ABSTRACT
Background
Although previous work has shown that children with older siblings tend to have poorer diet quality, no study has directly compared diets of infant siblings.
Objective
The goals of this analysis were to examine birth-order differences in dietary intake between firstborn (FB) and secondborn (SB) siblings, and to determine whether a responsive parenting (RP) intervention modified birth-order effects on diet.
Methods
The Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) study randomly assigned first-time mothers to an RP intervention, which included guidance on feeding, sleep, soothing, and interactive play, or control. INSIGHT mothers who delivered a second child enrolled in an observation-only study of their SB infant (SIBSIGHT). Mothers completed FFQs for both children at ages 6 (n = 97 sibling pairs) and 12 (n = 100) mo. FB compared with SB intake of food groups of interest were compared, and the moderating effect of the RP intervention on birth-order differences was tested using generalized linear mixed models.
Results
Though FBs and SBs had similar diets, more FBs than SBs consumed 100% fruit juice at both 6 (13.8 compared with 3.2%, P = 0.006) and 12 mo (46.0 compared with 32.0%, P = 0.01). SBs consumed fruit more frequently (FB 2.8 compared with SB 3.2 times/d, P = 0.01), and were more likely to consume fried potatoes (FB 38.4 compared with SB 57.6%, P = 0.0009) and processed meats (FB 43.0 compared with SB 58.0%, P = 0.02) than FBs at 12 mo. There were no differences by birth order in intake of sweets, snacks, or sugar-sweetened beverages at 12 mo. At 12 mo, RP-group SBs ate vegetables more times per day (3.2) than control SBs (2.2, P = 0.01). RP-SBs also consumed a greater variety of vegetables (10.2) than control-SBs (7.9, P = 0.01).
Conclusions
Birth order is not consistently associated with healthy or unhealthy infant dietary intake. However, an RP intervention delivered to first-time mothers may benefit subsequent infants’ vegetable intake. This trial was registered at clinicaltrials.gov as NCT01167270.
Keywords: infant, dietary intake, siblings, birth order, responsive parenting
Introduction
Dietary intake in infancy shapes dietary patterns and food preferences throughout the life course (1, 2). Exposure to a variety of fruits and vegetables in infancy promotes acceptance, liking, and intake of these foods (3, 4). Similarly, intake of energy-dense foods [e.g. sugar-sweetened beverages (SSBs) and foods high in added sugar or fat] in infancy is associated with liking and intake of these foods later in childhood (4, 5). However, most infants and toddlers consume less than optimal diets. Data from the Feeding Infants and Toddlers Study (FITS) 2016 found that among children aged 6–24 mo, ∼1 in 5 children did not consume any fruit and ∼1 in 4 did not consume a vegetable on the day of a 24-h recall. Among children aged 12–18 mo, 73% consumed sweets and 27% consumed SSBs on the day of the recall (6).
Family structure is a factor that may impact child dietary quality. In particular, the presence of older siblings in the household appears to affect the types of foods that children are fed (7–12). Children with older siblings have less healthy dietary patterns (10, 13–20) and greater exposure to energy-dense foods (21–24) and sweetened beverages (22, 25–28). Despite these findings, there are no published studies that have directly compared infant diet intake in first- and secondborn infant siblings within the same family.
The Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) study is a randomized controlled trial of a responsive parenting (RP) intervention for the primary prevention of obesity in infancy (29). We previously reported that infants of mothers receiving the INSIGHT RP intervention, which included guidance on responsive feeding and age-appropriate dietary intake, were more likely than control infants to have dietary patterns characterized by greater fruit and vegetable intake and lower intake of energy-dense foods (e.g. French fries, sweet foods, and sweet drinks) at age 9 mo (30). Because infants included in the INSIGHT trial were all firstborn (FB) children, their secondborn (SB) siblings were recruited into an observation-only cohort, SIBSIGHT (29). The objectives of SIBSIGHT were to study spillover effects of the INSIGHT intervention onto the SB child, as well as sibling differences in obesity-related parenting practices within families.
As FBs and SBs were each studied at the same points in development, the INSIGHT/SIBSIGHT sample provides an opportunity to directly compare diets of FB and SB siblings in later infancy, during the time when children are transitioning from an infant diet to a pattern more aligned with adult/family intake. The purpose of this analysis was to compare dietary intake and variety of key foods and food groups between FB and SB siblings at ages 6 and 12 mo, and to determine whether the INSIGHT intervention for FBs moderated sibling differences in diet. We hypothesized that: 1) dietary intake and variety would be correlated between FB and SB siblings; 2) SBs would have greater exposure to energy-dense foods (i.e. sweets, salty snacks, processed meats, fried foods, sweets, and SSBs) than their FB sibling; and 3) SBs would have less frequent intake of healthy foods, such as fruits and vegetables, than their FB sibling did at the same age. We also hypothesized that the INSIGHT RP intervention delivered to parents with their FB child would moderate differences in dietary intake between FB and SB child.
Methods
Participants
INSIGHT intervention study—FB children
First-time mothers and their infants were recruited shortly after birth from a single maternity ward in Central Pennsylvania. Recruitment spanned January 2012 through to March 2014. Mothers were eligible if they were aged ≥20 y, generally healthy, English-speaking, and had given birth to a full-term, generally healthy, singleton newborn weighing ≥2500 g at birth. Participants (n = 279) were randomly assigned to an RP intervention or child safety control intervention and completed the first study visit at 3–4 wk postpartum (Figure 1). Randomization was performed using permuted blocks of 6 and stratified on birth weight for gestational age (<50th percentile or ≥50th percentile) and intended feeding mode (breastfeeding or formula feeding). Nurses delivered intervention curricula at home visits at infant age 3, 16, 28, and 40 wk. At age 12 mo, participants (n = 250) attended a research center visit. The RP intervention included guidance on infant feeding, sleep, fussing and soothing, and interactive play. Within the feeding domain, examples of specific messaging included recognizing hunger and fullness cues, not using food to soothe distress unrelated to hunger, age-appropriate foods and portion sizes, and strategies for promoting acceptance of novel foods. The control group received a child safety intervention. Further study details have been previously published (29, 31).
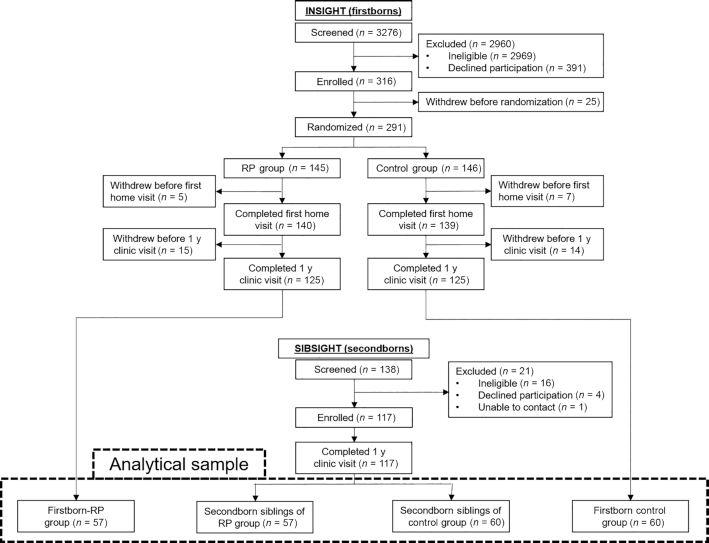
FIGURE 1.
Study participant flow diagram for INSIGHT (firstborn) and SIBSIGHT (secondborn) participants. First-time mothers were randomly assigned to the INSIGHT responsive parenting intervention or control group. Mothers who delivered a second child were invited to also participate in SIBSIGHT, an observation-only cohort. INSIGHT, Intervention Nurses Start Infants Growing on Healthy Trajectories; RP, responsive parenting.
SIBSIGHT observational study—SB children
INSIGHT mothers who gave birth to a second child between June 2013 and March 2017 were invited to participate in the observational cohort, SIBSIGHT. SBs were eligible if they were singleton, ≥36 weeks of gestation and ≥2250 g at birth, were generally healthy, and if families had no plans to move from Central Pennsylvania in the year following the second child's birth. A total of 117 SBs were enrolled and all remained in the study through to an age of 12 mo (Figure 1). Further details on recruitment and eligibility have been published elsewhere (29, 32). SB data was collected at home visits at 3, 16, and 28 wk and a research center visit at 12 mo. No intervention was delivered at SIBSIGHT study visits. Both INSIGHT and SIBSIGHT studies were approved by the Human Subjects Protection Office of the Penn State College of Medicine and registered at www.clinicaltrials.gov (NCT01167270) prior to enrollment of the first participant. Mothers provided written consent for their and their children's participation.
Measures
For both FBs and SBs, mothers completed a modified FFQ (33) at child ages 6 and 12 mo. Mothers were asked to indicate whether their child had ever consumed 121 food and beverage items, as well as how often they had consumed them in the previous week. The 8 response options included 0, 1, 2–3, 4–6 times per week, 1, 2, 3, and 4 or more times per day. Mothers also reported the number of feedings of breastmilk and formula per day. Demographic characteristics were collected at birth. Surveys were completed on paper or online using Research Electronic Data Capture (REDCap) (34).
Analysis
Data were analyzed using SAS 9.4 (SAS Institute). Total frequency of intake was calculated for several food categories, including fruit (18 items, 100% fruit juice not included), vegetables (22 items), dairy (12 items), meat/poultry/fish (13 items), processed meats (4 items), fried chicken/fish (2 items), grains (15 items), salty snacks (5 items), sweets (12 items), and SSBs (7 items). Fried potatoes and 100% fruit juice were analyzed as single item categories. At 6 mo, dietary variety was operationalized as the number of total foods, fruits, and vegetables that had ever been introduced to the infant. At 12 mo, fruit and vegetable variety was calculated by counting the total number of different items consumed in the past week. For energy-dense foods that were consumed less frequently, including 100% fruit juice, fried potatoes, processed meats, fried chicken/fish, salty snacks, sweets, and SSBs, infants were categorized by whether they did or did not consume any items from that category in the past week. Breastfeeding was categorized at 6 mo as predominantly breastfed if ≥80% of milk feedings were breastmilk, or not predominantly breastfed if <80% of milk feedings were breastmilk. At 12 mo, participants were categorized as breastfed if they reported any breastmilk feedings in the previous week.
Due to the nonnormality of frequency/count variables, these data were analyzed using nonparametric or negative binomial distributed tests. Similarity between siblings was assessed using Spearman correlations for frequency/count variables and chi-square tests for categorical variables. Preliminary investigation of differences between FB and SB siblings was conducted using Wilcoxon signed rank tests of the FB-SB difference for frequency/count variables and McNemar's test for categorical variables. Further examination of birth-order effects was performed using generalized linear mixed models (SAS PROC GLIMMIX) using the adaptive Gauss–Hermite quadrature estimation method and a random intercept to account for correlation within sibling pairs. A negative binomial distribution and log link function was used for frequency/count outcomes, and a binary distribution and logit link function was used for categorical outcomes (35). Study group and infant age in days at the time of survey completion was included as a covariate in these models. Birth weight for gestational age z-score and infant sex were also considered as covariates, but they did not affect the results, so simpler models without these covariates are presented. Finally, birth order by study group interactions were evaluated in the generalized linear mixed models to determine whether the RP intervention affected differences in dietary intake between FBs and SBs. Four sibling dyads at 6 mo and 1 dyad at 12 mo were excluded from analyses of frequency of fruit intake due to 1 sibling having extreme outlier/implausible values. Statistical significance was defined as P < 0.05 and adjusted for multiple comparisons using Tukey's test where appropriate.
Results
Demographic characteristics for mothers, FBs, and SBs are presented in Table 1. As expected, mothers were older at the birth of their second child, with a mean of 2.5 y (SD: 0.8, range 0.9–5.1) between births. Spacing between FB and SB births did not differ by study group. No mothers reported a change in their educational status between births, and the majority were college-educated and married with household incomes ≥$50,000 at both births. There was no difference between FBs and SBs in birth weight or sex distribution. Out of 117 enrolled families with siblings, 97 mothers (46 RP and 51 control) completed FFQs for both FB and SB infants at age 6 mo, and at 12 mo, 100 mothers (48 RP and 52 control) completed FFQs for both FB and SB children.
TABLE 1.
Demographic characteristics of mothers and firstborn infants enrolled in the INSIGHT RP intervention and control groups and their secondborn infants enrolled in the SIBSIGHT observational cohort
| RP | Control | |||
|---|---|---|---|---|
| FB (n = 57) | SB (n = 57) | FB (n = 60) | SB (n = 60) | |
| Maternal characteristics | ||||
| Age at delivery, y | 29.8 ± 4.4 | 32.1 ± 4.2* | 28.4 ± 4.0 | 31.1 ± 4.1* |
| Prepregnancy BMI, kg/m2 | 24.8 ± 4.7 | 25.8 ± 5.1* | 25.3 ± 5.4 | 26.0 ± 6.0* |
| Gestational weight gain, kg | 14.9 ± 5.6 | 11.1 ± 5.5* | 14.6 ± 5.1 | 11.3 ± 6.5* |
| Non-Hispanic white, n (%) | 52 (92.9) | 52 (92.9) | 57 (95.0) | 57 (95.0) |
| College graduate, n (%) | 43 (75.4) | 43 (75.4) | 46 (76.7) | 46 (76.7) |
| Married, n (%) | 53 (93.0) | 55 (96.5) | 54 (90.0) | 56 (93.3) |
| Household income, n (%) | ||||
| <$50,000 | 6 (10.5) | 3 (5.3) | 11 (18.3) | 5 (8.3) |
| ≥$50,000 | 51 (89.4) | 51 (89.4) | 46 (76.7) | 50 (83.3) |
| Don't know/refuse to answer/missing | 0 (0) | 3 (5.3) | 3 (5.0) | 5 (8.3) |
| Infant characteristics | ||||
| Birth weight, g | 3388 ± 424 | 3431 ± 442 | 3449 ± 412 | 3512 ± 432 |
| Sex, female, n (%) | 30 (52.6) | 35 (61.4) | 32 (53.3) | 32 (53.3) |
Values are mean ± SD or n (%). *Different from FB in same group. There were no significant differences by study group. FB, firstborn; INSIGHT, Intervention Nurses Start Infants Growing on Healthy Trajectories; RP, responsive parenting; SB, secondborn.
Dietary intake at 6 mo
At 6 mo, 43.3% of FBs and 49.5% of SBs were predominantly breastfed; these rates did not differ significantly. Predominant breastfeeding status was significantly associated between FB and SB siblings (chi-square P < 0.0001). Both siblings were predominantly breastfed at 6 mo in 37.1% of dyads, whereas 44.3% were both not predominantly breastfed. A greater percentage of FBs than SBs consumed fruit juice in the previous week (FB 13.8 compared with SB 3.2%, McNemar's test P = 0.006; generalized linear mixed model adjusted for covariates, P = 0.04). All but 1 FB (99.0%) and 4 SBs (95.9%) had been introduced to solid foods. The main foods consumed were fruits, vegetables, and infant cereal. Only a small percentage of infants in the total sample consumed meats (8.3%), salty snacks (2.6%), sweets (6.7%), fried foods (1.0%), or SSBs (1.6%), so these variables were not further analyzed. Mothers reported having introduced a greater total number of foods to their SB child than they did for the FB (FB 8.2 compared with SB 9.5, P = 0.04), as well as a greater number of fruits (FB 2.5 compared with SB 3.3 fruits introduced, P = 0.01, Table 2). However, in the models controlling for study group and age at the time of survey completion, these differences were no longer statistically significant. There was no difference in frequency of fruit, vegetable, or grain intake. A greater percentage of SBs (22.7%) than FBs (11.3%) consumed dairy foods (McNemar's test P = 0.01; generalized linear mixed model adjusted for covariates, P = 0.06), mostly in the form of yogurt. The number of foods introduced and frequency of intake of fruits and vegetables was modestly correlated between siblings (ranging from ρ = 0.21 to ρ = 0.46).
TABLE 2.
Dietary intake in firstborn and secondborn siblings at age 6 mo
| P value for sibling difference | Spearman correlation between siblings | ||||||
|---|---|---|---|---|---|---|---|
| Category | Dyads, n | FB1 | SB1 | Wilcoxon signed rank test | Generalized linear mixed model2 | ρ | P |
| Frequency of intake, times per day | |||||||
| Fruit | 933 | 0.6 ± 0.8 | 0.9 ± 0.9 | 0.07 | 0.50 | 0.29 | 0.004 |
| Vegetables | 97 | 1.2 ± 1.2 | 1.2 ± 1.1 | 0.54 | 0.70 | 0.29 | 0.004 |
| Grains | 97 | 0.9 ± 0.9 | 1.0 ± 1.0 | 0.47 | 0.56 | 0.21 | 0.04 |
| Number of foods introduced | |||||||
| Total | 97 | 8.2 ± 5.9 | 9.5 ± 6.8 | 0.04 | 0.88 | 0.42 | <0.0001 |
| Fruits | 97 | 2.5 ± 2.9 | 3.3 ± 3.2 | 0.01 | 0.41 | 0.46 | <0.0001 |
| Vegetables | 97 | 3.6 ± 2.4 | 4.1 ± 3.0 | 0.15 | 0.91 | 0.41 | <0.0001 |
Values are mean ± SD.
Adjusted for study group and exact age at FFQ completion.
Excluded 2 FB and 2 SB (n = 4 dyads total) with reported fruit intake >6 times per day (>4 SD above mean).
FB, firstborn; SB, secondborn.
Dietary intake at 12 mo
At age 12 mo, a significantly greater percentage of SBs (41.4%) compared with FBs (33.3%) were fed any breastmilk in the previous week (McNemar's test P = 0.02; generalized linear mixed model adjusted for covariates, P = 0.06, n = 99, 1 dyad missing SB data). Breastfeeding status was highly related within sibling pairs (chi square P < 0.0001); in 31.3% of dyads, both siblings were breastfed, and in 55.6% of dyads, neither sibling was breastfed. Regardless of adjustment for study group and exact age at survey completion, SBs consumed fruit more frequently than FBs (FB 2.6 compared with SB 3.2 times per day, Table 3). In the unadjusted Wilcoxon signed rank test analysis, SBs also consumed a greater variety of fruits than FBs (FB 7.3 compared with SB 8.1 unique items in past week); this difference fell just short of statistical significance in the covariate adjusted model (P = 0.051). There were no statistically significant differences between siblings in the frequency of intake of vegetables, dairy, meat/poultry/fish, or grains, or in the variety of vegetables consumed. For all food groups, frequency of intake and variety were modestly correlated between siblings (ranging from ρ = 0.27 to ρ = 0.46).
TABLE 3.
Dietary intake and variety in firstborn and secondborn siblings at age 12 mo
| P value for birth-order difference | Spearman correlation between siblings | ||||||
|---|---|---|---|---|---|---|---|
| Category | Dyads, n | FB1 | SB1 | Wilcoxon signed rank test | Generalized linear mixed model2 | ρ | P |
| Frequency of intake, times per day | |||||||
| Fruit | 993 | 2.6 ± 1.6 | 3.2 ± 2.0 | 0.04 | 0.03 | 0.37 | 0.0002 |
| Vegetables | 100 | 2.8 ± 1.6 | 2.9 ± 1.8 | 0.89 | 0.91 | 0.39 | <0.0001 |
| Dairy | 100 | 3.0 ± 2.1 | 3.1 ± 2.5 | 0.68 | 0.72 | 0.44 | <0.0001 |
| Meat/poultry/fish | 100 | 0.8 ± 0.7 | 1.0 ± 0.8 | 0.11 | 0.20 | 0.40 | <0.0001 |
| Grains | 100 | 2.3 ± 1.3 | 2.4 ± 1.4 | 0.71 | 0.90 | 0.46 | <0.0001 |
| Variety, number of unique items consumed in past week | |||||||
| Fruit | 100 | 7.3 ± 3.4 | 8.1 ± 3.6 | 0.03 | 0.05 | 0.27 | 0.007 |
| Vegetables | 100 | 9.1 ± 3.4 | 9.2 ± 3.9 | 0.70 | 0.79 | 0.31 | 0.002 |
Values are mean ± SD.
Adjusted for study group and exact age at FFQ completion.
Excluded 1 SB reporting fruit >16 times per day (>5 SDs above mean).
FB, firstborn; SB, secondborn.
Fewer SBs (32.0%, Figure 2) than FBs (46.0%) consumed 100% fruit juice at least once in the past week (McNemar's test P = 0.01). A greater percentage of SBs than FBs consumed fried potatoes (FB 38.4 compared with SB 57.6%, McNemar's test P = 0.0009) and processed meats (FB 43.0 compared with SB 58.0%, McNemar's test P = 0.02). There were no significant differences in the percentage of FBs and SBs who consumed fried chicken/fish, salty snacks, sweets, or SSBs (Figure 2). Similar results were obtained in models adjusted for study group and exact age at survey completion. For all of these items, infants were more likely to have consumed them if their sibling also did during infancy, as indicated by significant chi-square tests (sweets, P < 0.05; salty snacks, processed meats, and fried chicken/fish, P < 0.01; fruit juice, fried potatoes, and SSBs, P < 0.0001).
FIGURE 2.

Percent of FB and SB siblings consuming energy-dense food and beverage items at age 12 mo. N = 100 dyads, except for fried potatoes and fried chicken/fish where n = 99 dyads. *Significant difference between FB and SB, McNemar's test P < 0.05. Similar results were obtained from generalized linear mixed model including study group and age at questionnaire completion as covariates. FB, firstborn; SB, secondborn; SSB, sugar-sweetened beverage.
Sibling differences by study group
At 6 mo, there were no differences by study group in effect of birth order on dietary outcomes. At 12 mo, there was no interaction between study group and birth order for frequency of intake of fruit, dairy, meat, and grains (Table 4). However, for frequency of vegetable intake, the sibling difference pattern differed by study group (birth order × study group interaction P = 0.001). SBs from RP group families had greater frequency of vegetable intake than SBs in the control group (P = 0.02). A similar pattern of difference was seen between FBs and SBs within the RP group, with RP SBs consuming vegetables more frequently than their FB sibling, though this difference did not achieve statistical significance after adjustment for multiple comparisons (P = 0.07). There was also an interaction between birth order and study group for vegetable variety (P = 0.04) and a nearly significant interaction for fruit variety (P = 0.051). Posthoc tests of differences showed that SBs in the RP group consumed a greater variety of fruit than FBs in the RP group (P = 0.03) and a greater variety of vegetables than SBs in the control group (P = 0.01). For the energy-dense food categories, there were no statistically significant interactions between birth order and study group.
TABLE 4.
Dietary intake and variety at 12 mo in firstborn and secondborn infants of mothers receiving the INSIGHT responsive parenting or control intervention during their firstborn's infancy1
| RP (n = 48 dyads) | Control (n = 52 dyads)2 | Birth order | Study group | Birth order × study group | |||
|---|---|---|---|---|---|---|---|
| FB | SB | FB | SB | P | P | P | |
| Intake, times per day | |||||||
| Fruit | 2.5 ± 0.2 | 3.2 ± 0.3 | 2.5 ± 0.2 | 2.9 ± 0.3 | 0.02 | 0.50 | 0.51 |
| Vegetables | 2.5 ± 0.2 | 3.2 ± 0.3# | 2.8 ± 0.2 | 2.2 ± 0.2 | 0.82 | 0.17 | 0.001 |
| Dairy | 3.0 ± 0.4 | 3.1 ± 0.4 | 3.0 ± 0.4 | 2.6 ± 0.4 | 0.72 | 0.40 | 0.67 |
| Meat/poultry/fish | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.20 | 0.20 | 0.35 |
| Grains | 2.2 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.1 ± 0.2 | 0.88 | 0.72 | 0.48 |
| Variety, number of different items consumed in past week | |||||||
| Fruit | 6.9 ± 0.5 | 8.6 ± 0.5* | 7.2 ± 0.5 | 7.2 ± 0.5 | 0.05 | 0.32 | 0.05 |
| Vegetables | 9.1 ± 0.5 | 10.2 ± 0.6# | 8.7 ± 0.5 | 7.9 ± 0.5 | 0.84 | 0.02 | 0.04 |
Values are least squared means ± SE. Main effect and interaction P values from generalized linear mixed model that also included exact age at FFQ as a covariate.
n = 51 for fruit intake. *Different from FB in same group, # different from control at same birth order (P < 0.05 after Tukey adjustment).
FB, firstborn; INSIGHT, Intervention Nurses Start Infants Growing on Healthy Trajectories; RP, responsive parenting; SB, secondborn.
Discussion
In this analysis of FB and SB infant sibling dyads, we observed birth-order differences in dietary intake; however, these differences did not consistently indicate healthier or less healthy diets in SB siblings compared with their older sibling. The RP intervention had a beneficial effect on SB fruit and vegetable feeding. In particular, SBs from RP families had a higher frequency of vegetable intake at 12 mo than control SBs did. The RP intervention also resulted in greater fruit and vegetable variety among SBs.
In support of our first hypothesis, SB diets in infancy were similar to their older sibling's diet at the same age. The correlations observed between siblings were similar in magnitude to those seen in children aged 2–10 y (36). Previous research has demonstrated similarity between siblings’ food preferences (37). Parental diet has also been established as a strong predictor of child dietary patterns (9, 18, 38) and preferences (39). Because adult dietary patterns are relatively stable over periods of a few years (40, 41), FB and SB infants were likely similarly influenced by their parents’ dietary choices and the family food environment. Intervening on dietary intake in the family as a whole rather than a specific target child may be 1 strategy for improving infant dietary intake.
In partial support for our second hypothesis that SBs would have greater exposure to energy-dense foods than FBs, we found that SBs were more likely than their FB siblings to consume fried potatoes and processed meats at age 12 mo. Potatoes and meats provide important shortfall nutrients for infants and young children [e.g. potassium and fiber in potatoes (42) and iron in meat (43)], but providing energy-dense fried and processed versions of these foods may increase the risk of excess energy and sodium intake. However, contrary to our hypothesis, no statistically significant differences were observed in the percentage of infants consuming fried chicken/fish, salty snacks, sweets, and SSBs between FB and SB children.
Contrary to our hypothesis, SBs were less likely to consume 100% fruit juice than their FB siblings at both 6 and 12 mo. Only 3% of SB infants consumed fruit juice at 6 mo, compared with 13% of FB infants. At age 12 mo, though the percentage of children drinking juice increased in both FBs and SBs, only around a third of SB infants compared with nearly half of FB infants consumed juice. This finding could be reflective of population-level trends showing a decline in 100% fruit juice consumption. In the FITS studies, the percentage of children aged 6–12 mo children consuming 100% fruit juice decreased from 31 to 41% in FITS 2008 to 22–33% in FITS 2016 (6). Similar declines have been reported in NHANES analyses (44). In addition, in June 2017, the American Academy of Pediatrics released an updated position statement on fruit juice, recommending that introduction of juice be delayed until 12 mo of age (45). However, all FB data and the majority of SB data (94% of 6 mo data and 83% of 12 mo data) were collected prior to the release of this guideline.
We also unexpectedly found that fruit intake at 12 mo was greater among SBs than FBs, with a similar trend for fruit variety at 12 mo. Infants are born with an innate preference for sweet flavors, such as those found in fruits (46), and thus parents may perceive that sweet fruits may be more readily accepted than bitter vegetables (47). In addition, children tend to have greater liking of fruits than vegetables in preschool and middle childhood (48). When faced with the added challenge of managing feeding 2 children, parents may have opted to offer their SB foods that they felt would be more easily accepted by both their SB infant and older FB child. Qualitative research exploring strategies that parents use to manage feeding multiple children at different life-stages with differing dietary needs could shed light on this hypothesis.
Upon examining FB-SB differences by INSIGHT treatment group, we found that the RP intervention delivered to mothers with their FB infant had a significant impact on vegetable intake and variety of their SB infant. We found that SBs in the RP group consumed vegetables significantly more frequently than SBs in the control group. In contrast, SBs in the control group had the lowest frequency of intake of vegetables, ∼0.6 fewer times per day than their FB sibling. Though this difference did not reach statistical significance after adjustment for multiple comparisons (P = 0.12), it does suggest that vegetable intake tends to decrease from FB to SB. A similar advantage among SBs in the RP group was observed for fruit and vegetable variety. For vegetable variety, a significant difference was observed between SBs in the RP group and SBs in the control group. Though the birth order × study group interaction term for fruit variety fell just short of the P < 0.05 threshold, posthoc comparisons suggest that SBs in the RP group had greater fruit variety than their FB siblings. Though the INSIGHT RP intervention content included strategies to promote acceptance and intake of fruits and vegetables, these findings are surprising because there was no significant impact of the RP intervention on fruit or vegetables in FBs included in the subsample in this analysis, and only limited impact on dietary intake in the full INSIGHT sample (30, 49). INSIGHT encouraged parents to use a number of evidence-based strategies to promote fruit and vegetable liking and intake, such as introducing vegetables before fruits (50), offering tastes of new foods repeatedly over several days (51), offering a variety of flavors (3), and modeling healthy eating behaviors by consuming these foods themselves (52). It is possible that parental use of these strategies with the FB beyond 1 y of age may have resulted in greater modeling of vegetable intake by older siblings, in turn influencing the intake of the younger sibling.
This study has some limitations to note. First, the sample was small and was largely non-Hispanic white with few low-income participants, and therefore these findings may not be applicable to broader populations. Infants from families with lower socioeconomic status are at greater risk of poor dietary quality (7, 12, 13, 15, 17, 26). The effects of birth order on diet could be different in families with more limited resources. In addition, families who have a second child may differ from those who do not, and thus FB intake in these families may not be representative of FBs who remain only children. Infant dietary intake was reported by mothers. Self-reported (or proxy-reported, in the case of parents reporting for their children) dietary intake is known to have limited accuracy, in part due to underreporting of energy-dense, nutrient-poor foods and overreporting of fruit and vegetable intake (53, 54). However, because our primary comparisons were between FB and SB siblings within families, and dietary intake for both siblings was reported by the same individual (mother), the effect of self-reporting bias may not have been as large as in cross-sectional studies. The FFQ used in this study did not include information on portion sizes; thus, we were unable to estimate energy or nutrient intake. Rather, our goal was to describe general food patterns, an appropriate use of self-reported dietary data (55). Though the modified FFQ used in this study has not been validated against 24-h recall data, similar FFQs have been shown to be valid measures of dietary intake in infants aged 6–12 mo (56, 57).
Finally, this study would have been strengthened by collecting data on concurrent FB diet at the time of the SB assessments. Though the INSIGHT/SIBSIGHT study design allowed us to compare how parents feed FBs and SBs at the same points in their development through infancy, it is likely that the SB's diet is also influenced by what their older sibling is consuming at the same moment in time. The FBs in this sample were aged between 2 and 6 y at the time of the SB 12-mo dietary assessment. During the toddler and preschool years, dietary quality may decline due to picky eating and neophobia which peak during these years (58). Parents may choose to offer a more limited variety of foods that are acceptable to their picky eater to avoid mealtime struggles, which may also have implications for the kinds of foods that their younger sibling is exposed to. Further research exploring how families manage the dietary needs and preferences of multiple young children is needed to clarify this.
In summary, this research shows that SB infants tend to have more varied diets than FBs, but this includes exposure to both healthy and unhealthy foods. An RP intervention delivered to parents with their FB child appears to alter the effect of birth order on vegetable intake and variety but did not prevent greater exposure to some energy-dense foods seen in SBs. Intervening on first-time parents in a way that can improve outcomes for both FB and subsequent children is a cost-effective way to reach a larger number of children. These data suggest that the INSIGHT RP intervention is an effective tool for improving fruit variety and vegetable intake and variety in SB infants, but further optimization of the intervention is needed to affect other foods and food groups in both FB and SB infants.
Acknowledgments
We acknowledge Michele Marini, Lindsey Hess, Jessica Beiler, Jennifer Stokes, Patricia Carper, Amy Shelly, and Cara Ruggiero for their assistance with this project.
The authors’ contributions were as follows–EEH, JSS, LLB, and IMP: designed and conducted the research; EEH: analyzed the data, wrote the manuscript, and had primary responsibility for final content; and EEH, JSS, and IMP: read and approved the final manuscript.
Notes
This study was supported by NIH, National Institute of Diabetes and Digestive and Kidney Diseases, R01DK088244 and R01DK099364; Research Electronic Data Capture (REDCap) support was received from the Penn State Clinical & Translational Sciences Institute, NIH UL1 TR002014.
Author disclosures: The authors report no conflicts of interest.
LLB is deceased.
Abbreviations used: FB, firstborn; FITS, Feeding Infants and Toddlers Study; INSIGHT, Intervention Nurses Start Infants Growing on Healthy Trajectories; RP, responsive parenting; SB, secondborn; SSB, sugar-sweetened beverage.
References
- 1. Birch LL. Development of food acceptance patterns in the first years of life. Proc Nutr Soc. 1998;57:617–24. [DOI] [PubMed] [Google Scholar]
- 2. Savage JS, Fisher JO, Birch LL. Parental influence on eating behavior: conception to adolescence. J Law Med Ethics. 2007;35:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerrish CJ, Mennella JA. Flavor variety enhances food acceptance in formula-fed infants. Am J Clin Nutr. 2001;73:1080–5. [DOI] [PubMed] [Google Scholar]
- 4. Mallan KM, Fildes A, Magarey AM, Daniels LA. The relationship between number of fruits, vegetables, and noncore foods tried at age 14 months and food preferences, dietary intake patterns, fussy eating behavior, and weight status at age 3.7 years. J Acad Nutr Diet. 2016;116:630–7. [DOI] [PubMed] [Google Scholar]
- 5. Park S, Pan L, Sherry B, Li R. The association of sugar-sweetened beverage intake during infancy with sugar-sweetened beverage intake at 6 years of age. Pediatrics. 2014;134(Suppl 1):S56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roess AA, Jacquier EF, Catellier DJ, Carvalho R, Lutes AC, Anater AS, Dietz WH. Food consumption patterns of infants and toddlers: findings from the feeding infants and toddlers study (FITS) 2016. J Nutr. 2018;148:1525S–35S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Betoko A, Charles MA, Hankard R, Forhan A, Bonet M, Saurel-Cubizolles MJ, Heude B, de Lauzon-Guillain B, EDEN Mother-Child Cohort Study Group . Infant feeding patterns over the first year of life: influence of family characteristics. Eur J Clin Nutr. 2013;67:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kristiansen AL, Lande B, Sexton JA, Andersen LF. Dietary patterns among Norwegian 2-year-olds in 1999 and in 2007 and associations with child and parent characteristics. Br J Nutr. 2013;110:135–44. [DOI] [PubMed] [Google Scholar]
- 9. Robinson S, Marriott L, Poole J, Crozier S, Borland S, Lawrence W, Law C, Godfrey K, Cooper C, Inskip H et al. Dietary patterns in infancy: the importance of maternal and family influences on feeding practice. Br J Nutr. 2007;98:1029–37. [DOI] [PubMed] [Google Scholar]
- 10. Smithers LG, Brazionis L, Golley RK, Mittinty MN, Northstone K, Emmett P, McNaughton SA, Campbell KJ, Lynch JW. Associations between dietary patterns at 6 and 15 months of age and sociodemographic factors. Eur J Clin Nutr. 2012;66:658–66. [DOI] [PubMed] [Google Scholar]
- 11. Verger EO, Eussen S, Holmes BA. Evaluation of a nutrient-based diet quality index in UK young children and investigation into the diet quality of consumers of formula and infant foods. Public Health Nutr. 2016;19:1785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wen X, Kong KL, Eiden RD, Sharma NN, Xie C. Sociodemographic differences and infant dietary patterns. Pediatrics. 2014;134:e1387–98. [DOI] [PubMed] [Google Scholar]
- 13. Brazionis L, Golley RK, Mittinty MN, Smithers LG, Emmett P, Northstone K, Lynch JW. Characterization of transition diets spanning infancy and toddlerhood: a novel, multiple-time-point application of principal components analysis. Am J Clin Nutr. 2012;95:1200–8. [DOI] [PubMed] [Google Scholar]
- 14. Lioret S, Betoko A, Forhan A, Charles MA, Heude B, de Lauzon-Guillain B; EDEN Mother-Child Cohort Study Group . Dietary patterns track from infancy to preschool age: cross-sectional and longitudinal perspectives. J Nutr. 2015;145:775–82. [DOI] [PubMed] [Google Scholar]
- 15. Camara S, de Lauzon-Guillain B, Heude B, Charles MA, Botton J, Plancoulaine S, Forhan A, Saurel-Cubizolles MJ, Dargent-Molina P, Lioret S et al. Multidimensionality of the relationship between social status and dietary patterns in early childhood: longitudinal results from the French EDEN mother-child cohort. Int J Behav Nutr Phy. 2015;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. North K, Emmett P, Team AS. Multivariate analysis of diet among three-year-old children and associations with socio-demographic characteristics. Eur J Clin Nutr. 2000;54:73–80. [DOI] [PubMed] [Google Scholar]
- 17. Rose CM, Savage JS, Birch LL. Patterns of early dietary exposures have implications for maternal and child weight outcomes. Obesity (Silver Spring). 2016;24:430–8. [DOI] [PubMed] [Google Scholar]
- 18. Fisk CM, Crozier SR, Inskip HM, Godfrey KM, Cooper C, Robinson SM, Southampton Women's Survey Study Group . Influences on the quality of young children's diets: the importance of maternal food choices. Br J Nutr. 2011;105:287–96. [DOI] [PubMed] [Google Scholar]
- 19. Leventakou V, Sarri K, Georgiou V, Chatzea V, Frouzi E, Kastelianou A, Gatzou A, Kogevinas M, Chatzi L. Early life determinants of dietary patterns in preschool children: Rhea mother-child cohort, Crete, Greece. Eur J Clin Nutr. 2016;70:60–5. [DOI] [PubMed] [Google Scholar]
- 20. Luque V, Escribano J, Closa-Monasterolo R, Zaragoza-Jordana M, Ferre N, Grote V, Koletzko B, Totzauer M, Verduci E, ReDionigi A et al. Unhealthy dietary patterns established in infancy track to mid-childhood: The EU Childhood Obesity Project. J Nutr. 2018;148:752–9. [DOI] [PubMed] [Google Scholar]
- 21. Brekke HK, van Odijk J, Ludvigsson J. Predictors and dietary consequences of frequent intake of high-sugar, low-nutrient foods in 1-year-old children participating in the ABIS study. Br J Nutr. 2007;97:176–81. [DOI] [PubMed] [Google Scholar]
- 22. Vilela S, Oliveira A, Pinto E, Moreira P, Barros H, Lopes C. The influence of socioeconomic factors and family context on energy-dense food consumption among 2-year-old children. Eur J Clin Nutr. 2015;69:47–54. [DOI] [PubMed] [Google Scholar]
- 23. Koh GA, Scott JA, Oddy WH, Graham KI, Binns CW. Exposure to non-core foods and beverages in the first year of life: results from a cohort study. Nutrition & Dietetics. 2010;67:(3):137–42. [Google Scholar]
- 24. Grummer-Strawn LM, Scanlon KS, Fein SB. Infant feeding and feeding transitions during the first year of life. Pediatrics. 2008;122(Suppl 2):S36–42. [DOI] [PubMed] [Google Scholar]
- 25. Pawellek I, Grote V, Theurich M, Closa-Monasterolo R, Stolarczyk A, Verduci E, Xhonneux A, Koletzko B. Factors associated with sugar intake and sugar sources in European children from 1 to 8 years of age. Eur J Clin Nutr. 2017;71:25–32. [DOI] [PubMed] [Google Scholar]
- 26. Hendricks K, Briefel R, Novak T, Ziegler P. Maternal and child characteristics associated with infant and toddler feeding practices. J Am Diet Assoc. 2006;106:S135–48. [DOI] [PubMed] [Google Scholar]
- 27. Lande B, Andersen LF, Veierod MB, Baerug A, Johansson L, Trygg KU, Bjorneboe GE. Breast-feeding at 12 months of age and dietary habits among breast-fed and non-breast-fed infants. Public Health Nutr. 2004;7:495–503. [DOI] [PubMed] [Google Scholar]
- 28. Northstone K, Rogers I, Emmett P, Team AS. Drinks consumed by 18-month-old children: are current recommendations being followed?. Eur J Clin Nutr. 2002;56:236–44. [DOI] [PubMed] [Google Scholar]
- 29. Paul IM, Williams JS, Anzman-Frasca S, Beiler JS, Makova KD, Marini ME, Hess LB, Rzucidlo SE, Verdiglione N, Mindell JA et al. The Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) study. BMC Pediatr. 2014;14:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hohman EE, Paul IM, Birch LL, Savage JS. INSIGHT responsive parenting intervention is associated with healthier patterns of dietary exposures in infants. Obesity (Silver Spring). 2017;25:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: a randomized clinical trial. JAMA Pediatr. 2016;170:742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruggiero CF, Hohman EE, Birch LL, Paul IM, Savage JS. The Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) responsive parenting intervention for firstborns impacts feeding of secondborns. Am J Clin Nutr. 2020;111:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blum RE, Wei EK, Rockett HR, Langeliers JD, Leppert J, Gardner JD, Colditz GA. Validation of a food frequency questionnaire in Native American and Caucasian children 1 to 5 years of age. Matern Child Health J. 1999;3:167–72. [DOI] [PubMed] [Google Scholar]
- 34. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kiernan K. Insights into using the GLIMMIX procedure to model categorical outcomes with random effects. Proceedings of the SAS Global Forum 2018 Conference; 2018: SAS Institute; 2018. [Google Scholar]
- 36. Bogl LH, Silventoinen K, Hebestreit A, Intemann T, Williams G, Michels N, Molnar D, Page AS, Pala V, Papoutsou S et al. Familial resemblance in dietary intakes of children, adolescents, and parents: does dietary quality play a role?. Nutrients. 2017;9(8):E892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pliner P, Pelchat ML. Similarities in food preferences between children and their siblings and parents. Appetite. 1986;7:333–42. [DOI] [PubMed] [Google Scholar]
- 38. Cooke LJ, Wardle J, Gibson EL, Sapochnik M, Sheiham A, Lawson M. Demographic, familial and trait predictors of fruit and vegetable consumption by pre-school children. Public Health Nutr. 2004;7:295–302. [DOI] [PubMed] [Google Scholar]
- 39. Howard AJ, Mallan KM, Byrne R, Magarey A, Daniels LA. Toddlers’ food preferences. The impact of novel food exposure, maternal preferences and food neophobia. Appetite. 2012;59:818–25. [DOI] [PubMed] [Google Scholar]
- 40. Borland SE, Robinson SM, Crozier SR, Inskip HM; Group SWSS . Stability of dietary patterns in young women over a 2-year period. Eur J Clin Nutr. 2008;62:119–26. [DOI] [PubMed] [Google Scholar]
- 41. Weismayer C, Anderson JG, Wolk A. Changes in the stability of dietary patterns in a study of middle-aged Swedish women. J Nutr. 2006;136:1582–7. [DOI] [PubMed] [Google Scholar]
- 42. Storey ML, Anderson PA. Nutrient intakes and vegetable and white potato consumption by children aged 1 to 3 years. Adv Nutr. 2016;7:241S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Obbagy JE, English LK, Psota TL, Wong YP, Butte NF, Dewey KG, Fox MK, Greer FR, Krebs NF, Scanlon KS et al. Complementary feeding and micronutrient status: a systematic review. Am J Clin Nutr. 2019;109:852S–71S. [DOI] [PubMed] [Google Scholar]
- 44. Miles G, Siega-Riz AM. Trends in food and beverage consumption among infants and toddlers: 2005–2012. Pediatrics. 2017;139:e20163290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heyman MB, Abrams SA; Section on Gastroenterology, Hepatology, and Nutrition and Committee on Nutrition . Fruit juice in infants, children, and adolescents: current recommendations. Pediatrics. 2017;139:e20170967. [DOI] [PubMed] [Google Scholar]
- 46. Ventura AK, Mennella JA. Innate and learned preferences for sweet taste during childhood. Curr Opin Clin Nutr Metab Care. 2011;14:379–84. [DOI] [PubMed] [Google Scholar]
- 47. Spyreli E, McKinley MC, Allen-Walker V, Tully L, Woodside JV, Kelly C, Dean M. “The one time you have control over what they eat”: a qualitative exploration of mothers’ practices to establish healthy eating behaviours during weaning. Nutrients. 2019;11:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Skinner JD, Carruth BR, Bounds W, Ziegler PJ. Children's food preferences: a longitudinal analysis. J Am Diet Assoc. 2002;102:1638–47. [DOI] [PubMed] [Google Scholar]
- 49. Savage JS, Hohman EE, Marini ME, Shelly A, Paul IM, Birch LL. INSIGHT responsive parenting intervention and infant feeding practices: randomized clinical trial. Int J Behav Nutr Phys Act. 2018;15:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Barends C, de Vries J, Mojet J, de Graaf C. Effects of repeated exposure to either vegetables or fruits on infant's vegetable and fruit acceptance at the beginning of weaning. Food Qual Preference. 2013;29:157–65. [Google Scholar]
- 51. Birch LL, Marlin DW. I don't like it; I never tried it: effects of exposure on two-year-old children's food preferences. Appetite. 1982;3:353–60. [DOI] [PubMed] [Google Scholar]
- 52. Gregory JE, Paxton SJ, Brozovic AM. Maternal feeding practices predict fruit and vegetable consumption in young children. Results of a 12-month longitudinal study. Appetite. 2011;57:167–72. [DOI] [PubMed] [Google Scholar]
- 53. Fisher JO, Butte NF, Mendoza PM, Wilson TA, Hodges EA, Reidy KC, Deming D. Overestimation of infant and toddler energy intake by 24-h recall compared with weighed food records. Am J Clin Nutr. 2008;88:407–15. [DOI] [PubMed] [Google Scholar]
- 54. Eck LH, Klesges RC, Hanson CL. Recall of a child's intake from one meal: are parents accurate?. J Am Diet Assoc. 1989;89:(6):784–9. [PubMed] [Google Scholar]
- 55. Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, Thompson FE, Potischman N, Guenther PM, Tarasuk V et al. Addressing current criticism regarding the value of self-report dietary data. J Nutr. 2015;145:2639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palacios C, Rivas-Tumanyan S, Santiago-Rodriguez EJ, Sinigaglia O, Rios EM, Campos M, Diaz B, Willett W. A semi-quantitative food frequency questionnaire validated in Hispanic infants and toddlers aged 0 to 24 months. J Acad Nutr Diet. 2017;117:526–35. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marriott LD, Robinson SM, Poole J, Borland SE, Godfrey KM, Law CM, Inskip HM; Southampton Women's Survey Study Group . What do babies eat? Evaluation of a food frequency questionnaire to assess the diets of infants aged 6 months. Public Health Nutr. 2008;11:751–6. [DOI] [PubMed] [Google Scholar]
- 58. Taylor CM, Wernimont SM, Northstone K, Emmett PM. Picky/fussy eating in children: review of definitions, assessment, prevalence and dietary intakes. Appetite. 2015;95:349–59. [DOI] [PubMed] [Google Scholar]