Abstract
Rationale:
Obstructive sleep apnea (OSA) is common among patients with acute ischemic stroke (AIS) and is associated with reduced functional recovery and an increased risk for recurrent vascular events.
Aims and/or hypothesis:
The Sleep for Stroke Management and Recovery Trial (Sleep SMART) aims to determine whether automatically-adjusting continuous positive airway pressure (aCPAP) treatment for OSA improves clinical outcomes after AIS or high-risk transient ischemic attack (TIA).
Sample size estimate:
3,062 randomized subjects for the prevention of recurrent serious vascular events, and among these, 1,362 stroke survivors for the recovery outcome.
Methods and design:
Sleep SMART is a phase III, multicenter, prospective randomized, open, blinded outcome event assessed (PROBE) controlled trial. Adults with recent AIS/TIA and no contraindication to aCPAP are screened for OSA with a portable sleep apnea test. Subjects with confirmed OSA but without predominant central sleep apnea proceed to a run-in night of aCPAP. Subjects with use (≥4 hours) of aCPAP and without development of significant central apneas are randomized to aCPAP plus usual care or care-as-usual for 6 months. Telemedicine is used to monitor and facilitate aCPAP adherence remotely.
Study outcomes:
Two separate primary outcomes (1) the composite of recurrent AIS, acute coronary syndrome, and all-cause mortality (prevention), and (2) the modified Rankin scale scores (recovery) at 6- and 3-months post-randomization, respectively.
Discussion:
Sleep SMART represents the first large trial to test whether aCPAP for OSA after stroke/TIA reduces recurrent vascular events or death, and improves functional recovery.
Keywords: stroke, apnea, sleep, continuous positive airway pressure, clinical trial
Introduction and rationale
Many studies have demonstrated the high frequency of sleep apnea in patients with cardiovascular disease (CVD), in particular those who have experienced an acute ischemic stroke (AIS). Obstructive sleep apnea (OSA), the predominant type of post-stroke sleep apnea,(1) is associated with increased mortality, poor functional outcome, and high risk of recurrent vascular events following AIS.(2;3) Use of the standard OSA treatment, continuous positive airway pressure (CPAP), is associated with better stroke outcomes and lower vascular risk,(4;5) but it remains uncertain whether CPAP treatment reduces CVD risk after stroke or improves stroke outcomes.
Several pilot trials of CPAP conducted in the acute and subacute phases of acute AIS(6;7) support its safety but have highlighted difficulties with adherence and uncertainties over effects. The clinical outcome findings are mixed and insufficient to support the routine use of CPAP for stroke patients. As equipoise exists and randomized trials are considered ethical,(8) the American Heart Association (AHA) / American Stroke Association (ASA) secondary prevention guidelines explicitly support the need for a definitive trial to assess the effects of CPAP on stroke recovery and recurrent ischemic events.(9)
The Sleep for Stroke Management and Recovery Trial (Sleep SMART) seeks to determine whether, in patients with OSA, CPAP initiated early after AIS or high-risk transient ischemic attack (TIA) reduces the risks of recurrent AIS, acute coronary syndrome (ACS), and all-cause mortality, and improves functional recovery from AIS.
Methods
Study design
Sleep SMART (NCT03812653) is an investigator-initiated, phase III, multicenter, prospective randomized, open, blinded outcome event assessed (PROBE) controlled trial. Enrollment over 3.5 years is planned within at least 110 of the sites that participate in StrokeNet, an NIH-funded stroke clinical trials network. Sleep SMART is a superiority trial that compares 6 months of OSA treatment with CPAP to usual care. The study maximizes efficiency through inclusion of two trials: a prevention study and an embedded recovery trial. These trials address separate questions as the underlying benefits of CPAP for each outcome may work through different mechanisms. Patients with recent (≤14 days) AIS or high-risk TIA (ABCD2 scores ≥4) are enrolled during their acute or rehabilitation hospitalization and treated for 6 months (Figure). After consent, a portable cardiopulmonary sleep apnea test is used to assess for OSA. Automatically-adjusting CPAP (aCPAP) is then used for one night to determine tolerability (the “run-in” night). Subjects who are able to use the device for ≥4 hours on that run-in night, do not have excessive treatment-emergent central sleep apnea, and are willing to move forward are randomized to receive either 6 months of aCPAP plus usual care, or usual care alone. aCPAP training is provided to subjects in the intervention group, and includes education about aCPAP to increase treatment adherence. Through centralized, telemedicine-based service provided by SleepCharge by Nox Health, formerly FusionHealth, (Johns Creek, Ga), adherence to aCPAP is monitored wirelessly in near real-time, and supported remotely throughout the treatment period. Nox T3 sleep apnea test results are provided to subjects at the conclusion of their participation in Sleep SMART.
Figure.
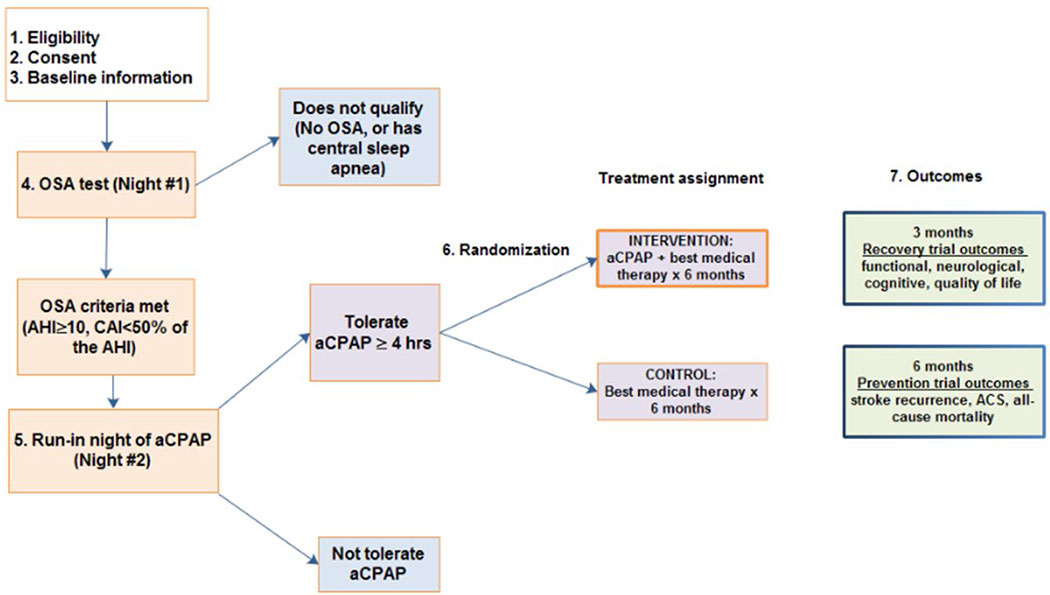
Sleep SMART study flow.
Patient population
Sleep SMART inclusion criteria are purposefully broad to enhance generalizability: adults (age ≥18 years) and consent obtained within 14 days of AIS or high risk TIA (ABCD2 score ≥4). AIS is defined as an episode of neurological dysfunction caused by focal cerebral ischemia. The definition includes: (1) symptoms that last ≥24 hours with evidence of associated cerebral infarction, (2) symptoms, thought to be from focal cerebral ischemia, that last ≥24 hours without evidence of infarction (e.g., no brain MRI performed), (3) transient symptoms lasting <24 hours with associated causative infarction on brain imaging, and (4) symptoms that last <24 hours thought to be due to cerebral ischemia, aborted by thrombolytic or endovascular treatment. The exclusion criteria are provided in Table 1. All randomized subjects contribute to the prevention analysis. However, only subjects enrolled within 7 days of AIS and with a National Institutes of Health Stroke Scale (NIHSS) score ≥1 contribute to the recovery analysis.
Table 1:
Sleep SMART enrollment criteria.
| Inclusion criteria |
| 1. Age ≥18 years |
| 2. Consent obtained within 14 days of AIS OR high risk TIA (ABCD2 score ≥4) |
| Exclusion criteria |
| 1. Pre-event inability to perform all of own basic activities of daily living (ADLs) |
| 2. Unable to obtain informed consent from subject or legally authorized representative |
| 3. Incarcerated |
| 4. Known pregnancy |
| 5. Current mechanical ventilation (can enroll later if this resolves) or tracheostomy |
| 6. Current use of positive airway pressure, or use within one month prior to stroke |
| 7. Anatomical or dermatologic anomaly that makes use of CPAP interface unfeasible |
| 8. Severe bullous lung disease |
| 9. History of prior spontaneous pneumothorax or current pneumothorax |
| 10. Hypotension requiring current treatment with pressors (can enroll later if this resolves) |
| 11. Other specific medical circumstances that conceivably, in the opinion of the site PI, could render the patient at risk of harm from use of CPAP |
| 12. Massive epistaxis or previous history of massive epistaxis |
| 13. Cranial surgery or head trauma within the past 6 months, with known or possible cerebrospinal fluid (CSF) leak or pneumocephalus |
| 14. Recent hemicraniectomy or suboccipital craniectomy (i.e. those whose bone has not yet been replaced), or any other recent bone removal procedure for relief of intracranial pressure |
| 15. Current receipt of oxygen supplementation >4 liters per minute |
| 16. Current contact, droplet, respiratory/airborne precautions |
Screening and randomization
The Nox T3™ sleep apnea test (Nox Medical, Reykjavίk, Iceland), a validated(10,11) ambulatory device that records nasal pressure, oxygen saturation, pulse, respiratory effort, snoring, position, and ECG, is used on the first available night after consent to screen for OSA. Nox T3™ data are reviewed by a sleep technologist; scoring is facilitated by the Nox software, Noxturnal. The Nox T3™ generates a respiratory event index (REIT3, number per hour of recording). Standard definitions of apneas and hyponeas are used.(12) Subjects who have OSA, defined by an REIT3 ≥10 events/hour and without significant central sleep apnea (central apnea index [CAI) ≥50% of the total REIT3), proceed to the aCPAP run-in night. Subjects who use aCPAP for ≥4 hours during the run-in night without treatment-emergent central sleep apnea (i.e., CAI remains <10 events/hour on aCPAP machine-generated estimates), and are willing to continue with the trial are eligible for randomization. Randomization (1:1) into the aCPAP plus usual care versus usual care only groups is performed centrally via a web-based system, using a minimal sufficient balance approach that prevents serious imbalances in the following important baseline covariates: site, age (continuous), NIHSS score (categorical), and use of intravenous thrombolysis or attempted endovascular therapy (dichotomous).
Intervention
aCPAP plus usual care
aCPAP is generally used, though other modalities such as bilevel positive airway pressure (PAP) or other PAP variants may be necessary at times for individual subjects. The aCPAP is delivered using the ResMed AirSense 10 AutoSet, successor versions of this model, or an equivalent device. Pressures can range from 4–20 cm H20 but can also be set to a narrower range if warranted once data on a subject’s use and response are available.
Following hospital discharge, CPAP care management is assumed by Nox Health. aCPAP adherence is monitored and maximized centrally through state-of-the-art, technology-enabled care management. The aCPAP devices used in Sleep SMART are equipped with modem-based adherence-tracking systems that monitor CPAP use, mask leak, and AutoSet machine-estimated residual REIAS. Device data are passively uploaded via cellular signals across the US on a daily basis and the SleepCharge workflow platform automatically identifies issues with a subject’s aCPAP hours of use or its effectiveness (e.g., residual REIAS, mask leak). By systematically flagging potential problems using standard-of-care algorithms, issues are immediately escalated for a care manager review and resolution at SleepCharge. Care managers provide behavioral solutions for subjects, caregivers, or bed partners, and utilize additional algorithms to escalate medical, PAP, and technical issues to the appropriate technology, respiratory therapy, or board-certified state licensed sleep medicine physician team members. New masks, alternative masks, and other supplies are mailed when needed directly to the subjects. Subjects receive contact from SleepCharge at a frequency determined by medical, behavioral, or equipment-based needs to optimize adherence. Subjects may also contact SleepCharge at any time.
aCPAP is begun with the settings noted in Table 2. However, SleepCharge may adjust the factors identified in Table 2 remotely, or may change treatments from aCPAP to bilevel PAP as clinically indicated. If aCPAP success remains insufficient, SleepCharge may ask the site’s research team to facilitate referral to a local sleep medicine expert for assistance in PAP care. At the end of subjects’ participation in Sleep SMART, they are offered referral to a sleep medicine provider, given a copy of their sleep apnea test results, and allowed to keep their aCPAP equipment.
Table 2.
Initial aCPAP settings, and capability for remote adjustment by SleepCharge.
| Initial default settings used in Sleep SMART | SleepCharge can adjust remotely | |
|---|---|---|
| Pressure | 5-20 cm water | × |
| Mode | Autoset | × |
| Expiratory pressure relief (EPR™) | On | × |
| EPR type | Full time | × |
| EPR level | 3 | × |
| Ramp time | Auto | × |
| SmartStart™/SmartStop™ | On | − |
| Humidity | 4 | − |
| ClimateLine™ tubing temperature | Auto | − |
Controls: care-as-usual arm
Subjects assigned to the care-as-usual group have the same 3- and 6-month assessments as the intervention group. Although we expect few care-as-usual subjects to cross over to CPAP, cross-overs are documented as a protocol violation. All care-as-usual subjects are offered, at the time they exit the study, referral to a sleep medicine provider. They are given a copy of their sleep apnea test report.
Blinding
Subjects, the local clinical team, and some of the local research team are not masked to treatment assignment. However, the local outcome assessors and central event adjudicators (DZ, DAL) are masked to treatment group. Subjects, the local clinical team, and the research team are masked to the sleep apnea test report details until the subject has completed participation in Sleep SMART.
Outcomes
Prevention outcome
The primary outcome for the prevention aim is the composite at 6 months of new AIS, acute coronary syndrome (ACS), and death from any cause. The definition of stroke used for eligibility is also used for the outcome. ACS includes acute myocardial infarction and unstable angina defined by ECG, diagnostic biomarkers, and cardiac symptoms or signs.(13) Stroke and ACS outcomes are determined based on blinded adjudicator review.
Recovery outcome
The recovery outcomes are measured at both 3 (primary) and 6 months. The primary outcome for the recovery aim is functional outcome as assessed by the modified Rankin scale (mRS)-9 question (9Q). The mRS-9Q is simple and quick, with very good inter-observer reliability and reproducibility, in face-to-face settings and by telephone.(14)
The secondary outcomes include stroke severity (NIH stroke scale), quality of life (Stroke Specific Quality of Life Scale (SS-QOL)), and cognitive function (5-minute protocol of the Montreal Cognitive Assessment (MoCA).
Data Monitoring Body
Oversight of safety is performed by a National Institute of Neurological Disorders and Stroke-appointed StrokeNet Data and Safety Monitoring Board (DSMB). The DSMB has approved the protocol and monitors trial progress, including recruitment and retention, performance of individual sites, data quality and timeliness, as well as other issues with special attention paid to patient safety, protection of trial data confidentiality, and maintenance of data integrity.
Sample size estimates
Prevention outcome:
The sample size calculation for the prevention trial is based on assumptions specified in the supplemental materials. The total number of events required is 233. The required sample size to observe this number of events, including an additional inflation factor of 1.1 to account for 10% loss to follow up by 6 months, is 3,062. We plan to conduct a blinded sample size re-estimation at the time of the single interim analysis.
Recovery outcome:
The sample size calculation for the recovery aim is based on the assumptions specified in the supplemental materials. The sample size needed at analysis is 872, which is inflated by a factor of 1.56 to account for 15% treatment non-use and 5% loss to follow-up by 3 months, yielding 1,362 subjects needed to be randomized. We anticipate that the required sample sizes for the recovery and prevention aims will be fulfilled simultaneously.
Statistical analyses
The study is designed to include, after 40% of subjects have adjudicated prevention outcomes available, one interim assessment of the prevention outcome for both efficacy and futility, and one assessment of the recovery outcome only for overwhelming efficacy. If stopping rules are met for either outcome, the DSMB will discuss potential protocol modifications to allow completion of the other aim.
Prevention outcome:
A cox-proportional hazards model will be used to assess time to first occurrence of recurrent stroke, ACS, or mortality by 6 months. The pre-specified primary analysis will adjust for age and baseline stroke severity (NIH stroke scale), as both are important predictors of stroke recurrence and mortality. For missing data, data will be censored at the last assessment date, date of consent withdrawal, or 180 days after randomization.
Recovery outcome:
A regression model will be implemented to test for mean differences in 3-month mRS (shift in the mean of the mRS distribution between groups). The pre-specified primary analysis will adjust for age, baseline NIH stroke scale, and thrombolytic use or endovascular treatment. Missing data will be addressed with multiple imputation. For the secondary outcomes, mean scores will be compared between the two treatment arms and adjusted for the same prognostic variables as the primary analysis.
Study organization and funding
Sleep SMART is funded by the National Institute of Neurological Disorders and Stroke (NINDS, U01NS099043) and is implemented through StrokeNet, an NINDS-funded clinical trials network. The National Coordinating Center for StrokeNet is at the University of Cincinnati and the National Data Management Center is at the Medical University of South Carolina. StrokeNet has Regional Coordinating Centers across the United States that each has affiliated clinical trial sites.
Discussion
Sleep SMART implements innovative approaches to enhance feasibility that are supported by existing literature. For example, the gold standard of in-laboratory polysomnography is not used in Sleep SMART to identify OSA, as this approach is often poorly tolerated in the AIS setting, may be logistically challenging during hospitalization, and is not necessary to identify AIS patients with OSA.(15) Home sleep apnea tests effectively identify OSA after stroke;(16,17) moreover, these devices have been commonly used in post-stroke research. The Nox T3™ device used in Sleep SMART has been validated against full polysomnography(10,11) and has advantages over some other portable sleep apnea tests in that it has both abdominal and thoracic abdominal effort belts, and uses respiratory inductance plethysmograph (RIP) technology.(18) These features would be expected to be particularly useful to distinguish central from obstructive apneas. The implementation of an aCPAP run-in night allows trial efforts to focus on subjects most likely to be adherent. However, requirements for first-night CPAP tolerability reduces trial generalizability.
aCPAP, in comparison to in-laboratory CPAP titration during polysomnography, has similar CPAP acceptance and adherence, administered pressures, event index <10 on CPAP, and improved sleepiness scores.(19) Use of aCPAP avoids formal titration studies that are often poorly tolerated just after stroke,(15) and further allows for automatic adjustment of pressure throughout the 6 months of treatment. This may be useful given that REI – and therefore, possibly the pressure required to normalize it – often decreases in the first several months after stroke.(20)
A telemedicine approach for Sleep SMART was adopted to standardize assessment and treatment across 110 sites with highly variable inpatient access to sleep medicine services. Telemedicine OSA management is gaining acceptance. Telemedicine-based strategies for CPAP management may be as effective as face-to-face visits, more cost-effective, and more convenient for patients.(21)
Summary and conclusions
If Sleep SMART hypotheses are confirmed, assessment and treatment for OSA will be an important consideration for the large majority of AIS/TIA patients. Treatment of OSA for stroke prevention and recovery represents a promising intervention, with strong epidemiological associations between OSA and stroke outcomes, plausible biological mechanisms,(22) observational evidence that OSA treatment is associated with an array of better outcomes, and availability of a relatively inexpensive, low risk treatment -- CPAP -- for a highly prevalent post-stroke/TIA condition.
Sleep SMART represents the first late-phase, definitive trial to address whether CPAP improves stroke recovery or prevents recurrences. The design efficiently addresses both questions, with two trial questions incorporated into one overall design. If the trial demonstrates better outcomes for the intervention group, the results would transform post-stroke management. AIS and high risk TIA patients would then require screening and treatment for OSA. Although CPAP at present may not be well tolerated by many stroke patients, data from Sleep SMART could motivate efforts to identify more acceptable alternatives.
Supplementary Material
Acknowledgements
Funding
Sleep SMART is funded by the National Institutes of Health (U01NS099043).
Footnotes
Conflict of interest
Brown: none
Durkalski: none
Durmer: Dr. Durmer is Chief Medical Officer of FusionHealth & Nox Medical.
Broderick: none
Zahuranec: none
Levine: none
Anderson: none
Bravata: none
Yaggi: none
Morgenstern: none
Moy: none
Chervin: Editor/author, UpToDate; member, board of directors, International Pediatric Sleep Association and the non-profit Sweet Dreamzzz, Inc.
Reference List
- (1).Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- (2).Brown DL, Shafie-Khorassani F, Kim S, et al. Sleep-disordered breathing is associated with recurrent ischemic stroke. Stroke 2019;50:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Turkington PM, Allgar V, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax 2004;59:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Martinez-Garcia MA, Galiano-Blancart R, Roman-Sanchez P, Soler-Cataluna JJ, Cabero-Salt L, Salcedo-Maiques E. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest 2005;128:2123–9. [DOI] [PubMed] [Google Scholar]
- (5).Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous Positive Airway Pressure Treatment Reduces Mortality in Patients with Ischemic Stroke and Obstructive Sleep Apnea: A 5-Year Follow-up Study. Am J Respir Crit Care Med 2009. July 1;180:36–41. [DOI] [PubMed] [Google Scholar]
- (6).Bravata DM, Sico J, Vaz Fragoso CA, et al. Diagnosing and Treating Sleep Apnea in Patients With Acute Cerebrovascular Disease. J Am Heart Assoc 2018;7(16):e008841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Brill AK, Horvath T, Seiler A, et al. CPAP as treatment of sleep apnea after stroke: A meta-analysis of randomized trials. Neurology 2019;90:e1222–e1230. [DOI] [PubMed] [Google Scholar]
- (8).Brown DL, Anderson CS, Chervin RD, et al. Ethical Issues in the Conduct of Clinical Trials in Obstructive Sleep Apnea. J Clin Sleep Med 2012;7:103–8. [PMC free article] [PubMed] [Google Scholar]
- (9).Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2014; 45:2160–236. [DOI] [PubMed] [Google Scholar]
- (10).Xu L, Han F, Keenan BT, et al. Validation of the Nox-T3 Portable Monitor for Diagnosis of Obstructive Sleep Apnea in Chinese Adults. J Clin Sleep Med 2017;13:675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Cairns A, Wickwire E, Schaefer E, Nyanjom D. A pilot validation study for the NOX T3(TM) portable monitor for the detection of OSA. Sleep Breath 2017;18:609–14. [DOI] [PubMed] [Google Scholar]
- (12).Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events RULES, TERMINOLOGY AND TECHNICAL SPECIFICATIONS, v2.4. Darien, IL:American Academy of Sleep Medicine 2017. [Google Scholar]
- (13).Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- (14).Patel N, Rao VA, Heilman-Espinoza ER, Lai R, Quesada RA, Flint AC. Simple and reliable determination of the modified rankin scale score in neurosurgical and neurological patients: the mRS-9Q. Neurosurgery 2012;71:971–5. [DOI] [PubMed] [Google Scholar]
- (15).Brown DL, Chervin RD, Kalbfleisch JD, et al. Sleep Apnea Treatment After Stroke (SATS) Trial: Is It Feasible? J Stroke Cerebrovasc Dis 2013;22:1216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Chernyshev OY, McCarty DE, Moul DE, et al. A pilot study: portable out-of-center sleep testing as an early sleep apnea screening tool in acute ischemic stroke. Nat Sci Sleep 2015;7:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Saletu MT, Kotzian ST, Schwarzinger A, Haider S, Spatt J, Saletu B. Home Sleep Apnea Testing is a Feasible and Accurate Method to Diagnose Obstructive Sleep Apnea in Stroke Patients During In-Hospital Rehabilitation. J Clin Sleep Med 2018;14:1495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13:479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rosen CL, Auckley D, Benca R, et al. A Multisite Randomized Trial of Portable Sleep Studies and Positive Airway Pressure Autotitration Versus Laboratory-Based Polysomnography for the Diagnosis and Treatment of Obstructive Sleep Apnea: The HomePAP Study. Sleep 2012;35:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Parra O, Abroix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med 2000;161:375–80. [DOI] [PubMed] [Google Scholar]
- (21).Isetta V, Negrín MA, Monasterio C, et al. A Bayesian cost-effectiveness analysis of a telemedicine-based strategy for the management of sleep apnoea: a multicentre randomised controlled trial. Thorax 2015;70:1054–61. [DOI] [PubMed] [Google Scholar]
- (22).McDermott M, Brown DL, Chervin RD. Sleep disorders and the risk of stroke. Expert Rev Neurother 2018;18:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


