ABSTRACT
Background
The intestinal epithelial cells, food molecules, and gut microbiota are continuously exposed to intestinal peristaltic shear force. Shear force may impact the crosstalk of human milk oligosaccharides (hMOs) with commensal bacteria and intestinal epithelial cells.
Objectives
We investigated how hMOs combined with intestinal peristaltic shear force impact intestinal epithelial cells and crosstalk with a commensal bacterium.
Methods
We applied the Ibidi system to mimic intestinal peristaltic shear force. Caco-2 cells were exposed to a shear force (5 dynes/cm2) for 3 d, and then stimulated with the hMOs, 2′-fucosyllactose (2′-FL), 3-FL, and lacto-N-triose II (LNT2). In separate experiments, Lactobacillus plantarumWCFS1 adhesion to Caco-2 cells was studied with the same hMOs and shear force. Effects were tested on gene expression of glycocalyx-related molecules (glypican 1 [GPC1], hyaluronan synthase 1 [HAS1], HAS2, HAS3, exostosin glycosyltransferase 1 [EXT1], EXT2), defensin β-1 (DEFB1), and tight junction (tight junction protein 1 [TJP1], claudin 3 [CLDN3]) in Caco-2 cells. Protein expression of tight junctions was also quantified.
Results
Shear force dramatically decreased gene expression of the main enzymes for making glycosaminoglycan side chains (HAS3 by 43.3% and EXT1 by 68.7%) (P <0.01), but did not affect GPC1 which is the gene responsible for the synthesis of glypican 1 which is a major protein backbone of glycocalyx. Expression of DEFB1, TJP1, and CLDN3 genes was decreased 60.0–94.9% by shear force (P <0.001). The presence of L. plantarumWCFS1 increased GPC1, HAS2, HAS3, and ZO-1 expression by 1.78- to 3.34-fold (P <0.05). Under shear force, all hMOs significantly stimulated DEFB1 and ZO-1, whereas only 3-FL and LNT2 enhanced L. plantarumWCFS1 adhesion by 1.85- to 1.90-fold (P <0.01).
Conclusions
3-FL and LNT2 support the crosstalk between the commensal bacterium L. plantarumWCFS1 and Caco-2 intestinal epithelial cells, and shear force can increase the modulating effects of hMOs.
Keywords: shear force, human milk oligosaccharides, Lactobacillus plantarumWCFS1, gut barrier function, intestinal epithelium
Introduction
Breastfeeding is considered to be the gold standard for infant nutrition. Mother milk contains many molecules responsible for preventing disease and support of health in infants. However, in ∼70% of neonates it is not possible to solely feed the infants on mother milk. In the majority of those cases, cow-milk-derived formula is given to infants. These cow-milk formulas lack, for example, human milk oligosaccharides (hMOs), which are essential for many gastrointestinal immune barrier processes. Up to now, the function of these hMOs was substituted by nondigestible carbohydrates such as inulins and galactooligosaccharides that can take over some but not all of the functions of hMOs (1, 2). However, during recent years, novel molecular approaches have led to the development of procedures to manufacture hMOs in sufficient, industrially relevant amounts to allow application of specific hMO molecules in infant formula (3).
HMOs have been reported to contribute to the development of gut barrier function (4), mucus production (5), glycocalyx development (6), immune maturation (7, 8), and shaping of the gut microbiota (9, 10). However, many of these functions are identified in in vitro studies in intestinal cells under static culture, without the shear force that intestinal epithelium and other components of the gut immune barrier experience during peristalsis (11). Intestinal epithelial cells, and in particular, the glycocalyx-containing apical brush border, have the ability to sense shear force and to provide downstream signals that are converted to biochemical responses (12). These mechanical forces induce signals that are considered to regulate cell-phenotype, ion-exchange, glycocalyx composition, and may modulate tight junction protein gene expression (11, 13, 14).
The mechanical forces on epithelial cells may also influence the adhesion of commensal bacteria (15). This is especially relevant in early life where adhesion of commensal bacteria is the first step to colonize the intestine, but also in later life where the adequacy of commensal bacterial adhesion is essential in prevention of pathogen adhesion and invasion of the host (16). Some commensal bacteria possess an exceptional resistance to mechanical forces. For instance, Lactobacillus rhamnosus GG persistently adheres to the gut epithelium even when exposed to a stepwise increase of shear force (15). It is unknown whether and how mother-milk-derived molecules such as hMOs contribute to the development of resistance to shear forces in the intestine, and whether they can enhance commensal bacterial adhesion to epithelial cells. This knowledge may be essential when developing hMO-containing infant formula that should support early life microbiota colonization or support gut barrier development.
As there is limited information available about how shear force influences the beneficial effects of hMOs on the development of the intestinal epithelium barrier and how it influences commensal bacterial adhesion, we applied a so-called Ibidi system to mimic the shear force during intestinal peristalsis. To this end, intestinal Caco-2 epithelial cells were exposed to a shear force of 5 dynes/cm2 for 3 d, followed by stimulation with hMOs. In separate experiments this was performed in the presence and absence of the commensal bacterium Lactobacillus plantarumWCFS1. We studied adhesion and changes in gene expression in the epithelial cells. Effects were tested on gene expression of glycocalyx-related molecules (glypican 1 [GPC1], hyaluronan synthase 1 [HAS1], HAS2, HAS3, exostosin glycosyltransferase 1 [EXT1], EXT2), antimicrobial peptide defensin β-1 (DEFB1), and tight junction (tight junction protein 1 [TJP1, also known as ZO-1], and claudin 3 [CLDN3]). The immunofluorescence intensity of tight junction proteins was also quantified. We tested the effects of 2′-fucosyllactose (2′-FL) and 3-FL, which are 2 of the most abundant hMOs in mother milk and can be produced in sufficient amounts to be included in infant formula. We also tested lacto-N-triose II (LNT2), which is a short-chain hMO, formed during passage through the gastrointestinal tract by acidic hydrolysis in the stomach.
Methods
hMOs
The hMO 2′-FL was provided by FrieslandCampina Domo, and 3-FL and LNT2 were provided by Glycosyn LLC. An overview of the structure is shown in Table 1. hMOs were dissolved into 2 mg/mL antibiotic-free cell culture medium before use. The medium without hMOs served as the control.
TABLE 1.
Overview of the structures of selected human milk oligosaccharides
| Name1 | Chemical composition | Structure2 |
|---|---|---|
| 2′-FL | Fucα1–2Galβ1–4Glc |
|
| 3-FL | Galβ1–4(Fucα1–3)Glc |
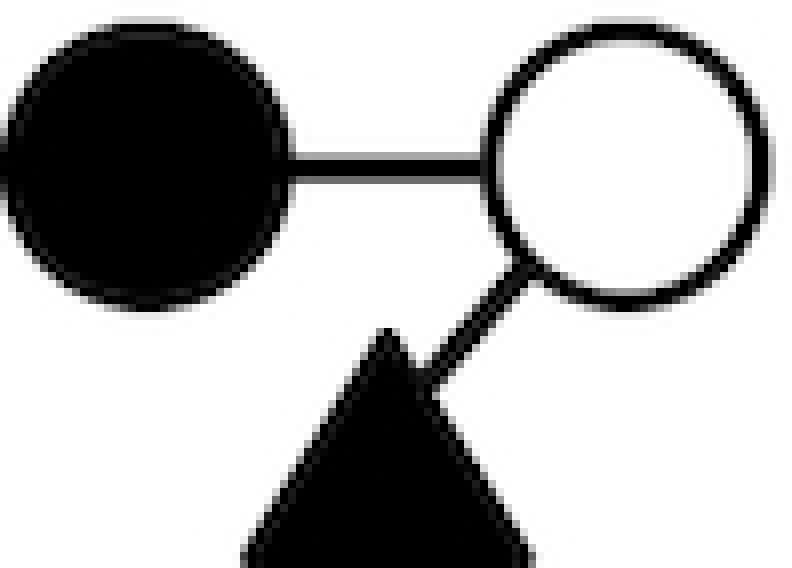
|
| LNT2 | GlcNAcβ1–3Galβ1–4Glc |
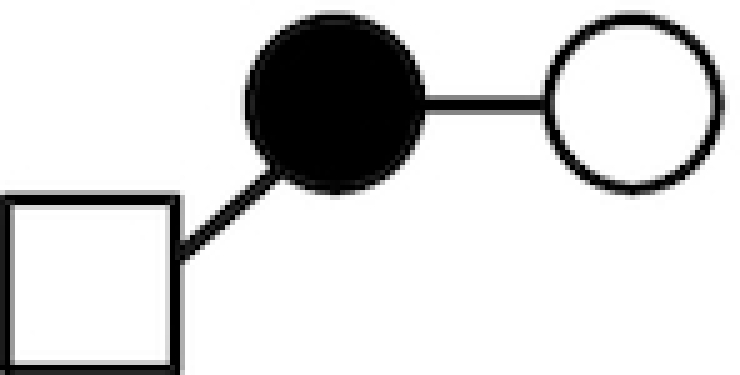
|
FL, fucosyllactose; LNT2, lacto-N-triose II.
○ Glucose; • Galactose; ▴ Fucose; □ N-acelylglucosamine.
Cell culture and test systems
Human colorectal adenocarcinoma Caco-2 cells were cultured with humidified 5% CO2 at 37°C, in DMEM (Lonza, Cat no. 12–604F) with glucose (4.5 g/L) and L-glutamine, supplemented with 10% fetal calf serum (Invitrogen), 1% nonessential amino acid solution (100×, Sigma), 0.5% penicillin-streptomycin (50 μg/mL-50 μg/mL, Sigma), and 10 mM HEPES (Sigma). The 5% CO2 is required for survival of Caco-2 cells and is the CO2 concentration in human blood and the intestinal tract as measured by ingestible gas-sensing capsules (17). The cells were used for hMO stimulation and commensal bacterial adhesion under 2 conditions. The first was a static incubation in which both gut epithelial cells and bacteria were not subjected to shear force, whereas the other was a culture in a so-called lbidi system in which shear force, mimicking the peristaltic forces in the intestine, was applied to both bacteria and gut epithelial cells. For static culture, the cell density was adjusted to 3*104/mL before seeding onto 24-well plates for bacterial adhesion assays, and 8-well chamber slides for immunofluorescence staining. The cells were cultured for 21 d until a transepithelial electrical resistance (TEER) value of 300 ± 100 Ω· cm2 was reached. For shear force culture, the cells were cultured in μ-Slide I 0.4 Luer ibiTreat flow chamber slides specially for the Ibidi pump system (ibidi GmbH). After 21 d of culture, the cells were stimulated with hMOs for another 2 h.
Before introducing Caco-2 cells into the Ibidi pump system, cells were grown on μ-Slide I 0.4 Luer for 18 d, followed by 3 d (18) exposure to a constant shear force of 5 dynes/cm2. During intestinal peristalsis, the shear force values vary between 0.02 and 35 dynes/cm2 as a consequence of various contraction frequencies in different parts of the intestine (19, 20). However, due to the presence of microvilli, shear forces are usually 5 dynes/cm2 or higher (13). For this reason 5 dynes/cm2 was chosen in our experimental setup.
Bacterial cultures
L. plantarumWCFS1 was cultured at 37°C in De Man, Rogosa and Sharpe (MRS) broth (Merck) from glycerol stock at −80°C. After overnight recovery and MRS agar plating, single colonies from the plate were inoculated in MRS broth for a second overnight culture at 37°C before application in the adhesion assay.
L. plantarum WCFS1 adhesion assay
L. plantarumWCFS1 was collected the next day after centrifugation at 2000 × g for 10 min and washed once with prewarmed PBS. The optical density (OD) was adjusted to OD540 = 0.6 in PBS and resuspended with a half volume of antibiotic-free cell culture medium containing 2′-FL, 3-FL, and LNT2, or only cell culture medium. After 2 h incubation at 37°C, the bacteria were brought onto the Caco-2 cells either under static or shear force culture for another 2 h at 37°C. Afterwards, the Caco-2 cells were gently washed with prewarmed PBS 3 times to remove the nonadherent bacteria. The adherent bacteria were released by adding 200 μL of 0.1% Triton-X100, followed by serial dilutions in PBS. Drop-plating (21) was applied to plate the adherent bacteria on MRS agar plates. The total CFUs were determined.
RNA isolation and reverse transcription
After stimulation with hMOs or bacteria, Caco-2 cells were lysed with TRIzol reagent (Life Technologies). RNA isolation and reverse transcription were performed as previously described (5).
Gene expression
GPC1, HAS1, HAS2, HAS3, EXT1, EXT2, DEFB1, TJP1, and CLDN3 expression was quantified with SYBR® Green Real time PCR Master Mix (Sigma). Reactions were carried out as previously described (5). The 2−ΔCt method was used to calculate relative mRNA expression normalized to the housekeeping gene GAPDH. The 2−ΔΔCt method was used for calculating fold change in gene expression levels versus untreated controls. All the primers were synthesized by Sigma-Aldrich as previously described in Supplemental Table 1 (22–27).
Immunofluorescence staining
The slides were collected after incubation with hMOs or hMO-treated bacteria. The cells were washed twice with 0.01% CaCl2, and fixed with ice-cold acetone/methanol (1:1, v/v) for 5 minutes at −20°C. Afterwards, cells were washed 3 times with PBS and blocked with 10% goat serum in 1% BSA for 1 h at room temperature. After overnight incubation with the primary antibody for ZO-1 (ZO-1 Polyclonal Antibody, 1:200, Thermo Fisher Scientific) and claudin-3 (claudin-3 Polyclonal Antibody, 1:50, Thermo Fisher Scientific) at 4°C, the cells were washed 3 times with PBS, and incubated with secondary antibody biotinylated goat anti-rabbit (1:500, Dako) for 1 h at room temperature. The cells were then washed 3 times with PBS and labeled with Streptavidin fluorescein isothiocyanate (FITC) (1:500, BioLegend) in the dark for 1 h. The cell nuclei were stained with DAPI (1:5000, Sigma) followed by 3 washes with PBS.
Confocal microscopy and Image J analysis
All the immunofluorescence images were captured with a Leica SP8 confocal laser microscope (Leica Microsystems) with the 40×/1.3 oil differentia linterference contrast (DIC) objective. FITC was excited at 488 nm and emitted at 500–600 nm (green); 4,6-diamidino-2-phenylindole (DAPI) was excited at 405 nm and emitted at 410–450 nm (blue). For analysis, Z-stack (512- × 512- pixel resolution × 8 bit) images were taken by a field of view 290.62 μm × 290.62 μm with step lengths of 1.0 μm from the bottom to the top of the Caco-2 cells monolayer. At least 3 images were captured for each sample in 1 experiment. To estimate the fluorescence intensity of the tight junction, the maximum intensity plane in the Z-stack of the FITC channel was chosen and analyzed by Image J procedure (Version 1.51n; NIH).
Statistical analysis
Statistical analysis was performed by GraphPad Prism 6 (GraphPad Prism Software Inc.). Results were expressed as mean ± SD. Normality of data distribution was confirmed using the Kolmogorov–Smirnov test. Unpaired t-test was applied to test the effect by shear force. All other data were analyzed using 1-factor ANOVA followed by Dunnett's multiple comparisons test. Nonparametrically distributed data were analyzed using the Kruskal–Wallis test with Dunn's multiple comparisons test. Significant difference was defined as P <0.05.
Results
Shear force modifies gene expression of glycocalyx-related molecules, antimicrobial peptide, and gene and protein expression of tight junction in gut epithelial Caco-2 cells
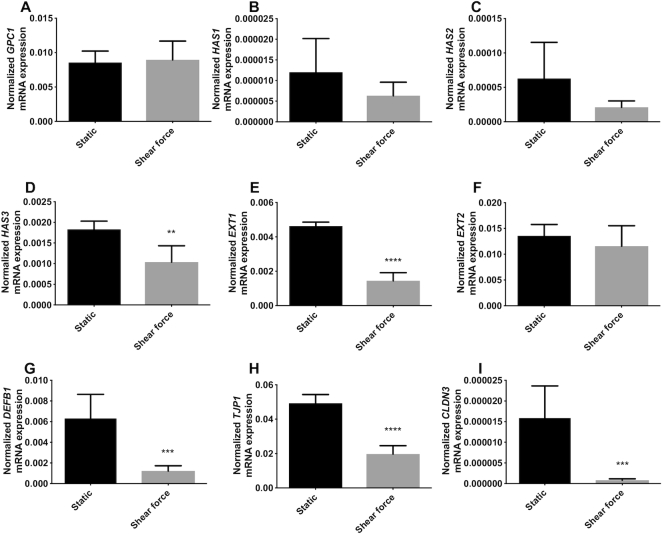
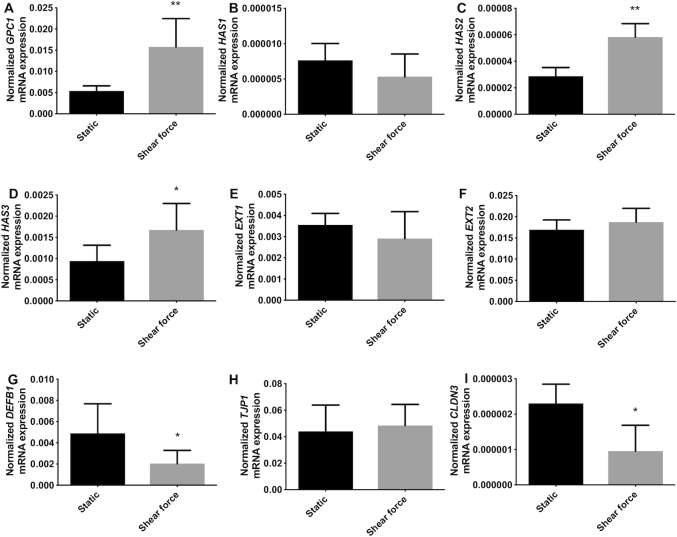
As shown in Figure 1, shear force exposure significantly downregulated gene expression of specific glycocalyx-related molecules compared with cells under static conditions. Shear force did not change the gene expression of GPC1, HAS1, HAS2, and EXT2 (Figure 1A–C, F), but significantly downregulated HAS3 (P <0.01, Figure 1D) and EXT1 (P <0.0001, Figure 1E), which decreased by 43.3% and 68.7%, respectively, compared with cells under static conditions.
FIGURE 1.
Shear force modifies gene expression of glycocalyx-related molecules, antimicrobial peptide, and tight junction in intestinal epithelial Caco-2 cells. (A) GPC1, (B) HAS1, (C) HAS2, (D) HAS3, (E) EXT1, and (F) EXT2 gene expression of the glycocalyx under static conditions and shear force; (G) DEFB1, 1 antimicrobial peptide gene expression under static conditions and shear force; (H) TJP1 and (I) CLDN3 gene expression of the tight junction protein under static conditions and shear force. Results are presented as relative values normalized to the housekeeping gene GAPDH. Values are means ± SDs, n = 6. Asterisks indicate different from static: **P <0.01, ***P <0.001, ****P <0.0001. CLDN3, claudin 3; DEFB1, defensin β-1; EXT, exostosin glycosyltransferase; GPC1, glypican 1; HAS, hyaluronan synthase; TJP1, tight junction protein 1.
Shear force exposure also induced a significant downregulation of gene expression of the antimicrobial peptide DEFB1 as shown in Figure 1G (P <0.001). This reduction was 80.5% compared with the cells cultured under static conditions.
The gene expression of tight junction proteins TJP1 and CLDN3 were significantly downregulated when the epithelial cells were exposed to shear force. TJP1 was downregulated 60.0% compared with cells under static culture (P <0.0001, Figure 1H). CLDN3 resulted in a more pronounced reduction (P <0.001, Figure 1I), with a decrease of 94.9% when the epithelial cells were exposed to shear force.
We also studied tight junction compositions on a protein level as shown in Supplemental Figure 1A, 1B, 1D, and 1E. Shear force tended to lower the fluorescence intensity of ZO-1, but this did not reach statistical significance (P = 0.07, Supplemental Figure 1C). Shear force did not change the fluorescence intensity of claudin-3 (Supplemental Figure 1F).
hMOs and hMO's acid hydrolysis product differently modulate gene expression of glycocalyx-related molecules, antimicrobial peptide, and gene and protein expression of tight junction in gut epithelial Caco-2 cells
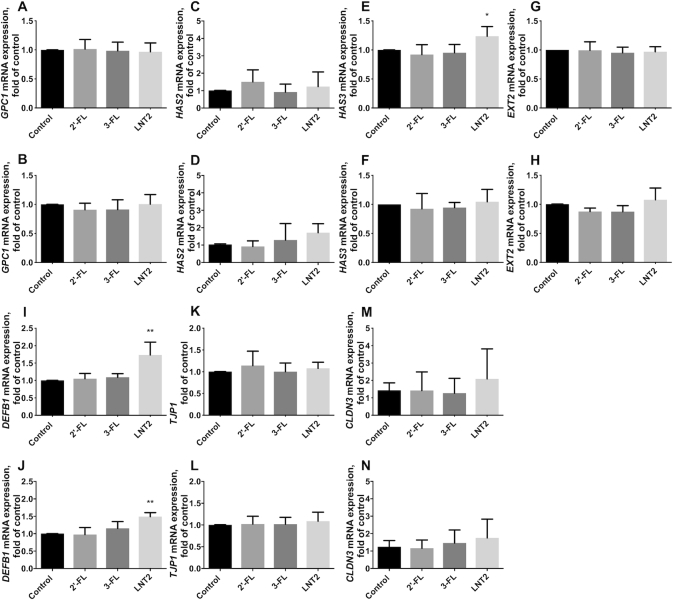
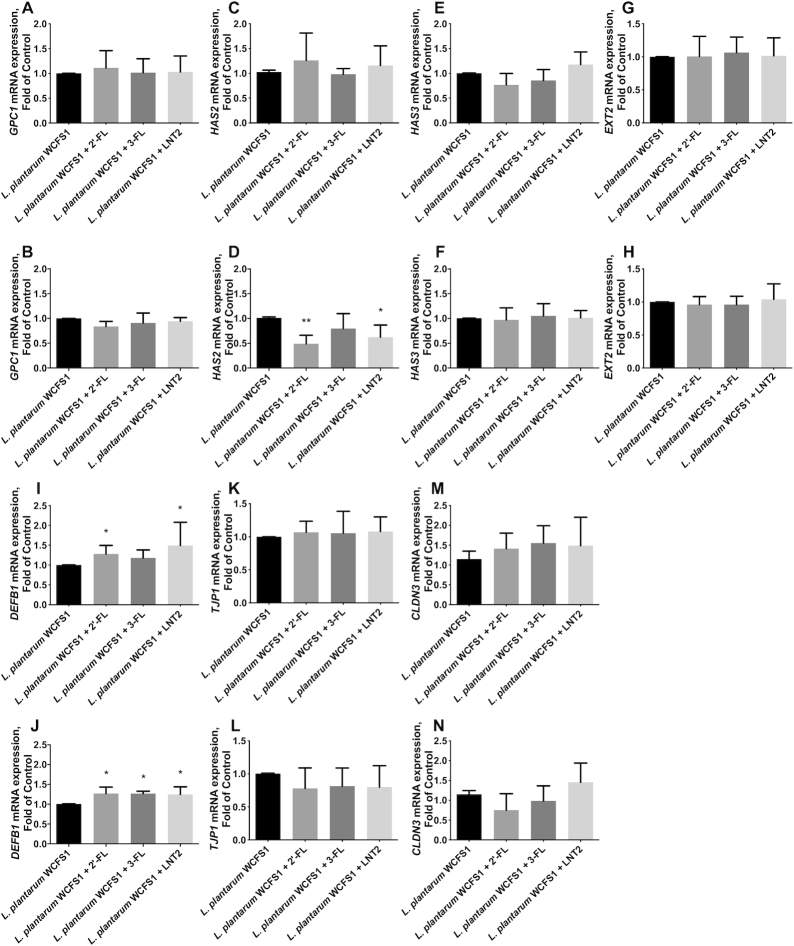
2′-FL, 3-FL, and LNT2 did not change GPC1 (Figure 2A, B) and HAS2 (Figure 2C, D) under either static conditions or shear force. LNT2 significantly upregulated HAS3 under static culture (P <0.05, Figure 2E), but this effect was not observed when the cells were exposed to shear force (Figure 2F). EXT2 was not influenced by the stimulation with hMOs under either shear force or static conditions (Figure 2G, H).
FIGURE 2.
hMOs (2′-FL and 3-FL) and hMO's acid hydrolysis product (LNT2) differently modulate gene expression of glycocalyx-related molecules, antimicrobial peptide, and tight junction in intestinal epithelial Caco-2 cells. (A, B) GPC1, (C, D) HAS2, (E, F) HAS3, and (G, H) EXT2 gene expression of the glycocalyx with 2′-FL, 3-FL, and LNT2 stimulation under static conditions and shear force, respectively; (I, J) DEFB1 gene expression of 1 antimicrobial peptide with 2′-FL, 3-FL, and LNT2 stimulation under static conditions and shear force, respectively; (K, L) TJP1 and (M, N) CLDN3 gene expression of the tight junction protein with 2′-FL, 3-FL, and LNT2 stimulation under static conditions and shear force, respectively. Values are means ± SDs, n = 6. Asterisks indicate different from control: *P <0.05, **P <0.01. CLDN3, claudin 3; DEFB1, defensin β-1; EXT, exostosin glycosyltransferase; FL, fucosyllactose; GPC1, glypican 1; HAS, hyaluronan synthase; hMO, human milk oligosaccharide; LNT2, lacto-N-triose II; TJP1, tight junction protein 1.
LNT2 but not 2′-FL or 3-FL significantly upregulated DEFB1 under static conditions, with an increase of 0.73-fold compared with cells cultured without hMOs (P <0.01, Figure 2I). This effect was also observed when the cells were exposed to shear force. Under shear force, LNT2 significantly increased DEFB1 to 1.49-fold compared with controls (P <0.01, Figure 2J).
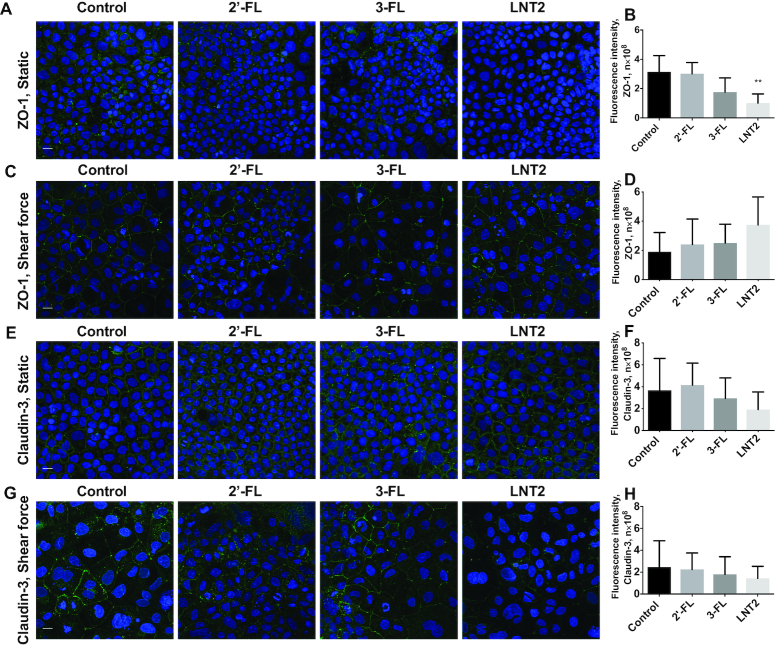
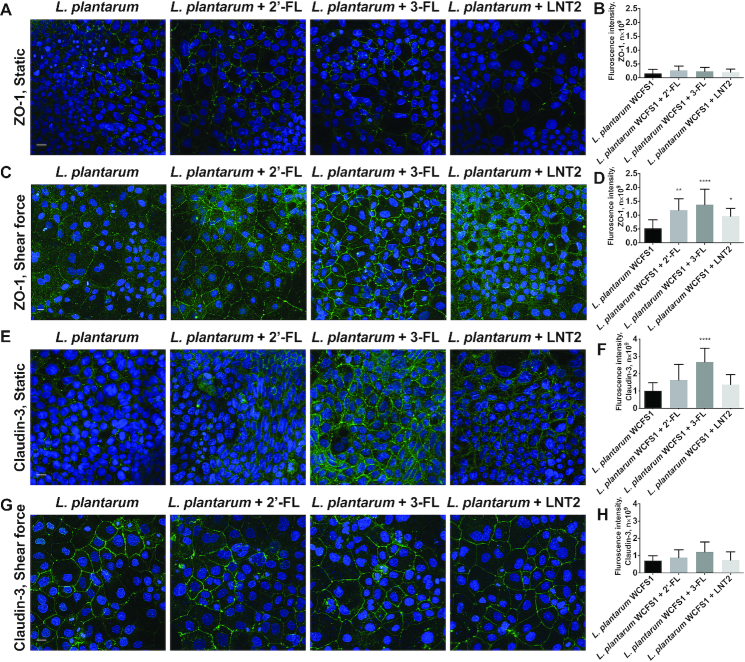
2′-FL, 3-FL, and LNT2 did not change the gene expression of tight junction TJP1 and CLDN3 (Figure 2K–N). However, at the protein level, LNT2 significantly decreased the fluorescence intensity of ZO-1 in cells under static culture (P <0.01, Figure 3A, B), whereas this downregulation was not observed when the cells were exposed to shear force (Figure 3C, D). There was no difference in the distribution and the fluorescence intensity of claudin-3 after exposure to hMOs under both static conditions or shear force exposure (Figure 3E–H).
FIGURE 3.
hMOs (2′-FL and 3-FL) and hMO's acid hydrolysis product (LNT2) differently modulate protein expression of tight junction in intestinal epithelial Caco-2 cells. (A, C) immunofluorescence images of tight junction protein ZO-1 (ZO-1, green; cell nuclei, blue) with 2′-FL, 3-FL, and LNT2 stimulation under static conditions and shear force, respectively; (B) and (D) immunofluorescence intensity analysis of (A) and (C), respectively. (E, G) immunofluorescence images of tight junction protein claudin-3 (claudin-3, green; cell nuclei, blue) with 2′-FL, 3-FL, and LNT2 stimulation under static conditions and shear force, respectively. (F) and (H) immunofluorescence intensity analysis of (E) and (G), respectively. Scale bar = 20 μm. Values are means ± SDs, n = 6. Asterisks indicate different from control: **P <0.01. FL, fucosyllactose; LNT2, lacto-N-triose II; ZO-1, zonula occludens.
hMOs exposure enhances L. plantarumWCFS1 adhesion to gut epithelial Caco-2 cells
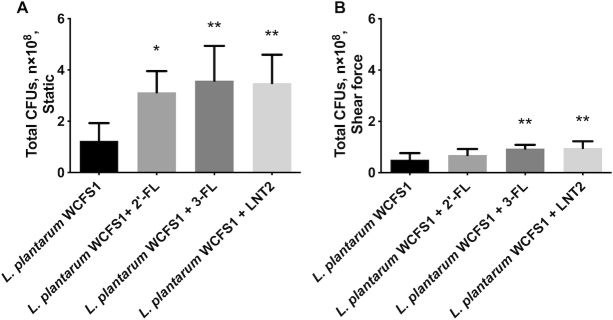
A pronounced increase of L. plantarumWCFS1 adhesion was induced by all hMOs studied under static culture (Figure 4A). 2′-FL, 3-FL, and LNT2 all significantly (P <0.05) enhanced the adhesion of L. plantarumWCFS1 to Caco-2 cells with an increase of 1.52-, 1.89-, and 1.81-fold, respectively.
FIGURE 4.
hMOs (2′-FL and 3-FL) and hMO's acid hydrolysis product (LNT2) exposure enhance Lactobacillus plantarumWCFS1 adhesion to intestinal epithelial Caco-2 cells. (A) L. plantarumWCFS1 adhesion to Caco-2 cells with 2′-FL, 3-FL, and LNT2 stimulation under static culture; (B) L. plantarumWCFS1 adhesion to Caco-2 cells with 2′-FL, 3-FL, and LNT2 stimulation exposed to shear force. Values are means ± SDs, n = 6. Asterisks indicate different from control: *P <0.05, **P <0.01. FL, fucosyllactose; LNT2, lacto-N-triose II.
Exposed to shear force, hMOs also enhanced the adhesion of L. plantarumWCFS1 but results were different from the static incubation. 3-FL and LNT2 enhanced L. plantarumWCFS1 adhesion to 1.85- and 1.90-fold (P <0.01, Figure 4B) but this was not observed with 2′-FL (Figure 4B).
Shear force modifies gene expression of glycocalyx-related molecules, antimicrobial peptide, and gene and protein expression of tight junction of gut epithelial Caco-2 cells in the presence of L. plantarumWCFS1
As shown in Figure 5, shear force exposure significantly upregulated gene expression of specific glycocalyx-related molecules compared with cells under static incubation in the presence of L. plantarumWCFS1. GPC1 was increased to 2.68-fold (P <0.01, Figure 5A) compared with cells in static culture. Shear force did not change HAS1 (Figure 5B), but significantly upregulated HAS2 (P <0.01, Figure 5C) and HAS3 (P <0.05, Figure 5D), with an increase to 2.03-fold and 1.78-fold, respectively, compared with epithelial cells in static conditions. Exposure to shear force did not impact EXT1 (Figure 5E) and EXT2 (Figure 5F) in Caco-2 cells.
FIGURE 5.
Shear force modifies gene expression of glycocalyx-related molecules, antimicrobial peptide, and tight junction with Lactobacillus plantarumWCFS1 in intestinal epithelial Caco-2 cells. (A) GPC1, (B) HAS1, (C) HAS2, (D) HAS3, (E) EXT1, and (F) EXT2 gene expression of the glycocalyx under static conditions and shear force with L. plantarumWCFS1; (G) DEFB1, 1 antimicrobial peptide gene expression under static conditions and shear force with L. plantarumWCFS1; (H) TJP1 and (I) CLDN3 gene expression of the tight junction protein under static conditions and shear force with L. plantarumWCFS1. Results are presented as relative values normalized to the housekeeping gene GAPDH. Values were means ± SDs, n = 6. Asterisks indicate different from static: *P <0.05, **P <0.01. CLDN3, claudin 3; DEFB1, defensin β-1; EXT, exostosin glycosyltransferase; GPC1, glypican 1; HAS, hyaluronan synthase; TJP1, tight junction protein 1.
Interestingly, shear force induced a significantly downregulated gene expression of the antimicrobial peptide DEFB1 (P <0.05, Figure 5G) in the presence of L. plantarumWCFS1. The reduction was 58.4% compared with epithelial cells cultured under static conditions.
The gene expression of tight junction TJP1 was not changed by exposure to shear force (Figure 5H), but CLDN3 was significantly downregulated to 41.5% when the cells were exposed to shear force (P <0.05, Figure 5I) combined with L. plantarumWCFS1.
Also, the tight junction ZO-1 and claudin-3 were tested on the protein level. Shear force significantly increased the fluorescence intensity of ZO-1 in the presence of L. plantarumWCFS1 (P <0.05, Supplemental Figure 2A–C). Claudin-3 was not influenced when the cells were exposed to shear force (Supplemental Figure 2D–F).
hMOs and an hMO's acid hydrolysis product differently modulate gene expression of glycocalyx-related molecules, antimicrobial peptide, and gene and protein expression of tight junction in the presence of L. plantarumWCFS1 and shear force
2′-FL, 3-FL, and LNT2 did not change the gene expression of GPC1 either in static culture (Figure 6A) or when the cells were exposed to shear force (Figure 6B) combined with exposure to L. plantarumWCFS1. Under shear force, 2′-FL and LNT2 significantly downregulated HAS2 by 51.7% (P <0.01, Figure 6D) and 38.5% (P <0.05, Figure 6D), respectively. This was unique for 2′-FL and LNT2 as it was not observed with 3-FL. No effects of 2′-FL, 3-FL, and LNT2 were observed on HAS3 (Figure 6E, F) and EXT2 (Figure 6G, H) independent of whether cells were cultured under static conditions or application of shear force.
FIGURE 6.
hMOs (2′-FL and 3-FL) and hMO's acid hydrolysis product (LNT2) differently modulate gene expression of glycocalyx-related molecules, antimicrobial peptide, and tight junction when intestinal epithelial Caco-2 cells are exposed to the commensal bacterium Lactobacillus plantarumWCFS1. (A, B) GPC1, (C, D) HAS2, (E, F) HAS3, and (G, H) EXT2 gene expression of the glycocalyx with 2′-FL, 3-FL, and LNT2 stimulation in the presence of L. plantarumWCFS1 under static conditions and shear force, respectively; (I, J) DEFB1 gene expression of 1 antimicrobial peptide with 2′-FL, 3-FL, and LNT2 stimulation in the presence of L. plantarumWCFS1 under static conditions and shear force, respectively; (K, L) TJP1 and (M, N) CLDN3 gene expression of the tight junction protein with 2′-FL, 3-FL, and LNT2 stimulation in the presence of L. plantarumWCFS1 under static conditions and shear force, respectively. Values are means ± SDs, n = 6. Asterisks indicate different from control: *P <0.05, **P <0.01. CLDN3, claudin 3; DEFB1, defensin β-1; EXT, exostosin glycosyltransferase; FL, fucosyllactose; GPC1, glypican 1; HAS, hyaluronan synthase; hMO, human milk oligosaccharide; LNT2, lacto-N-triose II; TJP1, tight junction protein 1.
Under static culture, 2′-FL and LNT2 significantly increased DEFB1 to 1.28-fold (P <0.05, Figure 6I) and 1.49-fold (P <0.05, Figure 6I), respectively, whereas 3-FL did not show such an effect on DEFB1 (Figure 6I) in epithelial cells exposed to L. plantarumWCFS1. When the cells were exposed to shear force and L. plantarumWCFS1, 2′-FL, 3-FL, and LNT2 induced a more pronounced DEFB1. As shown in Figure 6J, 2′-FL, 3-FL, and LNT2 all significantly (P <0.05) upregulated DEFB1 to 1.26-, 1.26-, and 1.24-fold, respectively.
The hMOs 2′-FL, 3-FL, and LNT2 did not change the gene expression of TJP1 (Figure 6K, L) and CLDN3 (Figure 6M, N) when Caco-2 cells were exposed to shear force and L. plantarumWCFS1. We also studied tight junction on the protein level. Under static culture, 2′-FL, 3-FL, and LNT2 did not influence ZO-1 production as all cells exposed to hMOs and L. plantarumWCFS1 had a relatively low intensity of staining, similar to the control (Figure 7A, B). A more pronounced fluorescence intensity of ZO-1 was obtained when the cells were exposed to shear force and L. plantarumWCFS1 as shown in Figure 7C. The fluorescence intensity of ZO-1 was statistically significantly higher when cells under shear force were exposed to 2′-FL, 3-FL, and LNT2 (P <0.05, Figure 7D). 3-FL had the most pronounced effect and increased ZO-1 to 2.66-fold.
FIGURE 7.
hMOs (2′-FL and 3-FL) and hMO's acid hydrolysis product (LNT2) differently modulate protein expression of tight junction in intestinal epithelial Caco-2 cells in the presence of Lactobacillus plantarumWCFS1. (A, C) immunofluorescence images of tight junction protein ZO-1 (ZO-1, green; cell nuclei, blue) with 2′-FL, 3-FL, and LNT2 stimulation in the presence of L. plantarumWCFS1 under static conditions and shear force, respectively; (B) and (D) immunofluorescence intensity analysis of (A) and (C), respectively. (E, G) immunofluorescence images of tight junction protein claudin-3 (claudin-3, green; cell nuclei, blue) with 2′-FL, 3-FL, and LNT2 stimulation in the presence of L. plantarumWCFS1 under static conditions and shear force, respectively. (F) and (H) immunofluorescence intensity analysis of (E) and (G), respectively. Scale bar = 20 μm. Values are means ± SDs, n = 6. Asterisks indicate different from control: *P <0.05, **P <0.01, ****P <0.0001. FL, fucosyllactose; LNT2, lacto-N-triose II; ZO-1, zonula occludens.
As shown in Figure 7E, the fluorescence intensity of claudin-3 was increased by hMOs in the presence of L. plantarumWCFS1. 3-FL significantly increased the claudin-3 of Caco-2 cells under static culture (P <0.0001, Figure 7F). This was not observed when the cells were exposed to shear force (Figure 7G, H).
Discussion
The intestinal epithelium barrier is the gatekeeper of the human body and protects the host against entrance of foreign antigens and pathogenic microorganisms (28). The epithelium is covered with a glycocalyx layer that is essential for colonization of the intestine by beneficial microbes in early life (10). Components in mother milk such as hMOs contribute to both the development and maintenance of the gut barrier function and colonization of the intestine but mechanisms by which this occurs are still largely unknown. It is also unknown how shear forces that occur in vivo during peristaltic movement impact epithelial cells and how shear force influences the efficacy by which hMOs induce modulation of gut epithelial cells. Here, we focused on the gene expression of glycocalyx-related molecules, antimicrobial peptide, and gene and protein expression of the tight junction in gut epithelial cells and found significant changes in cell behavior in the presence of shear force. In addition, we demonstrate that hMOs and shear force change the crosstalk between a commensal bacterial strain and epithelial cells as evidenced by distinct gene expression patterns in gut epithelial cells.
We observed that when exposed to shear force, specific genes for regulating glycocalyx synthesis, human β defensin secretion, as well as tight junction proteins synthesis were expressed lower than in cells cultured under static conditions. Glypican 1 proteoglycan is the protein backbone of the glycocalyx layer and an important carrier for glycosaminoglycan chains which include HA and heparan sulfate (HS) (29). Shear force did not change GPC1. Under shear force, HA synthase gene expression of HAS3 but not HAS1 and HAS2 was lower than in static conditions. HAS is essential for synthesis of HA (30), which is a highly viscous component of the intestinal mucus layer and responsible for tissue repair, stability, and anti-inflammatory effects (31). Compared with HAS1 and HAS2, HAS3 synthesizes low-molecular weight HA, and lower HAS3 gene expression implies reduced production of low-molecular weight HA, which is required for intestinal stem cell development (30) and protection of the liver from acute injury (32).
Another shear-force-induced downregulation was lowering of gene expression of EXT1. This gene is involved in the elongation of HS chains and responsible for integrating HS chains with nucleotide sugars in the Golgi apparatus (33). If it is lower, it may imply lowering of HS synthesis, which supports organogenesis, growth factor signaling, and bacterial adhesion (33). Overall, our data show that under static incubation, specific genes involved in glycocalyx synthesis and essential cellular processes might exhibit increased expression than under shear force such as occurs in vivo (11), implying shear force on gut epithelial cells influences cell development, cell signaling, but regulation seems to occur through downregulating glycosaminoglycan chains and not via synthesis of the protein backbone.
To gain insight into whether shear force impacts gut microbiota shaping defensins we studied DEFB1 as it is responsible for synthesis of the antimicrobial peptide human defensin beta-1, which is constitutively expressed by epithelial cells and is a key effector molecule of innate immunity (34). It is an essential molecule in defense against pathogens. Our results suggest that DEFB1 is decreased by shear force, which implies that shear force may influence epithelial responses against pathogens. We also studied the impact on the essential tight junction proteins ZO-1 and claudin-3. Both the intercellular tight junction protein ZO-1 and the transmembrane tight junction protein claudin-3 were decreased by shear force. These findings corroborate findings in tubular epithelial cells (35) that also demonstrate decreased expression of the tight junction proteins ZO-1 when shear force is applied.
Results and impact of shear force were different when cells were exposed to hMOs or breakdown products of hMO. The hMO acid hydrolysate LNT2 but not 2′-FL or 3-FL showed enhancing effects on gene expression of HAS3 under static culture. LNT2 significantly increased HAS3 but did not change HAS2 of the glycocalyx molecule HA under static conditions. This higher gene expression of HAS3 may indicate higher production of low-molecular weight HA under static conditions. However, none of the tested hMOs changed GPC1 or EXT2 in the intestinal epithelial Caco-2 cells glycocalyx layer. LNT2 also enhanced DEFB1 under both static conditions and shear force. This suggests that LNT2 may increase intestinal immunity as defensin DEFB1 is key in stimulating intestinal immunity (34). None of the hMOs changed TJP1 and CLDN3, but LNT2 decreased ZO-1 at protein level under static culture, which we did not observe in the presence of shear force. This demonstrates that the impact of hMOs and its hydrolysis product on Caco-2 cells is different under shear force and suggests that acid hydrolysis of hMOs in the stomach may enhance the effect of hMOs.
Our data also illustrate the positive impact of hMOs on the adhesion of the commensal bacterium L. plantarumWCFS1 to intestinal epithelial cells. Of the tested hMOs, 2′-FL, 3-FL, and LNT2 all significantly increased the adhesion of the commensal bacterium under static culture. This was different under shear force, where 3-FL and LNT2 but not 2′-FL significantly increased bacterial adhesion. Even though 2′-FL shares the same lactose core structure with 3-FL and LNT2 (Table 1), l-fucose of 2′-FL is fucosylated to galactose but not to glucose as in 3-FL. Our data might suggest this difference in fucosylation may make a difference on commensal bacterial adhesion under shear force by enhancing the capacity of intestinal cells to bind L. plantarumWCFS1. Similar structure-function effects of hMOs have been reported for pathogen adhesion (36) as well as for promoting production of MUC2 (5) in intestinal epithelial cells. Effects of hMOs under shear force seem to be very specific and corroborate findings with lectins that have also been shown to specifically, and not generally, block pathogenic intruders (37).
The enhanced bacterial adhesion of the commensal L. plantarumWCFS1 induced by 2′-FL, 3-FL, and LNT2 can be induced in multiple ways. It is possible that hMOs enhanced the expression of adhesion molecules on the bacteria such as glyceraldehyde-3-phosphate dehydrogenase (38). However, as the epithelial cells and not the bacteria were treated with hMOs it is likely that the induced changes in glycosylation and glycan diversity on the intestinal epithelium support adhesion of the commensal (10). This suggestion is corroborated by the observation that enhanced commensal adhesion induced by hMOs and shear force was associated with more profound changes in gene expression of intestinal epithelial glycocalyx, antimicrobial peptide, and tight junction proteins. 2′-FL for instance had lesser effects on these genes under shear force. This may be an explanation for the observation that 2′-FL had a lesser effect on L. plantarumWCFS1 adhesion under shear force than 3-FL and LNT2.
In the presence of the bacteria, shear force significantly increased glycocalyx-associated genes GPC1, HAS2, and HAS3, which is different from the lowering effects of shear force on HAS3 and EXT1 in the absence of bacteria. These data are corroborating findings in endothelial cells in which glypican 1 was shown to be the mechanosensitive glycocalyx core protein and HA is a transducer of the shear-induced changes in endothelial cells (14). The upregulating effects on the glycocalyx genes by bacteria may stimulate the synthesis of glypican 1 proteoglycan and HA glycosaminoglycan chains, which has been suggested to stimulate colonization of the bacteria by providing anchoring points (10). In the presence of the bacteria, shear force still downregulated the gene expression of DEFB1 and CLDN3. However, on the protein level, shear force significantly increased ZO-1 and did not change claudin-3. Our data strongly support that tight junction protein ZO-1 is a shear-sensitive protein in intestinal epithelial cells (11).
In our study, we selected essential glycocalyx and tight junction proteins as study parameters as they together make up the physical barrier of the intestine. Defensin beta-1 was studied as it is involved in shaping microbiota (34) and therewith also contributes to barrier function by preventing pathogens adhering to host epithelial cells. The studied hMOs strongly influence these genes and proteins but the impact was different in the presence and absence of shear force. An example of this is the observation that under shear force in the presence of L. plantarumWCFS1, 2′-FL, and LNT2, but not 3-FL, decreased HAS2. HA normally enhances viscosity and supports bacterial adhesion (31), and therefore it is not in line with the observation that L. plantarumWCFS1 can adhere better to epithelial cells exposed to 3-FL and LNT2 but consistent with the finding 2′-FL showed less bacterial adhesion under shear force. A possible explanation is that 3-FL and LNT2 stimulate L. plantarumWCFS1 adhesion in an HA-independent way. It cannot be excluded that other glycosylation structures are involved. It seems likely however, that HAS2 regulation by 2′-FL is involved in enhanced L. plantarumWCFS1 adhesion. Except for enhancing commensal bacterium adhesion, lowering of HAS2 by hMOs may also have an antipathogenic effect as it is associated with lower colonization of HA-degrading pathogenic bacteria via reducing the binding sites of the host. This was also shown for 3′-sialyllactose (3SL) that lowered expression of sialic acid and lactosamine, which was associated with reduced adhesion of Escherichia coli to intestinal epithelial cells (39).
Besides influencing the glycosylation of epithelial cells, hMOs and commensal bacteria can also stimulate the host to secrete antimicrobial peptides to inhibit pathogen growth (40). As shown in our study, under static culture in the presence of L. plantarumWCFS1, 2′-FL, and LNT2, but not 3-FL, significantly enhanced DEFB1 which was different under shear force as under these circumstances, 2′-FL, LNT2, as well as 3-FL stimulated gene expression of DEFB1. This demonstrates that the impact of some dietary molecules can only be observed when physiological shear force is applied. The enhancing effect on DEFB1, however, is much less in the absence of L. plantarumWCFS1. The finding that LNT2 under either static or shear force enhanced DEFB1 is also intriguing. It corroborates previous findings that hydrolysis of hMOs in the stomach enhance the biological efficacy of hMOs (41). Overall, our data suggest that hMOs support the crosstalk between commensal bacteria and epithelial cells, and commensal bacteria increase the impact of hMOs on epithelial cells under shear force.
3-FL had the most pronounced effects on the protein expression of tight junction ZO-1 when crosstalk was allowed between L. plantarumWCFS1 and gut epithelial cells under shear force. Expression of this tight junction protein was much higher than when cultured under static incubation. This was different for the protein expression of claudin-3, which was only significantly enhanced by 3-FL in static culture, and relatively lower expressed under shear force. Our data demonstrate that the efficacy of the tested hMOs to promote the adhesion of L. plantarumWCFS1 and to support the barrier-function-related tight junction proteins is dependent on the presence of shear force and influences regulation of essential genes involved in gut barrier function or crosstalk with L. plantarumWCFS1.
In summary, we show that shear force mimicking intestinal peristaltic shear forces has a relatively large impact on the intestinal epithelial cell characteristics such as gene expression of glycocalyx-related molecules, antimicrobial peptide, and gene and protein expression of tight junctions. It also changed the impact of hMOs on gut epithelial cells. The most profound changes were observed when crosstalk between a commensal bacterium and gut epithelial cells was allowed. We observed enhanced commensal bacterial adhesion and stronger gene expression of antimicrobial peptide and protein expression of tight junction ZO-1. 3-FL and LNT2 had such an effect, whereas 2′-FL did not have a significant effect.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—CK and PdV: designed the research; CK, LC, GK, and JF: conducted the research; CK, LC, and BJdH: analyzed data; CK, MTCW, and PdV: wrote the manuscript; CK: had primary responsibility for final content; and all the authors: read and approved the final manuscript.
Notes
Supported by China Scholarship Council (CSC), within the framework of the Sino-Dutch Doctoral Program on Sustainable Dairy coordinated by the Carbohydrate Competence Center (CCC, www.cccresearch.nl).
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CLDN3, claudin 3; DEFB1, defensin beta-1; EXT, exostosin glycosyltransferase; FITC, fluorescein isothiocyanate; GPC1, glypican 1; HAS, hyaluronan synthase; hMO, human milk oligosaccharide; HS, heparan sulfate; LNT2, lacto-N-triose II; TJP1, tight junction protein 1; ZO, zonula occludens; 2′-FL, 2′-fucosyllactose; 3-FL, 3-fucosyllactose.
References
- 1. Akkerman R, Faas MM, de Vos P. Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: effects on microbiota and gut maturation. Crit Rev Food Sci Nutr. 2018;59:1486–97. [DOI] [PubMed] [Google Scholar]
- 2. Comstock SS, Li M, Wang M, Monaco MH, Kuhlenschmidt TB, Kuhlenschmidt MS, Donovan SM. Dietary human milk oligosaccharides but not prebiotic oligosaccharides increase circulating natural killer cell and mesenteric lymph node memory T cell populations in noninfected and rotavirus-infected neonatal piglets. J Nutr. 2017;147:1041–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sprenger GA, Baumgärtner F, Albermann C. Production of human milk oligosaccharides by enzymatic and whole-cell microbial biotransformations. J Biotechnol. 2017;258:79–91. [DOI] [PubMed] [Google Scholar]
- 4. Perdijk O, van Baarlen P, Fernandez-Gutierrez MM, van den Brink E, Schuren FHJ, Brugman S, Savelkoul HFJ, Kleerebezem M, van Neerven RJJ. Sialyllactose and galactooligosaccharides promote epithelial barrier functioning and distinctly modulate microbiota composition and short chain fatty acid production in vitro. Front Immunol. 2019;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng L, Kong C, Walvoort MTC, Faas MM, de Vos P. Human milk oligosaccharides differently modulate goblet cells under homeostatic, proinflammatory conditions and ER stress. Mol Nutr Food Res. 2019;64(5):e1900976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kong C, Elderman M, Cheng L, de Haan BJ, Nauta A, de Vos P. Modulation of intestinal epithelial glycocalyx development by human milk oligosaccharides and non-digestible carbohydrates. Mol Nutr Food Res. 2019;63:e1900303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Triantis V, Bode L, van Neerven JRJ. Immunological effects of human milk oligosaccharides. Front Pediatr. 2018;6:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newburg DS, Ko JS, Leone S, Nanthakumar NN. Human milk oligosaccharides and synthetic galactosyloligosaccharides contain 3′-, 4-, and 6′-galactosyllactose and attenuate inflammation in human T84, NCM-460, and H4 cells and intestinal tissue ex vivo. J Nutr. 2016;146:358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gonia S, Tuepker M, Heisel T, Autran C, Bode L, Gale CA. Human milk oligosaccharides inhibit Candida albicans invasion of human premature intestinal epithelial cells. J Nutr. 2015;145:1992–8. [DOI] [PubMed] [Google Scholar]
- 10. Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. 2015;91:619–22. [DOI] [PubMed] [Google Scholar]
- 11. Delon LC, Guo Z, Oszmiana A, Chien CC, Gibson R, Prestidge C, Thierry B. A systematic investigation of the effect of the fluid shear stress on Caco-2 cells towards the optimization of epithelial organ-on-chip models. Biomaterials. 2019;225:119521. [DOI] [PubMed] [Google Scholar]
- 12. Kim SW, Ehrman J, Ahn MR, Kondo J, Lopez AAM, Oh YS, Kim XH, Crawley SW, Goldenring JR, Tyska MJ et al. Shear stress induces noncanonical autophagy in intestinal epithelial monolayers. Mol Biol Cell. 2017;28:3043–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo P, Weinstein AM, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol - Ren Physiol. 2000;279:698–712. [DOI] [PubMed] [Google Scholar]
- 14. Bartosch AMW, Mathews R, Tarbell JM. Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophys J. 2017;113:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eshrati M, Amadei F, Staffer S, Stremmel W, Tanaka M. Shear-enhanced dynamic adhesion of Lactobacillus rhamnosus GG on intestinal epithelia: correlative effect of protein expression and interface mechanics. Langmuir. 2019;35:529–37. [DOI] [PubMed] [Google Scholar]
- 16. van Tassell ML, Miller MJ. Lactobacillus adhesion to mucus. Nutrients. 2011;3:613–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalantar-Zadeh K, Berean KJ, Ha N, Chrimes AF, Xu K, Grando D, Ou JZ, Pillai N, Campbell JL, Brkljača R et al. A human pilot trial of ingestible electronic capsules capable of sensing different gases in the gut. Nat Electron. 2018;1:79–87. [Google Scholar]
- 18. Kim HJ, Lee J, Choi JH, Bahinski A, Ingber DE. Co-culture of living microbiome with microengineered human intestinal villi in a gut-on-a-chip microfluidic device. J Vis Exp. 2016;114:54344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Avvisato CL, Yang X, Shah S, Hoxter B, Li W, Gaynor R, Pestell R, Tozeren A, Byers SW. Mechanical force modulates global gene expression and beta-catenin signaling in colon cancer cells. J Cell Sci. 2007;120:2672–82. [DOI] [PubMed] [Google Scholar]
- 20. Trujillo-de Santiago G, Lobo-Zegers MJ, Montes-Fonseca SL, Zhang YS, Alvarez MM. Gut-microbiota-on-a-chip: an enabling field for physiological research. Microphysiological Syst. 2018;2:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herigstad B, Hamilton M, Heersink J. How to optimize the drop plate method for enumerating bacteria. J Microbiol Methods. 2001;44:121–9. [DOI] [PubMed] [Google Scholar]
- 22. Nahás-Scocate ACR, de Moraes GFA, Nader HB, Vicente CM, Toma L. Analysis of proteoglycan expression in human dental pulp. Arch Oral Biol. 2018;90:67–73. [DOI] [PubMed] [Google Scholar]
- 23. Kim E, Hwang K, Lee J, Han SY, Kim EM, Park J, Cho JY. Skin protective effect of epigallocatechin gallate. Int J Mol Sci. 2018;19(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo X, Lin M, Yan W, Chen W, Hong G. A novel splice mutation induces exon skipping of the EXT1 gene in patients with hereditary multiple exostoses. Int J Oncol. 2019;54:859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu R, Zeng X, Han Q, Lin M, Long L, Dan H, Zhou G, Chen Q. Overexpression and selectively regulatory roles of IL-23/IL-17 axis in the lesions of oral lichen planus. Mediators Inflamm. 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamaguchi H, Kojima T, Ito T, Kimura Y, Imamura M, Son S, Koizumi JI, Murata M, Nagayama M, Nobuoka T et al. Transcriptional control of tight junction proteins via a protein kinase C signal pathway in human telomerase reverse transcriptase-transfected human pancreatic duct epithelial cells. Am J Pathol. 2010;177:698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pahle J, Menzel L, Niesler N, Kobelt D, Aumann J, Rivera M, Walther W. Rapid eradication of colon carcinoma by Clostridium perfringens enterotoxin suicidal gene therapy. BMC Cancer. 2017;17:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kang H, Fan Y, Zhao P, Ren C, Wang Z, Deng X. Regional specific modulation of the glycocalyx and smooth muscle cell contractile apparatus in conduit arteries of tail-suspended rats. J Appl Physiol. 2016;120:537–45. [DOI] [PubMed] [Google Scholar]
- 30. Fujimoto K, Hasebe T, Kajita M, Ishizuya-Oka A. Expression of hyaluronan synthases upregulated by thyroid hormone is involved in intestinal stem cell development during Xenopus laevis metamorphosis. Dev Genes Evol. 2018;228:267–73. [DOI] [PubMed] [Google Scholar]
- 31. Filpa V, Bistoletti M, Caon I, Moro E, Grimaldi A, Moretto P, Baj A, Giron MC, Karousou E, Viola M et al. Changes in hyaluronan deposition in the rat myenteric plexus after experimentally-induced colitis. Sci Rep. 2017;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCracken JM, Jiang L, Deshpande KT, O'Neil MF, Pritchard MT. Differential effects of hyaluronan synthase 3 deficiency after acute vs chronic liver injury in mice. J Pharm Policy Pract. 2016;9:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Busse M, Kusche-Gullberg M. In vitro polymerization of heparan sulfate backbone by the EXT proteins. J Biol Chem. 2003;278:41333–7. [DOI] [PubMed] [Google Scholar]
- 34. Raschig J, Mailänder-Sánchez D, Berscheid A, Berger J, Strömstedt AA, Courth LF, Malek NP, Brötz-Oesterhelt H, Wehkamp J. Ubiquitously expressed human beta defensin 1 (hBD1) forms bacteria-entrapping nets in a redox dependent mode of action. PLoS Pathog. 2017;13:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maggiorani D, Dissard R, Belloy M, Saulnier-Blache JS, Casemayou A, Ducasse L, Grès S, Bellière J, Caubet C, Bascands JL et al. Shear stress-induced alteration of epithelial organization in human renal tubular cells. PLoS One. 2015;10:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shang J, Piskarev VE, Xia M, Huang P, Jiang X, Likhosherstov LM, Novikova OS, Newburg DS, Ratner DM. Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance. Glycobiology. 2013;23:1491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bode L, Jantscher-Krenn E. Structure-function relationships of human milk oligosaccharides. Adv Nutr. 2012;3:383S–91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang G, Zhang M, Zhao J, Xia Y, Lai PFH, Ai L. A surface protein from Lactobacillus plantarum increases the adhesion of lactobacillus strains to human epithelial cells. Front Microbiol. 2018;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Angeloni S, Ridet JL, Kusy N, Gao H, Crevoisier F, Guinchard S, Kochhar S, Sigrist H, Sprenger N. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology. 2005;15:31–41. [DOI] [PubMed] [Google Scholar]
- 40. Khan R, Petersen FC, Shekhar S. Commensal bacteria: an emerging player in defense against respiratory pathogens. Front Immunol. 2019;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jantscher-Krenn E, Marx C, Bode L. Human milk oligosaccharides are differentially metabolised in neonatal rats. Br J Nutr. 2013;110:640–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.