ABSTRACT
Background
Dietary polyphenols including anthocyanins target multiple organs.
Objective
We aimed to assess the involvement of glucagon-like peptide 1 (GLP-1), leptin, insulin and fibroblast growth factor 21 (FGF21) in mediating metabolic beneficial effects of purified anthocyanin cyanidin-3-glucoside (Cy3G).
Methods
Intestinal proglucagon gene (Gcg; encoding GLP-1) and liver Fgf21 expression were assessed in 6-wk-old male C57BL-6J mice fed a low-fat-diet (LFD; 10% of energy from fat), alone or with 1.6 mg Cy3G/L in drinking water for 3 wk [experiment (Exp.) 1; n = 5/group]. Similar mice were fed the LFD or a high-fat diet (HFD; 60% energy from fat) with or without Cy3G for 20 wk. Half of the mice administered Cy3G also received 4 broad-spectrum antibiotics (ABs) in drinking water between weeks 11 and 14, for a total of 6 groups (n = 8/group). Metabolic tolerance tests were conducted between weeks 2 and 16. Relevant hormone gene expression and plasma hormone concentrations were assessed mainly at the end of 20 wk (Exp. 2).
Results
In Exp. 1, Cy3G administration increased ileal but not colonic Gcg level by 2-fold (P < 0.05). In Exp. 2, Cy3G attenuated HFD-induced body-weight gain (20.3% at week 16), and improved glucose tolerance (26.5% at week 15) but not insulin tolerance. Although Cy3G had no effect on glucose tolerance in LFD mice, LFD/Cy3G/AB mice showed better glucose tolerance than LFD/Cy3G mice (23%). In contrast, HFD/Cy3G/AB mice showed worse glucose tolerance compared with HFD/Cy3G mice (15%). Beneficial effects of Cy3G in HFD mice were not associated with changes in plasma leptin, insulin or GLP-1 concentrations. However, Cy3G increased hepatic Fgf21 expression in mice in Exp. 1 by 4-fold and attenuated Fgf21 overexpression in HFD mice (Exp. 2, 22%), associated with increased expression of genes that encode FGFR1 and β-klotho (>3-fold, P < 0.05).
Conclusions
Dietary Cy3G may reduce body weight and exert metabolic homeostatic effects in mice via changes in hepatic FGF21.
Keywords: anthocyanin, Cy3G, dietary intervention, FGF21, GLP-1, OGTT
Introduction
The prevalence of diabetes and other metabolic disorders has reached epidemic levels in developed as well as developing countries (1). In addition to the facilitation of new drug development with modern biotechnological approaches, efforts have been made globally in testing the preventative and treatment effects of dietary interventions, including the utilization of various dietary polyphenols and other phytochemicals (2). Anthocyanin, curcumin, and resveratrol are the 3 most-studied dietary polyphenols (3–6).
With the development of the “chronic inflammation and oxidative stress” theory of aging-related diseases (7, 8), intensive studies have focused on establishing a link between the beneficial metabolic effects of dietary polyphenols and their anti-inflammatory and antioxidative actions (9–13). Since different dietary polyphenols may possess both common and unique functions, it is also necessary to assess features of these phytochemicals that may extend beyond these pathways. One such demonstrated action common to anthocyanin and curcumin is their stimulation on expression of proglucagon (Gcg), a gene that encodes the incretin hormone glucagon-like peptide 1 (GLP-1) in the gut enteroendocrine L cell (14–16). We also reported that the insulin-sensitizing effect of curcumin in mice may not always be secondary to its anti-inflammatory and body-weight–lowering effects (17). We found that in a mouse model of dexamethasone-induced insulin resistance, daily curcumin administration for 6 d improved insulin sensitivity, without affecting body weight (17). Our further investigations revealed a novel hepatic target of curcumin, fibroblast growth factor 21 (FGF21) (17, 18), which is a paracrine and endocrine regulator of metabolic homeostasis (19). In vitro curcumin treatment of hepatocytes, or 4–8-d curcumin administration in mice fed a low-fat diet (LFD), increases hepatic FGF21 production, whereas long-term curcumin intervention (>12 wk) attenuates high-fat-diet (HFD)–induced hepatic FGF21 overexpression, in association with improved hepatic FGF21 sensitivity (18). Beneficial effects of curcumin on FGF21 production and sensitivity have also been observed in a female Wistar rat model of nonalcoholic steatohepatitis development (20). Similarly, hepatic FGF21 expression is stimulated by resveratrol treatment (6) and by dietary betaine supplementation (21).
Another common feature of dietary polyphenols is that they target multiple organs. In the current study, we first assessed the effect of a purified anthocyanin, cyanidin-3-glucoside (Cy3G), in regulating expression of the gut incretin hormone gene Gcg that encodes GLP-1 and hepatic Fgf21 in mice fed an LFD. We then investigated the metabolic beneficial effects of Cy3G in an HFD-fed mouse model on attenuating body-weight gain, improving glucose as well as insulin tolerance, and the relation between glucose tolerance and insulin tolerance, focusing on assessing the involvement of the gut hormone GLP-1, the adipose tissue–driven hormone leptin, the hepatic hormone FGF21, and insulin signaling sensitization.
Methods
Reagents
Cy3G was provided by Polyphenols AS (Sandnes, Norway) as we have reported (22). The cAMP-enhancing agents 3-isobutyl-1-methylxanthine (IBMX) and forskolin were purchased from Sigma Aldrich (Oakville, Canada). The 4 broad-spectrum antibiotics (ABs; detailed below) were also the products of Sigma Aldrich (22).
Animals, animal experimental design, and ethical approval
Five-week-old male C57BL/6J mice were purchased from Charles River (Montreal, Canada) and allowed to acclimatize for 1 wk before conducting the experiments.
Experiment 1
Mice (n = 5 in each of the 2 groups) were all fed an LFD [10% of energy from fat; Harlan Teklad LM-485 Mouse, code 7912; diet information as previously described (17)]. Feedings were administered with water (control) or Cy3G with the drinking water bottle (1.6 mg/mL, ∼6.4 mg/d) for 3 wk. At week 3, overnight (18 h) feed-deprived blood and re-feeding blood (2 h after food was provided) were taken for plasma insulin and GLP-1 measurements. Mice were then killed for intestinal and liver tissue sample collection to measure expression of the gut incretin hormone genes Gcg and gastric inhibitory polypeptide (Gip) and hepatic Fgf21.
Experiment 2
Forty-eight mice were randomly divided into 6 groups (n = 8/group), designated as LFD, LFD/Cy3G, LFD/Cy3G/AB, HFD (60% of energy from fat; BioServ F3282) (23), HFD/Cy3G, and HFD/Cy3G/AB. Cy3G administration (1.6 mg/mL in the drinking water) was started together with LFD or HFD feeding in week 1. A mixture of 4 ABs was provided in the same drinking water bottle between week 11 and week 14, as we have reported previously (22). Between weeks 2 and 16, 7 metabolic tolerance tests were conducted for determining the relation between glucose tolerance and insulin tolerance. All mice were allowed to rest for 3 wk (to minimize the effect of AB utilization) before collecting blood samples (150 μL, with 24-h fasting) for plasma FGF21 concentration measurement. All mice were killed at the end of week 20 without feed deprivation for blood and tissue collection. The blood samples were utilized for measuring random plasma FGF21, GLP-1, and leptin concentrations, as well as for plasma triglyceride (TG) concentrations. Gut tissues were utilized for assessing Gcg and Gip expression, the hepatic tissue for Fgf21 expression and hepatic TG content measurement, and epididymal fat tissue for leptin gene (Lep) expression.
Freshly prepared Cy3G-water or the Cy3G/AB-water were provided twice per week in the 2 sets of experiments to ensure that utilized reagents were relatively fresh. All mice were maintained at room temperature with a relative humidity of 50%, with free access to food and water under a 12-h light/dark cycle. The protocols for animal use and euthanasia were approved by the University Health Network Animal Care Committee (1560.26) and were performed in accordance with the guidelines of the Canadian Council of Animal Care.
Metabolic tolerance tests in experiment 2
Four intraperitoneal insulin tolerance tests (IPITTs) and 3 oral-glucose-tolerance tests (OGTTs) were conducted. For IPITTs, mice were feed deprived for 6 h prior to the injection of insulin (1.0 or 0.5 U/kg body weight). For OGTTs, mice were feed deprived for 16 h prior to oral gavage of glucose (2 g/kg body weight) (24).
RNA extraction and qRT-PCR
Total RNAs were isolated from mouse tissues (liver, gut segments, or epididymal fat tissue) or cultured GLUTag cell line (detailed below) using the TRIzol reagent (Sigma Aldrich). qRT-PCR was performed as previously described (17). Nucleotide sequence of the primers utilized in this study and sizes of the PCR products are listed in Supplemental Table 1.
Plasma hormone assessment
Mouse plasma FGF21 concentrations were determined using the FGF21 immunoassay kit (catalog no. 32180; Antibody and Immunoassay Services, The University of Hong Kong) (18). Plasma leptin concentrations were assessed utilizing the Duoset mouse leptin kit (catalog no. DY498; R&D Systems). Plasma insulin and plasma total GLP-1 concentrations were determined using the Meso Scale Discovery kit (Meso Scale Diagnostics, Rockville, MD), as previously described (24).
Plasma and hepatic TG measurement
To measure mouse hepatic TG contents in experiment (Exp.) 2, 10 mg liver tissue was lysed and saponified in ethanoic potassium hydroxide at 55°C overnight, followed by neutralization with MgCl2. Glycerol from liver TG supernatant or mouse serum (25 μL) was then measured using the Free Glycerol Reagent (Sigma Aldrich), as we previously described (25).
Cell lines and luciferase reporter analysis
The mouse endocrine L-cell line GLUTag and the hamster pancreatic α-cell line InR1G9, described previously (16, 26), were utilized to determine whether Cy3G can directly regulate Gcg expression and Gcg promoter activity. Cells were cultured in 24-well tissue culture plates in DMEM with 4500 mg/L glucose and 10% FBS. For luciferase (LUC) reporter assay, cells were maintained in the above medium for 24 h, followed by transfection with 1 μg Gcg-LUC reporter plasmid (1.1 kb Glu-LUC) (27) using Lipofectamine 2000. Twenty-four hours after the plasmid transfection, the cells were treated with the indicated concentration of Cy3G for 4 h, in the absence of serum. The cells were then collected for LUC reporter assay as we have previously described (28).
Statistical analyses
Results are expressed as means ± SEMs. For Exp. 1, comparisons between 2 sets of samples were analyzed by Student's t test. For multiple-group comparisons, Bonferroni post hoc tests were conducted following 1-factor or 2-factor ANOVA (Refed × Cy3G or Fat × Cy3G) using GraphPad Prism 5 (GraphPad Software, Inc.) to include the main effects tested. Data with unequal variances were log transformed before statistical analysis. AUC data were utilized for statistical comparison to assess body-weight gain and metabolic tolerance tests. Statistical significance was determined at P < 0.05.
Results
Three-week dietary Cy3G consumption in LFD-fed mice increases expression of intestinal incretin hormone encoding genes
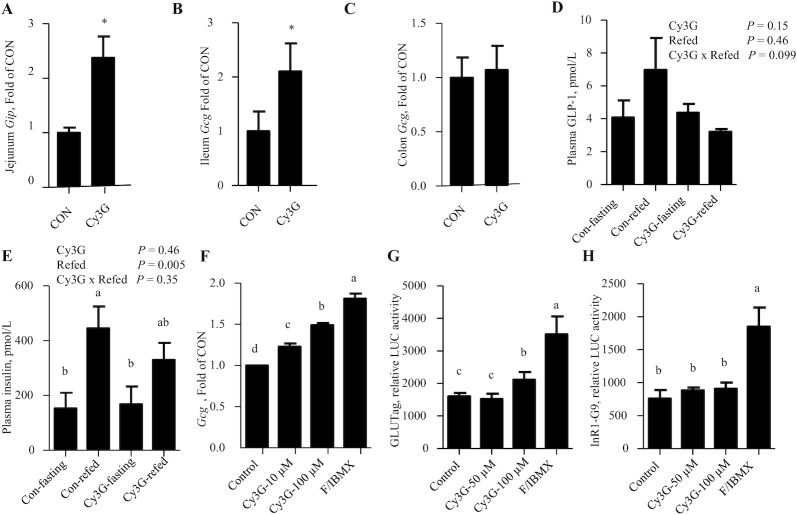
In Exp. 1, dietary administration of Cy3G for 3 wk resulted in a >2-fold elevation in expression of the gut incretin hormone genes Gip (which encodes GIP) in mouse jejunum and Gcg (which encodes GLP-1) in mouse ileum (Figure 1A, B). Unexpectedly, there was no stimulation of Gcg levels in mouse colon (Figure 1C). Furthermore, Cy3G consumption did not alter plasma concentrations of GLP-1 or insulin, regardless of whether blood samples were collected after overnight feed deprivation or 2 h after food was provided (Figure 1D, E).
FIGURE 1.
Three-week Cy3G consumption in LFD-fed mice increases mouse intestinal incretin gene expression. (A–C) Gut Gip or Gcg levels in the indicated gut regions after 3 wk of Cy3G administration (Exp. 1). (D, E) Overnight feed deprivation and 2-h refed plasma GLP-1 and insulin concentrations after 3 wk of Cy3G administration (Exp. 1). (F) Gcg level in GLUTag cells after 4 h of Cy3G treatment. (G, H) Gcg-LUC activities in the GLUTag cell line (G) and the pancreatic α-cell line InR1-G9 (H) 8 h after Cy3G treatment. Student's t test was applied in panels A–C. For panels D and E, 2-factor ANOVA was applied. For panels F–H, 1-factor ANOVA was applied. n = 5 for panels A–C, n = 4 for panels D and E, n = 3 for panels F–H. For panels A and B: *Different from CON, P < 0.05. For panels E–H: labeled means without a common letter differ, P < 0.05. CON, control; Cy3G, cyanidin-3-glucoside; Exp., experiment; F/IBMX, forskolin/3-isobutyl-1-methylxanthine (10 μM for forskolin and 10 μM for IBMX); Gcg, proglucagon; Gip, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide 1; LFD, low-fat diet; LUC, luciferase.
We then assessed whether Cy3G directly regulates Gcg expression in a Gcg-expressing gut cell line GLUTag. It was shown that 100 μM Cy3G treatment in GLUTag cells for 4 h increased expression of Gcg to 1.4-fold (Figure 1F). Gcg-LUC reporter activity was also stimulated by 100 μM Cy3G when the fusion gene plasmid was transfected into the GLUTag cell line but not in the InR1-G9 pancreatic α-cell line (Figure 1G, H). The cAMP-promoting agents, forskolin and IBMX (positive control), however, demonstrated stimulatory effects on Gcg-LUC reporter activity in both GLUTag and InR1-G9 cell lines (Figure 1G, H).
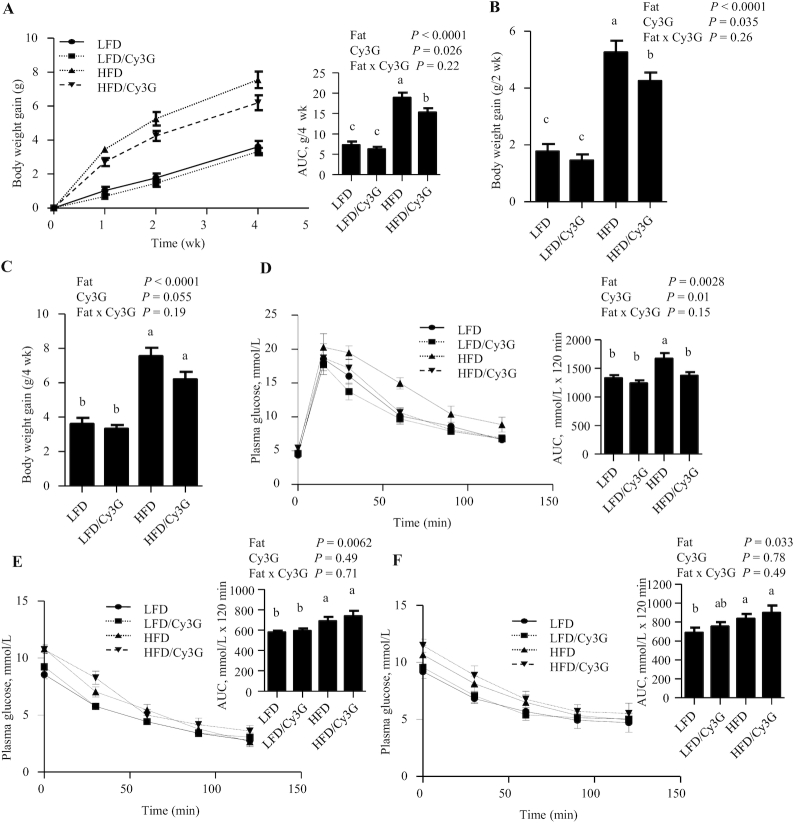
Body-weight–lowering and glucose-disposal–improvement effects of Cy3G intervention in HFD-fed mice are observed as early as 2–4 wk
In Exp. 2, with the experimental design illustrated in Figure 2, Cy3G intervention did not alter body-weight gain in mice fed an LFD during the first 4 wk (Figure 3A). However, in mice fed the HFD, Cy3G reduced body-weight gain during the first 4 wk by ∼22% (Figure 3A). Further analyses show that, in HFD mice, at the time point of wk 2, Cy3G reduced body-weight gain by 19% (Figure 3B), whereas at wk 4, Cy3G showed a trend in reducing body-weight gain (P = 0.052; Figure 3C). HFD feeding impaired glucose disposal, as reflected by increased glycemic excursions in both OGTTs and IPITTs (by 26% and 19%, respectively; Figure 3D–F). Cy3G intervention attenuated HFD-induced impairment in the OGTT (by 18%; Figure 3D) but did not affect the IPITT results, regardless of whether the IPITT was conducted with 1.0 or 0.5 U insulin/kg body weight (Figure 3E, F).
FIGURE 2.
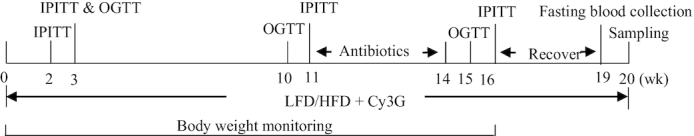
Illustration of the experimental design for Exp. 2. LFD, HFD, or Cy3G were provided for the treatment groups within the entire 20-wk experimental period. IPGTTs and OGTTs were conducted at the indicated time point. Antibiotics were administrated for the treatment groups between week 11 and week 14. Feed-deprived blood samples were collected at week 19 for plasma feed-deprived FGF21 concentration measurement, and all mice were killed at the end of week 20 for blood and tissue sample collection. Body weights were monitored for the first 16 wk. Cy3G, cyanidin-3-glucoside; Exp., experiment; FGF21, fibroblast growth factor 21; HFD, high-fat diet; IPITT, intraperitoneal insulin tolerance test; LFD, low-fat diet; OGTT, oral-glucose-tolerance test.
FIGURE 3.
Body-weight–lowering and glucose-tolerance–improvement effects are observed within 4 wk in HFD-fed mice with the Cy3G intervention. (A) Body-weight gain monitored within the first 4 wk after Cy3G dietary intervention, with the AUC presented at the right. (B, C) Body-weight gain after 2 or 4 wk of Cy3G administration. (D–F) OGTT results at week 3 (D) and IPITT results at week 2 (E) and week 3 (F) of Cy3G intervention, with AUCs on the right. For the IPITT experiment, 1.0 U (E) or 0.5 U (F) of insulin per kilogram mouse body weight was utilized. n ≥ 8 for panels A–C, n ≥ 4 for panels D–F. For panels A–F, 2-factor ANOVA was applied. Labeled means without a common letter differ, P < 0.05. Cy3G, cyanidin-3-glucoside; HFD, high-fat diet; IPITT, intraperitoneal insulin tolerance test; LFD, low-fat diet; OGTT, oral-glucose-tolerance test.
Body weight and body-weight gain were lower in the HFD/Cy3G group compared with the HFD group throughout the 20-wk experimental period. At the end of week 16, Cy3G administration attenuated HFD-induced body-weight gain by ∼20.3% (comparing HFD vs HFD/Cy3G in Figure 4B). The body-weight–lowering effect was associated with improved OGTT assessed at week 10 (by 15%; Figure 4C), but again, not with improved IPITT assessed at week 11 (Figure 4D).
FIGURE 4.
Body-weight–lowering and glucose-disposal–improving effects of the Cy3G intervention in mice are sustained beyond 10 wk (Exp. 2). (A) Body-weight changes during the 16-wk experimental period. (B) Body-weight gain changes during the 16-wk experimental period. (C) OGTT at week 10 and (D) IPITT at week 11. Panels on the right show the respective AUCs. Two-factor ANOVA was applied for all panels. n ≥ 8 for panels A and B. For panels C and D, n ≥ 4. For all panels, labeled means without a common letter differ, P < 0.05. Cy3G, cyanidin-3-glucoside; Exp., experiment; HFD, high-fat diet; IPITT, intraperitoneal insulin tolerance test; LFD, low-fat diet; LUC, luciferase; OGTT, oral-glucose-tolerance test.
Utilization of broad-spectrum ABs along with Cy3G further improves glucose tolerance in LFD-fed mice but not in HFD-fed mice
As shown in Figure 5A, B, LFD/Cy3G/AB mice exhibited improved OGTT (by 23% at week 15) but not IPITT (at week 16) when compared with LFD/Cy3G mice. In contrast, HFD/Cy3G/AB mice exhibited worse OGTT when compared with HFD/Cy3G mice (by 15% at week 15; Figure 5A).
FIGURE 5.
Use of the broad-spectrum antibiotics further improves glucose disposal in LFD-fed mice with the Cy3G intervention but not in HFD-fed mice (Exp. 2). (A) OGTT results at week 15. (B) IPITT results at week 16. (C, D) Hepatic TG content (C) and plasma TG concentration (D) at week 20. For panels A and B, panels on the right show the respective AUCs. Two-factor ANOVA was applied for all panels. For panels C and D, n ≥ 6. For all panels, labeled means without a common letter differ, P < 0.05. AB, 4 broad-spectrum antibiotics; Cy3G, cyanidin-3-glucoside; Exp., experiment; HFD, high-fat diet; IPITT, intraperitoneal insulin tolerance test; LFD, low-fat diet; OGTT, oral-glucose-tolerance test; TG, triglyceride.
Elevated hepatic and plasma TG concentrations (in mice killed at week 20) in response to HFD feeding were also reduced with Cy3G intervention (by 27% and 37%, respectively; Figure 5C, D). In HFD-fed mice, the attenuating effect of Cy3G on hepatic TG concentrations was reversed by the utilization of ABs (by 61% when comparing HFD/Cy3G to HFD/Cy3G/AB; Figure 5C).
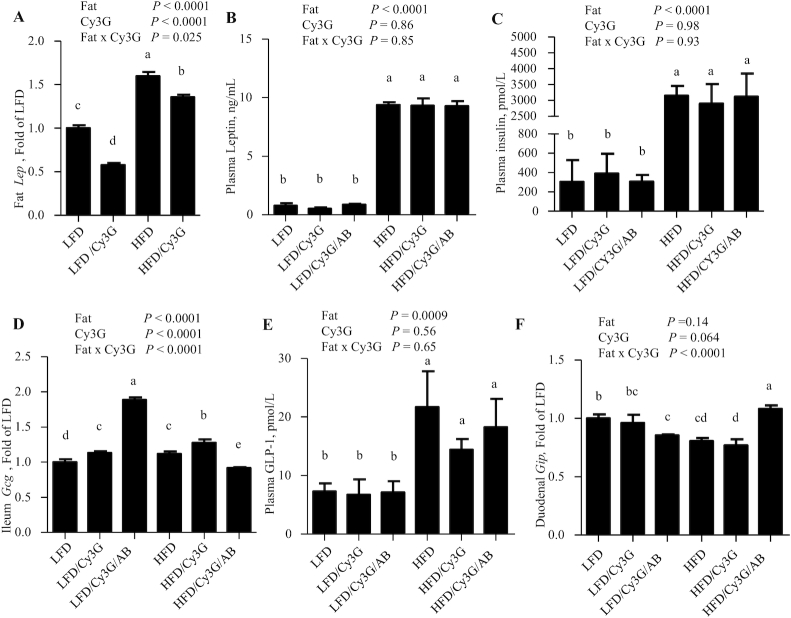
Cy3G intervention affects mouse white adipose tissue Lep and gut Gcg expression but not plasma concentrations of insulin, leptin, or GLP-1
HFD feeding increased mouse epididymal fat-pad Lep levels by ∼60%, while Cy3G intervention led to reduced Lep levels by 42% or 12% with LFD- or HFD-fed mice, respectively (Figure 6A). HFD feeding increased plasma leptin concentrations >8-fold, but this increase was not attenuated by Cy3G intervention, with or without the use of ABs (Figure 6B). The lack of attenuation of hyperleptinemia in HFD-fed mice with Cy3G administration was also associated with a lack of amelioration of the hyperinsulinemia (Figure 6C).
FIGURE 6.
Twenty-week Cy3G intervention in mice affects white adipose tissue Lep and gut Gcg expression but not the plasma concentrations of leptin or GLP-1. (A, B) Epididymal fat tissue Lep levels (A) and random plasma leptin concentrations (B) in mice at week 20. (C) Plasma insulin concentrations in mice at week 20. (D, E) Gut ileal Gcg levels (D) and random plasma total GLP-1 concentrations (E) in mice at week 20. (F) Duodenal Gip levels in mice at week 20. Two-factor ANOVA was applied for all panels. n ≥ 6 for all panels. For all panels, labeled means without a common letter differ, P < 0.05. AB, 4 broad-spectrum antibiotics; Cy3G, cyanidin-3-glucoside; Gcg, proglucagon; Gip, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide-1; HFD, high-fat diet; Lep, leptin; LFD, low-fat diet.
With LFD feeding, mouse ileal Gcg levels were increased by ∼1.2-fold by the Cy3G intervention (Figure 6D). HFD feeding itself moderately increased ileal Gcg levels, which were also further increased by the Cy3G intervention. However, the utilization of ABs in the HFD mice reduced gut Gcg expression by −28% (HFD/Cy3G vs HFD/Cy3G/AB; Figure 6D). HFD feeding also increased random plasma GLP-1 concentrations by >2-fold, although no further statistical changes were observed with Cy3G intervention or following the administration of ABs (Figure 6E). In contrast, effects on duodenal Gip mRNA levels were moderate for each of the treatments and diets, and the patterns were generally opposed to those observed for ileal Gcg expression (Figure 6F).
Cy3G administration affects plasma FGF21 hormone concentration, hepatic Fgf21 expression, and FGF21 receptor/co-receptor gene expression
Finally, possible contributions of hepatic FGF21 were assessed. In mice fed the LFD with Cy3G administration for 3 wk (Exp. 1), the hepatic Fgf21 level increased by >4-fold (Figure 7A), while plasma FGF21 hormone levels were not changed, regardless of whether the assessment was conducted after 24 h of feed deprivation or after 2 h of refeeding (Figure 7B). Furthermore, in Exp. 2, long-term Cy3G intervention increased hepatic Fgf21 levels by ∼74% in mice fed the LFD, and attenuated HFD-induced Fgf21 overexpression by 22% (Figure 7C). Importantly, 20 wk of the Cy3G intervention (in Exp. 2) also attenuated HFD-induced increases in circulating FGF21 concentrations by ∼37% (Figure 7D). In HFD-fed mice with the Cy3G intervention, feed-deprived circulating FGF21 concentrations were markedly increased by ∼70% (Figure 7E).
FIGURE 7.
Cy3G intervention affects mouse circulating FGF21 hormone concentration, hepatic Fgf21 expression, and Fgf21 receptor/co-receptor gene expression. (A, B) Hepatic Fgf21 (A) and plasma FGF21 concentration with overnight feed deprivation and 2 h refeeding (B) in mice (Exp. 1). (C) Fgf21 level in mouse liver at week 20 of Exp. 2. (D, E) Random (D) and feed-deprived (E) plasma FGF21 concentrations in mice (Exp. 2) at week 19 or week 20. (F, G) Fgfr1 (F) or Klb (G) levels in mouse liver at week 20 of Exp. 2. (H, I) Fgfr1 (H) or Klb(I) levels in white adipose tissue at week 20 of Exp. 2. Student's t test was applied for panel A, and 2-factor ANOVA was applied for all other panels. n = 5 for panels A and B, n ≥ 6 for panels C–E, and n ≥ 4 for panels F–I. For panel A: *Different from CON, P < 0.05. For panels B–G: labeled means without a common letter differ, P < 0.05. CON, control; Cy3G, cyanidin-3-glucoside; Exp., experiment; FGF21, fibroblast growth factor 21; Fgfr1, Fibroblast growth factor receptor 1. HFD, high-fat diet; Klb, beta-klotho; LFD, low-fat diet.
Elevated hepatic Fgf21 expression with long-term Cy3G intervention in LFD-fed mice was associated with moderate repression on the expression of fibroblast growth factor receptor 1 (Fgfr1) and beta-Klotho (Klb) (Figure 7F, G). However, HFD feeding profoundly reduced Fgfr1 and Klb levels by 85% and 75%, respectively (Figure 7F, G). In HFD-fed mice with the Cy3G intervention, Fgfr1 and Klb levels were increased to 284% and 216%, respectively (Figure 7F, G). In mouse white adipose tissue, HFD feeding also reduced Fgfr1 and Klb levels (Figure 7H, I). The reduction in Klb, but not in that of Fgfr1, was partially reversed with long-term Cy3G administration.
Discussion
Investigations have been conducted for decades to assess beneficial metabolic effects of dietary polyphenols, including various anthocyanins (4–6, 29–32). Previous studies have mainly focused on the anti-inflammatory and antioxidative stress features of these polyphenols (17, 33). In addition, many investigations on anthocyanins have been conducted with genetically modified mouse models or in HFD-fed rodent models with crude extracts from berries or black rice (9, 30, 34, 35). Herein, we aimed to determine the obesity preventative and glucose disposal improvement effects of a purified polyphenol compound, Cy3G, in HFD-fed wild-type mice, along with a mechanistic exploration, targeting hormones produced by the gut, pancreas, liver, and adipose tissue. The daily dose of Cy3G in the current study was ∼6.4 mg/d and was selected to be nutritionally relevant, similar to other studies with various berry extracts (30, 34), but much lower than that in certain other investigations (36, 37).
Gcg encodes the incretin hormone GLP-1, produced in distal ileum and colon (16, 38, 39). Kato et al. (14) found that the anthocyanin delphinidin 3-rutinoside (D3R) stimulates Gcg expression in the GLUTag cell line. We confirmed here the in vitro stimulation of Cy3G on Gcg expression in this cell line and expanded the findings to mice fed an LFD for 3 wk. Curiously, the stimulation of Cy3G on Gcg was observed in the ileum but not in the colon. This, along with the fact that the stimulation of Gcg expression and Gcg promoter activity was observed in vitro only when the dosage of Cy3G reached the supraphysiological concentration of 100 μM, prompted us to speculate whether the in vivo regulation is mediated by microbial metabolites of Cy3G or by other means involving gut microbiota. Indeed, using the same approach with ABs, we demonstrated previously that gut microbiota metabolites of anthocyanin can promote reverse cholesterol transport in mice (22). Why the effects of Cy3G on gut Gcg were observed only in the ileum remains unclear, but the transcriptome of L cells in the ileum differs from that of L cells in the colon, as reported recently (40). Thus, it is possible that certain metabolites of Cy3G demonstrate activity in the small intestine only, which deserves further investigation.
Despite the physiological importance of native GLP-1 and the pharmacological applications of GLP-1–based drugs in diabetes treatment (41), plasma GLP-1 concentrations have not been developed as a clinical diagnosis or prognosis parameter to date. Studies have suggested decreased postprandial GLP-1 concentrations in patients with diabetes (42–44). In rodents with HFD-induced insulin resistance and obese models, different outcomes with respect to both basal and glucose-stimulated GLP-1 secretion have been reported (45, 46), possibly due to different experimental settings and animal species utilized. Anini and Brubaker (45) found that mice with 8-wk HFD feeding showed reduced basal plasma GLP-1 concentrations and a diminished GLP-1 response to oral-glucose challenge, likely due to the development of leptin resistance after HFD challenge. Wang et al. (46) reported that, in Sprague-Dawley rats, an HFD challenge with pair feeding increased secretion of GLP-1 into the lymphatics following a duodenal Ensure (Abbott Laboratories, North Chicago, IL) challenge. With our current experimental paradigm, plasma GLP-1 concentrations were increased in the 3 groups of mice with HFD challenge, regardless of Cy3G intervention or the use of ABs (Figure 6E). Nevertheless, basal plasma GLP-1 concentrations were tightly associated with an ∼8-fold elevation in plasma leptin concentrations, which, along with the increased body weight and hyperinsulinemia, suggest the development of leptin resistance (45). Interestingly, the use of ABs along with Cy3G did reduce the glycemic response to an OGTT in LFD-fed mice but attenuated the effect of Cy3G intervention on glucose intolerance in HFD-fed mice. As these changes were unrelated to changes in plasma GLP-1 concentrations, possible roles for other metabolic hormones were therefore considered.
Anthocyanin from various sources inhibits 3T3-L1 cell differentiation towards adipocytes and reduces plasma leptin concentrations in HFD-fed mice (47–49). Cy3G or anthocyanin-rich fruit extracts were also shown to repress 3T3-L1 cell differentiation toward adipocytes (50). A previous study further demonstrated that, in HFD (45% kcal from fat)-fed male C57BL/6J mice, purified blueberry anthocyanins (0.2 mg/mL) decreased serum leptin concentrations (51). In the current study, we did not observe any attenuating effect of Cy3G on HFD-induced plasma leptin elevation. This could be because the HFD utilized in this study was composed of 60% total kcal from fat (23). Alternatively, the dosage of this specific anthocyanin, Cy3G, may not be high enough to achieve such an effect. Thus, although we observed the repressive effect of in vivo Cy3G administration on epididymal fat pad Lep expression in both LFD-fed and HFD-fed mice (Figure 6A), HFD-induced leptin resistance was not corrected with Cy3G intervention, along with a lack of appreciable improvement in insulin sensitivity. In contrast, curcumin intervention in HFD-fed mice did cause an improvement in the glycemic response to an IPITT (18). Whether these differences are due to the relatively low but physiological dose of Cy3G utilized in the present study or are a feature of this specific synthetic anthocyanin needs to be further studied.
Four IPITTs and 3 OGTTs were conducted from week 2 to week 16 in the current study to determine whether the improved glucose disposal and body-weight–lowering effect of Cy3G in response to HFD challenge can be independent of insulin tolerance improvement. In HFD-fed mice, despite the lack of correction of insulin resistance, Cy3G treatment attenuated body-weight gain and improved glucose disposal. These occurred as early as 3 wk after Cy3G administration and were sustained to week 16 when the last IPITT was conducted (Figure 5B), in association with reduced hepatic and plasma TGs measured at week 20 (Figure 5C, D). It needs to be pointed out that, following the last IPITT at week 16, we let the mice rest for 4 wk to minimize the effect of AB administration. Thus, IPITTs may not be among the initial or prime mechanisms improving metabolic homeostasis with Cy3G intervention, at least in our current experimental settings.
Circulating FGF21 is produced from the liver, although Fgf21 can be detected in adipose tissues and pancreas (52–54). We hence did not assess hepatic FGF21 protein concentrations by Western blotting in the current study. Instead, random and feed-deprived plasma FGF21 concentrations were measured in each group of mice either at week 19 (24-h feed-deprivation concentration) or week 20 (random concentration). Nevertheless, HFD-induced hepatic FGF21 overexpression has been demonstrated by our previous study on another dietary polyphenol, curcumin, with the similar protocol and the same HFD (18). In LFD-fed mice, curcumin treatment increases Fgf21 transcription and FGF21 hormone production. However, in HFD-fed mice, curcumin intervention attenuates HFD-induced hepatic Fgf21 overexpression, associated with increased FGF21 sensitivity (18). Hepatic FGF21 expression was also found to be stimulated by resveratrol and dietary betaine supplementation (6, 21). We show here that this regulatory pathway is shared by Cy3G. Thus, in HFD-fed mice, Cy3G intervention reduced random FGF21 concentrations, in association with reduced hepatic Fgf21 levels and increased receptor/co-receptor gene expression. These findings are in good agreement with the improvement in FGF21 sensitivity that we observed with curcumin intervention (18). However, curiously, in HFD-fed mice, Cy3G intervention increased feed-deprived circulating FGF21 concentrations, indicating a potential role for Cy3G in stimulating FGF21 secretion, which deserves to be explored further.
As illustrated in Supplemental Figure 1, Cy3G exerts beneficial metabolic effects in HFD-fed mice, at least in part via regulating FGF21 production and sensitivity. Although the beneficial effects occurred in the absence of detectable changes in circulating GLP-1 concentrations, dramatic effects on the expression of the incretin hormone genes were observed. A role for the involvement of the microbiota in the ileum, but not in the colon, is suggested by these findings, in agreement with a recent report that the transcriptome of L cells in the ileum differs from that of L cells in the colon (40). It also remains to be determined whether interactions between Cy3G or other dietary polyphenols and gut microbiota influence GLP-1 signaling via yet-to-be-explored mechanisms, such as hormone sensitivity, which would mask the effect on circulating GLP-1 concentrations. Furthermore, we did not see a systematic effect of Cy3G on improving leptin resistance, in association with a lack of improvement in insulin sensitivity. If this is due to the nature of the purified anthocyanin Cy3G, further chemistry studies are needed to identify the more active molecule(s) in anthocyanin-rich fruits, vegetables, and food. Finally, as the metabolic beneficial effects with Cy3G were observed in the absence of improvements in insulin sensitivity, our observations indicate the existence of insulin-independent metabolic actions of FGF21.
Supplementary Material
Acknowledgments
The authors thank Alexandre Martchenko for assistance with the GLP-1 assay. The authors’ responsibilities were as follows—TJ, PLB, WL, LT, and WS: were responsible for the conception and design of the research; LT, HN, WS, YB and ZS: conducted the experiments; WL: provided reagents and the experimental protocol for the use of antibiotics; BBY: provided advice on the luciferase assay and was the co-recipient for the supporting grant from BBDC; PLB: provided the experimental protocol for GLP-1 measurement and related experiments; LT: drafted the manuscript; HL: drafted part of the Methods and Results sections; TJ, WS, and PLB: read and approved the final manuscript..
Notes
Supported by the Canadian Institutes of Health Research (CIHR; PJT159735 to TJ) and Banting and Best Diabetes Center (BBDC; pilot grant to TJ and BBY). LT was supported by a BBDC fellowship from 2015 to 2017; ZS was supported by a BBDC graduate studentship; YB was supported by CIHR graduate studentship and BBDC–Novo Nordisk Studentship; PLB was supported by a Canada Research Chair. Some of the equipment used in this study was supported by the 3D (Diet, Digestive Tract, and Disease) Centre funded by the Canadian Foundation for Innovation and Ontario Research Fund, project numbers 19442 and 30961.
Author disclosures: The authors report no conflicts of interest. The funding agencies (CIHR and BBDC) played no role in the experimental design or manuscript publication.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AB, antibiotic (4 antibiotics utilized in this study); Cy3G, cyanidin-3-glucoside; Exp., experiment; FGF21, fibroblast growth factor 21; Fgfr1, fibroblast growth factor receptor 1; Gcg, proglucagon; Gip, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide 1; HFD, high-fat diet; IBMX, 3-isobutyl-1-methylxanthine; IPITT, intraperitoneal insulin tolerance test; Lep, Klb, beta-Klotho; leptin; LFD, low-fat diet; LUC, luciferase; OGTT, oral-glucose-tolerance test; TG, triglyceride.
References
- 1. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang L, Ling W, Du Z, Chen Y, Li D, Deng S, Liu Z, Yang L. Effects of anthocyanins on cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2017;8:684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39:2–13. [DOI] [PubMed] [Google Scholar]
- 4. Ray Hamidie RD, Yamada T, Ishizawa R, Saito Y, Masuda K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism. 2015;64:1334–47. [DOI] [PubMed] [Google Scholar]
- 5. Ferguson JJA, Stojanovski E, MacDonald-Wicks L, Garg ML. Curcumin potentiates cholesterol-lowering effects of phytosterols in hypercholesterolaemic individuals: a randomised controlled trial. Metabolism. 2018;82:22–35. [DOI] [PubMed] [Google Scholar]
- 6. Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, Kharitonenkov A, Yang Q, Gao B, Guarente L et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146:539–49. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 8. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. [DOI] [PubMed] [Google Scholar]
- 9. Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, Lu H, Fantus IG, Jin T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One. 2012;7:e28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yekollu SK, Thomas R, O'Sullivan B. Targeting curcusomes to inflammatory dendritic cells inhibits NF-kappaB and improves insulin resistance in obese mice. Diabetes. 2011;60:2928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan Y, Wang Y, Zhao Y, Peng K, Li W, Wang Y, Zhang J, Zhou S, Liu Q, Cai L et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes. 2014;63:3497–511. [DOI] [PubMed] [Google Scholar]
- 13. Li D, Zhang Y, Liu Y, Sun R, Xia M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J Nutr. 2015;145:742–8. [DOI] [PubMed] [Google Scholar]
- 14. Kato M, Tani T, Terahara N, Tsuda T. The anthocyanin delphinidin 3-rutinoside stimulates glucagon-like peptide-1 secretion in murine GLUTag cell line via the Ca2+/calmodulin-dependent kinase ii pathway. PLoS One. 2015;10:e0126157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takikawa M, Kurimoto Y, Tsuda T. Curcumin stimulates glucagon-like peptide-1 secretion in GLUTag cells via Ca2+/calmodulin-dependent kinase II activation. Biochem Biophys Res Commun. 2013;435:165–70. [DOI] [PubMed] [Google Scholar]
- 16. Drucker DJ, Jin T, Asa SL, Young TA, Brubaker PL. Activation of proglucagon gene transcription by protein kinase-A in a novel mouse enteroendocrine cell line. Mol Endocrinol. 1994;8:1646–55. [DOI] [PubMed] [Google Scholar]
- 17. Tian L, Zeng K, Shao W, Yang BB, Fantus IG, Weng J, Jin T. Short-term curcumin gavage sensitizes insulin signaling in dexamethasone-treated C57BL/6 mice. J Nutr. 2015;145:2300–7. [DOI] [PubMed] [Google Scholar]
- 18. Zeng K, Tian L, Patel R, Shao W, Song Z, Liu L, Manuel J, Ma X, McGilvray I, Cummins CL et al. Diet polyphenol curcumin stimulates hepatic Fgf21 production and restores its sensitivity in high-fat-diet-fed male mice. Endocrinology. 2017;158:277–92. [DOI] [PubMed] [Google Scholar]
- 19. Lewis JE, Ebling FJP, Samms RJ, Tsintzas K. Going back to the biology of FGF21: new insights. Trends Endocrinol Metab. 2019;30:491–504. [DOI] [PubMed] [Google Scholar]
- 20. Cunningham RP, Moore MP, Moore AN, Healy JC, Roberts MD, Rector RS, Martin JS. Curcumin supplementation mitigates NASH development and progression in female Wistar rats. Physiol Rep. 2018;6:e13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ejaz A, Martinez-Guino L, Goldfine AB, Ribas-Aulinas F, De Nigris V, Ribo S, Gonzalez-Franquesa A, Garcia-Roves PM, Li E, Dreyfuss JM et al. Dietary betaine supplementation increases Fgf21 levels to improve glucose homeostasis and reduce hepatic lipid accumulation in mice. Diabetes. 2016;65:902–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111:967–81. [DOI] [PubMed] [Google Scholar]
- 23. Ip BC, Liu C, Smith DE, Ausman LM, Wang XD. High-refined-carbohydrate and high-fat diets induce comparable hepatic tumorigenesis in male mice. J Nutr. 2014;144:647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chiang YT, Ip W, Shao W, Song ZE, Chernoff J, Jin T. Activation of cAMP signaling attenuates impaired hepatic glucose disposal in aged male p21-activated protein kinase-1 knockout mice. Endocrinology. 2014;155:2122–32. [DOI] [PubMed] [Google Scholar]
- 25. Tian L, Shao W, Ip W, Song Z, Badakhshi Y, Jin T. The developmental Wnt signaling pathway effector beta-catenin/TCF mediates hepatic functions of the sex hormone estradiol in regulating lipid metabolism. PLoS Biol. 2019;17:e3000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jin T, Drucker DJ. Activation of proglucagon gene transcription through a novel promoter element by the caudal-related homeodomain protein cdx-2/3. Mol Cell Biol. 1996;16:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin T, Drucker DJ. The proglucagon gene upstream enhancer contains positive and negative domains important for tissue-specific proglucagon gene transcription. Mol Endocrinol. 1995;9:1306–20. [DOI] [PubMed] [Google Scholar]
- 28. Ip W, Shao W, Song Z, Chen Z, Wheeler MB, Jin T. Liver-specific expression of dominant-negative transcription factor 7-like 2 causes progressive impairment in glucose homeostasis. Diabetes. 2015;64:1923–32. [DOI] [PubMed] [Google Scholar]
- 29. Jin TR. Curcumin and dietary polyphenol research: beyond drug discovery. Acta Pharmacol Sin. 2018;39:779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anhe FF, Nachbar RT, Varin TV, Vilela V, Dudonne S, Pilon G, Fournier M, Lecours MA, Desjardins Y, Roy D et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab. 2017;6:1563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song Z, Revelo X, Shao W, Tian L, Zeng K, Lei H, Sun HS, Woo M, Winer D, Jin T. Dietary curcumin intervention targets mouse white adipose tissue inflammation and brown adipose tissue UCP1 expression. Obesity (Silver Spring). 2018;26:547–58. [DOI] [PubMed] [Google Scholar]
- 32. Zeng K, Tian L, Sirek A, Shao W, Liu L, Chiang YT, Chernoff J, Ng DS, Weng J, Jin T. Pak1 mediates the stimulatory effect of insulin and curcumin on hepatic ChREBP expression. J Mol Cell Biol. 2017;9:384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jin T, Song Z, Weng J, Fantus IG. Curcumin and other dietary polyphenols: potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am J Physiol Endocrinol Metab. 2018;314:E201–E5. [DOI] [PubMed] [Google Scholar]
- 34. Anhe FF, Varin TV, Le Barz M, Pilon G, Dudonne S, Trottier J, St-Pierre P, Harris CS, Lucas M, Lemire M et al. Arctic berry extracts target the gut-liver axis to alleviate metabolic endotoxaemia, insulin resistance and hepatic steatosis in diet-induced obese mice. Diabetologia. 2018;61:919–31. [DOI] [PubMed] [Google Scholar]
- 35. Xia X, Ling W, Ma J, Xia M, Hou M, Wang Q, Zhu H, Tang Z. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. J Nutr. 2006;136:2220–5. [DOI] [PubMed] [Google Scholar]
- 36. Iizuka Y, Ozeki A, Tani T, Tsuda T. Blackcurrant extract ameliorates hyperglycemia in type 2 diabetic mice in association with increased basal secretion of glucagon-like peptide-1 and activation of AMP-activated protein kinase. J Nutr Sci Vitaminol (Tokyo). 2018;64:258–64. [DOI] [PubMed] [Google Scholar]
- 37. Tani T, Nishikawa S, Kato M, Tsuda T. Delphinidin 3-rutinoside-rich blackcurrant extract ameliorates glucose tolerance by increasing the release of glucagon-like peptide-1 secretion. Food Sci Nutr. 2017;5:929–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jin T. Mechanisms underlying proglucagon gene expression. J Endocrinol. 2008;198:17–28. [DOI] [PubMed] [Google Scholar]
- 39. Yi F, Sun J, Lim GE, Fantus IG, Brubaker PL, Jin T. Cross talk between the insulin and Wnt signaling pathways: evidence from intestinal endocrine L cells. Endocrinology. 2008;149:2341–51. [DOI] [PubMed] [Google Scholar]
- 40. Arora T, Akrami R, Pais R, Bergqvist L, Johansson BR, Schwartz TW, Reimann F, Gribble FM, Backhed F. Microbial regulation of the L cell transcriptome. Sci Rep. 2018;8:1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jin T, Weng J. Hepatic functions of GLP-1 and its based drugs: current disputes and perspectives. Am J Physiol Endocrinol Metab. 2016;311:E620–7. [DOI] [PubMed] [Google Scholar]
- 42. Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vaag AA, Holst JJ, Volund A, Beck-Nielsen HB. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)—evidence for decreased glucagon-like peptide 1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol. 1996;135:425–32. [DOI] [PubMed] [Google Scholar]
- 44. Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–13. [DOI] [PubMed] [Google Scholar]
- 45. Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–9. [DOI] [PubMed] [Google Scholar]
- 46. Wang F, Yoder SM, Yang Q, Kohan AB, Kindel TL, Wang J, Tso P. Chronic high-fat feeding increases GIP and GLP-1 secretion without altering body weight. Am J Physiol Gastrointest Liver Physiol. 2015;309:G807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han MH, Kim HJ, Jeong JW, Park C, Kim BW, Choi YH. Inhibition of adipocyte differentiation by anthocyanins isolated from the fruit of Vitis coignetiae pulliat is associated with the activation of AMPK signaling pathway. Toxicol Res. 2018;34:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Graf D, Seifert S, Jaudszus A, Bub A, Watzl B. Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in Fischer rats. PLoS One. 2013;8:e66690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo H, Guo J, Jiang X, Li Z, Ling W. Cyanidin-3-O-beta-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem Toxicol. 2012;50:3040–7. [DOI] [PubMed] [Google Scholar]
- 50. Kim HK, Kim JN, Han SN, Nam JH, Na HN, Ha TJ. Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr Res. 2012;32:770–7. [DOI] [PubMed] [Google Scholar]
- 51. Prior RL, S EW, T RR, Khanal RC, Wu X, Howard LR. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010;58:3970–6. [DOI] [PubMed] [Google Scholar]
- 52. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–37. [DOI] [PubMed] [Google Scholar]
- 53. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–25. [DOI] [PubMed] [Google Scholar]
- 54. Kharitonenkov A, DiMarchi R. FGF21 revolutions: recent advances illuminating FGF21 biology and medicinal properties. Trends Endocrinol Metab. 2015;26:608–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.