ABSTRACT
Background
Protein ingestion increases skeletal muscle protein synthesis rates during recovery from endurance exercise.
Objectives
We aimed to determine the effect of graded doses of dietary protein co-ingested with carbohydrate on whole-body protein metabolism, and skeletal muscle myofibrillar (MyoPS) and mitochondrial (MitoPS) protein synthesis rates during recovery from endurance exercise.
Methods
In a randomized, double-blind, parallel-group design, 48 healthy, young, endurance-trained men (mean ± SEM age: 27 ± 1 y) received a primed continuous infusion of l-[ring-2H5]-phenylalanine, l-[ring-3,5-2H2]-tyrosine, and l-[1-13C]-leucine and ingested 45 g carbohydrate with either 0 (0 g PRO), 15 (15 g PRO), 30 (30 g PRO), or 45 (45 g PRO) g intrinsically l-[1-13C]-phenylalanine and l-[1-13C]-leucine labeled milk protein after endurance exercise. Blood and muscle biopsy samples were collected over 360 min of postexercise recovery to assess whole-body protein metabolism and both MyoPS and MitoPS rates.
Results
Protein intake resulted in ∼70%–74% of the ingested protein-derived phenylalanine appearing in the circulation. Whole-body net protein balance increased dose-dependently after ingestion of 0, 15, 30, or 45 g protein (mean ± SEM: −0.31± 0.16, 5.08 ± 0.21, 10.04 ± 0.30, and 13.49 ± 0.55 μmol phenylalanine · kg−1 · h−1, respectively; P < 0.001). 30 g PRO stimulated a ∼46% increase in MyoPS rates (%/h) compared with 0 g PRO and was sufficient to maximize MyoPS rates after endurance exercise. MitoPS rates were not increased after protein ingestion; however, incorporation of dietary protein–derived l-[1-13C]-phenylalanine into de novo mitochondrial protein increased dose-dependently after ingestion of 15, 30, and 45 g protein at 360 min postexercise (0.018 ± 0.002, 0.034 ± 0.002, and 0.046 ± 0.003 mole percentage excess, respectively; P < 0.001).
Conclusions
Protein ingested after endurance exercise is efficiently digested and absorbed into the circulation. Whole-body net protein balance and dietary protein–derived amino acid incorporation into mitochondrial protein respond to increasing protein intake in a dose-dependent manner. Ingestion of 30 g protein is sufficient to maximize MyoPS rates during recovery from a single bout of endurance exercise.
This trial was registered at trialregister.nl as NTR5111.
Keywords: myofibrillar protein synthesis, mitochondrial protein synthesis, skeletal muscle, dose-response, dietary protein, carbohydrate, endurance exercise, young men
Protein ingestion increases muscle protein synthesis rates and is important in facilitating muscle reconditioning after exercise. However, the dose-response relationship between ingested protein and both whole-body and skeletal muscle myofibrillar (MyoPS) and mitochondrial (MitoPS) protein synthesis rates during recovery from endurance exercise remains unclear. In the present study, participants received either 0, 15, 30, or 45 g of protein following a single session of endurance exercise. Blood and muscle samples were collected over 360 min of postexercise recovery to assess whole-body protein metabolism and both MyoPS and MitoPS. Protein intake resulted in ~70-74% of the ingested protein-derived phenylalanine appearing in the circulation. Whole-body net protein balance was negative with 0 g protein but was positive and increased dose-dependently after ingestion of 15, 30, and 45 g of protein. Ingestion of 30 g protein increased MyoPS rates by ~46% compared to 0 g protein and was sufficient to maximize MyoPS rates after exercise. MitoPS rates were not increased following protein ingestion, however incorporation of dietary protein-derived L-[1-13C]-phenylalanine into de novo mitochondrial protein increased dose-dependently after protein intake at 360 min postexercise. These results may have important implications for endurance athletes interested in nutritional strategies to support postexercise recovery and training efforts.
See corresponding editorial on page 249.
Introduction
Endurance exercise training is characterized by improvements in muscle oxidative capacity, secondary to an increase in the size and density of mitochondria, and accumulation of myosin heavy chain I (1). A single bout of endurance exercise (e.g., cycling or running) in the fasted state increases mixed muscle (MPS) (2–5), myofibrillar (MyoPS) (6), and mitochondrial (MitoPS) (6) protein synthesis rates. Endurance exercise also enhances protein breakdown and stimulates a marked increase in amino acid oxidation (7, 8). It is recognized that adequate carbohydrate and protein intakes are important to replenish substrate stores and provide amino acids as building blocks to support skeletal muscle reconditioning after exercise (9). Protein ingestion during recovery from resistance exercise further increases skeletal muscle protein synthesis rates (10) and augments exercise-induced increases in muscle mass and strength (11, 12). However, less is known regarding the impact of protein ingestion on whole-body protein metabolism and MyoPS and MitoPS rates during recovery from endurance exercise. The majority of (13–17), but not all (2, 18), studies to date examining the effect of protein ingestion on MPS rates during recovery from an acute bout of endurance exercise have found that protein intake results in higher mixed MPS (15, 16) and MyoPS (13, 14, 17) rates than does a nonprotein control treatment. Intravenously infused amino acids (19, 20) and protein ingestion (21) stimulate increased MitoPS rates at rest; however, protein ingestion does not appear to enhance MitoPS rates during recovery from endurance exercise (13, 14, 18) or combined endurance and resistance exercise (22–24). The dose of ingested protein is a key factor determining the magnitude of increase in whole-body net protein balance (25) and MPS rates (26, 27) during recovery from resistance exercise. For example, mixed MPS (26) and MyoPS (27) rates are increased in a dose-dependent manner after protein ingestion during recovery from resistance exercise in young men, and are maximally stimulated with ∼20 g of a high-quality protein (26, 27). However, no study to date has determined the dose–response relation between the amount of ingested protein and changes in MyoPS and MitoPS rates during recovery from a bout of endurance exercise.
The present study examined the effects of co-ingesting 0 (0 g PRO), 15 (15 g PRO), 30 (30 g PRO), and 45 g (45 g PRO) of milk protein with 45 g carbohydrate on postprandial protein digestion and absorption kinetics, whole-body protein metabolism (breakdown, synthesis, oxidation, and net balance), MyoPS and MitoPS rates, and the utilization of dietary protein–derived phenylalanine for de novo MyoPS and MitoPS during recovery from a single bout of endurance exercise in young, healthy, endurance-trained men. We hypothesized that co-ingestion of protein with carbohydrate would result in greater postexercise MyoPS rates than 0 g PRO, and that 30 g PRO would maximally stimulate MyoPS rates during recovery from endurance exercise. Further, we hypothesized that 45 g PRO would best support postexercise increases in whole-body net protein balance and be required to stimulate increased MitoPS rates over 360 min of recovery from endurance exercise.
Methods
Participants
Forty-eight healthy, endurance-trained (cyclists and triathletes), young men (mean ± SEM age: 27 ± 1 y; height: 1.82 ± 0.01 m; weight: 74.9 ± 0.9 kg; V̇O2 peak: 58 ± 1 mL · kg−1 · min−1) volunteered to participate in this parallel-group, double-blind, randomized controlled trial. Supplemental Figure 1 presents the CONSORT flowchart. The trial was registered at the Nederlands Trial Register (NTR5111) and conducted between February 2016 and February 2017 at Maastricht University in Maastricht, Netherlands. Table 1 presents the participants’ characteristics.
TABLE 1.
Characteristics of male study participants who ingested nutritional treatments consisting of carbohydrate only, or carbohydrate co-ingested with 15 g, 30 g, or 45 g milk protein during recovery from a single bout of endurance exercise1
| Nutritional treatment group | |||||
|---|---|---|---|---|---|
| Characteristic | 0 g PRO | 15 g PRO | 30 g PRO | 45 g PRO | P |
| Age, y | 27 ± 1 | 26 ± 1 | 26 ± 1 | 29 ± 1 | 0.28 |
| Height, m | 1.83 ± 0.02 | 1.82 ± 0.01 | 1.82 ± 0.01 | 1.80 ± 0.01 | 0.50 |
| Weight, kg | 74.5 ± 2.2 | 74.1 ± 1.6 | 73.6 ± 1.2 | 77.5 ± 1.6 | 0.37 |
| BMI, kg/m2 | 22.4 ± 0.6 | 22.4 ± 0.4 | 22.3 ± 0.4 | 24.0 ± 0.5 | 0.04 |
| Fat + bone-free mass, kg | 59.9 ± 1.4 | 58.8 ± 1.3 | 58.2 ± 0.8 | 60.3 ± 1.2 | 0.64 |
| % Fat | 16.5 ± 1.0 | 17.6 ± 0.9 | 17.6 ± 0.6 | 19.5 ± 0.7 | 0.09 |
| Resting heart rate, bpm | 60 ± 2 | 59 ± 3 | 62 ± 4 | 59 ± 4 | 0.92 |
| V̇O2 peak, mL · kg−1 · min−1 | 58 ± 2 | 59 ± 1 | 57 ± 1 | 57 ± 2 | 0.75 |
| Wmax, W/kg | 4.9 ± 0.1 | 5.0 ± 0.1 | 4.8 ± 0.1 | 4.8 ± 0.1 | 0.52 |
Values are means ± SEMs. n = 12 per group. Data were analyzed using a 1-factor ANOVA. bpm, beats per minute; V̇O2 peak, peak oxygen consumption; Wmax, maximal workload capacity; 0 g PRO, 45 g carbohydrate with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g milk protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g milk protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g milk protein.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Maastricht University Medical Center+, Netherlands (METC 153010). The procedures followed were in accordance with the ethical standards of the Medical Ethics Committee of Maastricht University Medical Center+ on human experimentation and in accordance with the Helsinki Declaration of 1975 as revised in October 2013. Clinical Trial Center Maastricht independently monitored the study. All participants were informed about the purpose of the study, the experimental procedures, and possible risks before providing written informed consent to participate.
Preliminary testing
Participants aged 18–35 y with a BMI > 18.5 and < 30.0 underwent an initial screening session to assess height, weight, blood pressure, and body composition (by DXA; Discovery A, Hologic). Participants were deemed healthy based on their responses to a medical questionnaire and screening results. Subsequently, participants underwent supervised testing of their peak oxygen consumption (V̇O2 peak; mL · kg−1 · min−1) and maximal workload capacity (Wmax; W/kg) during an incremental test to volitional fatigue on a cycle ergometer (Lode Excalibur Sport). Participants began cycling at a workload equivalent to 100 W for 5 min, after which the workload was increased by 50 W every 150 s until volitional fatigue was reached, defined as the inability to maintain a cadence > 60 revolutions/min. V̇O2 peak was defined as the median of the highest consecutive values over 30 s. All settings on the ergometer were noted and replicated during the experimental test day. The pretesting and experimental trials were separated by ≥5 d.
Diet and physical activity
All participants refrained from strenuous physical activities and alcohol consumption for 3 d before the experimental trial. In addition, all participants were instructed to fill out food intake and physical activity questionnaires for 3 d immediately before the experimental trial. Dietary intake before the experimental trial is shown in Table 2 and was analyzed using Mijn Eetmeter (https://mijn.voedingscentrum.nl/nl/eetmeter/), online software available from the Dutch Health Council. On the evening before the experimental trial, all participants were provided with a prepackaged standardized meal containing 55% energy as carbohydrate, 30% energy as fat, and 15% energy as protein and instructed to consume it no later than 20:00, after which they remained fasted.
TABLE 2.
Average 3-d dietary intake of male study participants who ingested nutritional treatments consisting of carbohydrate only, or carbohydrate co-ingested with 15 g, 30 g, or 45 g milk protein during recovery from a single bout of endurance exercise1
| Nutritional treatment group | |||||
|---|---|---|---|---|---|
| 0 g PRO | 15 g PRO | 30 g PRO | 45 g PRO | P | |
| Energy, kJ/d | 11,310 ± 824 | 11,520 ± 979 | 10,867 ± 678 | 10,225 ± 717 | 0.77 |
| Carbohydrate, g | 340 ± 25 | 348 ± 33 | 314 ± 21 | 309 ± 21 | 0.63 |
| Fat, g | 94 ± 12 | 92 ± 11 | 97 ± 9 | 82 ± 8 | 0.77 |
| Protein, g | 100 ± 6 | 114 ± 10 | 103 ± 6 | 100 ± 9 | 0.62 |
| Protein, g · kg−1 · d−1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 | 0.33 |
| Carbohydrate, % total energy | 51 ± 2 | 50 ± 2 | 48 ± 2 | 51 ± 1 | 0.77 |
| Fat, % total energy | 30 ± 2 | 30 ± 2 | 33 ± 1 | 30 ± 1 | 0.58 |
| Protein, % total energy | 16 ± 1 | 17 ± 1 | 16 ± 1 | 16 ± 1 | 0.88 |
Values are means ± SEMs. n = 12 per group. Data were analyzed using a 1-factor ANOVA. 0 g PRO, 45 g carbohydrate with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g milk protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g milk protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g milk protein.
Study design
Participants were randomly assigned to ingest a beverage (590 mL) containing 45 g carbohydrate with 0 g protein, or 45 g carbohydrate with either 15, 30, or 45 g intrinsically l-[1-13C]-phenylalanine and l-[1-13C]-leucine labeled milk protein (described below). The carbohydrate powder was supplied by PepsiCo Inc. and was composed of dextrose and maltodextrin. Table 3 shows details of the amino acid, protein, and carbohydrate contents of the nutritional treatments. Randomization was performed using a computerized list randomizer (http://www.randomization.com/), and participants were sequentially allocated to a treatment according to the randomized list. An independent person was responsible for random assignment (n = 12 per group) and preparation of the study treatment beverages, which were sequentially numbered according to subject number. The beverages were prepared in nontransparent plastic containers and had a similar taste and smell.
TABLE 3.
Amino acid (l-form), protein, and carbohydrate contents of nutritional treatments consisting of carbohydrate only, or carbohydrate co-ingested with 15 g, 30 g, or 45 g milk protein, ingested by male participants during recovery from a single bout of endurance exercise1
| Nutritional treatment group | ||||
|---|---|---|---|---|
| 0 g PRO | 15 g PRO | 30 g PRO | 45 g PRO | |
| Amino acid content | ||||
| Alanine, g | — | 0.45 | 0.90 | 1.35 |
| Arginine, g | — | 0.50 | 1.00 | 1.50 |
| Aspartic acid, g | — | 0.92 | 1.84 | 2.76 |
| Glutamic acid, g | — | 2.51 | 5.02 | 7.53 |
| Glycine, g | — | 0.23 | 0.46 | 0.69 |
| Histidine, g | — | 0.33 | 0.66 | 0.99 |
| Isoleucine, g | — | 0.66 | 1.32 | 1.98 |
| Leucine, g | — | 1.44 | 2.88 | 4.32 |
| Lysine, g | — | 1.19 | 2.38 | 3.57 |
| Methionine, g | — | 0.18 | 0.36 | 0.54 |
| Phenylalanine, g | — | 0.63 | 1.26 | 1.89 |
| Proline, g | — | 1.38 | 2.76 | 4.14 |
| Serine, g | — | 0.70 | 1.40 | 2.10 |
| Threonine, g | — | 0.57 | 1.14 | 1.71 |
| Tyrosine, g | — | 0.78 | 1.56 | 2.34 |
| Valine, g | — | 0.84 | 1.68 | 2.52 |
| Totals | ||||
| ƩNEAA, g | — | 7.47 | 14.94 | 22.41 |
| ƩEAA, g | — | 5.84 | 11.68 | 17.52 |
| ƩAA, g | — | 13.31 | 26.62 | 39.93 |
| Protein, g | — | 15.00 | 30.00 | 45.00 |
| Carbohydrate, g | 45.00 | 45.00 | 45.00 | 45.00 |
Total protein was calculated as nitrogen content × 6.25. Cysteine and tryptophan were not measured. 0 g PRO, 45 g carbohydrate with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g milk protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g milk protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g milk protein; ƩAA, sum total amino acids; ƩEAA, sum total essential amino acids; ƩNEAA, sum total nonessential amino acids.
Production of intrinsically labeled milk protein
Intrinsically l-[1-13C]-phenylalanine and l-[1-13C]-leucine labeled milk protein concentrate (MPC80) was extracted from whole bovine milk obtained during the constant intravenous infusion of l-[1-13C]-phenylalanine (455 μmol/min) and l-[1-13C]-leucine (200 μmol/min) for 96 h into a lactating Holstein cow. The milk was collected and processed into the MPC80 in a manner similar to what has been previously described by our laboratory (28, 29). The l-[1-13C]-phenylalanine and l-[1-13C]-leucine enrichments in MPC80 averaged 38.3 mole percentage excess (MPE) and 10.8 MPE, respectively. The protein met all chemical and bacteriological specifications for human consumption.
Experimental protocol
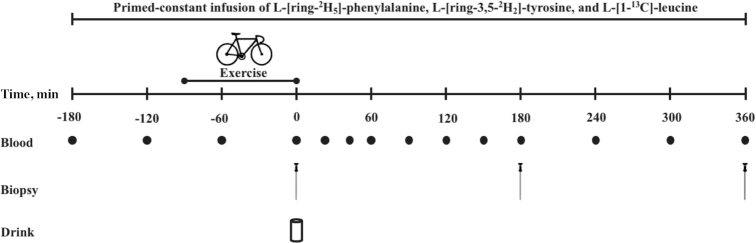
At ∼07:45, participants arrived at the laboratory in the overnight postabsorptive state. A catheter was inserted into an antecubital vein for stable isotope amino acid infusion, then a second catheter was subsequently inserted into a dorsal hand vein on the contralateral arm for arterialized venous blood sampling. To obtain arterialized blood samples, the hand was placed in a hot box (60°C) for 10 min before blood sample collection (30). After taking a baseline blood sample (t = −180 min), the plasma phenylalanine and leucine pools were primed with a single intravenous dose (priming dose) of l-[ring-2H5]-phenylalanine (2.25 µmol/kg), l-[ring-3,5-2H2]-tyrosine (0.92 µmol/kg), and l-[1-13C]-leucine (5.99 µmol/kg). After priming, a continuous intravenous infusion of l-[ring-2H5]-phenylalanine (0.050 μmol ⋅ kg−1 ⋅ min−1), l-[ring-3,5-2H2]-tyrosine (0.015 μmol ⋅ kg−1 ⋅ min−1), and l-[1-13C]-leucine (0.10 μmol ⋅ kg−1 ⋅ min−1) was initiated and maintained using a calibrated IVAC 598 pump. After resting in a supine position for 60 min, a second arterialized blood sample was drawn (t = −120 min). After resting for another 30 min, participants initiated (t = −90 min) the endurance exercise intervention (described below). A third blood sample was drawn (t = −60 min) during exercise while participants were cycling. Immediately after the exercise intervention (t = 0 min), an arterialized blood sample was obtained, and a muscle biopsy sample was collected from the vastus lateralis muscle of a randomly selected leg. Subsequently, participants received a 590-mL beverage corresponding to their randomly assigned treatment allocation [i.e., 0 g PRO (n = 12), 15 g PRO (n = 12), 30 g PRO (n = 12), or 45 g PRO (n = 12)]. Arterialized blood samples were then collected at t = 20, 40, 60, 90, 120, 150, 180, 240, 300, and 360 min in the postprandial period. Second and third muscle biopsy samples were collected at t = 180 and t = 360 min to determine postprandial MyoPS and MitoPS rates at t = 0–180, 180–360, and 0–360 min. Blood samples were collected into EDTA-containing tubes and centrifuged at 1000 × g for 15 min at 4°C. Aliquots of plasma were frozen in liquid nitrogen and stored at −80°C. Biopsy samples were collected using a 5-mm Bergström needle custom-adapted for manual suction. Samples were obtained from separate incisions from the middle region of the vastus lateralis, ∼15 cm above the patella and ∼3 cm below entry through the fascia, under 1% xylocaine local anesthesia with adrenaline (1:100,000). Muscle samples were freed from any visible nonmuscle material, immediately frozen in liquid nitrogen, and stored at −80°C until further processing. When the experimental protocol was complete, cannulas were removed, and participants ate and were assessed for ∼30 min before leaving the laboratory. For a schematic representation of the primed continuous infusion protocol, see Figure 1.
FIGURE 1.
Schematic representation of the experimental design.
Endurance exercise protocol
Participants performed 90 min continuous endurance exercise on a cycle ergometer at ∼60% of their previously determined Wmax. Participants were allowed ad libitum access to water during cycling. Visual feedback for pedal frequency (revolutions/min) and elapsed time were provided to participants and strong verbal encouragement was provided by 1 of the study investigators.
Plasma and muscle tissue analyses
The Supplemental Methods present details of the analysis relating to the determination of plasma glucose, insulin, and amino acid concentrations as well as plasma l-[ring-2H5]-phenylalanine, l-[ring-3,5-2H2]-tyrosine, l-[ring-2H4]-tyrosine, l-[1-13C]-leucine, l-[1-13C]-phenylalanine, and l-[1-13C]-tyrosine enrichments. A piece of wet muscle (∼100 mg) was homogenized on ice using a Teflon pestle in ice-cold homogenization buffer (10 μL/mg; 67 mM sucrose, 50 mM Tris/HCl, 50 mM KCl, 10 mM EDTA) containing protease/phosphatase inhibitor cocktail tablets (Complete Protease Inhibitor Mini-Tabs, Roche; and PhosSTOP, Roche Applied Science). After ∼5–10 min of hand homogenization, the homogenate was centrifuged at 700 × g for 15 min at 4°C to pellet a myofibrillar protein–enriched fraction. The supernatant was transferred to another tube and centrifuged at 12,000 × g for 20 min at 4°C to pellet a mitochondrial protein–enriched fraction. The Supplemental Methods present additional details regarding the preparation and analysis of skeletal muscle samples for measurement of myofibrillar and mitochondrial protein-bound l-[ring-2H5]-phenylalanine, l-[1-13C]-phenylalanine, and/or l-[1-13C]-leucine enrichments.
Calculations
The Supplemental Methods present details of the calculations used to assess whole-body amino acid (phenylalanine) kinetics and skeletal muscle myofibrillar and mitochondrial protein fractional synthesis rates (FSRs).
Outcome measures
The primary outcome measure was myofibrillar FSR based on l-[1-13C]-leucine incorporation into myofibrillar protein over the aggregate (i.e., 0–360 min) postprandial period. Whole-body phenylalanine kinetics [exogenous rate of appearance (Ra), endogenous Ra, total Ra, total rate of disappearance (Rd)], whole-body protein metabolism (breakdown, synthesis, oxidation, net balance), the time-course (i.e., 0–180 and 180–360 min) of myofibrillar FSR based on l-[1-13C]-leucine, and myofibrillar FSR based on l-[ring-2H5]-phenylalanine were secondary outcomes. Mitochondrial FSR based on l-[1-13C]-leucine, and both myofibrillar and mitochondrial l-[1-13C]-phenylalanine enrichments (MPE) as a measure of de novo protein synthesis were also secondary outcome measures. Plasma glucose, insulin, and amino acid (leucine, phenylalanine, and tyrosine) concentrations; l-[1-13C]-leucine, l-[ring-2H5]-phenylalanine, and l-[1-13C]-phenylalanine enrichments (MPE); and bi-phase linear regression analysis of l-[1-13C]-leucine-determined myofibrillar FSR (%/h) plotted against the ingested protein dose normalized to body mass were tertiary outcomes.
Statistical analysis
All data are expressed as means ± SEM except Figure 8, which is expressed as mean and 95% CI. Participant characteristics and average 3-d dietary intake data were analyzed using a 1-factor (treatment) ANOVA. Plasma glucose, insulin, and amino acid (leucine, phenylalanine, and tyrosine) concentrations were analyzed using a 2-factor (treatment × time) repeated-measures ANOVA. Maximum leucine concentrations were analyzed using a 1-factor (treatment) ANOVA. Whole-body phenylalanine kinetics (exogenous Ra, endogenous Ra, total Ra, total Rd) were analyzed by a 2-factor (treatment × time) repeated-measures ANOVA. Dietary protein–derived plasma phenylalanine availability calculated as a fraction of the total amount of ingested phenylalanine was analyzed by a 1-factor (treatment) ANOVA. Plasma enrichments were analyzed using a 2-factor (treatment × time) repeated-measures ANOVA. Whole-body postprandial protein metabolism (breakdown, synthesis, oxidation, and net balance) and myofibrillar and mitochondrial FSRs during early and late recovery (i.e., 0–180 and 180–360 min) were analyzed using a 2-factor (treatment × time) repeated-measures ANOVA. Aggregate whole-body protein metabolic responses and myofibrillar and mitochondrial FSRs (i.e., 0–360 min) were analyzed using a 1-factor (treatment) ANOVA. To determine the dose–response relation, l-[1-13C]-leucine-determined myofibrillar FSR (%/h) was plotted against the ingested protein dose normalized to body mass and analyzed with bi-phase linear regression. With the slope of the second portion of the bi-phase linear regression constrained to 0, the mean (95% CI) protein intake required to maximize postprandial MyoPS (over 0–360 min) was determined by breakpoint analysis. Myofibrillar and mitochondrial l-[1-13C]-phenylalanine enrichments (MPE) were analyzed using a 2-factor (treatment × time) repeated-measures ANOVA. The t = 0 time point was included as a repeated measure in the ANOVA models. A power calculation was performed based on previous studies (26, 27, 31) with differences in postprandial myofibrillar protein FSR as the primary outcome measure, using an SD of 0.0065%/h in all treatments and a difference in FSR of 0.008%/h between treatments (or ∼20% when expressed as the relative difference between treatments). With a power of 80%, a significance level of 0.05, and accounting for a dropout rate of 10% during testing, the final number of participants to be included was calculated as n = 12 per group. Assumptions of the statistical methods were assessed using Levene's test (for 1-factor ANOVA), Mauchley's test, and the D'Agostino–Pearson omnibus normality test at a significance of P < 0.05. If a significant Levene's test was determined, Welch's ANOVA was used. If a significant Mauchley's test was determined, the Greenhouse–Geisser correction factor was used to adjust the df accordingly. For data that did not pass the normality test, values were transformed with the ln or square root of the value. The statistical analysis was performed on transformed data, but nontransformed data are presented in graphical or tabular form for clarity. Significant main effects and interactions from ANOVA testing were further analyzed using Tukey's post hoc test to isolate specific differences between means. Statistical analyses were performed with the software packages IBM SPSS Statistics for Windows version 21.0 (IBM Corp.) and GraphPad Prism version 6 (GraphPad Software). Means were considered to be significantly different for P values < 0.05.
Results
Participants’ characteristics
There were no significant differences between treatment groups for any of the participants’ characteristics (Table 1). Results of the 1-factor (treatment) ANOVA for BMI were significant at P = 0.04; however, none of the treatment groups were significantly different (all comparisons showed P > 0.05) based on Tukey's post hoc analysis.
Dietary intake
There were no significant differences between treatment groups for any of the dietary intake data (Table 2).
Plasma concentrations
Plasma glucose concentrations (Figure 2A) increased after beverage intake (interaction: P < 0.001) and were higher in 0 g PRO than in both 45 g PRO and 30 g PRO at 40–60 min after beverage intake, respectively. Plasma glucose concentrations were higher in 45 g PRO than in both 0 g PRO and 30 g PRO at 120–180 min after beverage intake and were also higher than in 15 g PRO at 180 min. Plasma insulin concentrations (Figure 2B) increased after beverage intake (interaction: P < 0.001) and were significantly higher in 30 g PRO than in 0 g PRO at 20–120 min after beverage intake. Similarly, plasma insulin concentrations in 45 g PRO were significantly higher than in 0 g PRO and 15 g PRO at 40–180 min and 60–120 min after beverage intake, respectively. Plasma leucine concentrations (Figure 3A) rapidly increased in response to the ingestion of protein-containing beverages, displaying maximum concentrations of 221 ± 10, 359 ± 12, and 402 ± 16 μmol/L in 15 g PRO, 30 g PRO, and 45 g PRO, respectively (45 g PRO > 30 g PRO > 15 g PRO > 0 g PRO; P < 0.001). Plasma leucine concentrations were significantly (interaction: P < 0.001) higher in 45 g PRO than in 15 g PRO and 30 g PRO at 20–300 min and at 120–240 min after beverage intake, respectively. Similarly, plasma leucine concentrations were significantly higher in 30 g PRO than in 15 g PRO at 20–180 min after beverage intake. Ingestion of protein-containing beverages resulted in plasma leucine concentrations that were significantly higher than 0 g PRO, with a less protracted increase in plasma leucine concentrations in 15 g PRO (at 20–240 min) than in 30 g PRO and 45 g PRO (at 20–360 min). Plasma phenylalanine concentrations (Figure 3B) rapidly increased in response to the ingestion of protein-containing beverages and were significantly (interaction: P < 0.001) higher in 45 g PRO than in both 15 g PRO and 30 g PRO at 20–180 min and at 150–240 min after beverage intake, respectively. Similarly, plasma phenylalanine concentrations were significantly higher in 30 g PRO than in 15 g PRO at 20–120 min after beverage intake. Ingestion of protein-containing beverages resulted in plasma phenylalanine concentrations that were significantly higher than 0 g PRO, with a less protracted increase in 15 g PRO (at 20–120 min) than in 30 g PRO (at 20–150 min) and 45 g PRO (at 20–180 min). Plasma tyrosine concentrations (Figure 3C) rapidly increased in response to the ingestion of protein-containing beverages and were significantly (interaction: P < 0.001) higher in 45 g PRO than in both 15 g PRO and 30 g PRO at 20–360 min and at 120–360 min after beverage intake, respectively. Similarly, plasma tyrosine concentrations were significantly higher in 30 g PRO than in 15 g PRO at 20–240 min after beverage intake. Ingestion of protein-containing beverages resulted in plasma tyrosine concentrations that were significantly higher than 0 g PRO, with a less protracted increase in 15 g PRO (at 20–180 min) than in 30 g PRO and 45 g PRO (at 20–360 min).
FIGURE 2.
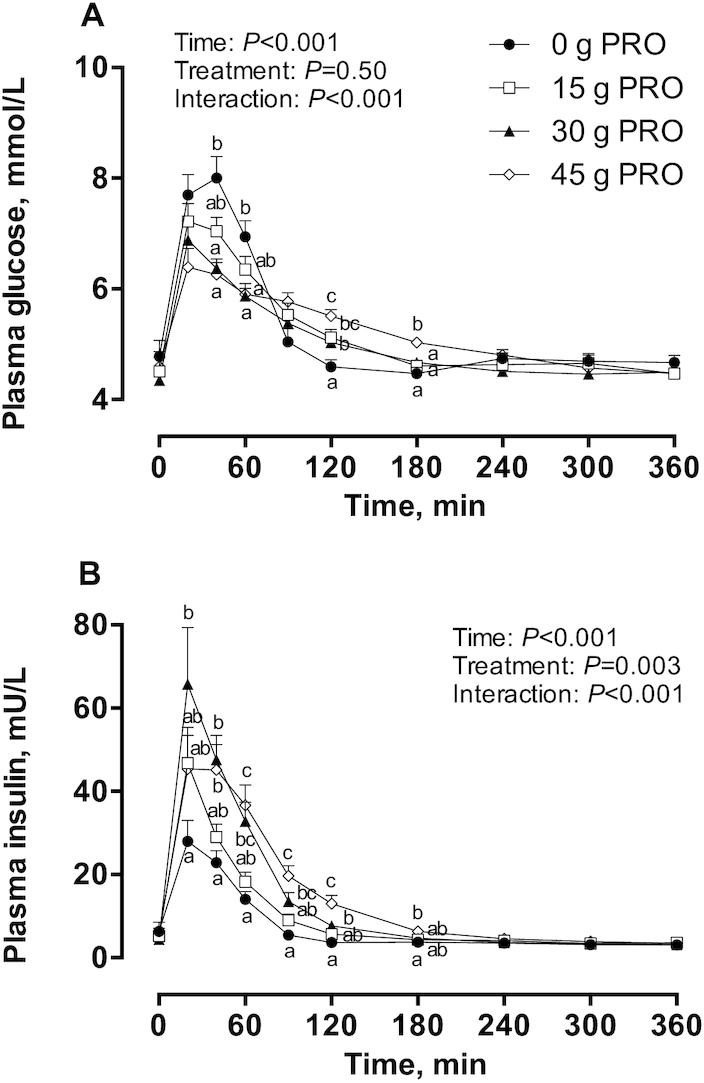
Plasma glucose (A) and insulin (B) concentrations during postabsorptive conditions (t = 0 min) and during postprandial conditions (t = 20–360 min) after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. Data for glucose and insulin were analyzed by a 2-factor repeated-measures ANOVA. Tukey post hoc testing was used to detect differences between groups. Labeled means within a time without a common letter differ, P < 0.05. 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
FIGURE 3.

Plasma leucine (A), phenylalanine (B), and tyrosine (C) concentrations during postabsorptive conditions (t = 0 min) and during postprandial conditions (t = 20–360 min) after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. Data for plasma leucine, phenylalanine, and tyrosine were analyzed by a 2-factor repeated-measures ANOVA. Tukey post hoc testing was used to detect differences between groups. Labeled means within a time without a common letter differ, P < 0.05. 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
Plasma stable isotope tracer enrichments
Plasma l-[1-13C]-leucine (Figure 4A) enrichments (MPE) were increased after beverage intake and were significantly (interaction: P < 0.001) higher in 45 g PRO than in both 0 g PRO and 15 g PRO at 20–360 min, and 30 g PRO at 150–240 min. Similarly, plasma l-[1-13C]-leucine enrichments were significantly higher in 30 g PRO than in both 0 g PRO and 15 g PRO at 20–180 min, respectively. Plasma l-[1-13C]-leucine enrichments were also significantly higher in 15 g PRO than in 0 g PRO at 20–180 min after beverage intake. Plasma l-[ring-2H5]-phenylalanine (Figure 4B) enrichments were reduced in response to the ingestion of protein-containing beverages, being significantly (interaction: P < 0.001) lower in 45 g PRO than in 0 g PRO and 15 g PRO at 20–180 min and at 40–150 min after beverage intake, respectively. Similarly, plasma l-[ring-2H5]-phenylalanine enrichments were significantly lower in 30 g PRO than in 0 g PRO and 15 g PRO at 20–150 min and 20–120 min after beverage intake, respectively. Plasma l-[ring-2H5]-phenylalanine enrichments were significantly lower in 15 g PRO than in 0 g PRO at 20–120 min after beverage intake. Plasma l-[1-13C]-phenylalanine (Figure 4C) enrichments were increased in response to the ingestion of protein-containing beverages and were significantly (interaction: P < 0.001) higher in 45 g PRO than in 0 g PRO and 15 g PRO at 20–360 min, and higher than in 30 g PRO at 120–360 min. Similarly, plasma l-[1-13C]-phenylalanine enrichments were significantly higher in 30 g PRO than in 0 g PRO and 15 g PRO at 20–360 min. Plasma l-[1-13C]-phenylalanine enrichments were significantly higher in 15 g PRO than in 0 g PRO at 20–360 min after beverage intake.
FIGURE 4.

Plasma l-[1-13C]-leucine (A), l-[ring-2H5]-phenylalanine (B), and l-[1-13C]-phenylalanine (C) enrichments (MPE) during postabsorptive conditions (t = 0 min) and during postprandial conditions (t = 20–360 min) after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. Data for plasma l-[1-13C]-leucine, l-[ring-2H5]-phenylalanine, and l-[1-13C]-phenylalanine enrichments were analyzed by a 2-factor repeated-measures ANOVA. Tukey post hoc testing was used to detect differences between groups. Labeled means within a time without a common letter differ, P < 0.05. MPE, mole percentage excess; 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
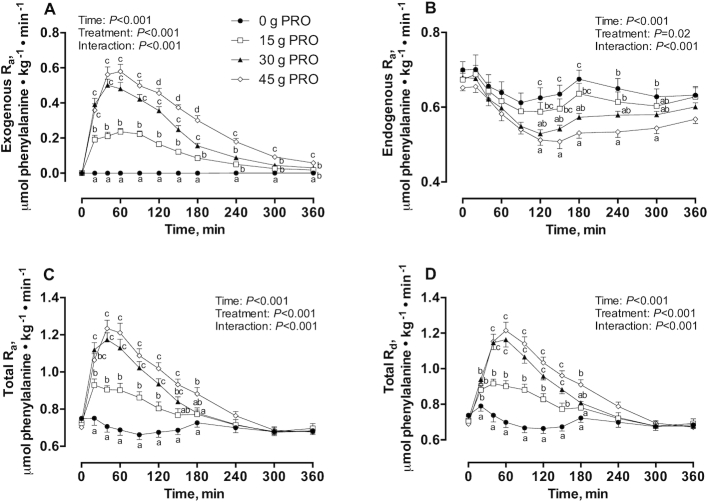
Whole-body phenylalanine kinetics
Exogenous phenylalanine Ra (Figure 5A) rapidly increased in a dose-dependent manner after the ingestion of protein-containing beverages. Exogenous phenylalanine Ra were significantly (interaction: P < 0.001) higher in 45 g PRO than in 0 g PRO and 15 g PRO at 20–360 min, and were higher than in 30 g PRO at 120–360 min. Similarly, exogenous phenylalanine Ra were significantly higher in 30 g PRO than in 0 g PRO and 15 g PRO at 20–360 min and 20–180 min, respectively, and higher in 15 g PRO than in 0 g PRO at 20–360 min after beverage intake. Endogenous phenylalanine Ra (Figure 5B) decreased after beverage intake (interaction: P < 0.001) and were lower in 45 g PRO than in 0 g PRO at 120–300 min after beverage intake. Endogenous phenylalanine Ra were also reduced in 45 g PRO compared with 15 g PRO at 120–240 min. Similarly, endogenous phenylalanine Ra were significantly reduced in 30 g PRO compared with 0 g PRO at 120–180 min after beverage intake. Total phenylalanine Ra (Figure 5C) increased after the ingestion of protein-containing beverages and were significantly (interaction: P < 0.001) higher in 45 g PRO than in 0 g PRO and 15 g PRO at 20–180 min and 40–180 min after beverage intake, respectively. Similarly, total phenylalanine Ra were significantly higher in 30 g PRO than in 0 g PRO and 15 g PRO at 20–150 min and 20–120 min, respectively, whereas 15 g PRO were higher than 0 g PRO at 20–120 min after beverage intake. Total phenylalanine Rd (Figure 5D) increased after the ingestion of protein-containing beverages and were significantly (interaction: P < 0.001) higher in 45 g PRO than in 0 g PRO at 20–180 min and in 15 g PRO at 40–180 min into the postprandial period. Similarly, total phenylalanine Rd were significantly higher in 30 g PRO than in 0 g PRO and 15 g PRO at 20–150 min and at 40–150 min after beverage intake, respectively. Total phenylalanine Rd were also higher in 15 g PRO than in 0 g PRO at 20–150 min after beverage intake. Dietary protein–derived plasma phenylalanine availability calculated over 360 min postexercise recovery averaged 74.2% ± 3.1%, 73.9% ± 1.2%, and 70.2% ± 1.5% in 15 g PRO, 30 g PRO, and 45 g PRO, respectively, and was not different between groups (P = 0.33).
FIGURE 5.
Whole-body phenylalanine kinetics. Exogenous Ra (A), endogenous Ra (B), total Ra (C), and total Rd (D) (μmol phenylalanine · kg−1 · min−1) during postabsorptive conditions (t = 0 min) and during postprandial conditions (t = 20–360 min) after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. Data for exogenous Ra, endogenous Ra, total Ra, and total Rd were analyzed by a 2-factor repeated-measures ANOVA. Tukey post hoc testing was used to detect differences between groups. Labeled means within a time without a common letter differ, P < 0.05. Ra, rate of appearance; Rd, rate of disappearance; 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
Whole-body protein metabolism
Figure 6A presents whole-body protein metabolism (breakdown, synthesis, oxidation, and net balance) assessed during early (0–180 min) and late (180–360 min) postexercise recovery. Whole-body protein breakdown rates were lower in 45 g PRO than in 0 g PRO (treatment: P < 0.01). Whole-body protein synthesis rates were higher in 15 g PRO, 30 g PRO, and 45 g PRO than in 0 g PRO at 0–180 min but were not different between treatment groups at 180–360 min (interaction: P < 0.001). Whole-body protein oxidation rates were increased in response to protein ingestion over 0–180 min but were not different between treatment groups over 180–360 min of postexercise recovery (interaction: P < 0.001). Whole-body net protein balance was negative in 0 g PRO but was positive in response to the ingestion of protein-containing treatments over both 0–180 min and 180–360 min of postexercise recovery. The positive whole-body net protein balance displayed a clear dose–response relation over 0–180 min, with 45 g PRO > 30 g PRO > 15 g PRO (interaction: P < 0.001). Figure 6B presents whole-body protein metabolism assessed over the entire 0–360 min postexercise recovery period. Whole-body protein breakdown rates were lower in 45 g PRO than in 0 g PRO (P < 0.01). Whole-body protein synthesis rates were higher in 30 g PRO and 45 g PRO than in 0 g PRO (all P < 0.001). Whole-body protein oxidation rates were higher in 15 g PRO than in 0 g PRO (P = 0.021), and higher in 30 g PRO and 45 g PRO than in both 0 g PRO (all P < 0.001) and 15 g PRO (all P < 0.01). Whole-body net protein balance was negative in 0 g PRO but was positive in response to the ingestion of protein-containing beverages. The positive whole-body net protein balance displayed a clear dose–response relation, with 45 g PRO > 30 g PRO > 15 g PRO (all P < 0.001).
FIGURE 6.

Whole-body protein metabolism (B, S, O, and NB; μmol phenylalanine · kg−1 · h−1) over 0–180 and 180–360 min (A) and over 0–360 min (B) after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. (A) Time-course data were analyzed by a 2-factor repeated-measures ANOVA. (B) Aggregate data were analyzed by a 1-factor ANOVA. Tukey post hoc testing was used to detect differences between groups. Within each outcome measure, labeled means within a time without a common letter differ, P < 0.05. B, breakdown; NB, net balance; O, oxidation; S, synthesis; 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
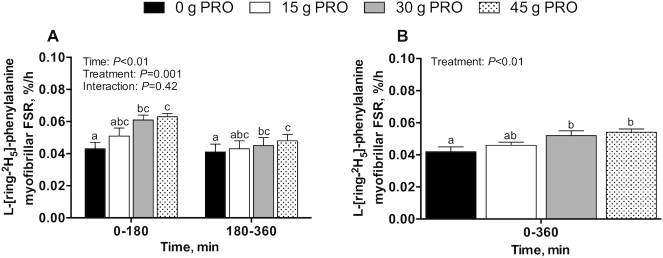
Myofibrillar FSRs, bi-phase linear regression and breakpoint analysis, and protein-bound enrichments
Postprandial l-[1-13C]-leucine MyoPS rates, assessed during early (0–180 min) and late (180–360 min) postexercise recovery (Figure 7A), were significantly higher in 30 g PRO (P < 0.01) and 45 g PRO (P < 0.001) than in 0 g PRO. Postprandial l-[1-13C]-leucine MyoPS rates assessed over the entire 0–360 min postexercise recovery period (Figure 7B) averaged 0.046 ± 0.004%/h in 0 g PRO, 0.054 ± 0.004%/h in 15 g PRO, 0.067 ± 0.003%/h in 30 g PRO, and 0.070 ± 0.004%/h in 45 g PRO (P < 0.001). Post hoc analysis revealed that MyoPS rates were higher in 30 g PRO (P < 0.01) and 45 g PRO (P < 0.001) than in 0 g PRO but were not significantly different from each other (P = 0.91). MyoPS rates were not higher in 15 g PRO than in 0 g PRO (P = 0.42). Bi-phase linear regression model characteristics (Figure 8) were as follows: mean (95% CI) slope (%/h per g/kg): 0.05 (0.024, 0.077); breakpoint (g/kg): 0.49 (0.26, 0.72); goodness of fit (r2): 0.37; df: 45. Postprandial l-[ring-2H5]-phenylalanine MyoPS rates, assessed during early (0–180 min) and late (180–360 min) postexercise recovery (Figure 9A), were significantly higher in both 30 g PRO (P = 0.01) and 45 g PRO (P = 0.001) than in 0 g PRO. Postprandial l-[ring-2H5]-phenylalanine MyoPS rates assessed over the entire 0–360 min postexercise recovery period (Figure 9B) averaged 0.042 ± 0.003%/h in 0 g PRO, 0.046 ± 0.002%/h in 15 g PRO, 0.052 ± 0.003%/h in 30 g PRO, and 0.054 ± 0.002%/h in 45 g PRO (P < 0.01). Post hoc analysis revealed that MyoPS rates were higher in 30 g PRO (P < 0.05) and 45 g PRO (P < 0.01) than in 0 g PRO but were not different from each other (P = 0.91). MyoPS rates were not higher in 15 g PRO than in 0 g PRO (P = 0.63). The incorporation of dietary protein–derived amino acids (l-[1-13C]-phenylalanine enrichments) into de novo myofibrillar protein was 0.000 ± 0.001 MPE in 0 g PRO, 0.011 ± 0.002 MPE in 15 g PRO, 0.025 ± 0.002 MPE in 30 g PRO, and 0.027 ± 0.002 MPE in 45 g PRO during 0–180 min after beverage intake. During 0–360 min after beverage intake, the incorporation of l-[1-13C]-phenylalanine into de novo myofibrillar protein was 0.001 ± 0.001 MPE in 0 g PRO, 0.013 ± 0.002 MPE in 15 g PRO, 0.032 ± 0.002 MPE in 30 g PRO, and 0.042 ± 0.002 MPE in 45 g PRO (interaction: P < 0.001) (Figure 10). Post hoc analysis revealed that l-[1-13C]-phenylalanine myofibrillar protein-bound enrichments were higher in 15 g PRO (P = 0.001), 30 g PRO (P < 0.001), and 45 g PRO (P < 0.001) than in 0 g PRO over both 0–180 min and 0–360 min (all P < 0.001) after beverage intake. Similarly, l-[1-13C]-phenylalanine myofibrillar protein-bound enrichments were higher in 30 g PRO and 45 g PRO than in 15 g PRO over both 0–180 min (all P < 0.001) and 0–360 min (all P < 0.001) after beverage intake. l-[1-13C]-phenylalanine myofibrillar protein-bound enrichments were higher in 45 g PRO than in 30 g PRO over 0–360 min (P < 0.01) after beverage intake.
FIGURE 7.
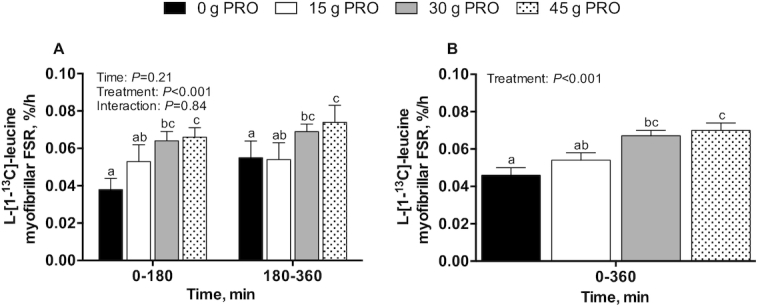
l-[1-13C]-leucine-determined myofibrillar FSR over 0–180 and 180–360 min (A) and over 0–360 min (B) after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. (A) Time-course data were analyzed by a 2-factor repeated-measures ANOVA. (B) Aggregate data were analyzed by a 1-factor ANOVA. Tukey post hoc testing was used to detect differences between groups. Labeled means without a common letter differ, P < 0.05. FSR, fractional synthesis rate; 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
FIGURE 8.

l-[1-13C]-leucine-determined myofibrillar FSR over 0–360 min after beverage intake during recovery from a single bout of endurance exercise in young men plotted against the ingested protein dose normalized to body mass. Values represent individual participant data, mean, and 95% CI, n = 48. Data were analyzed by bi-phase linear regression to determine the mean (95% CI) protein intake to maximize postprandial myofibrillar protein synthesis as determined by breakpoint analysis. FSR, fractional synthesis rate.
FIGURE 9.
l-[ring-2H5]-phenylalanine-determined myofibrillar FSR over 0–180 and 180–360 min (A) and over 0–360 min (B) after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. (A) Time-course data were analyzed by a 2-factor repeated-measures ANOVA. (B) Aggregate data were analyzed by a 1-factor ANOVA. Tukey post hoc testing was used to detect differences between groups. Labeled means without a common letter differ, P < 0.05. FSR, fractional synthesis rate; 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
FIGURE 10.
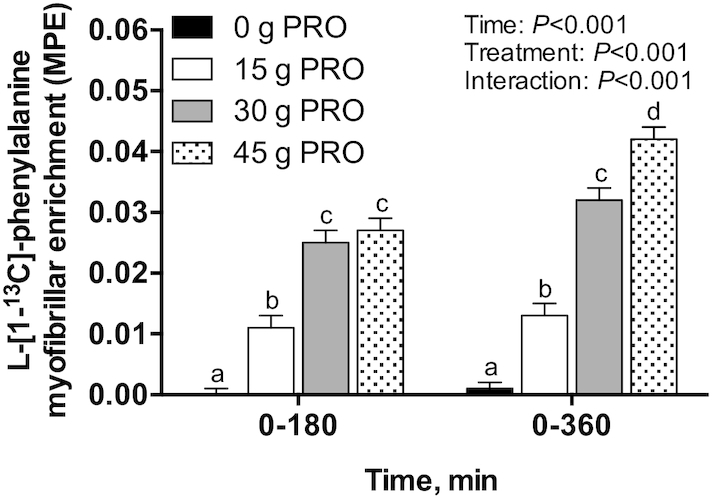
Change in l-[1-13C]-phenylalanine enrichment (MPE) in myofibrillar protein over 0–180 and 0–360 min after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. Data were analyzed by a 2-factor repeated-measures ANOVA. Tukey post hoc testing was used to detect differences between groups. Labeled means within a time without a common letter differ, P < 0.05. MPE, mole percentage excess; 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
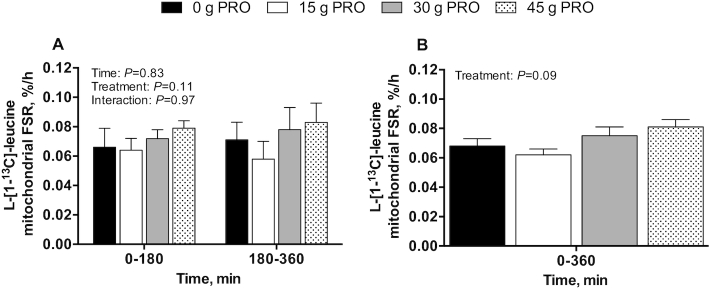
Mitochondrial FSRs and protein-bound enrichments
Postprandial l-[1-13C]-leucine MitoPS rates, assessed during early (0–180 min) and late (180–360 min) postexercise recovery (Figure 11A), did not differ between groups (P = 0.11). Similarly, MitoPS rates over the entire 360-min postexercise recovery period (Figure 11B) did not differ between groups (P = 0.09). Postprandial l-[ring-2H5]-phenylalanine MitoPS rates were not analyzed because all of the available mitochondrial sample was utilized to measure mitochondrial protein-bound l-[1-13C]-leucine and l-[1-13C]-phenylalanine enrichments. The incorporation of dietary protein–derived amino acids (l-[1-13C]-phenylalanine enrichments) into de novo mitochondrial protein was 0.001 ± 0.002 MPE in 0 g PRO, 0.013 ± 0.002 MPE in 15 g PRO, 0.024 ± 0.003 MPE in 30 g PRO, and 0.029 ± 0.003 MPE in 45 g PRO over 0–180 min, and 0.002 ± 0.002 MPE in 0 g PRO, 0.018 ± 0.002 MPE in 15 g PRO, 0.034 ± 0.002 MPE in 30 g PRO, and 0.046 ± 0.003 MPE in 45 g PRO over 0–360 min after beverage intake (interaction: P = 0.001) (Figure 12). Post hoc analysis revealed that l-[1-13C]-phenylalanine mitochondrial protein-bound enrichments were higher in 15 g PRO (P = 0.02), 30 g PRO (P < 0.001), and 45 g PRO (P < 0.001) than in 0 g PRO over 0–180 min after beverage intake. l-[1-13C]-phenylalanine mitochondrial protein-bound enrichments were also higher in 15 g PRO, 30 g PRO, and 45 g PRO than in 0 g PRO over 0–360 min after beverage intake (all P < 0.001). Similarly, l-[1-13C]-phenylalanine mitochondrial protein-bound enrichments were higher in 30 g PRO ( P = 0.01) and 45 g PRO ( P < 0.001) than in 15 g PRO over 0–180 min and 0–360 min after beverage intake, respectively (all P < 0.001). l-[1- 13C]-phenylalanine mitochondrial protein-bound enrichments were higher in 45 g PRO than in 30 g PRO over 0–360 min ( P < 0.01) after beverage intake.
FIGURE 11.
l-[1-13C]-leucine-determined mitochondrial FSR over 0–180 and 180–360 min (A) and over 0–360 min (B) after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. (A) Time-course data were analyzed by a 2-factor repeated-measures ANOVA. (B) Aggregate data were analyzed by a 1-factor ANOVA. FSR, fractional synthesis rate; 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
FIGURE 12.
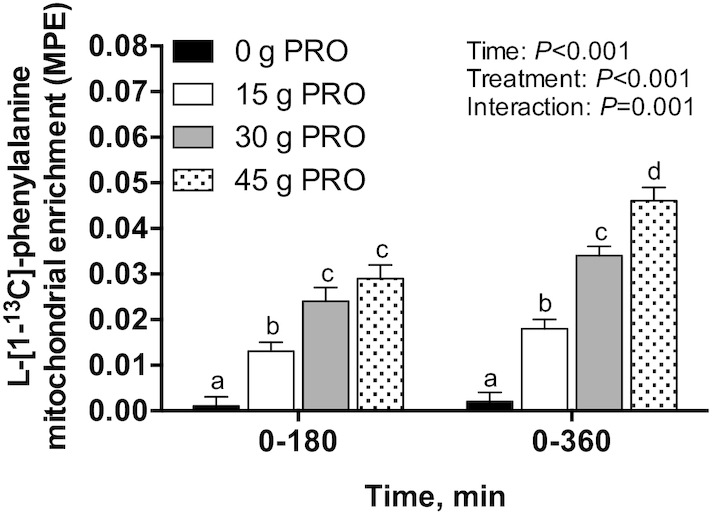
Change in l-[1-13C]-phenylalanine enrichment (MPE) in mitochondrial protein over 0–180 and 0–360 min after beverage intake during recovery from a single bout of endurance exercise in young men. Values represent means ± SEMs, n = 12 per group. Data were analyzed by a 2-factor repeated-measures ANOVA. Tukey post hoc testing was used to detect differences between groups. Labeled means within a time without a common letter differ, P < 0.05. MPE, mole percentage excess; 0 g PRO, 45 g carbohydrate co-ingested with 0 g protein; 15 g PRO, 45 g carbohydrate co-ingested with 15 g protein; 30 g PRO, 45 g carbohydrate co-ingested with 30 g protein; 45 g PRO, 45 g carbohydrate co-ingested with 45 g protein.
Discussion
In the present study, we assessed postprandial protein digestion and amino acid absorption kinetics, whole-body protein metabolism, and both MyoPS and MitoPS rates after co-ingestion of different quantities of milk protein (0, 15, 30, or 45 g) with carbohydrate (45 g) after a bout of endurance exercise in endurance-trained young men. Protein co-ingested with carbohydrate was efficiently digested and absorbed, with ∼70%–74% of protein-derived phenylalanine appearing in the circulation over 360 min of postexercise recovery. Whole-body net protein balance was positive only after protein co-ingestion, and greatest after ingestion of 45 g protein. Alternatively, MyoPS rates over 360 min of postexercise recovery were maximally stimulated after ingestion of 30 g protein (or a mean of 0.49 g protein/kg). Protein co-ingestion did not result in higher MitoPS rates but ingesting greater amounts of protein allowed more dietary protein–derived phenylalanine to be directed toward de novo MyoPS and MitoPS.
This study is the first to our knowledge to assess dietary protein digestion and amino acid absorption after the ingestion of different quantities of protein during recovery from endurance exercise. Protein co-ingestion resulted in a rapid increase in plasma amino acid concentrations (Figure 3A–C) and l-[1-13C]-phenylalanine enrichments (Figure 4C), demonstrating rapid protein digestion and absorption of dietary protein–derived phenylalanine. An estimated 11.1 ± 0.5 g, 22.2 ± 0.4 g, and 31.6 ± 0.7 g protein-derived amino acids became available in the circulation over 360 min after the ingestion of 15, 30, and 45 g protein. Altogether, our data demonstrate that ingestion of greater quantities of protein results in greater exogenous plasma amino acid availability and higher peak leucine concentrations during recovery from endurance exercise, both of which are important in the regulation of whole-body and muscle protein synthesis rates with feeding.
In the present study, whole-body net protein balance during postexercise recovery was negative in 0 g PRO but positive after protein co-ingestion (Figure 6A, B). These results are congruent with studies demonstrating that protein intake during and/or after endurance exercise leads to a positive whole-body net protein balance during postexercise recovery (8, 15, 32, 33). Our results also demonstrate the presence of a dose–response relation between protein ingestion and whole-body net protein balance during recovery from endurance exercise. This finding aligns with previous work demonstrating that whole-body net protein balance increases more when greater amounts of protein are ingested either at rest (31, 34, 35) or after resistance exercise (25, 35). The amount of protein required to maximize whole-body net protein balance during recovery from endurance exercise is unclear. Mazzulla et al. (33) reported that whole-body net protein balance reached a plateau in response to increasing protein intakes and may be maximized in response to ∼0.15 g protein · kg−1 · h−1 in young men after exercise. In the present study, subjects in 45 g PRO received ∼0.58 g protein/kg or ∼0.097 g protein · kg−1 · h−1 over 360 min of postexercise recovery. Although this amount is below the ∼0.15 g protein · kg−1 · h−1 that may maximize whole-body net protein balance during recovery from endurance exercise, any increase in whole-body net protein balance that may have been achieved by ingesting greater quantities of dietary protein would likely be attributed to an enhanced retention of dietary protein–derived amino acids within nonmuscle (e.g., splanchnic) tissue (36). Because skeletal muscle accounts for only 25%–30% of whole-body protein turnover (37), changes in protein metabolism at the whole-body level are not necessarily reflective of the skeletal muscle protein synthesis or proteolytic response to feeding.
We combined the ingestion of l-[1-13C]-leucine-labeled milk protein with a continuous intravenous infusion of l-[1-13C]-leucine to assess both MyoPS and MitoPS rates during recovery from endurance exercise (Figures 7, 11A, B). In agreement with previous studies (8, 13–17), protein intake enhanced MyoPS rates during recovery from endurance exercise when compared with 0 g PRO. In the present study, 15 g PRO resulted in l-[1-13C]-leucine MyoPS rates that were ∼17% higher than 0 g PRO over 360 min of recovery. However, 30 g PRO and 45 g PRO resulted in MyoPS rates that were ∼46% and ∼52% greater than 0 g PRO, respectively. Whereas studies conducted within the context of resistance exercise demonstrate that mixed MPS (26) and MyoPS (27) rates are maximized after the ingestion of 20 g protein in young men, our data suggest that 30 g is sufficient to maximize MyoPS rates after endurance exercise in young men. The mean relative protein requirement to maximally stimulate MyoPS rates (Figure 8) during recovery from endurance exercise in the current study (∼0.49 g protein/kg) also appears greater than that recently reported for resistance exercise (∼0.31 g protein/kg) (38). The reason for the potentially greater protein requirement after endurance exercise is unclear but may relate to the need to remodel both muscle and splanchnic proteins owing to increased protein breakdown (39), and to replace exercise-induced amino acid losses incurred via direct oxidation (7, 40) and/or hepatic gluconeogenesis (41).
In the present study, protein co-ingestion did not result in greater MitoPS rates than 0 g PRO (Figure 11A, B). MitoPS rates increase in response to intravenous infusion of amino acids (19, 20), and the ingestion of larger (i.e., 36 g) (21), but not smaller (i.e., 18 g) (18) doses of protein at rest. Our finding that protein co-ingestion did not result in greater MitoPS rates than 0 g PRO aligns with previous studies that reported no further increase in MitoPS rates after protein intake compared with a nonprotein control during recovery from endurance exercise (13, 14) or combined endurance and resistance exercise (22–24). Studies to date examining the impact of protein ingestion on MitoPS rates after an acute bout of endurance exercise or combined endurance and resistance exercise have provided protein doses < 30 g (13, 14, 18, 22–24). Our results show that even relatively large doses of protein (45 g or ∼0.58 g protein/kg) do not increase MitoPS rates more than the ingestion of carbohydrate only during the first few hours of recovery from a single bout of endurance exercise.
Participants in our study ingested protein intrinsically labelled with l-[1-13C]-phenylalanine at a very high enrichment (∼38 MPE), allowing us to directly assess the metabolic fate of ingested protein-derived phenylalanine. Previous studies from our laboratory (25, 42, 43) have shown that l-[1-13C]-phenylalanine from ingested protein is used for de novo MyoPS during recovery from resistance exercise. In the present study, l-[1-13C]-phenylalanine from the ingested protein was used for de novo MyoPS during recovery from endurance exercise (Figure 10). Furthermore, there was a dose-dependent increase in dietary protein–derived l-[1-13C]-phenylalanine incorporation into myofibrillar protein over 0–360 min after exercise. The present data are the first, as far as we know, to evaluate whether ingested protein is used for de novo MitoPS during recovery from exercise. Our data show that l-[1-13C]-phenylalanine from ingested protein is used for de novo MitoPS during recovery from endurance exercise, and that ingestion of greater amounts of protein results in a greater increase in dietary protein–derived l-[1-13C]-phenylalanine incorporation into mitochondrial protein (Figure 12). Therefore, amino acids from ingested protein contribute to mitochondrial protein remodeling after endurance exercise.
The present study evaluated the dose–response relation of MyoPS and MitoPS during recovery from a bout of endurance exercise after the ingestion of nutritional treatments differing in energy and protein content by participants in the overnight postabsorptive state. Differences in the energy content of the nutritional treatments would not be expected to influence the stimulation of postprandial MyoPS rates because previous studies have shown that co-ingesting additional energy with isolated protein does not further increase postprandial MPS rates when compared with the ingestion of protein or amino acids only (44–46). Although providing protein after exercise performed in the overnight postabsorptive state allowed us to isolate the effects of our nutritional treatments on whole-body and skeletal muscle protein metabolic responses, individuals typically undertake exercise in the fed state and may ingest carbohydrate and/or protein during exercise. Given that there appears to be little difference in the dose–response relation between ingested protein and MPS during recovery from resistance exercise in young men examined in the overnight postabsorptive state (26) or ∼3 h after a mixed-macronutrient energy-rich breakfast (27), our results may also translate to individuals who undertake exercise in the fed state. An additional consideration is whether the current results, obtained utilizing milk protein, may also translate to the use of other high-quality dietary proteins. Given the results of our recent work demonstrating no differences in postprandial MyoPS or MitoPS rates between the ingestion of milk protein, whey, and micellar casein (24), or whey, soy, and leucine-enriched soy protein (23) with carbohydrate during recovery from concurrent exercise, the current findings may extend to the use of other high-quality proteins in isolated or whole-food form. Finally, the current study included only men as research participants and involved testing of multiple outcomes. Because there is evidence for sex-based differences in protein metabolism within the context of endurance exercise (40, 47, 48), additional research is necessary to determine whether the current findings apply to endurance-trained women. The testing of multiple outcomes may have increased the risk of type I statistical errors.
In conclusion, dietary protein ingested after endurance exercise is efficiently digested and absorbed, with ∼70%–74% of the ingested protein-derived phenylalanine appearing in the circulation over 360 min of postexercise recovery. Whole-body net protein balance and dietary protein–derived amino acid incorporation into myofibrillar and mitochondrial protein respond to increasing protein intake in a dose-dependent manner. Whereas protein co-ingestion did not result in greater MitoPS rates than carbohydrate only, ingestion of 30 g of a high-quality protein (or a mean of ∼0.49 g protein/kg) is sufficient to maximize MyoPS rates after endurance exercise in young, endurance-trained men.
Supplementary Material
Acknowledgments
We thank Wouter M Peeters for his assistance with data collection during some of the experimental tests.
The authors’ responsibilities were as follows—TAC-V, IR, and LJCvL: conceived and designed the research; TAC-V, PJMP, JSJS, MWB, JMS, JPBG, and APG: conducted the research; TAC-V, PJMP, MWB, JPBG, APG, LBV, and LJCvL: analyzed the data; TAC-V and LJCvL: interpreted the results of the experiments, edited and revised the manuscript, and hold primary responsibility for the final content; TAC-V: prepared the figures and drafted the manuscript; and all authors: read and approved the final manuscript. IR is an employee of the Gatorade Sports Science Institute, a division of PepsiCo Inc. IR contributed to the study design of the project through regular discussion and review of the final manuscript but was not involved in the collection, analysis, or interpretation of the data. LJCvL and LBV have received research grants, consulting fees, speaking honoraria, or a combination of these, from Friesland Campina and PepsiCo. All other authors report no conflicts of interest.
Notes
Supported by PepsiCo/Gatorade Sports Science Institute (to LJCvL).
The views expressed in this manuscript are those of the authors and do not necessarily reflect the position or policy of PepsiCo Inc.
Supplemental Figure 1 and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article (in deidentified form), code book, and analytic code will be made available upon request pending application and approval from the corresponding author.
Abbreviations used: FSR, fractional synthesis rate; MitoPS, mitochondrial protein synthesis; MPC80, milk protein concentrate; MPE, mole percentage excess; MPS, muscle protein synthesis; MyoPS, myofibrillar protein synthesis; Ra, rate of appearance; Rd, rate of disappearance; V̇O2 peak, peak oxygen consumption; Wmax, maximal workload capacity; 0 g PRO, 45 g carbohydrate with 0 g protein; 15 g PRO, 45 g carbohydrate with 15 g protein; 30 g PRO, 45 g carbohydrate with 30 g protein; 45 g PRO, 45 g carbohydrate with 45 g protein.
Contributor Information
Tyler A Churchward-Venne, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
Philippe J M Pinckaers, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
Joey S J Smeets, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
Milan W Betz, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
Joan M Senden, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
Joy P B Goessens, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
Annemie P Gijsen, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
Ian Rollo, Gatorade Sports Science Institute, Leicester, United Kingdom.
Lex B Verdijk, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
Luc J C van Loon, NUTRIM School of Nutrition and Translational Research in Metabolism, Department of Human Biology, Maastricht University Medical Center+, Maastricht, Netherlands.
References
- 1. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–82. [PubMed] [Google Scholar]
- 2. Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1254–62. [DOI] [PubMed] [Google Scholar]
- 3. Mascher H, Ekblom B, Rooyackers O, Blomstrand E. Enhanced rates of muscle protein synthesis and elevated mTOR signalling following endurance exercise in human subjects. Acta Physiol (Oxf). 2011;202:175–84. [DOI] [PubMed] [Google Scholar]
- 4. Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol. 1990;259:E470–6. [DOI] [PubMed] [Google Scholar]
- 5. Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–22. [DOI] [PubMed] [Google Scholar]
- 6. Di Donato DM, West DW, Churchward-Venne TA, Breen L, Baker SK, Phillips SM. Influence of aerobic exercise intensity on myofibrillar and mitochondrial protein synthesis in young men during early and late postexercise recovery. Am J Physiol Endocrinol Metab. 2014;306:E1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowtell JL, Leese GP, Smith K, Watt PW, Nevill A, Rooyackers O, Wagenmakers AJ, Rennie MJ. Effect of oral glucose on leucine turnover in human subjects at rest and during exercise at two levels of dietary protein. J Physiol. 2000;525(Pt 1):271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levenhagen DK, Carr C, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise protein intake enhances whole-body and leg protein accretion in humans. Med Sci Sports Exerc. 2002;34:828–37. [DOI] [PubMed] [Google Scholar]
- 9. Thomas DT, Erdman KA, Burke LM. American College of Sports Medicine joint position statement. Nutrition and athletic performance. Med Sci Sports Exerc. 2016;48:543–68. [DOI] [PubMed] [Google Scholar]
- 10. Reidy PT, Rasmussen BB. Role of ingested amino acids and protein in the promotion of resistance exercise–induced muscle protein anabolism. J Nutr. 2016;146:155–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 12. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, Baar K, Tipton KD. The influence of carbohydrate–protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. J Physiol. 2011;589:4011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, Phillips SM, Hawley JA. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints. Eur J Appl Physiol. 2011;111:1473–83. [DOI] [PubMed] [Google Scholar]
- 15. Howarth KR, Moreau NA, Phillips SM, Gibala MJ. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J Appl Physiol. 2009;106:1394–402. [DOI] [PubMed] [Google Scholar]
- 16. Lunn WR, Pasiakos SM, Colletto MR, Karfonta KE, Carbone JW, Anderson JM, Rodriguez NR. Chocolate milk and endurance exercise recovery: protein balance, glycogen, and performance. Med Sci Sports Exerc. 2012;44:682–91. [DOI] [PubMed] [Google Scholar]
- 17. Rowlands DS, Nelson AR, Phillips SM, Faulkner JA, Clarke J, Burd NA, Moore D, Stellingwerff T. Protein–leucine fed dose effects on muscle protein synthesis after endurance exercise. Med Sci Sports Exerc. 2015;47:547–55. [DOI] [PubMed] [Google Scholar]
- 18. Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, Philp A, Li Z, Paluska SA, Burd NA et al. Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise. Physiol Rep. 2018;6:e13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol. 2003;552:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beals JW, Mackenzie RWA, van Vliet S, Skinner SK, Pagni BA, Niemiro GM, Ulanov AV, Li Z, Dilger AC, Paluska SA et al. Protein-rich food ingestion stimulates mitochondrial protein synthesis in sedentary young adults of different BMIs. J Clin Endocrinol Metab. 2017;102:3415–24. [DOI] [PubMed] [Google Scholar]
- 22. Camera DM, West DW, Phillips SM, Rerecich T, Stellingwerff T, Hawley JA, Coffey VG. Protein ingestion increases myofibrillar protein synthesis after concurrent exercise. Med Sci Sports Exerc. 2015;47:82–91. [DOI] [PubMed] [Google Scholar]
- 23. Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Peeters WM, Zorenc AH, Schierbeek H, Rollo I, Verdijk LB, van Loon LJC. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with whey, soy, or leucine-enriched soy protein after concurrent resistance- and endurance-type exercise. J Nutr. 2019;149:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Peeters WM, Zorenc AH, Schierbeek H, Rollo I, Verdijk LB, van Loon LJC. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise. J Nutr. 2019;149:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holwerda AM, Paulussen KJM, Overkamp M, Goessens JPB, Kramer IF, Wodzig W, Verdijk LB, van Loon LJC. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. J Nutr. 2019;149:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89:161–8. [DOI] [PubMed] [Google Scholar]
- 27. Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr. 2014;99:86–95. [DOI] [PubMed] [Google Scholar]
- 28. van Loon LJ, Boirie Y, Gijsen AP, Fauquant J, de Roos AL, Kies AK, Lemosquet S, Saris WH, Koopman R. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci. 2009;92:4812–22. [DOI] [PubMed] [Google Scholar]
- 29. Pennings B, Pellikaan WF, Senden JM, van Vuuren AM, Sikkema J, van Loon LJ. The production of intrinsically labeled milk and meat protein is feasible and provides functional tools for human nutrition research. J Dairy Sci. 2011;94:4366–73. [DOI] [PubMed] [Google Scholar]
- 30. Abumrad NN, Rabin D, Diamond MP, Lacy WW. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981;30:936–40. [DOI] [PubMed] [Google Scholar]
- 31. Pennings B, Groen B, de Lange A, Gijsen AP, Zorenc AH, Senden JM, van Loon LJ. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302:E992–9. [DOI] [PubMed] [Google Scholar]
- 32. Koopman R, Pannemans DL, Jeukendrup AE, Gijsen AP, Senden JM, Halliday D, Saris WH, van Loon LJ, Wagenmakers AJ. Combined ingestion of protein and carbohydrate improves protein balance during ultra-endurance exercise. Am J Physiol Endocrinol Metab. 2004;287:E712–20. [DOI] [PubMed] [Google Scholar]
- 33. Mazzulla M, Volterman KA, Packer JE, Wooding DJ, Brooks JC, Kato H, Moore DR. Whole-body net protein balance plateaus in response to increasing protein intakes during post-exercise recovery in adults and adolescents. Nutr Metab (Lond). 2018;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kouw IWK, Holwerda AM, Trommelen J, Kramer IF, Bastiaanse J, Halson SL, Wodzig WKWH, Verdijk LB, van Loon LJC. Protein ingestion before sleep increases overnight muscle protein synthesis rates in healthy older men: a randomized controlled trial. J Nutr. 2017;147:2252–61. [DOI] [PubMed] [Google Scholar]
- 35. Kim IY, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, Wolfe RR. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am J Physiol Endocrinol Metab. 2016;310:E73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52:1377–85. [DOI] [PubMed] [Google Scholar]
- 37. Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol. 1988;254:E208–13. [DOI] [PubMed] [Google Scholar]
- 38. Moore DR. Maximizing post-exercise anabolism: the case for relative protein intakes. Front Nutr. 2019;6:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howarth KR, Phillips SM, MacDonald MJ, Richards D, Moreau NA, Gibala MJ. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J Appl Physiol. 2010;109:431–8. [DOI] [PubMed] [Google Scholar]
- 40. Lamont LS, McCullough AJ, Kalhan SC. Gender differences in leucine, but not lysine, kinetics. J Appl Physiol. 2001;91:357–62. [DOI] [PubMed] [Google Scholar]
- 41. Tarnopolsky M. Protein requirements for endurance athletes. Nutrition. 2004;20:662–8. [DOI] [PubMed] [Google Scholar]
- 42. Trommelen J, Holwerda AM, Kouw IW, Langer H, Halson SL, Rollo I, Verdijk LB, Van Loon LJ. Resistance exercise augments postprandial overnight muscle protein synthesis rates. Med Sci Sports Exerc. 2016;48:2517–25. [DOI] [PubMed] [Google Scholar]
- 43. Trommelen J, Kouw IWK, Holwerda AM, Snijders T, Halson SL, Rollo I, Verdijk LB, van Loon LJC. Presleep dietary protein-derived amino acids are incorporated in myofibrillar protein during postexercise overnight recovery. Am J Physiol Endocrinol Metab. 2018;314:E457–E67. [DOI] [PubMed] [Google Scholar]
- 44. Churchward-Venne TA, Snijders T, Linkens AM, Hamer HM, van Kranenburg J, van Loon LJ. Ingestion of casein in a milk matrix modulates dietary protein digestion and absorption kinetics but does not modulate postprandial muscle protein synthesis in older men. J Nutr. 2015;145:1438–45. [DOI] [PubMed] [Google Scholar]
- 45. Gorissen SH, Burd NA, Hamer HM, Gijsen AP, Groen BB, van Loon LJ. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin Endocrinol Metab. 2014;99:2250–8. [DOI] [PubMed] [Google Scholar]
- 46. Gorissen SHM, Burd NA, Kramer IF, van Kranenburg J, Gijsen AP, Rooyackers O, van Loon LJC. Co-ingesting milk fat with micellar casein does not affect postprandial protein handling in healthy older men. Clin Nutr. 2017;36:429–37. [DOI] [PubMed] [Google Scholar]
- 47. Phillips SM, Atkinson SA, Tarnopolsky MA, MacDougall JD. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J Appl Physiol. 1993;75:2134–41. [DOI] [PubMed] [Google Scholar]
- 48. McKenzie S, Phillips SM, Carter SL, Lowther S, Gibala MJ, Tarnopolsky MA. Endurance exercise training attenuates leucine oxidation and BCOAD activation during exercise in humans. Am J Physiol Endocrinol Metab. 2000;278:E580–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.