease progression, starting with a mild-to-moderate presentation, followed by a secondary respiratory worsening 9–12 days after the first onset of symptoms.4, 6, 7 Respiratory deterioration is concomitant with extension of ground-glass lung opacities on chest CT scans, lymphocytopenia, and high prothrombin time and D-dimer levels.4
Early evidence supports the hypothesis that some survivors might develop long-term respiratory sequelae. Fibrotic abnormalities of the lung have been detected as early as 3 weeks after the onset of symptoms regardless of whether the acute illness was mild, moderate, or severe.3, 8, 9, 10 Abnormal lung function (ie, restrictive abnormalities, reduced diffusion capacity, and small airways obstruction) has also been identified at the time of discharge from hospital and 2 weeks after discharge.11, 12, 13 These lung function abnormalities appear to be more common among patients whose acute COVID-19 was severe with high levels of inflammatory markers, and are often accompanied by evidence of pulmonary fibrosis including interstitial thickening, coarse reticular patterns, and parenchymal bands.12
It is too soon to determine which patients with COVID-19 are at greatest risk for developing long-term pulmonary abnormalities, if such sequelae will resolve, improve, or become permanent, and how the pulmonary abnormalities might be affected by therapeutics such as remdesivir, dexamethasone, and others under investigation. We hypothesise that most COVID-19 survivors will manifest early pulmonary abnormalities, which could range from being asymptomatic, to mild to severe, and debilitating. We further hypothesise that among patients without pre-existing lung disease, the duration of pulmonary abnormalities will be related to the severity of their acute COVID-19 course, with complete or near complete resolution within 6 months in patients who had a mild course (ie, did not require admission to hospital) and within 12 months in patients who had a moderate course (ie, admitted to hospital but did not require intensive care). However, persistent lung function abnormalities, including restrictive lung disease, decreased diffusing capacity, and fibrosis, are expected in patients who had a severe course, particularly those who required mechanical ventilation. These hypotheses need to be tested, which requires a systematic approach. We call on the pulmonary community to work together to develop a uniform and systematic approach to follow-up of COVID-19 survivors. Such an approach should facilitate research and knowledge generation and, ultimately, improve patient outcomes.
An approach to deciding when it is safe to schedule COVID-19 survivors for elective in-person visits has been published.14 However, no empirical evidence or consensus exists on how patients should be followed-up. Here, we propose an approach for consideration, which is based upon evolving clinical knowledge, clinical experience and rationale.
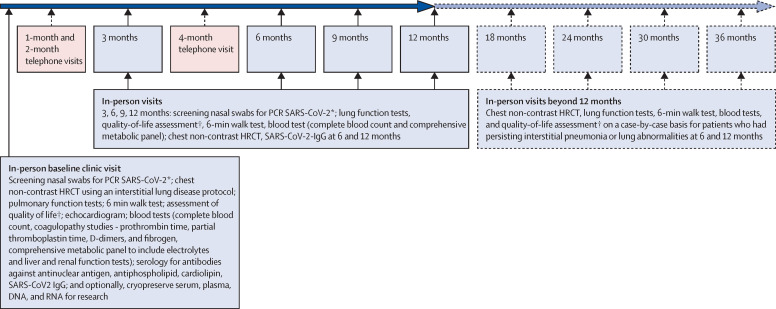
The initial in-person visit should target the establishment of a patient's baseline after COVID-19. This process would require a thorough investigation of present and past medical, social, and family history, physical examination, and blood testing, including the following: a complete blood count; comprehensive metabolic panel; coagulopathy studies (prothrombin time, partial thromboplastin time, D-dimers, and fibrinogen); serology for antiphospholipid and anticardiolipin antibodies; SARS-CoV-2 IgG antibody levels; and cryopreservation of serum and plasma, including RNA and DNA for genotype research studies. Additionally, a baseline non-contrast high-resolution CT scan (HRCT), pulmonary function tests (spirometry, lung volumes, and diffusion capacity), 6-min walk test, assessment of quality of life (including fatigue, anxiety and depression) by patient reported outcomes, pulse oximetry on room air at rest and during the 6-min walk test, pulse oximetry with supplemental oxygen if the pulse oximetry on room air is less than 88%, and an echocardiogram should be considered, if resources permit.
Once the COVID-19 survivor's baseline has been established, a follow-up evaluation during a structured protocol visit should aim to better understand the natural course of disease and identify new abnormalities early. A reasonable plan would be to follow-up patients with mild impairment of lung function by phone visits or videoconferencing, or both, at 1, 2, and 4 months and in-person at 3 and 6 months, and subsequently at 9, 12, 18, 24, 30, and 36 months based on the degree and extent of lung involvement and impairment on a case-by-case basis (figure ). During the initial 12 months of follow-up, the in-person visits could be accompanied by repeat testing for COVID-19 infectivity, repeat pulmonary testing, 6-min walk test, monitoring of quality of life, fatigue, and some blood testing (eg, complete blood count, comprehensive metabolic panel, coagulopathy studies, and SARS-CoV-2 IgG antibody levels). Imaging by non-contrast HRCT of the chest at the 6-month and 12-month in-person visits could be done to assess improvement, resolution, persistence, or worsening of any fibrosis. Beyond 12 months, most tests could be ordered on a case-by-case basis, although patients with fibrosis on their 6-month or 12-month HRCT of the chest might warrant additional scans at 24 and 36 months to understand long-term sequelae of interstitial pneumonia or pulmonary fibrosis.
Figure.
Suggested follow-up care for COVID-19 survivors
HRCT=high-resolution CT. SARS-CoV-2= severe acute respiratory syndrome coronavirus 2. *Nasal swab testing during the 3–5 days before visit is to make sure that the survivors are not shedding the virus particles and thus ascertain the status of infectivity at baseline and during follow-up visits. The intended in-person baseline and follow-up visits could then be converted to telemedicine visits if found to be positive for SARS-CoV-2, on a case-by-case basis, or appropriate precautionary measures could be taken with personal protective equipment by health-care workers. †Quality of life assessment via patient reported outcomes with standard questionnaires used for respiratory diseases, fatigue, anxiety, and depression.
In summary, the varying extent of pulmonary fibrosis and lung function impairment among survivors of COVID-19, and the unknown course of such abnormalities, highlight the need for pulmonary clinicians to closely monitor disease course in survivors. Such follow-up will generate knowledge about the natural course of disease and facilitate enrolment in clinical trials assessing the treatment of abnormalities with immune modulating drugs and antifibrotic drugs.15 A standard approach from institution to institution will facilitate research and could improve outcomes.

© 2020 Lea Paterson/Science Photo Library
Acknowledgments
GR has provided consultation services to Boerhinger Ingelheim, Roche-Genentech, Blade therapeutics, PureTech Health, and Humanetics corporation. KCW declares no competing interests.
References
- 1.WHO . World Health Organization; Geneva: Jan 12, 2020. Novel coronavirus — China.https://www.who.int/csr/don/12–january-2020–novel–coronavirus-china/en/ [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Zeng W, Li X. CT imaging changes of corona virus disease 2019(COVID-19): a multi-center study in Southwest China. J Transl Med. 2020;18:154. doi: 10.1186/s12967-020-02324-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salje H, Tran Kiem C, Lefrancq N. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Guan X, Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed H, Patel K, Greenwood D. Long-term clinical outcomes in survivors of coronavirus outbreaks after hospitalisation or ICU admission: a systematic review and meta-analysis of follow-up studies. medRxiv. 2020 doi: 10.1101/2020.04.16.20067975. published online April 22. (preprint) [DOI] [PubMed] [Google Scholar]
- 9.British Thoracic Society; May 11, 2020. British Thoracic Society guidance on respiratory follow up of patients with a clinico-radiological diagnosis of COVID-19 pneumonia. [Google Scholar]
- 10.Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo X, Jian W, Su Z. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020 doi: 10.1183/13993003.01217-2020. published online May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol. 2020;21:746–755. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dongqing Lv XC, Mao L, Sun J. Pulmonary function of patients with 2019 novel coronavirus induced pneumonia: a retrospective cohort study. Research Square. 2020 doi: 10.21203/rs.3.rs-24303/v1. published online April 27. (preprint) [DOI] [PubMed] [Google Scholar]
- 14.Wilson KC, Kaminsky DA, Michaud G. Restoring pulmonary and sleep services as the COVID-19 pandemic lessens: from an Association of Pulmonary, Critical Care, and Sleep Division Directors and American Thoracic Society-coordinated task force. Ann Am Thorac Soc. 2020 doi: 10.1513/AnnalsATS.202005-514ST. published online July 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30225-3. published online May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]