ABSTRACT
Background
Factor VIIc, fibrinogen, and plasminogen activator inhibitor 1 (PAI-1) are cardiovascular disease (CVD) risk factors and are modulated, in part, by fat type and amount.
Objective
We evaluated fat type and amount on the primary outcomes: factor VIIc, fibrinogen, and PAI-1.
Methods
In the Dietary Effects on Lipoproteins and Thrombogenic Activity (DELTA) Trial, 2 controlled crossover feeding studies evaluated substituting carbohydrate or MUFAs for SFAs. Study 1: healthy participants (n = 103) were provided with (8 wk) an average American diet [AAD; designed to provide 37% of energy (%E) as fat, 16% SFA], a Step 1 diet (30%E fat, 9% SFA), and a diet low in SFA (Low-Sat; 26%E fat, 5% SFA). Study 2: participants (n = 85) at risk for CVD and metabolic syndrome (MetSyn) were provided with (7 wk) an AAD, a step 1 diet, and a high-MUFA diet (designed to provide 37%E fat, 8% SFA, 22% MUFA).
Results
Study 1: compared with AAD, the Step 1 and Low-Sat diets decreased mean factor VIIc by 1.8% and 2.6% (overall P = 0.0001), increased mean fibrinogen by 1.2% and 2.8% (P = 0.0141), and increased mean square root PAI-1 by 0.0% and 6.0% (P = 0.0037), respectively. Study 2: compared with AAD, the Step 1 and high-MUFA diets decreased mean factor VIIc by 4.1% and 3.2% (overall P < 0.0001), increased mean fibrinogen by 3.9% and 1.5% (P = 0.0083), and increased mean square-root PAI-1 by 2.0% and 5.8% (P = 0.1319), respectively.
Conclusions
Replacing SFA with carbohydrate decreased factor VIIc and increased fibrinogen in healthy and metabolically unhealthy individuals and also increased PAI-1 in healthy subjects. Replacing SFA with MUFA decreased factor VIIc and increased fibrinogen but less than carbohydrate. Our results indicate an uncertain effect of replacing SFA with carbohydrate or MUFA on cardiometabolic risk because of small changes in hemostatic factors and directionally different responses to decreasing SFA. This trial was registered at https://clinicaltrials.gov/ct2/show/NCT00000538?term=NCT00000538&rank=1 as NCT00000538.
Keywords: DELTA (Dietary Effects on Lipoproteins and Thrombogenic Activity), factor VIIc, fibrinogen, plasminogen activator inhibitor, PAI-1, dietary fat
Introduction
Cardiovascular diseases (CVDs) account for 31% of all deaths globally (1). Myocardial infarction and stroke, caused by occlusive arterial thrombosis, are the most common CVDs (1). In the United States, communities of color continue to have disproportionately higher rates of death due to coronary artery disease (CAD) and stroke (2). Several hemostatic factors have been identified that affect CVD risk (3), notably fibrinogen, factor VIIc, and plasminogen activator inhibitor 1 (PAI-1) (4). The Fibrinogen Studies Collaboration, a meta-analysis on 31 prospective studies with 154,211 participants, reported that the relative risk of coronary heart disease (CHD) and stroke increased by 1.8 (95% CI: 1.6, 2.0) units per each 1-g/L increase in plasma fibrinogen after adjustment for several CVD risk factors (5). The association between CVD and factor VIIc coagulant activity (factor VIIc) is less robust; most prospective studies have not reported an association, although in the Northwick Heart Park Study an elevated factor VIIc was associated with increased CHD mortality (6). The association between elevated PAI-1 plasma concentrations and vascular thrombosis has also been reported (7).
Epidemiologic evidence suggests that dietary total fat and SFA are positively associated with prothrombotic factors (8, 9), whereas fish, fish oil, dietary fiber, and alcohol consumption (light to moderate) are associated with lower concentrations of prothrombotic factors (10–13). Some clinical studies have suggested that reducing dietary fat and SFA and increasing dietary fiber lowers factor VIIc concentrations (14–16); however, the Women's Health Initiative reported no effect of a low-fat diet on factor VIIc activity and concentration in postmenopausal women (17). In contrast, the effects of diet composition on fibrinogen and PAI-1 concentrations have been small and inconsistent (14–16, 18), although weight loss achieved by caloric restriction has been shown to improve markers of fibrinolytic activity (19–21). Based on the research to date, Forouhi et al. (22) concluded that there is insufficient evidence on the effects of fatty acids on thrombosis. This agrees with the conclusion by Siri-Tarino et al. (23) that the effects of SFA on thrombosis are unclear.
The 2013 American Heart Association (AHA)/American College of Cardiology (ACC) Guideline on Lifestyle Management to Reduce Cardiovascular Risk (24) recommended the Dietary Approaches to Stop Hypertension (DASH) healthy, food-based dietary pattern that is low in SFA, trans fat, and sodium to reduce elevated LDL cholesterol and blood pressure. The recent ACC/AHA Primary Prevention Guideline (25) reinforces this healthy dietary pattern message to decrease atherosclerotic CVD risk factors. The Mediterranean-style dietary pattern, a variation of DASH (i.e., it is low in SFA and high in unsaturated fat) has remarkable cardiovascular benefits (26). There also is some evidence that a Mediterranean-style dietary pattern (26) has a protective effect on thrombosis (27, 28), which may be due to dietary unsaturated fat. We have an understanding of the effects of substituting carbohydrates and unsaturated fats for SFA on lipids and lipoproteins (29); however, little is known about the effects of macronutrient variations on hemostatic factors.
The Dietary Effects on Lipoproteins and Thrombogenic Activity (DELTA) Study was conducted to evaluate the effects of diet on the following primary outcomes: lipids, lipoproteins, and hemostatic factors in different population groups, including those at high risk of CVD (e.g., African Americans and individuals with or at risk for metabolic syndrome) (30). A major goal was to identify whether dietary carbohydrate or MUFA was the preferred caloric substitute for SFA for minimization of CVD risk factors. Study 1 evaluated the effects of a stepwise reduction in dietary total fat and SFA on plasma lipids, lipoproteins, and hemostatic factors; SFA was replaced with carbohydrate (31). Study 2 evaluated the effects on lipids, lipoproteins, and hemostatic factors of replacing SFA with either MUFA or carbohydrate in participants with an accentuated metabolic and cardiovascular risk profile (32). Including both studies enabled us to address a key nutrition question, which is whether dietary carbohydrate or unsaturated fat is the preferred macronutrient substitute for SFA?
Methods
Study design
Detailed descriptions of the protocols, diet designs, and treatment effects on plasma lipids and lipoproteins have been reported previously (31, 32). The studies were conducted at the following research centers: Columbia University, Pennington Biomedical Research Center, Pennsylvania State University, and the University of Minnesota; the Coordinating Center was at the University of North Carolina. Both study 1 and study 2 used a randomized double-blind crossover design with 3 feeding periods and 6 diet sequences. After a brief run-in period to assess protocol compliance, each participant was randomly assigned to 1 of 6 diet sequences. The randomization procedure provided and controlled by the Coordinating Center was stratified by center. The duration of each feeding period was 8 wk in study 1 and 7 wk in study 2. Each period was followed by a 4- to 6-wk washout interval (or “rest interval”) during which time participants resumed their habitual dietary patterns. All food was provided to participants during the feeding periods for both studies. Participants’ meals were prepared individually, with all items containing fat weighed to the nearest 0.1 g; other foods were weighed to the nearest gram. For each protocol, all foods and all of the 8-d-cycle menus were identical among the 4 research centers. Diet composition was monitored by chemical analysis of duplicate meal homogenates collected daily at all research centers (33). Chemical analysis confirmed that target nutrient goals of the designed diets were met (31–33). Study participants were required to eat 2 meals on-site each weekday (either breakfast and dinner, or lunch and dinner, depending on the research center). Other meals and snacks were packed and distributed to participants for consumption at a time and place of convenience. Dietary compliance was assessed by observation of 2 meals/d, daily records, and interviews. Participants were blinded to the test diets. In addition, all samples were assayed in blinded manner. The research centers began feeding on the same date and followed identical feeding schedules and study procedures for both studies. In study 1, the 3 feeding periods spanned 27 September 1993 to 4 May 1994. In study 2, the 3 feeding periods spanned 30 September 1994 to 19 May 1995.
Energy requirements were calculated using the Harris-Benedict equation with the physical activity factor derived from self-reported activity levels. If necessary, the calculated energy level was adjusted to maintain body weight. Participants were instructed not to modify exercise or other physical activity habits. These factors, the occurrence of illness, and use of medications were monitored weekly. Protocols for both studies were approved by the institutional review boards at each research center. This study is registered at Clinicaltrials.gov as NCT00000538.
Study cohorts
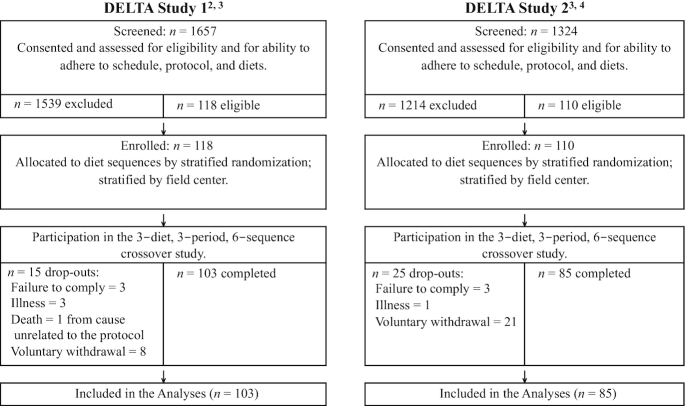
Participant recruitment for both studies is presented in a CONSORT (Consolidated Standards of Reporting Trials) diagram (Figure 1). For study 1 and study 2, we estimated that we needed targeted samples sizes that would yield ≥96 participants and 110 participants with complete data. The lengths of feeding periods were designed to allow physiological stabilization on each diet. Primary considerations in choosing the target sample sizes, the crossover experimental design, the number of longitudinal evaluations (blood draws) per period, and the lengths of the feeding periods included cost and time requirements, as well as reasonable conjectures about the anticipated levels of precision of the statistical estimators of interest, and the levels of power for the hypothesis tests of interest. The conjectures about anticipated levels of precision and power were informed by point and interval estimates of variance components obtained from our previous studies. The estimates of statistical power were obtained only for the primary hypothesis tests regarding diet effects on the lipids, lipoproteins, and hemostatic factors.
FIGURE 1.
CONSORT diagrams for DELTA study 1 and study 2. 1Methods, diets, and other information as described in Ginsberg et al. (31). 2Each participant was randomly assigned to a sequence of 3 protocol-specific experimental diets. 3Methods and diets as described in Berglund et al. (32); other information from unpublished data (Collaborative Studies Coordinating Center, School of Public Health, University of North Carolina, Chapel Hill, NC, 1995). CONSORT, Consolidated Standards of Reporting Trials; DELTA, Dietary Effects on Lipoproteins and Thrombogenic Activity.
We note that all calculations of anticipated power and anticipated precision, including those used in the planning stage, have no valid role in the interpretation of study results. Once a study is completed, those historical conjectures provide no valid guidance in the analysis of the results (34–36). Rather, the CI estimates, point estimates, and hypothesis tests we have reported provide all the appropriate information and guidance for interpretation of study results.
Study 1 (amount of total and saturated fat)
In study 1, a diverse population (by age, gender, and race) with lipid/lipoprotein concentrations representative of the US population was recruited. Healthy, normolipidemic participants between 22 and 67 y of age who were taking no medications known to affect lipids or hemostatic factors were recruited. Participants were eligible if their mean fasting total cholesterol concentrations (measured at 2 separate visits) were between the 25th and 90th percentile for age, race, and gender based on NHANES III (1998–2004) (37), and their plasma triglycerides (TGs) and HDL cholesterol were between the 10th and 90th percentiles. To achieve a sample size of ≥96 participants with complete data, each research center enrolled 25–32 participants. A goal was to have ∼40% males and 60% females (with similar numbers of pre- and postmenopausal women and males > and <40 y of age; i.e., older men = men ≥40 y; younger men = men <40 y of age) and 25% African Americans. Postmenopausal status was self-reported based on 12 consecutive months without a menses (38).
Study 2 (type of fat for SFA replacement)
In study 2, males and females 21–68 y of age were eligible to participate if the average of 2 screening measurements met any of the following age-, race-, and gender-adjusted criteria based on NHANES III data: HDL cholesterol ≤30th percentile, TGs ≥70th percentile, and insulin ≥70th percentile. Participants were ineligible if their 1) average screening total cholesterol was <25th percentile or >90th percentile, 2) LDL cholesterol was >190 mg/dL, 3) fasting TG concentrations were <30th percentile or >500 mg/dL, or 4) HDL cholesterol was >70th percentile. For enrolled participants, insulin resistance was defined as a baseline homeostasis model assessment (HOMA) index >3. Established criteria for MetSyn were defined after we conducted study 2 (39). In the present report, we have classified participants as having MetSyn using the Adult Treatment Panel III criteria described in a previous DELTA paper by Berglund et al. (32), as defined by Grundy et al. (39). Participants were classified as having MetSyn if any 3 of the 5 defined characteristics of MetSyn were present at baseline: elevated waist circumference, elevated TGs, reduced HDL cholesterol, elevated blood pressure, and elevated fasting glucose (37).
Diet composition
Study 1 (amount of total fat)
The study diets we designed were as follows: a high-SFA average American diet (AAD), a Step 1 diet, and a low-SFA diet (Low-Sat) (Table 1). The proportion of each SFA in the AAD studied was representative of the United States at the time, as well as the current US diet. trans Fatty acids were maintained at <1.5% of calories for each diet. Since the goal of study 1 was to evaluate the effects of reducing dietary SFA, dietary cholesterol was maintained at <300 mg/d (assayed value was 275 mg/d) for all 3 diets for all calorie levels. Dietary carbohydrate replaced SFA on the Step 1 and Low-Sat diets. The dietary carbohydrate was a mixture of whole and refined grains and included a variety of fruits and vegetables. Diets were designed to be isocaloric, and unit foods (i.e., muffins) that were compositionally similar to the experimental diet being evaluated were used to adjust calories to achieve weight maintenance.
TABLE 1.
Target nutrient composition of the experimental diets1
| Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|
| Nutrient, % of energy | AAD | Step 1 | Low-Sat | AAD | Step 1 | MUFA |
| Total fat | 37 | 30 | 26 | 37 | 30 | 37 |
| SFA | 16 | 9 | 5 | 16 | 8 | 8 |
| MUFA | 14 | 14 | 14 | 14 | 15 | 22 |
| PUFA | 7 | 7 | 7 | 7 | 7 | 7 |
| Protein | 16 | 16 | 16 | 16 | 16 | 16 |
| Carbohydrate | 47 | 54 | 58 | 47 | 54 | 47 |
| Sugar | 18 | 21 | 23 | 19 | 19 | 18 |
| Cholesterol (mg/d) | 300 | 300 | 300 | 300 | 300 | 300 |
| Fiber (g/1000 kcal) | 9 | 10 | 11 | 8 | 15 | 8 |
1AAD, average American diet; Low-Sat, low-SFA diet.
Study 2 (type of fat)
The study diets designed were as follows: an AAD, a Step 1 diet, and a high-MUFA diet (Table 1). The high-MUFA diet was designed to match the saturated fat and polyunsaturated fat content of the Step 1 diet, and to maintain the total fat content of the AAD. For the high-MUFA diet, we replaced ∼7% of calories from carbohydrate on the Step 1 diet with an equivalent proportion of calories from monounsaturated fat. All 3 diets provided ∼300 mg/d of cholesterol.
Hemostasis measurement methods
The studies were designed to provide several consecutive weekly blood samples in order to 1) assess the time required to achieve a stable state for hemostatic factors and 2) reduce the effects of intraindividual variation on these outcome measures. In study 1, during each of the three 8-wk feeding periods, fasting blood samples were obtained once weekly during weeks 5, 6, 7, and 8. Because plasma concentrations of hemostatic factors stabilized by week 4 in study 1, study 2 was shortened by 1 wk, and fasting blood samples were obtained once weekly during weeks 5, 6, and 7 of each feeding period. Plasma samples were collected in the morning between 06:00 and 10:00 h from the antecubital vein into citrated evacuated tubes. Tourniquet time was limited by protocol to no more than 2 min to avoid stasis. Blood was centrifuged immediately after collection at 2000 × gfor 20 min at room temperature (factor VIIc) or 4°C (fibrinogen, PAI-1) to remove platelets and ensure that PAI-1 measurement reflected circulating plasma concentrations and not contamination from platelet PAI-1. Processing of plasma for factor VIIc was carried out at room temperature and the isolated plasma was rapidly frozen at −80°C to avoid cold activation.
Plasma aliquots were stored at −80°C until the end of each study. Immediately after each study was completed, samples were assayed at the Laboratory for Clinical Biochemistry Research, University of Vermont. Hemostatic factor assays were conducted as follows: the factor VIIc–one-stage clot-rate assay was based on the prothrombin time using human placental thromboplastin, standardized using WHO reference plasma, and performed on a semiautomated Coag-A-Mate X2 instrument (Organon-Technika Co.); PAI-1 antigen concentration was quantified using the ELISA method adapted by Cushman et al. (40), and fibrinogen was assayed using the method described by Clauss (41). Interassay CVs, based on assays of split specimens, for fibrinogen, factor VIIc, and PAI-1, were 3.1%, 5.3%, and 9.0%, respectively.
Study 1 and study 2 also included secondary outcome measures of coagulation: D-dimer (DD; ng/mL), prothrombin fragments (F1.2; nM), plasmin-antiplasmin complex (PAP; nM), C-reactive protein (CRP; μg/mL), and B-thromboglobulin (BTG; IU/mL).
In study 2, for each diet, BTG was measured only during weeks 5 and 6, while CRP, F1.2, PAP, and DD were measured only during weeks 6 and 7. In study 1, for each diet, F1.2, PAP, and DD were measured during weeks 6 and 7.
The F1.2 concentrations were measured using the Behring kit (Behringwerke) with a CV of 15% (42); PAP was measured using 2-site ELISA with murine monoclonal antibodies specific for PAP complex (43) with a CV of 3.6%; CRP immunoassay (44) used specific anticlonal antibodies and purified CRP (Calbiochem) in a competitive ELISA with a CV of 6%; and DD concentrations were measured using an ELISA 2-site immunoassay with a CV of 6% (45); BTG was measured using an immunoassay kit from Diadnotica Stago with a CV of 9%. Each of these secondary hemostasis measures was transformed to log10 scale for the statistical analyses.
Statistical analyses
Primary analyses
The primary hemostasis measures for study 1 and study 2 were fibrinogen, plasma factor VIIc, and PAI-1. In planning study 1, it was anticipated that each of these measures would tend to decrease as dietary intake of total fat and saturated fat decreased. Informed by the surprising results from study 1, study 2 was designed to differentiate the effect of total fat from the effect of saturated fat. In both studies, longitudinal statistical analyses using linear mixed-effects models were performed separately for each of the hemostasis outcome variables. For fibrinogen, for example, each participant contributed 9 fibrinogen values indexed by period (1, 2, or 3), diet within period, and week (fifth, sixth, or seventh) within period. This analysis strategy closely paralleled that previously described (31, 32) for the analyses of the lipid and lipoprotein outcome measures. To enable a more exact statistical analysis, the square-root scale was used for PAI-1 (ng/mL) to reduce skewness and improve distributional properties (46); similarly, the log10 scale had been used (31, 32) for TG concentration (mg/dL). Those transformation decisions were made a priori based on previous studies. The linear mixed-effects model represented mean response as a function of diet, gender-by-age group, race, center, amount of energy intake, feeding period, and interactions of diet with group, race, center, and caloric intake. The 2 categories for race were “African American” and “all other.” The gender-by-age groups were “premenopausal women,” “postmenopausal women,” “men under 40 y of age,” and “men, 40 y and older.” Dietary energy intake categories were 1500, 2000, 2500, 3000, and 3500 kcals/d. The “baseline” characteristics (group, race, center, energy intake) were included to potentially improve the precision of the estimators of treatment effects and period effects; however, the magnitudes of those estimators are invariant to their inclusion or exclusion. The a priori analysis plans specified criteria for dropping from the model energy intake (if no improvement in the precision of the treatment main-effect estimators) and for dropping all the interaction terms from the model (the magnitudes of the estimates of their regression coefficient being <10% of the estimate of the AAD mean, along with negligible impact on the estimates of the diet treatment main effects, i.e., the estimates changed by <10%). For both studies, total variance of the conditional distribution of assay values was assumed to be constant across all factor levels and occasions. Correlation between any 2 of an individual's assay values was assumed to be larger for same-diet pairs, smaller for different-diet pairs, but otherwise invariant. This model was represented and interpreted as a components-of-variance model with 3 components: interindividual variance of the individuals’ overall mean concentrations (“subject”), interindividual variance of individuals’ diet-specific mean concentrations (“diet-by-subject”), and intraindividual variance (“within subject”). In these models, missing values (if any) for the dependent variable were not imputed; the linear mixed-effects model is designed to cope with incomplete longitudinal data. Participants with some missing data values were not excluded from the computations; however, participants who dropped out of the study during the first few weeks of period #1 were excluded from the primary analyses. No baseline covariate data values were missing in these studies. Estimates of the parameters of the linear mixed-effects models were used to compute point estimates and CI estimates of diet-specific means and mean differences. In terms of mean differences, the pairwise null hypotheses (“no difference between the 2 diets in the target population”) were tested using the Marcus-Peritz-Gabriel closed hierarchical multiple-comparisons procedure (47); specifically, each pairwise null hypothesis is rejected only if its F-test P value is less than α = 0.01 and the F-test P value for the overall null hypothesis (“no differences among the 3 diets in the target population”) is also less than α = 0.01. Tests observed to be not statistically significant are inconclusive; all statistical test procedures are, by design, incapable of establishing that the null hypothesis is true. In contrast, the point and interval estimates are always informative to some degree.
The decision to choose α = 0.01 for the primary hypothesis tests in these studies was made a priori by the research team based on investigator preferences regarding interpretation of evidence and consideration of the large number of co–primary outcome variables involved in the studies (31, 32). For consistency, 99% CIs are reported; however, the main purpose of the reported CIs is interval estimation and not service to hypothesis testing. The CIs are informative regarding the treatment effect in the target population as they identify a range of candidate values that is most compatible with the observed data. In contrast, hypothesis tests yielding a P value > α are entirely inconclusive as to whether the (null) hypothesis is true or false—that is, the result cannot be used to infer that the treatment effect is zero in the target population from which the sample of participants was drawn.
Secondary analyses of heterogeneity of treatment effects
Subpopulation differences in diet effects (i.e., heterogeneity of treatment effects; HTEs) were investigated. For study 1 and study 2, these secondary analyses focused on interactions of diet with age-gender group and interactions of diet with race. Also, for study 2, the primary model was modified to include additional covariates representing risk categories and their interactions with diet: categories defined by the baseline eligibility criteria or risk categories defined by MetSyn (yes, no), HDL cholesterol (high, low), TGs (high, low), HOMA (high, low).
Analyses of secondary hemostasis measures
Similar analysis strategies were applied to prothrombin fragment F1.2, PAP, DD, and BTG in studies 1 and 2, and to CRP in study 2.
Sensitivity analyses
To guide our trust in the main results, auxiliary diagnostic procedures and variations on the analysis methods were applied to evaluate the sensitivity or robustness of the main results to reasonable perturbations of methods and assumptions used; for example, we examined 1) temporal stabilization of hemostatic variables during the 5–8 or 5–7 wk; 2) first-order carryover effects were investigated; 3) variations in the number of variance-covariance parameters assumed; 4) graphical representations of within-subject residuals, between-subject residuals for evidence of departures from modeling assumptions (via Q-Q plots, histograms, scatterplots); 5) diagnostics for identification of extreme or influential values; and 6) analysis of agreement between the participant's observed responses (e.g., fibrinogen values) and model-based estimates of the participant's responses—the estimates being computed by best empirical linear unbiased prediction (eBLUP) methods (48).
Computations
All statistical computations were performed using SAS System software version 9.1 and were updated using version 9.4 (SAS Institute).
Results
The experimental diets were well accepted. Good dietary compliance, assessed by observation of 2 meals/d, daily records and interviews, was achieved as noted previously (31). In addition, participants in both studies maintained their weight (31). As verified by chemical assays, the fat and fatty acid composition of each experimental diet met protocol specifications (within 1–2% of the target value; data previously reported) (31–33). In study 1 and study 2, the estimates of diet treatment effects reported here were obtained from the reduced model, accounting only for race, gender-by-age, research center, and feeding period. We focus on the reduced model because the estimates for the interaction terms and caloric intake were negligible and had negligible impact on the estimates of the diet effects. Although not part of the criterion for removing those terms, the CIs were wide, and the hypothesis tests were inconclusive regarding the null hypotheses that those parameters are zero in the target population.
Study 1 results (amount of total fat)
Of the 118 participants who were enrolled and randomly assigned, 103 completed the study (Figure 1). Reasons for drop-out and incomplete data included scheduling issues, adherence problems with the study protocol, or short-term illness (Figure 1). The participants who dropped out did so during the first few weeks of feeding period #1 (except for the 1 death that occurred during period #2); they were excluded from the primary statistical analyses. Males and females of different ages and ethnicities were studied (Table 2). The demographic profiles of participants in study 1 were representative of the population at large in the United States when the study was conducted. Baseline characteristics of study participants are shown in Table 3. In addition, baseline percentiles of TGs and insulin are presented in Supplemental Table 1.
TABLE 2.
Study 1: categorization of participants by sex, age, and race
| African American | Non–African American | Total | |
|---|---|---|---|
| Premenopausal women1 | 11 | 28 | 392 |
| Postmenopausal women3 | 6 | 12 | 18 |
| Men aged <40 y | 8 | 22 | 30 |
| Men aged ≥40 y | 1 | 15 | 16 |
| Total | 26 | 77 | 103 |
Premenopausal status was self-reported based on the presence of menses, either regular or irregular periods.
Because the total (n = 103) is very close to 100, the column percentages approximate the counts; for example, 37.9% (39/103) were premenopausal women.
Postmenopausal status was self-reported based on 12 consecutive months without a menses.
TABLE 3.
Baseline characteristics of study participants1
| Study 1 (amount of total fat) | Study 2 (type of fat) | |||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Men (n = 46) | Women (n = 57) | Total (n = 103) | Men (n = 52) | Women (n = 33) | Total (n = 85) |
| Age, y | 36.0 ± 12.0 | 39.4 ± 14.8 | 37.9 ± 13.6 | 33.3 ± 8.6 | 39.0 ± 9.7 | 35.5 ± 9.4 |
| BMI, kg/m2 | 24.7 ± 3.6 | 24.4 ± 3.0 | 24.5 ± 3.3 | 27.4 ± 4.2 | 27.9 ± 4.6 | 27.6 ± 4.4 |
| Total cholesterol, mg/dL | 198.9 ± 29.3 | 201.2 ± 31.8 | 200.1 ± 30.6 | 199.5 ± 34.8 | 199.2 ± 30.9 | 199.5 ± 30.9 |
| LDL cholesterol, mg/dL | 132.3 ± 29.6 | 128.2 ± 30.2 | 130.1 ± 29.9 | 128.8 ± 23.2 | 127.2 ± 27.1 | 128.4 ± 23.2 |
| HDL cholesterol, mg/dL | 44.5 ± 9.3 | 55.1 ± 9.9 | 50.4 ± 11.0 | 39.1 ± 7.7 | 45.6 ± 11.6 | 41.8 ± 7.7 |
| Triglycerides, log10 mg/dL | 2.01 ± 0.2 | 1.91 ± 0.2 | 1.96 ± 0.2 | 2.14 ± 0.3 | 2.08 ± 0.2 | 2.11 ± 0.2 |
| Glucose, mg/dL | ND | ND | ND | 93.3 ± 12.6 | 92.4 ± 10.8 | 93.0 ± 12.6 |
| Insulin, log10 μU/mL | ND | ND | ND | 1.06 ± 0.2 | 1.01 ± 0.2 | 1.04 ± 0.2 |
Values are means ± 1 SD. All analytes were measured in plasma. ND, not done.
Primary analyses
The effects of each diet on hemostatic responses are shown in Table 4. In the study 1 cohort, we observed differences in the mean concentrations of factor VIIc, fibrinogen, and PAI-1 when comparing the AAD with the Step 1 and the Low-Sat diets. In terms of their estimated effects (with 99% CIs), the Step 1 and Low-Sat diets decreased the mean concentration of factor VIIc by 1.6 (0.2, 3.0) and 2.3 (0.9, 3.7) units (1.8% and 2.6%), respectively; increased mean fibrinogen by 3.3 (−3.6, 10.2) and 7.7 (0.9, 14.6) units (1.2% and 2.8%); and increased the mean of square-root PAI-1 by 0.001 (−0.15, 0.15) and 0.17 (0.01, 0.31) units (0.0% and 6.0%). For factor VIIc and for fibrinogen, but not for PAI-1, the hypothesis test procedure comparing Step 1 with Low-Sat was inconclusive as to whether the mean differences are exactly zero in the target population.
TABLE 4.
Study 1: overall effects of diet on hemostatic factors in healthy and metabolically unhealthy participants1
| Factor VIIc (n = 103), % | Fibrinogen (n = 103), mg/dL | PAI-1 square root (n = 102), ng/mL | |
|---|---|---|---|
| Study 1 diets | |||
| AAD | 89.1 ± 1.5 | 276.6 ± 4.4 | 2.81 ± 0.15 |
| Step 1 | 87.5 ± 1.5 | 279.9 ± 4.4 | 2.81 ± 0.15 |
| Low-Sat | 86.8 ± 1.5 | 284.3 ± 4.4 | 2.97 ± 0.15 |
| Difference: Step 1 – AAD | |||
| Estimate ± SE | −1.6 ± 0.5 | 3.3 ± 2.7 | 0.00 ± 0.06 |
| 99% CI | (−3.0, −0.2) | (−3.6, 10.2) | (−0.15, 0.15) |
| Pairwise P value | 0.00322 | 0.2153 | 0.9925 |
| Percentage change of means | −1.8 ± 0.63 | 1.2 ± 1.0 | 0.0 ± 2.0 |
| Difference: Low-Sat – AAD | |||
| Estimate ± SE | −2.3 ± 0.5 | 7.7 ± 2.7 | 0.17 ± 0.06 |
| 99% CI | (−3.7, −0.9) | (0.9, 14.6) | (0.02, 0.31) |
| Pairwise P value | ≤0.00012 | 0.0036 | 0.0037 |
| Percentage change of means | −2.6 ± 0.6 | 2.8 ± 1.0 | 6.0 ± 2.0 |
| Difference: Low-Sat – Step 1 | |||
| Estimate ± SE | −0.7 ± 0.5 | 4.4 ± 2.7 | 0.16 ± 0.06 |
| 99% CI | (−2.1, 0.68) | (−2.4, 11.3) | (0.02, 0.31) |
| Pairwise P value | 0.18532 | 0.0938 | 0.0038 |
| Percentage change of means | −0.8 ± 0.6 | 1.6 ± 1.0 | 6.0 ± 2.0 |
| Overall P value | 0.00012 | 0.0141 | 0.0037 |
Each entry is the estimated mean ± 1 SE obtained via a linear mixed-effects model accounting for diet, feeding period, center, race, and gender/age group. All PAI-1 values were missing for 1 participant. All analytes were measured in plasma. AAD, average American diet; Low-Sat, low-SFA diet; PAI-1, plasminogen activator inhibitor 1.
In terms of mean differences, the pairwise null hypotheses (“no difference between the 2 diets in the target population”) were tested using the Marcus-Peritz-Gabriel closed hierarchical multiple-comparisons procedure (47): each pairwise null hypothesis is rejected only if its F-test P value is less than α = 0.01 and the F-test P value for the overall null hypothesis (“no differences among the 3 diets in the target population”) is also less than α = 0.01. Tests observed to be not statistically significant are inconclusive; all statistical test procedures are, by design, incapable of establishing that the null hypothesis is true. In contrast, the point and interval estimates are always informative to some degree.
Percentage change in the mean from AAD ± 1 SE.
Sensitivity analyses
In examining the robustness of the diet effect estimates, similar results were obtained with and without inclusion of energy intake and terms representing interactions of diet with gender-age group, center, race, and energy intake. For example, similar results are shown in Supplemental Table 2 in terms of unadjusted means ± SDs for factor VIIc and fibrinogen, and as unadjusted medians and IQRs for PAI-1. Analyses of the model's residuals (total residuals, within-subject residuals, between-subject residuals) adequately supported the assumptions of the linear mixed-effects models. Identification and removal of extreme or influential observations did not occur. Model diagnostics indicated satisfactory goodness-of-fit. Stabilization of the hemostatic measures by week 4 was verified by longitudinal linear mixed-effects model analyses of the outcomes at weeks 5–8 accounting for “week” and diet-by-week interactions with use of from 6 to 12 variance-covariance parameters. For the models with 9 variance-covariance parameters, in terms of week-specific estimates of the Step 1 main-effect (relative to AAD), week 5 differed from week 8 by 0.8 units for factor VIIc, by 10 units for fibrinogen, by 0.1 units for square-root PAI-1; and similarly, for the Low-Sat main effect, these differences were 0.2, 10, and 0.2 units, respectively. Point estimates of first-order carryover effects were negligible (<5% of the estimate of the AAD mean), their CIs were wide, and the hypothesis tests were inconclusive regarding the null hypotheses that those parameters are zero in the target population. These results were obtained by inclusion of carryover terms in the models used for the main analyses. Collectively, the results of these various sensitivity analyses strengthened our trust in the main results.
Secondary analyses of HTEs
Treatment effects were generally consistent across the subpopulations of interest and across the 4 centers, as illustrated in Supplemental Figures 1–3.
Analysis of secondary hemostasis measures
For DD, F1.2, PAP, CRP, and BTG, the diet treatment effect estimates were small with wide CIs and the primary hypothesis tests were inconclusive (data not shown).
Study 2 results (type of fat)
Of the 110 participants who were enrolled and randomly assigned, 85 completed all 3 feeding periods (Figure 1). Reasons for missing data and drop-out in study 2 were similar to those reported for study 1. The participants were metabolically unhealthy as defined by their baseline characteristics (presented in Table 3) and risk factors for cardiometabolic disease (Table 5). The 2 most common eligibility criteria were low HDL cholesterol and low HDL cholesterol plus high TGs (Table 5). The study 2 cohort differed from the study 1 cohort in their responses to the AAD: mean factor VIIc was ∼25% higher, mean fibrinogen was 6% higher, and mean square-root PAI-1 was >100% higher.
TABLE 5.
Study 2: categorization of participants by eligibility criteria at baseline1
| Criteria | n | Percentage |
|---|---|---|
| ↓ HDL-C (≤ 30th percentile)2 | 26 | 30.6 |
| ↑ TGs (≥70th percentile) 2 | 7 | 8.2 |
| ↑ Insulin (≥70th percentile) 2 | 5 | 5.9 |
| ↓ HDL-C + ↑ TGs | 25 | 29.4 |
| ↓ HDL-C + ↑ insulin | 5 | 5.9 |
| ↑ TGs + ↑ insulin | 2 | 2.4 |
| ↓ HDL-C + ↑ TGs + ↑ insulin | 15 | 17.6 |
| Total | 85 | 100 |
All analytes were measured in plasma. HDL-C, HDL cholesterol; TG, triglyceride.
Based on the third NHANES (NHANES III) specific for age, sex, and race.
Primary analyses
The effects of each diet on hemostatic responses are shown in Table 6.
TABLE 6.
Study 2: overall effects of diet on hemostatic factors in healthy and metabolically unhealthy participants1
| Factor VIIc (n = 85), % | Fibrinogen (n = 85), mg/dL | PAI-1 square root (n = 85), ng/mL | |
|---|---|---|---|
| Study 2 diets | |||
| AAD | 111.1 ± 4.3 | 308.0 ± 9.7 | 4.36 ± 0.37 |
| Step 1 | 106.5 ± 4.3 | 319.9 ± 9.7 | 4.44 ± 0.37 |
| MUFA | 107.5 ± 4.3 | 312.5 ± 9.7 | 4.81 ± 0.37 |
| Difference: Step 1 – AAD | |||
| Estimate ± SE | −4.6 ± 1.0 | 11.9 ± 3.9 | 0.09 ± 0.13 |
| 99% CI | (−7.1, −2.0) | (1.9, 21.9) | (−0.24, 0.42) |
| Pairwise P value | ≤0.00012 | 0.0022 | 0.4942 |
| Percentage change of means | −4.1 ± 0.93 | 3.9 ± 1.3 | 2.0 ± 2.9 |
| Difference: MUFA − AAD | |||
| Estimate ± SE | −3.6 ± 1.0 | 4.6 ± 3.9 | 0.25 ± 0.13 |
| 99% CI | (−6.1, −1.0) | (−5.5, 14.6) | (−0.08, 0.58) |
| Pairwise P value | ≤0.00042 | 0.2392 | 0.0477 |
| Percentage change of means | −3.2 ± 0.9 | 1.5 ± 1.3 | 5.8 ± 2.9 |
| Difference: MUFA – Step 1 | |||
| Estimate ± SE | 1.0 ± 1.0 | −7.4 ± 3.9 | 0.17 ± 0.13 |
| 99% CI | (−1.6, 3.6) | (−17.4, 2.7) | (−0.16, 0.50) |
| Pairwise P value | ≤ 0.31242 | 0.0578 | 0.1929 |
| Percentage change of means | 0.9 ± 0.9 | −2.4 ± 1.3 | 3.8 ± 2.9 |
| Overall P value | <0.00012 | 0.0083 | 0.1319 |
Each entry is the estimated mean ± 1 SE obtained via a linear mixed-effects model accounting for diet, feeding period, center, race, and gender/age group. All analytes were measured in plasma. AAD, average American diet; PAI-1, plasminogen activator inhibitor 1.
In terms of mean differences, the pairwise null hypotheses (“no difference between the 2 diets in the target population”) were tested using the Marcus-Peritz-Gabriel closed hierarchical multiple-comparisons procedure (47): each pairwise null hypothesis is rejected only if its F-test P value is less than α = 0.01 and the F-test P value for the overall null hypothesis (“no differences among the 3 diets in the target population”) is also less than α = 0.01. Tests observed to be not statistically significant are inconclusive; all statistical test procedures are, by design, incapable of establishing that the null hypothesis is true. In contrast, the point and interval estimates are always informative to some degree.
Percentage change in the mean for AAD ± 1 SE.
The Step 1 and high-MUFA diets, respectively, decreased the mean concentration of factor VIIc by 4.6 (2.0, 7.1) and 3.6 (1.0, 6.1) units (4.1% and 3.2%), increased mean fibrinogen by 11.9 (1.9, 21.9) and 4.6 (−5.5, 14.6) units (3.9% and 1.5%), and increased mean square-root PAI-1 by 0.09 (−0.24, 0.42) and 0.25 (−0.08, 0.58) units (2.0% and 5.8%) (Table 6). The high-MUFA diet and the Step 1 diet were similar in improving factor VIIc. The high-MUFA diet raised mean fibrinogen less than the Step 1 diet, but the Step 1 diet raised PAI-1 less than the high-MUFA diet. The hypothesis tests comparing MUFA and Step 1 were inconclusive as to whether the mean differences are exactly zero in the target population (Table 6).
Sensitivity analyses
In Supplemental Table 2, similar results are presented as unadjusted means ± SDs for factor VIIc and fibrinogen, and as medians and IQRs for PAI-1. The study conclusions were essentially the same regardless of choice of methods and model assumptions used. As in study 1, stabilization of the hemostatic measures by week 4 was verified by longitudinal analyses for weeks 1–7, estimates of first-order carryover effects were negligible, and analyses of residuals adequately supported the assumptions of the models. Collectively, the results of these various sensitivity analyses strengthened our trust in the main results.
Secondary analyses of HTEs
Estimation of subpopulation differences in treatment effects showed consistency in the directions and magnitudes of the diet effects across categories of gender, age, race, baseline eligibility criteria, and risk categories defined by MetSyn, low HDL cholesterol, high TGs, and high HOMA. Estimates of selected potential interactions are illustrated in Supplemental Figures 4–6.
For factor VIIc (Supplemental Figure 4) the interaction effect estimates ranged from 1.0 to 4.0. The observed estimates of the effect of MUFA (vs. AAD) were slightly better for categories “TG high,” “HDL low,” and “HOMA high,” but slightly worse for “MetSyn yes.” The effects for the Step 1 diet were very similar to those of MUFA.
For fibrinogen (Supplemental Figure 5), the treatment effects for Step 1 and MUFA (vs. AAD) were consistent across subpopulations.
For PAI-1 (Supplemental Figure 6), the diet effect estimates for the MUFA and Step 1 diets (vs. AAD) were generally consistent across subpopulations, with 1 potential exception: the estimates suggest that individuals with MetSyn may be different from those without MetSyn. For those with MetSyn, mean square-root PAI-1 was not increased but rather was decreased by 0.15 units on the MUFA diet and was increased by 0.52 units on the Step 1 diet (Table 7).
TABLE 7.
Study 2 heterogeneity of diet effects in PAI-1: participants with or without MetSyn1
| All (n = 85) | With MetSyn (n = 20) | Without MetSyn (n = 65) | Difference: with vs. without | Difference: P value | |
|---|---|---|---|---|---|
| AAD | 4.54 ± 0.372 | 5.04 ± 0.482 | 4.03 ± 0.392 | 1.02 ± 0.473 | 0.03174 |
| MUFA | 4.65 ± 0.37 | 4.90 ± 0.48 | 4.41 ± 0.39 | 0.49 ± 0.47 | 0.2956 |
| Step 1 | 4.77 ± 0.37 | 5.56 ± 0.48 | 3.99 ± 0.39 | 1.57 ± 0.47 | 0.0009 |
| MUFA − AAD | 0.12 ± 0.153 | −0.15 ± 0.25 | 0.38 ± 0.14 | −0.52 ± 0.295 | 0.0732 |
| Step 1 − AAD | 0.24 ± 0.15 | 0.52 ± 0.26 | −0.04 ± 0.14 | 0.56 ± 0.29 | 0.0570 |
| Step 1 − MUFA | 0.12 ± 0.15 | 0.66 ± 0.26 | −0.42 ± 0.14 | 1.08 ± 0.29 | 0.0003 |
All analytes were measured in plasma. AAD, average American diet; MetSyn, metabolic syndrome; PAI-1, plasminogen activator inhibitor 1.
Entry is the estimated mean ± 1 SE obtained via a linear mixed-effects model accounting for diet, feeding period, center, race, gender/age group, MetSyn, and terms representing diet-by-MetSyn interactions.
Entry is the estimated mean difference ± 1 SE obtained from the same model.
The P value for the test of the null hypothesis that the difference is zero in the target population from which the sample was drawn.
Entry is the estimated difference of mean differences ± 1 SE obtained from the same model.
Analysis of the secondary hemostasis measures
For DD, F1.2, PAP, and BTG, the diet treatment effect estimates were small with wide CIs, and the primary hypothesis tests were inconclusive (data not shown).
Discussion
The 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk (24), as well as the 2019 ACC/AHA Prevention Guideline (24) recommend a healthy dietary pattern that is low in SFA and sodium to decrease CVD morbidity and mortality, which aligns with previous recommendations (25, 49–51). Higher concentrations of fibrinogen and factor VIIc have been reported with consumption of diets high in total fat and SFA and low in fiber, fruit and vegetables, and fish (52–55). In our study, type and amount of dietary fat had a very modest effect and directionally different responses on 3 hemostatic factors that affect CVD risk.
Overall findings
In both studies, decreasing saturated fat, irrespective of the total fat content of the diet, modestly lowered mean factor VIIc in both healthy participants and participants at risk for CVD. The mean fibrinogen response was adversely affected by higher-carbohydrate, lower-fat diets (i.e., the Low-Sat diet in study 1 and the Step 1 diet in study 2) but increased modestly with the high-MUFA diet in study 2. Mean square-root PAI-1 increased slightly with the Low-Sat diet in study 1 versus the AAD and increased slightly with the high-MUFA diet in study 2. As noted above, these effects were very small.
In both studies, there was little or no evidence of HTEs across subpopulations; generally, the overall and subpopulation-specific treatment effects were consistently in the same direction and were of similar magnitudes. The tests of null hypotheses of the form “no HTE” were inconclusive. We noted that the point estimates and CIs in study 2 suggested that some HTE is plausible. For example, in study 2, the high-MUFA diet (vs. AAD) reduced factor VIIc slightly more for the participants with high TGs, low HDL cholesterol, and/or high HOMA, but slightly less for MetSyn participants. We found similarly weak evidence of a possible diet-by-metabolic syndrome interaction for square-root PAI-1; the MUFA diet decreased the mean concentration of square-root PAI-1, whereas the Step 1 diet increased the mean (Table 7). If so, then for individuals at risk for cardiometabolic diseases, a moderate-fat diet high in MUFA versus a lower-fat diet higher in carbohydrate may confer benefits on hemostatic factors that contribute to CVD risk. However, this needs to be studied further. Nonetheless, as we reported previously for the lipid and lipoprotein responses (30, 31), the MUFA diet provided the greatest risk reduction as a replacement for SFA because it lowered TGs and increased HDL cholesterol (vs. dietary carbohydrate).
Based on our findings from both studies, decreasing SFA would be expected to lower factor VIIc. A lower-fat and -saturated-fat diet, however, tends to increase fibrinogen in healthy and at-risk individuals. However, the complexity of this finding relates to the PAI-1 responses. PAI-1 increased with the Low-Sat diet in study 1 and with the high-MUFA diet in study 2. In contrast, the Step 1 diets in both studies had little effect on PAI-1, suggesting that the lower and upper ranges of total fat recommended for heart health might not benefit PAI-1. Our secondary analyses of diet-by-subpopulation interactions suggested that the high-MUFA diet may decrease PAI-1, whereas the Step 1 diet may increase PAI-1 in individuals with MetSyn. If this hypothesis is confirmed, individuals with MetSyn might benefit from a diet low in SFA and higher in MUFA. However, further research is needed to evaluate this.
Factor VIIc
Controlled clinical studies have shown that factor VIIc activity is altered by changes in dietary fat, saturated fat, and fiber (14, 56–58). The results of a 2- to 3-wk feeding study with normolipidemic individuals demonstrated a decrease in factor VIIc of ∼5–10% with a reduction in dietary total and SFA (16). In a longer-term study (20 wk), mean factor VIIc concentrations likewise decreased (11%) when total and SFA decreased (57). The findings of study 1 are consistent with these reports; however, the magnitudes of the factor VIIc responses (reductions of 1.8% and 2.6%) were smaller than previously reported (16, 57). In study 2, the decreases were somewhat higher but still modest when reducing SFA (3.6% and 4.6%). The differences compared with other studies may be explained by differences in the diets used in the other studies (16, 58).
Fibrinogen
Fat reduction has been shown to decrease fibrinogen (18). In contrast, weight loss (19, 59, 60) has not had an effect on fibrinogen concentrations. In the current study, we observed small increases in mean fibrinogen concentrations when SFA was reduced in conjunction with total fat on the Low-Sat diet in study 1 and the Step 1 diet in study 2 [along with increases in TG concentrations and a decrease in HDL-cholesterol concentrations that we reported previously (31, 32)]. Collectively, the increase in mean fibrinogen along with adverse changes in TG and HDL-cholesterol concentrations in participants following a reduced-fat diet (32) may increase the CVD risk burden. Moreover, fibrinogen appears to be more responsive to the total fat versus the SFA content of the diet.
PAI-1
In our study, PAI-1 concentrations were 2-fold higher in participants at increased risk for cardiometabolic diseases (in study 2) compared with healthy participants (in study 1). We found that the lower and upper ranges of total fat recommended for heart health might not benefit PAI-1, except for individuals with MetSyn who had the best PAI-1 response with the high-MUFA diet, indicating the need for further research to better understand this observation. Moreover, the need for further research in individuals with MetSyn is reinforced by a recent study conducted by Teng et al. (61), which reported no effect of replacing SFA with MUFA or dietary carbohydrate on PAI-1. Since PAI-1 was associated with acute coronary syndrome and ischemic stroke in the European Prospective Investigation into Cancer and Nutrition–Italy Cohort (EPICOR) Study (62), a better understanding is needed about how diet, especially type and amount of dietary fat, affects PAI-1 and, in turn, CVD risk, particularly in persons with MetSyn who are at especially high CVD risk.
Strengths and limitations
Strengths of both studies were that they used a randomized, multicenter, double-blind longitudinal crossover design and very carefully prepared and monitored diets supported by central laboratories, managed by a collaborative-studies coordinating center, and funded by the NIH. The sample sizes were carefully chosen based on our previous studies. Adequacy of the repeated-measures designs and sample sizes is indicated in this report by the widths of the CIs and magnitudes of SEs. The duration of feeding periods and the numbers of weekly measurements were sufficient to ensure temporal stabilization of hemostatic factors during the feeding periods. The repeated blood sampling minimized the effects of large intraindividual variation in the hemostatic factor measures (63). Also, we have shown that the variability in hemostatic factors in premenopausal women is similar to (or better than) that for men and postmenopausal women, indicating that the menstrual cycle is not a source of variance that needed to be controlled in this study (46). In study 1, the heterogeneity of the population (45% female, 25% African American, and 17% postmenopausal females) enhanced the generalizability of the results. Study 2 addressed an important population, participants with cardiometabolic risk factors, in whom there are questions about the ideal macronutrient profile of diets that decrease the risk of thrombosis. Another strength of both studies is the carefully planned statistical analysis strategy and methods finalized prior to data collection. In the analyses of the results, we have used the current (2019) recommendations from the American Statistical Association for our statistical inferences (64–66). Specifically, we have focused on the point and interval estimates of the population parameters of interest and interpreted P values on continuous scale. We noted that a P value that is not small (e.g., P > α) cannot be interpreted to mean that the difference in question is zero in the target population from whom the sample of participants was drawn. When a hypothesis test yields a P value > α, the test is entirely inconclusive with regard to whether the null hypothesis is true or false; i.e., no conclusion can be drawn and it is incorrect to say “there is no difference.” In contrast, CIs are always informative to some degree. CIs identify a range of candidate values for the population parameter that is highly compatible with the observed data.
One limitation of both studies was the duration of the intervention. Although 7–8 wk of controlled feeding is a substantial period of time, this is relatively brief compared with lifestyle nutrition trials (and lifelong dietary patterns). Also, many potential dietary and lifestyle permutations were not tested. One example of this is that the type of carbohydrate that was used in the test diets was a mix of refined grains, added sugars, and some whole grains, as well as fruits and vegetables. As noted, however, our study focused on type and amount of fat and not specifically type of dietary carbohydrate. Further research is needed to understand the effect of type and amount of dietary carbohydrate on hemostatic factors. Despite a relatively higher than desired dropout rate in both studies (13% and 23%), the widths of the reported CIs indicate there were still enough participants to provide high levels of precision for the primary analyses. Furthermore, it is reasonable to assume that the reasons for dropout did not induce selection bias. It can be argued fairly that the hemostatic factor differences reported are small, but we believe that, especially in conjunction with the lipid and lipoprotein responses reported previously, long-term CVD risk might be affected; however, we hasten to add this requires further study because of the small and bidirectional changes in hemostatic factors. The changes we saw in hemostatic factor responses ranged between 0% and 6%. To put this in context, with respect to PAI-1, one study reported that cases (coronary events) had a 23% higher PAI-1 versus control participants (18.2 vs. 14.8 ng/mL) in the European Concerted Action on Thrombosis and Disabilities (ECAT) Study (67). Similarly, in the Framingham Heart Study, Jung et al. (68), reported that PAI-1 antigen concentrations were higher in those with major adverse cardiac events, with a mean difference of 6.11 ng/mL (95% CI: [3.27, 8.96]; P < 0.001). Also, in the Framingham cohort, Tofler et al. (69) reported a 32% increase in PAI-1 for participants with incident CVD (i.e., 29.1 vs. 22.1 ng/mL for those without incident CVD). For fibrinogen, the Northwick Park Heart Study reported a 10.3% higher concentration of fibrinogen in men and a 3.3% higher concentration in women for CHD death versus participants with no history of CHD (70). The Fibrinogen Studies Collaboration reports an HR of 2.42 for a 100-mg/dL increase in fibrinogen (5). Assuming a typical mean value for fibrinogen of ∼300 mg/dL, a 100-mg/dL change corresponds to a 33% difference in risk of major CVDs. For factor VIIc, in the study by De Stavola and Meade (70), men and women who experienced CHD death had 10.9% and 16.5% higher concentrations, respectively. However, not all research shows a relation for factor VIIc and CHD (71). Finally, while the DELTA studies were conducted many years ago, the findings are still relevant because the American diet has not changed much in the past 25 years (72). Furthermore, the cohorts studied are still germane to topics of public health significance, including effects of race on diet responses and dietary management of dyslipidemia, especially given current population trends in overweight and obesity.
Conclusions
We have shown that reducing SFA modestly lowers factor VIIc in healthy and at-risk individuals. A diet low in total fat and SFA elicited a greater mean increase in fibrinogen than a moderate fat diet that was low in SFA and high in MUFA. These results indicate that, as a substitute for SFA, MUFA compared with carbohydrate results in less of an increase in fibrinogen. The results regarding the diet effects on PAI-1 were inconclusive. In secondary analyses of potential heterogeneity of diet effects, results for PAI-1 suggested that for individuals with MetSyn it is plausible that it may be beneficial to replace SFA with MUFA instead of replacing SFA with carbohydrate. Further research is needed to clarify the effect of the total fat content and the fatty acid profile of the diet on hemostatic factors in different population groups including those at high CVD risk.
Supplementary Material
Acknowledgments
We thank Joyce Merkel for her editorial assistance in the preparation of the manuscript. The authors’ responsibilities were as follows—PMK-E, HNG, RPT, ML, PWS, PJE, LB, AGE, TAP, and BHD: designed both studies and had oversight for all aspects of the research conducted; PWS, RR, and SFH: conducted the data analyses; CMC, WK, PMK-E, BHD, and AGE: designed the experimental diets; PMK-E, HNG, ML, PWS, and AGE: had primary responsibility for the final content of the manuscript; and all authors: contributed to writing the manuscript and read and approved the final manuscript.
DELTA Research Group—Columbia University: Henry Ginsberg, MD (Principal Investigator); Rajasekhar Ramakrishnan, DSc, Wahida Karmally, DrPH, RD, CDE; Lars Berglund, MD, PhD; Maliha Siddiqui, MS, RD; Niem-Tzu Chen, MS; Steve Holleran, BS; Colleen Johnson, RD; Roberta Holeman, Karen Chirgwin, Kellye Stennett, Lencey Ganga, Tajsudeen Towolawai, MBA; Minnie Myers, BS; Colleen Ngai, BS; Nelson Fontenez, BS; Jeff Jones, BS; Carmen Rodriguez, Norma Useche. Pennington Biomedical Research Center: Michael Lefevre, PhD, and Paul S Roheim, MD (deceased) (Co-Principal Investigators); Donna Ryan, MD; Marlene Most, PhD, RD; Catherine Champagne, PhD, RD; Donald Williamson, PhD; Richard Tulley, PhD; Ricky Brock, RN; Deonne Bodin, BS, MT; Betty Kennedy, MPA; Michelle Barkate, MS, RD; Elizabeth Foust, BS, Deshoin York, BS. Pennsylvania State University: Penny Kris-Etherton, PhD, RD (Principal Investigator); Satya Jonnalagadda, PhD; Janice Derr, PhD; Abir Farhat-Wood, MS; Vikkie Mustad, PhD; Kate Meaker, MS; Edward Mills, PhD; Mary-Ann Tilley, MS, RD; Helen Smiciklas-Wright, PhD; Madeleine Sigman-Grant, PhD, RD; Shaomei Yu, MS, PhD; Jean-Xavier Guinard, PhD; Pamela Sechevich, MS, C Channa Reddy, PhD; Andrea M Mastro, PhD; Allen D Cooper, MD. University of Minnesota: Patricia Elmer, PhD (Principal Investigator); Aaron Folsom, MD; Nancy Van Heel, MS, RD; Christine Wold, RD; Kay Fritz, MA, RD; Joanne Slavin, PhD; David Jacobs, PhD. University of North Carolina at Chapel Hill: Barbara Dennis, PhD (First Principal Investigator); Paul Stewart, PhD (Second Principal Investigator); C Davis, PhD; James Hosking, PhD; Nancy Anderson, MSPH; Susan Blackwell, BS; Lynn Martin, MS; Hope Bryan, MS; W Brian Stewart, BS; Jeffrey Abolafia, MA; Malachy Foley, BS; Conroy Zien, BA; Szu-Yun Leu, MS; Marston Youngblood, MPH; Thomas Goodwin, MAT; Monica Miles, Jennifer Wehbie. Mary Imogene Bassett Hospital: Thomas Pearson, MD, PhD, and Roberta Reed, PhD. University of Vermont: Russell Tracy, PhD, and Elaine Cornell, BS. Virginia Polytechnic and State University: Kent Stewart, PhD, and Katherine Phillips, PhD. Southern University: Bernestine McGee, PhD, RD, and Brenda Williams, BS. Beltsville Agricultural Research Center: Gary Beecher, PhD; Joanne Holden, MS; Carol Davis, BS. National Heart, Lung, and Blood Institute: Abby Ershow, ScD; David Gordon, MD, PhD; Michael Proschan, PhD; Basil Rifkind, MD, FRCP (deceased).
The DELTA investigators express thanks to the following companies for their in-kind contributions: AARHUS, Bertolli USA, Best Foods, Campbell Soup Company, Del Monte Foods, General Mills, Hershey Foods Corporation, Institute of Edible Oils and Shortenings, Kraft General Foods, Land O'Lakes, McCormick Incorporated, Nabisco Foods Group, Neomonde Baking Company, Palm Oil Research Institute, Park Corporation, Proctor and Gamble, Quaker Oats, Ross Laboratories, Swift-Armour and Eckrick, Van Den Bergh Foods, Cholestech® Corporation, LifeLines Technology Incorporated.
Notes
Supported by National Heart, Lung, and Blood Institute grants 5-U01-HL49644, 49648, 49649, 49651, and 49659. Supported in part by grants MO1-RR00400 and MO1-RR00645 from the National Center for Research Resources.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–6 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
See the Acknowledgments for a list of the DELTA investigators.
Abbreviations used: AAD, average American diet; ACC, American College of Cardiology; AHA, American Heart Association; BTG, B-thromboglobulin; CHD, coronary heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DD, D-dimer; DELTA, Dietary Effects on Lipoproteins and Thrombogenic Activity; F1.2, prothrombin fragments; HOMA, homeostasis model assessment; HTE, heterogeneity of treatment effect; MetSyn, metabolic syndrome; PAI-1, plasminogen activator inhibitor 1; PAP, plasmin-antiplasmin complex; TG, plasma triglyceride.
Contributor Information
for the DELTA Investigators:
Henry Ginsberg, Rajasekhar Ramakrishnan, Wahida Karmally, Lars Berglund, Maliha Siddiqui, Niem-Tzu Chen, Steve Holleran, Colleen Johnson, Roberta Holeman, Karen Chirgwin, Kellye Stennett, Lencey Ganga, Tajsudeen Towolawai, Minnie Myers, Colleen Ngai, Nelson Fontenez, Jeff Jones, Carmen Rodriguez, Norma Useche, Michael Lefevre, Paul S Roheim, Donna Ryan, Marlene Most, Catherine Champagne, Donald Williamson, Richard Tulley, Ricky Brock, Deonne Bodin, Betty Kennedy, Michelle Barkate, Elizabeth Foust, Deshoin York, Penny Kris-Etherton, Satya Jonnalagadda, Janice Derr, Abir Farhat-Wood, Vikkie Mustad, Kate Meaker, Edward Mills, Mary-Ann Tilley, Helen Smiciklas-Wright, Madeleine Sigman-Grant, Shaomei Yu, Jean-Xavier Guinard, Pamela Sechevich, C Channa Reddy, Andrea M Mastro, Allen D Cooper, Patricia Elmer, Aaron Folsom, Nancy Van Heel, Christine Wold, Kay Fritz, Joanne Slavin, David Jacobs, Barbara Dennis, Paul Stewart, C Davis, James Hosking, Nancy Anderson, Susan Blackwell, Lynn Martin, Hope Bryan, W Brian Stewart, Jeffrey Abolafia, Malachy Foley, Conroy Zien, Szu-Yun Leu, Marston Youngblood, Thomas Goodwin, Monica Miles, Jennifer Wehbie, Thomas Pearson, Roberta Reed, Russell Tracy, Elaine Cornell, Kent Stewart, Katherine Phillips, Bernestine McGee, Brenda Williams, Gary Beecher, Joanne Holden, Carol Davis, Abby Ershow, David Gordon, Michael Proschan, and Basil Rifkind
References
- 1. World Health Organization. Cardiovascular diseases (CVDs). [Internet] 2017; [accessed 2020 May 18]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). [Google Scholar]
- 2. Pahigiannis K, Thompson-Paul AM, Barfield W, Ochiai E, Loustalot F, Shero S, Hong Y. Progress toward improved cardiovascular health in the United States. Circulation. 2019;139:1957–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lowe G. Can haemostatic factors predict atherothrombosis?. Intern Emerg Med. 2011;6:497–501. [DOI] [PubMed] [Google Scholar]
- 4. Kannel WB. Overview of hemostatic factors involved in atherosclerotic cardiovascular disease. Lipids. 2005;40:1215–20. [DOI] [PubMed] [Google Scholar]
- 5. Fibrinogen Studies Collaboration. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality. JAMA. 2005;294:1799–809. [DOI] [PubMed] [Google Scholar]
- 6. Donati M, Iacoviello L. Fibrinogen and factor VIIc levels: independent risk factors or markers of coronary disease risk?. J Thromb Haemost. 2007;5:458–60. [DOI] [PubMed] [Google Scholar]
- 7. Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28:e72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lefevre M, Kris-Etherton PM, Zhao G, Tracy RP. Dietary fatty acids, hemostasis, and cardiovascular disease risk. J Am Diet Assoc. 2004;104:410–9.; quiz 92. [DOI] [PubMed] [Google Scholar]
- 9. Miller GJ. Dietary fatty acids and the haemostatic system. Atherosclerosis. 2005;179:213–27. [DOI] [PubMed] [Google Scholar]
- 10. Steffen LM, Folsom AR, Cushman M, Jacobs DR, Rosamond WD. Greater fish, fruit, and vegetable intakes are related to lower incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. Circulation. 2007;115:188–95. [DOI] [PubMed] [Google Scholar]
- 11. Varraso R, Kabrhel C, Goldhaber SZ, Rimm EB, Camargo CA. Prospective study of diet and venous thromboembolism in US women and men. Am J Epidemiol. 2012;175:114–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pomp ER, Rosendaal FR, Doggen CJ. Alcohol consumption is associated with a decreased risk of venous thrombosis. Thromb Haemost. 2008;99:59–63. [DOI] [PubMed] [Google Scholar]
- 13. Mukamal KJ, Jadhav PP, D'Agostino RB, Massaro JM, Mittleman MA, Lipinska I, Sutherland PA, Matheney T, Levy D, Wilson PW. Alcohol consumption and hemostatic factors: analysis of the Framingham Offspring cohort. Circulation. 2001;104:1367–73. [DOI] [PubMed] [Google Scholar]
- 14. Marckmann P, Sandström B, Jespersen J. Fasting blood coagulation and fibrinolysis of young adults unchanged by reduction in dietary fat content. Arterioscler Thromb. 1992;12:201–5. [DOI] [PubMed] [Google Scholar]
- 15. Allman-Farinelli MA, Gomes K, Favaloro EJ, Petocz P. A diet rich in high-oleic-acid sunflower oil favorably alters low-density lipoprotein cholesterol, triglycerides, and factor VII coagulant activity. J Am Diet Assoc. 2005;105:1071–9. [DOI] [PubMed] [Google Scholar]
- 16. Kristensen M, Bugel S. A diet rich in oat bran improves blood lipids and hemostatic factors, and reduces apparent energy digestibility in young healthy volunteers. Eur J Clin Nutr. 2011;65:1053–8. [DOI] [PubMed] [Google Scholar]
- 17. Rajpathak SN, Xue X, Wassertheil-Smoller S, Van Horn L, Snetselaar L, Martin LW, Rohan TE. Effect of long term low-fat dietary intervention on change in hemostatic factors: results from the Women's Health Initiative. Nutr Metab Cardiovasc Dis. 2012;22:337–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bladbjerg EM, Larsen TM, Due A, Jespersen J, Stender S, Astrup A. Long-term effects on haemostatic variables of three ad libitum diets differing in type and amount of fat and carbohydrate: a 6-month randomised study in obese individuals. Br J Nutr. 2010;104:1824–30. [DOI] [PubMed] [Google Scholar]
- 19. Murakami T, Horigome H, Tanaka K, Nakata Y, Ohkawara K, Katayama Y, Matsui A. Impact of weight reduction on production of platelet-derived microparticles and fibrinolytic parameters in obesity. Thromb Res. 2007;119:45–53. [DOI] [PubMed] [Google Scholar]
- 20. Hankey CR, Lean ME, Lowe GD, Rumley A, Woodward M. Effects of moderate weight loss on anginal symptoms and indices of coagulation and fibrinolysis in overweight patients with angina pectoris. Eur J Clin Nutr. 2002;56:1039–45. [DOI] [PubMed] [Google Scholar]
- 21. Morel O, Luca F, Grunebaum L, Jesel L, Meyer N, Desprez D, Robert S, Dignat-George F, Toti F, Simon C. Short-term very low-calorie diet in obese females improves the haemostatic balance through the reduction of leptin levels, PAI-1 concentrations and a diminished release of platelet and leukocyte-derived microparticles. Int J Obes. 2011;35:1479–86. [DOI] [PubMed] [Google Scholar]
- 22. Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 2018;361:k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siri-Tarino PW, Chiu S, Bergeron N, Krauss RM. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu Rev Nutr. 2015;35:517–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–99. [DOI] [PubMed] [Google Scholar]
- 25. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Circulation. 2019;140:e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 27. Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA study. J Am Coll Cardiol. 2004;44:152–8. [DOI] [PubMed] [Google Scholar]
- 28. Ros E, Martinez-Gonzalez MA, Estruch R, Salas-Salvado J, Fito M, Martinez JA, Corella D. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv Nutr. 2014;5:330S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang DD, Hu FB.. Dietary fat and risk of cardiovascular disease: recent controversies and advances. Annu Rev Nutr. 2017;37:423–46. [DOI] [PubMed] [Google Scholar]
- 30. Ginsberg HN. New directions in dietary studies and heart disease: the National Heart, Lung and Blood Institute sponsored Multicenter Study of Diet Effects on Lipoproteins and Thrombogenic Activity. In: Longenecker JB, Kritchevsky D, Drezner MK editors. Nutrition and biotechnology in heart disease and cancer. Boston: Springer; 1995. pp. 241–7. [DOI] [PubMed] [Google Scholar]
- 31. Ginsberg HN, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, Pearson T, Roheim P, Ramakrishnan R, Reed R. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the Delta Study, protocol 1. Arterioscler Thromb Vasc Biol. 1998;18:441–9. [DOI] [PubMed] [Google Scholar]
- 32. Berglund L, Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, Stewart PW, Ershow A, Pearson TA, Dennis BH, Roheim PS. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am J Clin Nutr. 2007;86:1611–20. [DOI] [PubMed] [Google Scholar]
- 33. Dennis BH, Stewart P, Champagne C, Windhauser M, Ershow A, Karmally W, Phillips K, Stewart K, Heel NV, Farhat-Wood A. Diet design for a multicenter controlled feeding trial: the DELTA program. J Am Diet Assoc. 1998;98:766–76. [DOI] [PubMed] [Google Scholar]
- 34. Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121:200–6. [DOI] [PubMed] [Google Scholar]
- 35. Senn SJ. Power is indeed irrelevant in interpreting completed studies. BMJ. 2002;325:1304. [PMC free article] [PubMed] [Google Scholar]
- 36. Hoenig JM, Heisey DM.. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat. 2001;55:19–24. [Google Scholar]
- 37. Johnson CL, Rifkind BM, Sempos CT, Carroll MD, Bachorik PS, Briefel RR, Gordon DJ, Burt VL, Brown CD, Lippel K. Declining serum total cholesterol levels among US adults. JAMA. 1993;269:3002–8. [PubMed] [Google Scholar]
- 38. US Food and Drug Administration. Menopause & hormones [Internet]. 2019; Available from: https://www.fda.gov/media/130242/download. [Accessed 2020 May 20]. [Google Scholar]
- 39. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- 40. Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–70. [PubMed] [Google Scholar]
- 41. Clauss A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens [Rapid physiological coagulation method in determination of fibrinogen]. Acta Haematol. 1957;17:237–46. [DOI] [PubMed] [Google Scholar]
- 42. Teitel JM, Bauer KA, Lau HK, Rosenberg RD. Studies of the prothrombin activation pathway utilizing radioimmunoassays for the F2/F1+ 2 fragment and thrombin–antithrombin complex. Blood. 1982;59:1086–97. [PubMed] [Google Scholar]
- 43. Holvoet P, de Boer A, Verstreken M, Collen D. An enzyme-linked immunosorbent assay (ELISA) for the measurement of plasmin-alpha 2-antiplasmin complex in human plasma—application to the detection of in vivo activation of the fibrinolytic system. Thromb Haemost. 1986;56:124–7. [PubMed] [Google Scholar]
- 44. Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–8. [PubMed] [Google Scholar]
- 45. Declerck P, Mombaerts P, Holvoet P, De Mol M, Collen D. Fibrinolytic response and fibrin fragment D-dimer levels in patients with deep vein thrombosis. Thromb Haemost. 1987;58:1024–9. [PubMed] [Google Scholar]
- 46. Hill AM, Stewart PW, Fung MK, Kris-Etherton PM, Ginsberg HN, Tracy RP, Pearson TA, Lefevre M, Reed RG, Elmer PJ et al. Monthly haemostatic factor variability in women and men. Eur J Clin Invest. 2014;44:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analyses of variance. Biometrika. 1976;63:655–60. [Google Scholar]
- 48. Harville DA. BLUP (best linear unbiased prediction) and beyond. In: Gianola D, Hammond K editors. Advances in statistical methods for genetic improvement of livestock. Heidelberg (Germany): Springer; 1990. pp. 239–76. [Google Scholar]
- 49. NHLBI. Lifestyle interventions to reduce cardiovascular risk: systematic evidence review from the Lifestyle Work Group. [Internet] 2013. Available from: https://www.nhlbi.nih.gov/sites/default/files/media/docs/lifestyle.pdf. [Accessed 2020 May 20]. [Google Scholar]
- 50. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–23. [DOI] [PubMed] [Google Scholar]
- 51. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Iso H, Folsom AR, Sato S, Wu KK, Shimamoto T, Koike K, Iida M, Komachi Y. Plasma fibrinogen and its correlates in Japanese and US population samples. Arterioscler Thromb. 1993;13:783–90. [DOI] [PubMed] [Google Scholar]
- 53. Weststrate JA, van het Hof KH, van den Berg H, Velthuis-te-Wierik EJ, de Graaf C, Zimmermanns NJ, Westerterp KR, Westerterp-Plantenga MS, Verboeket-van de Venne WP. A comparison of the effect of free access to reduced fat products or their full fat equivalents on food intake, body weight, blood lipids and fat-soluble antioxidants levels and haemostasis variables. Eur J Clin Nutr. 1998;52:389–95. [DOI] [PubMed] [Google Scholar]
- 54. Freitas RN, Luben R, Wareham NJ, Khaw KT. Relationship between plasma fibrinogen and fiber intake in the EPIC-Norfolk cohort. Eur J Clin Nutr. 2012;66:443–51. [DOI] [PubMed] [Google Scholar]
- 55. Shahar E, Folsom AR, Wu KK, Dennis BH, Shimakawa T, Conlan MG, Davis C, Williams OD. Associations of fish intake and dietary n-3 polyunsaturated fatty acids with a hypocoagulable profile: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1993;13:1205–12. [DOI] [PubMed] [Google Scholar]
- 56. Tholstrup T, Miller GJ, Bysted A, Sandstrom B. Effect of individual dietary fatty acids on postprandial activation of blood coagulation factor VII and fibrinolysis in healthy young men. Am J Clin Nutr. 2003;77:1125–32. [DOI] [PubMed] [Google Scholar]
- 57. Brace LD, Gittler-Buffa C, Miller GJ, Cole TG, Schmeisser D, Prewitt TE, Bowen PE. Factor VII coagulant activity and cholesterol changes in premenopausal women consuming a long-term cholesterol-lowering diet. Arterioscler Thromb. 1994;14:1284–9. [DOI] [PubMed] [Google Scholar]
- 58. Bladbjerg E, Marckmann P, Sandström B, Jespersen J. Non-fasting factor VII coagulant activity (FVII: C) increased by high-fat diet. Thromb Haemost. 1994;71:755–58. [PubMed] [Google Scholar]
- 59. Rissanen P, Vahtera E, Krusius T, Uusitupa M, Rissanen A. Weight change and blood coagulability and fibrinolysis in healthy obese women. Int J Obes. 2001;25:212–8. [DOI] [PubMed] [Google Scholar]
- 60. Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3:85–101. [DOI] [PubMed] [Google Scholar]
- 61. Teng KT, Chang LF, Vethakkan SR, Nesaretnam K, Sanders TAB. Effects of exchanging carbohydrate or monounsaturated fat with saturated fat on inflammatory and thrombogenic responses in subjects with abdominal obesity: a randomized controlled trial. Clin Nutr. 2017;36:1250–8. [DOI] [PubMed] [Google Scholar]
- 62. Iacoviello L, Agnoli C, De Curtis A, di Castelnuovo A, Giurdanella MC, Krogh V, Mattiello A, Matullo G, Sacerdote C, Tumino R et al. Type 1 plasminogen activator inhibitor as a common risk factor for cancer and ischaemic vascular disease: the EPICOR study. BMJ Open. 2013;3:e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chambless LE, McMahon R, Wu K, Folsom A, Finch A, Shen Y-L. Short-term intraindividual variability in hemostasis factors the ARIC study. Ann Epidemiol. 1992;2:723–33. [DOI] [PubMed] [Google Scholar]
- 64. Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305–7. [DOI] [PubMed] [Google Scholar]
- 65. Wasserstein RL, Lazar NA.. The ASA's statement on P-values: context, process, and purpose. Am Stat. 2016;70:129–33. [Google Scholar]
- 66. Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “P < 0.05”. Am Stat. 2019;73:1–19. [Google Scholar]
- 67. Juhan-Vague I, Pyke SD, Alessi MC, Jespersen J, Haverkate F, Thompson SG. Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. ECAT Study Group. European Concerted Action on Thrombosis and Disabilities. Circulation. 1996;94:2057–63. [DOI] [PubMed] [Google Scholar]
- 68. Jung RG, Motazedian P, Ramirez FD, Simard T, Di Santo P, Visintini S, Faraz MA, Labinaz A, Jung Y, Hibbert B. Association between plasminogen activator inhibitor-1 and cardiovascular events: a systematic review and meta-analysis. Thrombosis J. 2018;16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tofler GH, Massaro J, O'Donnell CJ, Wilson PWF, Vasan RS, Sutherland PA, Meigs JB, Levy D, D'Agostino RB Sr. Plasminogen activator inhibitor and the risk of cardiovascular disease: the Framingham Heart Study. Thromb Res. 2016;140:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. De Stavola BL, Meade TW. Long-term effects of hemostatic variables on fatal coronary heart disease: 30-year results from the first prospective Northwick Park Heart Study (NPHS-I). J Thromb Haemost. 2007;5:461–71. [DOI] [PubMed] [Google Scholar]
- 71. Tracy RP, Arnold AM, Ettinger W, Fried L, Meilahn E, Savage P. The relationship of fibrinogen and factors VII and VIII to incident cardiovascular disease and death in the elderly: results from the cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:1776–83. [DOI] [PubMed] [Google Scholar]
- 72. Kuklina EV, Carroll MD, Shaw KM, Hirsch R. Trends in high LDL cholesterol, cholesterol-lowering medication use, and dietary saturated-fat intake: United States, 1976–2010. NCHS Data Brief No. 117. 2013:1–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.