Abstract
Current management of diabetic macular edema (DME) predominantly involves treatment with short-acting intravitreal injections of anti-vascular endothelial growth factors (anti-VEGFs) and/or corticosteroids; however, short-acting therapies (lasting between 1 and 6 months) require frequent injections to maintain efficacy, meaning a considerable treatment burden for diabetic patients with multiple comorbidities. Continuous injections needed in some cases are an economic burden for patients/healthcare system, so real-life clinical practice tends to adopt a reactive approach, ie, watch and wait for worsening symptoms, which consequently increases the risk of undertreatment and edema recurrence. On March 7th 2019, a group of experts in retinal medicine and surgery held a roundtable meeting in Madrid, Spain to discuss how to (1) optimize clinical outcomes through earlier use of fluocinolone acetonide (FAc) implant (ILUVIEN®) in patients with persistent or recurrent DME despite therapy; and, (2) to provide guidance to assist physicians in deciding which patients should be treated with ILUVIEN. In this regard, a 36-month follow-up consensus protocol is presented. In conclusion, patients that achieve a complete or partial anatomical, and preferably functional, response following one or two intravitreal dexamethasone implants, but with recurrence of edema after 3–4 months, are deemed by the authors most likely to benefit from ILUVIEN, and the switch to FAc implant should not be delayed more than 12 months after the initiation of at least the first dexamethasone implant.
Keywords: diabetic macular edema, DME, fluocinolone acetonide implant therapy, ILUVIEN
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) therapy, with or without adjunctive focal laser treatment, has generally become the global standard of care and used as a first-line therapy to improve visual outcomes in center-involved diabetic macular edema (DME).1 Not all eyes that receive anti-VEGF or laser photocoagulation show complete resolution of DME with improvement of vision, however. Despite applying “pro re nata” (PRN) or “treat and extend” (T&E) regimens, with a varying mean of 6–10 injections per year, approximately half of patients undergoing frequent anti-VEGF therapy do not improve, have persistent edema, or both.2–4 Several factors may contribute to suboptimal outcomes among DME patients treated with anti-VEGF therapy, such as delays in diagnosis and/or treatment, insufficient response to therapy,5 and more definitively, the impossibility of physicians to administer therapies according to the standard-of-care (SoC) in real-life practice.6 Factors that have been exacerbated by the current corona virus pandemic and also led to patients fearing traveling to and attending clinical appointments to receive therapy.
Following anti-VEGF treatment, the intravitreal dexamethasone implant OZURDEX®) and the fluocinolone acetonide (FAc) intravitreal implant (ILUVIEN®) are commonly used as second-line treatments in DME, although these therapies are increasingly being used earlier in the treatment algorithm in Spain due to their high efficacy and response rates, as well as their proven safety profile in real-life.7 In some particular cases, dexamethasone implants are even used as first-line therapy in naïve patients with positive functional outcomes.8,9
A single dexamethasone implant has a short-term action of up to 6 months,10,11 compared with up to 36 months of continuous treatment with the longer-acting FAc implant.7,12 Further advantages of sustained intravitreal release of the FAc implant include reduction in the frequency of injections and clinic visits, with subsequent lower rates of complications (such as retinal detachment, endophthalmitis and lens iatrogenic injury) related to injection procedure, higher patient compliance and lower healthcare costs.13–15
Based on the authors’ real-life experiences managing DME with the FAc implant in clinical practice, the current approach to DME treatment is challenged. To better identify and select the DME patients who would benefit from FAc implant, a consensus of the authors’ opinions was reached following a meeting of retinal specialists held on March 7th, 2019 in Madrid, Spain. The current review was designed to provide a general introduction to DME as well as an overview of the FAc technology, its pharmacokinetics and pharmacodynamics.
Comorbidities and Ocular Complications Associated with Diabetes
Diabetes is a group of inflammatory chronic metabolic diseases characterized by elevated blood glucose and degenerative damage to blood vessels.16,17 The pathogenesis of microvascular complications is complex, and involves metabolic and hemodynamic disturbances, extending beyond hyperglycemia to include insulin resistance, dyslipidemia, hypertension, and immune dysfunction.18 These disturbances initiate several damaging processes, such as increased reactive oxygen species (ROS) production, inflammation, and ischemia.18
Diabetic retinopathy (DR) is a frequent inflammatory and neurovascular complication of diabetes and the main cause of irreversible vision loss in the working-age population.19,20 A global meta-analysis study reported that 35.4% of patients with diabetes had some form of DR.21 It is also noted that 1 in 10 (11.7%) had potentially vision-threatening retinal changes such as DME.21
DME is a Chronic, Inflammatory and Recurrent Disease
DME is characterized by macula thickening due to accumulation of exudative extracellular fluid derived from hyperpermeable retinal capillaries in the macula.22 It is the most common form of vision-threatening retinopathy in people with diabetes, affecting approximately 7% (range 4–18%) of patients with diabetes and more than 20 million individuals worldwide.22–26 Alteration of the blood–retinal barrier, release of inflammatory mediators, and ischemic processes are possible mechanisms for vision loss due to DME.27
Inflammatory processes play an important role in DR pathogenesis.28–30 In DME patients, levels of inflammatory cytokines, VEGF, as well as the important modulator of angiogenesis, angiopoietin-2, are significantly elevated.31–33 However, the most compelling evidence pointing to inflammation as a critical contributor to the development of DR is the dramatic anti-inflammatory and anti-edematous effect of corticosteroid treatment in DME.34 Significant decreases in retinal thickness have been observed within 1 hour of intravitreal triamcinolone acetonide (IVTA) injection, though no change was seen with bevacizumab after 24 hours.35 In addition, corticosteroids reduce edema and prevent angiogenesis, which may be partly attributed to their ability to block VEGF gene expression.36 Treatment with a long-term intravitreal corticosteroid, which requires fewer injections than treatment with short-acting therapies, has been found to reduce inflammation and stabilize/improve vision in the majority of patients.37–39 Continuous daily treatment, eg, as with the FAc implant, may help to reduce under treatment for 36 months and, therefore, reduce the likelihood of edema recurrence that is stated to drive long-term damage to the retina due to the constant presence of intra and/or subretinal fluid accumulation, development of disorganization of the retinal inner layers (DRIL), and poor long-term functional outcomes.40,41
Consensus on DME Unmet Clinical Needs
Despite the available treatment options for DME, there are several unmet clinical needs that the FAc implant could address if it was to be included in routine clinical practice. Almost all the patients on dexamethasone intravitreal implant treatment have recurrent edema <5 months after an injection.13,42 Compared with FAc implants, dexamethasone implants have a short-term effect, resulting in a see-saw pattern of intermittent improvement and regression in macular thickness.43–45
DME management with reactive regimens leads to periods of undertreatment. The decision to re-treat patients involves “watching and waiting” for retinal signs or symptoms, which means frequent clinic visits periodically, based on a set of prespecified criteria, as determined by the physician (eg, visual acuity (VA) and optical coherence tomography (OCT)/fluorescein angiography assessments).46–48
There is a high treatment burden for patients with diabetes, along with additional time required from any accompanying carer6,49,50 There is also lack of a treatment that could improve patients’ quality of life, as well as family and healthcare system burden.51,52
Non-adherence to treatment and follow-up regimens is a common problem in the management of patients with DME and limits clinical treatment outcomes under real-life conditions.49,53 In real life, there is lower patient non-adherence to intravitreal treatment in DME (44.0%) compared with other retinal diseases, such as neovascular age-related macular degeneration (nAMD; 32.2%) or branch retinal vein occlusion (BRVO; 25.0%; p<0.01 between groups).49 Lower non-adherence in DME patients is associated with a higher risk of significant visual acuity loss.49
Anti-VEGF treatment in real life is not mirroring randomized clinical trials (RCTs) as the treatment regimens are impossible to follow, eg, in RCTs, a monthly injection regimen was typically followed, resulting in a mean best-corrected visual acuity (BCVA) gain of approximately 12 letters, while in real-life the mean BCVA gains are much lower, such as +4.7 letters if a PRN regimen is followed.2,6,54 Dexamethasone treatment is also difficult to follow in real-life and requires more frequent reinjection than in clinical trials.13,55,56 Although retreatment with dexamethasone implant for DME is recommended after 6 months, the therapeutic effect in most eyes lasts approximately 4 months.57 Indeed, in real-life DME studies, the mean number of dexamethasone injections administered in 12 months is typically more frequent than recommended (ie, >2 injections per year).13,55,56
Comparison of Corticosteroids
Short-acting intravitreal corticosteroid injections have a limited duration of action, which require frequent re-treatment (eg, dexamethasone implant). Intravitreal sustained-release implants (eg, FAc implant) have been developed to prolong the effect of fluocinolone acetonide and to lessen the need for repeated application.58 Overall, there is a progressive and continuous improvement of the macula and visual outcomes with the FAc implant, maintained over 36 months.59 A comparison of the available short- and long-acting intravitreal corticosteroids are shown in Table 1.
Table 1.
Comparison of Available Intravitreal Corticosteroid Agents
| Agent | Total Dose (Daily Release) |
Formulation | Procedure | Duration of Action of 1 Injection | Primary Endpoint | Number of Treatments Over 36 Months in RCTs | Number of Treatments Over 12 Months in Real Life | Licensed DME Indication | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| DEX | 700 μg (~6.25 μg/day) OZURDEX® |
Biodegradable | Injectable 22 gauge needle/stepped incision | ~4 months | % ≥15 letter gain in BCVA at year 3; 22% vs 12%; p<0.001 (OZURDEX vs sham group) | 5.0 | N/A | Adult patients with visual impairment due to DME who are pseudophakic or who are considered insufficiently responsive to, or unsuitable for, non-corticosteroid therapy | [11,57] |
| Systematic review of real-life studies | N/A | 2.6 | [13] | ||||||
| IVTA | 4 mg TA (unknown) | Preservative-free | Injectable 25 gauge needle/standard intravitreal injection | ~3 months | 3-year extension: mean BVCA = +5 with laser vs +0 with TA | 4.1 | N/A | Not licenced in the EU | [96] |
| FAc | 190 μg (0.2 μg/day) ILUVIEN® | Nonbioerodible (polyimide tube) | Injectable 25 gauge needle/standard intravitreal injection | Up to 3 years | ≥15 letter gain (chronic DME) 34% vs 13%; p<0.001 (ILUVIEN vs sham group) | 1.3 | 1 | Vision impairment associated with chronic DME considered insufficiently responsive to available therapies | [7,70] |
Notes: Adapted from Kane et al, 2008.66
Abbreviations: BCVA, best corrected visual acuity; DEX, dexamethasone; DME, diabetic macular edema; FAc, fluocinolone acetonide; IVTA, intravitreal triamcinolone acetonide; N/A, not applicable; RCT, randomized controlled trial; TA, triamcinolone acetonide.
Corticosteroid efficacy is dependent on release kinetics (eg, burst height of corticosteroid concentration in the vitreous and duration of release), which varies between different corticosteroid formulations (burst height is 1–20 ng/g for FAc vs >1,100 ng/g for dexamethasone and 10,000 ng/g for triamcinolone acetonide [TA]).59–61 Drugs with high burst heights, such as intravitreal TA and the dexamethasone implant, show the greatest effect during this initial burst, followed by exponential fall of drug concentration.10 In contrast, the FAc implant, with a low burst height and near-zero release kinetics, is better able to maintain efficacy over time.59
Efficacy differences may be partially explained by the fact that affinities for TA and FAc for human glucocorticoid receptor are higher than dexamethasone (dexamethasone [IC50 2.95 nM] > triamcinolone [IC50 0.27 nM] > FAc [IC50 0.19 nM]), which may explain the higher clinical dose of dexamethasone versus FAc.62–65 In addition, the water solubility is lower and lipophilicity is higher for FAc compared to dexamethasone and TA which increases the availability of the drug so a much lower pharmacokinetic (PK) dose of FAc is needed in the implant than dexamethasone or TA.64,66-69 Hence higher doses are required to produce an appropriate therapeutic effect due to weaker binding affinity and lower lipophilicity of dexamethasone and shorter duration of action of TA. The combination of high affinity to the glucocorticosteroid receptor and more efficient delivery mechanism means that an effective, low dose of FAc can be delivered to the retina for up to 36 months.7,12
Fluocinolone Implant Technology and Pharmacokinetics
The FAc implant is an injectable, non-bioerodible, intravitreal insert designed to provide stable, long-term delivery of FAc to the eye continuously every day for 36 months.70 It was specifically designed by ophthalmologists looking for more effective and efficient ways to treat inflammation-driven ocular diseases safely and locally.71
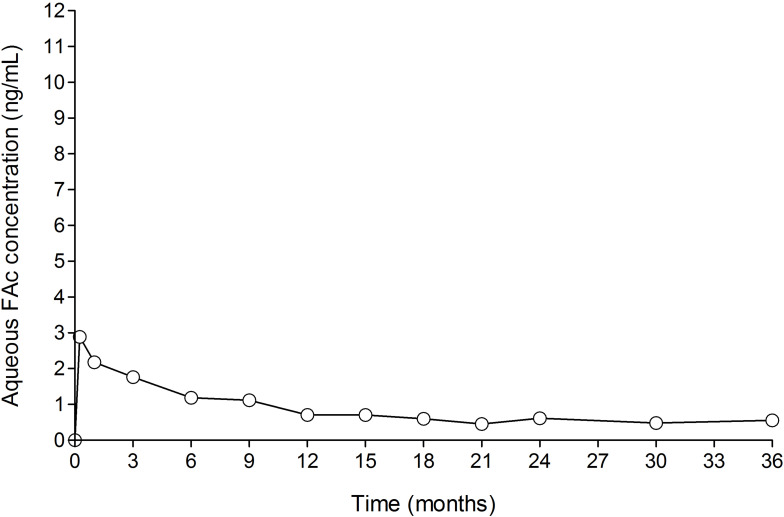
In a human PK study (the FAMOUS study), FAc concentrations in plasma were below the lower limit of quantitation of the assay (100 pg/mL) at all time points from Day 1 through Month 36.12 The fact that plasma levels of FAc were below the detectable limit supports its localized action at the site of injected eyes. Aqueous humor FAc concentrations remained stable from Month 6 to Month 36 for subjects who were not retreated (see Figure 1). Notably, there is no significant difference in FAc plasma concentration following the initial dose and following reinjection of a second dose after 12 months.70
Figure 1.
FA levels in human aqueous humor in subjects receiving one ILUVIEN® implant (FAMOUS Study).
Notes: Data from Campochiaro et al.12 FAc, 0.2 μg/day fluocinolone acetonide (ILUVIEN).
Phase III FAc Implant Studies – A Brief Overview
The FAc implant is indicated for the treatment of vision impairment associated with chronic DME, considered insufficiently responsive to available therapies.70 The 0.19 mg FAc implant (0.2 µg/day) is effective for the treatment of DME. Evidence of effectiveness is shown in the Fluocinolone Acetonide in Diabetic Macular Edema (FAME) studies (FAME A and B). FAME studies A and B were Phase III, multicenter RCTs designed to assess the efficacy and safety of a single injection of the FAc implant over a 3-year period versus the SoC (mainly laser photocoagulation; Table 2).72,73
Table 2.
Key Clinical Trial and Real-World Studies Demonstrating Effectiveness of 0.19 Mg Fluocinolone Acetonide Implant
| Study Name (Author) |
Region (Study Type) |
No. of Eyes | Mean no. Prior Intravitreal Injections | Follow-Up Period | Mean ETDRS Letter Gains | Mean Decrease in CRT | IOP-Lowering Medication | Mean Supplemental Therapies and Time to Administration |
|---|---|---|---|---|---|---|---|---|
| Phase III trials | ||||||||
| Fluocinolone Acetonide in Diabetic Macular Edema studies; FAME A and B7,75 | USA, Canada, EU, India (Two multicenter, randomized double-blind, parallel group studies) |
953 | N/A – laser given as a prior therapy | 36 months | At 36 months + 5.3 (full DME group) + 7.6 (chronic DME group) |
At 36 months -181 μm (full DME group) -186.8 µm (chronic DME group) |
At 36 months 38.4% (full DME group) 35.9% (chronic DME group) |
At 36 months 40.7% laser and 13.4% including anti-VEGF and IVTA (chronic DME group) |
| European real-world studies (≥81 eyes, multicenter) | ||||||||
| ILUVIEN® Registry Safety Study; IRISS38 | UK, Germany, Portugal (Observational study) |
593 | 5.3 ranibizumab (66.3%), 3.3 bevacizumab (28.8%), 4.0 aflibercept (5.2%), 1.4 dexamethasone implant (19.2%), 2.7 ocular corticosteroids (22.6%), (full group) | ~15 months (471.2 days; range, 1–1269 days) | At 12 months +3.7 (all DME eyes) Chronic DME (pseudophakic eyes): s hort-standing chronic DME = +8.2; and, l ong-standing chronic DME = +1.7 |
Not recorded | Overall 23.3% |
Overall Anti-VEGF, corticosteroid therapies, laser and ILUVIEN retreatment (31.0%) The mean time to additional treatment was 356.1±274.8 days. |
| MEDISOFT Audit Group39 | UK (Retrospective analysis) |
345 | Mean: 7.36 intravitreal treatments including ranibizumab (68.4%), IVTA (29.0%), dexamethasone implant (5.5%) (full group) | ~14.1 months (428 days; range, 0–919 days) | At 24 months +5.3 (all DME eyes) +5.8 (DME, pseudophakic eyes) |
Overall -95.7 μm |
Overall 13.9% |
Overall Anti-VEGF, corticosteroid therapies (ILUVIEN retreatment) and laser (35.7%) A mean of 4.4 intravitreal therapies were administered |
| Retro-IDEAL94 | Germany (Retrospective study) |
81 | 4.3 ranibizumab (91.1%), 4.3 bevacizumab (91.1%), 5.0 aflibercept (6.3%), 1.5 dexamethasone implant (24.1%), 1.1 IVTA (41.8%) (full group) | 30.8 months92 | At 36 months +2.7 At 30 months (ie mean follow-up period) +4.3 |
At 36 months -158.0 μm At 30 months -111.0 μm |
Overall 49.4% (27.2% were emergent IOP cases) |
Overall Anti-VEGF and corticosteroid therapies (30.9%) 16% of supplemental treatments were given by Month 12 with a further 40% administered between 12 and 24 months |
| European real-world studies reporting 3 years of follow-up (n>1, single center) | ||||||||
| Panos et al.76 | UK | 24 | 4.8 ranibizumab (58.3%); 1.1 IVTA (29.2%) | 36 months (n=9/24) | At 36 months +7.5 letters (from 0.62 to 0.47 logMAR) |
At 36 months -93.0 μm |
Overall 20.8% |
54.2% (n=13/24) patients received supplemental injections of anti-VEGF or corticosteroid. Mean time to supplementary treatment was 13.5 (range: 3–27) months |
| Rehak et al.77 | Germany | 49 | 6.74 anti-VEGF (100.0%); 1.27 dexamethasone implant (51.0%) (full group) | 36 months (n=19/49) | At 36 months Group A: +10.6 (switched from anti-VEGF to ILUVIEN) (n=12) Group B: +9.7 (switched from anti-VEGF to dexamethasone and then to ILUVIEN) (n=7) |
At 36 months Group A: −257.6 μm Group B: −245.6 μm |
At 36 months 28.6% |
Overall Group A: 37.5% including anti-VEGF, dexamethasone implant and a second ILUVIEN implant Group B: 36.0% including anti-VEGF and anti-VEGF Mean time to supplemental treatment was: Group A, 22.7 months; and, Group B, 21.8 months |
| Young et al.78 | UK | 21 | 4.7 bevacizumab (52.4%), 11.7 ranibizumab (66.7%), 7.7 aflibercept (14.3%), 1.3 dexamethasone implant (19.0), 1.4 IVTA (23.8%) (full group) | 36 months (n=10/21) | At 36 months +9.3 |
At 36 months -157.8 μm |
Overall 38.1% |
At 36 months 13.1 anti-VEGF (30.0% [n=3/10]) |
| Fusi-Rubiano et al.79 | UK | 29 | 4.3 bevacizumab (62.1%), 3.9 ranibizumab (58.6%), 2.3 IVTA (55.2%) (full group) | 36 months (n=6/29) |
At 36 months +11.0 |
At 36 months -137.0 μm |
Overall 6.9% |
At 36 months 5.0 anti-VEGF or laser (83.3% [n=5/6]) Mean time to supplementary treatment was 12.8 (range: 10–16 months) |
Notes: For the group “European real-world studies reporting 3 years of follow-up”, data is presented at the 36-month time point if it was reported in the paper, otherwise the overall numbers are reported and refer to the full group analysed.
Abbreviations: CRT, central retinal thickness; DEX, dexamethasone; DME, diabetic macular edema; ETDRS, Early Treatment Diabetic Retinopathy Study; IOP, intraocular pressure; IVTA, intravitreal triamcinolone acetonide; N/A, not applicable; SD, standard deviation; VEGF, vascular endothelial growth factors.
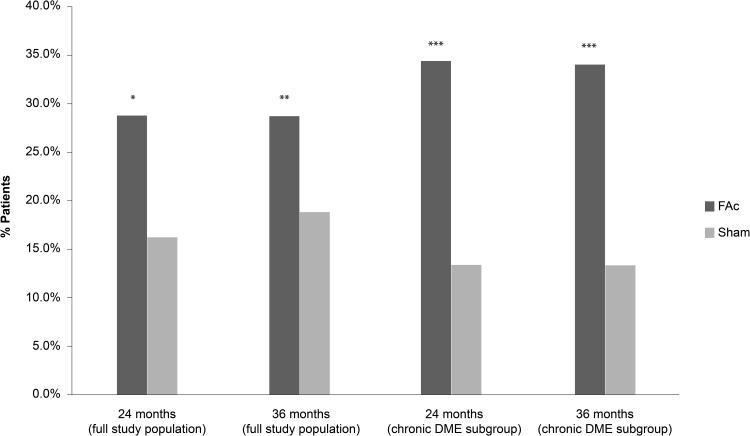
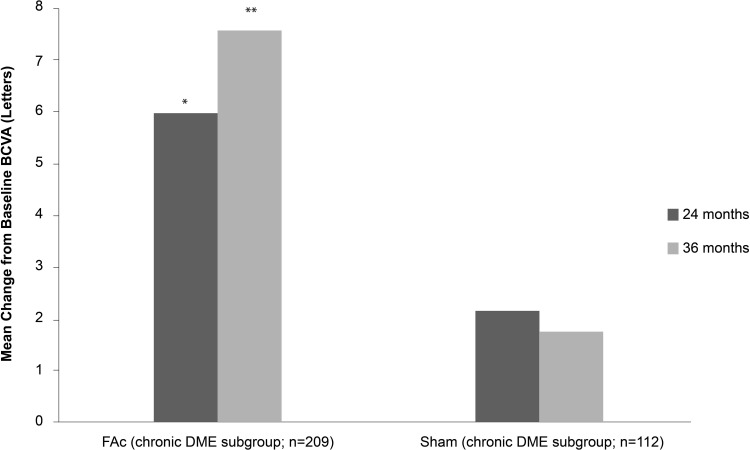
The primary endpoints of both studies were VA gain of ≥15 letters at 24 months (patients were followed up to 36 months), which was met in both FAME A and B. At both 24 and 36 months, chronic DME patients receiving FAc implants achieved greater improvements in VA compared with the sham group (Figures 2 and 3). These VA results from FAME at 36 months follow-up form the basis for the approved license of the FAc implant in Europe. Figure 2 shows the results at Months 24 and 36 of the proportion of chronic DME subjects with ≥15 letter improvement from baseline BCVA.7 Figure 3 shows the 24 and 36-month analyses for mean gains in BVCA from baseline for chronic DME patients.
Figure 2.
Percentage of subjects with ≥15 letter improvement from baseline in best corrected visual acuity (BCVA) at 24 and 36 months in patients with DME treated with 0.2 μg/day FAc implant versus sham injection.
Notes: Data from Campochiaro et al.7 , and ILUVIEN® Spanish Summary of Product Characteristics.70 *P = 0.002 FAc vs sham at Month 24; **P < 0.018 FAc vs sham at Month 36; ***P < 0.001 FAc vs sham for both at Month 24 and 36. Full study population, N= 376 (FAc), N = 185 (sham); Chronic DME subgroup, N = 207 (FAc), N = 111 (sham).
Abbreviation: DME, diabetic macular edema; FAc, 0.2 μg fluocinolone acetonide (ILUVIEN).
Figure 3.
Mean gains in best corrected visual acuity (BVCA) from baseline at 24 and 36 months in patients with chronic DME treated with 0.2 μg/day FAc implant.
Notes: *P = 0.35 vs sham at Month 24; **P = 0.04 vs sham at Month 36.; FAc, 0.2 μg fluocinolone acetonide.
Abbreviations: DME, diabetic macular edema; FAc, 0.2 μg fluocinolone acetonide (ILUVIEN®).
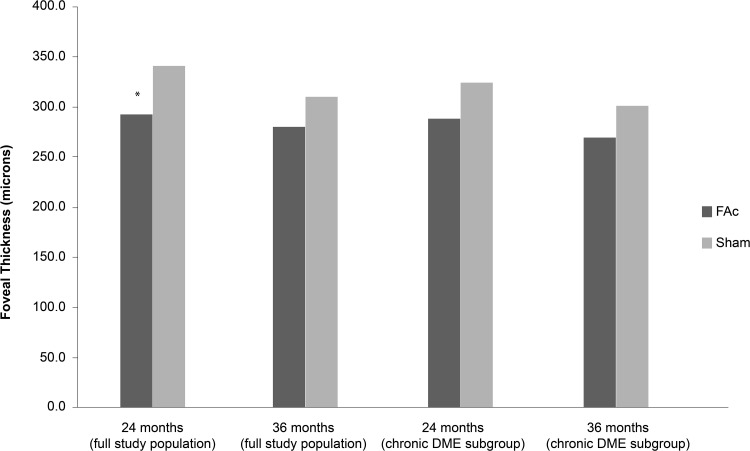
Sustained reduction in central foveal thickening (CFT) in the FAc-treated group from as early as the first follow-up visit (week 1) was maintained through to Month 36 (Figure 4).7
Figure 4.
Mean central foveal thickness (CFT) change in patients with chronic DME treated with 0.2 μg/day FAc implant.
Notes: Data from Campochiaro et al.7, and ILUVIEN® Spanish Summary of Product Characteristics.70 *P = 0.005 vs sham at Month 24.
Abbreviation: DME, diabetic macular edema; FAc, 0.2 μg fluocinolone acetonide (ILUVIEN).
Raised intraocular pressure and cataract formation are well-known side effects of intraocular corticosteroids. During the course of the 3-year FAME trial, 80.0% of eyes receiving the FAc implant (FAc 0.2 µg/day) required cataract surgery.7 However, the overall visual benefit after cataract surgery was similar to that in pseudophakic patients.74 Patients in the FAc implant group experienced more intraocular pressure (IOP)-related adverse events overall than patients in the sham group (FAc = 37.1% vs sham = 11.9%).7
Overall, there was a markedly better relative benefit for patients with chronic DME compared with sham patients, suggesting that patients with chronic DME who tend to respond poorly to many treatments, including focal/grid laser photocoagulation, respond well to administration of the FAc implant.7
Real-World FAc Implant Studies
Patient populations in real-world studies typically have more severe characteristics and are more heterogeneous than those in RCTs. In the FAME trial, all patients had received at least one prior focal/grid macular laser treatment, although all FAME participants were treatment-naïve for intravitreal injections.73 In the real-world setting, the FAc implant is chosen by clinical-decision for second- or third-line treatment. In contrast to FAME, all chronic DME patients in the real-world ILUVIEN Registry Safety Study (IRISS) study had demonstrated insufficient response to a wider range of prior DME therapies, mainly intravitreal anti-VEGFs but also corticosteroids in some cases. Furthermore, IOP was an exclusion criterion in the FAME study, but 5.2% of patients in IRISS had an IOP >21 mmHg at baseline.75
Table 2 compares FAME study results with key real-world study results that demonstrate the efficacy and safety of the FAc implant. Notably, the results from the largest European (ie, IRISS, MEDISOFT Audit, and Retro-IDEAL) studies represent the largest pool of real-world data on the usage of the FAc implant in clinical practice. The proportions of patients requiring IOP-lowering incisional glaucoma surgery were lower in the real-world studies compared with those observed in FAME (IRISS = 0.8%, MEDISOFT Audit = 0.8% at 18 months versus FAME at 18 months = 1.5%).7,38,39 IOP is maintained following FAc implant administration. After receiving one FAc implant, 86.1% of the patients did not require any intervention to manage IOP after 24 months of follow-up in the MEDISOFT Audit study.39 Other single-center studies have followed patients for three years after ILUVIEN therapy (Table 2) and report similar values (mean=23.6%; range, 6.9% to 38.1%).
Clinical-Decision Criteria to Identify Patients Suitable for ILUVIEN Therapy
Efficacy results with the FAc implant were observed in pharmacological-naïve patients in the Phase III RCT (FAME), with a mean gain of +7.6 letters vs +1.8 letters for the treated group compared with the sham group; of note, 34% of treated patients gained ≥15 letters at 36 months compared with only 13.4% of patients in the sham group.7
RCTs are valuable in understanding the efficacy and safety of the FAc implant; however, current real-world studies include all patients with DME treated with the FAc implant and provide a wider indication of the implant’s effectiveness and safety. Table 2 summarizes the largest real-world studies. It is notable that most (99%) patients have received the FAc implant as second- or third-line treatment in the Phase IV registry study, IRISS.38 Irrespective of this, an improvement of 3.7 letters (in all DME eyes) was recorded by Month 12 and similar improvements (~5 letters) have been reported in other studies (eg, MEDISOFT and Retro-IDEAL at 24 and 36 months) (Table 2) where patients have received the FAc implant as a second- or third-line therapy and where long-standing disease is evident. The IRISS study also suggested that patients with short-standing chronic DME may achieve better outcomes than those with long-standing chronic DME and supports better outcomes for patients when they are treated earlier in the disease (Table 2).38 The limitations of these studies have been reported, but one important point is the limited number of patients that have three years of follow-up which is needed to confirm the effectiveness and safety of the FAc implant in clinical practice. A number of smaller studies (see Table 2) have assessed effectiveness over three years and show improvements in visual acuity ranging from 7.5 to 11.0 letters. These studies are also important in trying to understand the effectiveness of the FAc implant in multi-ethnic populations,76 following anti-VEGF therapy,77 following a prior dexamethasone implant,77 in different regions within the same country and in different countries.76–79
Several investigators have concluded that, due to the clear IOP-related safety and visual acuity benefits for short-term DME patients, the FAc implant should be positioned earlier in the DME treatment algorithm,38 ie, a DME patient who has received one or two previous dexamethasone implant injections, showing the presence of DME from 3–4 months following injection, should be considered for switching to FAc implant. This would mean that this patient has recurrent DME and will continue requiring injections for a long period of time. Therefore, the dexamethasone implant test-time should be no longer than 12 months to avoid performing a high number of unnecessary injections of a suboptimal treatment. In clinical practice, it is observed that DME with a pattern of >2 edema recurrence events during the first year is, expectantly, repetitive and replicated during the second and third years.
Recommendation – Patients That May Benefit from FAc Implant Therapy
In our opinion, the main patient profile that could benefit from the FAc implant is for patients with a complete anatomical response after one injection of dexamethasone implant for those patients whom the retina layers are preserved. Regarding OCT biomarkers, there are signals which indicate that edema is not recent, such as subretinal fluid, presence of DRIL, disorganization/disruption of outer retinal layers and the presence of hyperreflective foci. These signals indicate a worse prognosis, irrespective of the chosen therapy. Indeed, the FAc implant has shown efficacy even in those patients that had insufficient response to previous therapies.38
It has also been previously stated that the FAc implant could be an alternative option for those DME patients that showed insufficient response to either anti-VEGF agents or short-lasting corticosteroids.80,81 Owing to the insufficient response to previous treatments in Phase IV studies, the FAc implant could also be an option for patients that do not have a complete anatomical response or vision improvement after one injection of dexamethasone implant, as has been demonstrated in real-world practices,82 because the FAc implant contains a different molecule that could induce a different response.
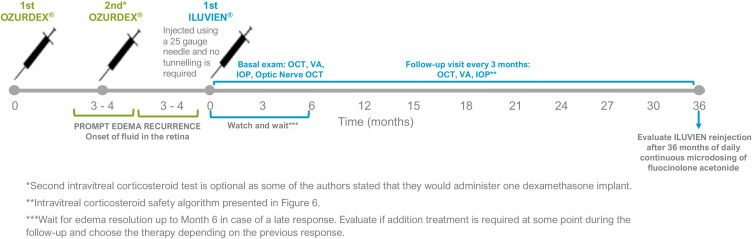
Protocol for the Management and Monitoring of DME Patients Treated with the FAc Implant
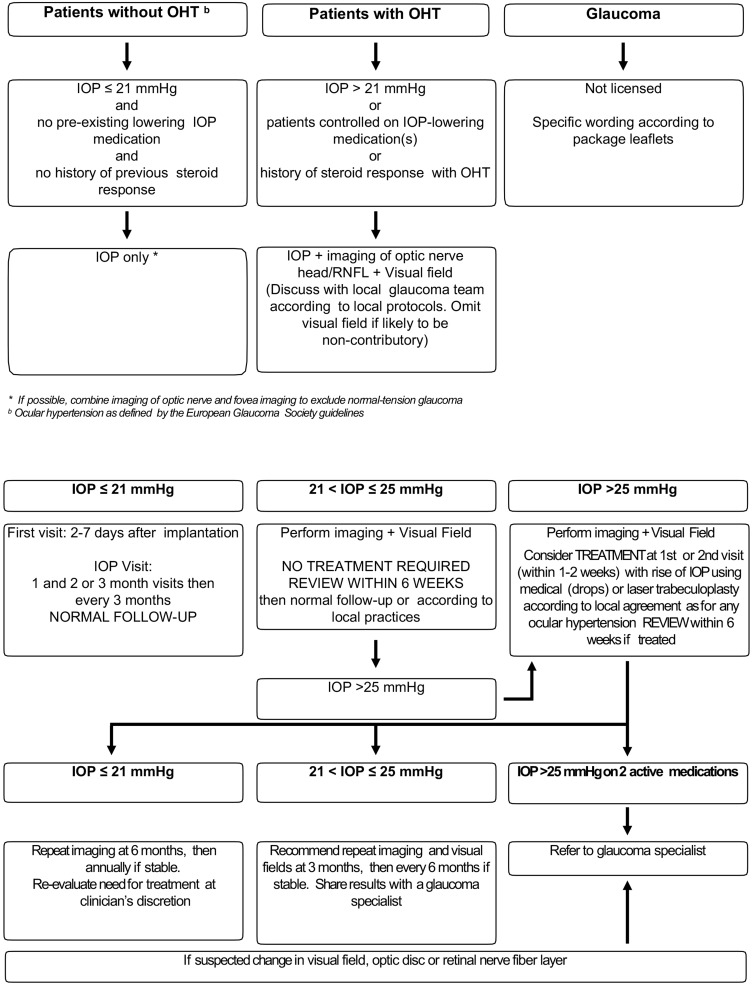
Based on the existing evidence on the effect of the FAc implant, as well as on the clinical experience of a group of medical and surgical retinal experts, a general protocol has been established to monitor FAc implant treatment in DME patients (see Table 3 and Figure 5). Further, pre-FAc and post-FAc implant safety considerations, as well as a post-injection algorithm when there is an IOP increase, have been proposed by glaucoma specialist, Dr Marta Pazos (see text below and Figure 6). These safety considerations are based on the Hospital Clínic de Barcelona’s glaucoma service experience of IOP events related to intravitreal corticosteroids (triamcinolone, dexamethasone implant and FAc implant) and on available medical literature.
Table 3.
General Pre- and Post-Injection Action Protocol for FAc Implant Treatment
| Medical Exam (Pre-injection) |
|
|
|
|
| Follow-up checks for 36 months (post-injection) |
|
|
Figure 5.
ILUVIEN® indication and follow-up pathway.
Figure 6.
Algorithm for the management of IOP elevation by retinal specialists: pre- (top panels) and post-corticosteroid injection (bottom panels).
Note: Adapted from Goni et al.93
Safety Considerations
1. Ocular hypertension (OHT) and glaucoma are not the same: Most of the time glaucoma will show as an increased “cupping” of the optic disc with a peripapillary nerve fiber layer thickness reduction by OCT, whereas OHT will appear as a healthy-looking optic disc with peripapillary RNFL thickness between normal limits.
Glaucoma is diagnosed based on the presence of a characteristic optic neuropathy (focal or generalized thinning of the neuroretinal rim with excavation and enlargement of the optic cup) with a corresponding visual field defect without regard of the level of IOP. Patients with elevated IOP in the absence of optic nerve damage are considered ocular hypertensives. It is known that between 30–50% of all subjects diagnosed with glaucoma do not have high IOP at diagnosis.83
However, IOP has been shown to be the most important risk factor for the disease, at all levels of pressure, all ages, and all stages of the neuropathy.84–86
In recent years, spectral domain optical coherence tomography (SD-OCT); especially peripapillary retinal nerve layer (pRNFL)-analysis is playing an increasing role in glaucoma diagnosis, discriminating very well between healthy and glaucomatous eyes.87
2. OCT to rule out glaucoma: Perform a peripapillary RNFL OCT at baseline; these should be repeated for an IOP increase of >21 mmHg, and suspected or diagnosed glaucoma, particularly with a family history of glaucoma. Consider visual field testing in high-risk patients or in cases of doubt.
3. Effect of corticosteroids on IOP: Corticosteroids induce changes in the trabecular extracellular matrix, which lead to decreased outflow facility. Elevated IOP usually develops 2–6 weeks after initiating therapy, but may occur at any time.88 Approximately 30% of healthy patients are corticosteroid-responders, but these percentages are different (11–79%), depending on several factors, such as dosage, chemical structure, time duration, administration route and individual susceptibility.89,90 There are several known risk factors that predispose to corticosteroid-induced IOP elevation: primary open angle glaucoma (with as much as 97% of patients responding), family history of glaucoma, diabetes, myopia, rheumatoid arthritis, uveitis, children, and elderly patients.89
4. Not all the corticosteroids are equal: Intravitreal dexamethasone is a free-floating biodegradable implant that consists of dexamethasone embedded in a degradable polymer, which results in a gradual release of medication for not more than 6 months. In a real-world setting, the cumulative probability of having an IOP ≥21 mmHg was 60% at 24 months after injection with intravitreal dexamethasone.95 At 24 months, 54% of the eyes required IOP-lowering topical treatment in the real-world study (n = 353).95 Glaucoma surgery was performed in 0.9% of eyes (4/429) in real-life.95
The FAc implant is a non-biodegradable intravitreal implant of 0.19 mg of FAc that releases the medication at a rate of 0.2 µg/day over 36 months. In the real-world IRISS, the mean IOP in the entire cohort remained in the normal range throughout the 24-month follow-up period, and the majority of DME patients (76.7%) did not require IOP-lowering therapy following treatment with the FAc implant.38 In IRISS, 23.3% of patients required IOP-lowering medication compared with 13.9% of patients in the Medisoft audit also at 24-month follow-up.38,39 The low proportion in the latter likely reflects clinical practice in the UK, as all Medisoft sites were UK-based.39
If an IOP-related event is not experienced with a previous dexamethasone implant, the risk that it will occur with the FAc implant may be reduced. This conclusion is based on a real-life study showing no adverse events in a subgroup of DME patients where a prior corticosteroid had been given and where there had been no prior IOP events.39
If there is an increase of IOP with short-acting intravitreal corticosteroid injections, and if IOP required at least two IOP-lowering medications to be under control, an increased risk for IOP-lowering surgery should be anticipated; therefore, a close follow-up with a glaucoma specialist is strongly recommended. This conclusion is based on a real-life study by Pessoa et al. (2018),96 where, in a retrospective analysis, all of the patients who needed surgery for OHT (3/28 patients; 11%) were on at least two IOP-lowering medications after short-acting intravitreal corticosteroid injection.92
5. Practical considerations to prevent/treat IOP elevation: if the increase in IOP during the pre-injection phase (see top panels in Figure 6), consider including RNFL OCT in the protocol for all patients at baseline and look to identify high-risk patients by closely following-up the patient.
Once the corticosteroid has been administered include the following: (a) the IOP needs to be checked at every appointment (every 3 months) and no extra tests are needed if IOP <21 mmHg; (b) if IOP is between 22 and 25 mmHg in a healthy eye (normal nerve, normal OCT RNFL), treatment is not needed, but check IOP and new OCT RNFL at 6 weeks (consider performing visual field) and if it is normal then control using standard follow-up (including OCT RNFL every 6 months); (c) if IOP is >25 mmHg in a healthy eye (normal nerve, normal OCT RNFL), start treatment with topical hypotensive drops (ideally avoiding prostaglandin analogues if possible) and perform an OCT RNFL and a visual field test. These recommendations are based on the management of IOP as proposed by Goñi et al. (2016).93 Further, if IOP is still high with two drugs or there are changes in the OCT RNFL or visual field, refer the patient to a glaucoma specialist. Finally, in eyes with controlled prior glaucoma, individualize action protocol with a glaucoma specialist.
Supplemental Treatments Following Treatment with the FAc Implant
Regarding additional treatments, the current literature the largest datasets (Table 2) show that around 30% of DME patients treated with the FAc implant need additional therapy.38,39,94 The IRISS study reported the mean time to supplemental treatment was 356.1 ± 274.8 days and that the majority (22.4%) of eyes received supplement anti-VEGF therapy.38 It is recommended that if a recurrence of DME is detected at any point during the follow-up, it is recommended to wait and observe the patient for up to 6 months, depending on the severity of the edema, to see if it has resolved. If not, a combination therapy with anti-VEGF, laser photocoagulation or an additional intravitreal injections of corticosteroid should be considered, depending on the previous response to each treatment.
Other real-world studies75–79 have reported 3-year outcomes and it is estimated from Table 2, that roughly 48.2% (range, 30.0% to 83.3%) of patient eyes will require supplemental treatments. On average, 7.9 (range, 5.0 to 13.1) treatments may be required with a mean time to administrated being around 16.0 (range, 12.8 to 21.8) months.
Conclusions
There is a gap between clinical decisions based on real medical needs and drug labeling or public health agency requirements. Based on the authors’ real-life experiences managing DME with the FAc implant in clinical practice, a consensus of clinical-decision criteria to help physicians identify recurrent DME patients has been proposed.
Overall, patients eligible for FAc implant treatment should be identified as those DME patients that exhibit an anatomical resolution of edema after one or two dexamethasone implants, but show edema recurrence, with intraretinal and/or subretinal fluid after 3–4 months following the injection. It is agreed that the switch to the FAc implant must be delayed no more than twelve months after the start of the short-acting corticosteroid therapy. It is consented that, during the treatment follow-up, OCT and BCVA tests must be performed every 3 months. In addition, a safety protocol adapted to the long-lasting treatment is proposed.
It is important to highlight that the current real-world reactive treatment approach is not only influenced by physician treatment decisions but also by hospital budgets for intravitreal injections, ie, ophthalmology service saturation, as well as low patient adherence to intravitreal treatment.
In the authors’ own clinical experience, it is observed that DME with a pattern of >2 edema recurrence events during the first year is repetitive and replicated during the following years. Thus, as discussed above, many patients could benefit from the FAc implant, a continuous daily micro-dose treatment that controls DME for up to 36 months with a single injection.
Acknowledgments
Editorial assistance was provided by Dr Klara Belzar (PhD), Prescript Communications Ltd., UK.
Disclosure
Alfredo Adán declares financial support from Allergan, Bayer, Brill Pharma, Alimera and Novartis; Francisco Cabrera and Marta S. Figueroa declare financial support from Alcon, Allergan, Bayer, Brill Pharma, Novartis, and Roche; Patricia Udaondo declares financial support from Alimera, Allergan, Bayer, Bausch and Lomb, Brill Pharma, Graybug, Novartis, Roche; Maximino Abraldes declares financial support from Allergan, Bayer, Brill Pharma, Boehringer Ingelheim, Novartis, Ophthotech and Roche; Miguel Ángel Reyes declares financial support from Alcon, Bayer, Novartis and Brill Pharma; Marta Pazos declares financial support from Allergan, Santen, Brill Pharma, VISUfarma, and Zeiss, including non-financial support from Heidelberg Engineering; and Félix Armadá declares financial support from Bayer, Novartis, Alcon, Medical Mix, Zeiss, Allergan, Bausch & Lomb and Dorc. The authors report no other conflicts of interest in this work.
References
- 1.Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222. doi: 10.1159/000458539 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 3.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. doi: 10.1016/j.ophtha.2010.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. JAMA Ophthalmol. 2012;130(8):972–979. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase iii trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–2022. doi: 10.1016/j.ophtha.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 6.Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti–vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retina. 2018;2(12):1179–1187. doi: 10.1016/j.oret.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 7.Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi: 10.1016/j.ophtha.2012.04.030 [DOI] [PubMed] [Google Scholar]
- 8.Escobar-Barranco JJ, Pina-Marin B, Fernandez-Bonet M. Dexamethasone implants in patients with naive or refractory diffuse diabetic macular edema. Ophthalmologica. 2015;233(3–4):176–185. doi: 10.1159/000371770 [DOI] [PubMed] [Google Scholar]
- 9.Menezo M, Roca M, Menezo V, Pascual I. Intravitreal dexamethasone implant Ozurdex in the treatment of diabetic macular edema in patients not previously treated with any intravitreal drug: a prospective 12-month follow-up study. Curr Med Res Opin. 2019;35:2111–2116. [DOI] [PubMed] [Google Scholar]
- 10.Chang-Lin JE, Burke JA, Peng Q, et al. Pharmacokinetics of a sustained-release dexamethasone intravitreal implant in vitrectomized and nonvitrectomized eyes. Invest Ophthalmol Vis Sci. 2011;52(7):4605–4609. doi: 10.1167/iovs.10-6387 [DOI] [PubMed] [Google Scholar]
- 11.Boyer DS, Yoon YH, Belfort R, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 12.Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120(3):583–587. doi: 10.1016/j.ophtha.2012.09.014 [DOI] [PubMed] [Google Scholar]
- 13.Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: a systematic review of real-world studies. J Pharmacol Sci. 2018;138(4):219–232. doi: 10.1016/j.jphs.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 14.Raman V. A cost analysis comparing continued 3-year aflibercept monotherapy versus a switch from aflibercept to the fluocinolone acetonide intravitreal implant in phakic patients with chronic diabetic macular edema. Expert Rev Ophthalmol. 2018;13(5):299–307. doi: 10.1080/17469899.2018.1523720 [DOI] [Google Scholar]
- 15.Quhill F, Beiderbeck A. Cost advantage of fluocinolone acetonide implant (ILUVIEN®) versus ranibizumab in the treatment of chronic diabetic macular oedema. Global Regional Health Technol Assess. 2017;4(1):grhta.5000268. doi: 10.5301/grhta.5000268 [DOI] [Google Scholar]
- 16.American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes. Diabetes Care. 2019;42(Supplement 1):S13–S28. doi: 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 17.Rask-Madsen C, King G. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17(1):20–33. doi: 10.1016/j.cmet.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456–480. doi: 10.4239/wjd.v6.i3.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 2012;47(4):171–188. doi: 10.1159/000329603 [DOI] [PubMed] [Google Scholar]
- 20.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 21.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17. doi: 10.1186/s40662-015-0026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan GS, Cheung N, Simó R, Cheung GCM, Wong TY. Diabetic macular oedema. Lancet Diabetes Endocrinol. 2017;5(2):143–155. doi: 10.1016/S2213-8587(16)30052-3 [DOI] [PubMed] [Google Scholar]
- 23.Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334–1340. doi: 10.1001/jamaophthalmol.2014.2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varma R, Choudhury F, Klein R, Chung J, Torres M, Azen SP. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):752–761. doi: 10.1016/j.ajo.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emanuele N, Moritz T, Klein R, et al. Ethnicity, race, and clinically significant macular edema in the Veterans Affairs Diabetes Trial (VADT). Diabetes Res Clin Pract. 2009;86(2):104–110. doi: 10.1016/j.diabres.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 27.Rubsam A, Parikh S, Fort PE. Role of inflammation in diabetic retinopathy. Int J Mol Sci. 2018;19(4). doi: 10.3390/ijms19040942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86(4):363–365. doi: 10.1136/bjo.86.4.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva PS, Sun JK, Aiello LP. Role of steroids in the management of diabetic macular edema and proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24(2):93–99. doi: 10.1080/08820530902800355 [DOI] [PubMed] [Google Scholar]
- 31.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116(1):73–79. doi: 10.1016/j.ophtha.2008.09.037 [DOI] [PubMed] [Google Scholar]
- 32.Patel JI, Hykin PG, Gregor ZJ, Boulton M, Cree I. Angiopoietin concentrations in diabetic retinopathy. Br J Ophthalmol. 2005;89(4):480–483. doi: 10.1136/bjo.2004.049940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734–1746. [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez-Ulla F, Marticorena J, Alfaro DV, Fernandez M, Mendez ER, Rothen M. Intravitreal triamcinolone for the treatment of diabetic macular edema. Curr Diabetes Rev. 2006;2(1):99–112. doi: 10.2174/157339906775473572 [DOI] [PubMed] [Google Scholar]
- 35.Sonoda Y, Arimura N, Shimura M, Sakamoto T. Early change of central macular thickness after intravitreous triamcinolone or bevacizumab in diabetic macular edema or retinal vein occlusion. Retina. 2011;31(2):290–297. doi: 10.1097/IAE.0b013e3181eef070 [DOI] [PubMed] [Google Scholar]
- 36.Nauck M, Karakiulakis G, Perruchoud AP, Papakonstantinou E, Roth M. Corticosteroids inhibit the expression of the vascular endothelial growth factor gene in human vascular smooth muscle cells. Eur J Pharmacol. 1998;341(2–3):309–315. doi: 10.1016/S0014-2999(97)01464-7 [DOI] [PubMed] [Google Scholar]
- 37.Eichenbaum DA, Buznego C, Weng CY, Dhoot DS, Wykoff CC, Sheth VS. When and how to incorporate steroids for persistent diabetic macular edema: a discussion of real-world treatment optimization strategies. Ophthalmic Surg Lasers Imaging Retina. 2018;49(7):S5–s15. doi: 10.3928/23258160-20180621-01 [DOI] [PubMed] [Google Scholar]
- 38.Chakravarthy U, Taylor SR, Koch FHJ, Castro de Sousa JP, Bailey C. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol. 2019;103:1072–1077. doi: 10.1136/bjophthalmol-2018-312284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J. Real-world experience with 0.2 μg/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye. 2017;31(12):1707–1715. doi: 10.1038/eye.2017.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun JK, Radwan SH, Soliman AZ, et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015;64(7):2560–2570. doi: 10.2337/db14-0782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joltikov KA, Sesi CA, de Castro VM, et al. Disorganization of retinal inner layers (DRIL) and neuroretinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59(13):5481–5486. doi: 10.1167/iovs.18-24955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Querques G, Darvizeh F, Querques L, Capuano V, Bandello F, Souied EH. Assessment of the real-life usage of intravitreal dexamethasone implant in the treatment of chronic diabetic macular edema in France. J Ocul Pharmacol Ther. 2016;32(6):383–389. doi: 10.1089/jop.2016.0010 [DOI] [PubMed] [Google Scholar]
- 43.Singer MA, Dugel PU, Fine HF, Capone CA, Maltman J. Real-world assessment of dexamethasone intravitreal implant in dme: findings of the prospective, multicenter REINFORCE study. Ophthalmic Surg Lasers Imaging Retina. 2018;49(6):425–435. doi: 10.3928/23258160-20180601-07 [DOI] [PubMed] [Google Scholar]
- 44.Danis RP, Sadda S, Li X-Y, Cui H, Hashad Y, Whitcup SM. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year phase III trials. Br J Ophthalmol. 2016;100(6):796–801. doi: 10.1136/bjophthalmol-2015-306823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatz K, Ebneter A, Tuerksever C, Pruente C, Zinkernagel M. Repeated dexamethasone intravitreal implant for the treatment of diabetic macular oedema unresponsive to anti-VEGF therapy: outcome and predictive SD-OCT features. Ophthalmologica. 2018;239(4):205–214. doi: 10.1159/000485852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanzetta P, Loewenstein A. Vision academy steering committee. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255(7):1259–1273. doi: 10.1007/s00417-017-3647-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holz FG, Amoaku W, Donate J, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118(4):663–671. doi: 10.1016/j.ophtha.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 48.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol. 2009;148(1):43–58.e41. doi: 10.1016/j.ajo.2009.01.024 [DOI] [PubMed] [Google Scholar]
- 49.Ehlken C, Helms M, Böhringer D, Agostini HT, Stahl A. Association of treatment adherence with real-life VA outcomes in AMD, DME, and BRVO patients. Clin Ophthalmol. 2017;12:13–20. doi: 10.2147/OPTH.S151611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss M, Sim DA, Herold T, et al. Compliance and adherence of patients with diabetic macular edema to intravitrael anti-vascular endothelial growth factor therapy in daily practice. Retina. 2018;38(12):2293–2300. doi: 10.1097/IAE.0000000000001892 [DOI] [PubMed] [Google Scholar]
- 51.Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol. 2016;10:939–946. doi: 10.2147/OPTH.S100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mourtzoukos S. The treatment of diabetic macular oedema (DMO) in UK real-life clinical practice with ILUVIEN® (fluocinolone acetonide) – its impact on current clinical practice. Expert Rev Ophthalmol. 2017;12(2):95–97. doi: 10.1080/17469899.2017.1285698 [DOI] [Google Scholar]
- 53.García Layana A, Adán A, Ascaso FJ, et al. Use of intravitreal dexamethasone implants in the treatment of diabetic macular edema: expert recommendations using a Delphi approach. Eur Jl Ophthalmol. 2019:1120672119861623. [DOI] [PubMed] [Google Scholar]
- 54.Holekamp NM, Campbell J, Almony A, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83–91. doi: 10.1016/j.ajo.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 55.Urbancic M, Gardasevic Topcic I. Dexamethasone implant in the management of diabetic macular edema from clinician’s perspective. Clin Ophthalmol. 2019;13:829–840. doi: 10.2147/OPTH.S206769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lam WC, Albiani DA, Yoganathan P, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:1255–1268. doi: 10.2147/OPTH.S80500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.OZURDEX® Spanish Summary of Product Characteristics. Available from: https://cima.aemps.es/cima/publico/detalle.html?nregistro=10638001. Accessed July 2, 2020.
- 58.Nentwich MM, Ulbig MW. The therapeutic potential of intraocular depot steroid systems: developments aimed at prolonging duration of efficacy. Dtsch Arztebl Int. 2012;109(37):584–590. doi: 10.3238/arztebl.2012.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang Y, Bailey C, Loewenstein A, Massin P. Intravitreal corticosteroids in diabetic macular edema: pharmacokinetic considerations. Retina. 2015;35(12):2440–2449. doi: 10.1097/IAE.0000000000000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zarranz-Ventura J, Escobar Barranco JJ, Marin P. Corticosteroids for diabetic macular edema. Ann D’oftalmologia. 2016;24(4). [Google Scholar]
- 61.Kuppermann BD, Chou C, Weinberg DV, Whitcup SM, Haller JA, Blumenkranz MS. Intravitreous dexamethasone effects on different patterns of diabetic macular edema. Arch Ophthalmol. 2010;128(5):642–643. doi: 10.1001/archophthalmol.2010.44 [DOI] [PubMed] [Google Scholar]
- 62.Whitcup SM, Cidlowski JA, Csaky KG, Ambati J. Pharmacology of corticosteroids for diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59(1):1–12. doi: 10.1167/iovs.17-22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz SG, Scott IU, Stewart MW, Flynn Jr. HW. Update on corticosteroids for diabetic macular edema. Clin Ophthalmol. 2016;10:1723–1730. doi: 10.2147/OPTH.S115546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eichenbaum DA. Fluocinolone acetonide (FAc) 0.19 mg: pharmacokinetics and clinical relevance (Poster B0343). Paper presented at: ARVO; May 7–11; 2017; Baltimore, MD, US. [Google Scholar]
- 65.Kane FE, Green KE, Weissman A. A comparison of in vitro receptor binding of glucocorticoids. Invest Ophthalmol Vis Sci. 2007;48(13):106. [Google Scholar]
- 66.Kane FE, Burdan J, Cutino A, Green KE. Iluvien: a new sustained delivery technology for posterior eye disease. Expert Opin Drug Deliv. 2008;5(9):1039–1046. doi: 10.1517/17425247.5.9.1039 [DOI] [PubMed] [Google Scholar]
- 67.O’Neil AJ. The Merck Index. 13th ed. 2001:518. [Google Scholar]
- 68.Florey K. Analytical Profiles of Drug Substances. Vol. 11 Academic Press; 1982:620. [Google Scholar]
- 69.Osol A, Hoover JE. Remington’s Pharmaceutical Sciences. 15th ed. Easton, Penn: Mack Publishing Co.; 1975:892. [Google Scholar]
- 70.ILUVIEN® Spanish Summary of Product Characteristics. Available from: https://cima.aemps.es/cima/publico/detalle.html?nregistro=76832. Accessed July 2, 2020.
- 71.Jaffe GJ. Sustained drug-delivery for retinal disease: current technologies include implanted and injected devices. Retina Today. 2010;59–61. [Google Scholar]
- 72.Campochiaro PA, Hafiz G, Shah SM, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010;117(7):1393–1399.e1393. doi: 10.1016/j.ophtha.2009.11.024 [DOI] [PubMed] [Google Scholar]
- 73.Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–635.e622. doi: 10.1016/j.ophtha.2010.12.028 [DOI] [PubMed] [Google Scholar]
- 74.Yang Y, Bailey C, Holz FG, et al. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye. 2015;29(9):1173–1180. doi: 10.1038/eye.2015.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cunha-Vaz J, Ashton P, Iezzi R, et al. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology. 2014;121(10):1892–1903.e1893. doi: 10.1016/j.ophtha.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 76.Panos GD, Arruti N, Patra S. The long-term efficacy and safety of fluocinolone acetonide intravitreal implant 190 μg (ILUVIEN®) in diabetic macular oedema in a multi-ethnic inner-city population. 2020. Eur J Ophthalmol. 1120672119898414. [DOI] [PubMed] [Google Scholar]
- 77.Rehak M, Busch C, Unterlauft JD, Wiedemann P. Outcomes in diabetic macular edema switched directly or after a dexamethasone implant to a fluocinolone acetonide intravitreal implant following anti-VEGF treatment. Acta Diabetol. 2020;57:469–478. doi: 10.1007/s00592-019-01439-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Young JF, Walkden A, Stone A, Mahmood S. Clinical effectiveness of intravitreal fluocinolone acetonide (FAc) (ILUVIEN™) in patients with diabetic macular oedema (DMO) refractory to prior therapy: the manchester experience. Ophthalmol Ther. 2019;8(3):477–484. doi: 10.1007/s40123-019-0197-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fusi-Rubiano W, Mukherjee C, Lane M, et al. Treating Diabetic Macular Oedema (DMO): real world UK clinical outcomes for the 0.19mg Fluocinolone Acetonide intravitreal implant (Iluvien™) at 2 years. BMC Ophthalmol 2018;18:62. doi: 10.1186/s12886-018-0726-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hall J. ILUVIEN in diabetic macular edema: the choice of second-line corticosteroid should be left to the clinical judgement of the treating physician. Ophthalmologica. 2018;239(4):234–237. [DOI] [PubMed] [Google Scholar]
- 81.Coelho J, Malheiro L, Melo Beirão J, Meireles A, Pessoa B. Real-world retrospective comparison of 0.19 mg fluocinolone acetonide and 0.7 mg dexamethasone intravitreal implants for the treatment of diabetic macular edema in vitrectomized eyes. Clin Ophthalmol. 2019;13:1751–1759. doi: 10.2147/OPTH.S201611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaz-Pereira S, Castro-de-Sousa JP, Martins D, et al. The outcomes of switching from short- to long term intravitreal corticosteroid implant therapy in patients with diabetic macular edema. Ophthalmic Res. 2020;63(2):114–121. doi: 10.1159/000503036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111(9):1641–1648. doi: 10.1016/j.ophtha.2004.03.029 [DOI] [PubMed] [Google Scholar]
- 84.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713; discussion 829–730. doi: 10.1001/archopht.120.6.701 [DOI] [PubMed] [Google Scholar]
- 85.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative normal-tension glaucoma study group. Am J Ophthalmol. 1998;126(4):487–497. doi: 10.1016/S0002-9394(98)00223-2 [DOI] [PubMed] [Google Scholar]
- 86.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268 [DOI] [PubMed] [Google Scholar]
- 87.Mwanza JC, Oakley JD, Budenz DL, Anderson DR. Ability of cirrus HD-OCT optic nerve head parameters to discriminate normal from glaucomatous eyes. Ophthalmology. 2011;118(2):241–248.e241. doi: 10.1016/j.ophtha.2010.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones R, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17(2):163–167. doi: 10.1097/01.icu.0000193079.55240.18 [DOI] [PubMed] [Google Scholar]
- 89.Kiddee W, Trope GE, Sheng L, et al. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Surv Ophthalmol. 2013;58(4):291–310. doi: 10.1016/j.survophthal.2012.08.003 [DOI] [PubMed] [Google Scholar]
- 90.Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics. II. The effect of dexamethasone on the glaucomatous eye. Arch Ophthalmol. 1963;70:492–499. doi: 10.1001/archopht.1963.00960050494011 [DOI] [PubMed] [Google Scholar]
- 91.Beck RW, Edwards AR, Aiello LP, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127(3):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pessoa B. Intraocular pressure after 0.19 mg fluocinolone acetonide implant in vitrectomized and non-vitrectomized eyes with diabetic macular edema. Presented at the European VitreoRetinal Society (EVRS) Congress; 2019; Lisbon, Spain. [Google Scholar]
- 93.Goni FJ, Stalmans I, Denis P, et al. Elevated intraocular pressure after intravitreal steroid injection in diabetic macular edema: monitoring and management. Ophthalmol Ther. 2016;5(1):47–61. doi: 10.1007/s40123-016-0052-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Augustin AJ, Bopp S, Fechner M, et al. Three-year results from the Retro-IDEAL study: real-world data from diabetic macular edema (DME) patients treated with ILUVIEN® (0.19 mg fluocinolone acetonide implant). Eur J Ophthalmol. 2020;30:382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zarranz-Ventura J, Sala-Puigdollers A, Velazquez-Villoria D, et al. Long-term probability of intraocular pressure elevation with the intravitreal dexamethasone implant in the real-world. PLoS One. 2019;14(1):e0209997. doi: 10.1371/journal.pone.0209997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pessoa B, Coelho J, Correia N, Ferreira N, Beirao M, Meireles A. Fluocinolone acetonide intravitreal implant 190 μg (ILUVIEN®) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthalmic Res. 2018;59(2):68–75. doi: 10.1159/000484091 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- ILUVIEN® Spanish Summary of Product Characteristics. Available from: https://cima.aemps.es/cima/publico/detalle.html?nregistro=76832. Accessed July 2, 2020.