ABSTRACT
Background
Dietary Guidelines for Americans recommend the consumption of 3 servings/d of low-fat/nonfat dairy. The effects of higher dairy consumption and its fat content are unknown in patients with type 2 diabetes.
Objective
Evaluate the impact of higher consumption of high- compared with low-fat dairy on glycated hemoglobin (HbA1c), body weight, and cardiovascular disease risk factors in patients with type 2 diabetes.
Methods
We enrolled 111 subjects with type 2 diabetes (aged 58.5 ± 8.9 y, 47% females, diabetes duration 13.2 ± 8.3 y, HbA1c 8.09 ± 0.96%) who consumed <3 servings of dairy/d. We randomly assigned them into 3 groups: control group maintained baseline dairy intake, low-fat (LF) group incorporated ≥3 servings/d of LF dairy, and the high-fat (HF) group incorporated ≥3 servings/d of HF dairy. We evaluated HbA1c, body weight, BMI, body composition parameters, blood pressure (BP), lipid parameters, homeostatic model assessment of insulin resistance (HOMA-IR), and total energy and macronutrient intake at baseline, and after 12 and 24 wk.
Results
At 24 wk, percent energy from saturated fat increased from baseline in the HF group by 3.6%, (95% CI: 2.2, 5.1) and decreased in the LF group by −1.9% (95% CI: −3.3, −0.4). The LF group increased their percent energy from protein by 4.5% (95% CI: 2.6, 6.4), whereas the HF group decreased their percent energy from carbohydrates by −3.4% (95% CI: −0.2, −6.7). There were no differences in the mean changes in HbA1c, body weight, BMI, body composition or lipid parameters, or BP between the 3 groups at 24 wk.
Conclusion
In patients with type 2 diabetes, increased dairy consumption to ≥3 servings/d compared with <3 servings/d, irrespective of its fat content, while maintaining energy intake has no effect on HbA1c, body weight, body composition, lipid profile, or BP. This trial was registered at clinicaltrials.gov as NCT02895867.
Keywords: dairy, high-fat, low-fat, type 2 diabetes, HbA1c, body weight
Introduction
Dairy products are rich in protein, vitamin D, calcium, potassium, and other nutrients, but they can also be rich in SFAs and calories. The Dietary Guidelines for Americans recommend 3 servings of low-fat (LF) dairy products per day for adults (1). Observational studies suggest that increased dairy consumption, particularly fermented dairy products, cheese, and LF dairy, particularly yogurt, are associated with a lower risk of type 2 diabetes whereas high-fat (HF) dairy was not associated with diabetes risk (2). Dairy products remain an integral component of a healthy diet (3–5). A few small-size clinical trials have looked at the effect of dairy intake on glycemic outcomes among people with obesity or metabolic syndrome. In overweight or obese adults, a high intake of low-fat dairy products (4 servings/d) improved insulin resistance compared with a lower intake of dairy products (1–2 servings/d) over a 6-mo period, without adverse effects on body weight or lipid profile (6). However, subjects with metabolic syndrome who consumed 3–5 servings of dairy products per day for 6 mo had no change in HOMA-IR or body weight compared with a control diet group (7). A meta-analysis of randomized clinical trials (RCTs) found that dairy intake, especially LF dairy products, has a beneficial effect on HOMA-IR, waist circumference, and body weight. A previous mixed-meal study demonstrated that among patients with type 2 diabetes, whey protein consumption led to a higher postprandial insulin excursion (8).
Cohort studies show that dietary fatty acids, such as trans-palmitoleic acid and SCFAs, both of which are present in dairy, are associated with lower type 2 diabetes risk, improved insulin sensitivity, and decreased adiposity (9–11). On the other hand, a meta-analysis of randomized controlled clinical trials showed that dairy consumption resulted in a decrease in body weight only in the setting of an energy-restricted diet (12). There is also a concern that SFAs, which are high in full-fat dairy, may have a detrimental effect on glucose and cardiovascular disease risk (13). It is unknown whether this effect is different when consumed as part of complex food matrices, such as those in dairy foods.
The prevalence of type 2 diabetes is ∼13% of adults in the USA (14). Despite the abundance of studies in individuals without diabetes with some evidence suggesting that higher serum markers of dairy fat may be positively associated with insulin sensitivity in healthy adults (11), research among individuals with type 2 diabetes is lacking. Understanding the effect of dairy consumption on glycemic control is crucial, given the importance of dietary modification in diabetes management. We hypothesized that increasing dairy consumption to ≥3 servings/d, irrespective of its fat content, will not negatively affect glycemic control or any of the common cardiovascular disease (CVD) risk factors in patients with type 2 diabetes.
Methods
Subjects
This is a randomized controlled single-center clinical trial that enrolled adults with type 2 diabetes with glycated hemoglobin (HbA1c) >7% on stable doses of antihyperglycemic medications for ≥3 mo. The institution's Committee on Human Studies approved the study protocol, and participants signed informed consent forms prior to enrollment in the study. This trial was registered at clinicaltrials.gov as NCT02895867. The study protocol was conducted in accordance with the principles described in the Declaration of Helsinki. Eligible participants for this study were: patients diagnosed with type 2 diabetes for ≥3 mo; between the age of 18 and 75 y; BMI ≥25 kg/m2; body weight maintained within a 10% weight loss or gain during the 6 mo leading up to study enrollment; and on stable doses of antihyperglycemic, antihypertensive, and lipid-lowering medications for ≥3 mo. Participants were asked during the screening process to recall their dairy intake over the last week and to report if it was more than, less than, or about the same as their usual dairy intake. Participants consuming ≥3 servings/d of dairy products were excluded. Exclusion criteria also included: pregnancy; lactose intolerance; cow milk allergy; use of orlistat; enrollment in weight management programs/trials; recent cardiovascular event (e.g. myocardial infarction, stroke) ≤6 mo prior to the screening visit; history of congestive heart failure, active malignancy (excluding carcinoma in situ of the cervix and the following dermal malignancies: basal cell carcinoma and squamous cell carcinoma); bariatric surgery; pancreatitis; and chronic gastrointestinal disease (e.g., Crohn's disease, ulcerative colitis, chronic malabsorption, chronic diarrhea, and gastroparesis).
Study design
Participants were randomly assigned, in a 1:1:1 ratio, to 1 of 3 dietary intervention groups. The control group was instructed to change neither their baseline dairy consumption nor any other aspect of their diets. The LF group was instructed to consume ≥3 servings of LF dairy products (<2% fat) per day. The HF group was instructed to consume ≥3 servings of HF dairy products (≥2% fat) per day. Subjects were followed for 24 wk. Randomization was performed centrally by means of computer-generated permuted blocks of size 3. A study coordinator or coinvestigator generated the random allocation sequence, enrolled participants, and assigned participants to interventions. All participants were instructed to maintain their stable doses of antihyperglycemic, antihypertensive, and cholesterol-lowering medications without any changes throughout the study duration. Subjects taking calcium supplements were asked to take ≤500 mg/d of elemental calcium throughout the study.
Dietary intervention
Study participants were instructed to complete a 3-d food log prior to baseline evaluation. All 3 groups received nutritional counseling from a registered dietitian aiming at maintaining baseline energy intake and body weight throughout the study. Participants in the control group were educated by the study's registered dietitian about what constitutes a dairy serving. They were also asked to maintain their baseline dairy intake. In the intervention groups, the study's registered dietitian worked individually with each participant to help them make isocaloric changes in their diet plan to increase dairy consumption without increasing overall caloric intake. Participants were instructed to consume milk, yogurt, and/or cheese as part of their 3+ servings/d of dairy products. Participants were advised that butter, dairy-based desserts such as ice cream, sour cream, cream cheese, cream, and half-and-half would not count towards their 3 daily servings of dairy products; however, they were still instructed to record intake of these foods if consumed. Participants in the LF and HF groups were educated about serving sizes and fat contents of different dairy products. A serving size of dairy was defined using the USDA definition of a serving size: 8 fluid ounces (237 mL)of milk, 8 fluid ounces of yogurt, and 1.5 ounces (42.5 g) of hard cheese (e.g., cheddar, Swiss, etc.) or 2 ounces (56.7 g) of processed cheese (e.g., American cheese) each counted as 1 serving of dairy (15). In order to increase dairy consumption to 3 servings/d without modifying daily total energy intake (TEI), participants in the LF group were asked to substitute other foods in their daily diets with LF dairy products. For example, a participant may have been asked to replace their usual 150 calorie snack of a granola bar with 8 ounces of LF yogurt. Similarly, a participant in the HF group may have been asked to replace a 150–200 calorie snack of a granola bar or protein bar with 1.5 ounces of hard cheese. To allow investigators to monitor dairy purchases, subjects in the LF and HF groups were provided with a prepaid debit card with which to purchase dairy products along with preaddressed envelopes to mail back receipts for all dairy product purchases every 4 wk. Participants in all 3 groups were asked to record their daily intake of dairy products, including type and amount, in a separate dairy log-book. In addition, all study participants were asked to record their total food intake for 3 d in a designated log-book before each study visit (baseline, 12 wk, and 24 wk). At each of these visits, the study dietitian reviewed the 3-d food log-book and daily dairy log-book with each participant in order to reconcile any missing data. A daily dairy log-book was used to estimate average daily dairy intake over 12 and 24 wk. Changes in TEI and dietary macronutrients were assessed by analyzing 3-d food logs collected at baseline and after 24 wk using Food Processor Diet & Nutrient Analysis Software (version 11.1.620, 2015, ESHA Research). To narrow the study's explanatory variables to those related to dairy consumption, study participants were asked to maintain their baseline physical activity level without any changes throughout the study period and were neither given specific exercise or behavioral recommendations nor asked to keep any exercise logs.
Study procedures
Each participant was instructed to arrive after an overnight fast for 3 study visits over a period of 24 wk. Visits occurred at baseline, 12 wk, and 24 wk. At each visit, participants met with the study's registered dietitian for counseling according to their assigned group. The registered dietitian also instructed participants on tracking and recording their daily dairy consumption in a dedicated dairy log-book. Similar procedures were done at the 12- and 24-wk study visits. In addition to the 3 in-person visits, each participant received 5 follow-up phone calls from 1 of the study investigators at weeks 2, 4, 8, 16, and 20 to answer any questions, to motivate participants, and to improve adherence.
Anthropometric measurements and venous blood samples were taken at each visit by a trained clinical research nurse/nurse practitioner or study team member following standard protocol. Venous whole blood samples for HbA1c measurement were collected using a BD Vacutainer® K2 EDTA 7.2 mg (BD, Becton Dickinson). Blood was collected until vacuum was exhausted and blood ceased to flow, then gently inverted 8–10 times to disperse anticoagulants. For fasting plasma glucose, fasting serum insulin, lipid profile, and C-reactive protein (CRP) measurements, blood was collected in 2 BD Vacutainers® serum separator tube (SST™, BD), gently inverted 5–6 times to disperse clot activator, placed in a vertical position for 30 min to clot, then centrifuged at 1100–1300 × g for 10 min. Tubes were properly labeled and shipped in ambient temperature to a central laboratory for analysis (LabCorp). HbA1c, glucose, and CRP were analyzed by immunoturbidimetric assay (instruments C513, C701, and 502, respectively, Roche Diagnostics). Insulin concentrations were analyzed by electrochemiluminescent immunoassay (ECLIA, instrument E602, Roche Diagnostics). The lipid profile was analyzed by enzymatic colorimetric assay (instrument C702, Roche Diagnostics). BP was measured in the seated position using a CARESCAPE™ V100 monitor (GE Medical Technologies). Body weight was measured using a calibrated scale (Tanita BWB–800). Body composition measurements were performed using a bioelectrical impedance analyzer (Tanita TBF–215). Visceral fat was measured using a validated bioelectrical impedance device (Tanita, Viscan AB–140) and was expressed in arbitrary units ranging from 1 to 59 (16). Height was measured without shoes. Waist circumference was measured just above the hip bone, and hip circumference was measured around the maximum circumference of the buttocks. Insulin sensitivity was calculated using the HOMA-IR equation from fasting glucose and insulin at baseline and after 12 and 24 wk.
Study endpoints
The primary endpoint of this study was the change in HbA1c concentrations from baseline to 24 wk in the 3 groups. Secondary endpoints included changes in body weight; BMI; body composition parameters; waist and hip circumferences; TEI from fat, saturated fat, carbohydrates, and protein; LDL cholesterol; HDL cholesterol; VLDL cholesterol; triglycerides; CRP; systolic and diastolic BP; changes in glycemic parameters including fasting plasma glucose and HOMA-IR; and changes in dietary macronutrient composition from baseline to 24 wk in the 3 groups.
Statistical analyses
In a study conducted previously by our group using the same design and analytic approach, and with an intervention expected to induce a similar magnitude of effect to the current trial, we observed between-group differences in change in HbA1c over time of >0.5%. Based on these data and assuming a linear mixed models approach to analyzing the data from the current trial, we estimated that 30 subjects in each group were needed to achieve 90% power to detect a significant (at a 2-sided 5% level) difference of 0.5% in mean HbA1c between any 2 groups at 24 wk compared with baseline, so 112 subjects were randomized to allow for a planned 25% attrition. These results were calculated using the longpower routine in R that implements the methods reported here (17). All study-related quantitative, qualitative, and clinical data were collected and managed using REDCap (Research Electronic Data Capture) (18). Primary and secondary endpoints were analyzed on an intent-to-treat (ITT) basis including all randomly assigned subjects as well as in the per-protocol (PP) population consisting of all randomly assigned subjects who completed the study without major protocol violations of inclusion/exclusion criteria (Figure 1) (1 subject was excluded for having positive pancreatic antibodies). Baseline, 12-wk, or, when available, clinic lab data were used to replace missing HbA1c (56/333), fasting glucose (56/333), body weight (56/333), lipid profile (308/1665), and BP (114/666) data in the ITT analysis. Missing data were handled using multiple imputations in the ITT analysis using the SAS PROC MI procedure. Primary and secondary endpoints were analyzed using a linear mixed-effects model (analogous to repeated-measures ANOVA; PROC MIXED) with group, visit, and group-by-visit interaction as fixed effects and subject as the random effect. We began the statistical analysis with an unadjusted model. Given the possible role of TEI as a mediator in the association of HF dairy consumption with weight gain, we added TEI to the model, and results were similar to the unadjusted model. We then added baseline HOMA-IR, which was higher in the HF group, and percent subjects on insulin treatment, which may possibly influence weight change, to the model, and results showed neither covariates had an effect on study outcomes. To explore whether consumption of different types of dairy products (fermented compared with nonfermented) would affect study outcomes, we added average servings per day of fermented dairy products to our model. We also added age and gender to our model, and neither covariates showed an effect on study outcomes. Thus, results for the PP and ITT populations are reported for the unadjusted model. Cross-sectional comparisons were performed using linear contrasts using the SAS Mixed Procedure (PROC MIXED). In addition to the end of trial data analysis, the data for this trial were analyzed when enrollment was 64% complete, and the results were presented at the American Diabetes Association's 78th Scientific Sessions. Because the outcome of this analysis was not intended to, and did not, influence the conduct of the trial or the final analyses, no multiplicity adjustments were made to either analysis (19, 20). A 2-sided P <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc.) (outcomes) or STATA SE 15.0 (StataCorp) (demographic data).
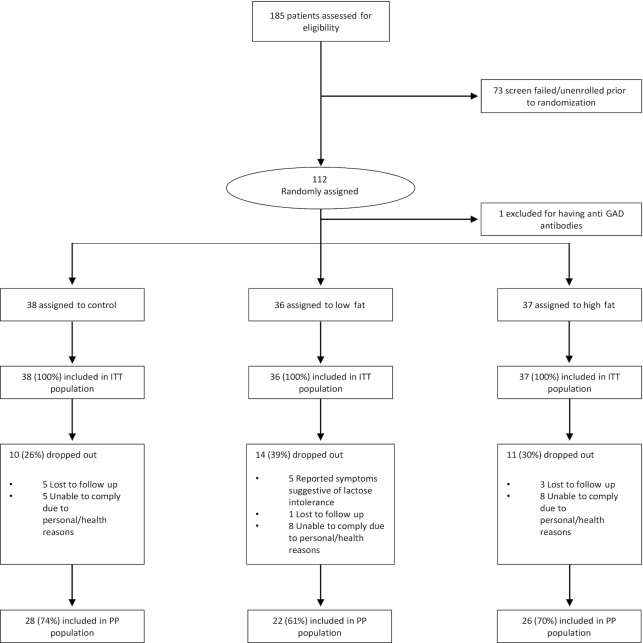
FIGURE 1.
Flow of study participants. GAD, glutamic acid decarboxylase; ITT, intent-to-treat; PP, per-protocol.
Results
Of 185 screened participants, 112 met the eligibility criteria and were randomly assigned to 1 of the 3 intervention groups (Figure 1). One participant was excluded after randomization for having positive pancreatic antibodies. Of the included participants, 32% dropped out at different intervals throughout the study. The dropout rate was not significantly different between groups (P = 0.5), and baseline characteristics of the completers were not different from noncompleters (Supplementary Table 1).
The mean (± SD) baseline HbA1c of the entire group was 8.09 (± 0.96)%, and their mean diabetes duration was 13.2(± 8.3) y. Demographic data of the study participants are shown in Table 1.
TABLE 1.
Baseline characteristics of the ITT population
| All participants (n = 111) | Control group (n = 38) | Low-fat group (n = 36) | High-fat group (n = 37) | |
|---|---|---|---|---|
| Age, y | 58.5 ± 8.9 | 58.3 ± 9.3 | 58.7 ± 7.6 | 58.4 ± 9.8 |
| Sex, F % | 47 | 44.7 | 44.4 | 51.4 |
| Duration of diabetes, y | 13.2 ± 8.3 | 12.1 ± 7.2 | 14.2 ± 7.8 | 13.4 ± 9.8 |
| Total daily energy intake, kcal | 1925 ± 553 | 2041 ± 600 | 1944 ± 553 | 1785 ± 480 |
| Energy from total fat, % | 37.8 ± 5.5 | 38.6 ± 6.0 | 37.3 ± 5.0 | 37.6 ± 5.6 |
| Energy from saturated fat, % | 13.1 ± 2.9 | 13.8 ± 3.1 | 12.7 ± 2.8 | 12.6 ± 2.8 |
| Energy from carbohydrate, % | 43.4 ± 7 | 43.6 ± 7.5 | 44.0 ± 6.9 | 42.7 ± 6.8 |
| Energy from protein, % | 18.2 ± 3.7 | 17.5 ± 3.4 | 18.2 ± 3.7 | 18.9 ± 3.9 |
| HbA1c, % | 8.09 ± 0.96 | 7.99 ± 0.97 | 8.09 ± 0.76 | 8.21 ± 1.14 |
| Body weight, kg | 93 ± 19 | 97.3 ± 20.5 | 91.0 ± 17.5 | 91.4 ± 18.4 |
| BMI, kg/m2 | 32.5 ± 5.7 | 33.24 ± 5.99 | 32.06 ± 6.47 | 32.09 ± 4.46 |
| Waist circumference, cm | 110 ± 14 | 109.1 ± 13.8 | 109.2 ± 13.6 | 111.7 ± 14.6 |
| Hip circumference, cm | 109 ± 12 | 110.4 ± 10.6 | 107.9 ± 14.9 | 110.2 ± 11.6 |
| Waist/hip ratio | 1 ± 0.08 | 0.99 ± 0.08 | 1.02 ± 0.07 | 1.01 ± 0.09 |
| Body fat, % | 36.7 ± 8.4 | 36.7 ± 7.6 | 35.1 ± 10.1 | 38.4 ± 7.3 |
| Fat mass, kg | 33.4 ± 12 | 34.6 ± 23.8 | 32.2 ± 13.3 | 33.3 ± 12 |
| Fat-free mass, kg | 56.5 ± 13.9 | 59.2 ± 13.8 | 57.6 ± 13.3 | 52.6 ± 13.9 |
| Total body water, kg | 41.3 ± 10.1 | 43.3 ± 10.1 | 42.1 ± 9.7 | 38.5 ± 10.2 |
| Trunk fat, % | 40.8 ± 7.6 | 41 ± 6.5 | 38.6 ± 8.6 | 42.6 ± 7.3 |
| Visceral fat, arbitrary units | 16.3 ± 6.1 | 17.1 ± 6.6 | 15.2 ± 4.8 | 16.7 ± 6.6 |
| CRP, mg/L | 4.7 ± 6.6 | 3.1 ± 3.8 | 6.0 ± 8.9 | 5.1 ± 6.1 |
| Fasting glucose, mg/dL | 163 ± 61 | 156 ± 45 | 160 ± 54 | 175 ± 78 |
| HOMA-IR | 7.3 ± 5.9 | 6.8 ± 5.3 | 5.9 ± 4.2 | 9.3 ± 7.2 |
| Systolic blood pressure, mmHg | 130 ± 16 | 132 ± 16 | 131 ± 17 | 129 ± 16 |
| Diastolic blood pressure, mmHg | 71 ± 9 | 73 ± 8 | 71 ± 11 | 70 ± 8 |
| Lipid profile | ||||
| Total cholesterol, mg/dL | 163 ± 37 | 155 ± 34 | 166 ± 36 | 167 ± 40 |
| HDL-C, mg/dL | 47 ± 15 | 48 ± 16 | 48 ± 16 | 45 ± 12 |
| LDL-C, mg/dL | 84 ± 27 | 78 ± 25 | 88 ± 31 | 86 ± 26 |
| VLDL-C, mg/dL | 28 ± 13 | 24 ± 10 | 29 ± 14 | 30 ± 13 |
| Triglycerides, mg/dL | 164 ± 155 | 150 ± 121 | 157 ± 93 | 183 ± 222 |
Data are mean + SD or %. Analyses were performed by linear contrasts using the SAS Mixed Procedure (PROC MIXED). CRP, C-reactive protein; HbA1c, glycated hemoglobin; ITT, intent-to-treat.
At the end of the study, the intake of dairy foods was 1.4 ± 0.8 servings/d in the control group, 3.0 ± 0.7 servings/d in the LF group, and 3.0 ± 0.7 servings/d in the HF group, which indicates that LF and HF groups increased their total dairy intake as instructed. The HF group consumed, on average, 9.6 ± 4.4 and 9.6 ± 5.6 servings/wk of fermented and nonfermented dairy products, respectively. The LF group consumed, on average, 9.5 ± 5 and 10.5 ± 6.8 servings/wk of fermented and nonfermented dairy products, respectively. The control group consumed, on average, 4.3 ± 2.9 and 4.2 ± 3.6 servings/wk of fermented and nonfermented dairy products, respectively.
There was no statistically significant difference in the change in TEI between the 3 groups. As expected, percent TEI from fat was significantly different between the 3 groups (P = 0.00007) at 24 wk, being significantly lower in the LF group by −6% (95% CI: −8.9, −3, P = 0.00004) compared with baseline. Similarly, percent TEI from saturated fat was significantly different between the 3 groups (P = 0.00002) at 24 wk, being significantly higher in the HF group by 3.6% (95% CI: 2.2, 5.1; P = 0.000002) compared with baseline. These changes indicate good compliance to the planned dietary intervention. The LF group compensated their reduction of fat intake by increasing carbohydrate and protein intake (Table 2). On the contrary, intake of carbohydrates and protein was lower in the HF group (Table 2). Of note, the control group increased their consumption of total fat at 24 wk; however, this increase was not paralleled by a change in percent TEI from saturated fat (Table 2).
TABLE 2.
Change from baseline in anthropometric, cardiometabolic, and dietary intake parameters in response to increase in dairy intake in the ITT population
| Control group (n = 38) | Low-fat group (n = 36) | High-fat group (n = 37) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 12 wk | 24 wk | 12 wk | 24 wk | 12 wk | 24 wk | P 4 | P5 | P6 | |
| Total daily energy intake, kcal | 67 (−97, 231) | 90 (−92, 273) | 124 (−45, 293) | 26 (−168, 220) | 272 (105, 439) | 255 (64, 446) | 0.33 | 0.6 | 0.006 |
| Energy from total fat, % | 0.2 (−2.3, 2.8) | 2.8 (0.2, 5.3)1 | −3.7 (−6.4, −1.1)2 | −6.0 (−8.9, −3.0)3 | 0.7 (−2.0, 3.3) | 2.5 (−0.4, 5.4) | 0.00007 | 0.0001 | 0.4 |
| Energy from saturated fat, % | −0.3 (−1.7, 1.1) | −0.1 (−1.5, 1.3) | −0.8 (−2.3, 0.6) | −1.9 (−3.3, −0.4)1 | 2.9 (1.4, 4.4)3 | 3.6 (2.2, 5.1)3 | 0.00002 | 0.00004 | 0.3 |
| Energy from carbohydrate, % | −0.3 (−3.1, 2.5) | −1.6 (−4.7, 1.5) | −0.8 (−3.6, 2.1) | 1.9 (−1.3, 5.2) | −1.8 (−4.6, 1.0) | −3.4 (−0.2, −6.7)1 | 0.03 | 0.08 | 0.23 |
| Energy from protein, % | −0.2 (−2.0, 1.6) | −0.7 (−2.5, 1.1) | 4.6 (2.7, 6.4)3 | 4.5 (2.6, 6.4)3 | 0.6 (−1.3, 2.4) | 0.8 (−1.1, 2.7) | 0.0014 | 0.00002 | 0.0058 |
| HbA1c, % | 0.1 (−0.19, 0.4) | 0.02 (−0.34, 0.39) | 0.31 (0.01, 0.6) | 0.37 (−0.02, 0.77) | 0.25 (−0.05, 0.54) | 0.23 (−1.16, 0.6) | 0.77 | 0.32 | 0.04 |
| Body weight, kg | 0.7 (0, 1.4) | 0.7 (−0.1, 1.6) | 0.1 (−0.7, 0.8) | −0.2 (−1, 0.6) | 0.6 (−0.1, 1.3) | 1 (0.1, 1.8) | 0.4 | 0.25 | 0.08 |
| BMI, kg/m2 | 0.2 (−0.03, 0.44) | 0.23 (−0.03, 0.5) | 0.02 (−0.21, 0.26) | −0.09 (−0.37, 0.2) | 0.18 (−0.06, 0.41) | 0.32 (0.04, 0.61) | 0.32 | 0.54 | 0.09 |
| Waist circumference, cm | 1.6 (−0.2, 3.4) | 2.0 (−0.3, 4.4) | −0.6 (−2.5, 1.2) | −0.4 (−3.0, 2.2) | 0.7 (−1.1, 2.5) | 0.6 (−1.9, 3.0) | 0.45 | 0.6 | 0.46 |
| Hip circumference, cm | 2.4 (0.6, 4.1)2 | 2.4 (0.1, 4.7)1 | 0.6 (−1.2, 2.4) | 1.3 (−1.3, 3.9) | −1.6 (−3.3, 0.2) | 0.4 (−2.0, 2.7) | 0.045 | 0.5 | 0.16 |
| Waist/hip ratio | 0 (−0.02, 0.01) | 0 (−0.02, 0.02) | −0.02 (−0.03, 0.003) | −0.02 (−0.04, 0.001) | 0.02 (0.0003, 0.04) | 0.001 (−0.02, 0.02) | 0.07 | 0.14 | 0.5 |
| Body fat, % | −0.9 (−2.6, 0.8) | 0.8 (−1.2, 2.7) | −0.9 (−2.6, 0.8) | −1.6 (−3.7, 0.6) | −0.8 (−2.5, 0.8) | −1.4 (−3.4, 0.9) | 0.48 | 0.23 | 0.06 |
| Fat mass, kg | −0.7 (−4.2, 2.7) | −1.3 (−5.3, 2.7) | −2.8 (−6.4, 0.7) | −2.6 (−7.2, 1.9) | −2.5 (−6, 1) | −1.3 (−5.5, 2.8) | 0.9 | 0.37 | 0.08 |
| Fat-free mas, kg | 2.2 (−4.2, 8.7) | −2.3(−8.5, 3.8) | −3.5 (−10.2, 3.2) | 0.8(−6.1, 7.7) | −1 (−7.4, 5.5) | 1.2 (−5.1, 7.5) | 0.44 | 0.12 | 0.9 |
| Total body water, kg | 1.7 (−3.1, 6.4) | −1.7 (−6.2, 2.7) | −2.6 (−7.5, 2.3) | 0.5 (−4.4, 5.6) | −0.7 (−5.4, 4) | 1.3 (−3.3, 5.9) | 0.4 | 0.14 | 0.9 |
| Trunk fat, % | 0.5 (−0.8, 1.8) | 0.1 (−1.3, 1.5) | 0.6 (−0.7, 2) | −0.2 (−1.7, 1.3) | −0.5 (−1.8, 0.7) | −0.4 (−1.8, 1) | 0.7 | 0.12 | 0.67 |
| Visceral fat, arbitrary units | −0.1 (−1.1, 0.9) | −0.3 (−1.6, 0.9) | 0.06 (−1, 1.1) | −0.2 (−1.5, 1.2) | −0.4 (−1.4, 0.6) | −0.2 (−1.5, 1) | 0.9 | 0.32 | 0.8 |
| CRP, mg/L | −0.3 (−2.2, 1.6) | 1.2 (−1.1, 3.6) | −2 (−0.1, −3.9) | −2 (−4.5, 0.5) | −1.6 (−3.4, 0.3) | −1.5 (−3.9, 0.9) | 0.33 | 0.33 | 0.03 |
| Fasting glucose, mg/dL | 3 (−18, 23) | −13 (−36, 10) | 12 (−9, 33) | −2 (−26, 22) | −3 (−24, 18) | −15 (−38, 9) | 0.88 | 0.2 | 0.03 |
| HOMA-IR | 0 (−9.4, 9.3) | −1.3 (−10.8, 8.3) | 0.4 (−9.2, 9.9) | −0.6 (−11.0, 9.8) | −1.0 (−10, 8) | 10.6 (0.8, 20.4) | 0.31 | 0.06 | 0.5 |
| Systolic blood pressure, mmHg | −2 (−7, 3) | −5 (−10, 1) | −5 (−10, 1) | −1 (−7, 5) | −1 (−7, 4) | 1 (−5, 6) | 0.39 | 0.8 | 0.3 |
| Diastolic blood pressure, mmHg | −2 (−5, 1) | −2 (−5, 1) | −1 (−4, 2) | 2 (−1, 5) | 2(−1, 5) | −1 (−4, 3) | 0.16 | 0.75 | 0.8 |
| Lipid profile | |||||||||
| Total cholesterol, mg/dL | −1 (−8, 7) | 1 (−10, 11) | −2 (−9, 6) | 4 (−8, 15) | 1 (−6, 8) | 13 (2, 24) | 0.6 | 0.22 | 0.16 |
| HDL-C, mg/dL | −1 (−3, 1) | 0 (−2, 2) | −4 (−6, −2) | −1 (−3, 1) | 1 (−1, 4) | 1 (−2, 3) | 0.07 | 0.9 | 0.33 |
| LDL-C, mg/dL | 0 (−7, 7) | 0 (−11, 10) | −1 (−8, 6) | 5 (−7, 16) | −3 (−10, 3) | 7 (−3, 18) | 0.68 | 0.23 | 0.2 |
| VLDL-C, mg/dL | 0 (−3, 4) | 2 (−2, 6) | 3 (−1, 7) | 4 (−1, 9) | 4 (0.3, 8) | 6 (1, 10) | 0.64 | 0.06 | 0.008 |
| Triglycerides, mg/dL | 14 (−32, 61) | 17 (−10, 43) | 51 (4, 99) | 10 (−19, 39) | 11 (−36, 58) | 18 (−10, 45) | 0.65 | 0.66 | 0.07 |
Data are mean (95% CI).
P < 0.05.
P < 0.01.
P < 0.001 compared with baseline.
P value for group*time interaction.
P value for group effect.
P value for time effect.
P values calculated from unadjusted analyses using a linear mixed-effects model (analogous to repeated-measures ANOVA; PROC MIXED) with group, visit, and group-by-visit interaction as fixed effects and subject as a random effect.
CRP, C-reactive protein; HbA1c, glycated hemoglobin.
Effect on HbA1c, body weight, BMI, and body composition parameters
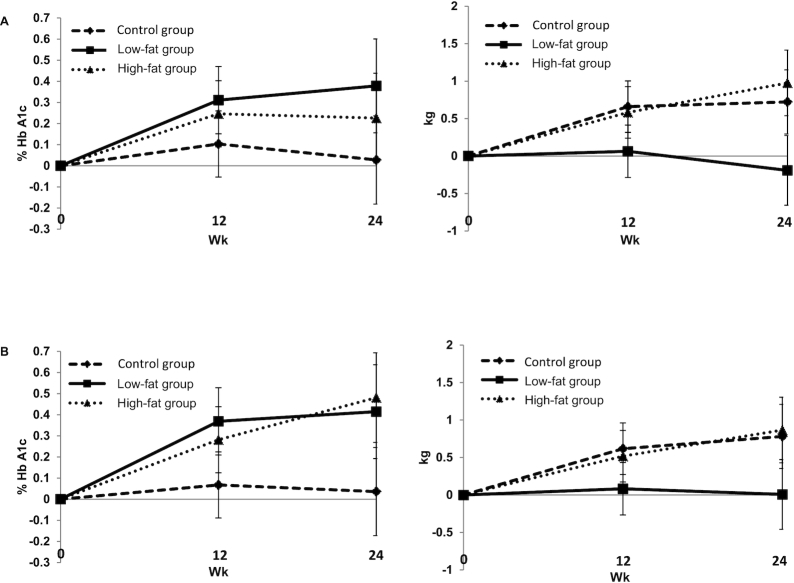
At 24 wk, there was no statistically significant difference in the mean change in HbA1c or body weight between the 3 groups (Figure 2). Similarly, there were no significant changes from baseline to 24 wk in HbA1C, body weight, BMI, waist circumference, waist-to-hip ratio, or body composition parameters between groups (Table 2). Adding type of dairy to our model did not affect the outcomes.
FIGURE 2.
Least-square mean difference ± SEM in HbA1c and body weight during the study by treatment group in the intent-to-treat population (A) and per-protocol population (B). Intent-to-treat population: control group (n = 38); low-fat group (n = 36); high-fat group (n = 37). Per-protocol population: control group (n = 28); low-fat group (n = 22); high-fat group (n = 26). There were no significant differences between the changes in HbA1c and body weight in the 3 groups. Analyses were performed using a linear mixed-effects model (analogous to repeated-measures ANOVA; PROC MIXED) with group, visit, and group-by-visit interaction as fixed effects and subject as a random effect. HbA1c, glycated hemoglobin.
Effect on inflammation, BP, glycemia, and lipid profile
At 24 wk, there were no significant differences in the mean changes in CRP, fasting glucose, HOMA-IR, or BP between the 3 groups. Despite the relative increase in percent TEI from total and saturated fat in the HF group, there were no significant differences in the mean changes in LDL cholesterol, HDL cholesterol, triglycerides, or VLDL cholesterol between the 3 groups or from baseline to 24 wk (Table 2).
Discussion
This study shows that advising subjects with type 2 diabetes to increase consumption of dairy foods to ≥3 servings/d, irrespective of dairy fat content, has no significant effect on HbA1c, body weight, body composition parameters, lipid profile, and BP compared with maintaining a diet with <3 servings/d. The LF group increased their energy intake from protein while decreasing their fat intake. On the other hand, the HF group increased their energy intake from fat while decreasing their carbohydrate intake. Previous studies have in general showed a beneficial effect on insulin resistance; higher dairy intake was positively associated with greater systemic and hepatic insulin sensitivity and better response to an oral-glucose-tolerance test (21), an effect that could be attributed to the insulinotropic effects of dairy amino acids (22). Similar to amino acids, dairy-derived fatty acids may have protective metabolic effects (10, 23), and has been inversely associated with fasting plasma glucose (20) and lower incidence of diabetes (24). The effect of dairy on glycemic markers in previous RCTs is consistent. In a meta-analysis of RCTs, HbA1c was inversely associated with an elevated intake of dairy products (2.7 servings/d, on average) in comparison to minimal intake (0.8 servings/d, on average) although the observed HbA1c reduction was small with a mean difference: −0.09%; 95% CI: −0.09%, −0.03%; P = 0.005, I2 = 0% (25). Several factors may explain this observed difference in the effects of dairy on glycemia. First, it is the difference in insulin resistance in people with diabetes compared with those without diabetes. Another difference is the relatively higher level of dairy consumption in this study (3 servings/d in both intervention groups compared with 1.4 servings/d in the control group) compared with the average dairy consumption in the meta-analysis (2.7 servings/d in the dairy groups compared with 0.8 servings/d in the minimal dairy intake groups) (25). In another meta-analysis of 14 RCTs, where the majority of subjects had BMI >25, HOMA-IR was lower with increased dairy intake (26). In our study, the average intake of dairy foods was 3.0 ± 0.7 servings/d in the LF group and 3 ± 0.7 servings/d in the HF group, which is slightly higher than the average consumption in the pooled meta-analyses. It is possible that dairy may have beneficial effects in individuals with prediabetes or at high risk of diabetes, as suggested by previous observational studies, and this benefit disappears when the disease has progressed to a stage where it is unlikely for lifestyle intervention to have an effect as seen in our study.
Meta-analyses of several large cohort studies have consistently shown that dairy intake was associated with a lower risk of diabetes (2, 25, 27, 28). For example, in a meta-analysis of 22 cohort studies comprised of 579,832 individuals, total dairy intake was inversely associated with type 2 diabetes risk (RR: 0.97 per 200-g/d increment; 95% CI: 0.95, 1.00; P = 0.04); however, there was heterogeneity (2). This effect was attributed to the consumption of LF dairy, particularly yogurt, whereas the effect of HF dairy was nonsignificant. When comparing HF to LF, the substitution of 1 serving/d of yogurt or reduced-fat milk for cheese was associated with a 16% (95% CI: 10%, 22%) or 12% (95% CI: 8%, 16%) lower type 2 diabetes risk, respectively (28). Another cohort study suggested that substituting 1 serving/d of HF yogurt with LF yogurt was associated with a higher risk of type 2 diabetes (HR 1.17; 95% CI: 1.06, 1.29), whereas substituting 1 serving/d of LF milk, HF milk, or buttermilk with HF yogurt was associated with a lower risk of type 2 diabetes (HR 0.89; 95% CI: 0.83, 0.96; HR 0.89; 95% CI: 0.82, 0.96; HR 0.89; 95% CI: 0.81, 0.97; per serving/d substituted of LF milk, HF milk, or buttermilk, respectively) (27). It is possible that the effects of dairy on cardiometabolic health may be product-specific possibly via matrix effects. In our study, adding average servings per day of fermented dairy products to the model had no effect on study outcomes.
In a randomized clinical trial of a weight maintenance diet for 12 wk that compared overweight or obese adults with metabolic syndrome who consumed high-dairy diets (3.5 daily servings) versus low-dairy diets (0.5 daily servings), there was no significant difference in fasting plasma glucose or body weight between the 2 groups. However, the high-dairy group had a significant reduction in plasma insulin and improvements in HOMA-IR (29). It is worth noting that previous studies have used different comparators or different weight-maintaining strategies, and none of them had a similar study design to ours for clear relevance. For example, a 6-wk crossover study among postmenopausal women with metabolic syndrome compared the consumption of ∼3 servings/d of 2% milk to a diet devoid of dairy (30). The study demonstrated that the consumption of 2% milk had no effect on insulin sensitivity (30–32). Whereas, in comparison to lower dairy intake (1–2 servings/d), high intake of LF dairy products (4 servings/d) improved insulin resistance as measured by HOMA-IR by 11% (P = 0.03) and had no effect on body weight and lipid profile over 6 mo (6). Similar to our study, participants were instructed to maintain their usual diet and physical activity levels and were advised to incorporate dairy by substitution so as not to increase energy intake. However, that intervention was limited to LF dairy and to adults with overweight and obesity. A study by Wennersberg et al. conducted among patients with metabolic syndrome showed that adding 3–5 servings of dairy products for 6 mo had no effect on body composition but was associated with improvement in HOMA-IR (7). However, a key difference is that we controlled energy intake.
Our study showed no significant benefit of increasing dairy consumption on CVD risk factors, rather the LF and HF dairy groups had a nonsignificant increase in HbA1c by 0.37% (95% CI: −0.02, 0.77) and 0.23% (95% CI: −1.16, 0.6) at 24 wk, respectively. It is possible that the effect of dairy on glycemia is different in people with diabetes compared with those without diabetes or those with high risk of diabetes. Our patient population had type 2 diabetes for a relatively long duration (13.2 ± 8.3 y). The longer duration of diabetes is associated with progressive loss of β-cell function (33). It is possible that this explains the absence of improvement in CVD risk factors.
Our study did not show a difference in weight change between the 3 groups; however, body weight was higher from baseline in the HF group. This observation might be explained by an increased intake of energy-dense fat. Twenty studies enrolling 1677 individuals showed that increased dairy intake was associated with modest weight gain (+0.60 kg, 95% CI: 0.30, 0.90 kg, P <0.0001). This effect was similar to LF and HF dairy (34). Our study confirms other observations that in the absence of caloric restriction, high-dairy consumption may be linked to increased body weight (12).
This study did not show differences in the impact of higher consumption of dairy products, irrespective of their fat content, on BP. However, increased consumption of LF and HF dairy products was associated with 5 mg/dL (95% CI: −7, 16) and 7 mg/dL (−10, 3) increase in LDL cholesterol, respectively, although these changes were not statistically significant. It is important to note that consumption of dairy food is frequently associated with a better lifestyle and an overall healthier eating pattern, which are consequently associated with more favorable cardiometabolic profiles (35).
Our study has several limitations. It was conducted at a single tertiary care facility, which limits generalization of the study. Participants were not directly observed while consuming dairy products; however, each participant completed a daily log-book in order to report accurate dairy intake, while frequent follow-up with a registered dietitian encouraged better adherence. Although it was difficult to confirm their proper consumption of dairy as instructed, participants provided us with purchasing receipts to confirm buying dairy products. The increase in energy intake in the HF group and the subsequent modest gain in body weight reflects a suboptimal ability to incorporate dairy food into a diet while maintaining baseline energy intake. It is important to recognize that the observed effect is not only related to the addition of dairy but also depends on the nutrients that were removed from the diet to replace dairy. To mitigate this bias, participants were asked to incorporate dairy by substitution with other foods with similar macronutrient composition. Another limitation is the study attrition rate, which was 32%. A higher attrition rate (49.3%) was seen in a relatively similar intervention (36). It is unlikely that attrition rates would have an impact on results from our study as attrition was not significantly different between groups.
Despite randomization, HOMA-IR was unbalanced at baseline. Although HOMA-IR is frequently used in clinical trials, it has limitations in reflecting true insulin sensitivity in comparison to the gold standard techniques of measuring insulin sensitivity (37). Nevertheless, adjusting for it showed no effect on the study outcomes. Due to possible mediation by TEI and the association with weight gain, we included TEI in our model, and this did not affect the results. The proportion of subjects on insulin treatment was numerically higher in the LF group compared with the HF and control groups; however, in our adjusted model, percentage of subjects on insulin had no effect on study outcomes. Epidemiologic data have shown strong inverse association of dairy intake with HbA1c in people without diabetes. It is possible that antihyperglycemic medications might have weakened the association of higher dairy intake and HbA1c.
In conclusion, this study suggests that within the same caloric intake, increased dairy consumption, irrespective of its fat content, to ≥3 servings/d in patients with type 2 diabetes has no effect on HbA1c, body weight, lipid profile, or BP compared with maintaining the same diet with dairy consumption of <3 servings/d.
Longer duration studies with larger sample sizes are needed to evaluate whether it is possible to maintain higher dairy intake for longer than 24 wk and whether the metabolic effect of high-dairy consumption in diabetes is sustainable. It would also be helpful to know whether specific dairy products have different effects (27).
Supplementary Material
Acknowledgments
We thank the nursing staff of the clinical research center (CRC) at Joslin Diabetes Center for their support in conducting this study. We are grateful to Taha Elseaidy for his substantial coordination efforts, Padraig Carolan for his initial administrative support and Ahmad Al Maradni; Khaled Alsibai; Noor Mahmoud; Brakatun Nisak Mohd Yusof; Sue Ellen Anderson-Haynes; and Cara Schrager who conducted study visits at different time periods but were not involved in study design, data analysis, or preparation of this manuscript for publication.
The authors’ responsibilities were as follows—JM: contributed to study design and prepared the first draft of the manuscript; ST: collected data, conducted statistical analysis, and prepared the first draft of the manuscript; AM: contributed to study design, collected data and reviewed and edited the manuscript; VS: contributed to study design, acquired dietary data, and reviewed and edited the manuscript; SA, AHE, and MWT: collected data and reviewed and edited the manuscript; DMP: conducted statistical analysis and reviewed and edited the manuscript; OH: contributed to study design, supervised the work, and reviewed and edited the manuscript; OH, JM, and ST: are guarantors of this work and, as such, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the statistical analysis; and all authors read and approved the final version of the manuscript.
JM consulted for Novo Nordisk, receives research support from the National Dairy Council, and has spoken on dietary patterns for local Dairy Councils; receives research support from KOWA, Inc., the NIH and the Juvenile Diabetes Research Foundation (JDRF). OH receives research support from the National Dairy Council; consults for Merck, Sanofi-Aventis and Abbott Nutrition; is on the advisory board of Astra Zeneca; and is a shareholder of Healthimation. ST, AM, VS, SA, DMP, AHE, and MWT report no conflicts of interest.
Notes
Data from this work were presented at the 78th and 79th Scientific Sessions of the American Diabetes Association, 2018, Orlando, FL, and 2019, San Francisco, CA, USA, respectively.
Supported by the National Dairy Council and NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Diabetes Research Center grant (P30DK036836).
The funding source had no role in the study design and conduct, data analysis, or manuscript preparation.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
The authors will make the data (in deidentified form, if human data) used in the manuscript, code book, and analytic code available to editors upon request either before or after publication.
Abbreviations used: BCAAs, lysine and branched-chain amino acids; BP, blood pressure; CRP, C-reactive protein; HbA1c, glycated hemoglobin; HF, high-fat; ITT, intent-to-treat; LF, low-fat; PP, per-protocol; TEI, total energy intake.
Contributor Information
Joanna Mitri, Research Division, Joslin Diabetes Center, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Shaheen Tomah, Research Division, Joslin Diabetes Center, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Adham Mottalib, Research Division, Joslin Diabetes Center, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA; Department of Medicine, Lahey Hospital and Medical Center, Burlington, MA, USA.
Veronica Salsberg, Research Division, Joslin Diabetes Center, Boston, MA, USA.
Sahar Ashrafzadeh, Research Division, Joslin Diabetes Center, Boston, MA, USA.
David M Pober, Research Division, Joslin Diabetes Center, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Ahmed H Eldib, Research Division, Joslin Diabetes Center, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Mhd Wael Tasabehji, Research Division, Joslin Diabetes Center, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Osama Hamdy, Research Division, Joslin Diabetes Center, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
References
- 1. US Department of Health and Human Services and US Department of Agriculture [Internet]. 2015–2020 Dietary Guidelines for Americans. 8th Edition Washington, DC: 2015. Available from: https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines/. [Google Scholar]
- 2. Mitri J, Yusof B-NM, Maryniuk M, Schrager C, Hamdy O, Salsberg V. Dairy intake and type 2 diabetes risk factors: a narrative review. Diabetes Metab Syndr. 2019;13(5):2879–87. [DOI] [PubMed] [Google Scholar]
- 3. Gijsbers L, Ding EL, Malik VS, De Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr. 2016;103(4):1111–24. [DOI] [PubMed] [Google Scholar]
- 4. Gao D, Ning N, Wang C, Wang Y, Li Q, Meng Z, Liu Y, Li Q. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PLoS One. 2013;8(9):e73965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rice BH, Quann EE, Miller GD. Meeting and exceeding dairy recommendations: effects of dairy consumption on nutrient intakes and risk of chronic disease. Nutr Rev. 2013;71(4):209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rideout TC, Marinangeli CP, Martin H, Browne RW, Rempel CB. Consumption of low-fat dairy foods for 6 months improves insulin resistance without adversely affecting lipids or bodyweight in healthy adults: a randomized free-living cross-over study. Nutr J. 2013;12(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wennersberg MH, Smedman A, Turpeinen AM, Retterstøl K, Tengblad S, Lipre E, Aro A, Mutanen P, Seljeflot I, Basu S. Dairy products and metabolic effects in overweight men and women: results from a 6-mo intervention study. Am J Clin Nutr. 2009;90(4):960–8. [DOI] [PubMed] [Google Scholar]
- 8. Tessari P, Kiwanuka E, Cristini M, Zaramella M, Enslen M, Zurlo C, Garcia-Rodenas C. Slow versus fast proteins in the stimulation of beta-cell response and the activation of the entero-insular axis in type 2 diabetes. Diabetes Metab Res Rev. 2007;23(5):378–85. [DOI] [PubMed] [Google Scholar]
- 9. Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2013;97(4):854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mozaffarian D, Cao H, King IB, Lemaitre RN, Song X, Siscovick DS, Hotamisligil GkS. trans-Palmitoleic acid, metabolic risk factors, and new-onset diabetes in US adults: a cohort study. Ann Intern Med. 2010;153(12):790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577. [DOI] [PubMed] [Google Scholar]
- 12. Geng T, Qi L, Huang T. Effects of dairy products consumption on body weight and body composition among adults: an updated meta‐analysis of 37 randomized control trials. Mol Nutr Food Res. 2018;62(1):doi:10.1002/mnfr.21700410. [DOI] [PubMed] [Google Scholar]
- 13. Drouin-Chartier J-P, Côté JA, Labonté M-È, Brassard D, Tessier-Grenier M, Desroches S, Couture P, Lamarche B. Comprehensive review of the impact of dairy foods and dairy fat on cardiometabolic risk. Adv Nutr. 2016;7(6):1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Control CfD, Prevention. National Diabetes Statistics Report, 2020. Atlanta: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2020. [Google Scholar]
- 15. US Department of Agriculture. ChooseMyPlate [Internet]. Available from: https://www.choosemyplate.gov/eathealthy/dairy.
- 16. Thomas EL, Collins AL, McCarthy J, Fitzpatrick J, Durighel G, Goldstone AP, Bell JDJ. Estimation of abdominal fat compartments by bioelectrical impedance: the validity of the ViScan measurement system in comparison with MRI. Eur J Clin Nutr. 2010;64(5):525–33. [DOI] [PubMed] [Google Scholar]
- 17. Liu G, Liang K-Y. Sample size calculations for studies with correlated observations. Biometrics. 1997;53(3):937–47. [PubMed] [Google Scholar]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armitage P, McPherson C, Rowe B. Repeated significance tests on accumulating data. J R Stat Soc A. 1969;132(2):235–44. [Google Scholar]
- 20. Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64(2):191–9. [Google Scholar]
- 21. Kratz M, Marcovina S, Nelson JE, Yeh MM, Kowdley KV, Callahan HS, Song X, Di C, Utzschneider KM. Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not β-cell function in humans. Am J Clin Nutr. 2014;99(6):1385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chartrand D, Da Silva MS, Julien P, Rudkowska I. Influence of amino acids in dairy products on glucose homeostasis: the clinical evidence. Can J Diabetes. 2017;41(3):329–37. [DOI] [PubMed] [Google Scholar]
- 23. Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2008;18(7):503–10. [DOI] [PubMed] [Google Scholar]
- 24. Yakoob MY, Shi P, Willett WC, Rexrode KM, Campos H, Orav EJ, Hu FB, Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation. 2016;133(17):1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Connor S, Turcotte AF, Gagnon C, Rudkowska I. Increased dairy product intake modifies plasma glucose concentrations and glycated hemoglobin: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10(2):262–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sochol KM, Johns TS, Buttar RS, Randhawa L, Sanchez E, Gal M, Lestrade K, Merzkani M, Abramowitz MK, Mossavar-Rahmani Y. The effects of dairy intake on insulin resistance: a systematic review and meta-analysis of randomized clinical trials. Nutrients. 2019;11(9):2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ibsen DB, Laursen ASD, Lauritzen L, Tjønneland A, Overvad K, Jakobsen MU. Substitutions between dairy product subgroups and risk of type 2 diabetes: the Danish Diet, Cancer and Health cohort. Br J Nutr. 2017;118(11):989–97. [DOI] [PubMed] [Google Scholar]
- 28. Drouin-Chartier J-P, Li Y, Ardisson Korat AV, Ding M, Lamarche B, Manson JE, Rimm EB, Willett WC, Hu FB. Changes in dairy product consumption and risk of type 2 diabetes: results from 3 large prospective cohorts of US men and women. Am J Clin Nutr. 2019;110(5):1201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stancliffe RA, Thorpe T, Zemel MB. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am J Clin Nutr. 2011;94(2):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Drouin-Chartier J-P, Gagnon J, Labonté M-È, Desroches S, Charest A, Grenier G, Dodin S, Lemieux S, Couture P, Lamarche B. Impact of milk consumption on cardiometabolic risk in postmenopausal women with abdominal obesity. Nutr J. 2015;14(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maki KC, Nieman KM, Schild AL, Kaden VN, Lawless AL, Kelley KM, Rains TM. Sugar-sweetened product consumption alters glucose homeostasis compared with dairy product consumption in men and women at risk of type 2 diabetes mellitus. J Nutr. 2015;145(3):459–66. [DOI] [PubMed] [Google Scholar]
- 32. Dugan CE, Aguilar D, Park Y-K, Lee J-Y, Fernandez ML. Dairy consumption lowers systemic inflammation and liver enzymes in typically low-dairy consumers with clinical characteristics of metabolic syndrome. J Am Coll Nutr. 2016;35(3):255–61. [DOI] [PubMed] [Google Scholar]
- 33. Bagust A, Beale S. Deteriorating beta‐cell function in type 2 diabetes: a long‐term model. QJM. 2003;96(4):281–8. [DOI] [PubMed] [Google Scholar]
- 34. Benatar JR, Sidhu K, Stewart RA. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLoS One. 2013;8(10):e76480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1998;280(4):356–62. [DOI] [PubMed] [Google Scholar]
- 36. Crichton GE, Howe PR, Buckley JD, Coates AM, Murphy KJ, Bryan J. Long-term dietary intervention trials: critical issues and challenges. Trials. 2012;13(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monzillo LU, Hamdy O. Evaluation of insulin sensitivity in clinical practice and in research settings. Nutr Rev. 2003;61(12):397–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.