ABSTRACT
Background
Longer-term feeding studies suggest that a low-carbohydrate diet increases energy expenditure, consistent with the carbohydrate-insulin model of obesity. However, the validity of methodology utilized in these studies, involving doubly labeled water (DLW), has been questioned.
Objective
The aim of this study was to determine whether dietary energy requirement for weight-loss maintenance is higher on a low- compared with high-carbohydrate diet.
Methods
The study reports secondary outcomes from a feeding study in which the primary outcome was total energy expenditure (TEE). After attaining a mean Run-in weight loss of 10.5%, 164 adults (BMI ≥25 kg/m2; 70.1% women) were randomly assigned to Low-Carbohydrate (percentage of total energy from carbohydrate, fat, protein: 20/60/20), Moderate-Carbohydrate (40/40/20), or High-Carbohydrate (60/20/20) Test diets for 20 wk. Calorie content was adjusted to maintain individual body weight within ± 2 kg of the postweight-loss value. In analyses by intention-to-treat (ITT, completers, n = 148) and per protocol (PP, completers also achieving weight-loss maintenance, n = 110), we compared the estimated energy requirement (EER) from 10 to 20 wk of the Test diets using ANCOVA.
Results
Mean EER was higher in the Low- versus High-Carbohydrate group in models of varying covariate structure involving ITT [ranging from 181 (95% CI: 8–353) to 246 (64–427) kcal/d; P ≤0.04] and PP [ranging from 245 (43–446) to 323 (122–525) kcal/d; P ≤0.02]. This difference remained significant in sensitivity analyses accounting for change in adiposity and possible nonadherence.
Conclusions
Energy requirement was higher on a low- versus high-carbohydrate diet during weight-loss maintenance in adults, commensurate with TEE. These data are consistent with the carbohydrate-insulin model and lend qualified support for the validity of the DLW method with diets varying in macronutrient composition. This trial was registered at clinicaltrials.gov as NCT02068885.
Keywords: obesity, dietary carbohydrate, dietary fat, carbohydrate-insulin model, energy requirement, energy expenditure, feeding study, metabolism
Introduction
The independent effect of dietary composition on energy expenditure remains a topic of controversy. According to the carbohydrate-insulin model of obesity, the high ratio of blood insulin-to-glucagon concentration in the postprandial period with consumption of a high-glycemic load diet partitions metabolic fuels toward fat storage (1, 2). As a result, hunger may increase and (under some conditions, such as postweight loss) energy expenditure may decrease relative to a low-glycemic load diet. Because reduced energy expenditure following weight loss may predispose to weight regain (3–5), research into the dietary determinants of metabolic rate holds both scientific and clinical significance.
A recent meta-analysis reported little effect of dietary carbohydrate-to-fat ratio on energy expenditure (6), but the included studies had a median duration of < 1 wk. As previously reviewed (2, 7), the adaptation to a low-carbohydrate diet takes ≥ 2 to 3 wk, limiting inferences about chronic macronutrient effects that can be drawn from these very short trials. A few prior studies of ≥ 2.5 wk duration suggest a numerical advantage favoring the low-carbohydrate diet (2), but each of these had important methodological limitations, such as low statistical power, lack of randomization, and physical confinement (e.g., in respiratory chambers) confounding activity-related energy expenditure.
In the longest feeding study addressing this question (8, 9), we reported that total energy expenditure (TEE) was about 250 kcal/d higher on a low- versus high-carbohydrate test diet throughout 20 wk of weight-loss maintenance, as determined using doubly labeled water (DLW) methodology. However, the validity of DLW methodology with diets varying in macronutrient composition has recently been called into question (10).
The aim of the present study was to assess the estimated energy requirement (EER) to maintain a mean 10.5% weight loss on diets containing 60%, 40%, and 20% of total energy as carbohydrate, controlled for protein (20%). If TEE increases with reduction in dietary carbohydrate and DLW methodology is valid for measuring TEE when comparing different macronutrient diets, we would expect to see dietary effects on energy requirement that correspond to effects on TEE.
Methods
Overview of parent study design and original findings
This study presents secondary and exploratory analyses from the Framingham State Food Study, a feeding trial for which the methods, participant flow, adverse events, and primary outcome were previously reported (8, 11, 12). Figure 1 depicts key design features, including outcome measurement time points. Briefly, 164 participants (in 3 cohorts over successive academic years) with overweight or obesity, who lost ≥ 10% of their body weight during the Run-in phase on a hypocaloric diet (45% of energy from carbohydrate, 35% fat, and 25% protein), were randomly assigned to Low-Carb (proportion of total energy: 20% carbohydrate, 60% fat), Moderate-Carb (40%, 40%), or High-Carb (60%, 20%) Test diets controlled for protein (20%). During the 20-wk Test phase, dietary energy provided to participants in prepared meals was adjusted with the aim of keeping weight within ± 2 kg of postweight loss, prerandomization baseline values. TEE was measured using DLW methodology at 4 time points: 1) preweight loss (PRE), 2) start of trial (START, weeks −2 to 0, postweight loss), 3) midpoint of Test phase (MID, weeks 8 to 10) and 4) end of Test phase (END, weeks 18 to 20). The primary finding of the trial was that TEE was significantly greater on Low-Carb compared with High-Carb in an intention-to-treat model (ITT: 209 kcal/d, n = 162, P = 0.002 for overall group effect) and a per protocol model (PP) that excluded participants who did not achieve weight stability at 10 or 20 wk (PP: 278 kcal/d, n = 120, overall P <0.001).
FIGURE 1.
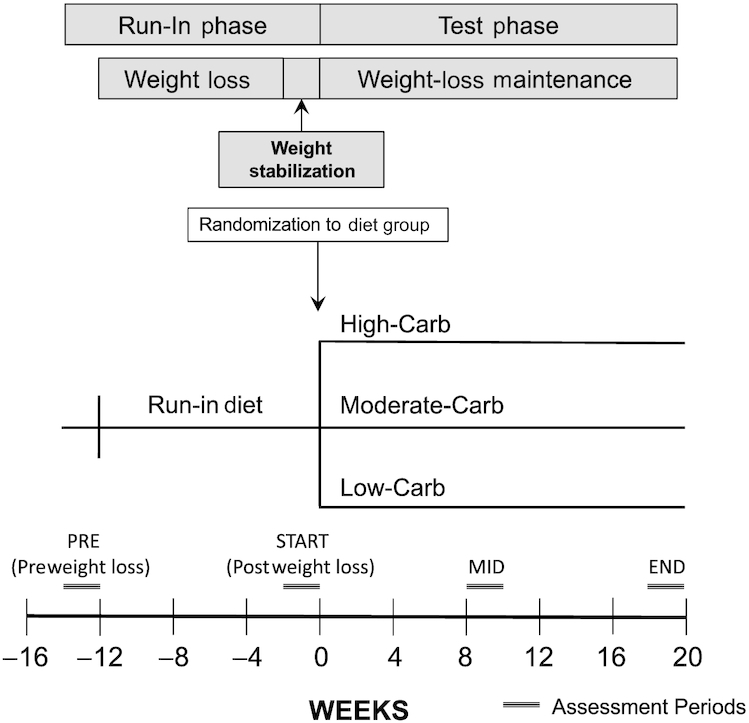
Study design of the Framingham State Food Study. Carb, carbohydrate; END, end of Test phase; MID, midpoint of Test phase; PRE, preweight loss; START, start of randomized trial (postweight loss, prerandomization).
We previously conducted a preliminary analysis in the PP group, comparing change in estimated energy intake from START to the average of MID (10 wk) and END (20 wk) using dietary data from the days when we assessed TEE (8). Change in energy intake increased in a pattern consistent with the dietary effect on TEE, though without significant group differences. However, as discussed in our initial report, these preliminary analyses were imprecise and inaccurate, with probable bias against those with higher energy requirement, thereby limiting scientific inference. (See Supplemental Methods for additional details on the conceptual approach to the current analyses.)
Assessment of Test diet energy
Details regarding the dietary interventions were published previously (11, 12). Standardized menus were calculated for 2000-kcal Run-in and Test diets using Food Processor Nutrition Analysis Software (ESHA Research Inc.) with energy distributed across breakfast (450 kcal), lunch (650 kcal), dinner (650 kcal), and an evening snack (250 kcal). Data for each menu item were exported from the ESHA Food Processor to Excel (Microsoft), and gram weights were imported from Excel into SAS (SAS Institute Inc.). In SAS, 2000-kcal menus were scaled to coincide with individualized energy levels, and food production sheets (1 sheet per participant per meal or snack) were generated to specify gram portions of each menu item. Details about supervising meals, estimating and adjusting dietary energy, quantifying unconsumed energy, and ensuring quality control are available in the Supplemental Methods.
The EER calculation included energy provided in weighed meals and snacks (as specified on food production sheets), with correction for unconsumed energy, and in ad libitum snacks (estimated at 200 kcal/d) and unit bars (based on the number provided, 100 kcal/bar). The first 10 wk of the Test phase was considered adequate time for physiological adaptations to the Test diets, that could affect energy metabolism and fluctuations in body weight (2), and fine-tuning initially imprecise estimates of energy levels for weight-loss maintenance. We also calculated EER for the first day of the Test phase (EER at START), to obtain insight regarding the level of imprecision and inaccuracy in the initial estimates of energy requirement, and for use as a baseline covariate in a statistical model.
Assessment of body composition
We assessed body composition by DXA (Discovery A, Hologic Inc.) and isotope dilution. Data from DXA, the more precise method, were available for PRE, START, and END. Data from isotope dilution were available for the same time points and also MID, allowing assessment of change in adiposity from weeks 10 through 20 of the Test phase which was the exact time frame of interest for determining EER (after the initial 10-wk period of physiological adaptation). Total body water was estimated using the isotope dilution space for 18O (calculated as previously described) (11), divided by 1.01 (to correct for binding to nonexchangeable sites) (13). Total body water was divided by 0.73 to estimate fat-free mass (FFM). Fat mass (FM) was calculated by subtracting FFM from total body weight. Percent body fat was calculated as: FM/body weight × 100.
Statistical analyses
The study was originally powered for TEE assessed using DLW methodology (8). For all summary and inferential computations for the present study, we used SAS 9.4 (SAS Institute).
Descriptive data
We inspected raw distributions of EER during the Test phase for the ITT and PP groups and compared raw distributions and descriptive data (mean and median) with those of TEE.
Variability in EER at START
We used partial correlation analysis to determine whether excessive variability in EER might have obscured the effect of diet on change in EER in our preliminary analyses (8). Controlling for diet group, we evaluated partial correlation of the residuals from models comparing EER at START with EER during the Test phase (MID through END), and TEE at START with TEE during the Test phase (average of MID and END).
Diet effect on EER
The analytic framework for statistical inference on EER was the general linear model (GLM) including ANCOVA. We evaluated EER at START (with body weight at START included as a covariate) and EER during the Test phase (with and without EER at START as a covariate). To be consistent with the approach in our prior study (8), the reported models include diet group assignment and a design variable [a polytomous covariate labeled cohort, which captured all combinations of study site (feeding location), cohort (denoting year of participation), and wave (denoting time frame of participation within a cohort), including 11 categories]. Because inclusion of this variable utilizes 10 degrees of freedom, and we have no reason to hypothesize confounding by cohort, a model without this adjustment was evaluated. Other variables in the primary model included sex, START age, weight loss during the Run-in phase (expressed as a percentage of PRE body weight), START weight, and START TEE. One participant who developed a medical condition (hypothyroidism) that affects energy expenditure was not included in the final analysis plan, consistent with the a priori plan for analyzing primary outcome data presented previously (8, 11). Here, we present analyses with (models 1 and 2) and without (models 3 and 4) inclusion of this individual. The outcome was the Test phase average of EER from 10 to 20 wk, modified from our original change analyses [Av(MID, END) – START] (8), using the conceptual approach presented in the Supplemental Methods.
From parameters of the fitted models, taking account of all data, we tested 2 null hypotheses: first, that the outcome was uniform across all diet groups, using an F test with 2 df; second, that the outcome did not differ between Low-Carb and High-Carb, using a 2-sided Student's t-test. The Low-Carb – High-Carb comparison was equivalent to a test for linear trend by carbohydrate, expressed as a percentage of total energy, given the equal increments of carbohydrate content (60%, 40%, 20%) across Test diets. The threshold for significance was P ≤0.05 when testing both of these hypotheses.
We conducted 4 sensitivity analyses using GLM (ANCOVA) to explore the potential effects of changes in body composition and nonadherence on EER during weight-loss maintenance. These analyses were based on our most conservative estimate of EER in the PP group that achieved weight-loss maintenance. For every kg increase or decrease in FM from START to END, assessed by DXA, we subtracted or added 55 kcal/d (7700 kcal/kg ÷ 140 d, the relevant time period). Similarly, for change in FM from MID to END, assessed by isotope dilution, we subtracted or added 110 kcal/d (7700 kcal/kg ÷ 70 d, the relevant time period). As a proxy measure of nonadherence, we defined energy discrepancy as EER-to-TEE ratio and excluded participants with energy discrepancy in the top quintile (those most likely to have underconsumed provided foods) and bottom quintile (those most likely to have consumed foods off protocol). In a final model, we excluded individuals in cohort 1 for whom unconsumed energy was not recorded in the online study portal, as noted in the Supplemental Methods.
Ethics
The study protocol was approved by the Institutional Review Board at Boston Children's Hospital and registered at clinicaltrials.gov NCT02068885.
Results
Descriptive data
Of the 164 randomly assigned participants, 148 completed the trial and were included in the ITT analyses for this report. Noncompleters comprised 3 (18.8%) in Low-Carb, 5 (31.3%) in Moderate-Carb, and 8 (50%) in High-Carb, with no significant difference in rate of drop-out by diet group (P = 0.26). Among the completers, 110 achieved weight-loss maintenance and were included in PP analyses. Table 1 summarizes baseline data describing all enrolled participants, those who completed the weight-loss Run-in phase (and were included in the ITT analyses), and those who achieved weight-loss maintenance (and were included in the PP analyses) for the trial outcomes. About two-thirds of the cohort were women, mean age was ∼39 y, and mean BMI at PRE was ∼32 kg/m2. Supplemental Figure 1 illustrates frequency distributions of EER for the ITT and PP analyses. The median and mean values of EER were 89.2% and 87.7% of TEE, respectively, for the ITT and 88.2% and 89.7% for the PP.
TABLE 1.
Characteristics of participants in the Framingham State Food Study1
| Characteristic | All enrolled 2 | Intention-to-treat 3 | Per protocol 4 |
|---|---|---|---|
| Categorical variables | |||
| Sex | |||
| Men | 49 (29.9) | 45 (30.4) | 33 (30.0) |
| Women | 115 (70.1) | 103 (69.6) | 77 (70.0) |
| Hispanic ethnicity | 25 (15.2) | 21 (14.2) | 18 (16.4) |
| Race | |||
| White | 128 (78.1) | 116 (78.4) | 84 (76.4) |
| Black | 17 (10.4) | 16 (10.8) | 11 (10.0) |
| Asian | 5 (3.0) | 5 (3.4) | 4 (3.6) |
| Unknown/Other | 14 (8.5) | 11 (7.4) | 11 (10.0) |
| Continuous variables | |||
| Weight at PRE, kg | 91.5 ± 18.2 | 91.2 ± 18.2 | 89.5 ± 16.6 |
| Height at PRE, cm | 167.7 ± 10.0 | 167.9 ± 10.0 | 167.3 ± 10.3 |
| BMI at PRE, kg/m2 | 32.4 ± 4.8 | 32.2 ± 4.8 | 31.8 ± 4.2 |
| Age at START, y | 38.0 ± 14.4 | 38.6 ± 14.4 | 39.8 ± 14.0 |
| Weight loss at START, % of PRE body weight | 10.5 ± 1.7 | 10.5 ± 1.6 | 10.5 ± 1.5 |
| Total energy expenditure at START, kcal/d | 2661 ± 547 | 2651 ± 557 | 2663 ± 559 |
For categorical variables, values are frequency (%). For continuous variables, values are mean ± SD. PRE, preweight loss; START, start of randomized trial (postweight loss, prerandomization).
N = 164. Two participants who were randomly assigned to a diet group had unusable START TEE data.
N = 148. Participants who completed the study were included in the ITT analyses. One participant (ITT only) had unusable START TEE data.
N = 110. Participants who completed the study and achieved weight-loss maintenance were included in the PP analyses.
ITT, intention-to-treat; PP, per protocol; TEE, total energy expenditure.
Variability in EER at START
We compared EER at START with EER measured from weeks 10 through 20 of the Test phase. As shown in Supplemental Figure 2, the partial correlation after adjusting for diet group (R2 = 0.54) was much weaker than that involving TEE (R2 = 0.85). These findings suggest that analytic models of change have adequate power for evaluating TEE but not EER (14, 15), providing rationale for using ANCOVA, as discussed in the Supplemental Methods.
Diet effect on EER
Table 2 shows EER by diet group in the ITT and PP analyses. At START, EER did not differ by diet. From weeks 10 through 20 of the Test phase, EER was significantly higher in Low-Carb compared with High-Carb, ranging from means of 181 to 323 kcal/d in models with varying covariate structure. Supplemental Figure 3 displays individual data by diet group in model 4 of the PP analysis.
TABLE 2.
Effects of Test diets varying in carbohydrate content on estimated energy requirement during weight-loss maintenance in the Framingham State Food Study1
| EER by diet group, kcal/d | Linear trend, kcal/d | |||||
|---|---|---|---|---|---|---|
| Analysis | Low-Carb | Moderate-Carb | High-Carb | P 2 | (Low-Carb) – (High-Carb) | P 3 |
| Baseline (START) 4 | ||||||
| ITT | 2284 (2214, 2354) | 2276 (2202, 2350) | 2229 (2152, 2305) | 0.54 | 56 (−50, 161) | 0.30 |
| PP | 2330 (2247, 2413) | 2309 (2219, 2398) | 2277 (2175, 2378) | 0.73 | 53 (−80, 187) | 0.43 |
| Diet effect, model 1 4 | ||||||
| ITT | 2517 (2396, 2639) | 2437 (2308, 2565) | 2303 (2170, 2435) | 0.07 | 215 (32, 398) | 0.02 |
| PP | 2565 (2432, 2698) | 2447 (2304, 2591) | 2289 (2127, 2452) | 0.04 | 276 (61, 490) | 0.01 |
| Diet effect, model 2 (model 1 additionally adjusted for START EER) 5 | ||||||
| ITT | 2505 (2391, 2620) | 2429 (2308, 2550) | 2324 (2199, 2450) | 0.12 | 181 (8, 353) | 0.04 |
| PP | 2552 (2427, 2677) | 2447 (2312, 2581) | 2308 (2155, 2460) | 0.06 | 245 (43, 446) | 0.02 |
| Diet effect, model 3 (model 2 excluding participant with hypothyroidism) 6 | ||||||
| ITT | 2528 (2414, 2642) | 2432 (2312, 2551) | 2323 (2200, 2447) | 0.07 | 204 (33, 376) | 0.02 |
| PP | 2582 (2458, 2706) | 2448 (2316, 2579) | 2309 (2160, 2458) | 0.03 | 272 (74, 471) | 0.008 |
| Diet effect, model 4 (model 3 without adjustment for the polytomous cohort variable) 7 | ||||||
| ITT | 2533 (2411, 2656) | 2460 (2333, 2587) | 2288 (2156, 2419) | 0.03 | 246 (64, 427) | 0.009 |
| PP | 2594 (2465, 2723) | 2467 (2331, 2602) | 2271 (2120, 2422) | 0.008 | 323 (122, 525) | 0.002 |
Values are means (95% CI). Data were calculated per kg and normalized to average weight of 82 kg at START. EER, estimated energy requirement; ITT, intention-to-treat analysis; PP, per protocol analysis; PRE, preweight loss; START, start of randomized trial (postweight loss, prerandomization); TEE, total energy expenditure measured using DLW methodology.
P value is for the overall diet group effect.
P value for (Low-Carb) – (High-Carb) contrast is equivalent to a test for linear trend across diet groups (with equal, 20% increments in the contribution of carbohydrate to total energy intake from Low-Carb to Moderate-Carb and from Moderate-Carb to High-Carb.)
N = 147 (ITT), N = 110 (PP). Covariates included cohort, sex, age, Run-in weight loss (% PRE body weight), START TEE, and START body weight. One participant (ITT only) had unusable START TEE data.
N = 147 (ITT), N = 110 (PP). Covariates included cohort, sex, age, Run-in weight loss (% PRE body weight), START TEE, START body weight, and START EER. One participant (ITT only) had unusable START TEE data.
N = 146 (ITT), N = 109 (PP). One participant (ITT and PP) developed hypothyroidism and was an a priori exclusion from analyses of the primary outcome (8, 11). Covariates included cohort, sex, age, Run-in weight loss (% PRE body weight), START TEE, START body weight, and START EER. One participant had unusable START TEE data (ITT only).
N = 146 (ITT), N = 109 (PP). One participant (ITT and PP) developed hypothyroidism and was an a priori exclusion from analyses of the primary outcome (8, 11). Covariates included sex, age, Run-in weight loss (% PRE body weight), START TEE, START body weight, and START EER. One participant (ITT only) had unusable START TEE data. Elimination of the polytomous cohort variable decreased predictor Df by 10 in ITT, and 9 in PP (because there were no participants in the PP analysis for 1 of the 11 categories of this variable).
DLW, doubly labeled water.
In sensitivity analyses (Table 3), this diet effect remained robust after accounting for concurrent change in body composition, excluding individuals for whom the EER-to-TEE ratio raised the possibility of nonadherence, and additional exclusion of individuals in cohort 1 lacking nonadherence data from the online portal. The nominal order of effect by group, with Moderate-Carb intermediate between Low-Carb and High-Carb, showed a pattern similar to that of TEE. The EER-to-TEE ratio did not differ by diet group (Supplemental Figure 4), indicating no selective nonadherence or bias in group comparisons.
TABLE 3.
Sensitivity analyses for effects of Test diets varying in carbohydrate content on estimated energy requirement during weight-loss maintenance in the Framingham State Food Study1
| EER by diet group, kcal/d | Linear trend, kcal/d | |||||
|---|---|---|---|---|---|---|
| Analysis | Low-Carb | Moderate-Carb | High-Carb | P 2 | (Low-Carb) – (High-Carb) | P 3 |
| Adjusted for change in body composition by DXA 4 | ||||||
| 2572 (2442, 2702) | 2452 (2313, 2592) | 2304 (2145, 2462) | 0.04 | 268 (58, 478) | 0.01 | |
| Adjusted for change in body composition by isotope dilution 4 | ||||||
| 2481 (2330, 2631) | 2394 (2234, 2553) | 2208 (2027, 2388) | 0.08 | 273 (32, 513) | 0.03 | |
| Accounting for possible dietary nonadherence 5 | ||||||
| 2631 (2488, 2775) | 2369 (2208, 2531) | 2347 (2198, 2495) | 0.02 | 285 (76, 493) | 0.008 | |
| As above, with additional elimination of participants lacking nonadherence data 6 | ||||||
| 2637 (2501, 2773) | 2456 (2278, 2634) | 2345 (2190, 2499) | 0.02 | 292 (84, 501) | 0.007 | |
Values are means (95% CI). Data were calculated per kg and normalized to average weight of 82 kg at START, using model 2 (Table 2, PP) to examine how changes in body composition and potential nonadherence could influence the diet effect on EER. EER, estimated energy requirement; PP, per protocol analysis; START, start of randomized trial (postweight loss, prerandomization); TEE, total energy expenditure measured using DLW methodology.
P value is for the overall diet group effect.
P value for (Low-Carb) – (High-Carb) contrast is equivalent to a test for linear trend across diet groups (with equal, 20% increments in the contribution of carbohydrate to total energy intake from Low-Carb to Moderate-Carb and from Moderate-Carb to High-Carb).
N = 110 (DXA), N = 109 (isotope dilution). Analyses were adjusted for change in body composition between weeks 10 and 20 of the Test phase. One participant had unusable isotope dilution data at MID and END.
N = 65. Participants (N = 45) were excluded if the EER-to-TEE ratio was in the top quintile (i.e., individuals most likely to have underconsumed provided foods) or bottom quintile (i.e., individuals most likely to have consumed foods off protocol).
N = 56. Additional participants (N = 9) from cohort 1 were excluded because data were missing for unconsumed energy.
DLW, doubly labeled water.
Body composition
As shown in Supplemental Table 1, there were no significant diet group differences in adiposity by DXA or isotope dilution during the Test (weight maintenance) phase of the study.
Discussion
In this analysis of a large feeding study, we observed higher estimated energy requirement on a low- compared with high-carbohydrate diet during weight-loss maintenance. The magnitude of this effect (about 200 to 300 kcal/d, or ∼50 kcal/d for every 10% decrease in carbohydrate as a proportion of total energy) and the numerical order across groups (Low-Carb > Moderate-Carb > High-Carb) are commensurate with previously reported changes in TEE (8), supporting the carbohydrate-insulin model.
If reproducible and generalizable, this finding may inform the scientific understanding of how dietary composition affects metabolism and the design of more efficacious long-term obesity treatment. Pharmaceutical agents to increase energy expenditure, or to prevent the fall in energy expenditure following weight loss, for obesity treatments have been sought for decades (16). Our study suggests that a low-carbohydrate diet may produce this metabolic effect, without the risks of chronic drug treatment.
The components of energy expenditure accounting for the higher observed energy requirement remain speculative and warrant further research but may include resting energy expenditure (17), spontaneous physical activity (18) (both of which were marginally higher on the low-carbohydrate diet in our study as previously reported [8]), sleeping energy expenditure (19), nutrient cycling (20), and better access to metabolic fuels in the late postprandial state (21). Hormonal changes accompanying a low-carbohydrate diet may mediate, to some degree, several of these components. Reduced insulin and ghrelin concentrations may increase energy expenditure in part through activation of brown-adipose tissue activity (22, 23), whereas high glucagon may increase energy expenditure through other mechanisms (24, 25). The lower leptin concentration on the low-carbohydrate diet (8) – a predictor of good long-term weight-loss maintenance (26–28) – may indicate improved hormone sensitivity (29). As such, the enhanced leptin signaling may not only lower hunger and food intake, but also confer metabolic benefits (30).
Results of the present study also have relevance to methods used to evaluate metabolism in outpatient settings. In a recent analysis of a nonrandomized pilot study, Hall et al. (10) questioned the validity of DLW methodology to compare diets differing in carbohydrate-to-fat ratio, in part due to the “theoretical possibility that … [differential] fluxes through biosynthetic pathways” could inflate measured energy expenditure on diets with lower carbohydrate content. However, their estimates of isotopic trapping through de novo lipogenesis, the pathway of greatest potential concern, appear overstated, and DLW methodology has worked well in animals with diets varying widely in macronutrient ratio, including obligate carnivores (8, 31). The congruence in dietary effect on EER and TEE from our trial provides qualified validation for the use of DLW methodology in human diet studies, though the possibility of other, unrecognized biases cannot be excluded. In contrast to the theoretical concerns involving use of DLW to measure TEE, whole room calorimetry – the other gold standard method – has been shown to underestimate adaptive thermogenesis (32) because of inherent constraints on physical activity energy expenditure [a confounding issue in the analyses of Hall et al. (10)]. Recognizing that reduction in dietary carbohydrate has been hypothesized to attenuate adaptive thermogenesis with weight loss (2, 8), macronutrient studies utilizing whole room calorimetry may yield results biased against low-carbohydrate diets. Indeed, the prior validation study (32) found a better correspondence between dietary calorie titration and TEE – the approach we used here – for DLW methodology compared with whole room calorimetry under several physiological conditions.
Strengths of this study include the relatively large sample size and long duration for a feeding trial, demonstration of weight stability during the Test phase, concurrent measurement of body composition, and sufficient power to conduct informative sensitivity analyses. The main limitation is the possibility of nonadherence to Test diets and, more generally, inaccuracy in the assessment of energy requirement, a methodological issue common to all long-term outpatient feeding studies.
Median and mean dietary energy were ∼10% to 12% lower than energy expenditure by DLW methodology, suggesting that our present analyses may have underestimated actual requirements modestly, although overestimation by DLW methodology is also possible. However, this relatively small discrepancy, irrespective of origin, would not threaten the validity of our findings unless there were selective bias in the preparation or consumption of Test diets between groups. Specifically, an overestimation of the diet effect on energy requirement might occur if individuals on the Low- versus High-Carb diet consumed less of the provided food than reported when not under direct observation; or if those on the High- compared with Low-Carb diet consumed more food off protocol. Either of these scenarios could arise if the Low-Carb diet were less palatable or more satiating. Conversely, because the High-Carb diet was substantially lower in energy density, the diet effect could be underestimated if participants in that group had difficulty consuming the larger volume of food. However, we designed the diets to be as similar as possible (types of foods included, cooking methods, and palatability) and employed state-of-the-art methods to monitor quality control (12). Moreover, we saw no discrepancy in the EER-to-TEE ratio across diet groups. Nor did we find evidence of overall bias in a sensitivity analysis excluding individuals with the EER-to-TEE ratio in the highest quintile (for whom energy intake might have been overestimated) and in the lowest quintile (for whom energy intake might have been underestimated). Furthermore, the findings strengthened in the PP analyses, involving participants who demonstrated successful weight-loss maintenance as an objective proxy measure of adherence (the opposite would be expected if nonadherence contributed importantly to the observed effect).
Other study limitations include the inherent imprecision of methods for measuring small changes in body composition during weight-loss maintenance, and possible inaccuracy arising from changes in body water on diets differing in macronutrient content. On the latter issue, any changes in body water resulting from reduction in dietary carbohydrate would stabilize after a few weeks, allowing for an unconfounded measurement of body composition between 10 and 20 wk of the Test phase, the relevant period for our calculations of energy requirement. Our estimates of energy requirement vary based on covariate structure of the analytic models and other assumptions. However, the comparison between the Low- and High-Carb diets was consistently significant as hypothesized in multiple models and sensitivity analyses. In light of the foregoing, our estimates of the magnitude of the diet effect on energy requirement should be interpreted cautiously.
Because of the inherent limitations of outpatient feeding studies discussed here, some suggest that the only informative diet studies are those conducted on metabolic wards (33), but these too have major limitations. For logistical and financial reasons, ward studies rarely exceed a few weeks in duration – too short to distinguish transient adaptive processes from the chronic metabolic effects of macronutrients (2, 34). Ward studies also entail an artificial environment, constraint on spontaneous physical activities, and the psychobiological effects of social isolation and other stresses. Even with presumably maximum control, substantial “unaccounted energy” – the basis of criticisms of our trial by Hall et al. (35) – may occur, as was seen in a recent trial by Hall et al. (9, 36). Discrepancies in energy balance are unsurprising, considering the cumulative error that would arise in comparisons encompassing 3 imprecise measures (energy intake, energy expenditure, and body energy stores), even with optimal conditions.
To elucidate underlying mechanisms involving diet and chronic disease, we will need a variety of complementary study designs, novel methods for ensuring high levels of dietary control for longer periods, multiple methods for measuring energy expenditure and substrate metabolism, and attention to effect modification by biological predisposition (2, 37, 38). Although research into more powerful behavioral and environmental interventions is also warranted, these approaches will be most effective when informed by accurate knowledge of the metabolic effects of dietary composition.
Supplementary Material
Acknowledgments
We thank Steven Heymsfield and Henry Feldman for critical feedback on the manuscript, Stephanie Dickinson and Shui Yu for helping to verify the data analyses, and Kimberly Greco for assistance with data analysis in the revision of the original manuscript.
The authors’ contributions were as follows—CBE: designed the study, interpreted data, and participated in drafting the manuscript; LB: helped design the study and analyzed the dietary records; PRL: conducted the statistical analysis, interpreted data, and participated in manuscript revision; GLK: directed the parent study and participated in manuscript revision; JMWW: codirected the parent study, helped design the Test diets, and participated in manuscript revision; PKL: codirected the parent study at the performance site and participated in manuscript revision; WWW: advised on double labeled water methodology and participated in manuscript revision; DSL: designed the study, interpreted data, and participated in drafting the manuscript; CBE and DSL: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Preprint posted: medRxiv 7/11/19: https://www.medrxiv.org/content/10.1101/19001248v1.
This work was conducted with grants from Nutrition Science Initiative (made possible by gifts from Arnold Ventures and Robert Lloyd Corkin Charitable Foundation), New Balance Foundation, Many Voices Foundation, and Blue Cross Blue Shield. DSL was supported by a midcareer mentoring award from the National Institute of Diabetes and Digestive and Kidney Diseases (K24DK082730). Nutrition Science Initiative monitored study progress and was given an opportunity to comment on the manuscript. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; approval of the manuscript; and decision to submit the manuscript for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the study sponsors.
Data presented in the manuscript, code used to analyze the data, and a corresponding codebook will be made publicly and freely available without restriction at Open Science Framework https://osf.io/rvbuy/.
Author disclosures: CBE and DSL have conducted research studies examining the carbohydrate-insulin model funded by the NIH and philanthropic organizations unaffiliated with the food industry; DSL received royalties for books on obesity and nutrition that recommend a low-glycemic load diet. All other authors report no conflicts of interest.
Supplemental Methods, Supplemental Table 1, and Supplemental Figures 1–4 are available from the ‘‘Supplementary data’’ link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: Carb, carbohydrate; DLW, doubly labeled water; END, end of Test phase; EER, estimated energy requirement for weight-loss maintenance; FFM, fat-free mass; FM, fat mass; ITT, intention-to-treat; MID, midpoint of Test phase; PP, per protocol; PRE, preweight loss; START, start of randomized trial (postweight loss, prerandomization); TEE, total energy expenditure.
References
- 1. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–23. [DOI] [PubMed] [Google Scholar]
- 2. Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out”. JAMA Intern Med. 2018;178(8):1098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Astrup A, Gotzsche PC, van de Werken K, Ranneries C, Toubro S, Raben A, Buemann B. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999;69(6):1117–22. [DOI] [PubMed] [Google Scholar]
- 4. Rosenbaum M, Hirsch J, Gallagher DA, Leibel RL. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am J Clin Nutr. 2008;88(4):906–12. [DOI] [PubMed] [Google Scholar]
- 5. Tremblay A, Royer MM, Chaput JP, Doucet E. Adaptive thermogenesis can make a difference in the ability of obese individuals to lose body weight. Int J Obes (Lond). 2013;37(6):759–64. [DOI] [PubMed] [Google Scholar]
- 6. Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152(7):1718–27. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sherrier M, Li H. The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. Am J Clin Nutr. 2019;110(3):562–73. [DOI] [PubMed] [Google Scholar]
- 8. Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, Luoto PK, Wolfe RR, Wong WW, Ludwig DS. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ludwig DS, Lakin PR, Wong WW, Ebbeling CB. Scientific discourse in the era of open science: a response to Hall et al. regarding the carbohydrate-insulin model. Int J Obes (Lond). 2019;43(12):2355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall KD, Guo J, Chen KY, Leibel RL, Reitman ML, Rosenbaum M, Smith SR, Ravussin E. Methodologic considerations for measuring energy expenditure differences between diets varying in carbohydrate using the doubly labeled water method. Am J Clin Nutr. 2019;109(5):1328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ebbeling CB, Klein GL, Luoto PK, Wong JMW, Bielak L, Eddy RG, Steltz SK, Devlin C, Sandman M, Hron B et al. A randomized study of dietary composition during weight-loss maintenance: rationale, study design, intervention, and assessment. Contemp Clin Trials. 2018;65:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong JM, Bielak L, Eddy RG, Stone L, Lakin PR, Sandman M, Devlin C, Seger-Shippee L, Wiroll D, Luoto PK et al. An academia-industry partnership for planning and executing a community-based feeding study. Curr Dev Nutr. 2018;2(9):nzy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Total body water measurement in humans with 18O and 2H labeled water. Am J Clin Nutr. 1980;33(12):2686–93. [DOI] [PubMed] [Google Scholar]
- 14. Van Breukelen GJ. ANCOVA versus change from baseline: more power in randomized studies, more bias in nonrandomized studies [corrected]. J Clin Epidemiol. 2006;59(9):920–5. [DOI] [PubMed] [Google Scholar]
- 15. Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9(6):465–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307(24):2627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scribner KB, Pawlak DB, Aubin CM, Majzoub JA, Ludwig DS. Long-term effects of dietary glycemic index on adiposity, energy metabolism, and physical activity in mice. Am J Physiol Endocrinol Metab. 2008;295(5):E1126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016;104(2):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bistrian BR. Some musings about differential energy metabolism with ketogenic diets. JPEN J Parenter Enteral Nutr. 2019;43(5):578–82. [DOI] [PubMed] [Google Scholar]
- 21. Walsh CO, Ebbeling CB, Swain JF, Markowitz RL, Feldman HA, Ludwig DS. Effects of diet composition on postprandial energy availability during weight loss maintenance. PLoS One. 2013;8(3):e58172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dallon BW, Parker BA, Hodson AE, Tippetts TS, Harrison ME, Appiah MMA, Witt JE, Gibbs JL, Gray HM, Sant TM et al. Insulin selectively reduces mitochondrial uncoupling in brown adipose tissue in mice. Biochem J. 2018;475(3):561–9. [DOI] [PubMed] [Google Scholar]
- 23. Mihalache L, Gherasim A, Nita O, Ungureanu MC, Padureanu SS, Gavril RS, Arhire LI. Effects of ghrelin in energy balance and body weight homeostasis. Hormones (Athens). 2016;15(2):186–96. [DOI] [PubMed] [Google Scholar]
- 24. Kim T, Nason S, Holleman C, Pepin M, Wilson L, Berryhill TF, Wende AR, Steele C, Young ME, Barnes S et al. Glucagon receptor signaling regulates energy metabolism via hepatic farnesoid X receptor and fibroblast growth factor 21. Diabetes. 2018;67(9):1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salem V, Izzi-Engbeaya C, Coello C, Thomas DB, Chambers ES, Comninos AN, Buckley A, Win Z, Al-Nahhas A, Rabiner EA et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab. 2016;18(1):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crujeiras AB, Goyenechea E, Abete I, Lage M, Carreira MC, Martinez JA, Casanueva FF. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 2010;95(11):5037–44. [DOI] [PubMed] [Google Scholar]
- 27. Erez G, Tirosh A, Rudich A, Meiner V, Schwarzfuchs D, Sharon N, Shpitzen S, Blüher M, Stumvoll M, Thiery J et al. Phenotypic and genetic variation in leptin as determinants of weight regain. Int J Obes (Lond). 2011;35(6):785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mavri A, Stegnar M, Sabovic M. Do baseline serum leptin levels predict weight regain after dieting in obese women?. Diabetes Obes Metab. 2001;3(4):293–6. [DOI] [PubMed] [Google Scholar]
- 29. Ye Z, Liu G, Guo J, Su Z. Hypothalamic endoplasmic reticulum stress as a key mediator of obesity-induced leptin resistance. Obes Rev. 2018;19(6):770–85. [DOI] [PubMed] [Google Scholar]
- 30. Pandit R, Beerens S, Adan RAH. Role of leptin in energy expenditure: the hypothalamic perspective. Am J Physiol Regul Integr Comp Physiol. 2017;312(6):R938–R47. [DOI] [PubMed] [Google Scholar]
- 31. Ludwig DS, Ebbeling CB, Wong JMW, Wolfe RR, Wong WW. Methodological error in measurement of energy expenditure by the doubly labeled water method: much ado about nothing?. Am J Clin Nutr. 2019;110(5):1253–4. [DOI] [PubMed] [Google Scholar]
- 32. Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, Heymsfield SB, Hirsch J, Leibel RL. A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol. 1996;270(3 Pt 2):R496–504. [DOI] [PubMed] [Google Scholar]
- 33. Polychronakos C. Too sweet? Macronutrients and energy expenditure; a word of caution. BMJ. 2018;363:k4583.30429127 [Google Scholar]
- 34. Ludwig DS, Astrup A, Bazzano LA, Ebbeling CB, Heymsfield SB, King JC, Willett WC. Ultra-processed food and obesity: the pitfalls of extrapolation from short studies. Cell Metab. 2019;30(1):3–4. [DOI] [PubMed] [Google Scholar]
- 35. Hall KD, Guo J, Speakman JR. Do low-carbohydrate diets increase energy expenditure? Int J Obes (Lond). 2019;43(12):2350–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, Chung ST, Costa E, Courville A, Darcey V et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2019;30(1):67–77. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hjorth MF, Zohar Y, Hill JO, Astrup A. Personalized dietary management of overweight and obesity based on measures of insulin and glucose. Annu Rev Nutr. 2018;38:245–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mendes-Soares H, Raveh-Sadka T, Azulay S, Ben-Shlomo Y, Cohen Y, Ofek T, Stevens J, Bachrach D, Kashyap P, Segal L et al. Model of personalized postprandial glycemic response to food developed for an Israeli cohort predicts responses in Midwestern American individuals. Am J Clin Nutr. 2019;110(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


