ABSTRACT
Background
The healthy human metabolome, including its physiological responses after meal consumption, remains incompletely understood. One major research gap is the limited literature assessing how human metabolomic profiles differ between fasting and postprandial states after physiological challenges.
Objectives
Our study objective was to evaluate alterations in high-resolution metabolomic profiles following a standardized meal challenge, relative to fasting, in Guatemalan adults.
Methods
We studied 123 Guatemalan adults without obesity, hypertension, diabetes, metabolic syndrome, or comorbidities. Every participant received a standardized meal challenge (520 kcal, 67.4 g carbohydrates, 24.3 g fat, 8.0 g protein) and provided blood samples while fasting and at 2 h postprandial. Plasma samples were assayed by high-resolution metabolomics with dual-column LC [C18 (negative electrospray ionization), hydrophilic interaction LC (HILIC, positive electrospray ionization)] coupled to ultra-high-resolution MS. Associations between metabolomic features and the meal challenge timepoint were assessed in feature-by-feature multivariable linear mixed regression models. Two algorithms (mummichog, gene set enrichment analysis) were used for pathway analysis, and P values were combined by the Fisher method.
Results
Among participants (62.6% male, median age 43.0 y), 1130 features (C18: 777; HILIC: 353) differed between fasting and postprandial states (all false discovery rate–adjusted q < 0.05). Based on differing C18 features, top pathways included: tricarboxylic acid cycle (TCA), primary bile acid biosynthesis, and linoleic acid metabolism (all Pcombined < 0.05). Mass spectral features included: taurine and cholic acid in primary bile acid biosynthesis; and fumaric acid, malic acid, and citric acid in the TCA. HILIC features that differed in the meal challenge reflected linoleic acid metabolism (Pcombined < 0.05).
Conclusions
Energy, macronutrient, and bile acid metabolism pathways were responsive to a standardized meal challenge in adults without cardiometabolic diseases. Our findings reflect metabolic flexibility in disease-free individuals.
Keywords: metabolomics, meal challenge, fasting, postprandial, metabolic pathways
Introduction
Cardiometabolic health, including the healthy human metabolome, remains incompletely understood (1–4). Cardiometabolic diseases are the leading cause of mortality worldwide (5). Elucidating the healthy metabolome is important to shed light on how to maintain more effectively optimal metabolic health over the life course.
One major research gap is characterizing the dynamic nature of systemic physiology in the metabolome of healthy individuals (3, 4, 6, 7). Energy homeostasis requires rapid adaptations of metabolic processes throughout heterogeneous energy states, including during meal consumption, exercise, and rest (1–4, 8). A solution is to use activity metabolomics (6, 9) to evaluate metabolic flexibility in a healthy state (3, 4).
Metabolic flexibility is a broad concept that describes physiological adaptability to differing energy and metabolic conditions (3, 4). A multitude of responses at the systemic, tissue, and cellular level in humans and other species have been characterized as different aspects of metabolic flexibility (3, 4). Two examples include the shifting energy metabolism of helminths in aerobic compared with anaerobic environments, and macronutrient fuel selection switching from lipids in a fasting state to glucose in a postprandial state in humans (3, 4).
Acknowledging the absence of a single standard definition for metabolic flexibility, in the present study we define metabolic flexibility as the changes in metabolomic features after a meal challenge, compared with while fasting. Metabolomic data reflect a plethora of influences, such as genetic and environmental factors, that affect physiological processes over the life course (10–13). Metabolic challenges, including meal consumption, represent a means to examine physiological perturbations in activity metabolomics studies in humans in vivo (14).
To date, few studies have assessed metabolomic changes before and after meal consumption in healthy individuals (Supplemental Table 1) (15–23). These previous studies have been characterized by small sample sizes (ranging from 9 to 21 participants) and extensive heterogeneity of mixed macronutrient composition meal challenges (15–23). Compared with a bolus dosage of glucose [oral-glucose-tolerance test (OGTT)], a mixed macronutrient intervention is more reflective of a physiological metabolic challenge, which is necessary to elucidate and characterize a healthy metabolome. Previous studies have shown differences between OGTTs and mixed meal challenges, including in insulin response (24), glucose concentration changes (25), and β-cell function (26). Our study objective was to evaluate high-resolution metabolomic profiles following a standardized meal challenge, relative to while fasting, in adults without cardiometabolic diseases.
Methods
Study population
We studied adult participants of the Institute of Nutrition of Central America and Panama (INCAP) Nutritional Supplementation Trial Longitudinal Study, which was established in Guatemala in 1969. Further details regarding the INCAP trial have been previously published (27, 28). In the present study, participants were enrolled in a follow-up data collection from 2015 to 2017 (29). Previous exclusion criteria included: 1) loss to follow-up (e.g., death, emigration from Guatemala, inability to contact, did not agree to voluntarily participate); 2) pregnancy or lactating; and 3) no data for the biological samples and key outcomes of interest. For this analysis, we additionally excluded any participants with obesity, hypertension, diabetes, metabolic syndrome, or any comorbidities of these cardiometabolic diseases, as defined elsewhere (24) (Supplemental Table 2).
Meal challenge
Following informed consent and an overnight fast, participants visited the research clinic in the morning. Participants were excluded from the meal challenge if their fasting blood glucose was ≥180 mg/dL or they self-reported having diabetes. Study participants received a standardized beverage, which was comprised of Incaparina (a vegetable protein mixture developed by INCAP), skim milk (lactose-free), safflower oil, and sugar. Each portion (259 mL) contained 520 kcal, 8.0 g protein, 24.3 g fat, and 67.4 g carbohydrate (Supplemental Table 3).
Data collection
Trained phlebotomists collected venous blood samples at baseline (fasting) and 120 min after the meal challenge (postprandial). Plasma samples for metabolomic analysis were collected in heparin tubes. Samples were stored on ice prior to centrifugation at 3000 rpm (906 × g) for 10 min, and subsequently at −80°C until assay.
Trained study staff interviewed study participants regarding sociodemographic and clinical information, including use of medications (29). Standard protocols were used for anthropometric measurements (29). Additional details regarding the 2015–2017 study data collection are in a prior publication (29).
High-resolution metabolomics
Plasma samples
Pairs of plasma samples (fasting, postprandial state) were sorted by sex, village, and birth year. A subset was randomly selected and ordered for metabolomic assay. Each sample was assayed in triplicate. After frozen samples were thawed, acetonitrile (2:1 v/v; HPLC grade; Millipore; Sigma Aldrich) was added to precipitate protein (30). Subsequently, samples were centrifuged (14,000 × g for 10 min at 4°C) and the supernatant was stored at 4°C in a refrigerated autosampler prior to assay (30, 31).
Fourteen commercially available, isotope-labeled internal standards were included: [13C6]-d-glucose, [15N]-indole, [2-15N]-l-lysine dihydrochloride, [13C5]-l-glutamic acid, [13C7]-benzoic acid, [3,4-13C2]-cholesterol, [15N]-l-tyrosine, [trimethyl-13C3]-caffeine, [15N2]-uracil, [3,3-13C2]-cystine, [1,2-13C2]-palmitic acid, [15N,13C5]-l-methionine, [15N]-choline chloride, and 2′-deoxyguanosine-[15N2,13C10]-5′-monophosphate (30, 31). Human reference plasma from the National Institute of Standards and Technology (NIST) [standard reference material (SRM) 1950] and a pooled reference plasma (Q-std3) prepared from commercial human plasma samples (Equitech Bio) were also included for quality control (32).
LC-MS
Plasma samples were assayed by LC Fourier transform MS (LC-FT-MS) with 2 chromatographic columns: C18 (Higgins Analytical, Targa, 2.1 × 50 mm) with negative electrospray ionization, and hydrophilic interaction LC (HILIC; Waters BEH Amide 2.1 × 50 mm) with positive electrospray ionization (31, 33). A Switchos control valve (LC Packings) allowed alternation between the 2 columns (31). Data acquisition occurred by mass spectrometer (Orbitrap Fusion; Thermo Fisher Scientific) with specifications as previously described (31, 34, 35). The scan range for the detection of m/z scan was 85–1250 m/z, and mass resolution was 120,000 (36).
Data extraction
Raw data (.raw files) were collected throughout the chromatographic separation and converted to .cdf files (Xcalibur software; Thermo Fisher Scientific). apLCMS (37) and xMSanalyzer (38) were used for the extraction and initial preprocessing of chromatographic data. Preprocessing included noise reduction, peak identification, retention time (RT) correction, peak alignment, feature quantification, weak signal detection, and batch effect adjustment with ComBat (37–40). A feature was defined as a unique combination of m/z and RT. The ion intensity (peak area) of each feature was integrated.
Standard operating procedures and quality control were based on prior studies, including via xMSanalyzer (38). For each sample, pairwise Pearson correlations between replicates were assessed and averaged. Prior to exclusions of participants and data filtering of features, the median averaged pairwise Pearson correlation between replicates was 98.7% (IQR: 97.0, 99.5%) across samples from C18 data, and 99.5% (IQR: 98.6, 99.8%) across all samples from HILIC data. Samples with mean Pearson correlation coefficients <0.75 across technical replicates were excluded.
In terms of data filtering, among data from each column (C18, HILIC), features observed in <80% of study participant samples were excluded (Supplemental Figure 1) (41). Subsequently, any feature peak area values that were missing or zero were assigned as half of the lowest observed value (limit of detection) of the feature peak area across all samples for each column.
Statistics and bioinformatics
Statistical analysis was conducted using R (version 3.5.1; R Foundation for Statistical Computing) and SAS (version 9.4; SAS Institute Inc). Statistical significance was based on 2-sided hypothesis tests, and α < 0.05. In feature-by-feature multivariable regressions with metabolomic data, a false discovery rate–adjusted q value <0.05 was considered significant. A complete case approach was used for all clinical and sociodemographic variables. Metabolic feature ion intensities (peak areas) were converted to log2-normalized values.
Descriptive analysis
Continuous and categorical variables were reported as median (IQR) or n (percentage). Subgroups were compared by parametric (t) or nonparametric (Wilcoxon) test statistics.
Feature selection approach via regressions
We used a feature selection approach based on multivariable linear regressions with repeated measurements. The model equation was:
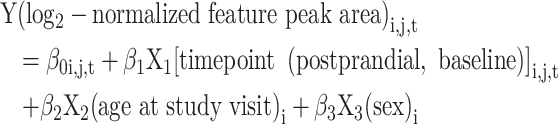 |
(1) |
where each study participant was denoted as i, feature was j, and timepoint was t. We included features with a β coefficient of the meal challenge timepoint (β1) of q < 0.05 in pathway enrichment analysis.
Functional pathway analysis and feature annotation
From the regression results (β coefficients, q values), functional pathway analysis was assessed by mummichog and gene set enrichment analysis (GSEA) algorithms [mummichog (version 1.0) in MetaboAnalyst] (42, 43). The Kyoto Encyclopedia of Genes and Genomes (KEGG) for Homo sapiens was the pathway reference library (44), and mass accuracy was 5 parts per million. To summarize the results from both algorithms (mummichog, GSEA), a combined P value (Pcombined) was calculated via the Fisher method (42, 43).
Features in the top pathways were matched with annotations in the KEGG (44) and Human Metabolome Database (HMDB) (45). We used xMSannotator, which considers multiple criteria in its algorithm (46) and incorporates HMDB reference database information (45). The xMSannotator algorithm includes several steps, including: 1) pairwise correlation analysis of all features (m/z, RT, peak area); 2) network modularity analysis; 3) RT-based clustering; 4) mass defect analysis; 5) database matching; and 6) score assignment (46). An xMSannotator score is assigned, based on correlation strength, RT difference, number of matching adducts and isotopes, and weighting based on more probable adducts and isotopes (46). A nonzero xMSannotator score is only assigned if 3 conditions are satisfied, based on the initial steps (46). We also assigned identification confidence scores to some features, based on the 5-level system proposed by the Metabolomics Standards Initiative (MSI) (47). Identities of select metabolites from each of the pathways presented were confirmed by coelution and MS/MS fragmentation patterns relative to authentic standards.
Quantification of metabolites in top pathways
We used a reference standardization approach (32) to estimate concentrations for selected, confirmed metabolites in top pathways based on metabolite concentrations in Q-Std3. Triplicates of 2 Q-Std3 samples were included as the beginning, middle, or end of every batch of 40 samples. Metabolite concentrations were calibrated either by: 1) NIST SRM 1950 for human plasma, if metabolite concentrations were available; or 2) external calibration by the method of standard additions (32, 48–51).
Ethical conduct of research
The Institutional Review Boards at Emory University and INCAP approved the study protocol. All study participants provided their written informed consent.
Results
Of the 123 study participants, 62.6% were men (Table 1). The median age was 43.0 y (IQR: 40.0, 46.0 y), and ages ranged between 37.0 and 52.0 y (Table 1). Median BMI was lower in men (24.7 kg/m2; IQR: 23.1, 26.6) than in women (25.6; IQR: 24.3, 27.9) (P < 0.05) (Table 1).
TABLE 1.
Sociodemographic and cardiometabolic indicators in Guatemalan adults (n = 123)1
| Overall (n = 123) | Males (n = 77) | Females (n = 46) | P | |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age at follow-up,5 y | 43.0 (40.0, 46.0) | 44.0 (40.0, 47.0) | 43.0 (40.0, 45.0) | 0.422 |
| Anthropometric | ||||
| BMI, kg/m2 | 25.0 (23.3, 27.0) | 24.7 (23.1, 26.6) | 25.6 (24.3, 27.9) | <0.052 |
| Biochemical | ||||
| Glucose profile | ||||
| Fasting blood glucose, mg/dL | 94.9 (90.9, 98.8) | 96.0 (93.0, 99.6) | 93.2 (89.7, 95.8) | <0.012 |
| Postprandial glucose, mg/dL | 99.4 (90.9, 112) | 94.7 (88.3, 104) | 105 (98.9, 118) | <0.012 |
| HbA1c, % | 5.6 (5.4, 5.8) | 5.6 (5.4, 5.8) | 5.6 (5.4, 5.8) | 0.333 |
| Plasma lipid concentrations | ||||
| Triglycerides, mg/dL | 108 (83, 142) | 115 (87, 157) | 102 (73, 125) | 0.062 |
| Total cholesterol, mg/dL | 167 (146, 194) | 162 (146, 187) | 174 (147, 199) | 0.243 |
| HDL cholesterol, mg/dL | 42.7 (36.8, 49.0) | 40.6 (35.9, 46.8) | 47.0 (40.4, 54.2) | <0.014 |
| Non-HDL cholesterol,6 g/dL | 0.13 (0.11, 0.14) | 0.12 (0.10, 0.14) | 0.13 (0.11, 0.15) | 0.923 |
| Clinical | ||||
| Systolic blood pressure, mm Hg | 115 (108, 121) | 117 (110, 122) | 110 (102, 119) | <0.014 |
| Diastolic blood pressure, mm Hg | 67.5 (63.0, 73.0) | 68.0 (64.0, 73.5) | 66.5 (61.5, 70.5) | 0.023 |
Study participants did not have any of the assessed cardiometabolic diseases, that is, obesity (52), hypertension (53), diabetes (54), or metabolic syndrome (55), including comorbidities (see Supplemental Table 2). Data values are median (IQR). HbA1c, glycated hemoglobin.
P values based on Wilcoxon rank-sum tests (for nonnormally distributed continuous variables).
P values from t tests (for normally distributed continuous variables) with equal variance (pooled).
P values from t tests (for normally distributed continuous variables) with unequal variance (Satterthwaite).
At study visit date (of biological sample collection) in 2015–2017 data collection.
Non-HDL cholesterol (mg/dL) calculated as the difference between total (mg/dL) and HDL cholesterol (mg/dL) plasma concentrations.
Overall, 9849 (C18) and 13,908 (HILIC) metabolomic features were observed across all study participant samples. After data filtering, 5085 C18 and 7444 HILIC features remained eligible for the feature selection process.
Metabolomic profiling in a standardized meal challenge
In total, 1130 features (C18: 777; HILIC: 353) differed following the meal challenge, compared with a fasting state (q < 0.05; Table 2). Among these features, 554 (49.4%) had putative annotations (MSI levels 2 and 3), based on xMSannotator (Table 2). Specifically, 43.6% of C18 features and 62.0% of HILIC features had annotations from xMSannotator (MSI levels 2 and 3; Table 2).
TABLE 2.
Summary of metabolomic features differing by meal challenge1
| Meal challenge | ||
|---|---|---|
| LC-FT-MS columns | ||
| C18 | HILIC | |
| No. of differing features | (n = 123) | (n = 123) |
| Feature selection | ||
| Stage 1: overall linear regression2 | ||
| q < 0.053 | 777 | 353 |
| Annotations | ||
| Features with putative annotations | 339 | 219 |
| Total no. of putative annotations | 1710 | 2463 |
Unless otherwise stated, values indicate the number of features with log2-normalized peak areas, which differed by the meal challenge timepoint (fasting, postprandial). FDR, false discovery rate; HILIC, hydrophilic interaction liquid chromatography; LC-FT-MS, liquid chromatography Fourier transform mass spectrometry.
After data filtering, 5085 (C18) and 7444 (HILIC) features remained eligible for the feature selection approach. A complete case approach was used in multivariable regressions. For each feature, a linear mixed model with repeated measurements was used (Proc Mixed in SAS). The model equation was: Y (log2-normalized feature peak area)i, j, t = β0i, j, t + β1X1 [timepoint (postprandial, baseline)] i, j, t + β2X2 (age at study visit)i + β3X3 (sex)i, where each study participant was denoted as i, feature was j, and timepoint was t. The numbers of features with a β coefficient of the meal challenge timepoint (β1) with an FDR-adjusted P value (q) < 0.05 were included in this table.
These features were subsequently eligible for annotations and functional pathway analysis.
C18 features that responded to the meal challenge were associated with 5 pathways, based on both GSEA and mummichog algorithms (all Pcombined < 0.05; Figure 1). These pathways were primary bile acid biosynthesis, citrate cycle [tricarboxylic acid (TCA) cycle], fatty acid metabolism, linoleic acid metabolism, and biotin metabolism (all Pcombined < 0.05; Figure 1B; Supplemental Table 4). A pathway for valine, leucine, and isoleucine biosynthesis was significant according to the mummichog algorithm (Pmummichog = 0.02), although not according to GSEA (PGSEA > 0.05; Figure 1A; Supplemental Table 4).
FIGURE 1.
Top pathways associated with metabolomic feature peak areas (C18) responsive to standardized meal challenge. Features were assayed by LC-Fourier transform-MS (C18 with negative electrospray ionization). Pathway analysis [mummichog and gene set enrichment algorithms via MetaboAnalyst (42, 43)] was conducted among features selected via a multivariable linear mixed regression model screening approach (q < 0.05; stage 1 in feature selection approach). (A) In this pathway analysis plot, KEGG pathways are visually represented. Each dot represents a pathway. The size and location of every dot reflect the enrichment factor and P value. Circle sizes indicate the most represented pathways, based on enrichment factor. The enrichment factor is the ratio between the number of hits in the pathway and the expected hits in the pathway. Quadrants indicate significant pathways based on the mummichog (y-axis, light gray), GSEA (x-axis, light gray), or both algorithms (dark gray). (B) Top pathways and their respective P values (mummichog and GSEA algorithms, combined) are shown. Pathways were ranked by the combined P values from the mummichog and GSEA algorithms, based on the selected features in stage 1 regressions and the Fisher method. GSEA, gene set enrichment analysis; KEGG, Kyoto Encyclopedia of Genes and Genomes; TCA, tricarboxylic acid cycle.
From HILIC features, linoleic acid metabolism was a significant pathway, according to both the mummichog (Pmummichog < 0.05) and GSEA (PGSEA < 0.05) algorithms (Pcombined < 0.05; Figure 2A; Supplemental Table 4). Primary bile acid biosynthesis was a significant pathway based on the mummichog algorithm (Pmummichog = 0.03), although not GSEA (PGSEA > 0.05; Supplemental Table 4). Conversely, 1-carbon pool by folate, cyanoamino acid metabolism, sphingolipid metabolism, ascorbate and aldarate metabolism, fatty acid elongation in mitochondria, and phenylalanine metabolism were significant pathways based on the GSEA algorithm (all PGSEA < 0.05), but not mummichog (Pmummichog > 0.05; Supplemental Table 4).
FIGURE 2.
Top pathways associated with metabolomic feature peak areas (HILIC) responsive to standardized meal challenge. Features were assayed by LC-Fourier transform-MS (HILIC with positive electrospray ionization). Pathway analysis [mummichog and gene set enrichment algorithms via MetaboAnalyst (42, 43)] was conducted among features selected via a multivariable linear mixed regression model screening approach (q < 0.05; stage 1 in feature selection approach). (A) In this pathway analysis plot, KEGG pathways are visually represented. Each dot represents a pathway. The size and location of every dot reflect the enrichment factor and P value. Circle sizes indicate the most represented pathways, based on enrichment factor. The enrichment factor is the ratio between the number of hits in the pathway and the expected hits in the pathway. Quadrants indicate significant pathways based on the mummichog (y-axis, light gray), GSEA (x-axis, light gray), or both algorithms (dark gray). (B) Top pathways and their respective P values (mummichog and GSEA algorithms, combined) are shown. Pathways were ranked by the combined P values from the mummichog and GSEA algorithms, based on the selected features in stage 1 regressions and the Fisher method. GSEA, gene set enrichment analysis; HILIC, hydrophilic interaction liquid chromatography; KEGG, Kyoto Encyclopedia of Genes and Genomes.
TCA
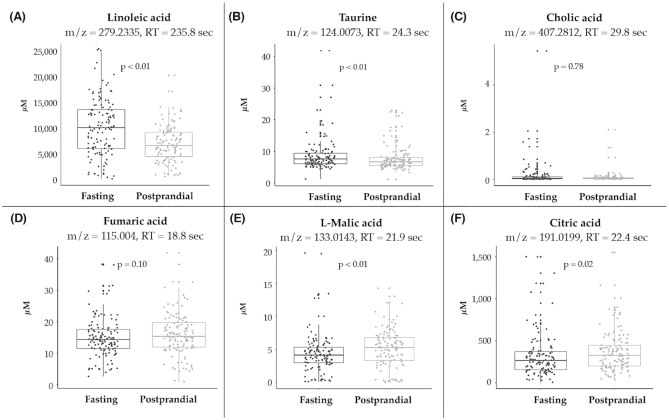
In C18 data, 8 spectral features were in the TCA cycle, including fumaric acid, l-malic acid, oxoglutaric acid, l-lysine, and citric acid (Supplemental Figure 2, Table 3, Figure 3, Supplemental Table 5A). Median plasma concentrations of l-malic acid (5.25 μM compared with 4.12 μM) and citric acid (327.58 μM compared with 266.22 μM) were higher following the prandial challenge, compared with baseline (both P ≤ 0.02).
TABLE 3.
Metabolomic features differing by meal challenge in top pathways1
| Feature | xMSannotator3 | Annotation score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathway2 | m/z | RT, s | Adduct | Log2 peak area (median; IQR) | Concentration, μM (median; IQR)4 | Column | Chemical compound | Formula | HMDB monoisotopic mass | HMDB ID | xMSannotator confidence score | In-house reference library | MSI level |
| Linoleic acid metabolism | 281.2473 | 25.6 | M + H | 16.71 (16.28, 17.14) | — | HILIC+ | Linoleic acid | C18H32O2 | 280.24023 | HMDB00673 | 3 | ✓ | 2 |
| 279.2335 | 235.8 | M-H | 30.19 (29.55, 30.79) | 7820.72 (5194.41, 11,637.75) | C18− | Linoleic acid | C18H32O2 | 280.24023 | HMDB00673 | 3 | ✓ | 2 | |
| Primary bile acid synthesis | 124.0073 | 24.3 | M-H | 20.51 (20.19, 20.85) | 7.12 (5.85, 8.74) | C18− | Taurine | C2H7NO3S | 125.014664 | HMDB00251 | 3 | ✓ | 2 |
| 407.2812 | 29.8 | M-H | 14.93 (7.02, 16.51) | 0.03 (0.02, 0.08) | C18− | Cholic acid | C24H40O5 | 408.287574 | HMDB00619 | 3 | — | 3 | |
| 433.3329 | 235.3 | M-H | 14.73 (13.72, 15.51) | — | C18− | 3α,7α-Dihydroxycoprostanic acid | C27H46O4 | 434.33961 | HMDB00359 | 2 | — | 3 | |
| 433.3329 | 235.3 | M-H | 14.73 (13.72, 15.51) | — | C18− | 3α,7α,12α-Trihydroxy-5β-cholestan-26-al | C27H46O4 | 434.33961 | HMDB03533 | 2 | — | 3 | |
| TCA cycle | 115.004 | 18.8 | M-H | 16.99 (16.35, 17.35) | 14.81 (11.65, 18.65) | C18− | Fumaric acid | C4H4O4 | 116.010959 | HMDB00134 | 2 | ✓ | 3 |
| 133.0143 | 21.9 | M-H | 20.41 (19.46, 21.05) | 4.67 (3.11, 6.11) | C18− | l-Malic acid | C4H6O5 | 134.021523 | HMDB00156 | 3 | ✓ | 2 | |
| 145.0143 | 20.6 | M-H | 21.80 (20.85, 22.39) | — | C18− | Oxoglutaric acid | C5H6O5 | 146.021523 | HMDB00208 | 2 | — | 3 | |
| 145.0982 | 28.2 | M-H | 16.83 (14.23, 17.65) | 176.31 (130.17, 264.13) | C18− | l-Lysine | C6H14N2O2 | 146.105528 | HMDB00182 | 2 | ✓ | 3 | |
| 173.0085 | 23.2 | M-H2O-H | 19.91 (19.37, 20.49) | — | C18− | Citric acid | C6H8O7 | 192.027003 | HMDB00094 | 3 | — | 3 | |
| 191.0199 | 22.4 | M-H | 24.37 (23.90, 24.87) | 286.35 (184.76, 416.27) | C18− | Citric acid | C6H8O7 | 192.027003 | HMDB00094 | 3 | — | 3 | |
C18-, C18 column with negative electrospray ionization; GSEA, gene set enrichment analysis; H, hydrogen; HILIC+, hydrophilic interaction liquid chromatography column with positive electrospray ionization; HMDB, Human Metabolome Database; KEGG, Kyoto Encyclopedia of Genes and Genomes; M, analyte molecule; MSI, Metabolomics Standards Initiative; RT, retention time; TCA, tricarboxylic acid.
Top pathways based on mummichog and GSEA algorithms (Pcombined < 0.05) in MetaboAnalystR (mummichog, v 1.0).
Features in top pathways. Annotations based on feature matches in KEGG (mummichog) and HMDB (xMSannotator).
Concentrations calculated based on the observed feature peak area, Q-std3 peak area, and known metabolite concentration. Only among features that matched with available calibrated reference data for metabolite.
FIGURE 3.
(A–F) Plasma concentrations of metabolites, stratified by prandial challenge. Horizontal lines in each boxplot indicate the median, 25th and 75th percentile values. Examples of selected features were included in this figure. Features were matched with calibrated reference data for metabolite. Metabolite plasma concentrations were calculated based on the observed feature peak area, Q-std3 peak area, and known metabolite concentration. The comparisons between median plasma concentrations at fasting compared with postprandial timepoints were based on Wilcoxon test statistics. RT, retention time.
Fatty acid metabolism
From C18 data, there were 4 features in fatty acid metabolism and 2 in linoleic acid metabolism (Supplemental Tables 4 and 5A). Linoleic acid was observed in both the C18 (m/z = 279.2335; RT = 235.8 s) and HILIC (m/z = 281.2473; RT = 25.6 s) columns (Table 3, Supplemental Tables 5A and 5B).
Bile acid metabolism
Observed C18 features in primary bile acid biosynthesis included: taurine, cholic acid, 3α,7α-dihydroxycoprostanic acid, and 3α,7α,12α-trihydroxy-5β-cholestan-26-al (Table 3, Figure 3, Supplemental Table 4). Median plasma concentration of taurine (m/z = 124.0073; RT = 24.3 s) was 7.58 μM while fasting, and 6.66 μM in a postprandial state (P < 0.01).
Discussion
In Guatemalan adults in our study, >1100 metabolomic features differed following a standardized meal challenge compared with the fasting state. From functional analysis, these features were associated with pathways of energy (TCA cycle), macronutrient, and bile acid metabolism. These changes in metabolomic profiling following a meal challenge reflect short-term metabolic flexibility in individuals without cardiometabolic diseases.
Metabolomic profiles differed between fasting and postprandial states, including in terms of functional pathways and individual features. Prior studies have elucidated differences between fasting compared with nonfasting states. For example, in the diagnoses of metabolic diseases such as diabetes and metabolic syndrome, the cutoff values and utility of key biomarkers (e.g., glucose, HDL cholesterol, triglycerides) depend on whether blood samples were collected while fasting or nonfasting (54, 56). Previous literature has raised questions about the strength of evidence for recommendations of fasting blood samples in cardiometabolic disease screening and diagnoses (57–60). Nonfasting triglycerides have been found to be predictive of cardiovascular disease outcomes (59, 61), and in 1 study to be more associated than fasting triglycerides (59). Based on our findings, future metabolomic studies should account for fasting or postprandial blood collection in study designs and harmonizing results across studies.
In our study participants, the TCA cycle was among the top pathways associated with features that changed following the standardized meal challenge. Two other studies also found that the TCA cycle (15) and energy metabolism (19) pathways differed after meal challenges. Previous evidence has demonstrated the role of the hepatic TCA cycle in the mitochondrial matrix, in which pyruvate oxidation occurs (62). Alterations in TCA cycle metabolism–related indicators have also been observed in people with metabolic diseases (63).
Fatty acid metabolism, particularly linoleic acid metabolism, was among the top pathways that were affected by the meal challenge. Median linoleic acid concentration in our participants was higher than in prior studies (64, 65), although possible explanations include higher dietary consumption of foods rich in linoleic acids (e.g., corn or maize oils) in Guatemala (66). Two prior metabolomic studies have shown that free fatty acids (16) and other lipids [sphingolipids (21)] responded to a meal challenge. However, direct comparisons with our results are difficult. One long-term study provided participants with olive oil, peanuts, Gruyère cheese, and butter, and had an initial overfeeding diet phase (days 5–34) followed by ad libitum food consumption (days 34–62) (16). The second study provided a single meal challenge comprised of refined wheat bread (50 g carbohydrates, 9 g protein, 4.2 g fat, 2.7 g dietary fiber), cucumber (40 g), and a noncaloric orange beverage (300 mL) (21).
Other previous studies have also found that amino acid pathways, including branched-chain amino acids and phenylalanine, responded to meal challenges (15–17, 21). Further metabolomic and mechanistic studies are needed to evaluate the extent that other key factors [e.g., dietary composition (carbohydrate-to-protein ratio), hepatic AMP-activated kinase activity (67)] affect macronutrient metabolism after meal consumption, given that these could provide insights regarding how to maintain metabolic flexibility over the life course.
The primary bile acid biosynthesis pathway was responsive to a meal challenge. Two previous small-scale studies have shown similar results (17, 68). In addition to the classical roles of bile acids in digestion, putative mechanisms explaining the link between bile acids and metabolism include endocrine signaling via bile acid–activated nuclear and membrane receptors (farnesoid X receptor, TGR5) (69–71), which can have downstream effects on glucagon-like peptide 1 and glucose metabolism (72). However, many preliminary studies have used murine models or have been in vitro experiments (70), and therefore further studies need to determine any clinical implications of the nonclassical roles of bile acids in metabolism (73). Separately, the potential influences of other factors, such as the microbiome (71) and tissue-specific receptor expression (69), on the link between bile acids and metabolism remain unclear.
This study had several limitations. One key challenge was metabolite identification, including the need for confirmation of putative feature annotations [MSI level 1 (47)]. Furthermore, improvements in reference databases are needed to address the substantial proportion of features with unknown identities. There is substantial diversity in metabolomic assay methodology, including the analytical technique (e.g., NMR, GC-MS, or LC-MS), instrumentation, sample collection and preparation protocols, preprocessing assumptions, data filtering, and bioinformatics (11). Our results should be interpreted while taking into account the specific methodological approaches we used. Metabolomic profiling encompasses small metabolites (generally <1.5 kDa) and related functional pathways; however, further studies with orthogonal techniques are needed to comprehensively characterize the healthy metabolome after meal consumption. For example, proteomic and lipidomic data are specific subsets of the metabolome, and based on different workflows and databases that are respectively optimized for proteins and lipids. We did not assess physiological factors, including circadian rhythms, genetics, and adipose and skeletal muscle tissues, that potentially affect metabolism following meal consumption.
One strength of this study was the standardized meal challenge, particularly given the heterogeneity of challenges in the literature to date. Additionally, we studied a larger sample size relative to study populations in prior studies (Supplemental Table 1). High-resolution untargeted metabolomics allows for more comprehensive coverage of features, compared with targeted metabolomics (11). In untargeted metabolomics, the wide range of observed metabolites have heterogeneous chemical properties (e.g., polarity, hydrophobicity) and interactions with different column types (11). Dual analytical columns (e.g., C18, HILIC) and different electrospray ionization modes (positive, negative) also increased feature coverage (31). We converted the relative ion intensities of selected features to plasma concentrations, based on a reference standardization approach. Reporting blood concentrations facilitates comparisons with other studies and addresses a major shortcoming of relative quantitation in many nontargeted metabolomic studies.
In conclusion, metabolomic features that differed following a standardized meal challenge were associated with energy (TCA cycle), macronutrient, and bile acid metabolism pathways in adults. Our results indicate short-term metabolic flexibility in disease-free individuals, which could suggest the need for consideration of metabolic flexibility in disease states such as cardiometabolic diseases.
Supplementary Material
Acknowledgments
We thank ViLinh Tran, Ken Liu, and Kristine Dennis for their technical assistance.
The authors’ responsibilities were as follows—ADS: conceived and designed the study; DPJ: developed the protocol for the high-resolution metabolomic assay and workflow; TY: designed the bioinformatic workflow for the initial preprocessing of MS data; EAY and ADS: analyzed data; EAY: wrote the initial manuscript draft; ADS: had primary responsibility for final content; and all authors: contributed to critically revising the paper, and read and approved the final manuscript.
Notes
This research was supported by the National Institutes of Health: HD075784 (ADS, Eunice Kennedy Shriver National Institute of Child Health and Human Development); HL007745 (EAY, National Heart, Lung, and Blood Institute); S10 OD018006 (DPJ, Office of the Director). DPJ was supported in part by R01 ES023485, U2C ES030163, and P30 ES019776 (National Institute of Environmental Health Sciences). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Heart, Lung, and Blood Institute, or the National Institute of Environmental Health Sciences.
Author disclosures: The authors report no conflicts of interest.
ADS is an Associate Editor on the Journal and played no role in the Journal's evaluation.
Supplemental Tables 1–5 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: C18, 18-carbon; GSEA, gene set enrichment analysis; HILIC, hydrophilic interaction liquid chromatography; HMDB, Human Metabolome Database; INCAP, Institute of Nutrition of Central America and Panama; KEGG, Kyoto Encyclopedia of Genes and Genomes; LC-FT-MS, liquid chromatography Fourier transform mass spectrometry; MSI, Metabolomics Standards Initiative; NIST, National Institute of Standards and Technology; OGTT, oral-glucose-tolerance test; RT, retention time; SRM, standard reference material; TCA, tricarboxylic acid.
Contributor Information
Elaine A Yu, Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Tianwei Yu, Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Dean P Jones, Clinical Biomarkers Laboratory, Division of Pulmonary, Allergy, and Critical Care Medicine, School of Medicine, Emory University, Atlanta, GA, USA.
Reynaldo Martorell, Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
Manuel Ramirez-Zea, Institute of Nutrition of Central America and Panama Research Center for the Prevention of Chronic Diseases, Institute of Nutrition of Central America and Panama, Guatemala City, Guatemala.
Aryeh D Stein, Hubert Department of Global Health, Rollins School of Public Health, Emory University, Atlanta, GA, USA.
References
- 1. Stefan N, Häring H-U, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–62. [DOI] [PubMed] [Google Scholar]
- 2. Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, Vikman P, Prasad RB, Aly DM, Almgren P et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–9. [DOI] [PubMed] [Google Scholar]
- 3. Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017;25:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith RL, Soeters MR, Wüst RCI, Houtkooper RH. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev. 2018;39:489–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Noncommunicable diseases progress monitor 2020. Geneva: WHO; 2020. [Google Scholar]
- 6. Rinschen MM, Ivanisevic J, Giera M, Siuzdak G. Identification of bioactive metabolites using activity metabolomics. Nat Rev Mol Cell Biol. 2019;20:353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muoio D M. Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell. 2014;159:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Ommen B, Keijer J, Heil SG, Kaput J. Challenging homeostasis to define biomarkers for nutrition related health. Mol Nutr Food Res. 2009;53:795–804. [DOI] [PubMed] [Google Scholar]
- 9. Guijas C, Montenegro-Burke JR, Warth B, Spilker ME, Siuzdak G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat Biotechnol. 2018;36:316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jang C, Chen L, Rabinowitz JD. Metabolomics and isotope tracing. Cell. 2018;173:822–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newgard CB. Metabolomics and metabolic diseases: where do we stand?. Cell Metab. 2017;25:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cirulli ET, Guo L, Leon Swisher C, Shah N, Huang L, Napier LA, Kirkness EF, Spector TD, Caskey CT, Thorens B et al. Profound perturbation of the metabolome in obesity is associated with health risk. Cell Metab. 2019;29:488–500..e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Suárez M, Caimari A, del Bas JM, Arola L. Metabolomics: an emerging tool to evaluate the impact of nutritional and physiological challenges. Trends Anal Chem. 2017;96:79–88. [Google Scholar]
- 15. Bondia-Pons I, Nordlund E, Mattila I, Katina K, Aura AM, Kolehmainen M, Oresic M, Mykkanen H, Poutanen K. Postprandial differences in the plasma metabolome of healthy Finnish subjects after intake of a sourdough fermented endosperm rye bread versus white wheat bread. Nutr J. 2011;10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Germain N, Galusca B, Caron-Dorval D, Martin JF, Pujos-Guillot E, Boirie Y, Khalfallah Y, Ling Y, Minnion JS, Bloom SR et al. Specific appetite, energetic and metabolomics responses to fat overfeeding in resistant-to-bodyweight-gain constitutional thinness. Nutr Diabetes. 2014;4:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathew S, Krug S, Skurk T, Halama A, Stank A, Artati A, Prehn C, Malek JA, Kastenmuller G, Romisch-Margl W et al. Metabolomics of Ramadan fasting: an opportunity for the controlled study of physiological responses to food intake. J Transl Med. 2014;12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moazzami AA, Shrestha A, Morrison DA, Poutanen K, Mykkanen H. Metabolomics reveals differences in postprandial responses to breads and fasting metabolic characteristics associated with postprandial insulin demand in postmenopausal women. J Nutr. 2014;144:807–14. [DOI] [PubMed] [Google Scholar]
- 19. Pantophlet AJ, Wopereis S, Eelderink C, Vonk RJ, Stroeve JH, Bijlsma S, van Stee L, Bobeldijk I, Priebe MG. Metabolic profiling reveals differences in plasma concentrations of arabinose and xylose after consumption of fiber-rich pasta and wheat bread with differential rates of systemic appearance of exogenous glucose in healthy men. J Nutr. 2017;147:152–60. [DOI] [PubMed] [Google Scholar]
- 20. Shi L, Brunius C, Lindelof M, Shameh SA, Wu H, Lee I, Landberg R, Moazzami AA. Targeted metabolomics reveals differences in the extended postprandial plasma metabolome of healthy subjects after intake of whole-grain rye porridges versus refined wheat bread. Mol Nutr Food Res. [Internet]2017;61 doi:10.1002/mnfr.201600924. [DOI] [PubMed] [Google Scholar]
- 21. Shrestha A, Mullner E, Poutanen K, Mykkanen H, Moazzami AA. Metabolic changes in serum metabolome in response to a meal. Eur J Nutr. 2017;56:671–81. [DOI] [PubMed] [Google Scholar]
- 22. Pan D, Mao C, Quattrochi B, Friedline RH, Zhu LJ, Jung DY, Kim JK, Lewis B, Wang Y-X. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat Commun. 2014;5:4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park Y, Kim SB, Wang B, Blanco RA, Le N-A, Wu S, Accardi CJ, Alexander RW, Ziegler TR, Jones DP. Individual variation in macronutrient regulation measured by proton magnetic resonance spectroscopy of human plasma. Am J Physiol Regul Integr Comp Physiol. 2009;297:R202–R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berthiaume N, Zinker BA. Metabolic responses in a model of insulin resistance: comparison between oral glucose and meal tolerance tests. Metabolism. 2002;51:595–8. [DOI] [PubMed] [Google Scholar]
- 25. Meier JJ, Baller B, Menge BA, Gallwitz B, Schmidt WE, Nauck MA. Excess glycaemic excursions after an oral glucose tolerance test compared with a mixed meal challenge and self-measured home glucose profiles: is the OGTT a valid predictor of postprandial hyperglycaemia and vice versa?. Diabetes Obes Metab. 2009;11:213–22. [DOI] [PubMed] [Google Scholar]
- 26. Rijkelijkhuizen JM, Girman CJ, Mari A, Alssema M, Rhodes T, Nijpels G, Kostense PJ, Stein PP, Eekhoff EM, Heine RJ et al. Classical and model-based estimates of beta-cell function during a mixed meal vs. an OGTT in a population-based cohort. Diabetes Res Clin Pract. 2009;83:280–8. [DOI] [PubMed] [Google Scholar]
- 27. Stein AD, Melgar P, Hoddinott J, Martorell R. Cohort profile: the Institute of Nutrition of Central America and Panama (INCAP) nutrition trial cohort study. Int J Epidemiol. 2008;37:716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martorell R, Habicht JP, Rivera JA. History and design of the INCAP longitudinal study (1969-77) and its follow-up (1988-89). J Nutr. 1995;125:1027s–41s. [DOI] [PubMed] [Google Scholar]
- 29. Ford ND, Behrman JR, Hoddinott JF, Maluccio JA, Martorell R, Ramirez-Zea M, Stein AD. Exposure to improved nutrition from conception to age 2 years and adult cardiometabolic disease risk: a modelling study. Lancet Glob Health. 2018;6:e875–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Go Y-M, Uppal K, Walker DI, Tran V, Dury L, Strobel FH, Baubichon-Cortay H, Pennell KD, Roede JR, Jones DP. Mitochondrial metabolomics using high-resolution Fourier-transform mass spectrometry. Methods Mol Biol. 2014;1198:43–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soltow QA, Strobel FH, Mansfield KG, Wachtman L, Park Y, Jones DP. High-performance metabolic profiling with dual chromatography-Fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics. 2013;9:S132–S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Go YM, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, Strobel F, Quyyumi AA, Ziegler TR, Pennell KD et al. Reference standardization for mass spectrometry and high-resolution metabolomics applications to exposome research. Toxicol Sci. 2015;148:531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernandes J, Chandler JD, Liu KH, Uppal K, Go YM, Jones DP. Putrescine as indicator of manganese neurotoxicity: dose-response study in human SH-SY5Y cells. Food Chem Toxicol. 2018;116:272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Johnson JM, Yu T, Strobel FH, Jones DP. A practical approach to detect unique metabolic patterns for personalized medicine. Analyst. 2010;135:2864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marshall AG, Hendrickson CL. High-resolution mass spectrometers. Annu Rev Anal Chem. 2008;1:579–99. [DOI] [PubMed] [Google Scholar]
- 36. Fernandes J, Chandler JD, Liu KH, Uppal K, Hao L, Hu X, Go YM, Jones DP. Metabolomic responses to manganese dose in SH-SY5Y human neuroblastoma cells. Toxicol Sci. 2019;169:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu T, Park Y, Johnson JM, Jones DP. apLCMS—adaptive processing of high-resolution LC/MS data. Bioinformatics. 2009;25:1930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics. 2013;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forsberg EM, Huan T, Rinehart D, Benton HP, Warth B, Hilmers B, Siuzdak G. Data processing, multi-omic pathway mapping, and metabolite activity analysis using XCMS Online. Nat Protoc. 2018;13:633–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27. [DOI] [PubMed] [Google Scholar]
- 41. Smilde AK, van der Werf MJ, Bijlsma S, van der Werff-van der Vat BJ, Jellema RH. Fusion of mass spectrometry-based metabolomics data. Anal Chem. 2005;77:6729–36. [DOI] [PubMed] [Google Scholar]
- 42. Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP, Pulendran B. Predicting network activity from high throughput metabolomics. PLoS Comput Biol. 2013;9:e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–W94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2011;40:D109–D14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E et al. HMDB 3.0—the Human Metabolome Database in 2013. Nucleic Acids Res. 2013;41:D801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uppal K, Walker DI, Jones DP. xMSannotator: an R package for network-based annotation of high-resolution metabolomics data. Anal Chem. 2017;89:1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TWM, Fiehn O, Goodacre R, Griffin JL et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics. 2007;3:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boyd RK, Basic C, Bethem RA. Tools of the trade VII: statistics of calibration, measurement and sampling. In: Boyd RK, Basic C, Bethem RAeditors. Trace quantitative analysis by mass spectrometry. Chichester, UK: John Wiley & Sons, Ltd; 2008. [Google Scholar]
- 49. Accardi CJ, Walker DI, Uppal K, Quyyumi AA, Rohrbeck P, Pennell KD, Mallon CT, Jones DP. High-resolution metabolomics for nutrition and health assessment of armed forces personnel. J Occup Environ Med. 2016;58:S80–8. [DOI] [PubMed] [Google Scholar]
- 50. Walker DI, Lane KJ, Liu K, Uppal K, Patton AP, Durant JL, Jones DP, Brugge D, Pennell KD. Metabolomic assessment of exposure to near-highway ultrafine particles. J Expo Sci Environ Epidemiol. 2019;29:469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu KH, Nellis M, Uppal K, Chunyu M, Tran VL, Liang Y, Walker DI, Jones DP. Reference standardization for quantification and harmonization of large-scale metabolomics. Anal Chem. 2020; doi: 10.1021/acs.analchem.0c00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO Technical Report Series 894. Geneva: WHO; 2000. [PubMed] [Google Scholar]
- 53. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 54. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Dia Care. 2019;42:S13–28. [DOI] [PubMed] [Google Scholar]
- 55. National Cholesterol Education Program. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 56. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- 57. Langsted A, Nordestgaard BG. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology. 2019;51:131–41. [DOI] [PubMed] [Google Scholar]
- 58. Nordestgaard BG. A test in context: lipid profile, fasting versus nonfasting. J Am Coll Cardiol. 2017;70:1637–46. [DOI] [PubMed] [Google Scholar]
- 59. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–16. [DOI] [PubMed] [Google Scholar]
- 60. Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, Watts GF, Sypniewska G, Wiklund O, Borén J et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37:1944–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 62. Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic β-cells. Nature. 2001;414:807–12. [DOI] [PubMed] [Google Scholar]
- 63. Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab. 2011;14:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kuriki K, Nagaya T, Tokudome Y, Imaeda N, Fujiwara N, Sato J, Goto C, Ikeda M, Maki S, Tajima K et al. Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: a cross-sectional study. J Nutr. 2003;133:3643–50. [DOI] [PubMed] [Google Scholar]
- 65. McNaughton SA, Hughes MC, Marks GC. Validation of a FFQ to estimate the intake of PUFA using plasma phospholipid fatty acids and weighed foods records. Br J Nutr. 2007;97:561–8. [DOI] [PubMed] [Google Scholar]
- 66. Bressani R, Benavides V, Acevedo E, Ortiz MA. Changes in selected nutrient contents and in protein quality of common and quality-protein maize during rural tortilla preparation. Cereal Chem. 1990;67:515–18. [Google Scholar]
- 67. Chalvon-Demersay T, Even PC, Chaumontet C, Piedcoq J, Viollet B, Gaudichon C, Tome D, Foretz M, Azzout-Marniche D. Modifying the dietary carbohydrate-to-protein ratio alters the postprandial macronutrient oxidation pattern in liver of AMPK-deficient mice. J Nutr. 2017;147:1669–76. [DOI] [PubMed] [Google Scholar]
- 68. Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, Jia W, Brouwer KL, Barritt AST. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:3318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Molinaro A, Wahlström A, Marschall H-U. Role of bile acids in metabolic control. Trends Endocrinol Metab. 2018;29:31–41. [DOI] [PubMed] [Google Scholar]
- 70. van Nierop FS, Scheltema MJ, Eggink HM, Pols TW, Sonne DP, Knop FK, Soeters MR. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 2017;5:224–33. [DOI] [PubMed] [Google Scholar]
- 71. Wahlstrom A, Sayin SI, Marschall HU, Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. [DOI] [PubMed] [Google Scholar]
- 72. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bronden A, Knop FK. Gluco-metabolic effects of pharmacotherapy-induced modulation of bile acid physiology. J Clin Endocrinol Metab. 2020;105(1):dgz025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.