Abstract
Introduction
Green pit vipers (GPV) are widely distributed throughout Thailand and are responsible for significant morbidity. The primary objective of this study was to characterize clinical presentations and treatment methods for GPV bites. The secondary objective was to demonstrate the earliest and latest onset of hematotoxicity.
Methods
GPV bites reported to the Ramathibodi Poison Center between July 1, 2016, and June 30, 2018, were analyzed.
Results
There were 288 GPV cases within the study period. Patients were predominantly male (62.8%), and the median age was 40 years (interquartile range (IQR) 22.8–58). Median time from envenomation to hospital presentation was 1 hour (IQR 0.5–2). Patients were primarily bitten on the finger (27.4%). Most patients reported swelling (90.3%). Necrosis and compartment syndrome occurred in 13 and 9 cases, respectively. Systemic effects occurred in 190 cases (65.9%), with median onset 15 hours (IQR 6–28.3) post-bite. Venous clotting time (VCT) showed the highest percentage of abnormalities. Systemic bleeding occurred in 13 cases (4.5%). Monitoring patients for 24, 48, and 72 hours after bites detected 62.7%, 85.9%, and 96.5% of cases with systemic effects, respectively. In total, 184 patients (62.5%) were treated, sometimes repeatedly, with antivenoms (285 courses, 949 vials). The most common indication was prolonged VCT (144 courses, 50.5%). Recurrent systemic effects after antivenom occurred in 11 cases (6.1% of patients received antivenom). No recurrence presented as systemic bleeding. Adverse reactions to antivenom were reported in 44 courses (15.4% of 285 courses), being anaphylaxis in 19 courses (6.7%). Other treatments included antibiotics (192 cases, 66.7%), surgical intervention (10, 34.7%), and blood components (4, 1.4%).
Conclusion
Most GPV bites result in envenomation. The most frequent local effect is mild swelling. Systemic bleeding is uncommon. The current recommendation of a 3-day follow-up can detect up to 96% of patients who may require antivenom. No severe morbidity or mortality is reported. Antivenoms are primarily indicated by prolonged VCT. Side effects of antivenom are minimal.
Keywords: green pit viper, Trimeresurus, presentation, manifestations, management
Introduction
Green pit vipers (GPV), Trimeresurus or Cryptelytrops species, which inflict injuries by infusing venom through front fangs, are widely distributed hematotoxic snakes that are responsible for most snake bites in Thailand.1 The venoms contain mostly enzymatic and non-enzymatic proteins that cause local and systemic effects.2,3 The usual local symptom is regional edema. Severe complications such as skin necrosis or digital gangrene are rare.4 Systemic effects are primarily hematotoxicity characterized by thrombocytopenia and mixed coagulopathy involving thrombin-like effects, hyperfibrinolysis,5 and elevated plasminogen activator activity.6 However, systemic bleeding occurs only in a minority of patients owing to the weak effects of the venom.7 Although mortality from GPV is uncommon,8 a Trimeresurus bite is considered a regional concern and is categorized as of high medical significance in Southeast Asia by the World Health Organization.9
The current treatment of GPV bites focuses mainly on timely antivenom administration10 together with appropriate antibiotics and surgical management. We use horse-derived F(ab′)2 GPV antivenom from Queen Saovabha Memorial Institute of the Thai Red Cross Society. Monovalent GPV antivenom was produced against T.albolabris, while polyvalent hematotoxin antivenom was produced against T.albolabris, Calloselasma rhodostoma, and Daboia russelli siamensis. Hematotoxicity warrants antivenom administration. The main challenges in clinical practice are the appropriate method and time of diagnosis as well as duration of follow-up, which varies extensively despite the current recommendation of at least 72 hours.7,10 Because the previous literature on clinical effects of GPV emanated from Bangkok, where Trimeresurus albolabris (white-lipped green pit viper) and Trimeresurus macrops (dark-green pit viper) dominated,7 our poison center database has established a bigger picture of GPV envenomation in Thailand, including more diverse Trimeresurus species11 such as T. purpureomaculatus (shore pit viper), T. wagleri (Wagler’s pit viper), and T. kanburiensis (Kanburi pit viper), and patient population, by retrieving cases reported to the Ramathibodi Poison Center (RPC).
The primary objective of this study was to characterize clinical presentations and treatment methods for GPV bites including antivenom, antibiotics, and surgical management. The secondary objective was to demonstrate the earliest and latest onset of hematotoxicity.
Methods
Data Source and Study Design
This is a retrospective study of cases of GPV bites across Thailand reported to the RPC during the period July 1, 2016 to June 30, 2018. The RPC provides information and evidence-based management advice about poisoning and envenomation through a 24-hour telephone service. The patient’s follow-up was done by calling the hospital where the patient was currently treated. The call was ensured until the patient’s discharge or significant clinical improvement. The current practice and recommendation are, regardless of edema, repeating laboratory investigations every 6 hours for 24 hours, then every 12–24 hours until 72 hours after the bite. This can be adjusted according to the patient’s clinical and coagulation status. For cases with antivenom allergy, we recommend withholding the antivenom, symptomatic treatment, premedication if antivenom is still indicated and reinstitution of antivenoms with a slower rate after symptoms subside. The treatment was based on clinical evaluation and decisions made by primary doctors in charge of the patients.
Population and Selection Criteria
We included all patients with suspected or confirmed GPV bites who were reported to the RPC. GPV was identified by snakes brought to the hospital, patients’ recognition of the snake, or unknown snakebite responsive to GPV antivenoms. Photos of snakes were confirmed by the RPC if requested by the primary health care provider. The exclusion criterion was information-related calls without the presence of GPV-bitten patients. The data extracted included age, gender, pregnancy status, region, medical history, bite site(s) and duration of exposure, time taken to reach hospital, clinical effects, laboratory results, clinical courses in terms of recovery and recurrence, treatment and adverse reactions, follow-up details, and clinical outcome.
Definitions
Dry bite was defined as a bite without local or systemic effects.9
Degree of swelling was defined according to the highest level of tissue swelling: 0, no edema; 1, local edema; 2, up to one articulation; 3, more than one articulation; 4, up to two articulations; 5, more than two articulations; 6, up to three articulations of the body.7 In our study, the degree was also defined as no swelling (grade 0), mild/local (grade 1–2), moderate/regional (grade 3–4), and severe/beyond regional (grade 5–6).
Hematotoxicity or systemic effects were defined as whole blood clotting time (WBCT) of more than 20 minutes, venous clotting time (VCT) of more than 20 minutes, international normalized ratio (INR) >1.2, platelet count of less than 50,000/µL, and systemic bleeding. These systemic effects are also the current indications for antivenom administration.10
Appropriate antivenom administration was defined as administration with indications based on the current criteria for hematotoxic snake bites in Thailand (mentioned above), appropriate dose (3–5 vials/course), duration (30–60 minutes), and interval (≥6 hours) according to the current guidelines.10
Anaphylaxis or early anaphylactic reactions from venom and antivenom were referenced from the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network.12
Recurrence was defined as evidence of systemic effects that reverted to normal and then recurred.13
Recovery was defined as evidence of systemic effects that returned to normal by the time of the last follow-up.
Time of onset, recurrence, and recovery referenced the time interval from being bitten to the studied outcome.
Statistical Analysis
Continuous data were presented as median, interquartile range (IQR), and range. Categorical data were presented as frequency and percentage. Ethical approval for the study was obtained from the Institutional Ethics Committee Board of Ramathibodi Hospital Faculty of Medicine, Mahidol University. The Ethics Approval Reference Number is MURA 2018/917.
Results
Among 1591 snake-bite inquiries, 291 cases were registered as GPV. Three cases were excluded because of the absence of envenomation (ie, asking about indications for antivenom, asking about effects after sucking the wound or external contact with snakes), leaving 288 cases for inclusion in the analysis.
General Characteristics of Patients and Their Exposure
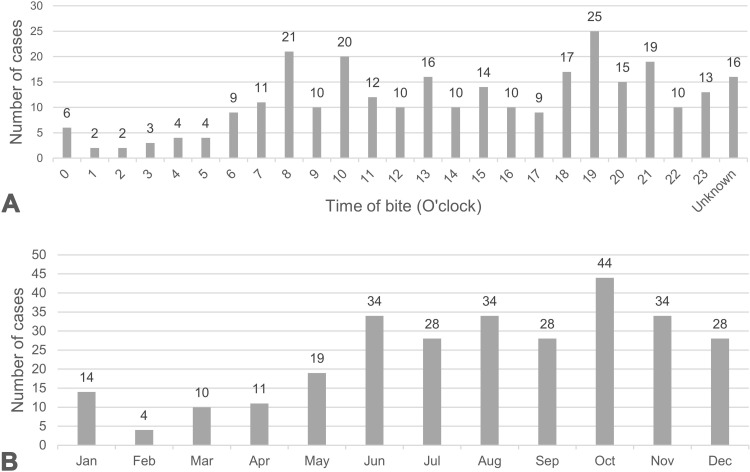
Characteristics of GPV bite cases are listed in Table 1. One hundred eighty-one patients (62.8%) were male. Two females were pregnant. The median age was 40 years (IQR 22.8–58, range 1–97). Fifty patients (17.4%) were children younger than 15 years. The most common time of day when patients were bitten was the evening (around 7 PM). Bites were most frequently reported from the northeastern region of Thailand. Figure 1A and B 1 describe the number of GPV-bitten patients reported each month and the time of day. The median time after being bitten to arrival at the health care facility was 1 hour (IQR 0.5–2, range 0.2–72). The most delayed presentation was 3 days, in two patients. One patient presented with worsening pain and the other with enlarging hematoma at the bite site.
Table 1.
Characteristics of GPV Bite Cases
| Characteristics | No. of Cases (% of Total 288 Cases) |
|---|---|
| Gender | |
| Male | 181 (62.8) |
| Female | 107 (37.2) |
| Age in years, median (IQR, range) | 40 (22.8–58, 1–97) |
| Region | |
| Northeast | 86 (29.9) |
| Central | 73 (25.3) |
| Bangkok | 59 (20.5) |
| North | 24 (8.3) |
| South | 18 (6.3) |
| West | 17 (5.9) |
| East | 11 (3.8) |
| Confirmation of GPV bite | |
| GPV or GPV carcass brought to the hospital | 162 (56.3) |
| Patient’s recognition of GPV | 102 (35.4) |
| Undetermined | 24 (8.3) |
Abbreviations: GPV, green pit viper; IQR, interquartile range.
Figure 1.
Numbers of cases of GPV bite reported each month (A) and the time of day (B).
Nine cases were confirmed to be Trimeresurus other than T. albolabris, including T. purpureomaculatus (shore pit viper) (3 cases), T. wagleri (Wagler’s pit viper) (2), T. kanburiensis (Kanburi pit viper) (2), T. macrops (large-eyed pit viper) (1), and T. venustus (beautiful pit viper) (1).
Clinical Effects
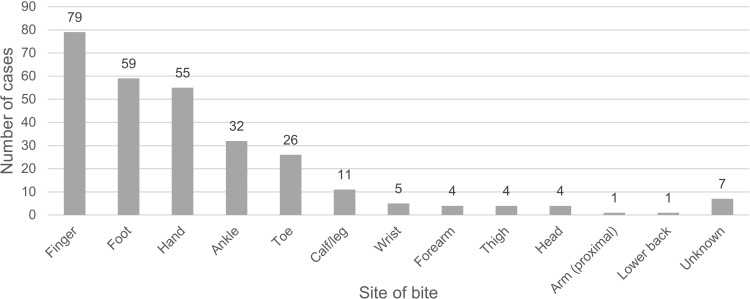
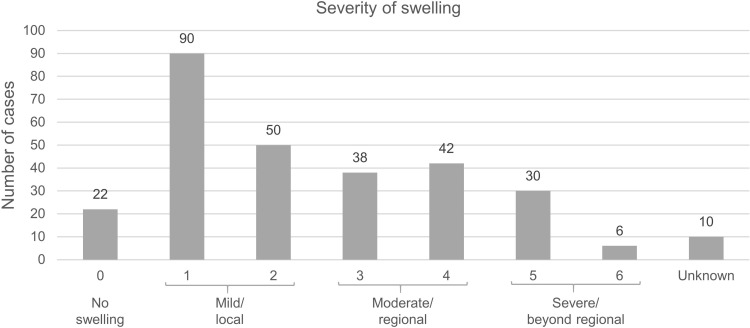
The most common bite site was the finger (79 cases, 27.4%). Frequencies of the bitten areas are shown in Figure 2. Fang marks were apparent in 188 cases (65.3%). Dry bites occurred in nine cases (3.1%). Two hundred sixty patients (90.3%) reported local effects. Most bites resulted in grade-1 swelling (90 cases, 31.2%) (Figure 3). Other local complications included pain (114, 39.6%), ecchymosis (50, 17.4%), blister (22, 7.6%), wound bleeding (16, 5.6%), wound infection (16, 5.6%), necrosis (13, 4.5%), compartment syndrome (9, 3.1%), and hematoma (5, 1.7%). One patient developed upper airway obstruction requiring intubation after being bitten on the forehead. Eighty-four patients (29.3%) reported local effects without any systemic effects.
Figure 2.
Frequency of each bite site.
Figure 3.
Frequency of local swelling severity.
Systemic effects occurred in 190 cases (65.9%). The number of cases with laboratory tests and cases with abnormal results are presented in Table 2. Platelet count and VCT were the two most performed tests. Systemic bleeding was reported in 13 cases (4.5%). Bleeding situations included hematemesis (fresh blood 3 cases, coffee ground 1 case), gross hematuria (3), bleeding from gums (2), melena (2), petechiae (2), hematochezia (2), bleeding from vagina (1), hemoptysis (1), and epistaxis (1). Three of these patients developed multiple sites of bleeding. Only 14 patients (4.9%) developed isolated systemic effects without local symptoms.
Table 2.
Number and Percentage of Cases with Abnormal Results for Each Lab Parameter, and Median Onset of Each Abnormal Test Result and Systemic Bleeding
| Parameters Tested (Number of Test Cases) | No. of Cases with Abnormal Results (%) | Time of Onset (h) (IQR, Range) |
|---|---|---|
| VCT (244) | 111 (45.5) | 13 (6–25, 0.2–120) |
| WBCT (157) | 69 (44.8) | 9 (2.5–21, 0.3–120) |
| INR (217) | 65 (30.0) | 19.5 (8–43, 0.5–120) |
| Platelets (280) | 43 (15.4) | 18.75 (10–37.5, 0.3–78) |
| Fibrinogen (10) | 3 (30) | 15 (10.5–46.5, 6–78) |
| Systemic bleeding (13) | 11 (2.3–37.5, 1–72) | |
| Overall onset of systemic effects | 15 (6–28.3, 0.2–120) | |
Abbreviations: IQR, interquartile range; INR, international normalized ratio; VCT, venous clotting time; WBCT, whole blood clotting time.
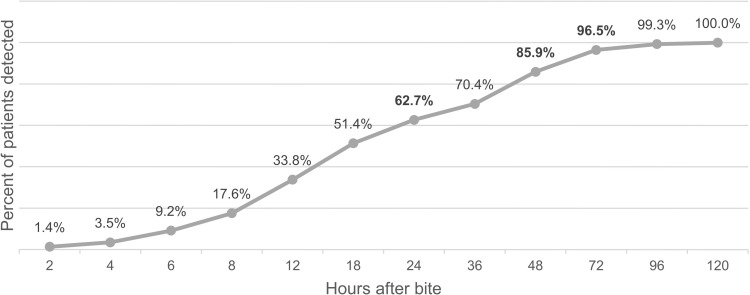
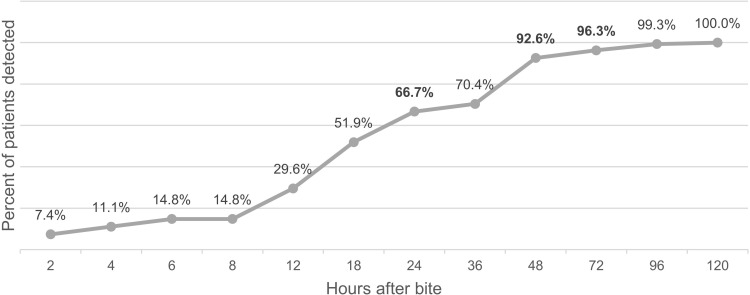
The median onset of each abnormal test result and systemic bleeding are shown in Table 2. The median onset of systemic effects was 15 hours (IQR 6–28.3, range 0.2–120). Figure 4 depicts the cumulative percentage of cases with systemic effect over time. Monitoring for 24, 48, and 72 hours after bites detected 62.7%, 85.9%, and 96.5% of the 190 cases with systemic effects, respectively.
Figure 4.
Cumulative percentage of cases detected at a specific time after bite.
Allergic reactions to venoms occurred in 10 patients (3.5%), all of whom developed systemic effects. The median onset was 1 hour (IQR 0.5–2.5, range 0.5–2.5). Symptoms included anaphylaxis (7 cases) and cardiovascular symptoms (3 cases). Other complications included secondary myocardial infarction (1 case).
Clinical characters of uncommon Trimeresurus spp. (other than T.albolabris) bite (9 cases) were generally milder. Dry bites occurred in 3 cases. Local symptoms occurred in 4 cases including mild swelling (4 cases) and ecchymosis (2 cases). Two patients developed systemic symptoms including prolonged VCT (1 case) and prolonged INR (1 case) and responded to monovalent GPV antivenoms (2 courses and 1 course, respectively). No allergic reactions to venoms and antivenoms were reported. All of them recovered fully before discharge.
Management
One hundred eighty patients (62.5%) received antivenoms. A total of 285 courses (949 vials) of antivenom were administered, including 255 courses (836 vials) of monovalent GPV antivenom and 30 courses (113 vials) of polyvalent hematotoxic snake antivenom. Median numbers of antivenom per case were one course (IQR 1–2, range 1–6) or three vials (IQR 3–6, range 2–19). Sixty-eight patients (23.6%) received more than one course of antivenom, as some of the courses resulted in insufficient recovery.
Two hundred fourteen courses of antivenom (75.1% of 285 courses) were deemed to be appropriate by meeting indications for antivenom. The most common indication of antivenom was prolonged VCT (144 courses, 50.5%). Sixty-nine courses of antivenom (23.9%) were given without indications. Antivenom indications and appropriateness of administration are presented in Table 3. A skin test, although not useful,9 was still performed in nine patients who received antivenom. Six of eight patients with a positive result (66.7%) and one with a negative result developed allergic symptoms.
Table 3.
Characteristics of Antivenom Administration
| Characteristics | No. of Cases (% of Total 180 Cases That Received AV) | No. of Courses (% of Total 285 Courses of AV) |
|---|---|---|
| Indication | ||
| VCT | 90 (50) | 144 (50.5) |
| WBCT | 55 (30.6) | 68 (23.9) |
| INR | 41 (22.8) | 46 (16.1) |
| Platelet | 39 (21.7) | 45 (15.8) |
| Systemic bleeding | 8 (4.4) | 9 (3.2) |
| Compartment syndrome, upper airway obstruction | 3 (1.7) | 3 (1.1) |
| Reasons for appropriateness | ||
| Appropriate dose | 178 (98.9) | 275 (96.5) |
| Appropriate duration and interval of administration | 148 (82.2) | 237 (83.2) |
| Slow administration due to allergic symptoms to AV | 12 (6.7) | 15 (5.3) |
Abbreviations: AV, antivenom; INR, international normalized ratio; VCT, venous clotting time; WBCT, whole blood clotting time.
Adverse reactions to antivenom were reported in 42 cases (23.3% of 180 patients received antivenom) and included 44 courses (15.4% of total 285 antivenom courses). Among these patients who developed allergy after the first course of antivenom, 23 cases required no further antivenom therapy, 17 cases were given subsequent courses of antivenom without allergic symptoms, and 2 cases developed allergic symptoms after subsequent courses of antivenom. Table 4 presents features of early reactions after administration of antivenoms and treatments. The median onset was 20 minutes (IQR 12.5–30, range 2–75) after administration. Anaphylaxis occurred in 19 courses (6.7%).
Table 4.
Features and Treatments of Early Reactions After Administration of Antivenoms
| Features of Early Reactions to AV | No. of Cases (% of Total 180 Cases That Received AV) | No. of Courses (% of Total 285 Courses of AV) |
|---|---|---|
| Cutaneous | ||
| Urticaria | 30 (16.7) | 31 (10.9) |
| Pruritus | 6 (3.3) | 6 (2.1) |
| Angioedema | 5 (2.8) | 5 (1.7) |
| Gastrointestinal | ||
| Nausea and vomiting | 2 (1.1) | 2 (0.7) |
| Respiratory | ||
| Chest tightness | 17 (9.4) | 17 (5.9) |
| Wheezing | 4 (2.2) | 4 (1.4) |
| Hypoxemia | 4 (2.2) | 5 (1.7) |
| Dyspnea | 2 (1.1) | 2 (0.7) |
| Cardiovascular | ||
| Tachycardia | 7 (3.9) | 7 (2.4) |
| Hypotension | 4 (2.2) | 4 (1.4) |
| Syncope | 1 (0.6) | 1 (0.3) |
| Others | ||
| Fever with chill | 3 (1.7) | 3 (1.1) |
| Drowsy | 1 (0.6) | 1 (0.3) |
| Treatments | ||
| Adrenaline | 14 (7.8) | 14 (4.9) |
| Dexamethasone IV | 16 (8.9) | 16 (5.6) |
| Chlorpheniramine IV | 31 (17.2) | 31 (10.9) |
| Ranitidine IV | 6 (3.3) | 6 (2.1) |
| Hydrocortisone IV | 4 (2.2) | 4 (1.4) |
| Meperidine IV | 1 (0.6) | 1 (0.3) |
Abbreviations: AV, antivenom; IV, intravenous.
Recurrent systemic effects after antivenom occurred in 11 cases (6.1% of patients received antivenom) including WBCT (5 cases), INR (3), VCT (1), platelet (1), and both WBCT and VCT (1). The median time to recurrence was 46.75 hours (IQR 38.5–63.5, range 19–98) after a bite. Median onset after the last dose of antivenom was 21 hours (IQR 15.75–28, range 13–66).
Other treatments included antibiotics (192 cases, 66.7%), surgical intervention (10, 34.7%), and blood components (4, 1.4%). Out of 9 patients diagnosed with compartment syndrome by primary doctors, six patients received antivenoms. Fasciotomy was indicated in 2 patients even after antivenom administration.
Outcomes
There were no amputations or mortality. The median follow-up duration was 3 days (IQR 2–4, range 0–10). Nineteen patients (10% of 190 patients with systemic effects) did not fully recover, with persistently abnormal INR (10 cases), platelet count (5), VCT (3), and WBCT (1). However, there were no reports of revisits or systemic bleeding after discharge.
Special Populations
Children
There were 50 children in the cohort with a median age of 8 years (IQR 4.4–11, range 1–14), none of whom had dry bites. Forty-eight patients (96%) reported local effects. Swelling was the most common symptom. There was no wound bleeding or compartment syndrome. Thirty-four patients (68%) showed systemic effects, none of whom reported allergic reactions to venoms. The median onset of each abnormal test result and systemic bleeding are presented in Table 5. The median onset of systemic effects was 12 hours (IQR 2–21.5, range 0.2–62). The cumulative percentage of cases with systemic effects over time is shown in Figure 5.
Table 5.
Median Onset of Each Abnormal Test Result and Systemic Bleeding in 34 Pediatric Patients
| Parameters Tested (No. of Cases with Abnormal Results) | Time of Onset (Hour) (IQR, Range) |
|---|---|
| VCT (23) | 13.5 (6–20.3, 0.2–48) |
| WBCT (15) | 8 (2–19, 1–77) |
| INR (13) | 21.5 (17–28.5, 0.5–60) |
| Platelets (5) | 23 (18–27, 12–62) |
| Systemic bleeding (2) | 67 (64.5–69.5, 62–72) |
| Overall (34) | 12 (3–21.5, 0.2–62) |
Abbreviations: IQR, interquartile range; INR, international normalized ratio; VCT, venous clotting time; WBCT, whole blood clotting time.
Figure 5.
Cumulative percentage of hematotoxicity (pediatric) cases detected.
Thirty-five patients (70%) received antivenom, including a total of 61 courses (205 vials). Allergic reactions occurred in eight cases (22.8% of 35 children received antivenom) including nine courses (14.8% of 61 total antivenom courses). Anaphylaxis was reported in four courses (6.5%).
Bleeding Tendency
Five patients reported concomitant use of antithrombotic drugs, namely aspirin and clopidogrel (2 patients), aspirin (2), and warfarin (1). For those on dual antiplatelets, one patient had prolonged INR and low platelets, and the other had prolonged INR and gross hematuria. For those taking aspirin, one patient had no systemic effects while the other had thrombocytopenia. The patient who took warfarin had prolonged INR (without systemic bleeding), which persisted until discharge. Two patients had underlying immune thrombocytopenia (baseline platelets 50,000–66,000/µL). One patient developed prolonged INR and the other had prolonged INR with thrombocytopenia. The INR normalized in both cases after antivenom administration.
Discussion
The results of this study show that most GPV envenomations result in local and/or systemic effects. The study demonstrates a lower incidence of systemic bleeding (6% compared with 20% in the previous study in 1996).7 This was probably due to different venom compositions and clinical effects among different Trimeresurus species in Thailand.14 Platelet count is the most frequently performed lab measurement because of the increased availability of tests. VCT shows the highest percentage of abnormalities. The most reliable lab test, fibrinogen, is the least performed.
The overall onset of hematotoxicity is 15 hours. Those with significant prognostic factors for severe systemic and local effects should be closely monitored.15 By following up for 3 days, 96.5% of patients with abnormality will be detected. This finding correlates with previous reports of cumulative percentage of onset of systemic effects (86.3% in 48–72 hours and 94.6% in 72–96 hours)7 and our current recommendation.10
Most patients respond to a three-vial-course of antivenom. Thai GPV antivenom was found to be effective against other Trimeresurus species.14 The most common indication is prolonged VCT. Some antivenoms were given without appropriate indication either before poison center consultation or against advice. They were usually prescribed to alleviate local symptoms, although previous studies showed only minimal reduction of limb circumference after a GPV bite.16 Currently, local edema is not an indication in our practice.
Most allergic reactions were mild and only 6.7% met the criteria for anaphylaxis. There were no differences in rates between adult and pediatric patients. The allergy rate is slightly higher than that in the previous study of GPV F(ab′)2 antivenom (15% versus 3.5%),17 which could be due to different doses and administration methods among hospitals. A previous report in Laos showed a higher incidence of antivenom allergy (53%) but did not mention specific snake type of antivenom contributing to the allergy.18 We believe that the allergic reactions are primarily non-IgE mediated,19,20 so the current guideline allows reinstitution of antivenoms (by slowing rate of infusion/giving premedication) if the symptoms subsided. However, data regarding the patient’s previous exposure to horse or sheep-derived products, which might induce IgE-mediated reactions, are limited in our study.
Antibiotics use is very common (66.7%) although it is controversial and only recommended in cases with infection.21 Prophylactic antibiotics are commonly prescribed by primary physicians to treat local swelling despite limited evidence regarding the prevention of infectious complications after envenomation.25 Although not reported herein, wound culture and hemoculture are currently encouraged in patients with a high risk of infection (ie, presence of blisters).12 Surgical complications are rare, and intervention is rarely indicated.
Our study reports recurrent coagulopathy with or without antivenom administration. There are at least four possible explanations for such recurrence: a longer half-life of venom compared with antivenom; circulating venom/antivenom complex separation after initial binding; late onset of different venom components; and host development of antibody to antivenom.22 In the present study, we found no recurrence presented as systemic bleeding, although reported previously.23 However, there are no established guidelines regarding the duration of follow-up after antivenom therapy in Thailand.
Ten percent of patients with systemic effects did not fully recover. Four of these patients were suspected to have other hematologic diseases or were on warfarin therapy. Persistent abnormalities seen in other cases could be explained by a long half-life of GPV venoms. The study by Rojnuckarin et al,24 demonstrated a GPV venom half-life of 27.5 hours during the first 3 days and >50 hours on days 5–7 after the bite. The postulation is different components of the venom may be detected by sandwich ELISA method at different time points, in that the smaller proteins may be excreted earlier and followed by higher molecular weight proteins.24
Reports of snake bites in children are limited. A prospective study of snake bites in Nakhon Ratchasima province included 72 cases (36.2%) of Trimeresurus sp. in children, 54 (27%) of whom were younger than 15 years. The subgroup analysis of pediatric patients showed no serious systemic effects except for local pain and swelling of the affected body parts,25 which is consistent with a study of envenomation by suspected T. albolabris in pediatric patients from Hong Kong.26 In our study, manifestations in pediatric patients were similar to those in adults except for the absence of dry bites and allergic symptoms to venoms. Complications were no more serious than in adults and only one patient required surgical intervention. Therefore, it is reasonable and expedient to manage pediatric snake envenomation in the same manner as for adults.
Limitations
As a retrospective study of poison center data, this study has some limitations. Because secondary data were acquired from telephone follow-up, these might lead to information bias such as recall bias and reporting bias. Cases referred to poison centers tend to be more severe and might also subject to selection bias. Moreover, identification of snakes is difficult because serologic confirmation is not practically available in Thailand.
Many hospitals prefer certain lab tests in accordance with resources. We found that VCT and WBCT are still widely performed, although these methods are subject to procedural and interpretational error. Their sensitivity and specificity are also limited in comparison with alternative tests such as prothrombin time with INR.27 Despite these impediments to the successful administration of antivenom, our study reflects real practices and situations in Thailand, although generalization of the results to other regions may be questionable.
Conclusions
Most GPV bites result in envenomation. The most frequent local effect is mild swelling. Systemic bleeding is uncommon. The current recommendation of a 3-day follow-up can detect up to 96% of patients who may require antivenom therapy. No severe morbidity or mortality are reported. Antivenoms are primarily indicated by prolonged VCT. The side effects of antivenom are minimal.
Acknowledgments
The abstract of this article was presented at the 18th Annual Scientific Congress of the Asia Pacific Association of Medical Toxicology (APAMT) on November 4–7 2019 in Putrajaya, Malaysia, as an oral presentation with interim findings. We thank Hugh McGonigle, from Edanz Group, for editing a draft of the manuscript.
Funding Statement
We declare no funding for this study.
Ethical Approval
This study was approved by the Institutional Ethics Committee Board of Ramathibodi Hospital Faculty of Medicine, Mahidol University. Because this was a retrospective study that used a pre-existing confidential database from the poison center, patient consent was not required by our hospital’s ethics committee board. The results of this study are reported anonymously and in compliance with the Declaration of Helsinki.
Disclosure
The authors report no conflict of interest.
References
- 1.Viravan C, Looareesuwan S, Kosakarn W, et al. A national hospital-based survey of snakes responsible for bites in Thailand. Trans R Soc Trop Med Hyg. 1992;86(1):100–106. doi: 10.1016/0035-9203(92)90463-M [DOI] [PubMed] [Google Scholar]
- 2.Sanhajariya S, Duffull SB, Isbister GK. Pharmacokinetics of snake venom. Toxins. 2018;10:2. doi: 10.3390/toxins10020073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rojnuckarin P, Muanpasitporn C, Chanhome L, Arpijuntarangkoon J, Intragumtornchai T. Molecular cloning of novel serine proteases and phospholipases A2 from green pit viper (Trimeresurus albolabris) venom gland cDNA library. Toxicon. 2006;47(3):279–287. doi: 10.1016/j.toxicon.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 4.Chotenimitkhun R, Rojnuckarin P. Systemic antivenom and skin necrosis after green pit viper bites. Clin Toxicol. 2008;46(2):122–125. doi: 10.1080/15563650701266826 [DOI] [PubMed] [Google Scholar]
- 5.Mitrakul C. Effects of green pit viper (Trimeresurus erythrurus and Trimeresurus popeorum) venoms on blood coagulation, platelets and the fibrinolytic enzyme systems: studies in vivo and in vitro. Am J Clin Pathol. 1973;60(5):654–662. doi: 10.1093/ajcp/60.5.654 [DOI] [PubMed] [Google Scholar]
- 6.Hutton RA, Looareesuwan S, Ho M, et al. Arboreal green pit vipers (genus Trimeresurus) of south-east Asia: bites by T. albolabris and T. macrops in Thailand and a review of the literature. Trans R Soc Trop Med Hyg. 1990;84(6):866–874. doi: 10.1016/0035-9203(90)90111-Q [DOI] [PubMed] [Google Scholar]
- 7.Rojnuckarin P, Mahasandana S, Intragumtornchai T, Swasdikul D, Sutcharitchan P. Moderate to severe cases of green pit viper bites in Chulalongkorn hospital. Thai J Hematol Transfus Med. 1996;6(3):199–205. [Google Scholar]
- 8.Looareesuwan S, Viravan C, Warrell DA. Factors contributing to fatal snake bite in the rural tropics: analysis of 46 cases in Thailand. Trans R Soc Trop Med Hyg. 1988;82(6):930–934. doi: 10.1016/0035-9203(88)90046-6 [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Regional Office for South-East Asia. Guidelines for the Management of Snakebites. 2nd ed New Delhi: World Health Organization; 2016. [Google Scholar]
- 10.Rojnuckarin P, Suteparak S, Sibunruang S. Diagnosis and management of venomous snakebites in Southeast Asia. Asian Biomed. 2012;6(6):795–805. doi: 10.5372/1905-7415.0606.125 [DOI] [Google Scholar]
- 11.Chanhome L, Cox MJ, Vasaruchapong T, Chaiyabutr N, Sitprija V. Characterization of venomous snakes of Thailand. Asian Biomed. 2011;5(3):311–328. doi: 10.5372/1905-7415.0503.043 [DOI] [Google Scholar]
- 12.Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303 [DOI] [PubMed] [Google Scholar]
- 13.Boyer LV, Seifert SA, Clark RF, et al. Recurrent and persistent coagulopathy following pit viper envenomation. Arch Intern Med. 1999;159(7):706–710. doi: 10.1001/archinte.159.7.706 [DOI] [PubMed] [Google Scholar]
- 14.Chanhome L, Khow O, Omori-Satoh T, Sitprija V. Capacity of Thai green pit viper antivenom to neutralize the venoms of Thai Trimeresurus snakes and comparison of biological activities of these venoms. J Nat Toxins. 2002;11(3):251–259. [PubMed] [Google Scholar]
- 15.Mahasandana S, Swasdikul D, Rojnuckarin P, Intragumthornchai T, Sutcharitchan P. Prognostic factors of green pit viper bites. Am J Trop Med Hyg. 1998;58(1):22–25. doi: 10.4269/ajtmh.1998.58.22 [DOI] [PubMed] [Google Scholar]
- 16.Rojnuckarin P, Chanthawibun W, Noiphrom J, Pakmanee N, Intragumtornchai T. A randomized, double-blind, placebo-controlled trial of antivenom for local effects of green pit viper bites. Trans R Soc Trop Med Hyg. 2006;100(9):879–884. doi: 10.1016/j.trstmh.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 17.Thiansookon A, Rojnuckarin P. Low incidence of early reactions to horse-derived F(ab′)2 antivenom for snakebites in Thailand. Acta Trop. 2008;105(2):203–205. doi: 10.1016/j.actatropica.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Vongphoumy I, Chanthilat P, Vilayvong P, et al. Prospective, consecutive case series of 158 snakebite patients treated at Savannakhet provincial hospital, Lao people’s democratic republic with high incidence of anaphylactic shock to horse derived F(ab’)2 antivenom. Toxicon. 2016;117:13–21. doi: 10.1016/j.toxicon.2016.03.011 [DOI] [PubMed] [Google Scholar]
- 19.Malasit P, Warrell DA, Chanthavanich P, et al. Prediction, prevention, and mechanism of early (anaphylactic) antivenom reactions in victims of snake bites. Br Med J. 1986;292(6512):17–20. doi: 10.1136/bmj.292.6512.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone SF, Isbister GK, Shahmy S, et al. Immune response to snake envenoming and treatment with antivenom; complement activation, cytokine production and mast cell degranulation. PLoS Negl Trop Dis. 2013;7(7):e2326. doi: 10.1371/journal.pntd.0002326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tagwireyi DD, Ball DE, Nhachi CF. Routine prophylactic antibiotic use in the management of snakebite. BMC Clin Pharmacol. 2001;1(1):4. doi: 10.1186/1472-6904-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyer LV, Seifert SA, Cain JS. Recurrence phenomena after immunoglobulin therapy for snake envenomations: part 2. Guidelines for clinical management with crotaline Fab antivenom. Ann Emerg Med. 2001;37(2):196–201. doi: 10.1067/mem.2001.113134 [DOI] [PubMed] [Google Scholar]
- 23.O’Brien NF, DeMott MC, Suchard JR, Clark RF, Peterson BM. Recurrent coagulopathy with delayed significant bleeding after crotaline envenomation. Pediatr Emerg Care. 2009;25(7):457–459. doi: 10.1097/PEC.0b013e3181ab7871 [DOI] [PubMed] [Google Scholar]
- 24.Rojnuckarin P, Banjongkit S, Chantawibun W, et al. Green pit viper (Trimeresurus albolabris and T. macrops) venom antigenaemia and kinetics in humans. Trop Doct. 2007;37(4):207–210. doi: 10.1258/004947507782332838 [DOI] [PubMed] [Google Scholar]
- 25.Buranasin P. Snakebites at Maharat Nakhon Ratchasima Regional Hospital. Southeast Asian J Trop Med Public Health. 1993;24(1):186–192. [PubMed] [Google Scholar]
- 26.Hon KL, Kwok LW, Leung TF. Snakebites in children in the densely populated city of Hong Kong: a 10-year survey. Acta Paediatr. 2004;93(2):270–272. doi: 10.1111/j.1651-2227.2004.tb00719.x [DOI] [PubMed] [Google Scholar]
- 27.Pongpit J, Limpawittayakul P, Juntiang J, Akkawat B, Rojnuckarin P. The role of prothrombin time (PT) in evaluating green pit viper (Cryptelytrops sp) bitten patients. Trans R Soc Trop Med Hyg. 2012;106(7):415–418. doi: 10.1016/j.trstmh.2012.04.003 [DOI] [PubMed] [Google Scholar]