ABSTRACT
Background
Nicotinamide riboside (NR) is an NAD+ precursor that boosts cellular NAD+ concentrations. Preclinical studies have shown profound metabolic health effects after NR supplementation.
Objectives
We aimed to investigate the effects of 6 wk NR supplementation on insulin sensitivity, mitochondrial function, and other metabolic health parameters in overweight and obese volunteers.
Methods
A randomized, double-blinded, placebo-controlled, crossover intervention study was conducted in 13 healthy overweight or obese men and women. Participants received 6 wk NR (1000 mg/d) and placebo supplementation, followed by broad metabolic phenotyping, including hyperinsulinemic-euglycemic clamps, magnetic resonance spectroscopy, muscle biopsies, and assessment of ex vivo mitochondrial function and in vivo energy metabolism.
Results
Markers of increased NAD+ synthesis—nicotinic acid adenine dinucleotide and methyl nicotinamide—were elevated in skeletal muscle after NR compared with placebo. NR increased body fat-free mass (62.65% ± 2.49% compared with 61.32% ± 2.58% in NR and placebo, respectively; change: 1.34% ± 0.50%, P = 0.02) and increased sleeping metabolic rate. Interestingly, acetylcarnitine concentrations in skeletal muscle were increased upon NR (4558 ± 749 compared with 3025 ± 316 pmol/mg dry weight in NR and placebo, respectively; change: 1533 ± 683 pmol/mg dry weight, P = 0.04) and the capacity to form acetylcarnitine upon exercise was higher in NR than in placebo (2.99 ± 0.30 compared with 2.40 ± 0.33 mmol/kg wet weight; change: 0.53 ± 0.21 mmol/kg wet weight, P = 0.01). However, no effects of NR were found on insulin sensitivity, mitochondrial function, hepatic and intramyocellular lipid accumulation, cardiac energy status, cardiac ejection fraction, ambulatory blood pressure, plasma markers of inflammation, or energy metabolism.
Conclusions
NR supplementation of 1000 mg/d for 6 wk in healthy overweight or obese men and women increased skeletal muscle NAD+ metabolites, affected skeletal muscle acetylcarnitine metabolism, and induced minor changes in body composition and sleeping metabolic rate. However, no other metabolic health effects were observed.
This trial was registered at clinicaltrials.gov as NCT02835664
Keywords: nicotinamide riboside, NAD, metabolic health, insulin sensitivity, mitochondrial function, acetylcarnitine, body composition, human, obesity
Nicotinamide riboside (NR) is a naturally occuring vitamin B-3 present in the human diet and acts as a NAD+-precursor. NAD+ plays a role in regulating oxidative metabolism. Preclinical data suggests that NR supplementation could improve mitochondrial function and insulin sensitivity, which are often impaired in humans with obesity or type 2 diabetes. So far, the number of human interventions with NR is small and the evidence that NR may have beneficial effects on human metabolic health is limited. For this reason a randomized controlled cross-over trial was conducted in which healthy overweight and obese participants received NR (1000 mg/d) and placebo supplementation for 6 wk, followed by broad metabolic phenotyping. In this edition of The American Journal of Clinical Nutrition, Remie et al. concluded that NR supplementation in healthy overweight and obese men and women did not affect muscle mitochondrial function or insulin sensitivity. However, it was observed that NR affected skeletal muscle acetylcarnitine metabolism and induced minor changes in body composition and sleeping metabolic rate.
See corresponding editorial on page 243.
Introduction
Nicotinamide riboside (NR) is a naturally occurring vitamin B-3 present in the human diet that acts as an NAD+ precursor (1) and is suggested to improve mitochondrial function and insulin sensitivity (2). NR acts via activation of the NAD+-dependent sirtuin enzyme family, thereby regulating oxidative metabolism (3–6, 2). In vitro experiments of NR supplementation have shown its successful NAD+-restoring capability and subsequent increased oxidative gene expression in skeletal muscle cells (7, 8). Results from in vivo mouse models have shown improvements in insulin sensitivity and oxidative energy metabolism, including enhanced metabolic flexibility, increased aerobic exercise capacity, and indications of improved mitochondrial biogenesis (8–12). In these mouse models NR seems to specifically act on muscle, liver, heart, and brown adipose tissue (8, 13). Reports indicate that NAD+ metabolism, including NAD+ concentrations, decreases in obese and older populations (14–16). The number of human interventions with NR is small and the evidence that NR may have beneficial effects in humans is limited. Human pharmacokinetic studies showed increased circulatory NAD+ metabolite concentrations in whole blood, peripheral blood mononuclear cells, and urine after various dosages of NR supplementation (17–22), varying from 300 to 2000 mg/d, indicating that NR supplementation is able to increase the NAD+ pool in humans. So far, 3 randomized placebo-controlled NR supplementation studies have been performed in humans, in which the effect of NR supplementation on human metabolic health was investigated. Dollerup et al. (20, 23) investigated the effect of 2000 mg NR/d for 12 wk in insulin-resistant middle-aged obese men. No effects of NR supplementation were found on insulin sensitivity, muscle mitochondrial function, or metabolic flexibility (20, 23). In addition, Martens et al. (19) investigated the effect of 1000 mg NR/d for 6 wk in healthy normal-weight middle-aged and older men and women. Besides a trend toward reduced arterial stiffness and lower blood pressure (BP) after NR supplementation, no effects were found on a wide variety of outcomes indicative of metabolic function, glucose metabolism, motor function, and exercise capacity (19). Furthermore, Elhassan et al. (24) investigated the effects of 1000 mg NR/d for 3 wk in older men and showed increased skeletal muscle NAD+ metabolite concentrations; however, again no effect on skeletal muscle mitochondrial function was observed. To date, the effect of NR supplementation on both insulin sensitivity and skeletal muscle mitochondrial function has only been recently published in 1 human clinical trial (20, 23), as far as we know. Based on the promising preclinical findings reported until 2017, we designed a double-blinded randomized placebo-controlled crossover study in which we aimed to investigate the effect of 1000 mg NR/d supplementation on metabolic health in healthy obese or overweight men and women. The primary focus was on insulin sensitivity and muscle mitochondrial function, and secondary outcomes were related to energy metabolism and skeletal muscle NAD+ metabolites. Therefore, our study expands the limited knowledge of the effect of NR supplementation on human metabolic health and aims to investigate the translational value of the previous promising preclinical findings.
Methods
The study (NCT02835664) was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Ethics Committee of the Maastricht University Medical Center. All participants provided written informed consent before screening.
Participants
Recruitment and data collection took place between December 2016 and December 2018 in Maastricht, Netherlands. Thirteen participants completed the study, whereas 2 dropped out because of personal or medical reasons (see Supplemental Figure 1). Men and postmenopausal women were included. Participants underwent a screening including assessment of blood biochemistry, electrocardiography, anthropometric measurements, and a questionnaire [including the Baecke physical activity questionnaire (25)] to evaluate eligibility. Inclusion criteria were 45–65 y of age, BMI (in kg/m2) 27–35, sedentary lifestyle (<3 h exercise/wk), nonsmoking for ≥6 mo, alcohol use of ≤2 servings/d, stable body weight for ≥6 mo, and having no active diseases.
Study design
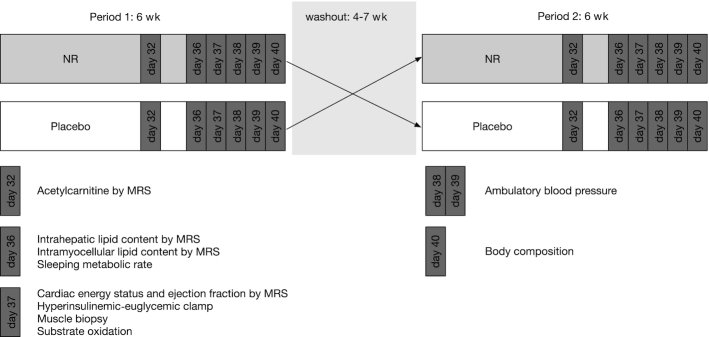
The study had a randomized, double-blinded, placebo-controlled, crossover design. Simple randomization was applied with the use of computer-generated random numbers. Each participant received 2 interventions, each of which lasted 6 wk. During the first 6 wk each participant was randomly assigned to receive either NR supplementation or placebo. This 6-wk period was followed by a washout period of 4–7 wk. At the end of the washout period each participant crossed over to the treatment they did not receive during the first 6 wk. In both intervention arms participants underwent exactly the same tests to determine the effect of the intervention (Figure 1). These tests were performed at the end of week 5 and during week 6. During the entire study period no changes in lifestyle (no change in diet, physical activity level, or medication or supplement use) were allowed. Compliance was checked on a weekly basis by pill count. Furthermore, a fasted blood sample was taken and body weight was monitored. During supplementation periods, any adverse effects and side effects were noted.
FIGURE 1.
Study design. In this crossover study, participants were randomly assigned to start with 6 wk NR supplementation or 6 wk placebo treatment. After a washout period of 4–7 wk, participants entered the other intervention arm such that all participants served as their own control. Participants were studied in week 5 (day 32) and in week 6 (day 36–40) of each of the 2 interventions. MRS, magnetic resonance spectroscopy; NR, nicotinamide riboside.
Determination of sleeping metabolic rate
On day 36 of each intervention, participants received a standardized dinner at 18:30, after which they remained fasted and entered a metabolic chamber at 19:30. The metabolic chamber is a small room with a bed, toilet, television, computer, and access to water in which oxygen consumption and carbohydrate production were measured continuously in sampled room air. During the overnight stay in the respiration chamber, sleeping metabolic rate was assessed. Participants decided themselves what time they went to sleep. At 06:00 the next morning participants were woken up and left the chamber in an overnight fasted state.
Hyperinsulinemic-euglycemic clamp
To determine insulin sensitivity, a 2-step hyperinsulinemic-euglycemic clamp with co-infusion of D-[6.6-2H2] glucose tracer (0.04 mg · kg−1 · min−1) was performed on day 37 of both interventions (26). Insulin was infused, starting at 10:30, at 10 mU · m−2 · min−1 for 2.5 h to assess hepatic insulin sensitivity and subsequently increased to 40 mU · m−2 · min−1 for 2 h to measure whole body insulin sensitivity. Blood was frequently sampled to measure glucose concentrations directly from arterialized blood and tracer-enriched 20% glucose was co-infused at a variable rate to maintain euglycemia (∼5.0 mmol/L) and reach a steady-state condition. During the steady state of 30 min, blood samples were collected and substrate utilization was measured using indirect calorimetry. Owing to technical failures, 1 participant was excluded from the clamp analysis.
Skeletal muscle biopsies
On day 37 of each intervention, a muscle biopsy was undertaken at 08:30 after an overnight stay in the respiration chamber from the m. vastus lateralis under local anesthesia (1% lidocaine, without epinephrine) using the Bergström technique (27). The muscle biopsy specimen was divided into several parts. One part was immediately frozen in melting isopentane for biochemical analyses. The remaining part was used for mitochondrial respiration analysis.
Skeletal muscle NAD+ metabolites
NAD+ content was determined in muscle biopsy specimens using an enzymatic spectrophotometric cycling assay based on the coupled reaction of malate and alcohol dehydrogenases, as previously described (28). NAD+ metabolites were measured in muscle biopsy specimens through metabolomics. Freeze-dried muscle tissue (2–4 mg) was transferred to 2-mL tubes, and then 425 µL water, 500 µL methanol, and 75 µL internal standards mixture (see Supplemental Table 1) were added to each sample. Samples were homogenized using TissueLyser II (Qiagen; 5 min at 30/s), followed by addition of 1000 µL chloroform and thorough mixing. After centrifugation (5 min, 16000 × g, 4°C), the top layer containing the polar phase was transferred to 1.5-mL tubes and dried in a vacuum evaporator at 60°C. Dried samples were reconstituted in 100 µL methanol/water (6:4, vol:vol) and analyzed in an Acquity UPLC system (Waters) coupled to an Impact IITM Ultra-High Resolution quadrupole time-of-flight mass spectrometer (Bruker). Chromatographic separation of the compounds was achieved using a SeQuant ZIC-cHILIC column (PEEK 100 × 2.1 mm, 3 µm particle size; Merck) at 30°C. The LC method consisted of a gradient running at 0.25 mL/min from 100% mobile phase B (9:1 acetonitrile:water with 5 mM ammonium acetate, pH 6.8) to 100% mobile phase A (1:9 acetonitrile:water with 5 mM ammonium acetate, pH 6.8) in 28 min, followed by a re-equilibration step at 100% B of 5 min. MS data were acquired in both negative and positive ionization modes in full scan mode over the range of m/z 50–1200. Owing to limited sample availability, NAD+ content was measured in muscle biopsy specimens from 8 participants and NAD+ metabolomics was measured in muscle biopsy specimens from 12 participants.
Skeletal muscle mitochondrial respiration and protein content
From the muscle biopsy specimens, permeabilized muscle fibers were prepared as described elsewhere (29). Thereafter, ex vivo mitochondrial respiration was determined by measuring oxygen consumption rate upon addition of several substrates using high-resolution respirometry (Oxygraph, OROBOROS Instruments) as described previously (30). All measurements were performed in quadruplicate and the integrity of the outer mitochondrial membrane was assessed in every experiment by the addition of cytochrome C (10 µmol/L) upon maximal coupled respiration. The mean ± SD cytochrome C response in the included traces was 1.7% ± 0.03% and traces with a cytochrome C response > 15% were excluded from statistical analyses. Data are expressed per mg wet weight. Oxidative phosphorylation (oxphos) complex protein content was measured in skeletal muscle biopsy specimens from 12 participants as previously described (31).
Skeletal muscle acylcarnitine concentrations
Two to 4 mg freeze-dried muscle tissue was homogenized in 1 mL 80% acetonitrile containing 50 µL internal standards. After centrifugation (5 min, 16000 × g, 4°C), the resulting supernatant was dried under a stream of nitrogen at 40°C and derivatized by addition of 1-propanol/acetylchloride (4:1, vol:vol) for 15 min at 60°C. After evaporation under nitrogen at 40°C, samples were redissolved in pure acetonitrile. Determination of the propylated acylcarnitines in the medium was performed by MS in an Acquity UPLC System (Waters) coupled to a Quattro Premier XE Tandem Quadrupole Mass Spectrometer (Waters).
Peak oxygen consumption
Peak oxygen consumption (V̇O2 peak) was assessed during an incremental cycling test on an ergometer (Lode Excalibur Sport) (32), in the first week of the first supplementation period. After a warm-up of 5 min at 75 Watt, the power was increased every 2.5 min by 50 Watt until 80% of the maximally calculated heart rate (HR) was reached, then the power was increased every 2.5 min by 25 Watt until exhaustion. The highest mean oxygen consumption over 25 s was used as the V̇O2 peak, reflecting the physical fitness of the participant.
Magnetic resonance spectroscopy
Proton magnetic resonance spectroscopy (MRS) was used to quantify intrahepatic lipid (IHL) content, intramuscular lipid (IMCL) content, and skeletal muscle acetylcarnitine concentrations. Phosphorus MRS (31P-MRS) was used for assessment of the ratio of phosphocreatine (PCr) to ATP as a marker of the energy status and in vivo mitochondrial function of the heart muscle. MRI was used for determination of the cardiac left ventricle ejection fraction (EF). All measurements were performed on a 3.0T whole body scanner (Achieva Tx, Philips Healthcare).
IHL and IMCL contents
IHL quantification took place on day 36 of each intervention at 17:00. Participants fasted for ≥3 h. Spectra were acquired as described previously (33). Values are given as T2-corrected ratios of the CH2 peak relative to the unsuppressed water peak, expressed as percentages. Consecutively, IMCL was measured in the m. tibialis anterior of the left leg, as reported previously (33). Values are given as T1- and T2-corrected ratios of the CH2 peak relative to the unsuppressed water peak, expressed as percentages. Owing to analytical problems only 9 participants could be included in the analyses of IMCL.
Acetylcarnitine
Acetylcarnitine concentrations in skeletal muscle were acquired on day 32 of each intervention at 17:00 in the evening. Participants fasted for ≥3 h and were asked to refrain from strenuous physical activity for 48 h before the measurement. Resting skeletal muscle acetylcarnitine concentrations were measured using a T1-editing method, as described previously (34). In addition, acetylcarnitine concentrations were measured after 30 min exercise (70% maximal output on an ergometer). Acetylcarnitine values were converted to absolute concentrations as described previously (35). The creatine peak was used as a reference.
Cardiac PCr:ATP ratio and EF
The PCr:ATP ratio was quantified by 31P-MRS on day 37 of each intervention at 06:30, using an image-selected in vivo spectroscopy sequence. Participants were positioned prone and head first in the MRI. A 1H31P surface heart coil was placed beneath the participant's chest. The voxel of interest was carefully placed around the left ventricle of the heart. Spectra were acquired during the end-systolic phase (number of signals averaged = 96, number of points = 2048, bandwidth = 3000 Hz) with a repetition time of 5–8 heartbeats, depending on HR. PCr and ATP resonances were quantified using a custom-written MATLAB (MATLAB version 2014b, The MathWorks, Inc.) script and values were corrected for T1 saturation and expressed as the ratio of PCr to γ-ATP. Owing to technical errors, only 11 participants were included in the analyses of PCr:ATP ratio. Left ventricular size was measured for 12 participants based on MRI images of the heart. Left ventricular EF was calculated from the end diastolic volume (EDV) and end systolic volume (ESV) according to the Biplane Ellipsoid Model, as described previously (36).
Ambulatory BP
Twenty-four-hour ambulatory BP was monitored (Mobil-O-Graph, IEM) on days 38 and 39 of each intervention for 2 d and 1 night (36 h). The device measured at 15-min intervals during the day and at 30-min intervals during the night on the nondominant arm. Subjects were asked to maintain their normal daily activities during all the recording periods and to avoid intensive exercise. From this measurement mean systolic (SBP) and diastolic (DBP) blood pressure during daytime and nighttime were determined. Nocturnal BP reductions (nighttime dipping) were calculated as continuous variables using the equation [(mean daytime BP − mean nighttime BP)/mean daytime BP] × 100, as previously described (37). Night was defined as the mean time for going to bed of all subjects (23:38) until the mean time for waking up of all subjects (08:05). Owing to technical failures nighttime BP could only be obtained from 12 participants.
Body composition
Body composition was determined on day 40 of each intervention at 08:30 after an overnight fast of ≥10 h. Body mass and body volume were assessed using air-displacement plethysmography using the BodPod device (Cosmed) according to the manufacturer's instructions (38) and as previously reported (39).
Blood sampling and analyses
Glucose (Hk-CP, Axonlab) and free fatty acids (FFAs) (NEFA-HR, WAKO Chemicals) were analyzed enzymatically in EDTA plasma using a Pentra 400 (Horiba). Triglycerides (Sigma), cholesterol (CHOD-PAP, Roche Diagnostics), and HDL cholesterol (CHOD-PAP, Roche Diagnostics), after precipitation of apoB-containing lipoproteins with phosphotungstic acid and magnesium ions, were analyzed in serum also using a Pentra 400. All samples from 1 subject were analyzed within 1 run. LDL cholesterol was calculated for 12 participants according to the Friedewald equation (40). In a subset of 7 participants (mean ± SD age: 60 ± 3 y; BMI: 30.0 ± 1.7; n = 2 women) inflammatory cytokine concentrations were measured on a Luminex® 200™ system using an inflammation 20-plex human Procartaplex panel (eBioscience, EPX200-12185-901) containing markers for sE-Selectin; intercellular adhesion molecule 1/CD54; IL-1α; IL-4; IL-12p70; IL-17A/cytotoxic T lymphocyte antigen -8; IP-10/CXC chemokine ligand 10; monocyte chemoattractant protein-1/CC chemokine ligand (CCL) 2; macrophage inflammatory protein (MIP) -1α/CCL3; MIP-1β/CCL4; sP-Selectin; and TNF-α.
Calculations
Energy expenditure was calculated based on the measured averaged oxygen and carbon dioxide concentrations in the inspired and expired gases with the assumption that protein oxidation was negligible, using the Weir equation (41, 42). Sleeping metabolic rate was calculated as the lowest average 3-h energy expenditure during the sleeping period. Glucose oxidation and fat oxidation rates were calculated according to Péronnet and Massicotte (41, 42). Steele's single-pool non–steady state equations were used to calculate the rate of glucose appearance (Ra) and the rate of glucose disappearance (Rd) during the clamp (43). Volume of distribution was assumed to be 0.160 L/kg for glucose. The change in insulin-stimulated glucose disposal (Δ Rd) was calculated by the difference between Rd under insulin-stimulated conditions and Rd under basal non-insulin-stimulated conditions. Endogenous glucose production (EGP) was calculated as Ra minus exogenous glucose infusion rate. Nonoxidative glucose disposal (NOGD) was calculated as Rd minus carbohydrate oxidation.
Sample size
The sample size was determined based on demonstrating the statistical superiority of NR on insulin-stimulated skeletal muscle glucose disposal compared with placebo. Twelve subjects were required to achieve 80% power with an α of 5%, an assumed treatment difference of within-person changes of 3.25 μmol · kg−1 · min−1, and an assumed SD of within-person changes of 3.60 μmol · kg−1 · min−1 for a 1-group paired t test for a hyperinsulinemic-euglycemic clamp. A dropout rate of 20% was taken into account, so 15 subjects were recruited. The expected effect size and SD were based on previous research within our research group (44).
Statistical analyses
Data are reported as mean ± SE, unless otherwise stated. Data are presented for n = 13, unless otherwise indicated. Differences between interventions were analyzed with a 2-tailed paired Student's t test for parametric data and with a Wilcoxon test for nonparametric data. A 2-tailed P < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS version 23.0 for MacOS.
Results
Participant population and study compliance
Thirteen healthy overweight or obese men and women (mean ± SD age: 59 ± 5 y; BMI: 30.2 ± 2.6; n = 7 women) participated in the study. Participants were nonsmokers, had no active diseases, used no medication or supplements interfering with the study outcomes, had a sedentary lifestyle according to the Baecke questionnaire [mean ± SD: 7.51 ± 1.16 arbitrary units (AU)], and a mean ± SD V̇O2 peak of 27.0 ± 5.7 mL · min−1 · kg−1 (see Supplemental Table 2). NR at 1000 mg/d was well tolerated and no adverse events or side effects were reported. Surplus NR and placebo supplements were returned by the participants and compliance rate was calculated as the proportion of capsules ingested relative to the prescribed number. The mean ± SD compliance rate during the 6-wk NR period was 99.3% ± 1.8% and during the 6-wk placebo period was 99.1% ± 2.2%. Participants were instructed to maintain their habitual diet and physical activity pattern during the entire study; this was confirmed by a stable mean ± SD body weight of 85.2 ± 3.8 kg after 6 wk NR supplementation compared with 85.5 ± 3.8 kg upon 6 wk placebo (P = 0.55).
NAD+ metabolites in skeletal muscle
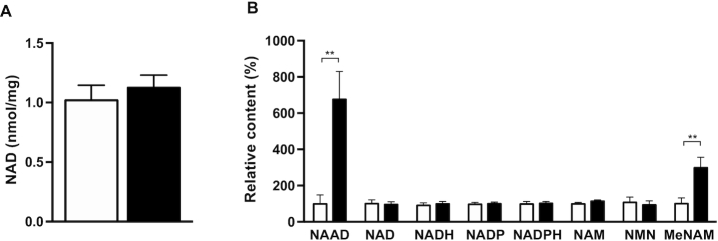
Compliance was further checked by analysis of NAD+-derived metabolites in muscle biopsy samples collected after 6 wk of NR and placebo, ∼14–16 h after supplement intake. First, skeletal muscle NAD+ content was measured. Quantitative analyses of NAD+ concentrations via enzymatic cycling assay in skeletal muscle showed that NAD+ content was not different between NR and placebo (1.019 ± 0.126 compared with 1.125 ± 0.106 nmol/mg dry weight in NR and placebo, respectively; change: 0.106 ± 0.105 nmol/mg dry weight, P = 0.34, n = 8) (Figure 2A). The lack of increase in NAD+ may indicate that NAD+ flux is increased without elevated steady-state NAD+ concentrations. Therefore, we next performed metabolomics to check if NAD+ and NAD+ metabolites in skeletal muscle were affected by NR. We confirmed that NR supplementation had no effect on skeletal muscle NAD+ content itself (P = 0.91, n = 12) (Figure 2B). However, oral NR supplementation significantly increased 2 main markers of enhanced NAD+ metabolism—nicotinic acid adenine dinucleotide (NAAD, 677 ± 155% increase in NR compared to placebo, P < 0.01, n = 12) (Figure 2B) and methylnicotinamide (MeNAM, 299 ± 62% increase in NR compared to placebo, P < 0.01, n = 12) (Figure 2B), confirming that indeed NR was amplifying skeletal muscle NAD+ metabolism without affecting the steady state. Moreover, NADH, NADP, NADPH, nicotinamide adenosine mononucleotide, and nicotinamide mononucleotide concentrations remained unchanged (P = 0.73, P = 0.79, P = 0.75, P = 0.25, and P = 0.97, respectively, n = 12; see Figure 2B).
FIGURE 2.
NAD+ metabolites in skeletal muscle after NR and placebo supplementation. (A) NAD+ concentrations measured in skeletal muscle biopsy specimens by enzymatic assay, n = 8. (B) NAD+ metabolites measured in skeletal muscle biopsy specimens by MS, n = 12. Black bars are NR, open bars are placebo. Data are expressed as mean ± SE. P values are derived from the analysis of the mean within-person changes and the SEM of the within-group changes. **P < 0.01. MeNAM, methylnicotinamide; NAAD, nicotinic acid adenine dinucleotide; NAM, nicotinamide adenosine mononucleotide; NMN, nicotinamide mononucleotide; NR, nicotinamide riboside.
Mitochondrial respiration in skeletal muscle
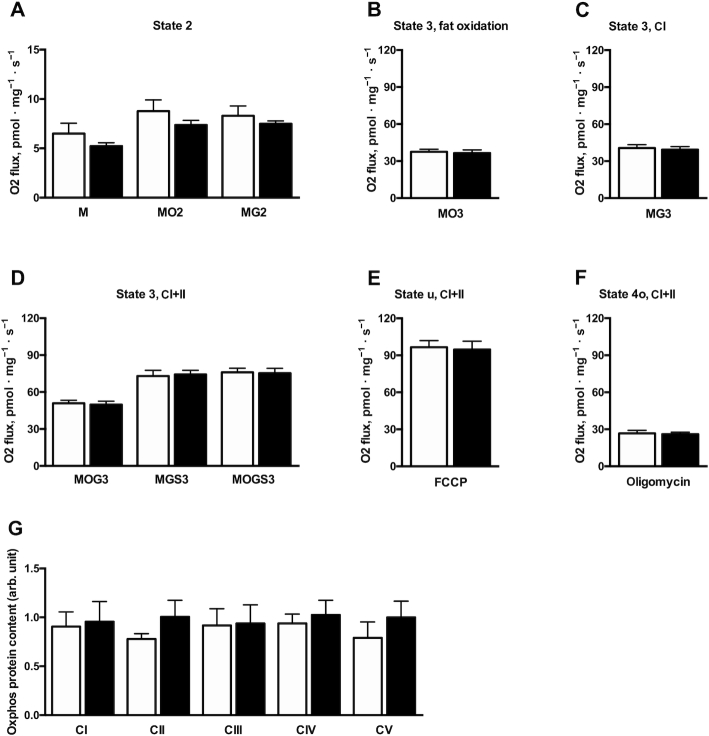
Supplementation with NR did not result in any change in mitochondrial respiration compared with the placebo state. Respiration in the presence of substrate alone (state 2) (malate, malate + octanoyl carnitine, or malate + glutamate) was not different between conditions (P = 0.34, P = 0.19, and P = 0.74, respectively) (Figure 3A). Furthermore, ADP-stimulated (state 3) respiration on lipid-derived substrate (malate + octanoyl carnitine + ADP) and upon complex I substrate (malate + glutamate + ADP) was unchanged (P = 0.67 and P = 0.64, respectively) (Figure 3B, C). Respiration upon parallel electron input to both complex I and II (malate + octanoyl carnitine + glutamate) was not different between conditions (49.87 ± 2.80 compared with 50.92 ± 2.44 pmol · mg−1 · s−1 in NR and placebo, respectively; change: −1.04 ± 2.58 pmol · mg−1 · s−1, P = 0.69) (Figure 3D). Similar results were observed when succinate was sequentially added in both experiments (malate + glutamate + succinate: 74.27 ± 3.27 compared with 72.90 ± 4.64 pmol · mg−1 · s−1 in NR and placebo, respectively; change: 1.37 ± 5.01 pmol · mg−1 · s−1, P = 0.79; malate + octanoyl carnitine + glutamate + succinate: 75.30 ± 3.92 compared with 76.09 ± 3.31 pmol · mg−1 · s−1 in NR and placebo, respectively; change: −0.79 ± 4.36 pmol · mg−1 · s−1, P = 0.86) (Figure 3D). Maximal carbonyl cyanide p-trifluoro-methoxyphenyl hydrazone–induced uncoupled respiration (state u), reflecting the maximal capacity of the electron transport chain, was also unchanged (P = 0.81) (Figure 3E). Finally, state 4o respiration (reflecting proton leak) was similar after NR and placebo conditions (P = 0.89) (Figure 3F). For a description of the different states, please see: https://www.bioblast.at/index.php/MitoPedia:_Respiratory_states. Mitochondrial OXPHOS protein concentrations (Complex I: 0.96 ± 0.21 compared with 0.91 ± 0.15 AU in NR and placebo, respectively; change: 0.05 ± 0.235 AU, P = 0.84; Complex II: 1.01 ± 0.17 compared with 0.78 ± 0.05 AU in NR and placebo, respectively; change: 0.23 ± 0.20 AU, P = 0.64; Complex III: 0.94 ± 0.19 compared with 0.92 ± 0.17 AU in NR and placebo, respectively; change: 0.02 ± 0.22 AU, P = 0.93; Complex IV: 1.02 ± 0.15 compared with 0.94 ± 0.10 AU in NR and placebo, respectively; change: 0.09 ± 0.14 AU, P = 0.55; Complex V: 1.00 ± 0.17 compared with 0.79 ± 0.16 AU in NR and placebo, respectively; change: 0.21 ± 0.17 AU, P = 0.24) were similar after NR and placebo supplementation, indicating no effect of NR on mitochondrial content (Figure 3G).
FIGURE 3.
Skeletal muscle ex vivo mitochondrial respiratory capacity after NR and placebo supplementation. (A) State 2 respiration upon M, MO2, and MG2. (B) ADP-stimulated state 3 respiration upon lipid-derived substrate, MO3. (C) ADP-stimulated state 3 respiration upon CI substrates, MG3. (D) ADP-stimulated state 3 respiration upon parallel electron input to both CI and II, MOG3, MGS3, and MOGS3. (E) State u: maximal FCCP-induced uncoupled respiration. (F) State 4o: oligomycin-induced respiration not coupled to ATP synthesis. (G) Protein content of individual complexes of the electron transport chain. Black bars are NR, open bars are placebo. n = 12. Data are expressed as mean ± SE. C, Complex; FCCP, carbonyl cyanide p-trifluoro-methoxyphenyl hydrazone; M, malate; MGS3, malate + glutamate + succinate; MG2, malate + glutamate; MG3, malate + glutamate; MOG3, malate + octanoyl carnitine + glutamate; MOGS3, malate + octanoyl carnitine + glutamate + succinate; MO2, malate + octanoyl carnitine; MO3, malate + octanoyl carnitine + glutamate; NR, nicotinamide riboside; Oxphos, oxidative phosphorylation.
Acetylcarnitine concentrations in skeletal muscle
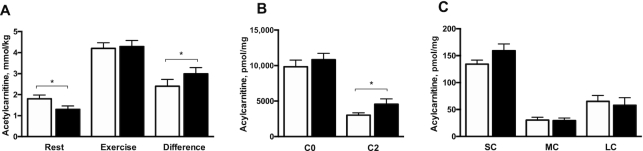
Quantification of acetylcarnitine concentrations in skeletal muscle, measured by MRS technique in the early evening at 17:00 after 3 h fasting, revealed significantly lower baseline acetylcarnitine concentrations under NR supplementation than under placebo (1.30 ± 0.16 compared with 1.80 ± 0.18 mmol/kg wet weight in NR and placebo, respectively; change: −0.53 ± 0.19 mmol/kg wet weight, P = 0.02) (Figure 4A). Maximally stimulated acetylcarnitine concentrations, measured upon exercise, were not different between conditions (4.29 ± 0.29 compared with 4.20 ± 0.27 mmol/kg wet weight in NR and placebo, respectively; change: 0.00 ± 0.20 mmol/kg wet weight, P = 0.67) (Figure 4A). Nonetheless, the capacity to increase acetylcarnitine formation, expressed as the change computed as the postexercise value minus the baseline value, was significantly higher in NR than in placebo (2.99 ± 0.30 compared with 2.40 ± 0.33 mmol/kg wet weight in NR and placebo, respectively; change: 0.53 ± 0.21 mmol/kg wet weight, P = 0.01) (Figure 4A). Based on these results, we decided to perform full acylcarnitine analysis in skeletal muscle biopsy specimens taken at 08:30 after an overnight fast. Remarkably, acetylcarnitine (C2) concentrations were significantly higher under NR supplementation than under placebo (4558 ± 749 compared with 3025 ± 316 pmol/mg dry weight in NR and placebo, respectively; change: 1533 ± 683 pmol/mg dry weight, P = 0.04) (Figure 4B). No differences were detected in free carnitine (C0), or other short-chain (C3–C5), medium-chain (C6–C12), or long-chain acylcarnitines (C13–C20) between NR and placebo (P = 0.25, P = 0.27, P = 0.99, and P = 0.45, respectively) (Figure 4C).
FIGURE 4.
Skeletal muscle acylcarnitine concentrations measured in the morning and evening after NR and placebo supplementation. (A) Acetylcarnitine concentrations measured by magnetic resonance spectroscopy in skeletal muscle in the evening during rest, after exercise, and the capacity to form acetylcarnitine expressed as the difference between rest and exercise. (B) C0 and C2 concentrations measured in muscle biopsy specimens taken in the morning during rest. (C) SC, MC, and LC concentrations measured in biopsy specimens taken during rest. Black bars are NR, open bars are placebo. n = 13. Data are expressed as mean ± SE. P values are derived from the analysis of the mean within-person changes and the SEM of the within-group changes. *P < 0.05. C0, free carnitine; C2, acetylcarnitine; LC, sum of long-chain acylcarnitines; MC, sum of medium-chain acylcarnitines; NR, nicotinamide riboside; SC, sum of short-chain acylcarnitines.
Insulin sensitivity and substrate kinetics
Potential effects of NR on whole body and tissue-specific insulin sensitivity were assessed by a 2-step hyperinsulinemic-euglycemic clamp. Whole body insulin stimulated glucose uptake, as expressed by the change in glucose disposal (Δ Rd) from baseline to high insulin dose, was not different between NR and placebo (P = 0.98) (Table 1). Hepatic insulin sensitivity, reflected by EGP suppression (EGP%) during the low insulin phase, was not affected by NR compared with placebo (P = 0.30) (Table 1). NR had no effect on baseline substrate oxidation (carbohydrate oxidation, P = 0.84; fat oxidation, P = 0.67). Furthermore, insulin-stimulated carbohydrate oxidation or suppression of fatty acid oxidation, reflecting metabolic flexibility, was not different between NR and placebo (P = 0.92 and P = 0.70, respectively) (Table 1). In addition, Δ NOGD during the low and high insulin phases of the clamp remained unchanged between conditions (P = 0.88 and P = 0.99, respectively) (Table 1). Plasma FFA concentrations were suppressed by insulin to a similar extent between NR and placebo (P = 0.97 and P = 0.99 during the low and high insulin phases, respectively) (Table 1), indicating similar white adipose tissue insulin sensitivity.
TABLE 1.
Insulin sensitivity and substrate kinetics1
| Placebo | NR | Change | P value | |
|---|---|---|---|---|
| Ra,2 μmol · kg−1 · min−1 | ||||
| Baseline | 9.32 ± 0.33 | 9.54 ± 0.78 | 0.22 ± 0.72 | 0.77 |
| Low insulin | 11.66 ± 0.93 | 11.74 ± 0.91 | 0.08 ± 0.42 | 0.85 |
| High insulin | 36.70 ± 3.40 | 37.60 ± 2.66 | 0.91 ± 2.24 | 0.69 |
| Rd,2 μmol · kg−1 · min−1 | ||||
| Baseline | 9.90 ± 0.53 | 9.52 ± 0.84 | −0.38 ± 0.97 | 0.70 |
| Low insulin | 12.06 ± 1.00 | 12.36 ± 0.94 | 0.30 ± 0.45 | 0.51 |
| High insulin | 36.76 ± 3.36 | 36.32 ± 2.62 | −0.45 ± 2.13 | 0.84 |
| Δ baseline–low insulin | 2.16 ± 1.07 | 2.84 ± 1.01 | 0.68 ± 1.03 | 0.47 |
| Δ baseline–high insulin | 26.86 ± 3.31 | 26.79 ± 2.86 | −0.07 ± 2.17 | 0.98 |
| EGP,2 μmol · kg−1 · min−1 | ||||
| Baseline | 9.32 ± 0.33 | 9.54 ± 0.78 | 0.22 ± 0.72 | 0.77 |
| Low insulin | 2.70 ± 0.40 | 3.38 ± 0.56 | 0.68 ± 0.48 | 0.18 |
| % suppression low insulin | 70.44 ± 4.57 | 61.02 ± 7.05 | −9.42 ± 7.13 | 0.30 |
| High insulin | −0.04 ± 0.26 | 0.51 ± 0.56 | 0.55 ± 0.51 | 0.30 |
| % suppression high insulin | 99.78 ± 2.52 | 96.82 ± 4.65 | −2.95 ± 4.28 | 0.50 |
| NOGD,2 μmol · kg−1 · min−1 | ||||
| Baseline | 5.27 ± 1.22 | 4.88 ± 1.06 | −0.40 ± 1.18 | 0.74 |
| Low insulin | 3.77 ± 0.92 | 3.56 ± 0.75 | −0.19 ± 0.63 | 0.76 |
| High insulin | 21.28 ± 2.84 | 20.85 ± 2.01 | −0.43 ± 2.03 | 0.84 |
| Δ baseline–low insulin | −1.51 ± 1.36 | −1.30 ± 0.85 | 0.21 ± 1.34 | 0.88 |
| Δ baseline–high insulin | 16.01 ± 2.81 | 15.97 ± 2.04 | −0.03 ± 1.83 | 0.99 |
| Carbohydrate oxidation, μmol · kg−1 · min−1 | ||||
| Baseline | 4.42 ± 0.81 | 4.58 ± 0.55 | 0.16 ± 0.61 | 0.84 |
| Low insulin | 7.84 ± 0.69 | 8.79 ± 0.78 | 0.95 ± 0.76 | 0.23 |
| High insulin | 14.90 ± 0.96 | 14.99 ± 1.15 | 0.09 ± 0.89 | 0.92 |
| Fat oxidation, μmol · kg−1 · min−1 | ||||
| Baseline | 3.78 ± 0.23 | 3.71 ± 0.20 | −0.07 ± 0.17 | 0.67 |
| Low insulin | 2.73 ± 0.19 | 2.55 ± 0.17 | −0.18 ± 0.21 | 0.41 |
| High insulin | 1.47 ± 0.23 | 1.38 ± 0.22 | −0.09 ± 0.22 | 0.70 |
| Plasma FFAs, μmol/L | ||||
| Baseline | 555.34 ± 30.87 | 581.16 ± 32.08 | 25.83 ± 35.55 | 0.48 |
| Low insulin | 128.55 ± 21.74 | 128.03 ± 19.26 | −0.52 ± 14.19 | 0.97 |
| High insulin | 49.84 ± 8.26 | 56.45 ± 14.80 | 6.61 ± 10.04 | 0.99 |
| Respiratory exchange ratio | ||||
| Baseline | 0.77 ± 0.01 | 0.77 ± 0.01 | 0.00 ± 0.01 | 0.83 |
| Low insulin | 0.82 ± 0.01 | 0.83 ± 0.01 | 0.01 ± 0.01 | 0.37 |
| High insulin | 0.91 ± 0.01 | 0.91 ± 0.01 | 0.00 ± 0.01 | 0.86 |
1Values are means ± SEs. P values are derived from the analysis of the mean within-person changes and the SE of the within-group changes. EGP, endogenous glucose production; FFA, free fatty acid; NOGD, nonoxidative glucose disposal; NR, nicotinamide riboside; Ra, rate of appearance; Rd, rate of disappearance.
2 n = 12.
Plasma biochemistry and inflammatory markers
NR supplementation did not affect fasting plasma glucose, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, or inflammatory markers, including chemokine, cytokine, or cell-adhesion molecule concentrations (Table 2). However, IL-1α tended to be lower after NR supplementation than after placebo (1.61 ± 0.28 compared with 2.11 ± 0.35 pg/mL, respectively, P = 0.06) (Table 2).
TABLE 2.
Blood biochemistry1
| Parameter | Placebo | NR | Change | P value |
|---|---|---|---|---|
| Glucose, mmol/L | 5.48 ± 0.14 | 5.44 ± 0.13 | −0.04 ± 0.10 | 0.70 |
| Triglycerides, mmol/L | 1.57 ± 0.35 | 1.63 ± 0.38 | 0.06 ± 0.08 | 0.24 |
| Total cholesterol, mmol/L | 5.54 ± 0.30 | 5.55 ± 0.35 | 0.01 ± 0.11 | 0.99 |
| HDL-C, mmol/L | 1.32 ± 0.12 | 1.32 ± 0.09 | −0.00 ± 0.04 | 0.99 |
| LDL-C,2 mmol/L | 3.42 ± 0.18 | 3.37 ± 0.17 | −0.05 ± 0.07 | 0.52 |
| sE-selectin,3 pg/mL | 32,726 ± 5298 | 34,765 ± 4354 | 686 ± 2068 | 0.99 |
| sP-selectin,3 pg/mL | 21,428 ± 3662 | 26,645 ± 4065 | 2624 ± 3739 | 0.58 |
| ICAM-1,3 pg/mL | 92,493 ± 19,326 | 129,236 ± 34,547 | 35,350 ± 27,518 | 0.58 |
| TNF-α,3 pg/mL | 29.80 ± 6.38 | 31.85 ± 6.14 | 2.70 ± 1.97 | 0.22 |
| IL-1α,3 pg/mL | 2.11 ± 0.35 | 1.61 ± 0.28 | −0.57 ± 0.24 | 0.06 |
| IL-4,3 pg/mL | 8.04 ± 1.03 | 8.86 ± 1.41 | 0.71 ± 3.63 | 0.69 |
| IL-12p70,3 pg/mL | 76.07 ± 2.73 | 78.89 ± 3.02 | 1.94 ± 2.82 | 0.81 |
| IL-17α,3 pg/mL | 6.36 ± 1.36 | 7.95 ± 2.09 | 1.76 ± 1.02 | 0.16 |
| CXCL10,3 pg/mL | 3.71 ± 0.55 | 3.39 ± 0.50 | −0.61 ± 0.40 | 0.22 |
| CCL2,3 pg/mL | 127.39 ± 24.54 | 144.34 ± 47.86 | 21.42 ± 40.12 | 0.69 |
| CCL3,3 pg/mL | 15.42 ± 8.59 | 17.04 ± 10.39 | 2.43 ± 1.92 | 0.30 |
| CCL4,3 pg/mL | 23.89 ± 5.98 | 28.49 ± 7.51 | 4.52 ± 4.45 | 0.38 |
1Values are means ± SEs. P values are derived from the analysis of the mean within-person changes and the SE of the within-group changes. Blood samples were taken in week 6 of NR supplementation and placebo after an overnight fast. CCL, CC chemokine ligand; CXCL, CXC chemokine ligand; HDL-C, HDL cholesterol; ICAM-1, intercellular adhesion molecule 1; LDL-C, LDL cholesterol; NR, nicotinamide riboside.
2 n = 12.
3 n = 7.
Body composition
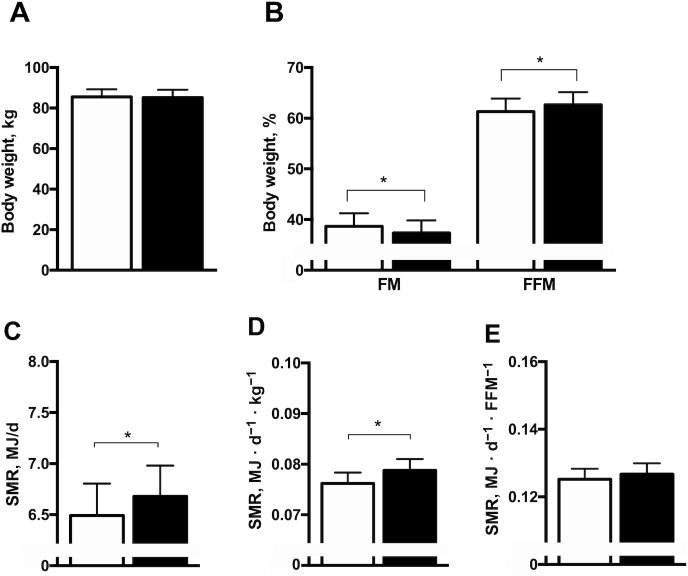
After 6 wk of NR and placebo supplementation, several changes in body composition were detected. Percentage fat-free mass (FFM) was significantly higher after NR than after placebo (62.65% ± 2.49% compared with 61.32% ± 2.58% in NR and placebo, respectively; change: 1.34% ± 0.50%, P = 0.02) (Figure 5B). In line with this, percentage fat mass (FM) was significantly lower after NR than after placebo (37.35% ± 2.49% compared with 38.68% ± 2.58% in NR and placebo, respectively; change: −1.34% ± 0.50%, P = 0.02) (Figure 5B). However, total body weight remained unchanged (P = 0.55) (Figure 5A).
FIGURE 5.
Body composition and SMR after NR and placebo supplementation. (A) Body weight. (B) FM and FFM. (C) SMR. (D) SMR corrected for body weight. (E) SMR corrected for FFM. Black bars are NR, open bars are placebo. Data are expressed as mean ± SE. n = 13. P values are derived from the analysis of the mean within-person changes and the SEM of the within-group changes. *P < 0.05. FFM, fat-free mass; FM, fat mass; NR, nicotinamide riboside; SMR, sleeping metabolic rate.
Sleeping metabolic rate
Sleeping metabolic rate, measured during an overnight stay in a respiration chamber, was higher upon 6 wk of NR than of placebo (6.68 ± 0.30 compared with 6.49 ± 0.31 MJ/d in NR and placebo, respectively; change: 0.19 ± 0.08 MJ/d, P = 0.05) (Figure 5C). This could be explained by the increase in FFM, and sleeping metabolic rate per percent of FFM was not significantly different (0.127 ± 0.003 compared with 0.125 ± 0.003 MJ · FFM−1 · d−1 in NR and placebo, respectively; change: 0.00 ± 0.00 MJ · FFM−1 · d−1, P = 0.48) (Figure 5E).
Ectopic lipid storage
IHL content, measured by MRS, was not different between the NR and placebo conditions (3.4% ± 1.2% compared with 3.4% ± 1.3% in NR and placebo, respectively; change: 0.0% ± 0.0%, P = 0.85). Furthermore, IMCL content, measured by MRS technique in the m. tibialis anterior, was not affected by NR supplementation (0.5% ± 0.1% compared with 0.5% ± 0.1% in NR and placebo, respectively; change: 0.0% ± 0.1%, P = 0.50).
Cardiac function
To investigate if NR supplementation could affect cardiac energetics, we determined cardiac PCr:ATP ratios, which however were not affected by NR (P = 0.90) (Table 3). No differences were observed in left ventricular ESV, EDV, stroke volume, and subsequently EF between NR and placebo (P = 0.23, P = 0.72, P = 0.69, and P = 0.24, respectively) (Table 3). NR supplementation had no effect on 24-h SBP, DBP, mean arterial pressure, pulse pressure, and HR (P = 0.56, P = 0.39, P = 0.40, P = 0.60, and P = 0.60, respectively) (Table 3). Separate analyses of daytime and nighttime measurements did not reveal an effect of NR supplementation (see Table 3). In addition, nighttime dipping of SBP and DBP was not affected by NR compared with placebo (P = 0.53 and P = 0.26, respectively) (Table 3).
TABLE 3.
Cardiometabolic health parameters1
| Placebo | NR | Change | P value | |
|---|---|---|---|---|
| MRS cardiac left ventricle2 | ||||
| Cardiac PCr:ATP ratio | 1.29 ± 0.11 | 1.22 ± 0.09 | −0.08 ± 0.14 | 0.90 |
| MRI cardiac left ventricle3 | ||||
| EF, % | 71.2 ± 2.2 | 68.2 ± 2.1 | −3.0 ± 2.4 | 0.24 |
| ESV, mL | 35.0 ± 4.2 | 38.9 ± 3.9 | 4.0 ± 3.6 | 0.23 |
| EDV, mL | 119.4 ± 8.2 | 121.2 ± 8.3 | 1.8 ± 5.1 | 0.72 |
| SV, mL | 84.4 ± 5.6 | 82.3 ± 5.6 | −2.1 ± 3.8 | 0.69 |
| Ambulatory BP 36-h3 | ||||
| SBP, mm Hg | 124.6 ± 2.4 | 126.6 ± 3.2 | 2.0 ± 2.3 | 0.56 |
| DBP, mm Hg | 77.1 ± 1.8 | 77.9 ± 2.0 | 0.8 ± 0.9 | 0.39 |
| MAP, mm Hg | 98.9 ± 1.7 | 100.2 ± 2.1 | 1.3 ± 1.5 | 0.40 |
| PP, mm Hg | 47.5 ± 2.6 | 48.6 ± 3.1 | 1.2 ± 2.2 | 0.60 |
| HR, bpm | 76.4 ± 3.3 | 75.5 ± 3.6 | −0.9 ± 1.7 | 0.60 |
| Nighttime dipping SBP, % | 10.6 ± 2.4 | 12.4 ± 0.8 | 1.8 ± 2.8 | 0.53 |
| Nighttime dipping DBP, % | 11.9 ± 2.4 | 14.8 ± 1.9 | 3.0 ± 2.5 | 0.26 |
| Ambulatory BP daytime | ||||
| SBP, mm Hg | 126.9 ± 2.3 | 129.0 ± 2.9 | 2.1 ± 2.3 | 0.54 |
| DBP, mm Hg | 79.5 ± 1.7 | 80.5 ± 1.9 | 1.0 ± 1.0 | 0.32 |
| MAP, mm Hg | 101.4 ± 1.5 | 102.7 ± 1.9 | 1.3 ± 1.4 | 0.39 |
| PP, mm Hg | 47.6 ± 2.7 | 48.5 ± 3.0 | 0.9 ± 2.1 | 0.68 |
| HR, bpm | 78.3 ± 3.2 | 77.5 ± 3.5 | −0.8 ± 1.7 | 0.67 |
| Ambulatory BP night-time3 | ||||
| SBP, mm Hg | 113.5 ± 3.4 | 113.0 ± 3.1 | −0.5 ± 3.7 | 0.90 |
| DBP, mm Hg | 69.4 ± 2.6 | 68.1 ± 2.3 | −1.3 ± 1.5 | 0.41 |
| MAP, mm Hg | 89.6 ± 2.7 | 88.8 ± 2.4 | −0.8 ± 2.5 | 0.75 |
| PP, mm Hg | 43.8 ± 2.5 | 45.2 ± 2.6 | 1.4 ± 2.3 | 0.57 |
| HR, bpm | 65.6 ± 2.5 | 66.6 ± 2.9 | 1.0 ± 1.9 | 0.60 |
1Values are means ± SEs. P values are derived from the analysis of the mean within-person changes and the SE of the within-group changes. BP, blood pressure; DBP, diastolic blood pressure; EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; HR, heart rate; MAP, mean arterial pressure; NR, nicotinamide riboside; PCr, phosphocreatin; PP, pulse pressure; SBP, systolic blood pressure; SV, stroke volume.
2 n = 11.
3 n = 12.
Discussion
We hypothesized that NR supplementation in humans would increase NAD+ availability and thereby would improve a broad range of metabolic health parameters, mainly via improving mitochondrial function. To investigate this hypothesis we performed a randomized, double-blinded, placebo-controlled, crossover study with detailed metabolic phenotyping in which we provided healthy overweight or obese men and women 1000 mg NR/d for 6 wk. In line with our hypothesis, NR supplementation did significantly increase markers of NAD+ metabolism—NAAD and MeNAM—in skeletal muscle. This effect was accompanied by small but significant improvements in body composition, sleeping metabolic rate, and skeletal muscle acetylcarnitine concentrations, and a trend toward increased circulatory IL-1α concentrations. No further effects on skeletal muscle mitochondrial function, hepatic and whole body insulin sensitivity, substrate oxidation, cardiovascular health markers, and ectopic lipid accumulation were observed. However, it should be noted that many outcomes have been tested in our study and no adjustments for multiple comparisons were performed, therefore the possibility of false positive findings cannot be excluded. These results suggest that NR, at the dosage of 1000 mg/d for 6 wk, did have, albeit relatively small, effects on metabolic parameters in humans, but was not effective in boosting muscle mitochondrial function or insulin sensitivity.
Animal studies showed that NR is able to increase plasma and tissue NAD+ concentrations (8–13). Also in humans, NR is able to increase circulatory NAD+ metabolites after several dosages ranging from 100 mg/d to 2000 mg/d (17–20). Here, we used a dosage of 1000 mg/d for 6 wk, and in line with other data presented we did not report side effects during 6 wk 1000 mg NR/d supplementation (17, 19). We investigated if NR supplementation was able to increase the NAD+ metabolome in skeletal muscle tissue. In agreement with findings by Elhassan et al. (19) and Dollerup et al. (23), we have shown that NR supplementation increased the NAD+ metabolites NAAD and MeNAM in skeletal muscle, but without an increase in total NAD+ content itself. NAAD is a highly sensitive biomarker of NR supplementation and an increased NAD+ synthesis rate in tissues (13). MeNAM is part of the NAD+ degradation pathway and is a marker for increased NAD+ flux. These results might suggest that NR increases NAD+ turnover rate, without affecting steady-state NAD+ concentrations in skeletal muscle.
We hypothesized that a NR-stimulated increase in NAD+ metabolism would lead to an increase in muscle mitochondrial function and a subsequent increase in human insulin sensitivity. However, in contrast to our hypothesis, skeletal muscle mitochondrial function was not elevated upon NR supplementation. This is in agreement with the findings of Elhassan et al. (24) and Dollerup et al. (23), who also reported no effect of NR on skeletal muscle mitochondrial function. Consistent with the lack of effect on mitochondrial function, we and others (20) did not observe improvements in insulin sensitivity upon NR supplementation. Although the limited duration of our and other studies may explain the lack of effect of NR on insulin sensitivity, we have previously shown that nutritional supplements like resveratrol can increase skeletal muscle mitochondrial function after 4 wk supplementation (44–46).
Trammel et al. (10) and Dollerup et al. (20) suggested that the underlying pathway of metabolic improvements observed in obese mice was a decrease in hepatic lipid accumulation. In addition, Dollerup et al. (20) described a decrease in hepatic lipid content in obese men with elevated baseline hepatic lipid content, although this was nonsignificant. Here, we did not observe an effect of NR on hepatic lipid accumulation, which may be attributed to our study population which had in general a healthy hepatic lipid content (i.e., <5% liver fat).
NR supplementation has also been suggested to improve cardiovascular health (19, 47). Therefore, we here examined the effect of NR on cardiovascular health via detailed cardiovascular phenotyping. In contrast to Martens et al. (19) but in accordance with Conze et al. (22), we did not observe an effect of NR on BP values. Martens et al. observed a decrease in resting SBP and DBP after NR, whereas we measured 36-h ambulatory BP, which gives a better estimation of BP values and gives a better indication of the risk of cardiovascular events (48, 49). Consistent with the lack of effect of NR on BP we did not find effects of NR on cardiac energy status or cardiac EF. Of note, the cardiac status of our participants was considered “healthy,” and in rodents also no change in cardiac function after NR supplementation in control mice with a healthy cardiac function could be observed (47).
We reported an improvement in body composition by an increase in percentage FFM mirrored by a decrease in percentage FM, whereas body weight remained unchanged. An effect of NR on body composition in humans has not been reported before (19, 20), to our knowledge. Interestingly, in 6 out of 7 women NR did increase FFM and reduce FM whereas this was only the case in 1 out of 6 men, suggesting that there might be a gender difference in the effect of NR supplementation on body composition. Consistent with the effect of NR on body composition, we have shown that the sleeping metabolic rate was also affected by NR supplementation and a higher metabolic rate could potentially lead to a reduction in FM. The increase in sleeping metabolic rate was due to an increase in FFM, suggesting that the primary effect of these findings could be an effect of NR on FFM. Interestingly, it has previously been shown that NAD+ metabolism and homeostasis are involved in maintaining muscle mass (12). Future studies should be designed to investigate if NR supplementation can indeed increase muscle mass in humans and investigate the underlying mechanisms.
Next to an effect of NR on muscle mass, NR supplementation enhanced the exercise-induced increase in acetylcarnitine. Moreover, acetylcarnitine concentrations, measured in skeletal muscle biopsy specimens obtained in the morning, were significantly increased after NR supplementation. These data suggest that NR is able to increase skeletal muscle acetylcarnitine metabolism, which has been associated with metabolic flexibility and improved metabolic health (35). Remarkably, however, resting acetylcarnitine concentrations measured using MRS 3 h after lunch and before the exercise session were significantly lower after NR than after placebo. It has previously been shown that meal consumption lowers acetylcarnitines in skeletal muscle (50, 51), but why NR would substantiate such meal-induced lowering in acetylcarnitine metabolism cannot be deduced from this study. Interestingly, a recently published study in obese mice showed an effect of combined supplementation of NR with l-carnitine and reported a reduction in FM percentage and hepatic steatosis (52), which matches with the positive outcome parameters of our and other studies (8, 20, 10). The exact link between NR metabolism and acetylcarnitine metabolism is, however, still unknown. Furthermore, the large gap between the clear metabolic improvements upon NR in mice and the lack of effects in humans might derive from the fact that the mouse studies applied a longer supplementation duration (8–15 wk) (8, 9, 11, 10) than the short-term supplementation in human trials (3–12 wk) (20, 23, 24, 19).
In conclusion, we here show that NR supplementation of 1000 mg/d for 6 wk in healthy overweight or obese men and women increased the NAD+ metabolites NAAD and MeNAM in human skeletal muscle and increased skeletal muscle acetylcarnitine metabolism. In addition, NR induced improvements in body composition and increased sleeping metabolic rate. However, no other metabolic health effects were observed. We conclude that NR, at this dosage and short duration, may not be beneficial in improving metabolic health in healthy overweight or obese men and women. However, further research is warranted into the effects of long-term NR supplementation on acetylcarnitine concentrations, sex-specific improvements in body composition, and metabolic health in humans.
Supplementary Material
Acknowledgments
We thank ChromaDex Inc. for providing NIAGEN and placebo capsules for the study.
The authors’ responsibilities were as follows—CMER, BH, JA, JH, VBS-H, EP, and PS: designed the study; CMER, KHMR, MPBM, NJC, and VHWdW: conducted the experiments; BH: provided medical responsibility; SABMA, BVS, HLE, and RZ-P: performed analysis on the plasma samples and muscle biopsy specimens; CMER, JM, VHWdW, TvdW, SABMA, RZ-P, and VBS-H: analyzed the data; CMER, SABMA, EL, RZ-P, RHH, JH, LL, VBS-H, EP, and PS: interpreted the data; CR, EP, and PS: wrote the paper; PS: had primary responsibility for the final content, is the guarantor of this work and as such had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by Dutch Heart Foundation, CVON Energise grant CVON2014-02 (to PS); the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement, postdoctoral grant 840110 (to RZ-P); and ERC starting grant 759161 (MRS diabetes) (to VBS-H). ChromaDex Inc. provided NIAGEN and placebo capsules for the study.
ChromaDex, Inc. had no role in the study design, data collection, analysis, or preparation of the manuscript.
Supplemental Tables 1 and 2 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article will be made available upon reasonable request pending discussion of the scientific aim.
Abbreviations used: AU, arbitrary units; BP, blood pressure; CCL, CC chemokine ligand; DBP, diastolic blood pressure; EDV, end diastolic volume; EF, ejection fraction; EGP, endogenous glucose production; ESV, end systolic volume; FFA, free fatty acid; FFM, fat-free mass; FM, fat mass; HR, heart rate; IHL, intrahepatic lipid; IMCL, intramyocellular lipid; MeNAM, methylnicotinamide; MIP, macrophage inflammatory protein; MRS, magnetic resonance spectroscopy; NAAD, nicotinic acid adenine dinucleotide; NOGD, nonoxidative glucose disposal; NR, nicotinamide riboside; oxphos, oxidative phosphorylation; PCr, phosphocreatine; Ra, rate of appearance; Rd, rate of disappearance; SBP, systolic blood pressure; V̇O2 peak, peak oxygen consumption; 31P-MRS, phosphor magnetic resonance spectroscopy.
Contributor Information
Carlijn M E Remie, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands.
Kay H M Roumans, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands.
Michiel P B Moonen, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands.
Niels J Connell, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands.
Bas Havekes, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands; Division of Endocrinology, Department of Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands.
Julian Mevenkamp, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands; Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands.
Lucas Lindeboom, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands; Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands.
Vera H W de Wit, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands.
Tineke van de Weijer, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands; Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands.
Suzanne A B M Aarts, Department of Medical Biochemistry, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands.
Esther Lutgens, Department of Medical Biochemistry, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands; Institute for Cardiovascular Prevention (IPEK), Ludwig Maximilian University, Munich, Germany.
Bauke V Schomakers, Laboratory Genetic Metabolic Diseases, Amsterdam Gastroenterology and Metabolism, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands; Core Facility Metabolomics, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands.
Hyung L Elfrink, Laboratory Genetic Metabolic Diseases, Amsterdam Gastroenterology and Metabolism, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands; Core Facility Metabolomics, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands.
Rubén Zapata-Pérez, Laboratory Genetic Metabolic Diseases, Amsterdam Gastroenterology and Metabolism, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands.
Riekelt H Houtkooper, Laboratory Genetic Metabolic Diseases, Amsterdam Gastroenterology and Metabolism, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands.
Johan Auwerx, Laboratory of Integrative and Systems Physiology, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland.
Joris Hoeks, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands.
Vera B Schrauwen-Hinderling, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands; Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands.
Esther Phielix, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands.
Patrick Schrauwen, Department of Nutrition and Movement Sciences, School for Nutrition and Translational Research in Metabolism, Maastricht University, Maastricht, Netherlands.
References
- 1. Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117(4):495–502. [DOI] [PubMed] [Google Scholar]
- 2. Connell NJ, Houtkooper RH, Schrauwen P. NAD+ metabolism as a target for metabolic health: have we found the silver bullet?. Diabetologia. 2019;62(6):888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houtkooper RH, Auwerx J. Exploring the therapeutic space around NAD+. J Cell Biol. 2012;199(2):205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17(11):679–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canto C, Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD+?. Pharmacol Rev. 2012;64(1):166–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fletcher RS, Ratajczak J, Doig CL, Oakey LA, Callingham R, Da Silva Xavier G, Garten A, Elhassan YS, Redpath P, Migaud ME et al. Nicotinamide riboside kinases display redundancy in mediating nicotinamide mononucleotide and nicotinamide riboside metabolism in skeletal muscle cells. Mol Metab. 2017;6(8):819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan NA, Auranen M, Paetau I, Pirinen E, Euro L, Forsström S, Pasila L, Velagapudi V, Carroll CJ, Auwerx J et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol Med. 2014;6(6):721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trammell SAJ, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep. 2016;6:26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi W, Hegeman MA, van Dartel DAM, Tang J, Suarez M, Swarts H, van der Hee B, Arola L, Keijer J. Effects of a wide range of dietary nicotinamide riboside (NR) concentrations on metabolic flexibility and white adipose tissue (WAT) of mice fed a mildly obesogenic diet. Mol Nutr Food Res. 2017;61(8):1600878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frederick DW, Loro E, Liu L, Davila A Jr, Chellappa K, Silverman IM, Quinn WJ 3rd, Gosai SJ, Tichy ED, Davis JG et al. Loss of NAD homeostasis leads to progressive and reversible degeneration of skeletal muscle. Cell Metab. 2016;24(2):269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016;7:12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7(7):e42357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Guia RM, Agerholm M, Nielsen TS, Consitt LA, Sogaard D, Helge JW, Larsen S, Brandauer J, Houmard JA, Treebak JT. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol Rep. 2019;7(12):e14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dellinger RW, Santos SR, Morris M, Evans M, Alminana D, Guarente L, Marcotulli E. Author correction: repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis. 2017;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Airhart SE, Shireman LM, Risler LJ, Anderson GD, Nagana Gowda GA, Raftery D, Tian R, Shen DD, O'Brien KD. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One. 2017;12(12):e0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, Stødkilde-Jørgensen H, Møller N, Brenner C, Treebak JT et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108(2):343–53. [DOI] [PubMed] [Google Scholar]
- 21. Conze D, Brenner C, Kruger CL. Safety and metabolism of long-term administration of NIAGEN (nicotinamide riboside chloride) in a randomized, double-blind, placebo-controlled clinical trial of healthy overweight adults. Sci Rep. 2019;9(1):9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Conze DB, Crespo-Barreto J, Kruger CL. Safety assessment of nicotinamide riboside, a form of vitamin B3. Hum Exp Toxicol. 2016;35(11):1149–60. [DOI] [PubMed] [Google Scholar]
- 23. Dollerup OL, Chubanava S, Agerholm M, Søndergård SD, Altintaş A, Møller AB, Høyer KF, Ringgaard S, Stødkilde-Jørgensen H, Lavery GG et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J Physiol. 2020;598(4):731–54. [DOI] [PubMed] [Google Scholar]
- 24. Elhassan YS, Kluckova K, Fletcher RS, Schmidt MS, Garten A, Doig CL, Cartwright DM, Oakey L, Burley CV, Jenkinson N et al. Nicotinamide riboside augments the aged human skeletal muscle NAD+ metabolome and induces transcriptomic and anti-inflammatory signatures. Cell Rep. 2019;28(7):1717–28..e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–42. [DOI] [PubMed] [Google Scholar]
- 26. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–23. [DOI] [PubMed] [Google Scholar]
- 27. Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71(2):140–50. [DOI] [PubMed] [Google Scholar]
- 28. Kato T, Berger SJ, Carter JA, Lowry OH. An enzymatic cycling method for nicotinamide-adenine dinucleotide with malic and alcohol dehydrogenases. Anal Biochem. 1973;53(1):86–97. [DOI] [PubMed] [Google Scholar]
- 29. van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, Livingstone R, Nowotny P, Sparks LM, Paglialunga S et al. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes. 2015;64(4):1193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoeks J, van Herpen NA, Mensink M, Moonen-Kornips E, van Beurden D, Hesselink MK, Schrauwen P. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes. 2010;59(9):2117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wefers J, van Moorsel D, Hansen J, Connell NJ, Havekes B, Hoeks J, van Marken Lichtenbelt WD, Duez H, Phielix E, Kalsbeek A et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci U S A. 2018;115(30):7789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuipers H, Verstappen FT, Keizer HA, Geurten P, van Kranenburg G. Variability of aerobic performance in the laboratory and its physiologic correlates. Int J Sports Med. 1985;6(4):197–201. [DOI] [PubMed] [Google Scholar]
- 33. Lindeboom L, Nabuurs CI, Hesselink MK, Wildberger JE, Schrauwen P, Schrauwen-Hinderling VB. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am J Clin Nutr. 2015;101(1):65–71. [DOI] [PubMed] [Google Scholar]
- 34. Lindeboom L, Bruls YM, van Ewijk PA, Hesselink MK, Wildberger JE, Schrauwen P, Schrauwen-Hinderling VB. Longitudinal relaxation time editing for acetylcarnitine detection with 1H-MRS. Magn Reson Med. 2017;77(2):505–10. [DOI] [PubMed] [Google Scholar]
- 35. Lindeboom L, Nabuurs CI, Hoeks J, Brouwers B, Phielix E, Kooi ME, Hesselink MK, Wildberger JE, Stevens RD, Koves T et al. Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. J Clin Invest. 2014;124(11):4915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van de Weijer T, van Ewijk PA, Zandbergen HR, Slenter JM, Kessels AG, Wildberger JE, Hesselink MK, Schrauwen P, Schrauwen-Hinderling VB, Kooi ME. Geometrical models for cardiac MRI in rodents: comparison of quantification of left ventricular volumes and function by various geometrical models with a full-volume MRI data set in rodents. Am J Physiol Heart Circ Physiol. 2012;302(3):H709–15. [DOI] [PubMed] [Google Scholar]
- 37. Joris PJ, Plat J, Bakker SJ, Mensink RP. Long-term magnesium supplementation improves arterial stiffness in overweight and obese adults: results of a randomized, double-blind, placebo-controlled intervention trial. Am J Clin Nutr. 2016;103(5):1260–6. [DOI] [PubMed] [Google Scholar]
- 38. Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27(12):1692–7. [PubMed] [Google Scholar]
- 39. Plasqui G, Soenen S, Westerterp-Plantenga MS, Westerterp KR. Measurement of longitudinal changes in body composition during weight loss and maintenance in overweight and obese subjects using air-displacement plethysmography in comparison with the deuterium dilution technique. Int J Obes (Lond). 2011;35(8):1124–30. [DOI] [PubMed] [Google Scholar]
- 40. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 41. Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16(1):23–9. [PubMed] [Google Scholar]
- 42. Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1–2):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–30. [DOI] [PubMed] [Google Scholar]
- 44. de Ligt M, Bruls YMH, Hansen J, Habets MF, Havekes B, Nascimento EBM, Moonen-Kornips E, Schaart G, Schrauwen-Hinderling VB, van Marken Lichtenbelt W et al. Resveratrol improves ex vivo mitochondrial function but does not affect insulin sensitivity or brown adipose tissue in first degree relatives of patients with type 2 diabetes. Mol Metab. 2018;12:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Timmers S, de Ligt M, Phielix E, van de Weijer T, Hansen J, Moonen-Kornips E, Schaart G, Kunz I, Hesselink MK, Schrauwen-Hinderling VB et al. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: a randomized controlled trial. Diabetes Care. 2016;39:2211–17. [DOI] [PubMed] [Google Scholar]
- 47. Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc J et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation. 2018;137(21):2256–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verdecchia P, Angeli F, Cavallini C. Ambulatory blood pressure for cardiovascular risk stratification. Circulation. 2007;115(16):2091–3. [DOI] [PubMed] [Google Scholar]
- 49. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19(3):243–50. [DOI] [PubMed] [Google Scholar]
- 50. Klepochová R, Valkovič L, Gajdošik M, Hochwartner T, Tschan H, Krebs M, Trattnig S, Krššák M. Detection and alterations of acetylcarnitine in human skeletal muscles by 1H MRS at 7 T. Invest Radiol. 2017;52(7):412–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Watt MJ, Heigenhauser GJ, Stellingwerff T, Hargreaves M, Spriet LL. Carbohydrate ingestion reduces skeletal muscle acetylcarnitine availability but has no effect on substrate phosphorylation at the onset of exercise in man. J Physiol. 2002;544(3):949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Salic K, Gart E, Seidel F, Verschuren L, Caspers M, van Duyvenvoorde W, Wong KE, Keijer J, Bobeldijk-Pastorova I, Wielinga PY et al. Combined treatment with l-carnitine and nicotinamide riboside improves hepatic metabolism and attenuates obesity and liver steatosis. Int J Mol Sci. 2019;20(18):4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.