ABSTRACT
Background
Concentrations of 25-hydroxyvitamin D [25(OH)D] tend to be lower in African Americans than in non-Hispanic whites, but whether adding information on parathyroid hormone (PTH) can help explain the higher cardiometabolic risk among African Americans is unknown.
Objectives
This study examined race (black/white)-specific independent and joint associations of 25(OH)D and PTH with cardiometabolic biomarkers including high-sensitivity C-reactive protein (hs-CRP), estimated glomerular filtration rate (eGFR), and homeostasis model assessment of insulin resistance (HOMA-IR) and β-cell function (HOMA-B).
Methods
Among 1500 white and 1300 black postmenopausal women without cardiovascular disease from the Women's Health Initiative Observational Study, a weighted linear regression analysis and a novel penalized spline-based semiparametric model with contour plots, accounting for possible nonlinear relations and interactions simultaneously, were used to investigate the race-specific independent and joint associations of 25(OH)D and PTH with each biomarker.
Results
Black women had lower concentrations of 25(OH)D and higher PTH, HOMA-IR, HOMA-B, hs-CRP, and eGFR than white women (all P values < 0.0001). Lower 25(OH)D and higher PTH were each independently and jointly associated with higher HOMA-IR in both white and black women, whereas a similar joint relation with HOMA-B was observed in white women only. In contrast, PTH was nonlinearly associated with HOMA-B in black women and positively associated with hs-CRP in white women, independently of 25(OH)D. Whereas there was an inverse linear relation between PTH and eGFR in white women after accounting for 25(OH)D, PTH and 25(OH)D were jointly and nonlinearly associated with eGFR in black women.
Conclusions
We found that the joint association of 25(OH)D and PTH with β-cell function, systemic inflammation, and kidney function apparently differed between white and black women. Further studies are needed to determine whether differences in the vitamin D–PTH endocrine system contribute to racial disparities in cardiovascular health.
Keywords: racial differences, cardiometabolic biomarkers, 25-hydroxyvitamin D, parathyroid hormone, joint associations, postmenopausal women
Introduction
Evidence from mechanistic studies indicates that the vitamin D and parathyroid hormone (PTH) endocrine system regulates diverse physiological functions, including insulin/glucose metabolism, the renin–angiotensin–aldosterone system (RAAS), vascular and cardiac cell function, inflammatory pathways, cell proliferation and differentiation, and immune response modulation (1–5). The hypothesized mechanisms underlying the relation between vitamin D and cardiometabolic health may operate through binding to the nuclear vitamin D receptor in a variety of tissues. Epidemiological studies suggest that vitamin D deficiency or PTH excess may be associated with intermediate cardiometabolic biomarkers, including HOMA-IR and homeostasis model assessment of β-cell function (HOMA-B), high-sensitivity C-reactive protein (hs-CRP), and estimated glomerular filtration rate (eGFR) (6–8), although the available evidence remains inconclusive (9–11). It has been consistently reported that, compared with whites, blacks have a higher prevalence of vitamin D deficiency, PTH excess, and the aforementioned cardiovascular disease (CVD) risk factors (12–15). There is also evidence for racial disparities in the associations of 25-hydroxyvitamin D [25(OH)D] or PTH with cardiometabolic biomarkers (16–18).
However, most previous studies have focused on independent associations of total 25(OH)D and PTH with CVD risk factors (6–11). Given the well-established interrelations between 25(OH)D and PTH, it remains unclear whether these patterns will extend to their joint associations with cardiometabolic biomarkers between whites and blacks.
By leveraging available core CVD biomarkers in the Women's Health Initiative Observational Study (WHI-OS), including HOMA-IR, HOMA-B, hs-CRP, and eGFR, we specifically evaluated the independent and joint associations of plasma total 25(OH)D and PTH with these 4 core cardiometabolic biomarkers among a random subcohort of US white and black postmenopausal women without CVD from the WHI-OS. Our aims were 1) to examine both linear and nonlinear independent associations of total 25(OH)D and PTH with each cardiometabolic biomarker and 2) to explore the joint association of 25(OH)D and PTH with each cardiometabolic biomarker, separately, for US white and black postmenopausal women.
Methods
Study population
We leveraged data from a case-cohort ancillary study conducted within the WHI-OS (19). The WHI-OS consisted of 93,676 ethnically diverse women aged 50–79 y recruited at 40 clinical centers across the United States between 1994 and 1998. With a 20% minority enrollment rate, the WHI-OS cohort roughly parallels the racial diversity of the US population (20). At baseline, all WHI participants self-reported their race and ethnicity, choosing from non-Hispanic white (referred to hereafter as white), non-Hispanic black (referred to hereafter as black), Hispanic, American Indian/Alaska Native, Asian (ancestry was Chinese, Indo-Chinese, Korean, Japanese, Pacific Islander, or Vietnamese), and other (21).
The ancillary case-cohort study included 2050 CVD cases and 2800 controls after excluding from the original WHI-OS cohort women with a history of stroke or myocardial infarction, or of receiving dialysis at baseline (19). We limited our study selection to blacks and whites in order to ensure adequate power for addressing black–white disparities in CVD risk and risk factors. All non-CVD controls, being representative samples of the entire WHI-OS cohort, were included in this study, yielding a final sample of 1500 white women and 1300 black women without baseline prevalent or incident CVD (Supplemental Figure 1). All participants provided written informed consent at study entry, and the study was approved by the institutional review boards of each participating center.
Biomarker assessment and outcomes
Blood samples were collected from all WHI-OS participants at baseline after ≥12 h of fasting and stored at −80°C before laboratory assays. All assays were performed in the laboratory of Nader Rifai (CERLab) at Boston Children's Hospital. Total 25(OH)D was measured by an enzyme immunoassay from Immunodiagnostic Systems Inc. PTH was determined by electrochemiluminescence immunoassay on the Roche E Modular system (Roche Diagnostics) with a lower limit of detection of 1.2 pg/mL. Plasma hs-CRP was measured using an immunoturbidimetric assay, creatinine by an enzymatic method, fasting glucose enzymatically, and fasting insulin by an electrochemiluminescence immunoassay; all assays were performed on the Roche E Modular system using Roche Diagnostic reagents (Roche Diagnostics). The mean intra-assay CVs for each assay were as follows: total 25(OH)D, 6.95%; PTH, 3.46%; hs-CRP, 3.34%; creatinine, 1.82%; fasting glucose, 3.26%; and fasting insulin, 2.49%. HOMA-IR was calculated by multiplying fasting plasma insulin (FPI) (μIU/mL) by fasting plasma glucose (FPG) (mmol/L), then dividing by the constant 22.5, i.e., HOMA-IR = (FPI × FPG)/22.5 (22). HOMA-B was computed using the following formula: HOMA-B = 20 × FPI (μIU/mL)/FPG (mmol/L) − 3.5 (22). We calculated eGFR using the well-validated Chronic Kidney Disease Epidemiology Collaboration equation, which has been shown to provide more accurate eGFR estimates than the Modification of Diet in Renal Disease equation (23):
Estimated GFR (in mL · min−1 · 1.73 m−2) = 141 × min(creatinine/κ, 1)α × max(creatinine/κ, 1)−1.209 × 0.993Age × 1.018(if female) × 1.159(if black)
where creatinine = standardized serum creatinine measures (mg/dL), κ = 0.7 for females or 0.9 for males, α = −0.329 for females or −0.411 for males, min = the minimum of creatinine/κ or 1, max = the maximum of creatinine/κ or 1, and age = years.
HOMA-IR, HOMA-B, hs-CRP, and eGFR, as the well-established risk factors for cardiometabolic health, had been chosen as core CVD biomarkers and were widely measured in a large cohort of >25,000 participants in the WHI-OS. They were included in the present study as primary outcomes.
Covariates
Information on demographics, lifestyle behaviors, and medication history was collected from each woman at study entry (i.e., baseline) via self-administered questionnaires, including age (y), race (white compared with black), clinical center (Southern: <35°N, Middle: 35–40°N, and Northern: >40°N), education (≤ high school graduate/General Educational Development, post–high school, and college graduate or higher), season of blood draw (spring, summer, autumn, and winter), cigarette smoking status (never, past, and current), alcohol consumption (never, past, and current), postmenopausal hormone therapy (never, past, and current), and physical activity levels (metabolic equivalent of task-h/wk). A physical examination was also performed, including height, weight, and other anthropometric measurements of each participant (20). BMI (in kg/m2) was calculated.
Statistical analysis
We compared white and black women in terms of total 25(OH)D, PTH, and other baseline characteristics using Wilcoxon's rank-sum test for continuous variables and the chi-square test for categorical variables. Age-adjusted Spearman partial correlation coefficients were computed to examine the correlations of vitamin D biomarkers with each of the following cardiometabolic biomarkers: eGFR, hs-CRP, HOMA-IR, HOMA-B, fasting glucose, and fasting insulin.
As a random subsample of the WHI-OS cohort, our study population represents the entire cohort. Therefore, we performed a weighted linear regression analysis to assess the independent associations between vitamin D biomarkers and each cardiometabolic biomarker at baseline. To reflect the WHI-OS population characteristics, we used an inverse probability weighting method based on Barlow's approach (24). Each vitamin D biomarker was parameterized as a continuous variable by assuming that it had a linear relation with the cardiometabolic biomarkers. Results for direct comparison of effect sizes between 25(OH)D and PTH were reported per 1-SD increment in biomarker concentrations.
HOMA-IR, HOMA-B, and hs-CRP measures were log transformed owing to their skewed distributions. For ease of interpretation, regression coefficients (β) obtained from these models were back-transformed to a relative difference, which can be interpreted in terms of percentage change. Because of the potential nonlinear associations between vitamin D biomarkers and cardiometabolic biomarkers, we also divided all participants according to quartiles of 25(OH)D and PTH concentrations. For separate analyses with 25(OH)D and PTH as a continuous variable and as a categorical variable by quartiles, covariates adjusted in the main models included age, race, clinical center, education, season of blood draw, cigarette smoking status, alcohol consumption, postmenopausal hormone therapy, physical activity levels, and BMI. We tested for linear trends across quartiles of vitamin D biomarkers by using the median values of each category as a continuous variable in the models. In addition, we used quadratic and cubic terms of each vitamin D biomarker as a continuous variable to capture potential nonlinear trends. To investigate black–white differences in the associations between vitamin D biomarkers and cardiometabolic biomarkers, we repeated our multivariable analyses, stratifying by race. We also tested for interaction between race and vitamin D biomarkers by including an interaction term in our main models. Statistical analyses were performed using SAS version 9.4 (SAS Institute), unless otherwise specified.
We applied a novel penalized spline-based semiparametric regression model, developed by Tu and colleagues (25, 26), to explore the joint associations of total 25(OH)D and PTH with each cardiometabolic biomarker. By accommodating possible nonlinear relations and interactions between the 2 independent variables (i.e., vitamin D and PTH), a nonlinear bivariate surface function was used to depict the simultaneous influences of 25(OH)D and PTH on the cardiometabolic biomarkers, including HOMA-IR, HOMA-B, hs-CRP, and eGFR, in blacks and whites. The estimated surface functions were presented in the form of colored contour plots, where the height of the surface function at each combination of 25(OH)D and PTH represented the mean value of each cardiometabolic biomarker. By contrasting the shapes of the contour surfaces between black and white participants, one could make inferences about the potentially differential influences of 25(OH)D and PTH on CVD in the 2 racial groups. The quantile-quantile (Q-Q) plots of the empirical distributions of the cardiometabolic biomarkers were used to examine the normality assumption. We implemented the analysis using the mgcv package in R software, version 3.4.2 (R Foundation for Statistical Computing).
Results
Table 1 presents the baseline characteristics of our study population by ethnicity. Briefly, compared with white women, black women had significantly higher BMI, lower levels of physical activity, education, and current alcohol consumption, and less hormone therapy use, and were more likely to be current smokers and have a history of diabetes or hypertension, but less likely to have a family history of CVD. Plasma concentrations of total 25(OH)D were significantly lower, but PTH, HOMA-IR, HOMA-B, hs-CRP, and eGFR were significantly higher, in black women than in white women (all P < 0.0001).
TABLE 1.
Baseline characteristics by ethnicity in our subsample of participants from the Women's Health Initiative Observational Study1
| Variables | Black women (n = 1300) | White women (n = 1500) | P value2 |
|---|---|---|---|
| Age, y | 62 ± 7.1 | 63 ± 7.1 | <0.0001 |
| BMI, kg/m2 | 30.6 ± 6.5 | 26.7 ± 5.4 | <0.0001 |
| 29.5 [26–34] | 25.6 [23–29.4] | ||
| Family history of CVD | 495 (42.2) | 739 (52.2) | <0.0001 |
| History of diabetes | 115 (18.4) | 81 (7.5) | <0.0001 |
| History of hypertension | 676 (52.8) | 413 (28.2) | <0.0001 |
| History of high cholesterol | 193 (15.2) | 206 (14.1) | 0.437 |
| Physical activity, MET-h/wk | 6.5 [1.3–16] | 10.5 [3.8–21] | <0.0001 |
| Cigarette smoking status | <0.0001 | ||
| Never | 628 (49.3) | 715 (48.5) | |
| Past | 513 (40.2) | 681 (46.2) | |
| Current | 134 (10.5) | 77 (5.2) | |
| Alcohol consumption status | <0.0001 | ||
| Never | 246 (19.2) | 128 (8.7) | |
| Past | 400 (31.2) | 249 (16.9) | |
| Current | 636 (49.6) | 1099 (74.5) | |
| Hormone therapy use | <0.0001 | ||
| Never | 758 (58.6) | 533 (35.8) | |
| Past | 169 (13.1) | 226 (15.2) | |
| Current | 366 (28.3) | 731 (49.1) | |
| Statin use | 186 (14.4) | 212 (14.2) | 0.914 |
| Educational levels | <0.0001 | ||
| ≤ High school graduate/GED | 342 (26.4) | 301 (20.2) | |
| Post–high school | 484 (37.4) | 512 (34.3) | |
| College graduate or higher | 469 (36.2) | 678 (45.5) | |
| Geographical latitudes (clinical center) | 0.001 | ||
| Southern: <35°N | 442 (32.6) | 444 (29.8) | |
| Middle: 35–40°N | 428 (33.1) | 434 (29.1) | |
| Northern: >40°N | 445 (34.4) | 613 (41.1) | |
| Season of blood draw | 0.74 | ||
| Spring | 381 (29.8) | 437 (29.4) | |
| Summer | 348 (27.2) | 431 (29.0) | |
| Autumn | 279 (21.8) | 318 (21.4) | |
| Winter | 271 (21.2) | 299 (20.1) | |
| Vitamin D biomarkers | |||
| Total 25(OH)D, nmol/L | 42.5 [33.4–54.7] | 63.3 [51.1–76.7] | <0.0001 |
| PTH, pg/mL | 40.2 [31.4–51.7] | 35.6 [28.4–44.2] | <0.0001 |
| Cardiometabolic biomarkers | |||
| Fasting glucose, mg/dL | 94.0 [87.0–105.0] | 93.0 [88.0–99.0] | 0.0003 |
| Fasting insulin, μIU/mL | 9.1 [5.6–13.7] | 6.6 [4.6–10.0] | <0.0001 |
| HOMA-IR | 2.2 [1.3–3.6] | 1.5 [1.0–2.4] | <0.0001 |
| HOMA-B | 98.1 [62.9–145.9] | 81.4 [57.3–116.4] | <0.0001 |
| hs-CRP, mg/L | 3.3 [1.4–7.2] | 2.2 [0.9–4.9] | <0.0001 |
| eGFR, mL · min−1 · 1.73 m−2 | 94.1 ± 18 | 86.2 ± 13 | <0.0001 |
Values are means ± SDs, medians [IQRs], or n (%), unless otherwise indicated. CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; GED, General Educational Development; HOMA-B, homeostasis model assessment of β-cell function; hs-CRP, high-sensitivity C-reactive protein; MET, metabolic equivalent of task; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
P values for differences between black and white women were obtained by Wilcoxon's rank-sum test for continuous variables and the chi-square test for categorical variables. Percentage calculations were based on completed data.
We examined the correlations of vitamin D biomarkers with each cardiometabolic biomarker (Table 2). Among all participants, total 25(OH)D was inversely correlated with PTH and all cardiometabolic biomarkers, whereas PTH was positively correlated with HOMA-IR, HOMA-B, hs-CRP, fasting insulin, and fasting glucose. Whereas total 25(OH)D was significantly and inversely correlated with hs-CRP (r = −0.08; P = 0.001) and HOMA-B (r = −0.19; P < 0.0001) in white women only, PTH showed a significant inverse correlation with eGFR only in black women (r = −0.07; P = 0.014). Although the correlations of 25(OH)D with eGFR were similar between white (r = −0.05) and black women (r = −0.06), white women had a stronger correlation between 25(OH)D and HOMA-IR (r = −0.23) than black women (r = −0.13). In contrast, the correlations of PTH with HOMA-IR and HOMA-B appeared to be stronger in black women (r = 0.13 for HOMA-IR; r = 0.15 for HOMA-B) than in whites (r = 0.11 for HOMA-IR; r = 0.08 for HOMA-B).
TABLE 2.
Age-adjusted Spearman correlation coefficients for vitamin D biomarkers and cardiometabolic biomarkers among postmenopausal women, stratified by race1
| Biomarkers | Total 25(OH)D | Fasting glucose | Fasting insulin | PTH | eGFR | hs-CRP | HOMA-IR | HOMA-B |
|---|---|---|---|---|---|---|---|---|
| All participants (n = 2800) | ||||||||
| Total 25(OH)D | 1 | −0.13** | −0.26** | −0.37** | −0.16** | −0.14** | −0.26** | −0.17** |
| Fasting glucose | 1 | 0.49** | 0.07** | 0.06** | 0.18** | 0.62** | −0.14** | |
| Fasting insulin | 1 | 0.17** | 0.03 | 0.36** | 0.98** | 0.73** | ||
| PTH | 1 | 0.01 | 0.08** | 0.16** | 0.14** | |||
| eGFR | 1 | 0.04 | 0.04* | −0.04* | ||||
| hs-CRP | 1 | 0.36** | 0.25** | |||||
| HOMA-IR | 1 | 0.61** | ||||||
| HOMA-B | 1 | |||||||
| American white women (n = 1500) | ||||||||
| Total 25(OH)D | 1 | −0.12** | −0.24** | −0.29** | −0.05* | −0.08** | −0.23** | −0.19** |
| Fasting glucose | 1 | 0.47** | 0.06* | 0.04 | 0.1** | 0.58** | −0.08** | |
| Fasting insulin | 1 | 0.11** | −0.01 | 0.31** | 0.99** | 0.81** | ||
| PTH | 1 | 0.003 | 0.05 | 0.11** | 0.08** | |||
| eGFR | 1 | 0.02 | −0.002 | −0.04 | ||||
| hs-CRP | 1 | 0.3** | 0.28** | |||||
| HOMA-IR | 1 | 0.71** | ||||||
| HOMA-B | 1 | |||||||
| American black women (n = 1300) | ||||||||
| Total 25(OH)D | 1 | −0.07** | −0.12** | −0.36** | −0.06* | −0.05 | −0.13** | −0.05 |
| Fasting glucose | 1 | 0.5** | 0.05 | 0.06* | 0.24** | 0.67** | −0.24** | |
| Fasting insulin | 1 | 0.16 | −0.03 | 0.35** | 0.97** | 0.64** | ||
| PTH | 1 | −0.07* | 0.04 | 0.13** | 0.15** | |||
| eGFR | 1 | −0.01 | 0.004 | −0.11** | ||||
| hs-CRP | 1 | 0.37** | 0.18** | |||||
| HOMA-IR | 1 | 0.46** | ||||||
| HOMA-B | 1 | |||||||
Age-adjusted Spearman partial correlation coefficients were calculated to examine the correlations of vitamin D biomarkers with each of the following cardiometabolic biomarkers. Among all participants, the SDs of vitamin D biomarkers and cardiometabolic biomarkers were 20.90 for 25(OH)D, 28.33 for fasting glucose, 8.11 for fasting insulin, 20.66 for PTH, 15.98 for eGFR, 6.76 for hs-CRP, 3.70 for HOMA-IR, and 62.19 for HOMA-B. *P < 0.05; **P < 0.01. eGFR, estimated glomerular filtration rate; HOMA-B, homeostasis model assessment of β-cell function; hs-CRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
We assessed the independent associations between total 25(OH)D concentrations and each cardiometabolic biomarker at baseline (Table 3). Higher total 25(OH)D concentrations were independently associated with lower HOMA-IR and eGFR in a dose-response manner among all participants, adjusting for age, race, clinical center, education, season of blood draw, cigarette smoking status, alcohol consumption, postmenopausal hormone therapy, physical activity, and BMI (model 3). The statistically significant associations of 25(OH)D with HOMA-B and hs-CRP attenuated to nonsignificance after additional adjustment for BMI. The interaction term between race and 25(OH)D was significant for HOMA-B only (P for interaction = 0.029). When stratified by race, the observed associations of 25(OH)D with HOMA-IR (6.59% lower per 1-SD higher in 25(OH)D; P for linear trend = 0.0001) and HOMA-B (3.21% lower per 1-SD higher in 25(OH)D; P for linear trend = 0.03) persisted in white women only. In contrast, an inverse association between 25(OH)D and eGFR persisted in black women only (β = −0.99; P = 0.028).
TABLE 3.
Multivariable weighted linear regression analysis between total 25(OH)D and cardiometabolic biomarkers among postmenopausal women1
| Least-squares mean2 (95% CI) or ±SE | P for linear trend | P for nonlinearity | P for interaction4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | β ± SE | RD,3 % | ||||
| HOMA-IR | ||||||||||
| All participants (n = 2800) | Model 15 | 2.23 (2.11, 2.35) | 1.99 (1.89, 2.10)** | 1.69 (1.60, 1.79)** | 1.45 (1.37, 1.54)** | <0.0001 | 0.098 | −0.27 ± 0.02** | −23.93 | 0.669 |
| Model 26 | 2.17 (2.03, 2.31) | 1.96 (1.84, 2.09)* | 1.71 (1.60, 1.83)** | 1.51 (1.40, 1.62)** | <0.0001 | 0.192 | −0.24 ± 0.02** | −21.12 | 0.653 | |
| Model 37 | 2.01 (1.90, 2.13) | 1.94 (1.83, 2.05) | 1.82 (1.71, 1.94)** | 1.72 (1.61, 1.83)** | <0.0001 | 0.221 | −0.11 ± 0.02** | −10.65 | 0.340 | |
| American white women (n = 1500) | Model 15 | 2.00 (1.87, 2.13) | 1.72 (1.61, 1.84)** | 1.54 (1.44, 1.64)** | 1.31 (1.22, 1.40)** | <0.0001 | 0.521 | −0.16 ± 0.02** | −14.77 | — |
| Model 26 | 1.97 (1.80, 2.14) | 1.71 (1.57, 1.86)** | 1.58 (1.45, 1.72)** | 1.36 (1.24, 1.49)** | <0.0001 | 0.643 | −0.14 ± 0.02** | −13.29 | — | |
| Model 37 | 1.82 (1.69, 1.96) | 1.73 (1.61, 1.86) | 1.65 (1.53, 1.78)* | 1.55 (1.43, 1.68)** | 0.0001 | 0.583 | −0.07 ± 0.02** | −6.59 | — | |
| American black women (n = 1300) | Model 15 | 2.43 (2.22, 2.66) | 2.29 (2.09, 2.50) | 2.23 (2.04, 2.44) | 1.85 (1.69, 2.02)** | <0.0001 | 0.783 | −0.12 ± 0.02** | −10.91 | — |
| Model 26 | 2.32 (2.10, 2.57) | 2.21 (1.99, 2.44) | 2.23 (2.01, 2.47) | 1.88 (1.70, 2.09)** | 0.002 | 0.826 | −0.08 ± 0.02** | −8.14 | — | |
| Model 37 | 2.22 (2.02, 2.44) | 2.13 (1.94, 2.35) | 2.26 (2.06, 2.49) | 2.03 (1.84, 2.23) | 0.196 | 0.626 | −0.04 ± 0.02 | −3.45 | — | |
| HOMA-B | ||||||||||
| All participants (n = 2800) | Model 15 | 93.44 (89.67, 97.37) | 93.44 (89.58, 97.55) | 83.27 (79.62, 87.09)** | 76.62 (73.14, 80.26)** | <0.0001 | 0.018 | −0.15 ± 0.02** | −13.58 | 0.065 |
| Model 26 | 92.26 (87.71, 97.03) | 93.08 (88.35, 98.05) | 84.48 (80.04, 89.17)** | 78.95 (74.52, 83.64)** | <0.0001 | 0.0497 | −0.12 ± 0.02** | −11.52 | 0.092 | |
| Model 37 | 88.18 (84.07, 92.49) | 92.28 (87.83, 96.94) | 87.41 (83.05, 92.01) | 85.41 (80.82, 90.26) | 0.103 | 0.037 | −0.05 ± 0.02* | −4.64 | 0.029 | |
| American white women (n = 1500) | Model 15 | 92.66 (87.95, 97.63) | 86.30 (81.94, 90.88) | 79.60 (75.59, 83.83)** | 72.78 (69.13, 76.64)** | <0.0001 | 0.231 | −0.09 ± 0.01** | −8.85 | — |
| Model 26 | 91.87 (85.81, 98.36) | 86.22 (80.66, 92.17) | 81.33 (75.92, 87.12)** | 75.16 (69.95, 80.76)** | <0.0001 | 0.317 | −0.08 ± 0.01** | −7.66 | — | |
| Model 37 | 88.02 (82.68, 93.70) | 87.06 (81.90, 92.55) | 83.94 (78.81, 89.40) | 81.93 (76.68, 87.54)* | 0.03 | 0.249 | −0.03 ± 0.01* | −3.21 | — | |
| American black women (n = 1300) | Model 15 | 95.97 (89.46, 103.0) | 92.32 (86.07, 99.03) | 95.21 (88.76, 102.1) | 90.65 (84.44, 97.31) | 0.353 | 0.859 | −0.03 ± 0.02 | −3.28 | — |
| Model 26 | 95.77 (88.14, 104.1) | 91.44 (84.15, 99.35) | 95.08 (87.38, 103.5) | 92.20 (84.67, 100.4) | 0.642 | 0.893 | −0.02 ± 0.02 | −2.42 | — | |
| Model 37 | 93.32 (86.11, 101.1) | 89.88 (82.94, 97.39) | 96.13 (88.58, 104.3) | 96.32 (88.69, 104.6) | 0.337 | 0.750 | 0.004 ± 0.02 | 0.35 | — | |
| hs-CRP | ||||||||||
| All participants (n = 2800) | Model 15 | 2.83 (2.59, 3.09) | 2.81 (2.57, 3.08) | 2.18 (1.98, 2.40)** | 2.33 (2.11, 2.57)** | 0.0002 | 0.457 | −0.17 ± 0.04** | −15.97 | 0.650 |
| Model 26 | 2.81 (2.53, 3.12) | 2.92 (2.62, 3.26) | 2.36 (2.11, 2.64)** | 2.42 (2.15, 2.73)* | 0.004 | 0.840 | −0.16 ± 0.04** | −14.64 | 0.453 | |
| Model 37 | 2.58 (2.33, 2.85) | 2.89 (2.61, 3.20) | 2.55 (2.29, 2.83) | 2.95 (2.63, 3.31)* | 0.170 | 0.921 | 0.02 ± 0.04 | 1.85 | 0.381 | |
| American white women (n = 1500) | Model 15 | 2.59 (2.30, 2.92) | 2.09 (1.86, 2.35)* | 1.92 (1.71, 2.17)** | 2.03 (1.80, 2.29)** | 0.004 | 0.357 | −0.10 ± 0.03** | −9.83 | — |
| Model 26 | 2.42 (2.08, 2.82) | 2.09 (1.80, 2.43) | 1.92 (1.65, 2.24)** | 1.93 (1.64, 2.27)** | 0.007 | 0.592 | −0.10 ± 0.03** | −9.81 | — | |
| Model 37 | 2.21 (1.91, 2.54) | 2.11 (1.83, 2.42) | 2.05 (1.77, 2.37) | 2.30 (1.97, 2.67) | 0.650 | 0.980 | 0.001 ± 0.03 | 0.12 | — | |
| American black women (n = 1300) | Model 15 | 3.33 (2.93, 3.79) | 3.10 (2.73, 3.53) | 3.01 (2.65, 3.43) | 2.91 (2.56, 3.32) | 0.163 | 0.492 | −0.06 ± 0.03 | −5.82 | — |
| Model 26 | 3.48 (3.00, 4.04) | 3.18 (2.74, 3.69) | 3.42 (2.94, 3.98) | 3.37 (2.89, 3.92) | 0.958 | 0.282 | −0.02 ± 0.03 | −2.05 | — | |
| Model 37 | 3.28 (2.86, 3.76) | 2.99 (2.61, 3.43) | 3.50 (3.05, 4.02) | 3.74 (3.26, 4.30) | 0.042 | 0.303 | 0.05 ± 0.03 | 5.46 | — | |
| eGFR | ||||||||||
| All participants (n = 2800) | Model 15 | 91.30 ± 0.51 | 89.54 ± 0.52* | 90.10 ± 0.55 | 88.98 ± 0.57** | 0.010 | 0.131 | −1.25 ± 0.46** | 0.006 | 0.572 |
| Model 26 | 92.04 ± 0.63 | 90.28 ± 0.65* | 90.76 ± 0.67 | 89.71 ± 0.72** | 0.016 | 0.139 | −1.25 ± 0.48** | 0.010 | 0.450 | |
| Model 37 | 92.07 ± 0.64 | 90.28 ± 0.66* | 90.93 ± 0.69 | 89.80 ± 0.74** | 0.018 | 0.384 | −1.21 ± 0.51* | 0.017 | 0.444 | |
| American white women (n = 1500) | Model 15 | 87.38 ± 0.62 | 86.50 ± 0.61 | 85.51 ± 0.61* | 85.80 ± 0.61 | 0.045 | 0.246 | −0.63 ± 0.31* | 0.040 | — |
| Model 26 | 88.16 ± 0.83 | 87.16 ± 0.81 | 86.24 ± 0.83* | 86.86 ± 0.87 | 0.125 | 0.227 | −0.50 ± 0.33 | 0.125 | — | |
| Model 37 | 88.20 ± 0.83 | 87.34 ± 0.81 | 86.24 ± 0.84* | 86.96 ± 0.88 | 0.137 | 0.505 | −0.47 ± 0.34 | 0.172 | — | |
| American black women (n = 1300) | Model 15 | 95.36 ± 0.92 | 93.97 ± 0.92 | 94.07 ± 0.91 | 93.06 ± 0.92 | 0.102 | 0.436 | −0.70 ± 0.46 | 0.130 | — |
| Model 26 | 96.14 ± 1.08 | 94.58 ± 1.08 | 95.00 ± 1.10 | 93.01 ± 1.10* | 0.032 | 0.543 | −0.97 ± 0.48* | 0.045 | — | |
| Model 37 | 96.09 ± 1.09 | 94.63 ± 1.09 | 94.96 ± 1.11 | 92.88 ± 1.11* | 0.028 | 0.507 | −0.99 ± 0.50* | 0.046 | — | |
Weighted linear regression models were performed to assess the independent associations between total 25(OH)D and each cardiometabolic biomarker. Least-squares mean was obtained when 25(OH)D was parameterized as quartiles; β coefficient was obtained when 25(OH)D was modeled as a continuous variable. *P < 0.05; **P < 0.01. eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; HOMA-B, homeostasis model assessment of β-cell function; RD, relative difference; 25(OH)D, 25-hydroxyvitamin D.
Values are least-squares means (95% CIs) for log-transformed HOMA-IR, HOMA-B, and hs-CRP, and least-squares means ± SEs for eGFR.
RD was obtained from back-transformed β and is interpreted as percentage lower (negative value) or higher (positive value) concentrations of HOMA-IR, HOMA-B, or hs-CRP on the original scale for every 1-SD increase in total 25(OH)D.
P for interaction was obtained by adding an interaction term between 25(OH)D and race into each model.
Model 1 adjusted for age, clinical center, and race.
Model 2 further adjusted for education, season of blood draw, cigarette smoking status, alcohol intake, postmenopausal hormone therapy use, and physical activity levels.
Model 3 further adjusted for BMI.
We also assessed the independent associations between PTH concentrations and each cardiometabolic biomarker at baseline (Table 4). After adjusting for the same covariates as aforementioned, higher PTH concentrations were independently associated with lower eGFR among all participants. There was also a significant interaction between race and PTH on hs-CRP (P for interaction = 0.035). PTH was nonlinearly associated with eGFR across racial groups (P for nonlinearity = 0.005 for white women and 0.036 for black women). In a race-stratified analysis, higher PTH concentrations were significantly associated with higher HOMA-IR in white women only (3.29% increase per 1-SD increase in PTH) and higher HOMA-B in black women only (4.55% higher per 1-SD higher in PTH; P for nonlinearity = 0.003). Whereas there was a linear trend toward higher PTH associated with lower hs-CRP in black women (P for linear trend = 0.01), we found a nonlinear relation between PTH and hs-CRP among white women (P for nonlinearity = 0.039).
TABLE 4.
Multivariable weighted linear regression analysis between PTH and cardiometabolic biomarkers among postmenopausal women1
| Least-squares mean2 (95% CI) or ±SE | P for linear trend | P for nonlinearity | P for interaction4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | β ± SE | RD,3 % | ||||
| HOMA-IR | ||||||||||
| All participants (n = 2800) | Model 15 | 1.72 (1.63, 1.82) | 1.78 (1.68, 1.88) | 1.88 (1.78, 1.99)* | 2.12 (2.01, 2.24)** | <0.0001 | 0.042 | 0.15 ± 0.02** | 15.6 | 0.883 |
| Model 26 | 1.77 (1.66, 1.89) | 1.78 (1.67, 1.91) | 1.91 (1.79, 2.04)* | 2.09 (1.96, 2.22)** | <0.0001 | 0.693 | 0.13 ± 0.02** | 14 | 0.699 | |
| Model 37 | 1.88 (1.77, 2.00) | 1.83 (1.72, 1.94) | 1.93 (1.82, 2.05) | 1.95 (1.84, 2.07) | 0.140 | 0.761 | 0.05 ± 0.02** | 5.41 | 0.332 | |
| American white women (n = 1500) | Model 15 | 1.49 (1.39. 1.59) | 1.59 (1.48, 1.70) | 1.60 (1.50, 1.72) | 1.81 (1.69, 1.94)** | <0.0001 | 0.621 | 0.08 ± 0.02** | 8.24 | — |
| Model 26 | 1.57 (1.44, 1.72) | 1.63 (1.49, 1.78) | 1.66 (1.52, 1.81) | 1.85 (1.69, 2.02)** | 0.001 | 0.453 | 0.08 ± 0.02** | 7.9 | — | |
| Model 37 | 1.70 (1.58, 1.83) | 1.68 (1.55, 1.81) | 1.68 (1.56, 1.81) | 1.76 (1.63, 1.89) | 0.352 | 0.370 | 0.03 ± 0.01* | 3.29 | — | |
| American black women (n = 1300) | Model 15 | 1.89 (1.73, 2.07) | 2.07 (1.90, 2.27) | 2.33 (2.13, 2.55)** | 2.52 (2.30, 2.75)** | <0.0001 | 0.183 | 0.10 ± 0.02** | 10.74 | — |
| Model 26 | 1.93 (1.74, 2.14) | 2.06 (1.86, 2.29) | 2.28 (2.05, 2.52)* | 2.36 (2.13, 2.61)** | 0.001 | 0.644 | 0.08 ± 0.02** | 8.16 | — | |
| Model 37 | 2.08 (1.89, 2.29) | 2.09 (1.90, 2.30) | 2.31 (2.10, 2.54) | 2.16 (1.97, 2.37) | 0.372 | 0.948 | 0.03 ± 0.02 | 2.65 | — | |
| HOMA-B | ||||||||||
| All participants (n = 2800) | Model 15 | 81.48 (78.00, 85.11) | 85.38 (81.77, 89.15) | 88.36 (84.67, 92.21)** | 94.73 (90.89, 98.73)** | <0.0001 | 0.012 | 0.09 ± 0.02** | 9.51 | 0.282 |
| Model 26 | 83.74 (79.41, 88.30) | 86.00 (81.57, 90.68) | 88.89 (84.42, 93.60)* | 94.18 (89.51, 99.09)** | <0.0001 | 0.606 | 0.08 ± 0.02** | 8.43 | 0.484 | |
| Model 37 | 86.72 (82.48, 91.18) | 86.92 (82.69, 91.37) | 89.06 (84.83, 93.50) | 90.97 (86.68, 95.46) | 0.075 | 0.988 | 0.04 ± 0.02* | 3.83 | 0.716 | |
| American white women (n = 1500) | Model 15 | 77.77 (73.79, 81.96) | 82.31 (78.12, 86.72) | 82.41 (78.20, 86.84) | 87.32 (82.86, 92.02)** | 0.0003 | 0.323 | 0.04 ± 0.01** | 4.37 | — |
| Model 26 | 80.90 (75.51, 86.68) | 83.93 (78.32, 89.95) | 83.99 (78.45, 89.92) | 88.56 (82.67, 94.87)* | 0.019 | 0.219 | 0.04 ± 0.01** | 4.14 | — | |
| Model 37 | 85.12 (79.93, 90.66) | 85.45 (80.23, 91.02) | 84.99 (79.88, 90.43) | 86.50 (81.23, 92.10) | 0.659 | 0.188 | 0.02 ± 0.01 | 1.51 | — | |
| American black women (n = 1300) | Model 15 | 80.24 (74.81, 86.06) | 91.43 (85.29, 98.01)** | 99.50 (92.84, 106.6)** | 105.0 (97.94, 112.6)** | <0.0001 | <0.0001 | 0.08 ± 0.02** | 8.33 | — |
| Model 26 | 81.91 (75.32, 89.09) | 90.72 (83.48, 98.58)* | 98.80 (90.99, 107.3) | 103.6 (95.50, 112.4) | <0.0001 | <0.0001 | 0.07 ± 0.02** | 7.34 | — | |
| Model 37 | 85.30 (78.60, 92.57) | 91.32 (84.27, 98.97) | 100.1 (92.32, 108.5)** | 98.92 (91.36, 107.1)** | 0.003 | 0.003 | 0.04 ± 0.02* | 4.55 | — | |
| hs-CRP | ||||||||||
| All participants (n = 2800) | Model 15 | 2.49 (2.27, 2.73) | 2.34 (2.14, 2.57) | 2.61 (2.39, 2.86) | 2.83 (2.59, 3.09)* | 0.010 | 0.999 | 0.12 ± 0.04** | 13.24 | 0.150 |
| Model 26 | 2.69 (2.41, 3.00) | 2.47 (2.21, 2.76) | 2.64 (2.37, 2.94) | 2.85 (2.56, 3.17) | 0.162 | 0.970 | 0.09 ± 0.04* | 9.83 | 0.133 | |
| Model 37 | 2.95 (2.66, 3.27) | 2.57 (2.32, 2.85)* | 2.72 (2.46, 3.01) | 2.61 (2.36, 2.88)* | 0.120 | 0.002 | −0.01 ± 0.03 | −1.24 | 0.035 | |
| American white women (n = 1500) | Model 15 | 2.08 (1.84, 2.34) | 2.00 (1.78, 2.25) | 2.19 (1.94, 2.47) | 2.34 (2.08, 2.64) | 0.089 | 0.111 | 0.09 ± 0.03** | 9.48 | — |
| Model 26 | 2.08 (1.78, 2.43) | 2.02 (1.74, 2.36) | 2.07 (1.78, 2.41) | 2.26 (1.94, 2.64) | 0.249 | 0.200 | 0.07 ± 0.03* | 7.31 | — | |
| Model 37 | 2.30 (1.99, 2.65) | 2.09 (1.81, 2.41) | 2.12 (1.84, 2.44) | 2.13 (1.84, 2.45) | 0.427 | 0.039 | 0.02 ± 0.03 | 1.6 | — | |
| American black women (n = 1300) | Model 15 | 3.14 (2.77, 3.58) | 2.58 (2.27, 2.94)* | 3.53 (3.10, 4.01) | 3.18 (2.80, 3.62) | 0.271 | 0.482 | 0.03 ± 0.03 | 3.46 | — |
| Model 26 | 3.61 (3.10, 4.20) | 2.83 (2.43, 3.28)** | 3.69 (3.18, 4.29) | 3.39 (2.93, 3.93) | 0.773 | 0.796 | 0.01 ± 0.03 | 0.64 | — | |
| Model 37 | 4.05 (3.53, 4.65) | 2.90 (2.53, 3.32)** | 3.75 (3.27, 4.30) | 2.95 (2.58, 3.38)** | 0.010 | 0.943 | −0.08 ± 0.03* | −7.56 | — | |
| eGFR | ||||||||||
| All participants (n = 2800) | Model 15 | 90.37 ± 0.53 | 90.42 ± 0.53 | 90.86 ± 0.52 | 89.11 ± 0.51 | 0.077 | 0.034 | −2.20 ± 0.42** | <0.0001 | 0.052 |
| Model 26 | 91.20 ± 0.66 | 91.22 ± 0.66 | 91.66 ± 0.64 | 89.88 ± 0.63 | 0.066 | 0.032 | −2.18 ± 0.43** | <0.0001 | 0.098 | |
| Model 37 | 91.23 ± 0.67 | 91.24 ± 0.67 | 91.84 ± 0.65 | 89.91 ± 0.65 | 0.040 | 0.043 | −2.24 ± 0.43** | <0.0001 | 0.128 | |
| American white women (n = 1500) | Model 15 | 85.88 ± 0.62 | 86.84 ± 0.61 | 86.90 ± 0.61 | 85.47 ± 0.62 | 0.504 | 0.002 | −0.97 ± 0.31** | 0.002 | — |
| Model 26 | 86.80 ± 0.83 | 87.77 ± 0.83 | 87.74 ± 0.82 | 86.30 ± 0.83 | 0.425 | 0.003 | −1.03 ± 0.31** | 0.001 | — | |
| Model 37 | 86.96 ± 0.83 | 87.90 ± 0.83 | 87.84 ± 0.82 | 86.27 ± 0.83 | 0.310 | 0.005 | −1.09 ± 0.31** | 0.001 | — | |
| American black women (n = 1300) | Model 15 | 95.80 ± 0.92 | 94.69 ± 0.91 | 94.50 ± 0.91 | 91.41 ± 0.91** | 0.001 | 0.006 | −2.14 ± 0.46** | <0.0001 | — |
| Model 26 | 96.36 ± 1.10 | 95.18 ± 1.08 | 95.41 ± 1.08 | 92.13 ± 1.06** | 0.001 | 0.025 | −1.98 ± 0.47** | <0.0001 | — | |
| Model 37 | 96.31 ± 1.11 | 95.15 ± 1.09* | 95.22 ± 1.10 | 92.20 ± 1.08** | 0.002 | 0.036 | −1.93 ± 0.48** | <0.0001 | — | |
Weighted linear regression models were performed to assess the independent associations between PTH and each cardiometabolic biomarker. Least-squares mean was obtained when 25-hydroxyvitamin D was parameterized as quartiles; β coefficient was obtained when PTH was modeled as a continuous variable. *P < 0.05; **P < 0.01. eGFR, estimated glomerular filtration rate; HOMA-B, homeostasis model assessment of β-cell function; hs-CRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone; RD, relative difference.
Values are least-squares means (95% CIs) for log-transformed HOMA-IR, HOMA-B, and hs-CRP, and least-squares means ± SEs for eGFR.
RD was obtained from back-transformed β and is interpreted as percentage lower (negative value) or higher (positive value) concentrations of HOMA-IR, HOMA-B, or hs-CRP on the original scale for every 1-SD increase in PTH.
P for interaction was obtained by adding an interaction term between PTH and race into each model.
Model 1 adjusted for age, clinical center, and race.
Model 2 further adjusted for education, season of blood draw, cigarette smoking status, alcohol intake, postmenopausal hormone therapy use, and physical activity levels.
Model 3 further adjusted for BMI.
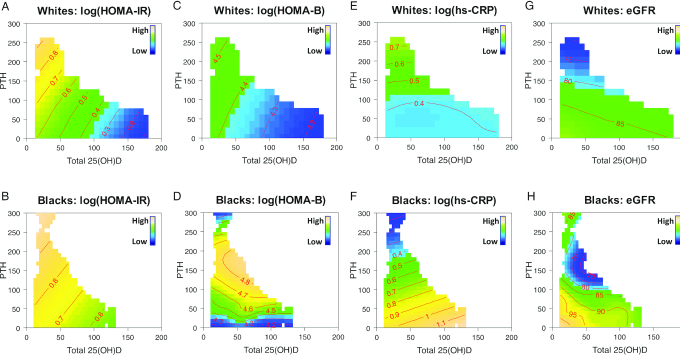
We further explored the joint associations of total 25(OH)D and PTH with each cardiometabolic biomarker (Figure 1A–H). In the color-filled contour plots, the mean concentrations of each cardiometabolic biomarker at all combinations of 25(OH)D and PTH concentrations were indicated by numbers on the contour lines after adjustment for confounding factors. On average, white women had lower HOMA-IR, HOMA-B, hs-CRP, and eGFR than black women. For example, in Figure 1A and B, contours with warmer color (yellow) indicate regions where the mean values of HOMA-IR were higher; contours with colder color (blue) indicate lower mean values. The parallel straight contour lines suggest linear relations of HOMA-IR with total 25(OH)D and PTH, simultaneously. In addition, closely spaced contour lines represent steeper slopes for the linear relation (Figure 1A) than those relatively spaced far apart (Figure 1B). Overall, the combination of lower total 25(OH)D and higher PTH was jointly associated with higher HOMA-IR in both white and black women with similar linear trends but varying slopes, suggesting a lack of racial differences. Specifically, in white women, when 25(OH)D ≥ 100 nmol/L and PTH ≤ 50 pg/mL, the mean levels of HOMA-IR on the logarithmic scale were ∼0.4 or less; in contrast, its mean levels were ∼0.7 or greater when 25(OH)D < 50 nmol/L and PTH ≥ 100 pg/mL (Figure 1A). Figure 1B shows a similar linear trend for the joint associations of 25(OH)D and PTH with HOMA-IR in black women. Conversely, black–white differences in the joint associations of 25(OH)D and PTH with HOMA-B, hs-CRP, and eGFR were evident (Figure 1C–H). Overall, the combination of lower 25(OH)D concentrations and higher PTH concentrations was jointly and linearly associated with higher HOMA-B in white women (Figure 1C), but there was a nonlinear association between PTH and HOMA-B in black women suggested by the curved contour lines mainly driven by PTH (Figure 1D). Whereas PTH alone was positively associated with hs-CRP in white women, there was a joint association of higher 25(OH)D and lower PTH with higher hs-CRP in black women (Figure 1E, F). To explore the unexpected results, we further performed a stratified analysis by BMI (<25 compared with ≥25) in black women. We found that 25(OH)D, jointly with PTH, was positively associated with hs-CRP in those with normal weight (Supplemental Figure 2). Among black women, the mean ± SD concentration of hs-CRP was lower in those with normal weight (2.91 ± 4.62 mg/L) than in those with overweight/obesity (6.29 ± 7.23 mg/L). We also observed some extreme values of hs-CRP in the scatterplot of PTH and hs-CRP in black women (Supplemental Figure 3). Although 25(OH)D and PTH were simultaneously associated with eGFR among both black and white women, there were notable racial differences in the shape of the associations (Figure 1G, H). Specifically, PTH appeared to be inversely and linearly associated with eGFR in white women, mainly confined to low 25(OH)D (Figure 1G); however, their joint association with eGFR took on a nonlinear and complex shape in black women and appeared to be predominantly driven by PTH concentrations (Figure 1H).
FIGURE 1.
Estimated concurrent associations of 25(OH)D and PTH on HOMA-IR (A–B), HOMA-B (C–D), hs-CRP (E–F), and eGFR (G–H) by race (blacks compared with whites). A penalized spline-based semiparametric model with contour plots was performed for each cardiometabolic biomarker among white (n = 1500) and black women (n = 1300). In the color-filled contour plot, the mean concentrations of each cardiometabolic biomarker at all 25(OH)D–PTH combinations in blacks and whites are indicated by the numbers on the contour lines, adjusting for age, clinical center, education, season of blood draw, BMI, cigarette smoking status, alcohol consumption, postmenopausal hormone therapy, and physical activity levels. The SDs for each outcome were 0.67 for log(HOMA-IR), 0.52 for log(HOMA-B), 1.17 for log(hs-CRP), and 12.97 for eGFR in white women; and 0.82 for log(HOMA-IR), 0.64 for log(HOMA-B), 1.18 for log(hs-CRP), and 18.03 for eGFR in black women. eGFR, estimated glomerular filtration rate; HOMA-B, homeostasis model assessment of β-cell function; hs-CRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
Discussion
In this large cohort of US postmenopausal women without CVD, total 25(OH)D was inversely correlated with PTH and all cardiometabolic biomarkers in both white and black participants but the joint association of 25(OH)D and PTH with β-cell function, systemic inflammation, and kidney function differed by race. However, higher PTH and lower 25(OH)D were independently and jointly associated with higher HOMA-IR in both white and black women, with similar linear patterns. Our findings suggest that the vitamin D–PTH endocrine system may play a role in explaining racial disparities in cardiometabolic health.
Our findings are consistent with most previous studies of the individual associations of 25(OH)D and PTH with either of these cardiometabolic biomarkers (27–34). Evidence from national surveys and recent observational studies suggested that 25(OH)D was inversely associated with HOMA-IR and HOMA-B and PTH was positively correlated with HOMA-IR and HOMA-B across different populations (27–31). No association between 25(OH)D and hs-CRP was observed in a cohort from the Framingham Offspring Study (n = 1381) (31). Several studies have demonstrated an inverse association of PTH and 25(OH)D with eGFR in patients with chronic kidney disease (CKD) and in general populations, respectively (32–34). However, inconsistent findings still exist (7, 34–37). Residual confounding due to different population characteristics, especially determinants of vitamin D status, may explain these null or contradictory findings. In addition, small sample size and different biomarker categorizations and model specifications may also explain these inconsistent results.
Contrary to studies of independent associations of 25(OH)D and PTH with cardiometabolic biomarkers, data on their joint associations are limited and have generally been analyzed using their ratio, subgroup analyses by broad categorizations of 25(OH)D and PTH, or adjusted parameter estimates. A case-control study consisting of 15 obese and 15 matched normal-weight adolescent girls suggested a joint association of high PTH and low 25(OH)D with high hs-CRP, which is similar to our results for black women, but in the opposite direction (38). In our stratified analysis by BMI (<25 compared with ≥25) in black women, the association of 25(OH)D with hs-CRP was positive in those with normal weight, synergistically with PTH. This could be, at least partly, due to 1) limited variability of hs-CRP among normal-weight black women compared with the women with overweight/obesity; and 2) the presence of extreme values. In line with our findings, the Korean national survey data showed a nonlinear trend for PTH across eGFR tertiles, after accounting for 25(OH)D and other covariates in Korean women (34). A recent hospital-based case-control study among 225 elderly Greek patients found that participants with vitamin D deficiency and high PTH (third tertile) had the highest HOMA-IR but no changes in HOMA-B compared with all other groups with either vitamin D sufficiency or lower PTH (39). The small sample size with grouped vitamin D/PTH data may have limited their ability to identify joint associations of vitamin D and PTH with HOMA-B.
The reciprocal relation between 25(OH)D and PTH is dynamic, complex, and very sensitive to racial background (13). To account for their nonlinear race-specific relations and their possible interactions with CVD biomarkers, we used novel model-based color contour plots to delineate their joint associations with each biomarker. We found consistent linear associations of higher PTH and lower 25(OH)D with higher HOMA-IR in both white and black women, but black–white differences in their associations with HOMA-B, hs-CRP, and eGFR. Although our results could be explained by the calcium-dependent effects of PTH on insulin release by pancreatic islets (40), the linear relation of higher PTH and lower 25(OH)D with higher HOMA-B is contrary to the existing biological evidence linking the vitamin D–PTH system to pancreatic β-cell function. HOMA-B may not be a reliable or sensitive surrogate of β-cell function alone (22, 41, 42). Its strong correlations with fasting glucose or insulin concentrations reflect insulin resistance to varying extents depending on population characteristics. Contrary to the joint associations of 25(OH)D and PTH with hs-CRP in black women, the independent association between PTH and hs-CRP in white women suggests that the anti-inflammatory property of PTH may be more active in white women than in black women.
Racial differences in associations of 25(OH)D and PTH with eGFR may explain racial disparities in inflammation-related cardiovascular health. The existing evidence, mainly focusing on their independent associations, cannot fully address the racial heterogeneity of their synergistic relations (18, 43, 44). CKD has been described as a state of stagnant vitamin D metabolism with decreased vitamin D catabolism; in this study, the joint associations of 25(OH)D and PTH with eGFR were linear in white women and nonlinear in black women. Our findings may suggest biological differences between whites and blacks in altered vitamin D catabolism related to impaired kidney function. Overall, our findings contribute to a better understanding of racial differences in complex associations of vitamin D and PTH with CVD risk and may thus inform the design of future clinical interventions to reduce racial disparities related to CVD.
Our findings may be explained by the pleiotropic effects of the vitamin D–PTH endocrine system on the cardiovascular system via their receptors in vascular smooth muscle and endothelium. Vitamin D stimulates insulin secretion and action, regulates the RAAS, and inhibits proinflammatory cytokine production, which can induce insulin resistance and inflammation-linked vascular endothelial dysfunction (1–3). PTH also plays an important role in the RAAS, endothelial function, and systemic inflammation independently or jointly with vitamin D (45, 46). Elevated PTH concentrations may increase hepatic production of C-reactive protein by stimulating the release of the cytokine IL-6 (47–49). PTH also affects glucose/insulin metabolism directly or indirectly (50).
This study has several strengths. The well-characterized biracial cohort allowed us to thoroughly examine racial disparities in the associations of total 25(OH)D and PTH with a panel of core cardiometabolic biomarkers. Further, we used a novel analytic approach to visually elucidate possible differences in their complex concurrent associations between whites and blacks, by simultaneously considering nonlinear relations and interactions, as well as controlling confounding. Our study also has some limitations. First, as surrogate measures of insulin resistance and β-cell function, the HOMA model may underestimate insulin sensitivity and overestimate β-cell function, without incorporating proinsulin secretion (22, 42). Further research with a more reliable and feasible marker is needed to confirm our results. Second, free and bioavailable 25(OH)D, which may better reflect vitamin D activity than total 25(OH)D, were not measured. However, their different assays have not been rigorously validated for large populations. Third, our cross-sectional design cannot address cause-to-effect relations. Finally, the lack of data on other racial groups limits the generalizability of the findings.
In conclusion, we found a similar pattern of joint associations of total 25(OH)D and PTH with insulin resistance between US postmenopausal white and black women, but black–white differences in their associations with biomarkers of β-cell function, systemic inflammation, and kidney function. Future longitudinal studies are warranted to determine race-specific thresholds of both 25(OH)D and PTH concentrations and their trajectories in relation to future risk of cardiometabolic diseases.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—YS: designed and implemented the research, acquired funding for the research, and had primary responsibility for the final content of the manuscript; JX and YS: developed the study conception and interpreted the data; YS and JEM: conducted data acquisition; JX and WT: analyzed the data; JX: drafted the manuscript; and all authors: made major contributions in revising the manuscript and read and approved the final manuscript. JEM was a recipient of NIH funding to conduct VITAL (Vitamin D and Omega-3 Trial), a large-scale randomized trial of vitamin D and omega-3s in the prevention of cancer and cardiovascular disease. The vitamin D study pills were donated by Pharmavite LLC (Northridge, CA). All other authors report no conflicts of interest.
Notes
Supported by NIH/National Heart, Lung, and Blood Institute (NHLBI) grant R01-HL113056 (to YS) as an ancillary study within the Women's Health Initiative (WHI) Observational Study. JX and YS were also supported by an Indiana University Health–Indiana University School of Medicine Strategic Research Initiative Grant. The WHI program is funded by the NHLBI, NIH, US Department of Health and Human Services, through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will be made available upon request pending application and approval from the corresponding author.
Abbreviations used: CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; FPI, fasting plasma insulin; HOMA-B, homeostasis model assessment of β-cell function; hs-CRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone; RAAS, renin–angiotensin–aldosterone system; WHI-OS, Women's Health Initiative Observational Study; 25(OH)D, 25-hydroxyvitamin D.
Contributor Information
Jin Xia, Department of Epidemiology, Indiana University Richard M Fairbanks School of Public Health, Indianapolis, IN, USA.
Wanzhu Tu, Department of Biostatistics, Indiana University School of Medicine, Indianapolis, IN, USA.
JoAnn E Manson, Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Hongmei Nan, Department of Epidemiology, Indiana University Richard M Fairbanks School of Public Health, Indianapolis, IN, USA.
Aladdin H Shadyab, Family Medicine and Public Health, School of Medicine, University of California, San Diego, La Jolla, CA, USA.
Jennifer W Bea, University of Arizona Cancer Center, College of Medicine, The University of Arizona, Tucson, AZ, USA.
Ting-Yuan D Cheng, Department of Epidemiology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, USA.
Lifang Hou, Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
Yiqing Song, Department of Epidemiology, Indiana University Richard M Fairbanks School of Public Health, Indianapolis, IN, USA.
References
- 1. Li YC, Kong J, Wei M, Chen Z-F, Liu SQ, Cao L-P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:419–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, McLachlan SM, Adams JS, Hewison M. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151(6):2423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ni W, Watts SW, Ng M, Chen S, Glenn DJ, Gardner DG. Elimination of vitamin D receptor in vascular endothelial cells alters vascular function. Hypertension. 2014;64(6):229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr. 2004;79(5):820–5. [DOI] [PubMed] [Google Scholar]
- 7. Jackson JL, Judd SE, Panwar B, Howard VJ, Wadley VG, Jenny NS, Gutiérrez OM. Associations of 25-hydroxyvitamin D with markers of inflammation, insulin resistance and obesity in black and white community-dwelling adults. J Clin Transl Endocrinol. 2016;5:21–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang W-H, Chen L-W, Lee C-C, Sun C-Y, Shyu Y-C, Hsu H-R, Chien R-N, Wu I-W. Association between parathyroid hormone, 25(OH) vitamin D, and chronic kidney disease: a population-based study. Biomed Res Int. 2017:7435657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bora K, Ruram AA. No association of 25-hydroxyvitamin D and parathormone levels with glucose homeostasis in type 2 diabetes – a study from Shillong, Meghalaya. Int J Vitam Nutr Res. 2019;89:285–92. [DOI] [PubMed] [Google Scholar]
- 10. Damasiewicz MJ, Magliano DJ, Daly RM, Gagnon C, Lu ZX, Ebeling PR, Chadban SJ, Atkins RC, Kerr PG, Shaw JE et al. 25-Hydroxyvitamin D levels and chronic kidney disease in the AusDiab (Australian Diabetes, Obesity and Lifestyle) study. BMC Nephrol. 2012;13:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haidari F, Zakerkish M, Karandish M, Saki A, Pooraziz S. Association between serum vitamin D level and glycemic and inflammatory markers in non-obese patients with type 2 diabetes. Iran J Med Sci. 2016;41(5):367–73. [PMC free article] [PubMed] [Google Scholar]
- 12. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. [DOI] [PubMed] [Google Scholar]
- 13. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int. 2011;22(6):1745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. Clin Chem. 2009;55(9):1627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Renal Data System. USRDS 2013 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: NIH, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 16. Scragg R, Sowers M, Bell C; Third National Health and Nutrition Examination Survey . Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–8. [DOI] [PubMed] [Google Scholar]
- 17. Ashraf AP, Fisher G, Alvarez J, Dudenbostel T, Calhoun DA, Szalai AJ, Gower BA. Associations of C-reactive protein to indices of vascular health and the influence of serum 25(OH)D status in healthy adults. J Nutr Metab. 2012:475975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ennis J, Worcester E, Coe F. Contribution of calcium, phosphorus and 25-hydroxyvitamin D to the excessive severity of secondary hyperparathyroidism in African-Americans with CKD. Nephrol Dial Transplant. 2012;27(7):2847–53. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Tu W, Manson JE, Tinker L, Liu S, Cauley JA, Qi L, Mouton C, Martin L, Hou L et al. Racial/ethnic differences in 25-hydroxy vitamin D and parathyroid hormone levels and cardiovascular disease risk among postmenopausal women. J Am Heart Assoc. 2019;8(4):e011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–77. [DOI] [PubMed] [Google Scholar]
- 21. Ma Y, Hébert JR, Manson JE, Balasubramanian R, Liu S, Lamonte MJ, Bird CE, Ockene JK, Qiao Y, Olendzki B et al. Determinants of racial/ethnic disparities in incidence of diabetes in postmenopausal women in the U.S.: the Women's Health Initiative 1993–2009. Diabetes Care. 2012;35(11):2226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Tu W. A semiparametric regression model for paired longitudinal outcomes with application in childhood blood pressure development. Ann Appl Stat. 2012;6:1861–82. [Google Scholar]
- 26. Li Z, Liu H, Tu W. A generalized semiparametric mixed model for analysis of multivariate health care utilization data. Stat Methods Med Res. 2017;26:2909–18. [DOI] [PubMed] [Google Scholar]
- 27. AI-Khalidi B, Kimball SM, Rotondi MA, Ardern CI. Standardized serum 25-hydroxyvitamin D concentrations are inversely associated with cardiometabolic disease in U.S. adults: a cross-sectional analysis of NHANES, 2001–2010. Nutr J. 2017;16(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon H, Jeon DJ, Park CE, You HS, Moon AE. Relationship between homeostasis model assessment of insulin resistance and beta cell function and serum 25-hydroxyvitamin D in non-diabetic Korean adults. J Clin Biochem Nutr. 2016;59(2):139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahmoudi T, Gourabi H, Ashrafi M, Yazdi RS, Ezabadi Z. Calciotropic hormones, insulin resistance, and the polycystic ovary syndrome. Fertil Steril. 2010;93(4):1208–14. [DOI] [PubMed] [Google Scholar]
- 30. Antonopoulou V, Grammatiki M, Rapti E, Koufakis T, Karras S, Yavropoulou M, Papavramidis T, Kotsa K. Glucose metabolism in primary hyperparathyroidism: the role of parathyroidectomy. Endocrine Abstracts. 2018;56:P226(abstr). [Google Scholar]
- 31. Shea MK, Booth SL, Massaro JM, Jacques PF, D'Agostino RB Sr, Dawson-Hughes B, Ordovas JM, O'Donnell CJ, Kathiresan S, Keaney JR Jr et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167(3):313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Evenepoel P, Meijers B, Viaene L, Bammens B, Claes K, Kuypers D, Vanderschueren D, Vanrenterghem Y. Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2010;5(7):1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phelps KR, Stote KS, Mason D. Tubular calcium reabsorption and other aspects of calcium homeostasis in primary and secondary hyperparathyroidism. Clin Nephrol. 2014;82(2):83–91. [DOI] [PubMed] [Google Scholar]
- 34. Han S-W, Kim S-J, Lee D-J, Kim K-M, Joo N-S. The relationship between serum 25-hydroxyvitamin D, parathyroid hormone and the glomerular filtration rate in Korean adults: the Korean National Health and Nutrition Examination Survey between 2009 and 2011. Korean J Fam Med. 2014;35(2):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Del Gobbo LC, Song Y, Dannenbaum DA, Dewailly E, Egeland GM. Serum 25-hydroxyvitamin D is not associated with insulin resistance or beta-cell function in Candian Cree. J Nutr. 2011;141(2):290–5. [DOI] [PubMed] [Google Scholar]
- 36. George JA, Norris SA, van Deventer HE, Crowther NJ. The association of 25 hydroxyvitamin D and parathyroid hormone with metabolic syndrome in two ethnic groups in South Africa. PLoS One. 2013;8(4):e61282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng S-P, Liu C-L, Liu T-P, Hsu Y-C, Lee J-J. Association between parathyroid hormone levels and inflammatory markers among US adults. Mediators Inflamm. 2014:709024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stanley T, Bredella MA, Pierce L, Misra M. The ratio of parathyroid hormone to vitamin D is a determinant of cardiovascular risk and insulin sensitivity in adolescent girls. Metab Syndr Relat Disord. 2013;11(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karras SN, Anagnostis P, Antonopoulou V, Tsekmekidou X, Koufakis T, Goulis DG, Zebekakis P, Kotsa K. The combined effect of vitamin D and parathyroid hormone concentrations on glucose homeostasis in older patients with prediabetes: a cross-sectional study. Diab Vasc Dis Res. 2018;15(2):150–3. [DOI] [PubMed] [Google Scholar]
- 40. Ni Z, Smogorzewski M, Massry SG. Effects of parathyroid hormone on cytosolic calcium of rat adipocytes. Endocrinology. 1994;135:1837–44. [DOI] [PubMed] [Google Scholar]
- 41. Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, Rifai N, Liu S. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women's Health Initiative Observational Study. Diabetes Care. 2007;30(7):1747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pfützner A, Derwahl M, Jacob S, Hohberg C, Blümner E, Lehmann U, Fuchs W, Forst T. Limitations of the HOMA-B score for assessment of β-cell functionality in interventional trials—results from the PIOglim study. Diabetes Technol Ther. 2010;12(8):599–604. [DOI] [PubMed] [Google Scholar]
- 43. Patel S, Barron JL, Mirzazedeh M, Gallagher H, Hyer S, Cantor T, Fraser WD. Changes in bone mineral parameters, vitamin D metabolites, and PTH measurements with varying chronic kidney disease stages. J Bone Miner Metab. 2011;29(1):71–9. [DOI] [PubMed] [Google Scholar]
- 44. Oh YJ, Kim M, Lee H, Lee JP, Kim H, Kim S, Oh KH, Joo KW, Lim CS, Kim S et al. A threshold value of estimated glomerular filtration rate that predicts changes in serum 25-hydroxyvitamin D levels: 4th Korean National Health and Nutritional Examination Survey 2008. Nephrol Dial Transplant. 2012;27(6):2396–403. [DOI] [PubMed] [Google Scholar]
- 45. Tomaschitz A, Ritz E, Pieske B, Rus-Machan J, Kienreich K, Verheyen N, Gaksch M, Grübler M, Fahrleitner-Pammer A, Mrak P et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014;63(1):20–31. [DOI] [PubMed] [Google Scholar]
- 46. Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways. Am J Physiol Renal Physiol. 2007;292(4):F1215–8. [DOI] [PubMed] [Google Scholar]
- 47. Mitnick MA, Grey A, Masiukiewicz U, Bartkiewicz M, Rios-Velez L, Friedman S, Xu L, Horowitz MC, Insogna K. Parathyroid hormone induces hepatic production of bioactive interleukin-6 and its soluble receptor. Am J Physiol Endocrinol Metab. 2001;280(3):E405–12. [DOI] [PubMed] [Google Scholar]
- 48. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. [DOI] [PubMed] [Google Scholar]
- 49. Murray TM, Rao LG, Divieti P, Bringhurst FR. Parathyroid hormone secretion and action: evidence for discrete receptors for the carboxyl-terminal region and related biological actions of carboxyl-terminal ligands. Endocr Rev. 2005;26(1):78–113. [DOI] [PubMed] [Google Scholar]
- 50. Baczynske R, Massry SG, Magott M, el Belbessi S, Kohan R, Brautbar N. Effect of parathyroid hormone on energy metabolism of skeletal muscle. Kidney Int. 1985;28:722–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.