ABSTRACT
Background
Infusion of a complete amino acid mixture into normal late-gestation fetal sheep potentiates glucose-stimulated insulin secretion (GSIS). Leucine acutely stimulates insulin secretion in late-gestation fetal sheep and isolated fetal sheep islets in vitro.
Objectives
We hypothesized that a 9-d leucine infusion would potentiate GSIS in fetal sheep.
Methods
Columbia-Rambouillet fetal sheep at 126 days of gestation received a 9-d leucine infusion to achieve a 50%–100% increase in leucine concentrations or a control infusion. At the end of the infusion we measured GSIS, pancreatic morphology, and expression of pancreatic mRNAs. Pancreatic islet endothelial cells (ECs) were isolated from fetal sheep and incubated with supplemental leucine or vascular endothelial growth factor A (VEGFA) followed by collection of mRNA. Data measured at multiple time points were compared with a repeated-measures 2-factor ANOVA. Data measured at 1 time point were compared using Student's t test or the Mann–Whitney test.
Results
Glucose-stimulated insulin concentrations were 80% higher in leucine-infused (LEU) fetuses than in controls (P < 0.05). In the pancreas, LEU fetuses had a higher proportion of islets >5000 μm2 than controls (75% more islets >5000 μm2; P < 0.05) and a larger proportion of the pancreas that stained for β cells (12% greater; P < 0.05). Pancreatic and pancreatic islet vascularity were both 25% greater in LEU fetuses (P < 0.05). Pancreatic VEGFA and hepatocyte growth factor (HGF) mRNA expressions were 38% and 200% greater in LEU fetuses than in controls (P < 0.05), respectively. In isolated islet ECs, HGF mRNA was 20% and 50% higher after incubation in supplemental leucine (P < 0.05) or VEGFA (P < 0.01), respectively.
Conclusions
A 9-d leucine infusion potentiates fetal GSIS, demonstrating that glucose and leucine act synergistically to stimulate insulin secretion in fetal sheep. A greater proportion of the pancreas being comprised of β cells and higher pancreatic vascularity contributed to the higher GSIS.
Keywords: leucine, insulin, pancreas, islet, β cell, fetus, pregnancy, VEGFA, hepatocyte growth factor, vascularity
Introduction
We previously demonstrated that an intravenous infusion of a complete amino acid mixture into the fetal circulation for 10–14 d potentiated fetal glucose-stimulated insulin secretion (GSIS) (1). This demonstrated that amino acids and glucose act synergistically to stimulate fetal insulin secretion. Previous studies have demonstrated the ability of various individual amino acids to acutely stimulate insulin secretion in fetal sheep (2–5). However, these studies did not test the capacity of individual amino acids to potentiate GSIS and it is not clear which amino acid within the infused mixture of complete amino acids was most critical for the increase in fetal insulin secretion in our previous study. Whether increasing the fetal supply of just a single amino acid also could potentiate fetal GSIS is unknown.
Among the various amino acids that might be responsible for potentiating fetal insulin secretion, leucine is a likely candidate for a variety of reasons. The ability of leucine to stimulate insulin secretion from adult pancreatic islets and β cells and the underlying mechanisms are well described. β Cells oxidize leucine to produce ATP. Leucine also stimulates the oxidation of glucose to produce ATP by activating glutamate dehydrogenase (6–11). The oxidative metabolism of cellular fuels to produce ATP is one of the main pathways linking nutrient concentrations to insulin secretion by the β cell (12). In addition, leucine stimulates intracellular signaling pathways that regulate cell growth and replication in multiple cell types, including β cells and endothelial cells (ECs) (13–15). By activating these pathways, leucine might increase pancreatic islet growth, development, and the β-cell population as a mechanism to increase insulin secretion (16, 17). Finally, leucine enhances EC signaling to the β cell by stimulating EC production of hepatocyte growth factor (HGF) (16, 17), which stimulates pancreatic β-cell growth and insulin secretion (18–23). We have demonstrated that leucine acutely stimulates insulin secretion in fetal sheep and isolated fetal sheep islets (5, 24). We also have demonstrated in fetal sheep ECs isolated from large vessels that leucine stimulates HGF mRNA expression (25).
The primary purpose of the current study was to test the impact of a 9-d leucine infusion into the late-gestation sheep fetus, adjusted to achieve a 50%–100% increase in fetal arterial plasma leucine concentrations, on fetal insulin concentrations, insulin secretion, and pancreatic morphology. We hypothesized that the leucine infusion would potentiate GSIS and increase pancreatic islet size and vascularity, the area of the pancreas which stains for insulin (β cells), and pancreatic expression of HGF and vascular endothelial growth factor A (VEGFA). We also tested the impact of leucine on the pancreatic expression of 2 β cell–specific genes that regulate glucose metabolism: SLC2A2 [glucose transporter-2 (GLUT2)] and GCK (glucokinase). Fetal glucagon concentrations increase with fetal amino acid infusions (26, 27). Glucagon can potentiate insulin secretion in perfused adult pancreases and isolated adult islets (28–31) and stimulates expansion of the pancreatic β-cell population (28). Therefore, we measured fetal glucagon concentrations, glucagon secretion, pancreatic glucagon expression, and the area of the pancreas that stains for glucagon (α cells) to test whether leucine increased glucagon secretion and thus could provide another mechanism by which leucine would stimulate fetal pancreatic development and insulin secretion.
Methods
Animal preparation
All experiments were conducted at the Perinatal Research Center, University of Colorado School of Medicine, in compliance with the Institutional Animal Care and Use Committee, and complied with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. This Center is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Studies were conducted in Columbia-Rambouillet sheep with singleton pregnancies. Animals were anesthetized at mean ± SD 120 ± 0 days of gestational age and surgeries were performed to place indwelling catheters into the fetal abdominal aorta, femoral vein, umbilical vein, and maternal femoral vein and artery as previously described (32). Before infusions, animals were allowed to recover for ≥5 d.
Study design
Fetal intravenous infusions were initiated at 126 ± 0 d of gestational age. Fetuses assigned to the leucine infusion [leucine-infused (LEU) fetuses] [n = 9; l-Leucine (Ajinomoto), 160 mmol/L in sterile water] were administered a variable-rate infusion adjusted daily to target a 50%–100% increase in fetal plasma leucine concentrations by day 3. Control (CON) fetuses (n = 8) were infused with a 0.9% saline at a constant rate of 0.3 mL/h to match the mean delivery of the LEU infusions. Infusions were maintained for 9 ± 0 d. All animals were included in all analyses with minor exceptions: n = 8 LEU for maternal biochemistry, fetal pancreatic weight, and β- and α-cell mass; and n = 6 CON for arginine-stimulated glucagon secretion.
On the second to last day of the infusions, GSIS, arginine-stimulated insulin secretion (ASIS), and arginine-stimulated glucagon secretion were measured with a fetal hyperglycemic clamp and an arginine infusion (1). All sample times are relative to the start of the fetal glucose infusion (time 0). Baseline plasma glucose and insulin concentrations were determined at −60, −45, −30, and −15 min. The hyperglycemic clamp was initiated with a 33% dextrose (wt:vol in saline) bolus (825 mg glucose) into the fetus followed by a variable infusion of 33% dextrose (wt:vol in saline) adjusted to maintain fetal arterial plasma concentration at ∼2–2.5 mmol/L, which elicits 90% maximal insulin concentrations in fetal sheep (33). The dextrose infusion was held constant beginning at minute 45. Fetal arterial plasma samples were collected at 5, 10, 15, 20, 30, 45, 60, 75, 90, and 105 min for glucose and insulin measurement. To measure glucose-potentiated ASIS, a bolus of arginine (0.5 mmol/kg estimated fetal weight) in 5 mL of 0.4 mol/L sodium acetate and 0.9% NaCl was injected over 4 min into the fetal circulation beginning at 110 min. Fetal arterial plasma samples were collected at 115, 120, 130, and 140 min for measurement of glucose, insulin, and glucagon concentrations. Fetal arterial plasma glucagon concentrations were also measured at 90 and 105 min (1). Chronic infusions were continued throughout the insulin secretion studies and through necropsy the following day. At necropsy the placenta and fetal organs were dissected and weighed and the pancreas was processed as previously described (24). A tracer study with 3H2O and 1-13C-leucine infused into the fetal circulation (34) was performed on the day of the GSIS test, before the hyperglycemic clamp. Results from this study will be reported in a separate article. Biochemical analyses for blood pH, blood gases, and hematocrit; and plasma glucose, lactate, amino acids, insulin, insulin-like growth factor I (IGF-I), cortisol, glucagon, and norepinephrine were performed as previously described (32).
Pancreatic mRNA analysis
RNA was extracted from pulverized −80°C fetal pancreas (100 mg), reverse transcribed into cDNA, and measured in triplicate by qPCR analysis for INS (insulin), GCG (glucagon), PDX1 (pancreatic and duodenal homeobox-1), GCK, SLC2A2, VEGFA, HGF, ACTA (actin), and GAPDH as previously described (25). Genes of interest were normalized to the geometric means of the reference genes ACTA and GAPDH, which were not different between groups. Results are presented relative to controls.
Pancreatic insulin analysis
Three aliquots (between 12 and 25 mg each) of pulverized hepatic pancreas per fetus were subjected to an acid ethanol extraction with 1 mL of 1 mol/L HCl in 70% ethanol (vol:vol) at −20°C for 18 h (35). The concentration of insulin was measured by ELISA and is presented as micrograms of insulin per gram of pancreas (32).
Histology of the fetal pancreas
Histological evaluation of the fetal pancreas was as previously described with minor modifications (25, 35–37). Frozen sections were incubated at 37°C for 30 min and then washed in deionized water. Sections were then heated in 10 mmol/L citric acid (pH 6.0) to 90°C for 10 min in a microwave. Sections were cooled for 20 min, washed, and then incubated in 0.1% Triton for 10 min. Sections were blocked for 60 min in 1.5% normal donkey serum (Sigma Life Science) in PBS. Incubations with primary antibodies and fluorescent-labeled Griffonia Simplicifolia Lectin 1 (GSL1) isolectin B4 (Vector Laboratories) were done overnight in 4°C. Incubations with secondary antibodies were done for 60 min at room temperature.
Pancreatic endocrine hormone identification
Endocrine hormones were identified in 4 sections of each pancreas with guinea pig anti-porcine insulin (Dako; 1:250; ref: A0564), mouse anti-porcine glucagon (Sigma; 1:500; ref: G2654), rabbit anti-human somatostatin (Dako; 1:500; ref: A0566), and rabbit anti-human pancreatic polypeptide (Abcam; 1:500; ref: AB113694). Immunocomplexes were detected with the following affinity-purified secondary antiserum (1:500): anti-rabbit IgG conjugated to Cy2, anti-mouse IgG conjugated to Alexa Fluor 549, and anti-guinea pig IgG conjugated to 7-amino-4-methylcoumarin-3-acetic acid (Jackson ImmunoResearch Laboratories).
Pancreatic vascularity identification
Pancreatic vascularity was evaluated in 2 sections of each pancreas with identification of endocrine hormones as aforementioned with the following modifications. Sections were blocked in a 0.5% tyramide signal amplification buffer (Perkin Elmer) and a 1% BSA solution (Fisher Scientific) was used for subsequent antibody incubations. Pancreatic ECs were identified using fluorescent-labeled GSL1 isolectin B4 (15 μg/mL). Endocrine hormones were identified as aforementioned except that somatostatin and pancreatic polypeptide immunocomplexes were detected using donkey anti-rabbit Alexa Flour 549 anti-serum (Jackson; 1:500).
Pancreatic β-cell replication
Pancreatic β-cell replication was evaluated in 2 sections of each pancreas as aforementioned with the following modifications. Insulin+ cells and Ki-67+ nuclei were identified with guinea pig anti-porcine insulin (Sigma; 1:100; ref: A0564) and Ki-67 (D3B5) Rabbit mAb (CellSignaling Technology; 1:500; ref: 9129S), respectively. Immunocomplexes were detected with affinity-purified secondary antiserum (Jackson; 1:500): anti-guinea pig IgG conjugated to Alexa Flour 680 and anti-rabbit IgG conjugated to Alexa Flour 488. Slides were washed in PBS before application of 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma; 1:1000).
Morphometric analysis
Images were captured with an Olympus IX-83 microscope (Olympus). Morphometric analysis for all parameters except Ki-67+ β cells was performed with CellSense software (Olympus). The entire pancreatic section was used to determine the percentage area of the pancreas which was insulin+ (β cells), glucagon+ (α cells), the pancreatic vascularity, and Ki-67+ β cells. The insulin+ and glucagon+ areas were used to calculate β-cell and α-cell mass, respectively, by multiplying the pancreas weight by the percentage of the total pancreas area positive for each target. Triple immunofluorescence with insulin, glucagon, and somatostatin plus pancreatic polypeptide was used to determine fetal pancreatic islet size in ≥15 fields of view (FOVs; FOV = 0.07 mm2) in each section (n = 100 ± 5 islets per animal). Insulin, glucagon, plus somatostatin plus pancreatic polypeptide, and lectin immunofluorescence were used to determine pancreatic islet vascularity in ≥20 FOVs (FOV = 0.07 mm2) in each section (n = 100 ± 7 islets per animal) by dividing the lectin+ area within an islet by the total islet area. The percentage of the islet area which was insulin+, glucagon+, or somatostatin+ and pancreatic polypeptide+ also was determined. Islets were defined as endocrine cell clusters containing ≥2 endocrine cell types that were >500 μm2 (36). Percentage areas and islet areas for each FOV or section from an animal were averaged to provide the value for that animal before summary and comparative analyses. FOVs were selected from endocrine hormone+ areas utilizing computerized random number generation. Triple fluorescence with insulin, DAPI, and Ki-67 was used to determine β-cell replication in entire sections (2 sections per animal, n = 84,526 ± 15,330 β cells per animal) using custom applications in Visiopharm image analysis software (Visiopharm). Scripts were developed to identify and quantify insulin+ areas within the endocrine pancreas and to identify and quantify DAPI and Ki-67 nuclear markers within insulin+ areas. Ki-67+ nuclei relative to the total nuclei (DAPI+) across all insulin+ areas were averaged per animal.
Pancreatic islet EC isolation and incubations
Pancreatic islet ECs were isolated as previously described from 4 late-gestation fetal sheep (37). Islets were isolated with collagenase digestion, then dispersed on collagen-coated 10-cm plates (Rat Tail Type 1, BD Biosciences) with DMEM with 10% FBS which was changed to Endothelial Cell Growth Medium MV2 (PromoCell) after 72 h. ECs were then allowed to propagate until the enriched EC population was ∼75% confluent. Islet ECs were then incubated with 10 mL of Roswell Park Memorial Institute (RPMI) Medium 1640 with 1% FBS (vol:vol) and 1.1 mmol/L glucose with either no further additions, 2.4 mmol/L leucine, or 50 ng/mL recombinant human VEGFA (R&D Systems) for 24 h. Islet ECs were then washed with ice-cold PBS and collected for RNA isolation. RNA was extracted from the samples, cDNA prepared, and analysis for HGF,RPS15(Ribosomal Protein S15), and GAPDH mRNA was performed as previously described (1, 25). Expression of HGF was normalized to the geometric means of the reference genes RPS15 and GAPDH, which were not different between the conditions. Results are expressed relative to incubation in nonsupplemented media.
Statistical analysis
Statistical analysis was performed using SAS version 9.2 (Statistical Analysis Software) and GraphPad Prism version 7.03 (GraphPad Software, Inc.). Results are expressed as means ± SEs. Maternal arterial biochemical values, gestational age, fetal weight, fetal lengths, placental weight, placentome number, organ weights, pancreas and pancreatic islet hormone areas, Ki67+ β cells, and pancreatic mRNAs were analyzed with Student's t test, except for maternal arterial plasma lactate, fetal kidney weight, somatostatin+-pancreatic polypeptide+ areas of the islet, and pancreatic SCL2A2and HGF mRNAs, which were analyzed with the Mann–Whitney test. Fetal arterial biochemical values, including insulin and glucagon secretion, were analyzed with a repeated-measures 2-factor ANOVA, which included terms for treatment group (LEU or CON), time, and interactions as main effects. If the interaction term of the ANOVA was P ≤ 0.1, then Fisher's protected least-squares difference was used for posttest comparisons. Data from isolated EC experiments were analyzed with the Kruskal–Wallis test. If the interaction term of the ANOVA was P ≤ 0.1, then Fisher's protected least-squares difference was used for posttest comparisons. Fetal sex was analyzed by a 2-sample test of equality of proportions. Because of the nonnormal distribution of islet size, these data were analyzed with a chi-squared test and the Van der Waerden and Savage tests for differences between the CON and LEU groups in the size distribution of the pancreatic islets. P values ≤ 0.05 were accepted as significant.
Results
Maternal and fetal metabolites, blood gases, and hormones during the infusion
LEU fetuses were infused with increasing rates of leucine (Figure 1A). This significantly increased fetal arterial plasma leucine concentrations in the LEU fetuses compared with baseline by day 2 (P < 0.05) (Figure 1B). Leucine concentrations were consistently higher in LEU than in CON fetuses by day 3 (P < 0.05) (Figure 1B). Fetal arterial plasma leucine concentrations were 100% higher in LEU fetuses than in controls at the end of the infusion (P < 0.0005). There were no differences between groups for maternal arterial biochemistry (Table 1). Fetal arterial blood hemoglobin, pH, partial pressure of carbon dioxide, partial pressure of oxygen, and plasma glucose and lactate were not different between the groups. However, fetal arterial hemoglobin oxygen saturation and oxygen concentration decreased in the LEU group and were ∼25% lower in LEU fetuses than in controls at the end of the study (P < 0.05) (Table 2). Except for fetal arterial plasma leucine concentrations, no other fetal amino acid concentration was significantly affected by the leucine infusion. Glutamate, isoleucine, and serine decreased and glutamine, histidine, and proline increased in both groups over time (P < 0.05) (Table 2). Asparagine and proline both started lower in LEU fetuses than in controls and stayed lower throughout the infusion period (P < 0.05) (Table 2). Fetal arterial plasma IGF-1, norepinephrine, and cortisol, as well as insulin before acute insulin secretion studies, were not affected by the leucine infusion (Table 2). Glucagon concentrations increased in the controls during the chronic infusion (P < 0.005) but not the LEU fetuses (Table 2).
FIGURE 1.
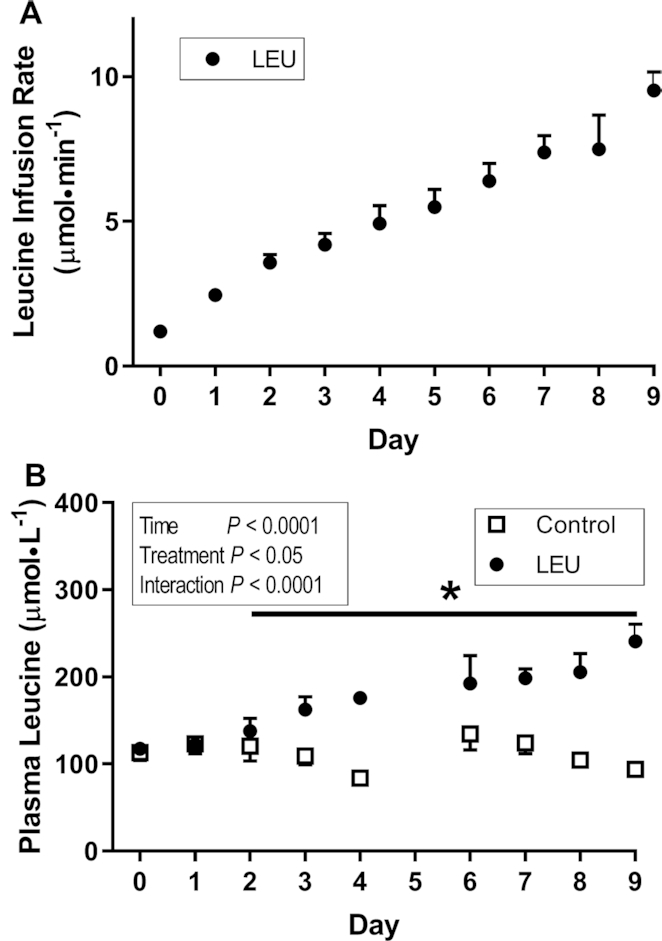
Fetal leucine infusion rates (A) and plasma leucine concentrations (B) in CON and LEU sheep. (A) Values are mean ± SE (n = 9) fetal leucine infusion rates in the LEU group. (B) Values are mean ± SE fetal arterial plasma leucine concentrations in CON (n = 8) and LEU (n = 9) animals. *Different from day 0 in the leucine group: P < 0.05. CON, control; LEU, leucine-infused.
TABLE 1.
Maternal arterial biochemistry in CON and LEU sheep1
| CON | LEU | P value | |
|---|---|---|---|
| Whole blood hemoglobin, g/dL | 5.95 ± 0.20 | 5.79 ± 0.13 | 0.51 |
| Whole blood pH | 7.46 ± 0.01 | 7.45 ± 0.01 | 0.70 |
| Whole blood pCO2, mm Hg | 34.7 ± 0.9 | 35.8 ± 0.9 | 0.40 |
| Whole blood pO2, mm Hg | 89.8 ± 2.2 | 91.4 ± 2.3 | 0.61 |
| Whole blood hemoglobin-O2 saturation, % | 97.4 ± 0.9 | 95.6 ± 0.9 | 0.18 |
| Whole blood O2 content, mmol/L | 5.6 ± 0.2 | 5.4 ± 0.1 | 0.34 |
| Plasma glucose, mmol/L | 3.5 ± 0.1 | 3.4 ± 0.1 | 0.14 |
| Plasma lactate, mmol/L | 0.7 ± 0.0 | 0.7 ± 0.1 | 0.88 |
| Plasma amino acids, μmol/L | |||
| Alanine | 145 ± 10 | 135 ± 11 | 0.50 |
| Arginine | 180 ± 11 | 169 ± 16 | 0.58 |
| Asparagine | 51 ± 5 | 48 ± 6 | 0.71 |
| Aspartate | 7 ± 1 | 7 ± 1 | 0.88 |
| Cystine | 23 ± 2 | 23 ± 2 | 0.88 |
| Glutamate | 56 ± 4 | 51 ± 4 | 0.42 |
| Glutamine | 337 ± 38 | 254 ± 17 | 0.06 |
| Glycine | 420 ± 43 | 461 ± 40 | 0.49 |
| Histidine | 55 ± 2 | 51 ± 3 | 0.32 |
| Isoleucine | 114 ± 9 | 101 ± 6 | 0.23 |
| Leucine | 142 ± 15 | 135 ± 7 | 0.69 |
| Lysine | 154 ± 16 | 157 ± 14 | 0.89 |
| Methionine | 33 ± 3 | 34 ± 2 | 0.84 |
| Phenylalanine | 63 ± 4 | 59 ± 4 | 0.44 |
| Proline | 119 ± 8 | 94 ± 9 | 0.07 |
| Serine | 82 ± 9 | 84 ± 9 | 0.88 |
| Taurine | 51 ± 6 | 50 ± 8 | 0.98 |
| Threonine | 172 ± 16 | 156 ± 20 | 0.53 |
| Tryptophan | 39 ± 3 | 39 ± 2 | 0.88 |
| Tyrosine | 77 ± 5 | 70 ± 5 | 0.30 |
| Valine | 225 ± 25 | 226 ± 12 | 0.98 |
Values are means ± SEs for CON (n = 8) and LEU (n = 9) groups. Measurements were made immediately before the fetal insulin secretion study. CON, control; LEU, leucine-infused; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen.
TABLE 2.
Fetal arterial biochemistry in CON and LEU sheep1
| CON | LEU | P values | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | Time | Treatment | Interaction | |
| Whole blood hemoglobin, g/dL | 6.59 ± 0.28 | 6.40 ± 0.32 | 6.78 ± 0.23 | 6.66 ± 0.21 | 0.03 | 0.69 | 0.39 |
| Whole blood pH | 7.37 ± 0.01 | 7.36 ± 0.01 | 7.37 ± 0.01 | 7.35 ± 0.01 | 0.08 | 0.80 | 0.642 |
| Whole blood pCO2, mm Hg | 51.1 ± 0.9 | 50.7 ± 0.7 | 53.4 ± 1.6 | 53.6 ± 1.1 | 0.64 | 0.09 | 0.90 |
| Whole blood pO2, mm Hg | 19.3 ± 1.6 | 19.6 ± 0.8 | 19.3 ± 1.2 | 17.7 ± 1.1 | 0.57 | 0.44 | 0.31 |
| Whole blood hemoglobin-O2 saturation, % | 43.6 ± 5.7a | 46.1 ± 3.1a | 42.6 ± 3.4ab | 34.1 ± 2.9b | 0.34 | 0.15 | 0.08 |
| Whole blood O2 content, mmol/L | 2.8 ± 0.3a | 2.9 ± 0.2a | 2.8 ± 0.2a | 2.2 ± 0.2b | 0.21 | 0.17 | 0.09 |
| Plasma glucose, mmol/L | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.1 | 0.9 ± 0.1 | <0.005 | 0.15 | 0.82 |
| Plasma lactate, mmol/L | 2.2 ± 0.4 | 2.3 ± 0.4 | 2.3 ± 0.1 | 2.6 ± 0.2 | 0.28 | 0.63 | 0.56 |
| Plasma amino acids, μmol/L | |||||||
| Alanine | 269 ± 36 | 291 ± 39 | 270 ± 15 | 295 ± 21 | 0.18 | 0.95 | 0.94 |
| Arginine | 76 ± 12 | 90 ± 14 | 63 ± 8 | 62 ± 6 | 0.28 | 0.14 | 0.24 |
| Asparagine | 38 ± 2 | 48 ± 7 | 34 ± 2 | 38 ± 2 | 0.12 | 0.13 | 0.48 |
| Aspartate | 19 ± 2 | 14 ± 1 | 13 ± 2 | 12 ± 2 | 0.08 | 0.03 | 0.21 |
| Cystine | 19 ± 2 | 15 ± 1 | 15 ± 2 | 15 ± 2 | 0.25 | 0.24 | 0.26 |
| Glutamate | 44 ± 6 | 34 ± 5 | 48 ± 4 | 41 ± 2 | <0.005 | 0.34 | 0.50 |
| Glutamine | 490 ± 49 | 498 ± 46 | 359 ± 25 | 377 ± 21 | 0.02 | 0.39 | 0.72 |
| Glycine | 257 ± 44 | 283 ± 37 | 316 ± 26 | 301 ± 19 | 0.80 | 0.35 | 0.34 |
| Histidine | 51 ± 5 | 64 ± 4 | 46 ± 4 | 53 ± 3 | <0.005 | 0.15 | 0.19 |
| Isoleucine | 85 ± 8 | 79 ± 8 | 84 ± 7 | 70 ± 4 | 0.03 | 0.54 | 0.33 |
| Lysine | 73 ± 10 | 78 ± 11 | 54 ± 4 | 59 ± 4 | 0.12 | 0.09 | 0.94 |
| Methionine | 76 ± 5 | 69 ± 7 | 94 ± 12 | 83 ± 8 | 0.22 | 0.14 | 0.75 |
| Phenylalanine | 106 ± 6 | 108 ± 7 | 110 ± 17 | 98 ± 8 | 0.46 | 0.84 | 0.25 |
| Proline | 155 ± 14 | 176 ± 15 | 122 ± 9 | 134 ± 7 | 0.02 | 0.02 | 0.43 |
| Serine | 585 ± 81 | 526 ± 71 | 632 ± 57 | 532 ± 35 | <0.005 | 0.76 | 0.36 |
| Taurine | 70 ± 13 | 67 ± 16 | 94 ± 20 | 74 ± 23 | 0.52 | 0.47 | 0.62 |
| Threonine | 244 ± 32 | 236 ± 29 | 206 ± 29 | 197 ± 21 | 0.68 | 0.28 | 0.97 |
| Tryptophan | 33 ± 4 | 39 ± 3 | 41 ± 2 | 40 ± 3 | 0.31 | 0.25 | 0.14 |
| Tyrosine | 123 ± 10 | 117 ± 9 | 122 ± 10 | 119 ± 12 | 0.41 | 0.97 | 0.74 |
| Valine | 335 ± 33 | 329 ± 39 | 361 ± 26 | 346 ± 10 | 0.53 | 0.57 | 0.78 |
| Plasma hormones | |||||||
| Insulin, ng/mL | 0.43 ± 0.11 | 0.38 ± 0.11 | 0.35 ± 0.05 | 0.40 ± 0.10 | 0.76 | 0.95 | 0.90 |
| Glucagon, pg/mL | 40.0 ± 10.0a | 61.0 ± 14.0b | 46.0 ± 4.0a | 48.0 ± 4.0ab | <0.01 | 0.73 | 0.02 |
| IGF-I, ng/mL | 102 ± 14 | 85 ± 12 | 83 ± 13 | 85 ± 10 | 0.20 | 0.56 | 0.13 |
| Norepinephrine, pg/mL | 587 ± 129 | 550 ± 104 | 468 ± 85 | 527 ± 104 | 0.89 | 0.59 | 0.54 |
| Cortisol, ng/mL | 6.8 ± 2.0 | 14.3 ± 3.3 | 14.1 ± 2.1 | 16.5 ± 2.2 | <0.005 | 0.15 | 0.11 |
Values are means ± SEs for CON (n = 8) and LEU (n = 9) fetuses. Measurements were made immediately before (Baseline) the fetal saline or leucine infusions started and immediately before fetal insulin secretion was measured 9 d after the infusions began (Final). Labeled values in a row without a common letter are significantly different, P < 0.05. CON, control; IGF-I, insulin-like growth factor I; LEU, leucine-infused; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen.
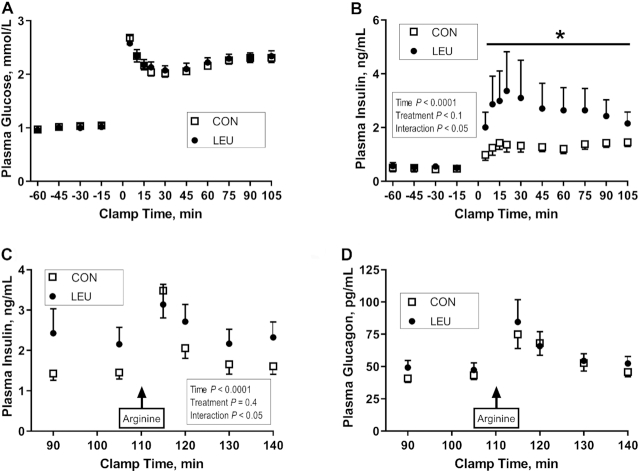
Fetal insulin and glucagon secretion at the end of the leucine infusion
Fetal insulin secretion was measured with a variable-rate, square-wave hyperglycemic clamp followed by a bolus arginine infusion. The baseline and hyperglycemic clamp glucose concentrations were similar in CON and LEU fetuses (Figure 2A). There was a tendency for higher glucose infusion rates required to achieve these hyperglycemic clamp glucose concentrations in LEU than in CON fetuses (P = 0.07; CON: 55.0 ± 4.3 μmol · min−1 · kg−1; LEU: 67.7 ± 4.8 μmol · min−1 · kg−1). Fetal insulin concentrations were significantly higher in the LEU group during the hyperglycemic clamp than in the control group (P < 0.05) (Figure 2B). After the arginine infusion, the expected increase in insulin concentrations between minute 105 and minute 115 was observed in CON fetuses (P < 0.0001) (Figure 2C). However, this was significantly attenuated in LEU fetuses (Figure 2C). After the arginine infusion, the mean glucagon concentration was higher than at baseline, but not different between the groups (Figure 2D).
FIGURE 2.
Fetal GSIS (A, B) and arginine-stimulated insulin (C) and glucagon (D) secretion in CON and LEU sheep. Fetal GSIS was measured with a fetal hyperglycemic clamp beginning at time 0. Values are means ± SEs in CON (n = 8 for insulin and glucose, and n = 6 for glucagon) and LEU (n = 9) fetuses. Fetal plasma glucose (A) and insulin (B) concentrations during measurement of GSIS. Fetal arginine-stimulated (C) insulin and (D) glucagon concentrations. *Difference between CON and LEU fetuses: P < 0.05. CON, control; GSIS, glucose-stimulated insulin secretion; LEU, leucine-infused.
Fetal and organ characteristics
There was a similar proportion of male fetuses in both groups. There were no differences between CON and LEU fetuses for fetal weight or length, any fetal organ weight measured, the number of placentomes, or the total placental weight (Table 3).
TABLE 3.
Fetal characteristics and organ weights in CON and LEU sheep1
| CON | LEU | P value | |
|---|---|---|---|
| Sex, % male | 78 | 50 | 0.23 |
| Gestational age, d | 134 ± 0 | 133 ± 0 | 0.24 |
| Fetal weight, g | 3331 ± 104 | 3086 ± 152 | 0.21 |
| Crown rump length, cm | 48.5 ± 0.9 | 46.9 ± 0.9 | 0.23 |
| Lower limb length, cm | 35.1 ± 0.5 | 35.9 ± 0.9 | 0.45 |
| Pancreas, g | 3.2 ± 0.2 | 3.6 ± 0.3 | 0.44 |
| Liver total, g | 90.7 ± 5.0 | 81.0 ± 4.6 | 0.17 |
| Kidneys, g | 21.2 ± 0.7 | 21.5 ± 2.0 | 0.74 |
| Perirenal adipose tissue, g | 10.8 ± 1.0 | 9.4 ± 0.6 | 0.25 |
| Spleen, g | 9.0 ± 0.7 | 7.7 ± 1.1 | 0.34 |
| Adrenal glands, g | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.66 |
| Lungs, g | 111.9 ± 6.9 | 110.6 ± 5.1 | 0.88 |
| Heart, g | 25.2 ± 0.9 | 25.9 ± 1.0 | 0.63 |
| Placentomes, n | 66 ± 6 | 69 ± 6 | 0.79 |
| Placental weight, g | 309.7 ± 21.6 | 276.4 ± 17.6 | 0.25 |
Values are means ± SEs for CON (n = 8) and LEU (n = 8–9) fetuses. CON, control; LEU, leucine-infused.
Fetal pancreatic histology
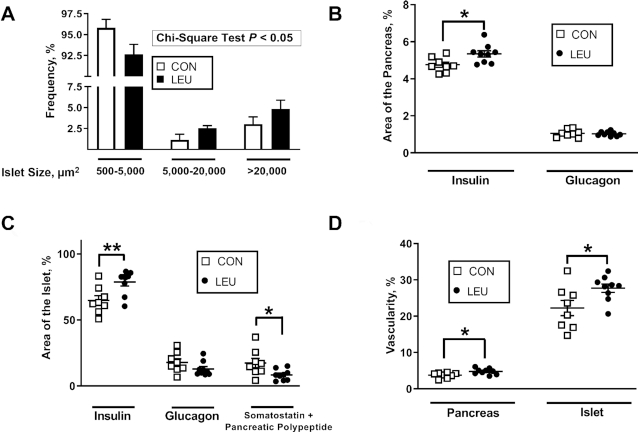
LEU pancreases were characterized by fewer small islets and more large islets (Figure 3A). Islet size was initially analyzed with a chi-squared test to demonstrate that the frequency distribution was statistically different between the 2 groups (P < 0.05). The Van der Waerden test (P < 0.0005) and the Savage test (P < 0.0001) for distributions confirmed a statistically significant difference in the distribution of islet size between the groups. The percentage area of the pancreas that stained for insulin mass was larger in LEU than in CON fetuses (P < 0.05) (Figure 3B). The percentage area of the pancreas that stained positive for glucagon was not different between CON and LEU fetuses (Figure 3B). The percentage of β cells that were Ki-67+ also was not different between CON and LEU fetuses (Table 4). The percentage area of the islet that stained positive for insulin was greater in LEU than in CON fetuses (P < 0.05) (Figure 3C). The percentage area of the islet that stained positive for glucagon was not different between groups, but the percentage area of the islet that stained for somatostatin and pancreatic polypeptide combined was lower in LEU fetuses than in controls (P < 0.05) (Figure 3C). Pancreatic vascularity and pancreatic islet vascularity were both greater in LEU than in CON fetuses (P < 0.05) (Figure 3D).
FIGURE 3.
Chronic leucine infusions in fetal sheep resulted in fetal pancreases with larger islets (A), larger insulin+ areas of the pancreas (B) and pancreatic islets (C), and more vascularization (D). (A) Values are means ± SEs in CON (n = 8) and LEU (n = 9) fetuses. The frequency, as a percentage of total islet number, of islets 500–5000, 5000–20,000, and >20,000 μm2 is shown for CON and chronically LEU fetuses. There was a higher proportion of larger islets and lower proportion of smaller islets in the LEU fetuses (P < 0.05). (B–D) Individual animal data with group means ± SEs are shown for CON (n = 8) and LEU (n = 9) fetuses. (B) Insulin+ and glucagon+ areas of the pancreas, (C) insulin+, glucagon+, and somatostatin+-pancreatic polypeptide+ areas of the islet, and (D) pancreatic and pancreatic islet vascularity. *,**Difference between CON and LEU fetuses: *P < 0.05; **P < 0.01. CON, control; LEU, leucine-infused.
TABLE 4.
Characteristics of the fetal pancreas in CON and LEU sheep1
| CON | LEU | P value | |
|---|---|---|---|
| β-Cell mass, mg | 154 ± 12 | 188 ± 28 | 0.13 |
| α-Cell mass, mg | 35 ± 4 | 36 ± 5 | 0.77 |
| Ki-67+ β cells, % | 3.78 ± 0.32 | 3.39 ± 0.47 | 0.51 |
| Insulin protein, μg/g of pancreas | 171 ± 30 | 172 ± 19 | 0.98 |
Values are means ± SEs for CON (n = 8) and LEU (n = 8–9) fetuses. CON, control; LEU, leucine-infused.
Leucine increases fetal pancreatic VEGFA and HGF
Pancreatic insulin protein content was similar between the 2 groups (Table 4). Figure 4 shows the pancreatic mRNA expressions of several different genes. Pancreatic INS and PDX1 mRNA expressions were similar in both groups. Pancreatic mRNA expressions of the 2 β cell–specific genes that allow for glucose entry into the cell and the initial phosphorylation of intracellular glucose, SLC2A2 and GCK, and of the 2 paracrine growth factors, VEGFA and HGF, were all greater in LEU fetuses than in controls (P < 0.05). GCG mRNA expression tended to be greater in LEU than in CON fetuses (P = 0.07). Next, we tested the role of leucine and VEGFA in regulating HGF expression in the isolated pancreatic islet ECs. HGF mRNA isolated from the ECs was consistently 20% higher after leucine supplementation compared with the nonsupplemented media (P < 0.05) (Figure 5). HGF mRNA after incubation with supplemental VEGFA was >50% higher than nonsupplemented media (P < 0.01) (Figure 5).
FIGURE 4.
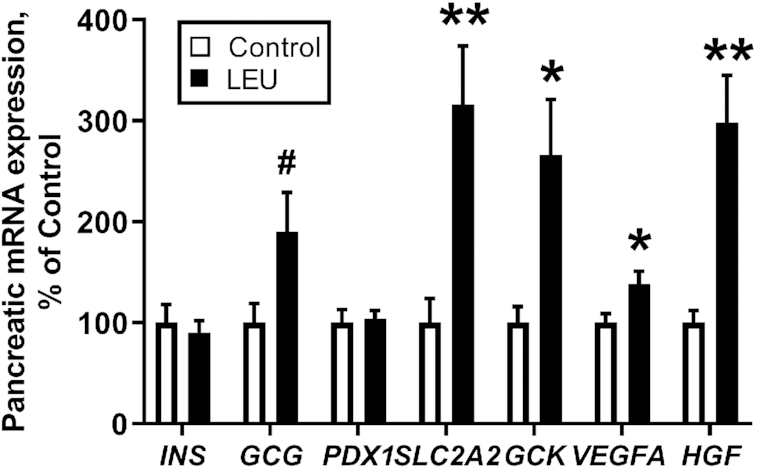
Chronic leucine infusions in fetal sheep resulted in more pancreatic mRNA expression of key metabolic and paracrine signaling genes. Values are means ± SEs in CON (n = 8) and LEU (n = 9) fetuses. Expression in LEU pancreases is shown relative to expression in CON pancreases. *,**Significant difference between CON and LEU fetuses: *P < 0.05; **P < 0.01. #P = 0.066. CON, control; LEU, leucine-infused.
FIGURE 5.
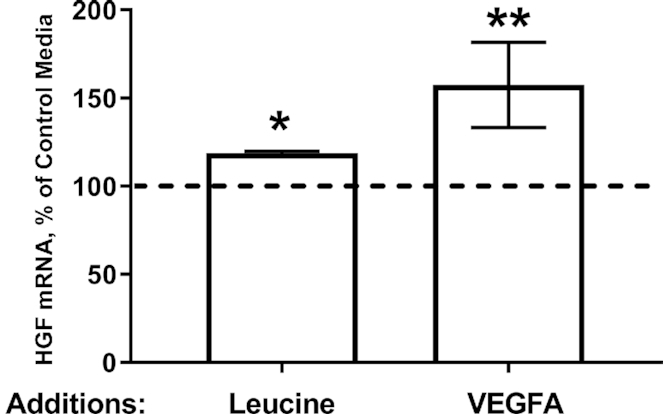
Supplemental leucine and VEGFA increase HGF mRNA expression in isolated fetal sheep islet ECs. Isolated fetal islet ECs were incubated with supplemental leucine (n = 4) or VEGFA (n = 3). HGF mRNA expression was then normalized to expression in control media. Values are means ± SEs. *,**Significant differences from nonsupplemented conditions: *P < 0.05; **P < 0.01. CON, control; EC, endothelial cell; GCG, glucagon; GCK, glucokinase; HGF, hepatocyte growth factor; INS, insulin; PDX1, pancreatic and duodenal homeobox-1; SLC2A2, glucose transporter-2; VEGFA, vascular endothelial growth factor A.
Discussion
Experimentally increasing fetal leucine concentrations for 9 d with a direct fetal leucine infusion in late-gestation normally growing fetuses potentiated fetal GSIS, replicating our previous findings that demonstrated potentiated fetal GSIS after chronically increasing the fetal amino acid supply with a complete amino acid mixture (1). Fetal insulin concentrations during the hyperglycemic clamp were twice as high after the chronic leucine infusion as in CON fetuses. The other novel and important results from the current study include the larger islet size, more vascularized islets, larger area of the pancreases that stained positive for insulin (β cells), and higher total pancreatic vascularity in the LEU fetuses. These histological features likely contributed to the higher GSIS in the LEU fetuses (16, 17). Moreover, we demonstrated that experimentally increasing fetal leucine concentrations for 9 d increased pancreatic HGF and VEGFA mRNA, important paracrine regulators of fetal pancreatic islet growth, vascularity, and function (17, 25, 37, 38). This result is supported by our in vitro experiments demonstrating that both leucine and VEGFA stimulate isolated fetal pancreatic islet EC HGF mRNA expression.
Leucine has been previously shown to stimulate insulin secretion and potentiate GSIS (5–11, 24). The mechanisms by which it does so in adult islets have been well described. Leucine can be metabolized as a cellular fuel and it also stimulates the metabolism of glucose by activating glutamate dehydrogenase (6–11). The metabolism of cellular fuels is one of the main pathways that links nutrient concentrations to insulin secretion by the β cell (12). LEU fetuses had higher pancreatic mRNA expression of the β cell–specific glucose transporter (GLUT2/SLC2A2) and hexokinase (GCK), which is consistent with an increase in β-cell glucose metabolism. This also is consistent with our previous demonstrations of leucine-potentiated GSIS in isolated fetal sheep islets (5, 24).
We targeted an increase in fetal leucine concentrations that was similar to the increase in fetal leucine concentrations achieved with the infusion of a complete amino acid mixture, which included leucine. Leucine concentrations by the end of the infusion period were similar in the LEU fetuses (∼200 μmol/L) compared with the experimental group in the previous study (∼250 μmol/L) (1, 26). The rate of leucine infusion in the current study (∼2.5 μmol · min−1 · kg−1) also was similar to that of the previous study (∼3 μmol · min−1 · kg−1) (1, 26). This rate is approximately two-thirds of the normal umbilical fetal leucine uptake rate from the placenta (∼4 μmol · min−1 · kg−1) (26). Importantly, unlike the previous study where the concentrations of most essential and many nonessential amino acids also increased in the amino acid–infused group, in the current study the leucine infusion had minimal impact on other amino acid concentrations or on glucose or lactate concentrations. In response to leucine, we identified larger islets with more β cells and a larger area of the pancreas that stained positive for insulin. This was not observed after a prolonged infusion of a complete amino acid mixture. We speculate that elevated concentrations or infusion of some other amino acid may have triggered a process which prevented the expansion of the pancreatic β cells during the complete amino acid infusion. Alternatively, other unidentified metabolic or hormonal changes might have been responsible for this difference between the response to leucine only and the response to the complete mixture of amino acids.
LEU fetuses had more vascularized pancreases and pancreatic islets than controls. These findings may have contributed to the larger islets with more β cells in the LEU fetuses. Paracrine signaling between β cells and ECs, specifically EC production of HGF and β-cell production of VEGFA, is required for normal islet development and function (17). Increasing HGF or VEGFA signaling pathways in the pancreas and pancreatic islet increases adult islet vascularity, β-cell mass, and/or insulin secretion (18, 39–41). Decreasing pancreatic and pancreatic islet HGF or VEGFA signaling reduces islet vascularity, β-cell mass, insulin secretion, and glucose tolerance in adult animals (19, 20, 42–45). We previously demonstrated that both a full complement of essential amino acids or just supplemental leucine stimulate HGF production in isolated large-vessel fetal sheep ECs (25), consistent with experiments in isolated adult rodent islet ECs and other cell types (22, 46). Consistently with these previous results, we found that pancreatic expression of HGF mRNA was 3-fold higher in LEU fetuses than in controls. Furthermore, LEU fetal pancreases contained almost 40% more VEGFA mRNA than controls. VEGFA stimulates EC production of HGF, including in isolated large-vessel fetal sheep ECs, and could therefore also be responsible for increased pancreatic HGF expression (25). Our in vitro studies confirm that both leucine and VEGFA have the capacity to stimulate HGF expression in isolated pancreatic islet ECs. Glucagon is another potential paracrine factor produced within the pancreatic islet that has the capacity to potentiate GSIS and stimulate pancreatic β-cell replication (28–31). However, after a chronic leucine infusion, we did not observe an increase in fetal glucagon concentrations, arginine-stimulated fetal glucagon secretion, or the area of the pancreas that was glucagon+.
This is the first study, to our knowledge, to demonstrate that increasing fetal leucine concentrations potentiates fetal GSIS and leads to increased fetal pancreatic islet size, vascularity, and β cells. Combined with previous results demonstrating that amino acids potentiate fetal GSIS (1), it is clear that glucose and amino acids act synergistically to stimulate fetal insulin secretion. Furthermore, given the current data and the previously published data demonstrating that leucine acutely increases fetal insulin concentrations and stimulates release of insulin from isolated fetal sheep islets (5, 24), leucine clearly has an important impact on the function of fetal pancreatic β cells. We have now shown that leucine has an important trophic effect on fetal pancreatic islet development and fetal β-cell function, independent of an increase in the concentrations of other amino acids or glucagon. The regulatory role of leucine involves stimulation of pancreatic paracrine growth factors such as VEGFA and HGF. It also may work through direct activation of intracellular signaling pathways. Future studies will determine the relative contribution of these various pathways to leucine's regulatory role for fetal insulin secretion and development of the pancreas and pancreatic islet.
Acknowledgments
The authors’ responsibilities were as follows—BHB: managed the software; BHB and PJR: performed the validation, conducted the formal analysis, curated the data, wrote the original draft, and performed the visualizationa; LDB, SRW, and WWH: reviewed and edited the manuscript; PJR: acquired the funding and is the guarantor of this work; and all authors: conceptualized the study, determined the methodology, and read and approved the final manuscript.
Notes
Portions of this work were presented at the Society of Reproductive Investigation 65th Annual Meeting, San Diego, California, March 6–10, 2018.
Supported by National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK088139 (to PJR) and R01DK108910 (to SRW), National Institute of Child Health and Human Development grants R01HD093701 (to PJR), T32HD007186 [to BHB (trainee) and PJR (principal investigator)], R01HD079404 (to LDB), and K12HD068372 (to WWH), and NIH grant S10OD023553 (to LDB).
Author disclosures: the authors report no conflicts of interest.
Abbreviations used: ACTA, actin; ASIS, arginine-stimulated insulin secretion; CON, control; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; EC, endothelial cell; FOV, field of view; GCG, glucagon; GCK, glucokinase; GLUT2, glucose transporter-2; GSIS, glucose-stimulated insulin secretion; GSL1, Griffonia Simplicifolia Lectin 1; HGF, hepatocyte growth factor; IGF-I, insulin-like growth factor I; INS, insulin; LEU, leucine-infused; PDX1, pancreatic and duodenal homeobox-1; RPS15, Ribosomal Protein S15; SLC2A2, glucose transporter-2; VEGFA, vascular endothelial growth factor A.
References
- 1. Gadhia MM, Maliszewski AM, O'Meara MC, Thorn SR, Lavezzi JR, Limesand SW, Hay WW Jr, Brown LD, Rozance PJ. Increased amino acid supply potentiates glucose-stimulated insulin secretion but does not increase β-cell mass in fetal sheep. Am J Physiol Endocrinol Metab. 2013;304:E352–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowden AL. Effects of adrenaline and amino acids on the release of insulin in the sheep fetus. J Endocrinol. 1980;87:113–21. [DOI] [PubMed] [Google Scholar]
- 3. Gresores A, Anderson S, Hood D, Zerbe GO, Hay WW Jr. Separate and joint effects of arginine and glucose on ovine fetal insulin secretion. Am J Physiol. 1997;272:E68–73. [DOI] [PubMed] [Google Scholar]
- 4. Philipps AF, Dubin JW, Raye JR. Alanine-stimulated insulin secretion in the fetal and neonatal lamb. Am J Obstet Gynecol. 1980;136:597–602. [DOI] [PubMed] [Google Scholar]
- 5. Rozance PJ, Limesand SW, Hay WW Jr. Decreased nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab. 2006;291:E404–11. [DOI] [PubMed] [Google Scholar]
- 6. Fajans SS, Floyd JC Jr, Knopf RF, Guntsche EM, Rull JA, Thiffault CA, Conn JW. A difference in mechanism by which leucine and other amino acids induce insulin release. J Clin Endocrinol Metab. 1967;27:1600–6. [DOI] [PubMed] [Google Scholar]
- 7. Gao Z, Young RA, Li G, Najafi H, Buettger C, Sukumvanich SS, Wong RK, Wolf BA, Matschinsky FM. Distinguishing features of leucine and α-ketoisocaproate sensing in pancreatic β-cells. Endocrinology. 2003;144:1949–57. [DOI] [PubMed] [Google Scholar]
- 8. Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Matschinsky FM, Stanley CA. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem. 2003;278:2853–8. [DOI] [PubMed] [Google Scholar]
- 9. Malaisse WJ, Hutton JC, Carpinelli AR, Herchuelz A, Sener A. The stimulus-secretion coupling of amino acid-induced insulin release: metabolism and cationic effects of leucine. Diabetes. 1980;29:431–7. [DOI] [PubMed] [Google Scholar]
- 10. McClenaghan NH, Barnett CR, O'Harte FP, Flatt PR. Mechanisms of amino acid-induced insulin secretion from the glucose-responsive BRIN-BD11 pancreatic B-cell line. J Endocrinol. 1996;151:349–57. [DOI] [PubMed] [Google Scholar]
- 11. Sener A, Malaisse WJ. l-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288:187–9. [DOI] [PubMed] [Google Scholar]
- 12. Malaisse WJ, Sener A, Herchuelz A, Hutton JC. Insulin release: the fuel hypothesis. Metabolism. 1979;28:373–86. [DOI] [PubMed] [Google Scholar]
- 13. Proud CG. Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans. 2007;35:1187–90. [DOI] [PubMed] [Google Scholar]
- 14. Draoui N, de Zeeuw P, Carmeliet P. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biol. 2017;7(12):170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocana A. Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes. 2014;63:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boehmer BH, Limesand SW, Rozance PJ. The impact of IUGR on pancreatic islet development and β-cell function. J Endocrinol. 2017;235:R63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rozance PJ, Hay WW Jr. Pancreatic islet hepatocyte growth factor and vascular endothelial growth factor A signaling in growth restricted fetuses. Mol Cell Endocrinol. 2016;435:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–7. [DOI] [PubMed] [Google Scholar]
- 19. Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–4. [DOI] [PubMed] [Google Scholar]
- 20. Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J et al. Pancreatic islet production of vascular endothelial growth factor-A is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–85. [DOI] [PubMed] [Google Scholar]
- 21. Johansson A, Lau J, Sandberg M, Borg LAH, Magnusson PU, Carlsson P-O. Endothelial cell signalling supports pancreatic beta cell function in the rat. Diabetologia. 2009;52:2385–94. [DOI] [PubMed] [Google Scholar]
- 22. Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson P-O. Islet endothelial cells and pancreatic β-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–24. [DOI] [PubMed] [Google Scholar]
- 23. Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N et al. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev Cell. 2006;10:397–405. [DOI] [PubMed] [Google Scholar]
- 24. Benjamin JS, Culpepper CB, Brown LD, Wesolowski SR, Jonker SS, Davis MA, Limesand SW, Wilkening RB, Hay WW Jr, Rozance PJ. Chronic anemic hypoxemia attenuates glucose-stimulated insulin secretion in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2017;312:R492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown LD, Davis M, Wai S, Wesolowski SR, Hay WW Jr, Limesand SW, Rozance PJ. Chronically increased amino acids improve insulin secretion, pancreatic vascularity and islet size in growth restricted fetal sheep. Endocrinology. 2016;157(10):3788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maliszewski AM, Gadhia MM, O'Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab. 2012;302:E1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rozance PJ, Crispo MM, Barry JS, O'Meara MC, Frost MS, Hansen KC, Hay WW Jr, Brown LD. Prolonged maternal amino acid infusion in late-gestation pregnant sheep increases fetal amino acid oxidation. Am J Physiol Endocrinol Metab. 2009;297:E638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gelling RW, Vuguin PM, Du XQ, Cui L, Rømer J, Pederson RA, Leiser M, Sørensen H, Holst JJ, Fledelius C et al. Pancreatic β-cell overexpression of the glucagon receptor gene results in enhanced β-cell function and mass. Am J Physiol Endocrinol Metab. 2009;297:E695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curry DL. Glucagon potentiation of insulin secretion by the perfused rat pancreas. Diabetes. 1970;19:420–8. [DOI] [PubMed] [Google Scholar]
- 30. Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43:1012–9. [DOI] [PubMed] [Google Scholar]
- 31. Moens K, Flamez D, Van Schravendijk C, Ling Z, Pipeleers D, Schuit F. Dual glucagon recognition by pancreatic β-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes. 1998;47:66–72. [DOI] [PubMed] [Google Scholar]
- 32. Culpepper C, Wesolowski SR, Benjamin J, Bruce JL, Brown LD, Jonker SS, Wilkening RB, Hay WW Jr, Rozance PJ. Chronic anemic hypoxemia increases plasma glucagon and hepatic PCK1 mRNA in late-gestation fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2016;311:R200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Green AS, Macko AR, Rozance PJ, Yates DT, Chen X, Hay WW Jr, Limesand SW. Characterization of glucose-insulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metab. 2011;300:E817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW Jr. Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab. 2012;303:E352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rozance PJ, Limesand SW, Zerbe GO, Hay WW Jr. Chronic fetal hypoglycemia inhibits the later steps of stimulus-secretion coupling in pancreatic β-cells. Am J Physiol Endocrinol Metab. 2007;292:E1256–64. [DOI] [PubMed] [Google Scholar]
- 36. Limesand SW, Jensen J, Hutton JC, Hay WW Jr. Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1297–305. [DOI] [PubMed] [Google Scholar]
- 37. Rozance PJ, Anderson M, Martinez M, Fahy A, Macko AR, Kailey J, Seedorf GJ, Abman SH, Hay WW Jr, Limesand SW. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes. 2015;64:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johansson M, Andersson A, Carlsson P-O, Jansson L. Perinatal development of the pancreatic islet microvasculature in rats. J Anat. 2006;208:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dai C, Li Y, Yang J, Liu Y. Hepatocyte growth factor preserves beta cell mass and mitigates hyperglycemia in streptozotocin-induced diabetic mice. J Biol Chem. 2003;278:27080–7. [DOI] [PubMed] [Google Scholar]
- 40. Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–32. [DOI] [PubMed] [Google Scholar]
- 41. García-Ocaña A, Vasavada RC, Cebrian A, Reddy V, Takane KK, López-Talavera JC, Stewart AF. Transgenic overexpression of hepatocyte growth factor in the β-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50:2752–62. [DOI] [PubMed] [Google Scholar]
- 42. Dai C, Huh CG, Thorgeirsson SS, Liu Y. β-Cell-specific ablation of the hepatocyte growth factor receptor results in reduced islet size, impaired insulin secretion, and glucose intolerance. Am J Pathol. 2005;167:429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–76. [DOI] [PubMed] [Google Scholar]
- 44. Mellado-Gil J, Rosa TC, Demirci C, Gonzalez-Pertusa JA, Velazquez-Garcia S, Ernst S, Valle S, Vasavada RC, Stewart AF, Alonso LC et al. Disruption of hepatocyte growth factor/c-Met signaling enhances pancreatic β-cell death and accelerates the onset of diabetes. Diabetes. 2011;60:525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reinert RB, Brissova M, Shostak A, Pan FC, Poffenberger G, Cai Q, Hundemer GL, Kantz J, Thompson CS, Dai C et al. Vascular endothelial growth factor-A and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes. 2013;62:4154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tomiya T, Nishikawa T, Inoue Y, Ohtomo N, Ikeda H, Tejima K, Watanabe N, Tanoue Y, Omata M, Fujiwara K. Leucine stimulates HGF production by hepatic stellate cells through mTOR pathway. Biochem Biophys Res Commun. 2007;358:176–80. [DOI] [PubMed] [Google Scholar]